Abstract
Background and Aims:
In addition to gastric sensorimotor dysfunctions, functional dyspepsia (FD) is also variably associated with duodenal microinflammation and epithelial barrier dysfunction, the pathogenesis and clinical significance of which are unknown. Our hypothesis was that miRNAs and/or inflammation degrade epithelial barrier proteins resulting in increased duodenal mucosal permeability in FD.
Methods:
We compared the duodenal mucosal gene expression and miRNAs, in vivo permeability (lactulose-mannitol excretion between 0-60 and 60-120 minutes after saccharide ingestion), ex vivo assessments (transmucosal resistance [TMR], FITC-dextran flux, and basal ion transport), and duodenal histology (light and electron microscopy) in 40 patients with FD and 24 controls.
Results:
Compared to controls, the mRNA expression of several barrier proteins (zona occludin-1, occludin, claudin-12, E-cadherin) was modestly reduced (ie, a fold change of 0.8-0.85) in FD with increased expression of several miRNAs (eg, miR-142-3p and miR-144-3-p), which suppress these genes. The urinary lactulose excretion and the lactulose:mannitol ratio between 60-120 minutes were greater in FD than in controls (P<0.05). The FITC-dextran flux, which reflects paracellular permeability, was inversely correlated (r=−0.32, P=0.03) with TMR and directly correlated (r = 0.4, P = 0.02) with lactulose:mannitol ratio. Other parameters (mucosal eosinophils, intraepithelial lymphocytes, and mast cells, TMR, FITC-dextran flux, average intercellular distance and proportion of dilated junctions) were not significantly different between groups.
Conclusions:
In FD, there is a modest reduction in the expression of several duodenal epithelial barrier proteins, which may be secondary to upregulation of regulatory miRNAs, and increased small intestinal permeability measured in vivo.
Keywords: intestinal barrier, duodenal epithelial permeability, inflammation, tight junction gene expression
Background
Functional dyspepsia (FD) is predominantly attributed to abnormal gastric emptying (GE), impaired gastric accommodation, and increased gastric sensation1. More recently, based on subtle duodenal inflammation, abnormal duodenal acid clearance and sensitivity, and reduced expression of duodenal epithelial barrier proteins, attention has shifted to the duodenum in FD2-9. In FD, the mRNA and protein levels for several duodenal epithelial barrier proteins were reduced while ex vivo epithelial permeability was increased3. For some barrier proteins, the decreased expression was correlated with duodenal mucosal mast cell counts and ex vivo epithelial permeability. Conceivably, low-grade inflammation may damage the duodenal epithelial barrier, thereby predisposing to symptoms and increased permeability in FD7, 8, 10.
9There are 4 questions about the concept that FD is a leaky gut disorder. First, since no study has evaluated in vivo intestinal permeability in FD. Second, there is variability among these studies. Duodenal microinflammation was observed in some2 but not in all studies5, 7 The duodenal mucosal expression of epithelial barrier proteins was reduced in some3 but preserved in other studies7. Third, “the correlation of duodenal pathology with symptom pattern and severity is highly variable”9. Four, the pathogenesis of an impaired duodenal epithelial barrier is unknown. microRNAs (miRNAs) are postulated to increase epithelial permeability in irritable bowel syndrome (IBS)11. miRNAs are noncoding RNAs that bind to the 3′-untranslated region of mRNAs, block translation, and degrade mRNAs; they thereby modulate gene expression, including genes at all 3 levels of the epithelial barrier (ie, tight junction proteins, adherens junction proteins, and desmosome proteins)12. The contribution of miRNAs to increased epithelial permeability in FD is unknown.
Our overarching hypothesis was that miRNAs and/or microscopic inflammation degrade epithelial barrier proteins resulting in duodenal barrier dysfunction in FD.
Methods
Design
Between June 2014 and November 2017, 40 patients with FD and 24 healthy controls who did not have any GI disorder were recruited from the clinical practice and by public advertisement. The exclusion criteria are detailed elsewhere (Supplementary Methods)13. The study was approved by the Mayo Clinic Institutional Review Board. Gastric emptying, small intestinal permeability and upper GI endoscopy were performed, in this order. Some findings have been published 6, 13,14. However, the findings of mRNA and miRNA expression of genes that regulate epithelial permeability, electron microscopy, and saccharide permeability test have not been published.
Symptoms
Symptoms were evaluated with the Nepean Dyspepsia Index questionnaire14, 15, the Patient Assessment of Upper Gastrointestinal Disorders-Symptom Severity Index16, and the Hospital Anxiety and Depression Scale (HADS) 17.
Urine Saccharide Test
Consumption of sweeteners such as sucralose, aspartame, lactulose, or mannitol was prohibited for 2 days before this test. After an overnight fast, participants ingested 1,000 mg of lactulose and 200 mg of mannitol (Sigma-Aldrich) in 500 mL water. Consistent with the focus on small intestinal permeability, urinary excretion of sugars from 0 to 2 hours was measured with high-performance liquid chromatography-mass spectrometry18.
Gastric Emptying and Enteral Lipid Infusion
GE of solids and liquids (296 kcal; 32% protein, 35% fat, and 33% carbohydrate) and small- bowel transit were simultaneously assessed with scintigraphy13.
Two hours after completing the lactulose-mannitol test, a 8F vinyl nasoduodenal feeding tube was placed under fluoroscopic guidance in the second part of the duodenum. The lipid infusion (Microlipid; Covidien AG) (66.7 mL diluted to 222 mL, 300 kcal) was administered over 2 hours at a rate which mimics the systemic delivery of calories after glucose ingestion4. At 15-minute intervals, 6 symptoms—nausea, fullness, bloating, abdominal pain, belching, and burning— were assessed on a Likert scale marked absent (0), light (1), moderate (2), severe (3), or intolerable (4)4.
Upper Gastrointestinal Endoscopy
Endoscopic duodenal (second part) mucosal biopsies were evaluated by pathologists who were blinded to the diagnosis after staining with hematoxylin-eosin and mast cells with anti-CD117 (KIT) antibodies (Rabbit Polyclonal, A4502; Dako) (Supplementary Methods).
Ex Vivo Small Intestinal Permeability
Within 30 minutes of collection, 4 biopsies were mounted on Ussing chambers (Physiological Instruments, San Diego, CA). The mean baseline TMR and Isc were measured in 1-3 biopsies from each participant13. Paracellular flux was studied in one biopsy from each subject using fluorescein isothiocyanate [FITC]-dextran (12.5mg/ml; 4 kDa, Sigma, MO) in the mucosal compartment. FITC-dextran flux was calculated from the slope of a linear fit to time versus concentration curve and reported as mg hr−1cm−2 (Supplementary Methods).
Electron Microscopy
Using established techniques, this assessment were performed in 37 FD patients and 21 healthy controls19 (Supplementary Methods).
Gene Expression
As detailed elsewhere, the duodenal mucosal gene expression was evaluated after extracting RNA from freshly frozen duodenal tissue13. Genes with a raw read count of at least 25 were included in the analysis to decrease the noise from genes with low expression.20 Differential gene expression analysis was performed using the Bioconductor edgeR21 package using the Benjamini-Hochberg procedure.22 Genes with false discovery rate (FDR) < 0.05 were considered to be significantly differentially expressed. This report is limited to genes related to the tight junction, adherens junction and desmosomes based on the KEGG database. The overlap between the genes in these pathways and our dataset was evaluated with a hypergeometric test, correcting for multiple comparisons.
miRNA Sequencing
miRNA data were analyzed with the CAP-miRSeq workflow23, which uses Cutadapt to trim adapter regions, then aligns the data to reference data using MiRDeep224 to detect novel and known miRNAs. The miRNA differential expression analysis was performed using Bioconductor edgeR21. Changes that were significant (P<.05) and exceeded a threshold (−1 > log2FoldChange >1) were considered differentially expressed in FD vs controls. An online miRNA database (miRDB, Washington University School of Medicine)25, 26 revealed miRNAs that regulate epithelial barrier genes.
Statistical Analyses
The Wilcoxon signed rank test assessed for differences between controls and FD patients. Spearman correlation coefficients analyzed correlations between variables. Linear regression models were used to test whether in vivo permeability variables were different between controls and FD after adjusting for anxiety and depression scores. P<.05 was considered statistically significant. Statistical analyses were performed with GraphPad Prism 7 (GraphPad Software) and JMP Pro 13 (SAS Institute Inc).
Results
Clinical features, gastrointestinal endoscopy, and gastric emptying
Demographic features were not significantly different between groups (Table 1). Patients had typical GI symptoms of FD (Table 1); six took acid-suppressive drugs. Anxiety and, to a lesser extent depression, were more prevalent in FD than controls. In 39 patients and 21 controls, upper endoscopy was normal (27 vs 12) or disclosed cystic fundic polyps (5 vs 1), antral erythema or minor erosions (6 vs 5), and mild esophagitis (1 vs 3). One control, but no FD patients had immunoglobulin A, but not immunoglobulin G, anti–tissue transglutaminase antibodies above normal (>4 U/mL); histology did not reveal celiac disease. The GE thalf for solids and liquids was longer in FD than in controls (P≤.02) (Table 1).
Table 1.
Demographic and Baseline Clinical Characteristics
| Group |
|||
|---|---|---|---|
| Characteristic | Control (n=24) | FD (n=40) | P Value |
| Age, y | 40 (13) | 42 (13) | .7 |
| Body mass index, kg/m2 | 26.1 (4.7) | 26.7 (6.6) | .9 |
| Women | 14 (58) | 31 (78) | .2 |
| Mean (SD) NDI dyspepsia symptom severity score a | 12.95 (12.5-13.0) | 10.8 (10.03-11.41) | <.001 |
| Mean (SD) NDI QOL score b | 100 (100-100) | 41.3 (18.6-62.1) | <.001 |
| PAGI-SYM subscores, Median (IQR) c | |||
| Heartburn | 0 (0-0.14) | 1.86 (1.14-2.55) | <.001 |
| Nausea, vomiting, and regurgitation | 0 (0-0) | 1 (0.33-2.33) | <.001 |
| Early satiety | 0 (0-0.25) | 3.1 (2.5-4.0) | <.001 |
| Bloating | 0 (0-0.13) | 3.5 (2.5-4.63) | <.001 |
| Upper abdominal pain | 0 (0-0) | 3.5 (2.38-4.0) | <.001 |
| Anxiety, n d | |||
| Borderline | 0 | 7 | .04 |
| Definite | 0 | 11 | .005 |
| Depression, n | |||
| Borderline | 0 | 8 | .02 |
| Definite | 0 | 3 | .3 |
| Mean (SD) GE thalf for solids, min | 103.8 (32.5) | 122.6 (35.0) | .02 |
| Mean (SD) GE thalf for liquids, min | 16 (5) | 22 (10) | .004 |
| Colonic filling at 6 hours, % | 56 (21) | 40(18) | .004 |
GE, gastric emptying; NDI, Nepean Dyspepsia Index; FD, nonulcer dyspepsia; PAGI-SYM, Patient Assessment of Upper Gastrointestinal Disorders-Symptom Severity; QOL, quality of life.
Least and most severe symptoms are 13 and 0
Best and worst QOL are 100 and 0
Least and most severe symptoms are respectively 0 and 5
Borderline and definite anxiety (or depression) are 8-10 and 11-21
Urinary saccharides and enteral infusion
Because the GE thalf of liquids was 22 (10) minutes in FD, we reasoned that the small intestinal exposure to sugars may be greater during the second than the first hour after ingestion and analyzed these periods separately. Between 60-120 minutes, lactulose excretion was greater (P=.02), mannitol excretion was not significantly different, and the lactulose:mannitol ratio was greater in FD than in controls (P=.008) (Figure 1). Subject status predicted (P<0.01) lactulose excretion between 60-120 minutes after adjusting for anxiety and depression scores. The intestinal permeability variables were not associated with postprandial distress syndrome or epigastric distress syndrome (data not shown).
Figure 1.
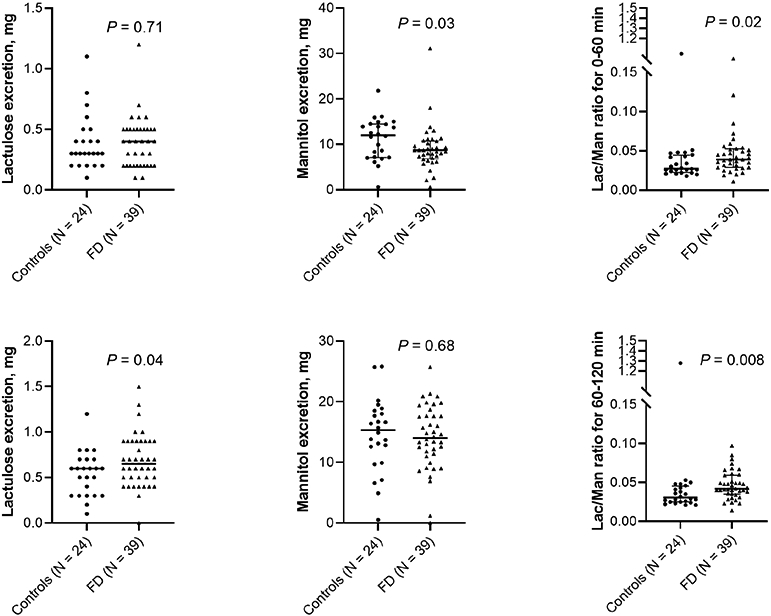
In Vivo Permeability. Lactulose and mannitol excretion between 0-60 minutes (upper panel) and 60-120 minutes (lower panel) in controls and functional dyspepsia (FD).
A feeding tube could not be placed in 3 controls and 5 patients. The infusion was terminated prematurely because of symptoms in 1 control and 8 patients. During enteral infusion, 3 of 21 controls (14%) and 22 of 35 patients (63%) reported at least 1 symptom of “severe” or “intolerable” intensity (P=.001).
Ex vivo permeability
Compared to controls, the basal short-circuit current (Isc) was greater (P<0.0001) but the TMR and FITC-dextran flux were not different in FD (Figure 2). The FITC-dextran flux, which reflects paracellular permeability, was inversely correlated (r=−0.32, P=0.03) with TMR and directly correlated with the lactulose:mannitol ratio at 60 (r=0.33, P=.02) and 120 minutes (r=0.38, P<.01) in all participants.
Figure 2.
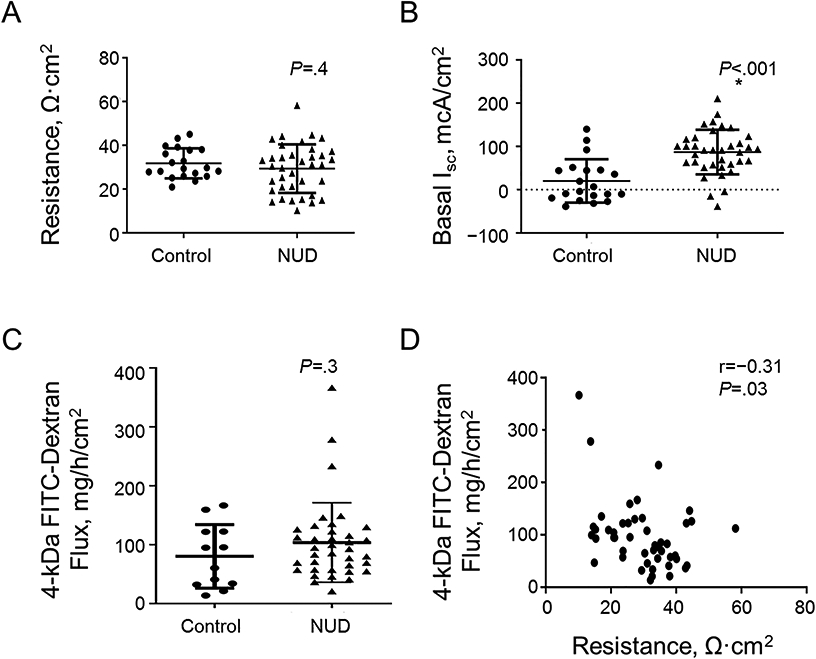
Ex-vivo assessments. Comparison of transmucosal resistance (TMR) (A), basal Isc (B), and rate of FITC–dextran flux measured over 3 hours (C) between controls and FD. Correlation between baseline duodenal transmucosal resistance (TMR) and FITC-dextran flux (D).
Expression of duodenal genes
The mapping percentages, total reads, gene body distribution, and other quality metrics were satisfactory in all 21 controls and 39 FD patients. Excluding non protein-coding RNAs, there were 2695 differentially expressed genes at a FDR of < 0.05 (Supplementary Table 1). Of these 2695 genes, the expression of 24 genes was reduced and 1 gene was increased in FD versus controls with a ∣log2(Fold Change)∣ ≥1.5.
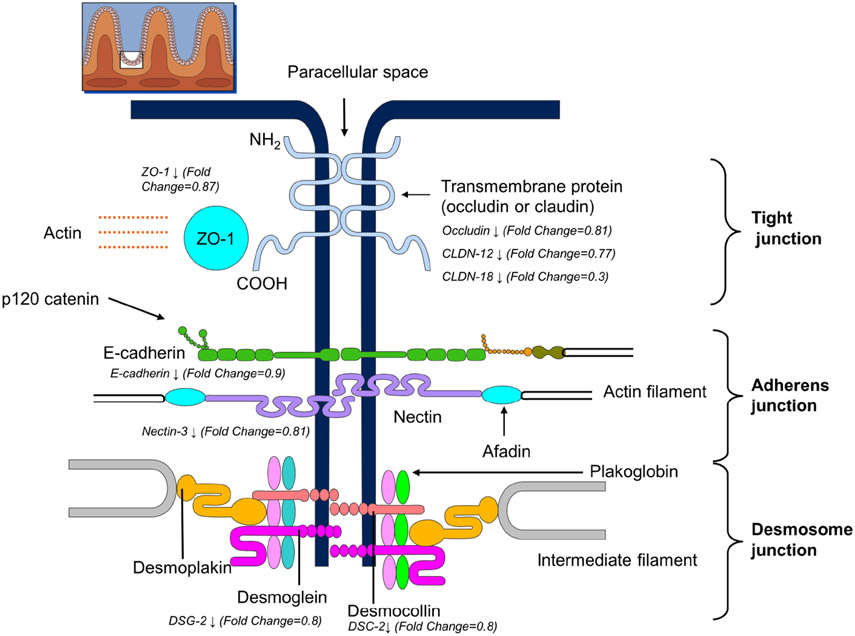
There was significant overlap between genes downregulated in FD and KEGG gene lists for tight junction proteins (hypergeometric test P=.003), adherens junction proteins (P=0.0003), and desmosomal proteins (P=0.006) (Supplementary Table 2). The expression of tight junction proteins, including zona occludens 1 (TJP1), occludin (OCLN), selected claudins (CLDN12 and CLDN18) was lower in FD than controls (Figure 3). At the adherens junction, the expression of E-cadherin and p120 catenin, which binds E-cadherin and prevents its endocytosis, was decreased in FD. Among desmosomal proteins, the expression of desmoglein 2, desmocollin 2, plakophilin-2, and plakoglobin was lower in FD than controls. However, other key barrier proteins were not differentially expressed (Supplementary table 1).
Figure 3.
Reduced Duodenal Mucosal Expression of Barrier Proteins in Functional Dyspepsia. (Used with permission, Mayo Foundation for Medical Education and Research.) All differentially expressed proteins are significant at a false discovery rate less than 0.05.
Comparison of findings
Among all participants, the mean symptom score during lipid infusion was correlated (1) inversely with the expression of OCLN (r=−0.33; P=.02), (2) with lactulose:mannitol ratio at 60 (r= 0.27; P<.05) and 120 minutes (r= 0.32; P=.02), and (3) with the Isc (r= 0.46; P=.0006) but not with FITC-dextran flux or with TMR (data not shown). The urinary mannitol excretion from 0-60 minutes was inversely correlated with the total PAGI-SYM score (r= 0.33; P=.007), and with the PAGI-SYM subscores (data not shown). After adjusting for multiple comparisons, these correlations were not significant. Other correlations between intestinal permeability variables and symptoms were not significant.
miRNAs
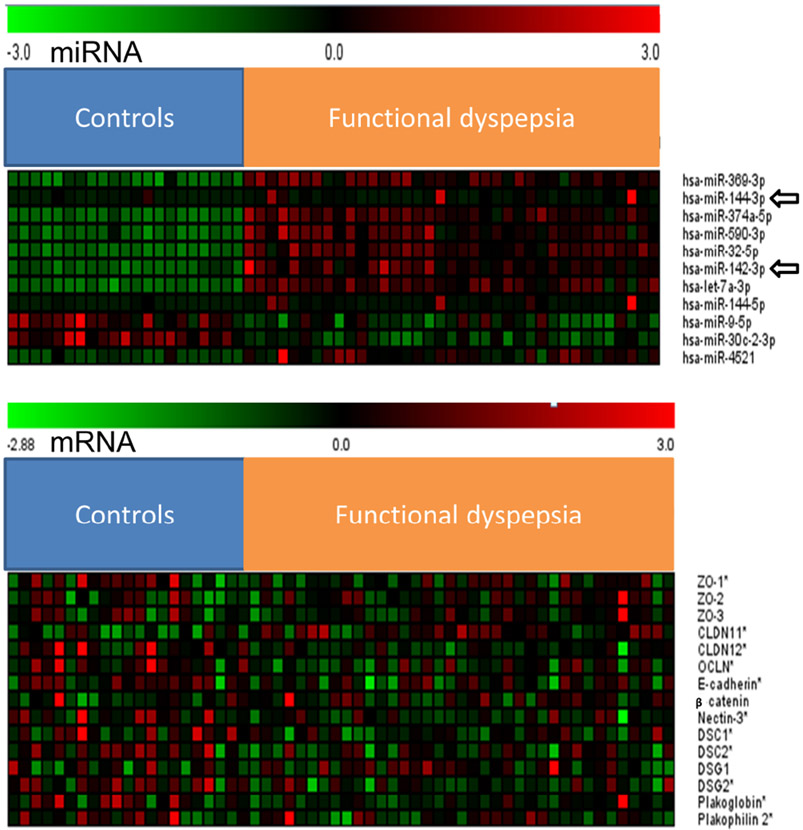
Compared with controls, 15 miRNAs were upregulated and 10 were downregulated in FD. For these 25 miRNAs, computational assessments suggested that 11 miRNAs (e.g., hsa-miR-369-3p, hsa-miR-144-3p, hsa-miR-590-3p) regulate epithelial barrier genes that were differentially expressed between FD and controls in our cohort (Figure 4, Table 2).
Figure 4.
Heat maps showing differentially expressed miRNAs (upper panel) and mRNAs (lower panel) between controls and FD.
Table 2.
Differentially Expressed Barrier Genes and miRNAs Implicated as Regulators of These Genes
| Expression, CPMa |
|||||||
|---|---|---|---|---|---|---|---|
| Gene | Control | FD | Fold Change |
FDR | Regulatory miRNAs |
Fold Change |
P Value |
| ZO-1 | 82.7, 157.9 | 95.4, 135.8 | 0.87 | 0.02 | hsa-miR-369-3p | 1.085 | 7.88e-25 |
| hsa-miR-144-3p | 1.163 | .002 | |||||
| hsa-miR-374a-5p | 1.36 | 3.18e-60 | |||||
| OCLN | 75.1, 146.3 | 75, 122.7 | 0.81 | 0.003 | hsa-miR-590-3p | 1.366 | 4.19e-26 |
| CLDN-12 | 38.9, 71.9 | 37.8, 50.8 | 0.77 | 0.00003 | hsa-miR-142-3p | 2 | 2.33e-22 |
| hsa-let-7a-3p | 1.005 | 7.89e-33 | |||||
| hsa-miR-369-3p | 1.085 | 7.88e-25 | |||||
| hsa-miR-144-5p | 1.91 | .0000003 | |||||
| E-cadherin | 513.9, 781.3 | 541.8, 765 | 0.9 | 0.04 | hsa-miR-9-5p | 0.5 | 1.26e-19 |
| β-catenin | 403.1, 600.1 | 456.4, 616.6 | 0.93 | 0.3 | hsa-let-7a-3p | 1.005 | 7.89e-33 |
| p120 catenin | 417.7, 597.7 | 464.4, 609.8 | 0.93 | 0.009 | hsa-miR-30c-2-3p | 0.45 | 6.26e-41 |
| DSG-2 | 300.4, 495 | 283.9, 461.4 | 0.8 | 0.0004 | hsa-miR-374a-5p | 1.357 | 3.18e-60 |
| hsa-let-7a-3p | 1.005 | 7.89e-33 | |||||
| hsa-miR-590-3p | 1.366 | 4.19e-26 | |||||
| DSC-2 | 245, 520.9 | 237.2, 459.8 | 0.8 | 0.006 | hsa-miR-32-5p | 1.13 | 4.13e-44 |
| hsa-miR-4521 | 1.16 | 8.93e-09 | |||||
CPM, counts per million; FDR, false discovery rate; miRNAs, microRNAs; FD, functional dyspepsia.
5th percentile, 95th percentile.
Mucosal histology
The eosinophil, intraepithelial lymphocyte and mast cell counts were not different between controls and FD (Supplementary Figure 1). Two of 6 controls (33%) and 7 of 36 (19%) of patients had more than 30 duodenal eosinophils/hpf. The mucosal gene expression of c-kit, eosinophil major basic protein, and lymphocyte markers (CD2, CD3, CD4, CD8, and CD19) was not different between FD vs controls (Supplementary Table 1). The mucosal eosinophil and lymphocyte counts were not significantly correlated with expression of epithelial barrier proteins, Ussing chamber findings, lactulose-mannitol excretion, or symptoms during enteral infusion (data not shown).
Electron microscopy
The average intercellular distance (FD, 26.7[6.1]nm, Controls, 25.7[7.4]nm, P=0.3) and proportion of dilated junctions (FD, 45.7[19.0] %, Controls, 40.0[21.8] %, P=0.3) were not different between FD vs controls. However, the proportion of junctions with perijunctional condensation was lower (P=0.002) in FD than in controls. The FITC-flux was correlated with the average intercellular distance (r = 0.4, P=0.005) and the proportion of dilated junctions (r = 0.4, P=0.002). The expression of TJP2 and TJP3 was negatively correlated with the average intercellular distance (TJP 2, r=−0.3, P=0.03, TJP3, r=−0.4, P=0.007) and the proportion of dilated junctions (TJP2, r = −0.3, P=0.03, TJP3, r=−0.3, P=0.01). TJP2 was also correlated with proportion of junctions with perijunctional condensation (r=−0.3, P=0.03).
Discussion
The duodenal mucosal expression of several epithelial barrier protein genes was reduced in FD. This may be partly explained by upregulation of regulatory miRNAs but not by mucosal inflammation. The excretion of lactulose and the lactulose:mannitol ratio were greater in FD than in controls, which suggests increased small intestinal permeability. Some correlations between in vivo and ex vivo parameters of permeability and sensation were significant before but not after adjusting for multiple comparisons.
Compared to controls, the expression of epithelial barrier proteins was modestly reduced in FD. Of the differentially expressed proteins, claudin-12, which is a member of the barrier forming group of claudins, OCLN, which regulates leak pathways at bicellular epithelial tight junctions, and ZO-1, which is a cytosolic scaffolding protein, and CDH1 ie, E-cadherin, are known to regulate permeability27. Loss of the desmosomal component DSG2, which regulates barrier properties, has been implicated in Crohn’s disease28. By contrast, other proteins that regulate the pore paracellular pathways (ie, CLDNs 1-4), and which together with transcellular pathways transport smaller molecules including ions, were not differentially expressed, which may explain why the TMR was not different in FD. As detailed previously, the resting duodenal ISC was greater in NUD than controls13, which suggests that an alteration of the cellular transporters and channels that contribute to ion fluxes.
While oral lactulose appeared in the cecum 30 minutes after ingestion, cecal radioactivity, indicating small intestinal transit, increased substantially between 60 and 120 minutes29. These findings probably explain why lactulose excretion was only increased between 60-120 minutes in FD. While the lactulose:mannitol ratio for 0-60 minutes was also increased in FD, this is driven by lower mannitol excretion, hence not explained by increased intestinal permeability30. Mannitol is typically used to correct for absorptive surface area. Here, small intestinal transit was slower in FD than in controls. Hence, numerically lower mannitol excretion in FD cannot be explained by mucosal inflammation, villous atrophy, or faster small intestinal transit.
Similar to this study, the jejunal31 and colonic32 expression of ZO-1 and the colonic expression of E-cadherin33 were reduced in diarrhea-predominant IBS (IBS-D). The decreased expression of barrier proteins in FD may be secondary to increased expression of miRNAs, which downregulate glutamine synthetase (ie, miRNA 29) and disorganize tight junction proteins (miR-125b and miR-16), and have been implicated to increase permeability in IBS-D34. In this study, of the 25 differentially expressed miRNAs, computational prediction suggests that 9 miRNAs, which were all upregulated, collectively modulate the expression of approximately 11,439 mRNAs. Four of the differentially expressed miRNAs in FD (miR-141-3p, miR-142-3p, miR-144-3-p, and miR-1291) have been associated with colitis in humans and/or mice models 12, 35. 36MiR-144, which was upregulated in our FD patients, markedly inhibited the expression of ZO-1 and OCLN in a rat model of IBS-D36. MiRs have also been linked to eosinophil and mast cell-mediated conditions. In a mouse model of allergic rhinitis, treatment with the miR let-7a increased nasal mucosal IL-5 production and eosinophilia 37; let-7a 3p was upregulated in this study. Mast cell-derived miR-223 destroyed intestinal barrier function by inhibiting CLDN8 expression in cultured intestinal epithelial cells38. Although widely used39, these predictions must be confirmed by in vitro studies that evaluate the effects of manipulating miRNAs on mRNA and protein levels. While inflammation, may also alter the duodenal epithelial barrier3, 40, the degree of inflammation was not correlated with ex vivo epithelial permeability3. In this study, markers for duodenal mucosal inflammation were not different between FD and controls. While a meta-analysis observed more duodenal mucosal eosinophils and mast cells in FD patients than controls, there was significant heterogeneity among studies and, for eosinophils, also evidence of publication bias5.
What are the clinical implications of these findings? The correlation between symptoms during enteral lipid infusion and the lactulose:mannitol ratio suggests that increased permeability, which can be identified by a simple 2hr test, may contribute to post-prandial symptoms in FD. Dietary glutamine supplementation restored the intestinal barrier and improved bowel habits in IBS-D41. A similar trial should be considered in FD. Similar to IBS42, the miRs may serve as biomarkers. In addition, they may offer drug targets. For example, in a pre-clinical model of IBS, the injectable miR-29a inhibitor alleviated intestinal barrier impairment induced by trinitro-benzene-sulfonic acid (TNBS)43. Treatment with exosomal miR-34a/c-5p and miR-29b-3p improved intestinal barrier function by upregulating barrier genes in Caco-2 cell lines and in animal models of ischemia/reperfusion induced intestinal damage44.
This study has some limitations. Duodenal histology was only evaluated in 6 controls. Although decreased mRNA levels probably decrease protein expression, this should be confirmed with protein analysis. The miRNAs may also target other, currently unknown, pathways. Environmental factors (eg, food allergies) that may perturb the barrier were not assessed. The sample size was larger than earlier epithelial permeability studies in FD3, 7 but smaller than epigenetic studies in cancer or immune-related diseases34.
In summary, FD is associated with decreased expression of several duodenal epithelial tight junction proteins, increased expression of several miRNAs that degrade the mRNA for epithelial barrier proteins, and increased small intestinal permeability in vivo.
Supplementary Material
Background and context
Some studies suggest that FD is associated with ex vivo duodenal epithelial microinflammation and barrier impairment; the pathogenesis of these findings is unclear. miRNAs reduce expression of epithelial barrier genes and have been postulated to increase epithelial permeability in irritable bowel syndrome.
Findings
Compared to controls, there is reduced mRNA expression of several barrier proteins (zona occludin-1), increased expression of several miRNAs (eg, miR-142-3p) that suppress the genes for barrier proteins in FD, and increased urinary lactulose excretion, which suggests increased small intestinal permeability in vivo.
Implications for Patient Care
Some patients with FD have increased permeability, which can be identified by a simple 2hr urine test, and may contribute to symptoms. Clinical trials to evaluate the effects of agents that restore the intestinal barrier (eg, dietary glutamine supplementation) should be considered in FD.
Grant support:
This study was supported by Grant Number R01 DK68055 from the National Institutes of Health to A.E.B.
Abbreviations
- CLDN
claudin
- FDR
false discovery rate
- GI
gastrointestinal tract
- HADS
Hospital Anxiety and Depression Scale
- IBS
irritable bowel syndrome
- miRNA
microRNA
- FD
nonulcer dyspepsia
- OCLN
occludin
- ZO
zona occludin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None
References
- 1.Vanheel H, Carbone F, Valvekens L, et al. Pathophysiological Abnormalities in Functional Dyspepsia Subgroups According to the Rome III Criteria. American Journal of Gastroenterology 2017;112:132–140. [DOI] [PubMed] [Google Scholar]
- 2.Talley NJ, Walker MM, Aro P, et al. Non-ulcer dyspepsia and duodenal eosinophilia: an adult endoscopic population-based case-control study. Clin Gastroenterol Hepatol 2007;5:1175–83. [DOI] [PubMed] [Google Scholar]
- 3.Vanheel H, Vicario M, Vanuytsel T, et al. Impaired duodenal mucosal integrity and low-grade inflammation in functional dyspepsia. Gut 2014;63:262–71. [DOI] [PubMed] [Google Scholar]
- 4.Bharucha AE, Camilleri M, Burton DD, et al. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. American Journal of Gastroenterology 2014;109:1910–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du L, Chen B, Kim JJ, et al. Micro-inflammation in functional dyspepsia: A systematic review and meta-analysis. Neurogastroenterology & Motility 2018;30:e13304. [DOI] [PubMed] [Google Scholar]
- 6.Chakraborty S, Desai A, Halland M, et al. Relationship between Symptoms during a Gastric Emptying Study and Intestinal Chemosensitivity with Daily Symptoms Neurogastroenterology & Motility 2019:e13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nojkov B, Zhou SY, Dolan RD, et al. Evidence of Duodenal Epithelial Barrier Impairment and Increased Pyroptosis in Patients With Functional Dyspepsia on Confocal Laser Endomicroscopy and "Ex Vivo" Mucosa Analysis. American Journal of Gastroenterology 2020;115:1891–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wauters L, Ceulemans M, Frings D, et al. Proton Pump Inhibitors Reduce Duodenal Eosinophilia, Mast Cells, and Permeability in Patients With Functional Dyspepsia. Gastroenterology 2020;18:18. [DOI] [PubMed] [Google Scholar]
- 9.Tack J, Schol J, Van den Houte K, et al. Paradigm Shift: Functional Dyspepsia-A "Leaky Gut" Disorder? American Journal of Gastroenterology 2021;116:274–275. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad R, Sorrell MF, Batra SK, et al. Gut permeability and mucosal inflammation: bad, good or context dependent. Mucosal immunology 2017;10:307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Q, Souba WW, Croce CM, et al. MicroRNA-29a regulates intestinal membrane permeability in patients with irritable bowel syndrome. Gut 2010;59:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tili E, Michaille JJ, Piurowski V, et al. MicroRNAs in intestinal barrier function, inflammatory bowel disease and related cancers-their effects and therapeutic potentials. Current Opinion in Pharmacology 2017;37:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puthanmadhom Narayanan S, Linden DR, Peters SA, et al. Duodenal mucosal secretory disturbances in functional dyspepsia. Neurogastroenterology & Motility 2021;33:e13955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talley NJ, Haque M, Wyeth JW, et al. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Alimentary Pharmacology and Therapeutics 1999;13:225–35. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Verlinden M, Jones M. Validity of a new quality of life scale for functional dyspepsia: a United States multicenter trial of the Nepean Dyspepsia Index. American Journal of Gastroenterology 1999;94:2390–7. [DOI] [PubMed] [Google Scholar]
- 16.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Quality of Life Research 2004;13:1737–49. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond A, Snaith R. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 18.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterology and Motility 2010;22:e15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puthanmadhom Narayanan S, O'Brien D, Sharma M, et al. Duodenal mucosal mitochondrial gene expression is associated with delayed gastric emptying in diabetic gastroenteropathy. JCI Insight 2021;6:e143596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sha Y, Phan JH, Wang MD. Effect of low-expression gene filtering on detection of differentially expressed genes in RNA-seq data. Conference proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference 2015;2015:6461–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 23.Sun Z, Evans J, Bhagwate A, et al. CAP-miRSeq: a comprehensive analysis pipeline for microRNA sequencing data. BMC Genomics 2014;15:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedlander MR, Mackowiak SD, Li N, et al. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res 2012;40:37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Research 2015;43:D146–D152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Zhang W, Wang Y, et al. Hypoxia-induced miR-214 expression promotes tumour cell proliferation and migration by enhancing the Warburg effect in gastric carcinoma cells. Cancer Lett 2018;414:44–56. [DOI] [PubMed] [Google Scholar]
- 27.Choi W, Yeruva S, Turner JR. Contributions of intestinal epithelial barriers to health and disease. Experimental Cell Research 2017;358:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spindler V, Meir M, Vigh B, et al. Loss of Desmoglein 2 Contributes to the Pathogenesis of Crohn's Disease. Inflammatory Bowel Diseases 2015;21:2349–59. [DOI] [PubMed] [Google Scholar]
- 29.Yu D, Cheeseman F, Vanner S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut 2011;60:334–40. [DOI] [PubMed] [Google Scholar]
- 30.Camilleri M Leaky gut: mechanisms, measurement and clinical implications in humans. Gut 2019;68:1516–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol 2012;107:736–46. [DOI] [PubMed] [Google Scholar]
- 32.Piche T, Barbara G, Aubert P, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut 2009;58:196–201. [DOI] [PubMed] [Google Scholar]
- 33.Wilcz-Villega E, McClean S, O'Sullivan M. Reduced E-cadherin expression is associated with abdominal pain and symptom duration in a study of alternating and diarrhea predominant IBS. Neurogastroenterology & Motility 2014;26:316–25. [DOI] [PubMed] [Google Scholar]
- 34.Wouters MM. Novel insight in diarrhoea-predominant IBS: miRNAs modulate barrier function. Gut 2017;66:1537–1538. [DOI] [PubMed] [Google Scholar]
- 35.Martinez C, Rodino-Janeiro BK, Lobo B, et al. miR-16 and miR-125b are involved in barrier function dysregulation through the modulation of claudin-2 and cingulin expression in the jejunum in IBS with diarrhoea. Gut 2017;66:1537–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou Q, Huang Y, Zhu S, et al. MiR-144 Increases Intestinal Permeability in IBS-D Rats by Targeting OCLN and ZO1. Cellular Physiology & Biochemistry 2017;44:2256–2268. [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Sun H, Wang Z, et al. MicroRNA let-7a up-regulates OPN expression in a mouse model of allergic rhinitis. Journal of Laryngology & Otology 2017;131:955–960. [DOI] [PubMed] [Google Scholar]
- 38.Li M, Zhao J, Cao M, et al. Mast cells-derived MiR-223 destroys intestinal barrier function by inhibition of CLDN8 expression in intestinal epithelial cells. Biological Research 2020;53:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokar T, Pastrello C, Rossos AEM, et al. mirDIP 4.1-integrative database of human microRNA target predictions. Nucleic Acids Res 2018;46:D360–d370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckley A, Turner JR. Cell Biology of Tight Junction Barrier Regulation and Mucosal Disease. Cold Spring Harbor perspectives in biology 2018;10:02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Q, Verne ML, Fields JZ, et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 2019;68:996–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahurkar-Joshi S, Rankin CR, Videlock EJ, et al. The Colonic Mucosal MicroRNAs, MicroRNA-219a-5p, and MicroRNA-338-3p Are Downregulated in Irritable Bowel Syndrome and Are Associated With Barrier Function and MAPK Signaling. Gastroenterology 2021;160:2409–2422.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu H, Xiao X, Shi Y, et al. Inhibition of miRNA-29a regulates intestinal barrier function in diarrhea-predominant irritable bowel syndrome by upregulating ZO-1 and CLDN1. Exp Ther Med 2020;20:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YY, Xu QW, Xu PY, et al. MSC-derived exosomal miR-34a/c-5p and miR-29b-3p improve intestinal barrier function by targeting the Snail/Claudins signaling pathway. Life Sci 2020;257:118017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.