Abstract
Background:
Mold sensitization and exposure are associated with asthma severity, but the specific species that contribute to difficult-to-control (DTC) asthma are unknown.
Objective:
To determine the association between overall and specific mold levels in the homes of urban children and DTC asthma.
Methods:
The Asthma Phenotypes in the Inner-City (APIC) study recruited participants, 6 to 17 years of age, from eight US cities and classified each participant as having either DTC asthma or easy-to-control (ETC) asthma based on treatment step level. Dust samples had been collected in each participant’s home (n=485) and any dust remaining (n=265 samples), after other analyses, were frozen at −20°C. The dust samples (n=265) were analyzed using qPCR to determine the concentrations of the 36 molds in the Environmental Relative Moldiness Index (ERMI). Logistic regression was performed to discriminate specific mold content of dust from homes of children with DTC versus ETC asthma.
Results:
Frozen-dust samples were available from 54% of homes of children with DTC (139/253) and ETC asthma (126/232). Only the average concentration of the mold Mucor was significantly (p<0.001) greater in homes of children with DTC asthma. In homes with window air-conditioning units, the Mucor concentration contributed about a 22% increase (1.6 odds ratio, 95% confidence interval = 1.2 to 2.2) in the ability to discriminate between cases of DTC versus ETC asthma.
Conclusion:
Mucor levels in the homes of urban youth were a predictor of DTC asthma and these higher Mucor levels were more likely in homes with a window air-conditioner.
Keywords: APIC, US cities, child, asthma treatment, mold, Mucor, air-conditioner
Capsule Summary
Previously, we reported that mold sensitization was significantly greater for children with difficult-to-control asthma. Now we have shown that higher levels of the mold Mucor in home dust samples were specifically associated with difficult-to-control asthma.
INTRODUCTION
Asthma is a heterogeneous disease and individuals with asthma vary widely in their presentation of symptoms, natural history, and response to treatment.1–3 Patients with difficult-to-control (DTC) asthma are defined as those for whom symptom control is not achieved despite high-dose inhaled corticosteroids and maximal add-on therapies.
Although patients with DTC asthma make up only a small fraction of those with asthma, they are more likely to suffer from significant asthma morbidity.4,5 Patients with DTC asthma have more frequent exacerbations, a poorer response to medications, and lower lung function compared to those with easy-to-control (ETC) asthma.4 Difficult-to-control asthma is more prevalent in urban, non-white, and under-resourced populations.6–8 These medical and demographic characteristics describe DTC asthma, but they do not provide insights into why some patients’ asthma is DTC.
Barsky et al.9 stated that understanding DTC asthma required an “assessment of medication delivery, the home environment, and, if possible, the school and other frequented locations, the psychosocial situation, and comorbid conditions.” Sheehan and Phipatanakul10 also noted the important link between DTC asthma and environmental factors. Zang et al11 showed that steroid resistance was associated with mold exposures. However, studies to date have not quantified mold exposures in the homes of children with DTC versus ETC asthma.
A previous analysis of the Asthma Phenotypes in the Inner City (APIC) study demonstrated that mold sensitization, but not sensitization to dust mites, roaches, rodents, pets, pollen/peanut or foods, was significantly more common in those participants with DTC asthma compared to those with ETC asthma.7 In this post-hoc analysis of APIC dust samples, we investigated whether mold exposure, assessed with the Environmental Relative Moldiness Index (ERMI) panel of 36 molds,12 might contribute to understanding DTC asthma for urban children in the cities of Baltimore, Boston, Chicago, Cincinnati, Denver, Detroit, New York City and Washington DC. Many studies have shown that the ERMI metric is useful in assessing the relationship between mold exposures and asthma.13 The ERMI metric was developed in a collaboration of the US Environmental Protection Agency and the Department of Housing and Urban Development to standardize mold quantification in homes.12
The ERMI metric classifies 36 indicator mold species into two groups. Group 1includes 26 molds indicative of water damage in the home. Group 2 includes 10 species commonly found indoors, even in homes without water damage, and originating primarily outdoors.14 The ERMI calculation takes the results from the concentrations of each of 36 molds and mathematically converts these into a single number, as shown in equation below.
The concentration of each of the 26 molds in Group 1 is log transformed and summed to calculate the “summed logs of the Group 1” (s1i) molds. Similarly, the concentration of each of the 10 molds in Group 2 is log transformed and summed to calculate the “summed logs of the Group 2” (s2j) molds. The arithmetic difference between the groups, s1i − s2j, determines the ERMI value for the home.12 Therefore, the higher the ERMI value, the greater the mold contamination in the home.
Prior reviews of the scientific literature have concluded that mold exposures are associated with asthma.15, 16 Therefore, we hypothesized that mold exposures might also be associated with DTC asthma.
To test this hypothesis, we conducted a post-hoc analysis of dust samples collected during the APIC study from the homes of children ages 6–17 years.7 Samples had been collected when it was practical for the investigators, and not limited to any specific season or time of day. Therefore, the relevance of differences in season, sampling time of day, temperature and humidity could not be distinguished in this study. In APIC, DTC asthma participants were defined as requiring a daily therapy of ≥ 500 μg of fluticasone (with or without a long acting β-agonist), and those with ETC asthma were defined as requiring ≤ 100 μg fluticasone. There were originally 485 dust samples collected in the homes of children with either DTC or ETC asthma. but a frozen-dust sample remained from only 265 of the homes. For this study, we analyzed all frozen-dust samples. The comparisons of the characteristics of the study subset of APIC participants (n=265) and the full APIC cohort (n=485) are shown in Table 1.
TABLE 1.
Comparison of the characteristics of the study subset (n=265) of APIC participants and the full APIC cohort (n=485).
| Site city | Study subset of APIC (N=265) | Overall APIC Population (N=485) |
|---|---|---|
| Baltimore | 53 (20.00%) | 81 (16.70%) |
| Boston | 32 (12.08%) | 65 (13.40%) |
| Chicago | 29 (10.94%) | 58 (11.96%) |
| Cincinnati | 29 (10.94%) | 49 (10.10%) |
| Dallas | 24 (9.06%) | 43 (8.87%) |
| Denver | 29 (10.94%) | 51 (10.52%) |
| Detroit | 24 (9.06%) | 44 (9.07%) |
| New York | 33 (12.45%) | 59 (12.16%) |
| Washington DC | 12 (4.53%) | 35 (7.22%) |
| Gender | ||
| Female | 104 (39.25%) | 205 (42.27%) |
| Male | 161 (60.75%) | 280 (57.73%) |
| Age (years) | ||
| Mean (SD) | 11.0 (3.05) | 10.9 (3.04) |
| Median | 11.0 | 11.0 |
| Q1, Q3 | 8.0, 13.0 | 8.0, 13.0 |
| Range | (6.0–17.0) | (6.0–17.0) |
| Participant race | ||
| Missing | 0 | 1 (0.21%) |
| Black (non-Hispanic) | 168 (63.40%) | 311 (64.12%) |
| Hispanic | 78 (29.43%) | 137 (28.25%) |
| Other/Mixed | 15 (5.66%) | 26 (5.36%) |
| White (non-Hispanic) | 4 (1.51%) | 10 (2.06%) |
| BMI Percentile at Screening | ||
| Number | 265 | 485 |
| Mean (SD) | 75.1 (27.64) | 75.1 (27.40) |
| Median | 88.2 | 87.3 |
| Q1, Q3 | 58.0, 97.5 | 58.0, 97.6 |
| Range | (0.0–99.9) | (0.0–99.9) |
| Income <$15,000 | ||
| Missing | 2 (0.75%) | 2 (0.41%) |
| No | 122 (46.04%) | 222 (45.77%) |
| Yes | 141 (53.21%) | 261 (53.81%) |
| Family history of asthma | ||
| Missing | 6 (2.26%) | 13 (2.68%) |
| No | 67 (25.28%) | 126 (25.98%) |
| Yes | 192 (72.45%) | 346 (71.34%) |
| Eczema diagnosis | ||
| No | 123 (46.42%) | 218 (44.95%) |
| Yes | 142 (53.58%) | 267 (55.05%) |
| Allergic rhinitis diagnosis | ||
| Allergic | 179 (67.55%) | 333 (68.66%) |
| Non-allergic | 86 (32.45%) | 152 (31.34%) |
| Age (months) asthma first diagnosed by doctor | ||
| Number | 264 | 483 |
| Mean (SD) | 42.9 (37.48) | 40.7 (37.34) |
| Median | 36.0 | 24.0 |
| Q1, Q3 | 12.0, 60.0 | 12.0, 60.0 |
| Range | (1.0–180.0) | (1.0–192.0) |
| Controller treatment step | ||
| Number | 265 | 485 |
| Mean (SD) | 3.3 (2.12) | 3.4 (2.06) |
| Median | 3.0 | 4.0 |
| Q1, Q3 | 2.0, 5.0 | 2.0, 5.0 |
| Range | (0.0–6.0) | (0.0–6.0) |
| Number of hospital stays - 12 months | ||
| Number | 265 | 485 |
| Mean (SD) | 0.2 (0.53) | 0.2 (0.55) |
| Median | 0.0 | 0.0 |
| Q1, Q3 | 0.0, 0.0 | 0.0, 0.0 |
| Range | (0.0–5.0) | (0.0–5.0) |
| Any steroid courses (in previous year) | ||
| No | 137 (51.70%) | 257 (52.99%) |
| Yes | 128 (48.30%) | 228 (47.01%) |
| eNO (ppb) at Enrollment | ||
| Number | 243 | 448 |
| Mean (SD) | 29.2 (27.34) | 29.2 (27.90) |
| Median | 19.0 | 19.0 |
| Q1, Q3 | 11.0, 35.5 | 11.0, 35.5 |
| Range | (2.5–137.0) | (2.5–179.0) |
| Baseline - Results of Best Effort - FEV1 (% predicted) at Enrollment | ||
| Number | 264 | 484 |
| Mean (SD) | 95.1 (16.70) | 93.7 (16.44) |
| Median | 94.5 | 94.0 |
| Q1, Q3 | 84.3, 106.0 | 82.8, 104.6 |
| Range | (44.0–136.5) | (39.7–136.5) |
| Baseline - Results of Best Effort - FEV1/FVC at Enrollment | ||
| Number | 259 | 476 |
| Mean (SD) | 80.1 (9.43) | 79.3 (9.25) |
| Median | 80.9 | 80.7 |
| Q1, Q3 | 75.0, 87.0 | 74.3, 85.6 |
| Range | (47.3–97.8) | (45.0–99.9) |
| Total IgE (kUA/L) | ||
| Number | 263 | 478 |
| Mean (SD) | 551.7 (754.12) | 625.0 (860.31) |
| Median | 213.0 | 248.0 |
| Q1, Q3 | 80.0, 719.0 | 91.0, 766.0 |
| Range | (1.0–3852.0) | (1.0–5001.0) |
| Number of allergen sensitivities (panel of 22) -skin test OR IgE - at least 1 non-missing | ||
| Number | 265 | 485 |
| Mean (SD) | 8.2 (6.02) | 8.7 (6.23) |
| Median | 8.0 | 8.0 |
| Q1, Q3 | 2.0, 13.0 | 3.0, 14.0 |
| Range | (0.0–21.0) | (0.0–21.0) |
| sIgE >= 0.35 to any aero allergen | ||
| Missing | 2 (0.75%) | 4 (0.82%) |
| No | 60 (22.64%) | 111 (22.89%) |
| Yes | 203 (76.60%) | 370 (76.29%) |
| sIgE >= 0.35 to any food allergen | ||
| Missing | 3 (1.13%) | 6 (1.24%) |
| No | 138 (52.08%) | 238 (49.07%) |
| Yes | 124 (46.79%) | 241 (49.69%) |
| Final protocol classi fication | ||
| Difficult-to-control | 139 (52.45%) | 253 (52.16%) |
| Easy-to-control | 126 (47.55%) | 232 (47.84%) |
The dust in each participant’s home had been collected by wiping horizontal, above floor surfaces, using a Swiffer cloth™ (Procter and Gamble, Cincinnati, OH) until the cloth was dark from the dust.17 The cloth was then placed in a Ziplock™ (Johnson and Johnson Co., Racine, WI) re-sealable, plastic bag and labeled. The samples were held at −20°C until the mold analysis was completed. Each of the 36 molds included in the ERMI was quantified by quantitative PCR (qPCR) assays.18 The analyses were performed by a commercial laboratory (Mycometrics LLC, Monmouth Junction, NJ).
RESULTS AND DISCUSSION
The Student t-test was used to compare the average summed logs of Group 1 or Group 2 molds and the average ERMI values of homes of children with DTC versus ETC asthma. There was no significant difference in the average summed logs of the Group 1 or Group 2 molds in homes of children with DTC versus ETC asthma (Table 2). The average ERMI values in the homes of children with DTC versus ETC asthma were also not significantly different (Table 2). Therefore, the total mold contamination was not a distinguishing factor in asthma-control difficulty. This finding is consistent with our earlier finding that “dampness in home” was not associated with DTC asthma.7
TABLE 2.
The average and standard deviation of the summed logs of the Group 1 and summed logs of the Group 2 molds and the Environmental Relative Moldiness Index (ERMI) values for the homes of children with difficult- versus easy-to-control asthma.
| (Number homes) | Difficult Average (n=139) | SD | Easy Average (n=126) | SD | Student T-test p-value |
|---|---|---|---|---|---|
| Group 1 | 17.01 | 8.1 | 16.53 | 7.4 | >0.2 |
| Group 2 | 12.29 | 4.2 | 11.46 | 3.7 | >0.2 |
| ERMI | 4.72 | 6.4 | 5.07 | 6.0 | >0.2 |
We then compared the average concentrations of each of the 36 ERMI molds in homes of children with DTC versus ETC asthma by using the Wilcoxon rank sum test, correcting for multiple comparisons using the Holms–Bonferroni test. After Bonferroni correction, Mucor was the only mold with a significantly greater average concentration in homes of those with DTC versus ETC asthma, average of 295 versus 67 cell equivalents per mg dust, respectively (p<0.001) (Table 3).
TABLE 3.
Comparison of average concentrations in cell equivalents (CE) per mg dust for each of the 36 molds in homes of children with difficult- versus easy-to-control asthma using the Wilcoxon rank sum test, corrected for multiple comparisons using the Holms–Bonferroni test. (Significant differences are bolded.)
| Molds | Difficult Average | Easy Average | Wilcoxon Test |
|---|---|---|---|
| Group 1 | CE/mg dust | CE/mg dust | p-value |
| Aspergillus flavus | 5.29 | 3.32 | 0.06 |
| Aspergillus fumigatus | 1.95 | 3.15 | 0.36 |
| Aspergillus niger | 173.67 | 162.70 | 0.12 |
| Aspergillus ochraceus | 9.04 | 27.39 | 0.89 |
| Aspergillus penicillioides | 1505.66 | 572.73 | 0.97 |
| Aspergillus restrictus | 1.57 | 5.42 | 0.99 |
| Aspergillus sclerotiorum | 1.65 | 21.07 | 0.94 |
| Aspergillus sydowii | 98.95 | 42.78 | 0.13 |
| Aspergillus unguis | 59.70 | 6.80 | 0.34 |
| Aspergillus versicolor | 86.06 | 326.56 | 0.71 |
| Aureobasidium pullulans | 698.88 | 441.93 | 0.45 |
| Chaetomium globosum | 9.03 | 4.15 | 0.002 |
| Cladosporium sphaerospermum | 40.12 | 36.95 | 0.82 |
| Eurotium amstelodami | 2078.36 | 503.92 | 0.44 |
| Paecilomyces variotii | 6.17 | 4.65 | 0.57 |
| Penicillium brevicompactum | 178.70 | 8.54 | 0.59 |
| Penicillium corylophilum | 19.05 | 5.22 | 0.39 |
| Penicillium crustosum | 30.27 | 16.40 | 0.08 |
| Penicillium purpurogenum | 4.12 | 0.15 | 0.03 |
| Penicillium spinulosum | 0.34 | 0.02 | 0.17 |
| Penicillium variabile | 6.19 | 4.96 | 0.38 |
| Scopulariopsis brevicaulis | 370.39 | 4.51 | 0.02 |
| Scopulariopsis chartarum | 4.17 | 10.31 | 0.39 |
| Stachybotrys chartarum | 1.73 | 1.76 | 0.49 |
| Trichoderma viride | 3.60 | 24.55 | 0.08 |
| Wallemia sebi | 579.76 | 792.05 | 0.98 |
| Group 2 | |||
| Acremonium strictum | 5.19 | 9.71 | 0.92 |
| Alternaria alternata | 245.57 | 133.27 | 0.19 |
| Aspergillus ustus | 6.58 | 2.99 | 0.03 |
| Cladosporium cladosporioides Type 1 | 1246.83 | 882.09 | 0.65 |
| Cladosporium cladosporioides Type 2 | 13.66 | 16.19 | 0.67 |
| Cladosporium herbarum | 787.84 | 449.30 | 0.32 |
| Epicoccum nigrum | 113.32 | 83.31 | 0.68 |
| Mucor group | 294.72 | 67.16 | <0.001 |
| Penicillium chrysogenum Type 2 | 694.65 | 80.38 | 0.01 |
| Rhizopus stolonifer | 70.64 | 12.94 | 0.09 |
Mucor is found worldwide in soil, vegetation and buildings.19 In buildings, Mucor is known to grow in and around air-conditioning (AC) systems and ducting due to moisture from condensation.20 If an AC unit is not cleaned, or the filter not changed regularly, dust and dampness can promote the growth of many organisms that can pose a health risk.21 Therefore, we examined the relationship between the occurrence of window AC units in homes of children with DTC versus ETC asthma.
Logistic regressions were performed for the log odds of finding a child with DTC in homes with and without a window air conditioner. Mold concentrations were used as candidate cut-off points to discriminate between DTC versus ETC asthma. The resulting points of true positive (sensitivity) versus false positive rates (1 – specificity) were plotted to produce empirical Receiver Operating Characteristics (ROC) curves for homes with or without a window AC unit.22
For homes with window AC units, the log transformed Mucor concentrations were found to be a significant (p=0.007) predictor of the probability of DTC asthma but not for homes without window AC units (p=0.148). Based on the ROC curve in those homes with window AC units (Figure 1), the Mucor concentration contributed about a 22% increase (1.6 odds ratio, 95% confidence interval = 1.2 to 2.2) in the ability to discriminate between cases of DTC versus ETC asthma.
Figure 1.
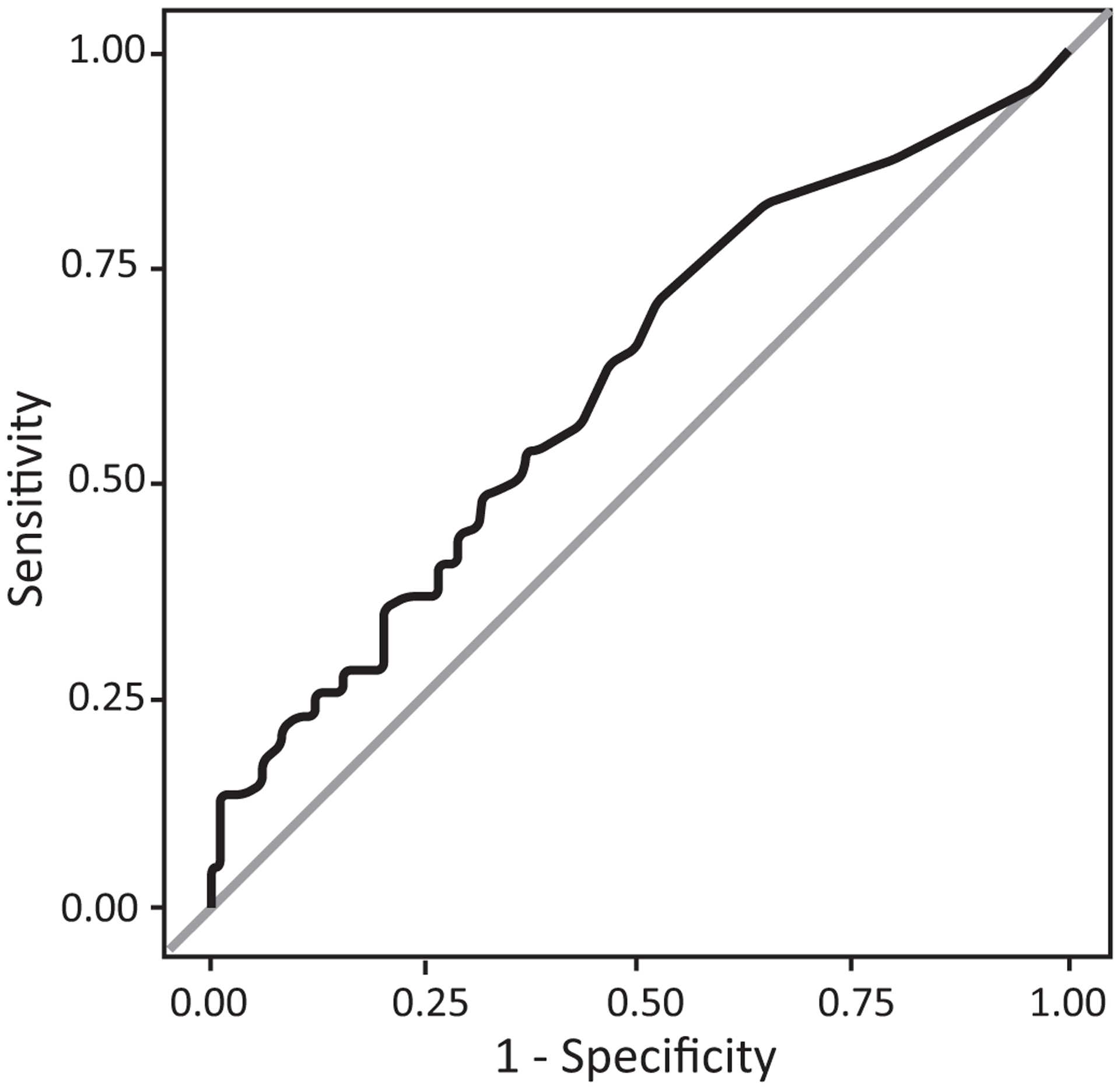
Receiver Operating Characteristic analysis (black, jagged-curved line) and area under the curve (AUC=0.6143) for homes with window air-conditioning units. Every potential Mucor concentration cut-off point plotted as a step function of the respective sensitivity (1-specificity) for difficult- versus easy-to-control asthma.
Mold exposures have been linked to poorly controlled asthma for children in other studies. For example, a prospective, cross-sectional study of children 5 to 15 years of age with poorly controlled asthma showed that allergic bronchopulmonary aspergillosis was diagnosed in 11.3% and aspergillus sensitization in 61.3% of children with poorly controlled asthma.23 Data for both adults and children suggests that severe asthma with fungal sensitization (SAFS) is associated with worse asthma control and greater susceptibility to asthma attacks than in non-sensitized patients.24 Therefore, our results are consistent with these studies in identifying mold exposures as relevant to the difficulty of controlling asthma.
Limitations to our study include the relatively small number of homes studied. However, as the homes we sampled were from cities across the US. Therefore, the findings have wide geographic application. Although frozen-dust samples were not available from all APIC homes, homes of children with DTC and ETC asthma were equally represented and the characteristics of the study subset of participants were comparable to the full APIC cohort (Table 1). Another limitation was that only the 36 ERMI-panel molds were quantified. We did not quantify other potential exposures, including other molds in the home, and other contaminants both inside and outside the home. Therefore, we are not suggesting there is a causal relationship between Mucor levels and DTC. Rather, a high concentration of Mucor in the home may be an “indicator” of higher levels of home contamination.
Standard treatments alleviate symptoms for most children with asthma, but new approaches are needed to help children who suffer from uncontrolled asthma.25 Cases of DTC asthma were more likely in homes with higher Mucor levels in dust samples and eliminating the conditions that contribute to high levels of Mucor might be appropriate to reduce DTC asthma.
Clinical Implications.
Quantifying molds, especially Mucor levels, in the dust in homes of children with difficult-to-control asthma might be helpful in guiding mitigation efforts.
Acknowledgements
We are grateful to the APIC study participants and their families.
Funding Statement
Funding Sources:
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract numbers HHSN272200900052C and HHSN272201000052I, and 1UM1AI114271-01.
Abbreviations used
- AC
Air-conditioner
- APIC
Asthma Phenotypes in the Inner City
- DTC
Difficult-to-control
- eNO
exhaled nitic oxide
- ERMI
Environmental Relative Moldiness Index
- ETC
Easy-to-control
- FEV1
Forced Expiratory Volume in one second
- FVC
Forced Vital Capacity
- kUA/L
Kilo units of allergen per liter
- Q
Quartile
- qPCR
Quantitative polymerase chain reaction
- ROC
Receiver Operating Characteristic
- SD
standard deviation
- slgE
specific IgE
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors report no conflict of interests.
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development collaborated in the research described here. It has been subjected to the Agency’s peer review and has been approved as an EPA publication. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use. The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the US EPA.
REFERENCES
- 1.Carr TF, Bleecker E. Asthma heterogeneity and severity. World Allergy Organ J 2016;9: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zoratti EM, Krouse RZ, Babineau DC, Pongracic JA, O’Connor GT, Wood RA, et al. Asthma phenotypes in inner-city children. J Allergy Clin Immunol 2016;138: 1016–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown KR, Krouse RZ, Calatroni A, Visness CM, Sivaprasad U, Kercsmar CM, et al. Endotypes of difficult-to-control asthma in inner-city African American children. PLoS ONE 2017;12(7): e0180778. doi: 0.1371/journal.pone.0180778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chipps BE, Haselkorn T, Paknis B, Ortiz B, Bleecker ER, Kianifard F, et al. More than a decade follow-up in patients with severe or difficult-to-treat asthma: The epidemiology and natural history of asthma: Outcomes and treatment regimens (TENOR) II. J Allergy Clin Immunol 2018;141: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 5.Licari A, Brambilla I, Marseglia A, De Filippo M, Paganelli V, Marseglia GL. Difficult vs. severe asthma: Definition and limits of asthma control in the pediatric population. Front Pediatr 2018;6: 170. doi: 10.3389/fped.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schatz M, Hsu JW, Zeiger RS, Chen W, Dorenbaum A, Chipps BE, et al. Phenotypes determined by cluster analysis in severe or difficult-to-treat asthma. J Allergy Clin Immunol 2014;133: 1549–56. [DOI] [PubMed] [Google Scholar]
- 7.Pongracic JA, Krouse RZ, Babineau DC, Zoratti EM, Cohen RT, Wood RA, et al. Distinguishing characteristics of difficult-to-control asthma in inner-city children and adolescents. J Allergy Clin Immunol 2016;138: 1030–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilbert T, Zeiger RS, Haselkorn T, Iqbal A, Alvarez C, Mink DR, et al. Racial disparities in asthma-related health outcomes in children with severe/difficult-to-treat asthma. J Allergy Clin Immunol Pract 2018;pii: S2213-2198(18)30546-4. doi: 10.1016/j.jaip.2018.07.050. [DOI] [PubMed] [Google Scholar]
- 9.Barsky EE, Giancola LM, Baxi SN, Gaffin JM. A practical approach to severe asthma in children. Ann Am Thorac Soc 2018;15: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheehan WJ, Phipatanakul W. Difficult-to-control asthma: epidemiology and its link with environmental factors. Curr Opin Allergy Clin Immunol 2015;15: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, Biagini-Myers JM, Brandt EB, Ryan PH, Lindsey M, Mintz-Cole RA, et al. Mold exposure promotes steroid resistant asthma through activation of TH17 responses. J Allergy Clin Immunol 2017;139: 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vesper SJ, McKinstry C, Haugland RA, Wymer L, Ashley P, Cox D, et al. Development of an environmental relative moldiness index for homes in the U.S. J Occup Environ Med 2007;49: 987–90. [DOI] [PubMed] [Google Scholar]
- 13.Vesper S, Wymer L. The relationship between Environmental Relative Moldiness Index values and asthma. Int J Hygiene Environ Health 2016;219: 233–8. [DOI] [PubMed] [Google Scholar]
- 14.Vesper S. Traditional mould analysis compared to a DNA-based method of mould analysis. Crit Rev Micro 2011:37: 15–24. [DOI] [PubMed] [Google Scholar]
- 15.Quansah R, Jaakkola MS, Hugg TT, Heikkinen SA, Jaakkola JJ. Residential dampness and molds and the risk of developing asthma: a systematic review and meta-analysis. PLoS ONE 2012; 7, e47526, 10.1371/journal.pone.0047526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe RA, Bearman N, Thornton CR, Husk K, Osborne NJ. Indoor fungal diversity and asthma: a meta-analysis and systematic review of risk factors. J Allergy Clin Immunol 2015;135: 110–122. [DOI] [PubMed] [Google Scholar]
- 17.Vesper S, Prill R, Wymer, Adkins L, Williams R, Fulk F. Mold contamination in schools with either high or low prevalence of asthma. Pediatr Allergy Immunol 2015; 26: 49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haugland RA, Varma M, Wymer LJ, Vesper SJ. Quantitative PCR of selected Aspergillus, Penicillium and Paecilomyces species. Sys Appl Microbiol 2004;27: 198–210. [DOI] [PubMed] [Google Scholar]
- 19.El-Herte RI, Baban TA, Kanj SS. Mucormycosis: A review on environmental fungal spores and seasonal variation of human disease. Adv Infect Dis 2012;2: 76–81. [Google Scholar]
- 20.Kelkar U, Kulkarni S. Contaminated air conditioners as potential source for contaminating operation theatre environment. Int J Infect Control 2011:8: 45–48. [Google Scholar]
- 21.Mendell MJ. Commentary: Air conditioning as a risk for increased use of health services. Int J Epi 2004;33: 1123–1126. [DOI] [PubMed] [Google Scholar]
- 22.Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39: 561–577. [PubMed] [Google Scholar]
- 23.Kumari J, Ram Jat K, Lodha R, Jana M, Xess I, Kabra SK. Prevalence and risk factors of allergic bronchopulmonary aspergillosis and Aspergillus sensitization in children with poorly controlled asthma. Trop Pediatr 2020;66: 275–283. doi: 10.1093/tropej/fmz066. [DOI] [PubMed] [Google Scholar]
- 24.Bush A. Kids, difficult asthma and fungus. J Fungi (Basel) 2020;6: 55. doi: 10.3390/jof6020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porcaro F, Ullmann N, Allegorico A, Di Marco, Cutrera R. Difficult and severe asthma in children. Children (Basel) 2020;7: E286. doi: 10.3390/children7120286. [DOI] [PMC free article] [PubMed] [Google Scholar]


