Abstract
Treatment of FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD) AML is restricted due to toxicity, drug resistance and relapse eventhough targeted therapies are clinically available. Resveratrol with its multi-targeted nature is a promising chemopreventive remaining limitedly studied in FLT3-ITD AML regarding to ceramide metabolism. Here, its cytotoxic, cytostatic and apoptotic effects are investigated in combination with serine palmitoyltransferase (SPT), the first enzyme of de novo pathway of ceramide production, inhibitor myriocin on MOLM-13 and MV4-11 cells. We assessed dose-dependent cell viability, flow cytometric cell death and cell cycle profiles of resveratrol in combination with myriocin by MTT assay, annexin-V/PI staining and PI staining respectively. Resveratrol’s dose-dependent effect on SPT protein expression was also checked by western blot. Resveratrol decreased cell viability in a dose- dependent manner whereas myriocin did not affect cell proliferation effectively in both cell lines after 48h treatments. Although resveratrol induced both apoptosis and a significant S phase arrest in MV4-11 cells, it triggered apoptosis and non-significant S phase accumulation in MOLM-13 cells. Co-administrations reduced cell viability. Increased cytotoxic effect of co-treatments was further proved mechanistically through induction of apoptosis via phosphatidylserine relocalization. The cell cycle alteration in co-treatment was significant with an S phase arrest in MV4-11 cells, however, it was not effective on cell cycle progression of MOLM-13 cells. Resveratrol also increased SPT expression. Overall, modulation of SPT together with resveratrol might be the possible explanation for resveratrol’s action. It could be an integrative medicine for FLT3-ITD AML after investigating its detailed mechanism of action in relation to de novo pathway of ceramide production.
Keywords: Apoptosis, Cell cycle, FLT3-ITD AML, Resveratrol, Serine palmitoyltransferase
Introduction
Sphingolipids and their metabolites possess membrane-related structural roles and important signaling functions in various intracellular processes including cell proliferation, cell death, cell migration and inflammation (Ogretmen 2018). Hence, their contribution to cancer development and therapy has attracted significant attention, recently. Each metabolite in the complex sphingolipid pathway has their own roles to regulate the critical pathways involved in carcinogenesis (Shaw et al. 2018). For instance, ceramide (Cer), central signaling lipid, is well known to inhibit cell proliferation by inducing apoptosis, autophagy and cell cycle arrest whilst its metabolites such as sphingosine-1-phosphate (S1P) produced by sphingosine kinases (SKs) and glucosylceramide (GC) generated by glucosylceramide synthase (GCS) favor cell survival and proliferation (Hannun and Obeid 2018). Sphingolipids could be produced by two different pathways named de novo pathway and salvage pathway (hydrolysis of sphingomyelin (SM) to Cer) (Shaw et al. 2018; Hannun and Obeid 2018).
De novo pathway is the common route to generate Cer via sequential functioning of different enzymes. Serine palmitoyltransferase (SPT) is the rate-limiting enzyme catalyzing the first step in de novo pathway, which is the condensation of L-serine and palmitoyl CoA to form 3-ketosphinganine. Then, this resulting intermediate is converted to Cer (Nganga et al. 2018). Although elevated levels of intracellular Cer in response to cellular stress inhibit cell growth and survival, inhibition of SPT, hence reducing Cer levels, is associated with growth suppression of cancer cells (Kojima et al. 2018; Bernhart et al. 2015). SPT’s roles in cellular functions seem to be context-dependent. In glioma cells, silencing or inhibition of SPT caused impaired cell growth and induced apoptosis (Bernhart et al. 2015). In in vitro and in vivo models of melanoma, SPT inhibition caused cell cycle arrest at G2/M phase, decreased sphingolipid levels and upregulated p53 and p21 expression (Lee et al. 2011; Lee et al. 2012). Therefore, targeting SPT could be an attractive strategy to interfere with proliferation of cancer cells and there are newly synthesized SPT inhibitors with promising anti-carcinogenic activities in addition to conventional SPT inhibitors such as myriocin (Kojima et al. 2018; Yaguchi et al. 2017).
Resveratrol is a plant-based pleiotropic polyphenol with well-defined and extensively reviewed anti-carcinogenic properties (Bhaskara et al. 2020). Although it affects cell proliferation, cell survival, cell cycle progression, invasion, metastasis, apoptosis and autophagy by activating or inhibiting various signaling pathways (Bhaskara et al. 2020; Berretta et al. 2020), it is clear that its functions in solid cancers, to a lesser extent, hematological malignancies, are also related to modulation of sphingolipid metabolism (Kisková and Kassayová 2019). In hepatocellular carcinoma, resveratrol treatment resulted in increased intracellular concentration of Cer by upregulating the enzymes of de novo pathway and downregulating Cer-catabolizing enzymes (Charytoniuk et al. 2019). In chronic myeloid leukemia (CML) and colon cancer cells, acid sphingomyelinase, responsible for degradation of sphingomyelin to Cer, was upregulated in response to resveratrol (Mizutani et al. 2016). Resveratrol downregulated Cer-metabolizing genes and upregulated Cer-producing genes in HL60 acute promyelocytic leukemia (APL) cells (Cakir et al. 2011). Resveratrol and its newly synthesized dimers were found to inhibit sphingosine kinase-1 (SK-1) expression and to induce PARP-dependent apoptosis in MCF-7 breast cancer cells (Lim et al. 2012). To our knowledge, there is no study investigating the therapeutic role of resveratrol in relation to de novo pathway of sphingolipid metabolism in FLT3-ITD positive acute myeloid leukemia (AML) although we recently showed it could affect Cer catabolism enzymes, SK-1 and GCS (Ersöz and Adan 2022)
FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD) positive AML is a subgroup of genetically complex AML classified based on the presence of ITD within the juxtamembrane domain of FLT3 receptor tyrosine kinase (Antar et al. 2020). FLT3 signaling is crucial for normal hematopoietic and immune system development, which leads to activation of downstream pathways involved in cell proliferation and suppression of apoptosis. In functionally differentiated cells, it is not expressed, however, majority of AML cases has overexpressed or mutated FLT3 (Antar et al. 2020; Daver et al. 2019). Two common mutations in FLT3 gene are ITD and tyrosine kinase domain mutations, resulting in constitutive activation of FLT3 kinase and its downstream proliferative pathways. Therefore, FLT3 is a therapeutic target for the development of FLT3 inhibitors, some of which are clinically approved for the treatment of FL3-ITD positive AML in combination therapies. However, the clinical results seem to be unsatisfactory due to off-target effects, toxicity and acquired drug resistance (Antar et al. 2020). Therefore, investigation of natural products as a chemopreventive or an adjuvant due to their less toxic behaviour and beneficial health effects could give promising results for the treatment of FLT3-ITD AML in combination approaches together with clinically used agents (Ha et al. 2020).
In this study, the possible cytotoxic, cytostatic and apoptotic effects of resveratrol on FLT3-ITD positive MOLM-13 and MV4-11 cells together with SPT inhibition were investigated. Resveratrol’s anti-proliferative effects through inducing apoptosis and cell cycle arrest were intensified by inhibiting SPT. Based on the results of this study, it would be suggested that resveratrol could be a part of integrative therapy when combined with clinically approved FLT3 inhibitors after further detailed in vitro and in vivo analyses of resveratrol’s sphingolipid metabolism-based actions.
Materials and methods
Chemicals
Resveratrol and MTT were purchased from Sigma-Aldrich (USA). Myriocin was obtained from Cayman Chemicals (Ann Arbor, MI, USA). 10 mM stock solutions were prepared in DMSO. The final concentration of DMSO did not exceed more than 0.1% in culture. Penicillin-streptomycin, RPMI 1640, and fetal bovine serum (FBS) were obtained from Invitrogen (Paisley, UK).
Cell lines and culture conditions
Heterozygous FLT3-ITD cell line MOLM-13 and FLT3- ITD loss of heterozygosity (LOH) cell line MV4-11 were obtained from DSMZ (German Collection of Microorganisms and Cell cultures). MOLM-13 and MV4-11 cells were cultured in RPMI 1640 (with L-glutamine) (GibcoTM) medium supplemented with 10-20% FBS and 1% penicillin-streptomycin in a 5% CO2 incubator at 37ºC.
Detection of cell viability
The effects of resveratrol (10-30 µM), myriocin (40-120 nM) and their combinations (10 µM resveratrol+40 nM myriocin, 20 µM resveratrol+80 nM myriocin and 30 µM resveratrol+120 nM myriocin) at a constant ratio on MOLM-13 and MV4-11 cells’ viability were assessed by MTT cell proliferation assay (Adan and Baran 2015; Oğuz and Adan 2021). Briefly, the cells were seeded into a 96 well plate at a density of 1 × 104 cells/well and incubated for 48 h. 20 µl MTT solution (5 mg/mL, Sigma Aldrich) was added to observe formazan crystals which were dissolved in 100 µl DMSO. Then, absorbance values (at 570 nm) were recorded and the cell proliferation/viability graphs were drawn. Based on the graphs, IC50 values (concentrations inhibiting cell viability by 50%) for resveratrol and myriocin were calculated using linear regression analysis in GraphPad software (San Diego, CA).
Combination index (CI) analysis
The method of Chou-Talalay was applied to determine whether there is synergistic/additive/antagonistic effects of combinational treatments in MOLM-13 and MV4-11 cells using CompuSyn software. This software generates CI values which were calculated from MTT cell viability results of aforementioned combinations. CI < 1 indicates synergism, CI = 1.0–1.1 indicates additive effect and CI> 1 indicates antagonism (Chou 2008).
Analysis of apoptosis using annexin-V/PI dual staining by flow cytometry
7.5 × 105 cells/well were treated with resveratrol (10-30 µM), myriocin (120 nM) and the combination (30 µM resveratrol plus 120 nM myriocin) for 48 h in a 6 well plate at 37 °C. The protocol based on Annexin V-FITC apoptosis detection kit (BioVision, USA) was followed with minor modifications. The cells were collected at 1800 rpm for 10 min, washed with cold 1X PBS and resuspended with 200 µl annexin binding buffer. Then, 2 µl propidium iodide and 2 µl Annexin V/FITC were added. Following incubation at room temperature for 15 min in the dark, apoptotic cells were detected using a BD FACSCalibur flow cytometer (BD Biosciences) within 1 h. The histograms were obtained and analyzed using BD FACSDivaTM (BD Biosciences) (Adan and Baran 2015; Oğuz and Adan 2021).
Analysis of cell cycle progression using PI staining by flow cytometry
MOLM-13 and MV4-11 cells (7.5 × 105 /well in a 6 well plate) were treated with resveratrol (10–30 µM) and myriocin (120 nM) alone or in combination (30 µM resveratrol plus 120 nM myriocin) for 48 h. After centrifugation at 260×g for 10 min, cell pellets were washed twice with cold PBS followed by incubation at − 20 °C with cold ethanol for 24 h. Samples were centrifuged at 260×g for 10 min and supernatant was removed. Pellets were homogenized in cold PBS and centrifuged. PBS-TritonX100 and RNase-A (200 µg/ml, Sigma Aldrich) were added and incubated at 37 °C for 30 min. Then, propidium iodide (1 mg/ml, Sigma Aldrich) staining was performed at room temperature for 10–15 min. Cell cycle analysis was performed using BD FACSCalibur flow cytometer with BD FACSDivaTM (BD Biosciences) (Adan and Baran 2015).
Western blot
4 × 106 cells were treated with with resveratrol (10-30 µM) for 48 h. Cells were lysed using RIPA buffer (Sigma-Aldrich, USA). Protein concentrations were calculated using RC DCTM Protein Assay Kit (Bio-Rad, USA). 30 µg/well total protein was separated by SDS-PAGE and transferred to PVDF membranes. The membranes were blotted with primary antibodies for SPT (1:3000, Abcam, Cambridge, UK) and Beta Actin (1:3000, Cell Signaling, USA) overnight at + 4 °C and conjugated with appropriate secondary antibodies (1:10000, Jackson Immuno Research, USA). The membranes were visualized with Pierce ™ ECL Western Blotting Substrate kit (Thermo ScientificTM, USA). Densitometric analysis of immunoreactive bands was performed by using the imaging software (Bio-Rad, ChemiDoc, Image LabTM 3.0).
Statistical analysis
GraphPad software (San Diego, CA) was used to analyze data and the results were expressed as the mean ± standard error (SEM) from at least three independent experiments. Comparisons among multiple groups were evaluated using student’s t test or one-way ANOVA followed by Dunnett’s test. P < 0.05 was considered as a statistically significant difference.
Results
Combined treatment with resveratrol and myriocin shows enhanced cytotoxic effects on FLT3-ITD AML
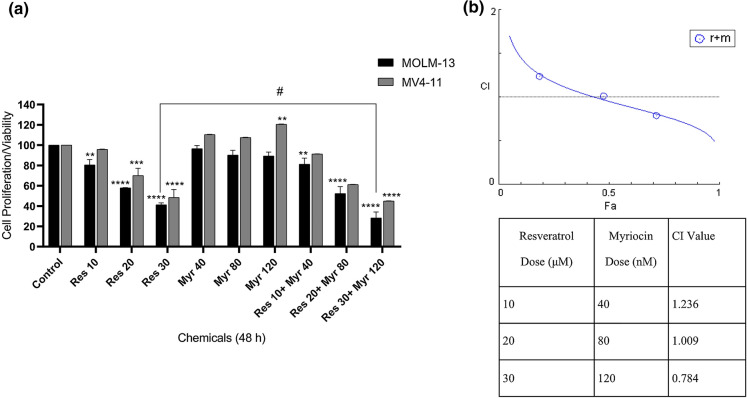
The viability of MOLM-13 and MV4-11 cells was checked after treating the cells with resveratrol (10-30 µM) and myriocin alone (40–120 nM) and in combinations by MTT assay. A significant decrease was observed in a concentration-dependent manner in response to increasing concentrations of resveratrol in both cell lines (Fig. 1). IC50 concentrations of resveratrol were calculated as 22 µM and 30 µM for MOLM-13 and MV4-11 cells, respectively. MOLM-13 cell was affected even at lowest concentration of resveratrol whereas the proliferation of MV4-11 cells affected at higher concentrations compared to untreated controls. Myriocin alone did not inhibit cell viability significantly in both cell lines. The increasing concentrations of resveratrol (10–30 µM) combined with those of myriocin (40-120 nM) resulted in inhibition of cell proliferation especially at higher concentrations of resveratrol (30 µM) and myriocin (120 nM) in MOLM-13 and MV4-11 cells as compared to untreated cells (Fig. 1a). However, MOLM-13 cells were more sensitive to the combinations and 30 µM resveratrol in combination with 120 nM myriocin suppressed cell viability significantly as compared to both control and resveratrol alone (p: 0.02). Based on these results, CI analysis was also performed on both MOLM-13 and MV4-11 cells. In accordance with cell viability results, the combination of 30 µM resveratrol with 120 nM myriocin showed synergistic effect (CI:0.78) in MOLM-13 cells (Fig. 1b) whilst 10 µM and 20 µM resveratrol in combination with 40 nM and 80 nM myriocin exhibited antagonistic (CI: 1.23) and additive effects (CI: 1.01), respectively. On the other hand, CIs could not be calculated for MV4-11 cells, which might be due to increasing trends in % cell viability after the treatments with myriocin’s individual concentrations relative to untreated control (Fig. 1a).
Fig. 1.
The % viability of MOLM-13 and MV4-11 cells treated with resveratrol plus myriocin after 48 h incubation (a). The data was expressed as a mean percentage ± SE relative to the untreated control from at least three replicates. **p < 0.005, ***p:0.0006, ****p < 0.0001 compared to the control, #p < 0.05 compared to 30 µM resveratrol in MOLM-13 cells. If there is no statistical difference between or among the groups, it is not depicted on the graphs. CI analysis of MOLM-13 cells in which C < 1, C:1.00-1.1 and CI > 1 indicate synergistic, additive and antagonistic effect, respectively (b). r: resveratrol, m: myriocin
Resveratrol plus myriocin showed increased apoptotic effects on FLT3-ITD AML cells
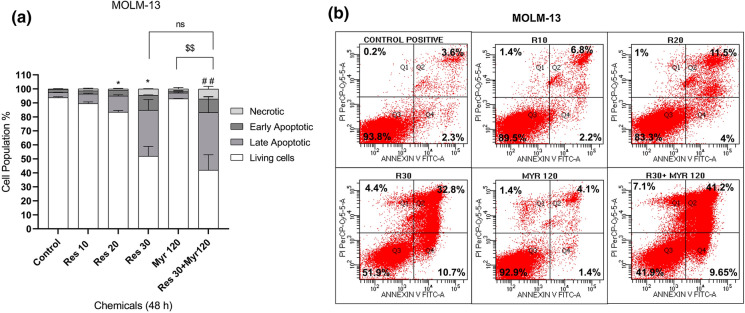
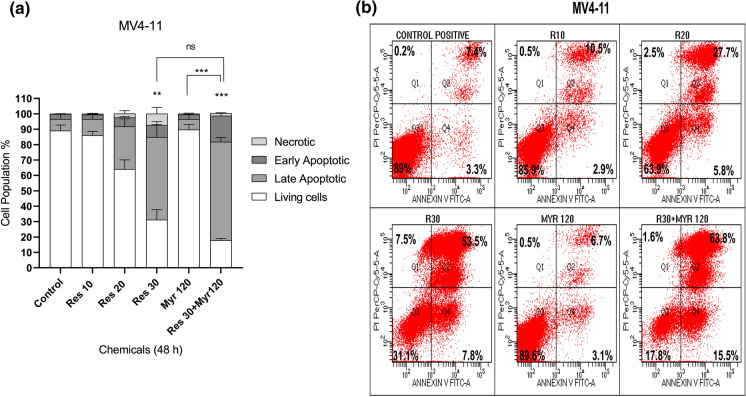
It was also explored whether resveratrol and myriocin may show an effect on FLT3-ITD AML cell apoptosis. Increasing concentrations of resveratrol (10-30 µM) individually increased apoptotic cell population (early (Q4) plus late (Q2) apoptosis) in MOLM-13 cells, compared with the control group (9% (10 µM), 15.5% (20 µM) and 43.5% (30 µM) vs. 5.9% (control), respectively) eventhough 20 and 30 µM resveratrol showed statistically significant effect (Fig. 2a and b). Similarly, MV4-11 cells underwent apoptosis in a dose-dependent manner after resveratrol treatment (10-30 µM) when compared to untreated control (13.4% (10 µM), 33.5% (20 µM), and 61.3% (30 µM), vs. 10.7% (control), respectively) while 30 µM resveratrol affected cell apoptosis significantly (Fig. 3a and b). Myriocin (120 nM) alone did not induce apoptosis in both cell lines. Combined resveratrol and myriocin (30 µM resveratrol+120 nM myriocin) significantly promoted MOLM-13 cell apoptosis as compared to the control (50.85 vs. 5.9%). Similarly, a combination of resveratrol and myriocin (30 µM resveratrol+120 nM myriocin) enhanced MV4-11 cell apoptosis significantly when compared to the control (79.3 vs. 10.7%). Comparison between 30 µM resveratrol and the combination treatment was not statistically significant although there were increases in both MOLM-13 (50.85% vs. 43.5%) and MV4-11 (79.3% vs. 61.3%) apoptotic cell population, respectively (Figs. 2 and 3). Comparison of apoptotic cell populations between 120 nM myriocin and co-treatment induced statistically significant apoptosis in both MOLM-13 (50.85% vs. 5%) (Fig. 2) and MV4-11 (79.3% vs. 9.8%) (Fig. 3). Collectively, these data suggested that the combination treatment decreased leukemia cell proliferation via induction of apoptosis relative to untreated cells or myriocin alone.
Fig. 2.
Apoptotic response of MOLM-13 cells to resveratrol in combination with myriocin for 48 h (a). Q4 quadrant shows Annexin-positive/PI negative, early apoptotic cells while Q2 quadrant shows Annexin-positive/PI positive, late apoptotic cells (b). The data was expressed as a mean percentage ± SE relative to the untreated control from at least three replicates. *p < 0.05, ##p:0.0079 versus control, $$ p: 0.0067 myr 120 versus res 30+ myr 120. If there is no statistical difference between or among the groups, it is not depicted on the graphs
Fig. 3.
Apoptotic response of MV4-11 cells to resveratrol in combination with myriocin for 48 h (a). Q4 quadrant shows Annexin-positive/PI negative, early apoptotic cells while Q2 quadrant shows Annexin-positive/PI positive, late apoptotic cells (b). The data was expressed as a mean percentage ± SE relative to the untreated control from at least three replicates. **p < 0.005, ***p < 0.0005 versus control or myr 120 versus res 30+ myr 120. If there is no statistical difference between or among groups, it is not depicted on the graphs
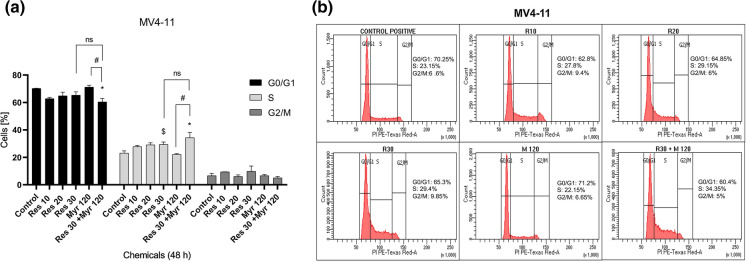
Differential effects of resveratrol in combination with myriocin on cell cycle distributions of MOLM-13 and MV4-11 cells
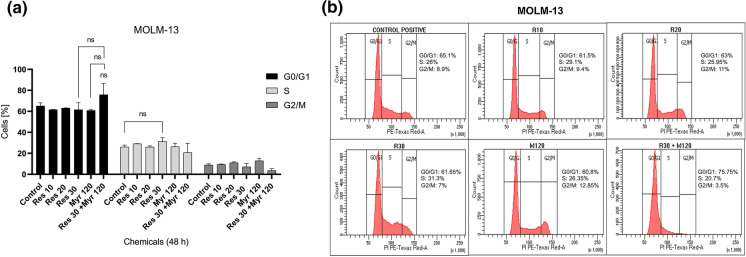
To investigate the probable mechanisms underlying the observed cytotoxic effects, cell cycle progression was also analyzed in MOLM-13 and MV4-11 cells treated with resveratrol, myriocin alone and resveratrol plus myriocin. The cells were accumulated at S phase when treated especially with 30 µM resveratrol for both MOLM-13 (31.3 vs. 26%) and MV4-11 (29.4 vs. 23.15%) cells, respectively as compared to untreated controls although MV4-11 cells only showed statistically significant arrest (Figs. 4 and 5). On the other hand, myriocin (120 nM) alone caused an increase at G2/M (12.85 vs. 8.9%) in MOLM-13 cells and a very slight rise in G0/G1 population in MV4-11 (71.2 vs. 70.25%) cells (Figs. 4 and 5), which were not statistically significant. 30 µM resveratrol in combination with 120 nM myriocin resulted in non-statistically significant G0/G1 arrest and statistically significant S phase arrest in MOLM-13 (75.75 vs. 65.01%) and MV4-11 (34.35 vs. 23.15%) cells when compared to the control, respectively (Figs. 4 and 5). Additionally, MV4-11 cells accumulated at S phase after the combination treatment relative to myriocin alone (22.15% vs. 34.35%). These data indicated that resveratrol in combination with myriocin showed a more significant suppressive effect on MV4-11 cell cycle progression than MOLM-13 cells.
Fig. 4.
Flow cytometric analysis of cell cycle kinetics in MOLM-13 cells exposed to resveratrol in combination with myriocin for 48 h (a, b). The data was expressed as a mean percentage ± SE relative to the untreated control from at least three replicates. If there is no statistical difference between or among groups, it is not depicted on the graphs
Fig. 5.
Flow cytometric analysis of cell cycle kinetics in MV4-11 cells exposed to resveratrol in combination with myriocin for 48 h (a, b). The data was expressed as a mean percentage ± SE relative to the untreated control from at least three replicates. *p < 0.05, $p< 0.05 versus control, #p < 0.05 myr 120 versus res 30+ myr 120
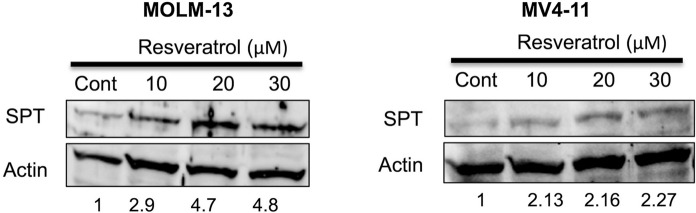
The effect of resveratrol on the protein expression level of SPT in FLT3-ITD positive AML cells
IC50 values or subtoxic concentrations (lower than IC50 values ) of resveratrol (10–30 µM) were chosen to analyze the alterations in protein levels of SPT in cultured MOLM-13 and MV4-11 cells using western blot. In both cell lines, SPT was upregulated in response to increasing concentrations of resveratrol (Fig. 6) compared to untreated cells. At 30 µM resveratrol, there were 4.8-fold and 2.27-fold increases in SPT levels for MOLM-13 and MV4-11 cells, respectively. Resveratrol could affect this enzyme more effectively in MOLM-13 cells as compared to MV4-11 cells.
Fig. 6.
Western blot analysis of SPT protein expression in MOLM-13 (a) and MV4-11 (b) cells exposed to increasing doses of resveratrol (10–30 µM). Actin was used as a loading control for both cell lines
Discussion
Resveratrol, a phytoalexin, has been recognized as a potential plant-based chemopreventive for cancers. Its anti-carcinogenic potential is quite complex and the common processes modulated by resveratrol include cell cycle progression, different modes of cell death, suppression of metastasis, invasion and angiogenesis through regulating different signaling pathways based on the context and cancer type (Ko et al. 2017). For instance, resveratrol induced apoptosis in HL60 and NB-4 APL cells by regulating PTEN/PI3K/AKT pathway (Meng et al. 2019). In lung and esophageal cancer cells, resveratrol suppressed invasion and metastasis by inhibiting ADAM9 expression (Lin et al. 2020). Additionally, it has been shown in limited studies that resveratrol’s anti-carcinogenic features could be also regulated via alterations in sphingolipid metabolism as extensively reviewed (Dei Gas and Ghidoni 2018).
Despite the recent advances in the treatment of FLT3-ITD AML by introduction of FLT3 inhibitors, an effective therapy is still missing due to primary or secondary resistance in monotherapy or in combination therapy and short duration of remission in patients (Scholl et al. 2020; Ambinder and Levis 2021). Therefore, in this particular study, the therapeutic potential and underlying mechanism of resveratrol's action was investigated by particularly focusing on inhibition of SPT involved in de novo generation of sphingolipids in FLT3-ITD expressing AML cells.
We firstly checked the effect of resveratrol in combination with SPT inhibitor myriocin on cell viability, cell death (necrosis and apoptosis) and cell cycle progression of FLT3-ITD cells. Resveratrol alone inhibited cell viability in a dose-dependent manner in both MOLM-13 and MV4-11 cells (Fig. 1a) in accordance with the results obtained from different leukemia types including APL, AML and CML (Siedlecka-Kroplewska et al. 2019; Ersöz and Adan 2022; Huang et al. 2019). On the other hand, myriocin alone used in sub-micromolar concentrations to inhibit enzyme activity (Chalfant et al. 2002) did not affect the cell viability in contrast to the literature in which µM concentrations of myriocin showed more cytotoxic effect (Choi et al. 2014; Sano et al. 2017). When increasing concentration of resveratrol combined with those of myriocin, there was inhibition of cell viability in both MOLM-13 and MV4-11 cells compared to untreated cells, however, MV4-11 cells’ lesser sensitivity to resveratrol was observed (Fig. 1a). To address whether the inhibition of cell viability is synergistic or not, CI values were obtained, which clearly showed that MOLM-13 cells responded to the combination treatment synergistically especially at highest concentrations of resveratrol and myriocin (Fig. 1b).
Cell cycle arrest and apoptosis are considered to be the main underlying mechanisms for the cytotoxic effects of most drugs and agents in cancers. Therefore, we next checked apoptosis induction and cell cycle progression in MOLM-13 and MV4-11 cells treated with resveratrol plus myriocin. Resveratrol induced apoptosis in both MOLM-13 and MV4-11 cells whilst myriocin was not effective (Figs. 2 and 3). On the other hand, co-administrations increased apoptotic cell population (early and late apoptotic cells) in both cells (Figs. 2 and 3) as compared to control or myriocin alone. As shown in Fig. 5, cell cycle progression was arrested at S phase in MV4-11 cells in response to resveratrol and resveratrol plus myriocin while there were non-statistically significant accumulation of MOLM-13 cells at S phase for resveratrol treatment and at G0/G1 for co-treatment (Fig. 4). Myriocin slightly but not significantly blocked cell cycle progression at G2/M and G0/G1 phases in MOLM-13 and MV4-11 cells, respectively (Figs. 4 and 5).
In these results, eventhough myriocin alone did not significantly contribute to apoptosis induction and cell cycle arrest, combination treatment resulted in increased apoptosis in both representative models of FLT3-ITD AML, however, cell cycle arrest seemed to be cell type specific. Resveratrol’s anti-proliferative effects were extensively studied in leukemias, which showed differences in mechanisms of action based on type of leukemia and leukemia cell context. In APL cells, resveratrol induced G0/G1 arrest and apoptosis (Siedlecka-Kroplewska et al. 2019). Resveratrol caused accumulation of acute lymphoblastic leukemia (ALL) cells at S phase and induced apoptosis (Opydo-Chanek et al. 2017), which is in accordance with S phase arrest in MV4-11 cells. The different sensitivity and response of MOLM-13 and MV4-11 cells to combination treatments could be expected due to the presence of additional epigenetic and genetic mutations, which might cause the expression of different set of genes (Razumovskaya et al. 2011; Lindblad et al. 2015).
We also checked the protein expression of SPT in MOLM-13 and MV4-11 cell lines after treating them with increasing concentrations of resveratrol and showed that resveratrol upregulated SPT expression in both cell lines (Fig. 6). This data is the first one showing the role of baseline expression of SPT and how its expression is altered by resveratrol in FLT3-ITD AML. To our knowledge, there are only limited studies investigating the role of SPT in resveratrol-mediated cytotoxicity in cancer. In metastatic breast cancer cells, combined activation of SPT and nSMase (neutral sphingomyelinase involved in salvage pathway) were responsible for Cer accumulation, leading to cell growth inhibition and apoptosis after resveratrol treatment (Scarlatti et al. 2003). Resveratrol showed anti-proliferative effect on colon cancer cells by inhibiting ornithine decarboxylase (ODC) activity, an enzyme of polyamine metabolism linked to cancer development and ODC inhibition by resveratrol was counteracted by inhibiting SPT (Ulrich et al. 2007). Chow et al. (2014) showed that resveratrol induced SPT activation resulting in endoplasmic reticulum (ER) stress and expansion, which was responsible for ER-mediated apoptosis in human nasopharyngeal carcinoma cells. Resveratrol sensitized DU145 prostate cancer cells to ionizing radiation by causing Cer accumulation. Cell death was decreased after resveratrol or resveratrol plus ionizing radiation treatment in the presence of myriocin, showing the role of de novo pathway (Scarlatti et al. 2007). Resveratrol upregulated SPT expression in hepatocellular carcinoma cells on lipid overload state (Charytoniuk et al. 2019). Resveratrol prevented the accumulation of breast cancer cells into multicellular tumor spheroids which was reversed partially in the presence of myriocin (Dolfini et al. 2007). In a recent study, SPT was upregulated in response to resveratrol and SPT inhibition counteracted resveratrol’s apoptotic activity in philadelphia chromosome positive acute lymphoblastic leukemia (Oğuz and Adan 2021).
Interestingly, all these results obtained in this study did not show what is expected in general regarding to the role of SPT inhibition, hence decreasing Cer accumulation, which could increase cell viability and suppress apoptosis in combination treatments to counteract or rescue resveratrol’s action as extensively discussed. However, inhibition of SPT was not able to reverse resveratrol’s cytotoxic and apoptotic effects, instead, it increased its anti-leukemic activities. It is thought that increased expression of SPT protein levels in response to resveratrol could not be related to increases in its enzymatic activity, which could result in discrepancy in the results relative to what is already known in literature. However, it is know from scarce studies that SPT inhibition alone or in combination treatments suppresses cell proliferation and induce cell death and cell cycle arrest, therefore, SPT inhibitors are though to be novel therapeutic agents based on the cell context (Bernhart et al. 2015; Kojima et al. 2018). In glioma cells, the major mechanism behind SPT inhibition-mediated growth suppression and apoptosis was related to impaired S1P receptor (S1PR) signaling due to decreased amount of S1P (Bernhart et al. 2015). Oppositely, in PL-21 AML cells, a novel SPT inhibitor led to growth suppression via arresting cells at G2/M and inducing apoptosis-independent cell death. This inhibitor reduced the levels of C16-, C24-Cer and C16-, C24-SM, which were suggested as main way of growth inhibition and reducing S1P levels by SPT inhibition was not related to its anti-proliferative effects (Yaguchi et al. 2017). Myriocin treatment decreased SM, Cer and S1P levels, induced both necrosis and apoptosis and suppressed pAKT levels in in vitro and in vivo models of merkel cell carcinoma (Bhat et al. 2019). Sano et al. (2017) proposed that a novel SPT inhibitor triggered necrosis-dependent cell death by upregulating COX-2 in lung cancer cells with an unknown mechanism. In lung cancer cells, myriocin induced cell death via activation of death receptor 4 (DR4) pathway alone or in combination with DR4 ligand TRAIL, docetaxel and cisplatin (Choi et al. 2014). Therefore, SPT’s activity should be evaluated to observe changes in the levels of sphingolipid species especially long carbon chain Cer, SM and S1P in response to resveratrol to understand why resveratrol plus myriocin treatment gave significant cytotoxic and apoptotic effects.
In conclusion, growth-inhibition of FLT3-ITD AML cells in the presence of resveratrol plus SPT inhibitor myriocin was mainly associated with induction of apoptosis. However, cell cycle arrest was also effective in MV4-11 cells. Nevertheless, the mechanism(s) leading to resveratrol-induced SPT upregulation and its role in resveratrol’s activity and the mechanisms underlying the response of cells to resveratrol plus myriocin remain to be determined. The activity of SPT in resveratrol treated cells and the quantification of Cer, SM and other sphingolipid species might be the future directions to confirm the involvement of SPT in resveratrol induced antileukemic effects. Overall, it could be suggested that resveratrol might be a chemopreventive in FLT3-ITD AML after its mechanism of action in relation de novo pathway is clarified in detail.
Acknowledgements
The authors acknowledge Genome and Stem Cell Center of Erciyes University for providing the flow cytometry facility. We are grateful to Esma Saraymen, the specialist, for flow cytometry measurements.
Author contributions
Conceptualization: AA; Acquisition, analysis or interpretation of data: NŞE, AA; Writing - review and editing: NŞE, AA; Approval of the final version: NŞE, AA; Supervision and principal author: AA.
Funding
This study was supported by Abdullah Gul University Scientific Research Projects Coordination Unit with Project No: FAB-2016-66.
Data availability
The manuscript has no associated data.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adan A, Baran Y. The pleiotropic effects of fisetin and hesperetin on human acute promyelocytic leukemia cells are mediated through apoptosis, cell cycle arrest, and alterations in signaling networks. Tumor Biol. 2015;36:8973–8984. doi: 10.1007/s13277-015-3597-6. [DOI] [PubMed] [Google Scholar]
- Ambinder AJ, Levis M. Potential targeting of FLT3 acute myeloid leukemia. Haematologica. 2021;106:671–681. doi: 10.3324/haematol.2019.240754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antar AI, Otrock ZK, Jabbour E, Mohty M, Bazarbachi A. FLT3 inhibitors in acute myeloid leukemia: ten frequently asked questions. Leukemia. 2020;34:682–696. doi: 10.1038/s41375-019-0694-3. [DOI] [PubMed] [Google Scholar]
- Bernhart E, Damm S, Wintersperger A, Nusshold C, Brunner AM, et al. Interference with distinct steps of sphingolipid synthesis and signaling attenuates proliferation of U87MG glioma cells. Biochem Pharmacol. 2015;96:119–130. doi: 10.1016/j.bcp.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta M, Bignucolo A, Di FranciaComello RF, Facchini G, Ceccarelli M, et al. Resveratrol in cancer patients: from bench to bedside. Int J Mol Sci. 2020;21:2945. doi: 10.3390/ijms21082945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara VK, Mittal B, Mysorekar VV, Amaresh N, Simal-Gandara J. Resveratrol, cancer and cancer stem cells: a review on past to future. Curr Res Food Sci. 2020;3:284–295. doi: 10.1016/j.crfs.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat VK, Bernhart E, Plastira I, Fan K, Ghaffari-Tabrizi-Wizsy N, Wadsack C, et al. Pharmacological inhibition of serine palmitoyl transferase and sphingosine kinase-1/-2 inhibits merkel cell carcinoma cell proliferation. J Invest Dermatol. 2019;139:807–817. doi: 10.1016/j.jid.2018.10.024. [DOI] [PubMed] [Google Scholar]
- Cakir Z, Saydam G, Sahin F, Baran Y. The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60: acute myeloid leukemia cells. J Cancer Res Clin Oncol. 2011;137:279–286. doi: 10.1007/s00432-010-0884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfant CE, Rathman K, Pinkerman RL, Wood RE, Obeid LM, Ogretmen B, Hannun YA. De novo ceramide regulates the alternative splicing of caspase 9 and Bcl-x in A549 lung adenocarcinoma cells. Dependence on protein phosphatase-1. J Biol Chem. 2002;277:12587–12595. doi: 10.1074/jbc.M112010200. [DOI] [PubMed] [Google Scholar]
- Charytoniuk T, Harasim-Symbor E, Polak A, Drygalski K, Berk K, Chabowski A, et al. Influence of resveratrol on sphingolipid metabolism in hepatocellular carcinoma cells in lipid overload state. Anticancer Agents Med Chem. 2019;19:121–129. doi: 10.2174/1871520619666181224161255. [DOI] [PubMed] [Google Scholar]
- Choi KE, Jung YS, Kim DH, Song JK, Kim JY, Jung YY, et al. Myriocin induces apoptotic lung cancer cell death via activation of DR4 pathway. Arch Pharm Res. 2014;37:501–511. doi: 10.1007/s12272-013-0315-z. [DOI] [PubMed] [Google Scholar]
- Chou TC. Preclinical versus clinical drug combination studies. Leuk Lymphoma. 2008;49:2059–2080. doi: 10.1080/10428190802353591. [DOI] [PubMed] [Google Scholar]
- Chow SE, Kao CH, Liu YT, Cheng ML, Yang YW, Huang YK, Hsu CC, Wang JS. Resveratrol induced ER expansion and ER caspase-mediated apoptosis in human nasopharyngeal carcinoma cells. Apoptosis. 2014;19:527–541. doi: 10.1007/s10495-013-0945-0. [DOI] [PubMed] [Google Scholar]
- Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33:299–312. doi: 10.1038/s41375-018-0357-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei Cas M, Ghidoni R. Cancer prevention and therapy with polyphenols: sphingolipid-mediated mechanisms. Nutrients. 2018;10:940. doi: 10.3390/nu10070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfini E, Roncoroni L, Dogliotti E, Sala G, Erba E, Sacchi N, Ghidoni R. Resveratrol impairs the formation of MDA-MB-231 multicellular tumor spheroids concomitant with ceramide accumulation. Cancer Lett. 2007;249:143–147. doi: 10.1016/j.canlet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Ha YN, Song S, Orlikova-Boyer B, Cerella C, Christov C, Kijjoa A, Diederich M. Petromurin c induces protective autophagy and apoptosis in flt3-itd-positive aml: synergy with gilteritinib. Mar Drugs. 2020;18:57. doi: 10.3390/md18010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XT, Li X, Xie ML, Huang Z, Huang YX, Wu GX, et al. Resveratrol: review on its discovery, anti-leukemia effects and pharmacokinetics. Chem Biol Interact. 2019;306:29–38. doi: 10.1016/j.cbi.2019.04.001. [DOI] [PubMed] [Google Scholar]
- Kisková T, Kassayová M. Resveratrol action on lipid metabolism in cancer. Int J Mol Sci. 2019;20:2704. doi: 10.3390/ijms20112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS. The role of resveratrol in cancer therapy. Int J Mol Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Asano Y, Kurasawa O, Hirata Y, Iwamura N, Wong TT, et al. Discovery of novel serine palmitoyltransferase inhibitors as cancer therapeutic agents. Bioorg Med Chem. 2018;26:2452–2465. doi: 10.1016/j.bmc.2018.04.008. [DOI] [PubMed] [Google Scholar]
- Lee YS, Choi KM, Choi MH, Ji SY, Lee S, Sin DM, et al. Serine palmitoyltransferase inhibitor myriocin induces growth inhibition of B16F10 melanoma cells through G2/M phase arrest. Cell Prolif. 2011;44:320–329. doi: 10.1111/j.1365-2184.2011.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Choi KM, Lee S, Sin DM, Lim Y, Lee YM, et al. Myriocin, a serine palmitoyltransferase inhibitor, suppresses tumor growth in a murine melanoma model by inhibiting de novo sphingolipid synthesis. Cancer Biol Ther. 2012;13:92–100. doi: 10.4161/cbt.13.2.18870. [DOI] [PubMed] [Google Scholar]
- Lim KG, Gray AI, Pyne S, Pyne NJ. Resveratrol dimers are novel sphingosine kinase 1 inhibitors and affect sphingosine kinase 1 expression and cancer cell growth and survival. Br J Pharmacol. 2012;166:1605–1616. doi: 10.1111/j.1476-5381.2012.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YS, Hsieh CY, Kuo TT, Lin CC, Lin CY, Sher YP. Resveratrol-mediated ADAM9 degradation decreases cancer progression and provides synergistic effects in combination with chemotherapy. Am J Cancer Res. 2020;10:3828–3837. [PMC free article] [PubMed] [Google Scholar]
- Lindblad O, Li T, Su X, Sun J, Kabir NN, Levander F, Zhao H, Lu G, Rönnstrand L, Kazi JU. BEX1 acts as a tumor suppressor in acute myeloid leukemia. Oncotarget. 2015;6:21395–21405. doi: 10.18632/oncotarget.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Liu GJ, Song JY, Chen L, Wang AH, Gao XX, Wang ZJ. Preliminary results indicate resveratrol affects proliferation and apoptosis of leukemia cells by regulating PTEN/PI3K/AKT pathway. Eur Rev Med Pharmacol Sci. 2019;23:4285–4292. doi: 10.26355/eurrev_201905_17933. [DOI] [PubMed] [Google Scholar]
- Mizutani N, Omori Y, Kawamoto Y, Sobue S, Ichihara M, Suzuki M, et al. Resveratrol-induced transcriptional up-regulation of ASMase (SMPD1) of human leukemia and cancer cells. Biochem Biophys Res Commun. 2016;470:851–856. doi: 10.1016/j.bbrc.2016.01.134. [DOI] [PubMed] [Google Scholar]
- Nganga R, Oleinik N, Ogretmen B. Mechanisms of ceramide-dependent cancer cell death. Adv Cancer Res. 2018;140:1–25. doi: 10.1016/bs.acr.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer. 2018;18:33–50. doi: 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oğuz O, Adan A. Involvement of sphingolipid metabolism enzymes in resveratrol-mediated cytotoxicity in philadelphia-positive acute lymphoblastic leukemia. Nutr Cancer. 2021 doi: 10.1080/01635581.2021.2005806. [DOI] [PubMed] [Google Scholar]
- Opydo-Chanek M, Rak A, Cierniak A, Mazur L. Combination of ABT-737 and resveratrol enhances DNA damage and apoptosis in human T-cell acute lymphoblastic leukemia MOLT-4 cells. Toxicol In Vitro. 2017;42:38–46. doi: 10.1016/j.tiv.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Razumovskaya E, Sun J, Rönnstrand L. Inhibition of MEK5 by BIX02188 induces apoptosis in cells expressing the oncogenic mutant FLT3-ITD. Biochem Biophys Res Commun. 2011;412:307–312. doi: 10.1016/j.bbrc.2011.07.089. [DOI] [PubMed] [Google Scholar]
- Sano O, Kazetani KI, Adachi R, Kurasawa O, Kawamoto T, Iwata H. Using a biologically annotated library to analyze the anticancer mechanism of serine palmitoyl transferase (SPT) inhibitors. FEBS Open Bio. 2017;7:495–503. doi: 10.1002/2211-5463.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. FASEB J. 2003;17:2339–2341. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Sala G, Ricci C, Maioli C, Milani F, Minella M, Botturi M, Ghidoni R. Resveratrol sensitization of DU145 prostate cancer cells to ionizing radiation is associated to ceramide increase. Cancer Lett. 2007;253:124–130. doi: 10.1016/j.canlet.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Scholl S, Fleischmann M, Schnetzke U, Heidel FH. Molecular mechanisms of resistance to FLT3 inhibitors in acute myeloid leukemia: ongoing challenges and future treatments. Cells. 2020;9(11):2493. doi: 10.3390/cells9112493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J, Costa-Pinheiro P, Patterson L, Drews K, Spiegel S, Kester M. Novel sphingolipid-based cancer therapeutics in the personalized medicine era. Adv Cancer Res. 2018;140:327–366. doi: 10.1016/bs.acr.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedlecka-Kroplewska K, Wozniak M, Kmiec Z. The wine polyphenol resveratrol modulates autophagy and induces apoptosis in MOLT-4 and HL-60 human leukemia cells. J Physiol Pharmacol. 2019 doi: 10.26402/jpp.2019.6.02. [DOI] [PubMed] [Google Scholar]
- Ulrich S, Huwiler A, Loitsch S, Schmidt H, Stein JM. De novo ceramide biosynthesis is associated with resveratrol-induced inhibition of ornithine decarboxylase activity. Biochem Pharmacol. 2007;74:281–289. doi: 10.1016/j.bcp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Yaguchi M, Shibata S, Satomi Y, Hirayama M, Adachi R, Asano Y, et al. Antitumor activity of a novel and orallyavailable inhibitor of serine palmitoyltransferase. Biochem Biophys Res Commun. 2017;484:493–500. doi: 10.1016/j.bbrc.2017.01.075. [DOI] [PubMed] [Google Scholar]
- Nur Şebnem, Ersöz Aysun, Adan (2022) Resveratrol triggers anti-proliferative and apoptotic effects in FLT3-ITD-positive acute myeloid leukemia cells via inhibiting ceramide catabolism enzymes. Medical Oncology 39(3) 10.1007/s12032-021-01627-2 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript has no associated data.