Abstract
Perilla frutescens (L.) Britton var. frutescens (egoma in Japan) is a traditional oilseed that has several varieties with different photoperiod responses. Although egoma pomace, industrial waste produced during oil extraction, is a rich source of macro- and micro-nutrients such as protein, fiber, minerals, and polyphenols, it has not yet been used for purposes other than livestock feeding. To find out a better use of perilla pomace and its function, we selected four varieties of egoma originating from different regions with different photoperiod responses: two varieties were from Japan, which are broadly cultivated for oilseed and are highly sensitive to light and temperature. The other two varieties from Nepal, which are tolerant to low light and low temperature. Rosmarinic acid-3-O-glucoside, rosmarinic acid, and apigenin-7-O-glucoside were detected as the main polyphenolic constituents in every variety, while apigenin and luteolin were present only in perilla pomace from Japan. In IgE-sensitized RBL-2H3 cells, polyphenols derived from two varieties of Japan suppressed degranulation of mast cells, but those derived from the two varieties of Nepal did not, indicating that apigenin and luteolin may be in part responsible for the anti-allergic response. In addition, it was found that proteins involved in the degranulation signaling pathway, such as PLCγ2, Syk, and Akt, were less phosphorylated in cells treated with the egoma pomace extracts of Japanese origin. Taken together, pomace from egoma varieties derived from different regions may differently modulate allergic response in part due to the difference in polyphenol composition and may be applied to develop nutraceuticals and functional foods fortified with anti-allergic properties.
Keywords: Anti-allergy, Perilla pomace, Apigenin, Luteolin, Rosmarinic acid, Degranulation
Introduction
Perilla frutescens (L.) Britton var. frutescens is an annual plant belonging to the mint family Lamiaceae, and has been used as a source of edible vegetables and traditional Chinese and Ayurvedic medicine to treat depression-related diseases, asthma and so on (Makino et al. 2003 and Dhyani et al. 2019). Perilla frutescens (L.) Britton var. frutescens (egoma in Japanese and silam in Nepal, hereafter referred to as egoma) has been widely cultivated in Asian countries such as China, Japan, South Korea, Vietnam, India, and Nepal, and has a broad swath of physiological functions (Hu et al. 2010; Zhou et al. 2014).
Seeds from egoma are mainly used for oil production and are rich in essential fatty acid, α-linolenic acid. Egoma oil has been recently reported to exert protective effects against high-fat diet-induced nonalcoholic fatty liver disease, gut dysbiosis, and neuronal cell death as well as anti-hyperlipidemic activity (Tian et al. 2016 and Lee et al. 2018). According to the Standard Tables of Food Composition in Japan 2015 (seventh revised edition), seeds from egoma are composed of 3.9% ash, 17.7% protein, and 20.8% fiber (1.7% soluble fiber and 19.1% insoluble fiber) in addition to 43.4% oil (Standard Tables of Food Composition in Japan). Egoma pomace is produced from cold-pressed oil extraction as a by-product and has been, for example, fed to pigs and laying hens to produce α-linolenic acid-rich meat and eggs. The percentages of minerals, proteins, and fibers are relatively greater in egoma pomace than in egoma seeds. Because of its nutrient content, egoma pomace could be utilized in different and better ways other than animal feed.
Since egoma has several varieties in Japan and other Asian countries and since these varieties respond differently to photoperiod, the timing of harvesting and choice of variety may also influence the levels and types of polyphenols, consequently resulting in different physiological functions. Accordingly, quantifying the polyphenols of egoma pomace obtained from different regions as well as different varieties may provide valuable information to seek egoma pomace with higher amounts of polyphenols and useful physiological functions. The aim of the current study was to quantify the content of major polyphenols in egoma pomace originating from different regions and to investigate functional differences in allergic responses in vitro. In this direction, we selected four varieties of egoma from Japan and Nepal.
Materials and methods
Reagents
Apigenin, apigenin-7-O-glucoside, caffeic acid, rosmarinic acid, and luteolin were purchased from Sigma-Aldrich (Tokyo, Japan) and rosmarinic acid-3-O-glucoside was purchased from Chemfaces (Wuhan, China). A23187 was procured from BioVision Inc. (Milpitas, CA, USA). Dulbecco’s modified Eagle medium (DMEM) was supplied by Nissui (Tokyo, Japan). Laemmli sample buffer and PVDF membranes were obtained from Bio-Rad (Hercules, CA USA). Akt (pan)(C67E7) rabbit mAb (#4691), P-Akt (T308)(C31E5E) rabbit mAb (#2965), phospho-Src family (Tyr416)(D49G4) rabbit mAb (#6943), Lyn (C13F9) rabbit mAb (#2796), phospho-PLCγ2 (Tyr1217) antibody (#3871), PLCγ2 antibody (#3872), phospho-Syk (Tyr525/526)(C87C1) rabbit mAb (#2710), Syk (D3Z1E) XP rabbit mAb (#13,198), and β-actin (13E5) rabbit mAb (HRP conjugate) (#5125) were purchased from Cell Signaling Technology (Danvers, MA, USA). HRP-labeled anti-rabbit IgG secondary antibody was acquired from Novus Biologicals (Centennial CO). Can Get Signal solutions 1 and 2 were purchased from Toyobo, Co., Ltd (Osaka, Japan), and TMB solution was obtained from Nacalai Tesque (Kyoto, Japan). Other reagents were of analytical grade and were used without further purification.
Plant materials
Egoma pomace used in the present study was derived from four locally grown varieties of egoma and obtained from Ecobito Farm & Company (Kanzaki, Saga, Japan). The name of the egoma variety was denoted according to the color of seeds created by the National Agriculture and Food Research Organization (NARO) of Japan. The four selected egoma varieties of different origins had different photoperiod responses. Egoma varieties originating from Nepal were denoted as cold resistant grayish white 1 and cold resistant grayish white 2, whereas those from Japan were blackish gray and grayish white. The photoperiod sensitivity of the two varieties from Nepal was different from those of the two Japanese varieties. The two varieties from Nepal were late blooming and relatively cold resistant, while those from Japan were not cold resistant and broadly cultivated for oilseed.
Preparation of egoma pomace extracts
Egoma seeds were crushed and ground to make a paste using a CMT Vibrating Sample Mill T1-100 (CMT Co., Ltd., Fukushima, Japan). An aliquot of egoma paste (1.0 g) was defatted twice with 20 mL of hexane at room temperature for 1 h. The resulting residue was dried and extracted twice with 10 mL of 80% ethanol by shaking at room temperature for 1 h using a tube mixer (CM-1000 Cute Mixer, EYELA, Tokyo, Japan), and centrifuged with a Kubota 5100 centrifuge (Kubota Corporation, Tokyo, Japan) at 1600×g for 15 min. The combined extract was transferred to a measuring flask and filled up to 20 mL. The extract was dried in a tube by a centrifugal evaporation (MiVac Quattro Concentrator, Genevac Ltd., Ipswitch, Suffolk, UK). The resultant dried egoma pomace extract was dissolved in 80% ethanol (50 mg/mL).
Total polyphenol measurement
The levels of total polyphenol were analyzed according to the method of Tsuruta et al. (2011) with a slight modification. In brief, the extract was diluted 50 times with 80% ethanol. Ten μL of the diluted samples was mixed with 75 μL of Folin-Ciocalteu's phenol reagent (Ainsworth and Gillespie 2007), diluted 10 times with water and incubated for 5 min after mixing well. The mixture was incubated in the dark for 15 min at room temperature after adding 75 μL of 2% anhydrous sodium carbonate and its absorbance was measured at 720 nm using a spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The concentrations were calculated using associated instrument software (SoftMax-Pro 5.4.1, Molecular Device, Sunnyvale, CA, USA). Rosmarinic acid was used as a standard compound and the total polyphenol content was expressed as rosmarinic acid equivalent (mg RE/g dry weight (dw)).
Determination of total flavonoid content
The total flavonoid content in egoma pomace was determined using the aluminum chloride colorimetric method of Chang et al. (2002) and Khanam and Oba (2013). In brief, 500 μL of a 1:50 dilution of the egoma pomace extracts was mixed with 1.5 mL of methanol, 0.1 mL of 10% aluminum chloride, 0.1 mL of 1 M potassium acetate, and 2.8 mL of distilled water. After incubating at room temperature for 30 min, the absorbance was measured at 415 nm using a spectrophotometer. Luteolin was used as a standard compound and the total flavonoid content was expressed as luteolin equivalent (mg LE/g dw).
Determination of phenolic compounds
HPLC analysis of the egoma pomace extracts was carried out using an HPLC System (Dionex™ UltiMate™ 3000, Thermo Fisher Scientific Inc., Sunnyvale, CA, USA). The egoma pomace extracts were filtered through a 0.45 µm filter (Toyo Roshi Kaisha, Ltd., Tokyo, Japan). Then, 3 µL of 50 times-diluted extract from egoma pomace was injected onto an analytical unison UK -C18 column (250 mm × 2, i.d., Imtakt Crop, Kyoto, Japan) at 40 °C. The mobile phase was composed of 0.1 volume of formic acid in water (eluent A) and 0.1 vol% of formic acid in acetonitrile (eluent B). The gradient program was as follows: 10% B (0 min), 50% B (20 min), 100% B (21 to 27 min), and 10% B (28 min and 40 min). The total run-time was 40 min. The absorbance at 330 nm was measured to detect phenolic compounds. A standard with known retention time was used to calculate the phenol content by comparing the peak area with that of the standard. The concentration of the standard ranged from 0.625 to 100 µg/mL.
Anti-allergic activity
Cells and cell culture
Rat basophilic leukemia RBL-2H3 cells were obtained from the Japanese Collection of Research Bioresources Cell Bank (Tokyo, Japan). The cells were maintained in DMEM supplemented with 10% fetal bovine serum (FBS) at 37 °C in humidified 5% CO2–95% air, as described elsewhere (Iwamoto et al. 2020).
Cell viability
Cell viability was measured using the CCK-8 assay (Dojindo, Kumamoto, Japan) according to the manufacturer’ instructions. RBL-2H3 cells (2.0 × 105 cells/mL) were cultured as described previously (Yoshioka et al. 2013). In brief, the culture medium, after 2 days’ incubation, was replaced with 10% FBS-DMEM containing 10% CCK-8 solution. The supernatant was collected and its absorbance at 450–650 nm was determined using a microplate reader (Molecular Devices, LLC, San Jose, CA, USA).
β-Hexosaminidase release
The anti-allergic activity of polyphenols derived from egoma pomace was evaluated by determining the inhibitory effects on the release of β-hexosaminidase from RBL-2H3 cells, as described previously (Yoshioka et al. 2013). In brief, RBL-2H3 cells in DMEM supplemented with 10% FBS were cultured at 6.0 × 104 cells/well in 96-well cell culture plates overnight at 37 °C in humidified 5% CO2–95% air. Next, the cells were treated with 33.3 ng/mL of anti-DNP IgE at 37 °C for 2 h and washed twice with 100 µL of modified Tyrode’s buffer with or without the egoma pomace extracts at 37 °C for 10 min. Then, 30 µL of DNP-HSA (200 µg/mL) was added to each well and incubated at 37 °C for 1 h. The supernatant (50 µL) was transferred into a 96-well microplate and 100 µL of 3.3 mM p-nitrophenyl-N-acetyl-β-d-glucosamide in 0.1 M citrate buffer (pH 4.5) was added and incubated at 37 °C for 25 min. The enzyme reaction was terminated by the addition of 100 µL of 0.1 M glycine buffer (pH 10) and the absorbance of the solution was determined at 405 nm using a microplate reader. β-Hexosaminidase release was calculated as follows: 100 x (Abs405sample/Abs405control).
Western blotting
RBL-2H3 cells were cultured at 6.0 × 104 cells/well in 24-well cell culture plates overnight at 37 °C in humidified 5% CO2-95% air and treated with anti-DNP IgE. After the anti-DNP IgE-treated cells were washed twice with modified Tyrode’s buffer, the cells were treated with 500 µL of the modified Tyrode’s buffer with or without the egoma pomace extracts at 37 °C for 10 min. Then, 150 µL of DNP-HSA (200 µg/mL) was added to each well followed by incubation at 37 °C for 1 h. After removing the reagent, the cells were lysed using Laemmli sample buffer and centrifuged at 15,000×g at 4 °C for 10 min. The supernatant was subjected to SDS-PAGE, and proteins were electrophoretically transferred to a PVDF membrane. The membrane was blocked with a PVDF blocking reagent for Can Get Signal at 4 °C overnight. After washing with phosphate-buffered saline with 0.1% Tween (PBS-T), the membrane was incubated with a primary antibody in Can Get Signal solution 1 at room temperature for 2 h. Next, the membrane was washed with PBS-T and incubated with HRP-labeled anti-rabbit IgG antibody in Can Get Signal solution 2 at room temperature for 1 h. After washing with PBS-T, blots were developed using TMB solution for western blotting (Nacalai Tesque, Kyoto, Japan). The band density was quantified using Image J software developed at the National Institute of Health (USA). Results were expressed as a ratio of target protein against a housekeeping protein β-actin.
Statistics
All values are expressed as mean ± SE. Statistical analysis was performed using one-way ANOVA with Tukey–Kramer’s post hoc test, and a p-value < 0.05 was considered statistically significant (Graph Pad Prism 6 version 6.07).
Results
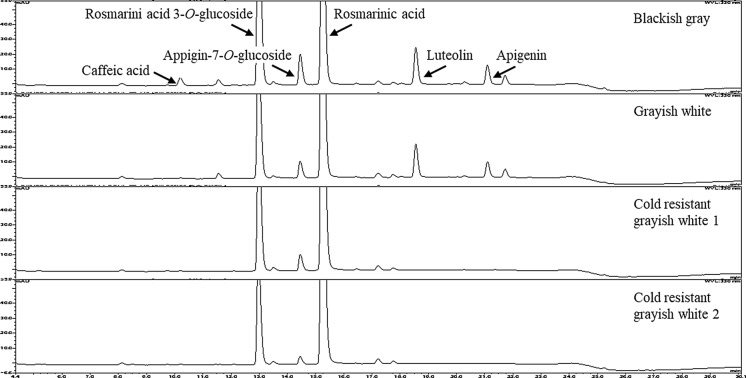
HPLC chromatograms of 80% ethanol extracts of pomace from four varieties of egoma showed the presence of rosmarinic acid-3-O-glucoside, rosmarinic acid, apigenin-7-O-glucoside, caffeic acid, luteolin, and apigenin (Fig. 1). The quantitative analysis of total polyphenol, total flavonoid, and individual major phenolic compounds was performed using HPLC, and the results are presented in Table 1. Varietal differences in the content of phenolic compounds were detected in the cold resistant grayish white 1 and 2 relative to blackish gray and grayish white varieties; three extra peaks were detected in the pomace of blackish gray and grayish white perilla, two of which were luteolin and apigenin. Cold resistant varieties 1 and 2 had a significantly higher rosmarinic acid content than the two Japanese varieties. The blackish gray variety had the highest amount of rosmarinic acid-3-O-glucoside among the four varieties. Caffeic acid was found only in blackish gray variety. Although the peak corresponding to caffeic acid was also evident in other varieties, its content was below the detection limit. Apigenin-7-O-glucoside was found in all varieties with blackish gray having the highest content. Luteolin and apigenin were detected only in Japanese varieties and the levels of them were relatively higher in the blackish gray variety.
Fig. 1.
HPLC spectra of polyphenols of egoma pomace derived from four different varieties
Table 1.
The amount of total polyphenol and total flavonoid in pomace of perilla frutescens var. frutecens varieties
| Variety | Rosmarinic acid-3-O-glucoside (mg/g) | Rosmarinic acid (mg/g) | Apigenin-7-O-glucoside (mg/g) | Apigenin(mg/g) | Luteolin (mg/g) | Caffeic acid (mg/g) | Total Polyphenol (mg RE/g) | Total flavonoid (mg LE/g) |
|---|---|---|---|---|---|---|---|---|
| Blackish gray | 7.21 ± 0.01a | 4.40 ± 0.04a | 0.41 ± 0.01a | 0.21 ± 0.00a | 0.48 ± 0.01a | 65.4 ± 1.2 | 12.26 ± 0.79a | 1.10 ± 0.00a |
| Grayish white | 3.64 ± 0.03b | 4.41 ± 0.07a | 0.19 ± 0.00b | 0.14 ± 0.01b | 0.37 ± 0.01b | – | 8.99 ± 0.32b | 0.97 ± 0.02b |
| Cold resistant grayish white 1 | 3.62 ± 0.02b | 5.95 ± 0.04b | 0.17 ± 0.00c | – | – | – | 9.34 ± 0.65b | 0.49 ± 0.00c |
| Cold resistant grayish white 2 | 2.16 ± 0.01c | 4.66 ± 0.04c | 0.05 ± 0.00d | – | – | – | 9.47 ± 1.15b | 0.44 ± 0.00d |
1n = 3
2Different character shows a significant difference (P < 0.05)
The total polyphenol and total flavonoid contents are also presented in Table 1. The total polyphenol content in the blackish gray variety was 12.26 ± 0.79 mg RE/g dw, which was significantly higher than that in the grayish white variety and cold-resistant grayish white 1 and 2. The total flavonoid content in the blackish gray variety was highest (1.10 ± 0.00 mg LE/g dw) among the four different varieties.
To determine the effects of varietal differences in egoma pomace on allergic responses, the β-hexosaminidase release was measured in RBL-2H3 cells in the presence or absence of the extracts (Fig. 2). Both Japanese and Nepalese egoma pomace did not affect cell viability: at a concentration of 500, 100 and 20 mg/mL of Grayish white, cell viability was 106.6 ± 0.3, 102.4 ± 6.0, and 100.0 ± 3.4, respectively, compared to the control. When compared to egoma pomace of cold resistant grayish white 1 and 2, blackish gray and grayish white significantly inhibited the release of β-hexosaminidase from RBL-2H3 cells. Egoma pomace originating from Japan decreased the release by 44.2–43.8%.
Fig. 2.
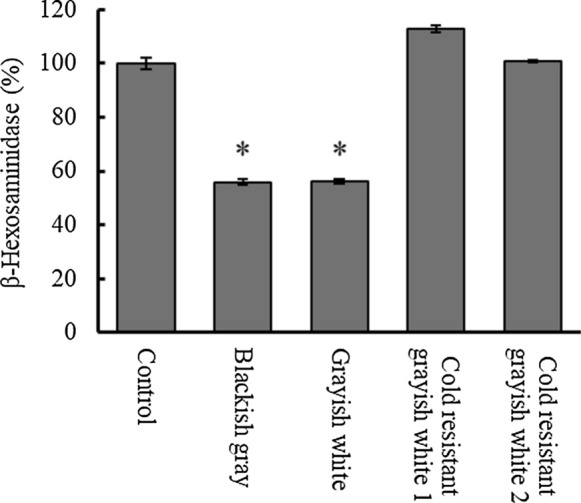
Effects of egoma pomace extracts on the degranulation of RBL-2H3 cells. RBL-2H3 cells were stimulated with A23187 (10 µM) in the presence of egoma pomace extracts to induce the release of b-hexosaminidase. Data are expressed as means ± SEM (n = 3). Statistically significant differences from the control are shown as *p < 0.05
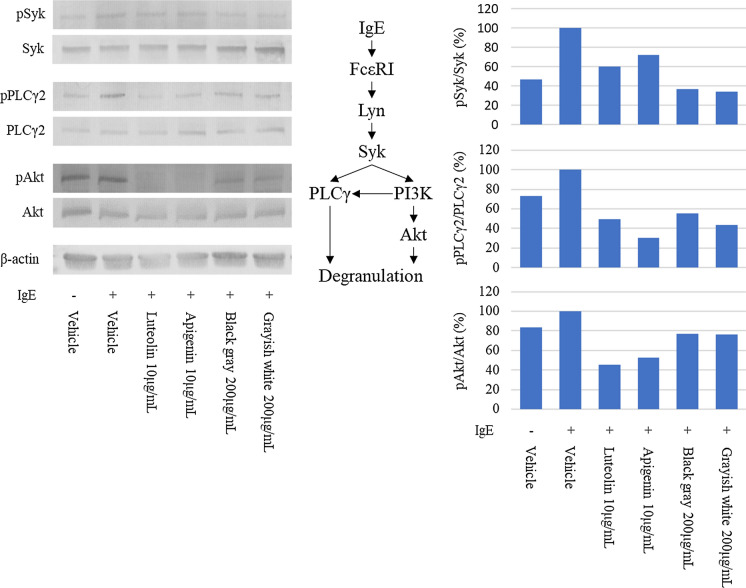
As apigenin and luteolin found in blackish gray and grayish white are known to suppress allergic response (Park et al. 2020; Ueda et al. 2002), to elucidate the mechanism by which egoma pomace from these two varieties alleviates degranulation, the effects of the extracts from Japanese egoma pomace as well as apigenin and luteolin were determined on the expression of proteins involved in degranulation cascade by using a western blot (Fig. 3). In Fig. 3A, 200 μg/mL of the extracts used contained 1.1 μg/mL and 1.06 μg/mL of apigenin and 2.50 μg/mL and 2.81 μg/mL of luteolin in Blackish gray and Grayish white, respectively. Although the level of pSyk was reduced in the presence of egoma pomace polyphenol, it was not affected much by luteolin or apigenin. Similarly, incubation with apigenin, luteolin, and polyphenols from blackish gray and grayish white decreased the ratio of pPLCγ2/PLCγ2. Although the addition of IgE enhanced the phosphorylation of Akt, luteolin and apigenin markedly inhibited the same, while polyphenols from egoma pomace exerted a negligible effect on Akt phosphorylation. In contrast, neither authentic polyphenols nor the extracts from egoma affected phosphorylation of Src (data not shown).
Fig. 3.
Effect of egoma pomace extracts on the expression of degranulation-related proteins in RBL-2H3 cells. (left) Representative images of proteins. (right) The relative densities of protein levels. Vehicle: anti-DNP IgE alone was added
Discussions
After oil extraction, egoma pomace becomes an industrial waste irrespective of being a rich source of proteins and dietary fiber. So far, it has not been used for purposes other than animal feed. Like shiso, which belongs to the mint family Lamiaceae and contains polyphenols such as apigenin, luteolin, and rosmarinic acid with beneficial physiological functions, egoma pomace has a wide variety of functional polyphenols, as shown in Table 1. Similar types of phenolic compounds are present in different varieties; however, the amounts appear to vary depending on environmental and genetic factors. Rosmarinic acid-3-O-glucoside, rosmarinic acid, and apigenin-7-O-glucoside were common among varieties whereas luteolin and apigenin were found only in Japanese varieties. Interestingly, caffeic acid was present only in the blackish gray variety from Japan. Thus, this variation in phenolic compounds such as apigenin, luteolin, and caffeic acid may determine varietal differences in the functional properties of egoma pomace. Polyphenols are naturally occurring secondary metabolites present in a wide range of plants and involved in defense against ultraviolet radiation or aggression by pathogens. In addition, numerous factors affect the contents of polyphenols in plants, some of which are the degree of ripeness, the time of harvesting, and factors related to food processing such as the length of storage and temperature (Pandey and Rizvi 2009). Here, we found varietal differences in the content of individual polyphenols associated with different physiological functions. Polyphenols are known as anti-allergic agents capable of influencing a wide range of cellular biological and immune pathways during allergic responses (Mlcek et al. 2016; Matsuda et al. 2016; Mwakalukwa et al. 2019). Among them, caffeic acid has been reported to be a widely distributed metabolite in plants that exerts anti-inflammatory and anti-cancer activities (Touaibia et al). Apigenin is also polyphenols that has been extensively studied for its anti-carcinogenic and anti-microbial activities (Yan et al. 2017). Thus, to investigate the novel function of egoma pomace, we focused on the effects of differences in the quality of polyphenols on the allergic response in RBH-2H3 cells.
In this study, we detected varietal differences in anti-allergic activity as shown in Fig. 2. Allergy-suppressing activity was observed in blackish gray and grayish white, but not in grayish white 1 and 2. In previous studies, plant extracts containing luteolin were found to be effective in inhibiting the degranulation of mast cells (Shimoda et al. 2006), which was partly due to a decreased inflammatory response (Ueda et al. 2002). Rosmarinic acid is also associated with reduced symptoms of allergic rhinitis (Takano et al. 2004). However, it is unlikely that rosmarinic acid is implicated in the anti-allergic activity of egoma pomace because the levels of rosmarinic acid in Japanese varieties were significantly lower than those in the Nepalese varieties (Table 1). This indicates that the anti-allergic activity of pomace from blackish gray and grayish white varieties could be at least in part due to the presence of luteolin and apigenin rather than rosmarinic acid. However, it cannot be ruled out that polyphenols other than apigenin, luteolin and their derivatives, which were not detected in our analysis system in the present study, may also account for anti-allergic response. It is also speculated that apigenin, luteolin, and rosmarinic acid synergistically and differently affect allergic pathway. Caffeic acid has been suggested as an active constituent to alleviate allergic reactions (Shimoda et al. 2006) and caffeic acid-containing plant (Aster yomena) has been shown to decrease degranulation in RBL-2H3 (Hwang et al. 2018). However, since only the blackish gray variety contained caffeic acid and both blackish gray and grayish white varieties exhibited inhibitory activity, caffeic acid is unlikely to be a strong candidate for anti-degranulation activity in the egoma pomace.
Considering the mechanism underlying the effect of egoma pomace on degranulation in RBL-2H3, proteins involved in the FcεRI signaling cascade were determined. As shown in Fig. 3A, once the antigen and IgE are attached to FcεRI, a signaling cascade of degranulation, which is modified by a variety of proteins through phosphorylation/dephosphorylation, triggers the release of β-hexosaminidase, resulting in allergic responses such as rhinitis and edema. Although to a different extent, polyphenol extracts from pomace of blackish gray and grayish white as well as apigenin and luteolin reduced phosphorylation of Syk, PLCγ2, and Akt, indicating that the suppression of allergic response by Japanese egoma pomace is at least in part due to decreased phosphorylation of proteins in a signaling cascade (Fig. 3). Compared to two authentic polyphenols, apigenin and luteolin, the extract from the two varieties decreased the phosphorylation of Syk to a greater extent, but that of Akt to a lesser extent, suggesting that polyphenols other than luteolin and apigenin may also account for the anti-allergic activity of egoma pomace. Alternatively, since apigenin is also present as apigenin-7-O-glucoside in nature (Wang et al. 2019), it is possible that apigenin derivatives are other candidates responsible for the anti-allergic potential. However, because apigenin-7-O-glucosde was also present in egoma pomace originating from Nepal which did not exhibit anti-allergic potential, it is unlikely that apigenin derivatives account for the anti-allergic activity of egoma pomace originating from Japan. To identify the polyphenols responsible for the anti-allergic action of egoma pomace and clarify the underlying mechanisms in detail, further studies are warranted in the future.
In conclusion, in addition to the fact that the degree of ripeness, the time of harvesting, and factors related to food processing such as the length of storage and temperature also determine the content of polyphenols in the seeds of plants (Pandey and Rizvi 2009), egoma pomace originating from different regions had differences in the content of phenolic compounds. Furthermore, egoma pomace from different regions modulated anti-inflammatory activity differently. Thus, this study will be helpful in expanding the application of egoma pomace to functional food and nutraceuticals.
Author contribution
YRG designed, executed the study and edited the manuscript. AI, KT, SO, and HH helped in the analysis. YN and TY supervised the study and edited the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ainsworth EK, Gillespie KM. Estimation of total phenolic and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Dhyani A, Chopra R, Garg M. A review on nutritional value, functional properties and pharmacological application of Perilla (Perilla frutescens L.) Biomed Pharmacol J. 2019;12:649–660. doi: 10.13005/bpj/1685. [DOI] [Google Scholar]
- Hu Y, Sun LW, Neo MC, Zhang YX, Wen CX, Xie XL, Liu YJ. Primary identifications and palynological observations of Perilla in China. J Syst Evol. 2010;48:133–145. doi: 10.1111/j.1759-6831.2010.00067.x. [DOI] [Google Scholar]
- Hwang K, Hwang Y-J, Song J. Anti-allergic effect of Aster yomena on ovalbumin-sensitized mouse RHL-2H3 cells via Th1/Th2 cytokine balance. J Funct Foods. 2018;44:1–8. doi: 10.1016/j.jff.2018.02.026. [DOI] [Google Scholar]
- Iwamoto A, Hamajima H, Tsuge K, Tsuruta Y, Nagata Y, Yotsumoto H, Yanagita Y. Inhibitory effects of green asparagus extract, especially phospholipids, on allergic response in vitro and in vivo. J Agric Food Chem. 2020;68:15199–15207. doi: 10.1021/acs.jafc.0c05615. [DOI] [PubMed] [Google Scholar]
- Khanam UKS, Oba S. Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and A. hypochondriacus. Can J Plant Sci. 2013;93:47–58. doi: 10.4141/cjps2012-117. [DOI] [Google Scholar]
- Lee AY, Choi JM, Lee MH, Lee J, Lee S, Cho EJ. Protective effects of perilla oil and alpha linolenic acid on SH-SY5Y neuronal cell death induced by hydrogen peroxide. Nutr Res Prac. 2018;12:93–100. doi: 10.4162/nrp.2018.12.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino T, Furuta Y, Wakushima H, Fujii H, Saito K, Kano Y. Anti-allergic effect of Perilla frutescens and its active constituents. Phytother Res. 2003;17:240–243. doi: 10.1002/ptr.1115. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Nakamura S, Yoshikawa M. Degranulation inhibitors from medicinal plants in antigen-stimulated rat basophilic leukemia (RBL-2H3) cells. Chem Pharm Bull. 2016;64:96–103. doi: 10.1248/cpb.c15-00781. [DOI] [PubMed] [Google Scholar]
- Mlcek J, Jurikova T, Skrovankova S, Sochor J. Quercetin and Its anti-allergic immune response. Molecules. 2016;21:623. doi: 10.3390/molecules21050623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwakalukwa R, Ashour A, Amen Y, Niwa Y, Tamrakar S, Miyamoto T, Shimizu K. Anti-allergic activity of polyphenolic compounds isolated from olive mill wastes. J Funct Foods. 2019;58:207–217. doi: 10.1016/j.jff.2019.04.058. [DOI] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxi Med Cell Long. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C-H, Min S-Y, Yu H-W, Kim K, Kim S, Lee H-J, Kim J-H, Park Y-J. Effects of apigenin on RBL-2H3, RAW264.7, and HaCat cells: anti-allergic, anti-inflammatory, and skin-protective activities. Int J Mol Sci. 2020;21:4620–4636. doi: 10.3390/ijms21134620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda H, Tanaka J, Yamada E, Morikawa T, Kasajima N, Yoshikawa M. Anti type I allergic property of Japanese butterbur extract and its mast cell degranulation inhibitor ingredients. J Agric Food Chem. 2006;54:2915–2920. doi: 10.1021/jf052994o. [DOI] [PubMed] [Google Scholar]
- Takano H, Osakabe N, Sanbongi C, Yanagisawa R, Inoue K, Yasuda A, Natsume M, Baba S, Ichiishi E, Yoshikawa T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp Biol Med. 2004;229:247–254. doi: 10.1177/153537020422900305. [DOI] [PubMed] [Google Scholar]
- Tian Y, Wang H, Yuan F, Li N, Huang Q, He L, Wang L, Liu Z. Perilla oil has similar protective effects of fish oil on high-fat diet-Induced nonalcoholic fatty liver disease and gut dysbiosis. BioMed Res Int. 2016;2016:1–11. doi: 10.1155/2016/9462571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touaibia M, Jean-Francois J, Doiron J. Caffeic acid, a versatile pharmacophore: an overview. Mini Review in Medicinal Chemistry. 2011;11:695–713. doi: 10.2174/138955711796268750. [DOI] [PubMed] [Google Scholar]
- Tsuruta Y, Nagao K, Kai S, Tsuge K, Yoshimura T, Koganemaru K, Yanagita T. polyphenolic extract of lotus root (edible rhizome of Nelumbo nucifera) alleviates hepatic steatosis in obese diabetic db/db mice. Lipid Health Dis. 2011;10:202–209. doi: 10.1186/1476-511X-10-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H, Yamazaki C, Yamazaki M. Luteolin as an anti-inflammatory and anti-allergic constituent of Perilla frutescens. Biol Pharm Bull. 2002;25:1197–1202. doi: 10.1248/bpb.25.1197. [DOI] [PubMed] [Google Scholar]
- Wang M, Firrman J, Liu L, Yam K. A review on flavonoid apigenin: dietary intake, ADME, antimicrobial effects, and interactions with human gut microbiota. BioMed Res Int. 2019;2019:7010467. doi: 10.1155/2019/7010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Qi M, Li P, Zhan Y, Shao H. Apigenin in cancer therapy: anti-cancer effects and mechanisms of action. Cell Biosci. 2017;7:50. doi: 10.1186/s13578-017-0179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Kamata A, Konishi T, Takahashi J, Oda H, Tamai T, Toyohara H, Sugahara T. Inhibitory effect of chlorophyll c2 from brown algae, Sargassum horneri, on degranulation of RBL-2H3 cells. J Funct Foods. 2013;5:204–210. doi: 10.1016/j.jff.2012.10.006. [DOI] [Google Scholar]
- Zhou X-J, Yan L-L, Yin P-P, Shi L-L, Zhang J-H, Liu Y-J, Ma C. Structural characterisation and antioxidant activity evaluation of phenolic compounds from cold-pressed Perilla frutescens var. arguta seed flour. Food Chem. 2014;164:150–157. doi: 10.1016/j.foodchem.2014.05.062. [DOI] [PubMed] [Google Scholar]