Abstract
A catalytic method for the enantioselective and C4-selective functionalization of pyridine derivatives is yet to be developed. Herein, we report an efficient method for the asymmetric β-pyridylations of enals that involve N-heterocyclic carbene (NHC) catalysis with excellent control over enantioselectivity and pyridyl C4-selectivity. The key strategy for precise stereocontrol involves enhancing interactions between the chiral NHC-bound homoenolate and pyridinium salt in the presence of hexafluorobenzene, which effectively differentiates the two faces of the homoenolate radical. Room temperature is sufficient for this transformation, and reaction efficiency is further accelerated by photo-mediation. This methodology exhibits broad functional group tolerance and enables facile access to a diverse range of enantioenriched β-pyridyl carbonyl compounds under mild and metal-free conditions.
Subject terms: Synthetic chemistry methodology, Photocatalysis
A catalytic method for the enantioselective and C4-selective functionalization of pyridine derivatives is yet to be developed. Here the authors report an efficient method for the asymmetric β-pyridylations of enals that involve N-heterocyclic carbene (NHC) catalysis with excellent control over enantioselectivity and pyridyl C4-selectivity.
Introduction
The pyridine moiety is an important structural unit found in many bioactive molecules, functional materials, and naturally occurring compounds1,2. Consequently, great effort has been devoted to functionalizing pyridine-containing compounds3–9. Recently, the photoinduced heteroarene modification has proven to be a powerful strategy for dealing with elusive radical transformations under mild conditions10–16. Despite the remarkable achievements of the Minisci-type radical-addition reaction, precise control over the regiochemical outcome of such a reaction has proven to be challenging owing to the presence of multiple reactive sites, which limits the site-selective applicability of this approach. Moreover, there is an increasing drive for higher degrees of three-dimensionality in pyridine-derived molecules for exploring chemical space, which necessitates the asymmetric installation of chiral centers adjacent to the pyridine core. Hou and collaborators reported a scandium-catalyzed enantioselective C–H alkylation of pyridines at the C2 position (Fig. 1a)17. Recently, the radical-mediated asymmetric approach has provided exciting strategies for the enantioselective syntheses of pyridines bearing adjacent stereogenic centers. The Phipps group reported the enantioselective additions of α-aminoalkyl radicals to the C2-positions of pyridines by dual photoredox and chiral phosphoric acid catalysis (Fig. 1a)18,19. Subsequently, Studer et al. reported an asymmetric three-component Minisci-type reaction using α-aminoalkyl radicals generated in situ20. High levels of asymmetric induction in this platform were achieved by exploiting favorable two-point interactions between protonated pyridines, phosphoric acid, and α-aminoalkyl radicals. Therefore, the scope of this approach is primarily confined to the ortho(C2)-selective radical addition to the pyridine core, and a catalytic method for the enantioselective and C4-selective functionalization of pyridine derivatives is yet to be developed. In this context, there remains a high demand for a new strategy that enables pyridine derivatives to be asymmetrically functionalized at the C4 position.
Fig. 1. Design plan.
a Enantioselective C2-alkylation of pyridines. b Breslow intermediate derivatives as chiral alkyl source. c Enantioselective NHC-catalyzed β-pyridylation of enals with pyridyl C4-selectivity.
N-Heterocyclic carbene (NHC) catalysis, a versatile organocatalytic process, has attracted significant interest from the synthetic community21–27. In particular, the development of NHC-catalyzed umpolung chemistry involving Breslow intermediates has provided unique approaches for the enantioselective construction of C–C bonds28–30. In recent years, single-electron NHC catalysis has emerged as a versatile tool for accessing new modes of radical reaction that were previously difficult to achieve31–39. Inspired by a biological system, Fukuzumi40 and Studer41 disclosed NHC-mediated radical reactions of Breslow intermediates involving two single-electron-transfer (SET) processes. Since these pioneering studies, important contributions that enable the direct assembly of functional molecules with broad utility have been made to this field. Recently, Ohmiya and coworkers reported that the NHC-catalyzed SET process has important potential for carbon–carbon bond formation through radical–radical coupling42–46. Moreover, asymmetric radical reactions under chiral NHC catalysis offer unique opportunities for unconventional approaches to the synthesis of chiral molecules. The groups of Rovis47,48 and Chi49 demonstrated the chiral NHC-catalyzed single-electron oxidation of NHC-bound homoenolates using nitroarenes for the enantioselective syntheses of β‑hydroxy esters. Despite significant achievements in NHC-catalyzed radical chemistry, the enantioselective coupling reaction between a homoenolate equivalent and a heteroarene remains a long-standing challenge but represents a straightforward approach that rapidly accesses value-added chiral β-heteroaryl carbonyl compounds. The requisite electron acceptors must be sufficiently electron-poor to chemically enable homoenolate oxidation50,51.
Herein, we describe an NHC-catalyzed radical reaction that converts various enals to value-added chiral β-pyridyl esters with excellent control over absolute stereochemistry and pyridyl C4-selectivity. As outlined in Fig. 1c, a pyridinium salt behaves as an effective oxidant for the SET oxidation of an enal under NHC catalysis, thereby enabling a radical-mediated transformation. The homoenolate radical formed in situ by the SET oxidation of a pyridinium salt readily adds to the C4 position of the pyridine core and impart chiral control.
Results
Reaction discovery and optimization
We propose that Breslow intermediate anion (Eo = −1.7–−1.8 V vs. SCE)39 has a sufficient reduction potential for a pyridinium salt (Eo = −0.75 to −0.82 V vs. SCE)16. At this juncture, we reasoned that Breslow intermediates would serve as efficient SET reductants for electron-deficient pyridinium salts to generate the corresponding homoenolate radicals. Intermolecular coupling of the homoenolate radical and the pyridinium salt would then forge a new C–C bond, with the resulting amidyl radical serving as an SET electron acceptor from the homoenolate to promote a chain process.
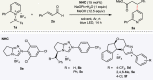
With these mechanistic considerations in mind, we first examined our proposed strategy by monitoring the reactivities of pyridinium salt 1a and enal 2a, as shown in Table 1. The β-pyridyl ester product 3a was obtained in 24% yield with an encouraging enantiomeric ratio (er) using a degassed solution of triazolium NHC precursor 5b in toluene. Switching the NHC catalyst from 5b to 5c resulted in an improved er (84:16) and a similar yield (entry 3). Further studies revealed that irradiation with visible light facilitated more efficient conversion (entries 4 and 5). We observed that mixing the pyridinium salt14,52–55 with pivalate resulted in a significant shift in the intensity of the UV/Vis absorption peak, suggestive of the formation of a photoactive electron donor–acceptor (EDA) complex (see Supplementary Information for details)56–63. Therefore, the intermediacy of the EDA complex further facilitates SET to generate amidyl radicals when irradiated by visible light, thereby promoting the radical chain pathway49,64–69. Screening chiral catalysts revealed that NHC scaffold 5f provided the product with improved reactivity and enantioselectivity (entry 8); therefore, 5f was selected for further optimization. Among the solvents screened, we found that hexafluorobenzene (HFB) provided higher enantioselectivity probably by establishing electrostatic interactions with the homoenolate during the radical addition to pyridine (entries 11 and 12)70. We were pleased to discover that excellent enantioselectivity (96:4, er) was obtained, together with very high regioselectivity (>20:1) for the pyridyl C4 position under our conditions (entry 12). Control experiments established the critical role of the NHC catalyst, as no desired reaction was observed in the absence of an NHC catalyst (entry 13). The reaction was completely inhibited by the addition of the 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO), which indicates that this reaction proceeds via a radical-mediated mechanism (entry 14).
Table 1.
Optimization of the reaction conditions.
 | |||||
|---|---|---|---|---|---|
| Entry | NHC | Solvent (M) | Light | Yield (%)a | erb |
| 1 | 5a | PhMe (0.05) | dark | 51 | – |
| 2 | 5b | PhMe (0.05) | dark | 24 | 77:23 |
| 3 | 5c | PhMe (0.05) | dark | 26 | 84:16 |
| 4 | 5b | PhMe (0.05) | blue LED | 47 | 78:22 |
| 5 | 5c | PhMe (0.05) | blue LED | 46 | 84:16 |
| 6 | 5d | PhMe (0.05) | blue LED | 55 | 85:15 |
| 7 | 5e | PhMe (0.05) | blue LED | 57 | 80:20 |
| 8 | 5f | PhMe (0.05) | blue LED | 58 | 89:11 |
| 9 | 5f | CHCl3 (0.05) | blue LED | 10 | 77:23 |
| 10 | 5f | EtOAc (0.05) | blue LED | 16 | 78:22 |
| 11 | 5f | HFB (0.05) | blue LED | 69 | 94:6 |
| 12 | 5f | HFB (0.025) | blue LED | 67 | 96:4 |
| 13c | 5f | HFB (0.025) | blue LED | n.d. | – |
| 14d | 5f | HFB (0.025) | blue LED | n.d. | – |
| 15 | 5f | HFB (0.025) | dark | 53 | 96:4 |
Reaction conditions: 1a (0.05 mmol), 2a (2.0 equiv), NaOPiv•H2O (1.0 equiv), NHC catalyst (15 mol%), MeOH (12.5 equiv) in solvent irradiated by blue LEDs (440 nm, 10 W) at rt under Ar for 14 h.
aYields were determined by 1H NMR analysis.
bEnantiomeric ratios were determined by HPLC.
cWithout NHC catalyst.
dAddition of TEMPO (3 equiv). n.d. not detected.
Substrate scope studies
With the optimal reaction conditions in hand, the substrate scope was next investigated to demonstrate the generality of this transformation. As shown in Fig. 2, a variety of α,β-unsaturated aldehydes serve as competent substrates under standard reaction conditions. For example, we were pleased to find that various electron-donating substituents at the para-position of the aryl ring, such as methyl (3b), methoxy (3c), and phenyl (3d), are well tolerated, with good-to-excellent ers obtained. Aldehydes bearing electron-deficient groups on the aryl ring are also suitable substrates, although slightly lower ers were observed (3e–3j). In addition, meta-substituted aryl enals were all found to be suitable substrates, irrespective of the electronic and steric effect of the substituent (3k–3n). A broad range of ortho-substituted enals, such as those bearing methyl (3o), methoxy (3p), chloro (3q), and bromo (3r) groups, readily participated in this reaction, with good enantioselectivities observed. Notably, the reactions of halogen-containing substrates readily produced the corresponding products, with the halogen moieties remaining intact. We also successfully used aldehydes bearing di-substituted aryl (3s), furan (3t), thiophene (3u), naphthalene (3v–3x) moieties as competent coupling partners with comparable reactivities and enantioselectivities. When β-alkyl enal was subjected to the standard reaction conditions, the desired product was not formed. We continued this study by evaluating the pyridinium salt scope. As shown in Fig. 2, this protocol is compatible with a broad range of N-amidopyridinium salts bearing either electron-donating or electron-withdrawing substituents.
Fig. 2. Substrate scope.
Reaction conditions: 1 (0.05 mmol), 2 (2.0 equiv), NaOPiv•H2O (1.0 equiv), and 5f (15 mol%) in HFB (2.0 mL) under light irradiation using blue LEDs (440 nm, 10 W) at rt under Ar for 14 h. aPyrazole (2 equiv) was used.
The unsubstituted pyridinium salt was a viable substrate, and product 4a was obtained with 94:6 er. The current method was amenable to the C2-substituted pyridinium salts bearing methyl (4b and 4c) group. Likewise, various substituents and substitution patterns on the benzene rings of the pyridinium substrates were compatible with this reaction to furnish their respective products; methyl (4d), methoxy (4e), trifluoromethyl (4f), thiophene (4g), pyridine (4h), and bromo (4i and 4j) groups were all tolerated. Importantly, enals were β-pyridylated exclusively at the C4 position of their pyridine cores in all cases, regardless of the substitution pattern. The absolute configurations of 3x and 4k were determined as (R) by X-ray diffraction analysis. The C3-substituted pyridinium salt was not compatible with this method, and the desired product was not obtained. We next showed that this transformation is applicable to a range of alcohol components. The reactions of linear (4l and 4m), branched (4n), propargyl (4o), benzylic (4p), allylic (4q), alkyl alcohols with different chain lengths (4r and 4s), and pyrazole (4t) were found to be effective with good-to-excellent enantioselectivities obtained.
To further demonstrate the broad applicability of the enantioselective transformation, we explored the late-stage modification of biorelevant molecules (Fig. 3). Pleasingly, pharmaceutically interesting compounds (estrone, L-phenylalanine, oxaprozin, and febuxostat derivatives) could be successfully employed in this enantioselective β‑pyridylation, which afforded desired products (6a–6d).
Fig. 3. Late-stage modification.
Standard reaction conditions.
Control experiments and proposed mechanism
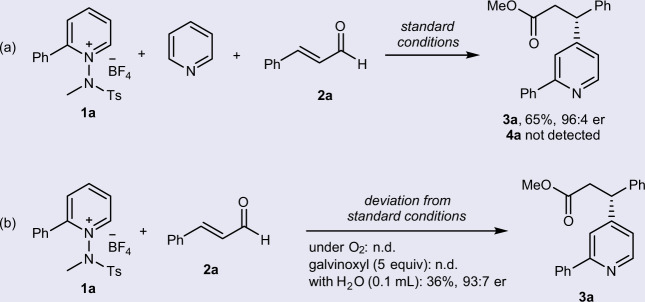
Several control experiments were performed to gain more insight into the reaction mechanism. First, a mixture of 1a and pyridine was reacted under our standard conditions to determine that the pyridinium salt is the only reactive radical acceptor in the reaction. The reaction proceeded only with pyridinium salt 1a, whereas pyridine was not engaged in the reaction (Fig. 4a). Next, we observed that the presence of oxygen or galvinoxyl completely inhibited the reaction, indicating the radical-involved reaction pathway (Fig. 4b). A decreased product yield was observed with the addition of H2O.
Fig. 4. Control experiments.
a Reaction with mixture of 1a and pyridine. b Reactions with the presence of O2, galvinoxyl free radical, and H2O.
Based on the above observations, a plausible NHC catalytic mechanism is illustrated in Fig. 5. The enolate form of Breslow intermediate I undergoes direct SET with the pyridinium salt to give the amidyl radical and homoenolate-centered radical II. At this point, the resultant β-radical II selectively adds to the C4 position of the N-amidopyridinium salt, which is the enantioselectivity-determining step. The resulting radical III undergoes facile deprotonation and subsequent homolytic cleavage of the N–N bond to give the amidyl radical and NHC-bound intermediate IV. The alcohol then reacts with intermediate IV to regenerate the NHC catalyst and eventually yield the desired β-pyridylated ester. The in-situ-generated amidyl radical is expected to engage in an SET event with homoenolate I, which propagates the radical chain. Since light-promoted reactivity and rate acceleration were observed, we propose an alternative mechanistic platform that involves a photon-absorbing pyridinium−pivalate EDA complex, which triggers the formation of an amidyl radical when irradiated with visible light, in turn, initiating a new radical chain pathway. During the process, there are two conceivable unproductive reaction pathways. Firstly, the redox esterification process is a prototypical reaction of enals in NHC catalysis, which provides methyl cinnamate as a side product. Remarkably, when NHC catalyst 5f was employed, the formation of the ester product was completely suppressed (see Supplementary Table 2 in the Supplementary Information). Secondly, the acyl radicals generated from the hydrogen atom abstraction (HAT) process between enals and amidyl radicals could add to pyridinium salts. Interestingly, the acylated pyridine was not detected in this reaction, and it is postulated that the amidyl radical reacts rapidly with I in a radical chain pathway.
Fig. 5. Proposed reaction mechanism.
The asymmetric β-pyridylation was achieved through single-electron NHC catalysis with excellent enantioselectivity.
Discussion
In summary, we report a catalytic method for the asymmetric β-pyridylations of enals through single-electron NHC catalysis. The method delivers excellent control over enantio- and site-selectivity. Mechanistically, the reaction is believed to proceed through SET involving a chiral Breslow-intermediate-derived homoenolate and a pyridinium salt, resulting in a homoenolate radical that adds to the C4 position of the pyridine core. Notably, the use of hexafluorobenzene as a solvent is the key to achieving excellent enantioselectivities. This methodology exhibits broad application scope and provides a new retrosynthetic disconnection for the straightforward syntheses of a diverse range of enantioenriched β-pyridyl carbonyl building blocks under mild and metal-free conditions. Considering the ubiquity of carbonyl and pyridyl groups in chemical and biological molecular systems, the developed protocol represents a powerful method that can broadly be applied in synthetic and medicinal chemistry.
Methods
Representative procedure for the enantioselective β-pyridylation
To a 16 mL test tube equipped with a Teflon-coated magnetic bar were added pyridinium salt (0.05 mmol), sodium pivalate hydrate (0.05 mmol), and NHC catalyst (0.0075 mmol). The test tube, anhydrous methanol, hexafluorobenzene degassed by freeze-pump-thaw, and cinnamaldehyde were placed into an argon-filled glovebox. In glovebox, stock solution of cinnamaldehyde (0.1 mmol) and MeOH (12.5 equiv) was prepared with 2.0 mL of hexafluorobenzene. The stock solution was added to the test tube. After adding solvent, the test tube was closed by cap and removed from the glovebox. The reaction mixture was stirred under 440 nm Kessil blue LEDs (10 W, 25% intensity) at room temperature for 14 h. After reaction completion, the mixture was diluted and extracted with dichloromethane three times. The organic layer was dried over sodium sulfate and filtered. The resulting mixture was concentrated under reduced pressure and purified by flash column chromatography on silica gel (ethyl acetate: n-hexane = 1:4 or CH2Cl2:MeOH = 30:1) to obtain the desired product 3a (67%, 10.6 mg).
Supplementary information
Acknowledgements
This research was supported financially by Institute for Basic Science (IBS-R010-A2). We thank Dr. Dongwook Kim (IBS) for XRD analysis.
Author contributions
H.C., G.R.M., and Seonghyeok Hong performed the experiments. Sungwoo Hong directed the project. All authors contributed to the preparation of the manuscript.
Peer review
Peer review information
Nature Communications thanks Yong Huang and the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Experimental procedure and characterization data of new compounds are available within Supplementary Information. Full crystallographic data of all structurally characterized compounds described herein are given in the Supplementary Information. Depository Number CCDC 2116151 (3x), 2116149 (4k), 2116154 (3d), 2116152 (3e), and 2116153 (4d) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-29462-7.
References
- 1.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 2.Zafar MN, et al. Pyridine and related ligands in transition metal homogeneous catalysis. Russ. J. Coord. Chem. 2016;42:1–18. [Google Scholar]
- 3.Pozharskii, A. F., Soldatenkov, A. T. & Katritzky, A. R. Heterocycles in Life and Society: An Introduction to Heterocyclic Chemistry, Biochemistry, and Applications, 2nd edn (John Wiley & Sons, 2011).
- 4.Ma X, Herzon SB. Intermolecular hydropyridylation of unactivated alkenes. J. Am. Chem. Soc. 2016;138:8718–8721. doi: 10.1021/jacs.6b05271. [DOI] [PubMed] [Google Scholar]
- 5.Lutz JP, Chau ST, Doyle AG. Nickel-catalyzed enantioselective arylation of pyridine. Chem. Sci. 2016;7:4105–4109. doi: 10.1039/c6sc00702c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fier PS. A bifunctional reagent designed for the mild, nucleophilic functionalization of pyridines. J. Am. Chem. Soc. 2017;139:9499–9502. doi: 10.1021/jacs.7b05414. [DOI] [PubMed] [Google Scholar]
- 7.Mathi GR, Kweon B, Moon Y, Jeong Y, Hong S. Regioselective C–H functionalization of heteroarene N-oxides enabled by a traceless nucleophile. Angew. Chem., Int. Ed. 2020;59:22675–22683. doi: 10.1002/anie.202010597. [DOI] [PubMed] [Google Scholar]
- 8.Choi J, Laudadio G, Godineau E, Baran PS. Practical and regioselective synthesis of C4-alkylated pyridines. J. Am. Chem. Soc. 2021;143:11927–11933. doi: 10.1021/jacs.1c05278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X, et al. Phosphorus-mediated sp2-sp3 couplings for C−H fluoroalkylation of azines. Nature. 2021;594:217–222. doi: 10.1038/s41586-021-03567-3. [DOI] [PubMed] [Google Scholar]
- 10.Jin J, MacMillan DWC. Direct α-arylation of ethers through the combination of photoredox-mediated C−H functionalization and the minisci reaction. Angew. Chem., Int. Ed. 2015;54:1565–1569. doi: 10.1002/anie.201410432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seath CP, Vogt DB, Xu Z, Boyington AJ, Jui NT. Radical hydroarylation of functionalized Olefins and mechanistic investigation of photocatalytic pyridyl radical reactions. J. Am. Chem. Soc. 2018;140:15525–15534. doi: 10.1021/jacs.8b10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim I, et al. Visible-light-induced pyridylation of remote C(sp3)−H bonds by radical translocation of N-alkoxypyridinium salts. Angew. Chem., Int. Ed. 2018;57:15517–15522. doi: 10.1002/anie.201809879. [DOI] [PubMed] [Google Scholar]
- 13.Moon Y, et al. Visible light induced alkene aminopyridylation using N-aminopyridinium salts as bifunctional reagents. Nat. Commun. 2019;10:4117. doi: 10.1038/s41467-019-12216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung S, Shin S, Park S, Hong S. Visible light-driven C4-selective alkylation of pyridinium derivatives with alkyl bromides. J. Am. Chem. Soc. 2020;142:11370–11375. doi: 10.1021/jacs.0c04499. [DOI] [PubMed] [Google Scholar]
- 15.Lee W, Jung S, Kim M, Hong S. Site-selective direct C–H pyridylation of unactivated alkanes by triplet excited anthraquinone. J. Am. Chem. Soc. 2021;143:3003–3012. doi: 10.1021/jacs.1c00549. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, You E, Park S, Hong S. Divergent reactivity of sulfinates with pyridinium salts based on one- versus two-electron pathways. Chem. Sci. 2021;12:6629–6637. doi: 10.1039/d1sc00776a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song G, Wylie WNO, Hou Z. Enantioselective C−H bond addition of pyridines to alkenes catalyzed by chiral half-sandwich rare-earth complexes. J. Am. Chem. Soc. 2014;136:12209–12212. doi: 10.1021/ja504995f. [DOI] [PubMed] [Google Scholar]
- 18.Proctor RSJ, Davis HJ, Phipps RJ. Catalytic enantioselective minisci-type addition to heteroarenes. Science. 2018;360:419–422. doi: 10.1126/science.aar6376. [DOI] [PubMed] [Google Scholar]
- 19.Proctor RSJ, Chuentragool P, Colgan AC, Phipps RJ. Hydrogen atom transfer-driven enantioselective minisci reaction of amides. J. Am. Chem. Soc. 2021;143:4928–4934. doi: 10.1021/jacs.1c01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng D, Studer A. Asymmetric synthesis of heterocyclic γ-amino-acid and diamine derivatives by three-component radical cascade reactions. Angew. Chem. Int. Ed. 2019;58:15803–15807. doi: 10.1002/anie.201908987. [DOI] [PubMed] [Google Scholar]
- 21.Bugaut X, Glorius F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012;41:3511–3522. doi: 10.1039/c2cs15333e. [DOI] [PubMed] [Google Scholar]
- 22.Sarkar SD, Biswas A, Samanta RC, Studer A. Catalysis with N-heterocyclic carbene under oxidative conditions. Chem. - Eur. J. 2013;19:4664–4678. doi: 10.1002/chem.201203707. [DOI] [PubMed] [Google Scholar]
- 23.Flanigan DM, Romanov-Michailidis F, White NA, Rovis T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 2015;115:9307–9387. doi: 10.1021/acs.chemrev.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong S, et al. Organocatalytic kinetic resolution of sulfoximines. J. Am. Chem. Soc. 2016;138:2166–2169. doi: 10.1021/jacs.6b00143. [DOI] [PubMed] [Google Scholar]
- 25.Murauski KJR, Jaworski AA, Scheidt KA. A continuing challenge: N-heterocyclic carbene-catalyzed syntheses of γ-butyrolactones. Chem. Soc. Rev. 2018;47:1773–1782. doi: 10.1039/c7cs00386b. [DOI] [PubMed] [Google Scholar]
- 26.Ohmiya H. N-Heterocyclic carbene-based catalysis enabling cross-coupling reactions. ACS Catal. 2020;10:6862–6869. [Google Scholar]
- 27.Bellotti P, Koy M, Hopkinson MN, Glorius F. Recent advances in the chemistry and applications of N-heterocyclic carbenes. Nat. Rev. Chem. 2021;5:711–725. doi: 10.1038/s41570-021-00321-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhao K, Enders D. Merging N-heterocyclic carbene catalysis and single electron transfer: a new strategy for asymmetric transformations. Angew. Chem., Int. Ed. 2017;56:3754–3756. doi: 10.1002/anie.201700370. [DOI] [PubMed] [Google Scholar]
- 29.Jang KP, et al. Asymmetric homoenolate additions to acyl phosphonates through rational design of a tailored N-heterocyclic carbene catalyst. J. Am. Chem. Soc. 2014;136:76–79. doi: 10.1021/ja410932t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bera S, Daniliuc CG, Studer A. Oxidative N-heterocyclic carbene catalyzed dearomatization of indoles to spirocyclic indolenines with a quaternary carbon stereocenter. Angew. Chem., Int. Ed. 2017;56:7402–7406. doi: 10.1002/anie.201701485. [DOI] [PubMed] [Google Scholar]
- 31.Regnier V, et al. What are the radical intermediates in oxidative N-heterocyclic carbene organocatalysis? J. Am. Chem. Soc. 2019;141:1109–1117. doi: 10.1021/jacs.8b11824. [DOI] [PubMed] [Google Scholar]
- 32.Ishii T, Nagao K, Ohmiya H. Recent advances in N-heterocyclic carbene-based radical catalysis. Chem. Sci. 2020;11:5630–5636. doi: 10.1039/d0sc01538e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Xing X-N, Huang J-H, Lu L-Q, Xiao W-J. Light opens a new window for N-heterocyclic carbene catalysis. Chem. Sci. 2020;11:10605–10613. doi: 10.1039/d0sc03595e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim I, Im H, Lee H, Hong S. N-Heterocyclic carbene-catalyzed deaminative cross-coupling of aldehydes with Katritzky pyridinium salts. Chem. Sci. 2020;11:3192–3197. doi: 10.1039/d0sc00225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mavroskoufis A, et al. N-Heterocyclic carbene catalyzed photoenolization/diels-alder reaction of acid fluorides. Angew. Chem., Int. Ed. 2020;59:3190–3194. doi: 10.1002/anie.201914456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bay AV, et al. Light-driven carbene catalysis for the synthesis of aliphatic and α-amino ketones. Angew. Chem., Int. Ed. 2021;60:17925–17931. doi: 10.1002/anie.202105354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu K, Studer A. Direct α-acylation of alkenes via N-heterocyclic carbene, sulfinate, and photoredox cooperative triple catalysis. J. Am. Chem. Soc. 2021;143:4903–4909. doi: 10.1021/jacs.1c01022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng Q-Y, Lezius L, Studer A. Benzylic C−H acylation by cooperative NHC and photoredox catalysis. Nat. Commun. 2021;12:2068. doi: 10.1038/s41467-021-22292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delfau L, et al. Critical assessment of the reducing ability of Breslow-type derivatives and implications for carbene-catalyzed radical reactions. Angew. Chem., Int. Ed. 2021;60:26783–26789. doi: 10.1002/anie.202111988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakanishi I, Itoh S, Fukuzumi S. Electron-transfer properties of active aldehydes of thiamin coenzyme models, and mechanism of formation of the reactive intermediates. Chem. Eur. J. 1999;5:2810–2818. [Google Scholar]
- 41.Guin J, De Sarkar S, Grimme S, Studer A. Biomimetic carbene-catalyzed oxidations of aldehydes using TEMPO. Angew. Chem., Int. Ed. 2008;47:8727–8730. doi: 10.1002/anie.200802735. [DOI] [PubMed] [Google Scholar]
- 42.Ishii T, Kakeno Y, Nagao K, Ohmiya H. N-Heterocyclic carbene-catalyzed decarboxylative alkylation of aldehydes. J. Am. Chem. Soc. 2019;141:3854–3858. doi: 10.1021/jacs.9b00880. [DOI] [PubMed] [Google Scholar]
- 43.Ishii T, Ota K, Nagao K, Ohmiya H. N-Heterocyclic carbene-catalyzed radical relay enabling vicinal alkylacylation of alkenes. J. Am. Chem. Soc. 2019;141:14073–14077. doi: 10.1021/jacs.9b07194. [DOI] [PubMed] [Google Scholar]
- 44.Kakeno Y, Kusakabe M, Nagao K, Ohmiya H. Direct synthesis of dialkyl ketones from aliphatic aldehydes through radical N-heterocyclic carbene catalysis. Aryl radical-mediated N-heterocyclic carbene catalysis. ACS Catal. 2020;10:8524–8529. [Google Scholar]
- 45.Matsuki Y, et al. Aryl radical-mediated N-heterocyclic carbene catalysis. Nat. Commun. 2021;12:3848. doi: 10.1038/s41467-021-24144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato Y, et al. Light-driven N-heterocyclic carbene catalysis using alkylborate. ACS Catal. 2021;11:12886–12892. [Google Scholar]
- 47.White NA, Rovis T. Enantioselective N-heterocyclic carbene-catalyzed β-hydroxylation of enals using nitroarenes: an atom transfer reaction that proceeds via single electron transfer. J. Am. Chem. Soc. 2014;136:14674–14677. doi: 10.1021/ja5080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White NA, Rovis T. Oxidatively initiated NHC-catalyzed enantioselective synthesis of 3,4-disubstituted cyclopentanones from enals. J. Am. Chem. Soc. 2015;137:10112–10115. doi: 10.1021/jacs.5b06390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Y, et al. N-Heterocyclic carbene-catalyzed radical reactions for highly enantioselective β-hydroxylation of enals. J. Am. Chem. Soc. 2015;137:2416–2419. doi: 10.1021/ja511371a. [DOI] [PubMed] [Google Scholar]
- 50.Chen X-Y, Chen K-Q, Sun D-Q, Ye S. N-Heterocyclic carbene-catalyzed oxidative [3 + 2] annulation of dioxindoles and enals: cross coupling of homoenolate and enolate. Chem. Sci. 2017;8:1936–1941. doi: 10.1039/c6sc04135c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Z, Huang M, Zhang X, Chen J, Huang Y. N-Heterocyclic carbene-catalyzed four-component reaction: chemoselective Cradical−Cradical relay coupling involving the homoenolate intermediate. ACS Catal. 2021;11:10123–10130. [Google Scholar]
- 52.Quint V, et al. Metal-free, visible light-photocatalyzed synthesis of benzo[b]phosphole oxides: synthetic and mechanistic investigations. J. Am. Chem. Soc. 2016;138:7436–7441. doi: 10.1021/jacs.6b04069. [DOI] [PubMed] [Google Scholar]
- 53.Quint V, et al. Visible-light-mediated α-phosphorylation of N-aryl tertiary amines through the formation of electron-donor–acceptor complexes: synthetic and mechanistic studies. Org. Chem. Front. 2019;6:41. [Google Scholar]
- 54.Kim I, Park S, Hong S. Functionalization of pyridinium derivatives with 1,4-dihydropyridines enabled by photoinduced charge transfer. Org. Lett. 2020;22:8730–8734. doi: 10.1021/acs.orglett.0c03347. [DOI] [PubMed] [Google Scholar]
- 55.Shin S, Lee S, Choi W, Kim N, Hong S. Visible-light-induced 1,3-aminopyridylation of [1.1.1]propellane with N-aminopyridinium salts. Angew. Chem., Int. Ed. 2021;60:7873–7879. doi: 10.1002/anie.202016156. [DOI] [PubMed] [Google Scholar]
- 56.Morack T, Muck-Lichtenfeld C, Gilmour R. Bioinspired radical stetter reaction: radical umpolung enabled by ion-pair photocatalysis. Angew. Chem., Int. Ed. 2019;58:1208–1212. doi: 10.1002/anie.201809601. [DOI] [PubMed] [Google Scholar]
- 57.Sandfort F, Strieth-Kalthoff F, Klauck FJR, James MJ, Glorius F. Deaminative borylation of aliphatic amines enabled by visible light excitation of an electron donor-acceptor complex. Chem. Eur. J. 2018;24:17210–17214. doi: 10.1002/chem.201804246. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Grant PS, Li X, Noble A, Aggarwal VK. Catalyst-free deaminative functionalizations of primary amines by photoinduced single-electron transfer. Angew. Chem., Int. Ed. 2019;58:5697–5701. doi: 10.1002/anie.201814452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu J, Bär RM, Guo L, Noble A, Aggarwal VK. Photoinduced deoxygenative borylations of aliphatic alcohols. Angew. Chem., Int. Ed. 2019;58:18830–18834. doi: 10.1002/anie.201910051. [DOI] [PubMed] [Google Scholar]
- 60.James MJ, et al. Visible-light-mediated charge transfer enables C−C bond formation with traceless acceptor groups. Chem. − Eur. J. 2019;25:8240–8244. doi: 10.1002/chem.201901397. [DOI] [PubMed] [Google Scholar]
- 61.Crisenza GEM, Mazzarella D, Melchiorre P. Synthetic methods driven by the photoactivity of electron donor−acceptor complexes. J. Am. Chem. Soc. 2020;142:5461–5547. doi: 10.1021/jacs.0c01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McClain EJ, Monos TM, Mori M, Beatty JW, Stephenson CRJ. Design and a implementation of a catalytic electron donor−acceptor complex platform for radical trifluoromethylation and alkylation. ACS Catal. 2020;10:12636–12641. [Google Scholar]
- 63.de Pedro Beato E, Spinnato D, Zhou W, Melchiorre P. A general organocatalytic system for electron donor−acceptor complex photoactivation and its use in radical processes. J. Am. Chem. Soc. 2021;143:12304–12314. doi: 10.1021/jacs.1c05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davies J, Morcillo SP, Douglas JJ, Leonori D. Hydroxylamine derivatives as nitrogen‐radical precursors in visible‐light photochemistry. Chem. Eur. J. 2018;24:12154. doi: 10.1002/chem.201801655. [DOI] [PubMed] [Google Scholar]
- 65.Chen J-R, Hu X-Q, Lu L-Q, Xiao W-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016;45:2044. doi: 10.1039/c5cs00655d. [DOI] [PubMed] [Google Scholar]
- 66.Xiong T, Zhang Q. New amination strategies based on nitrogen-centered radical chemistry. Chem. Soc. Rev. 2016;45:3069. doi: 10.1039/c5cs00852b. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen LQ, Knowles RR. Catalytic C–N bond-forming reactions enabled by proton-coupled electron transfer activation of amide N–H bonds. ACS Catal. 2016;6:2894. [Google Scholar]
- 68.Gentry EC, Knowles RR. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 2016;49:1546. doi: 10.1021/acs.accounts.6b00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu X-Y, Zhao Q-Q, Chen J, Xiao W-J, Chen J-R. When light meets nitrogen-centered radicals: from reagents to catalysts. Acc. Chem. Res. 2020;53:1066. doi: 10.1021/acs.accounts.0c00090. [DOI] [PubMed] [Google Scholar]
- 70.Lattanzi A, Fusco CD, Russo A, Poater A, Cavallo L. Hexafluorobenzene: a powerful solvent for a noncovalent stereoselective organocatalytic Michael addition reaction. Chem. Commun. 2012;48:1650–1652. doi: 10.1039/c2cc17488j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Experimental procedure and characterization data of new compounds are available within Supplementary Information. Full crystallographic data of all structurally characterized compounds described herein are given in the Supplementary Information. Depository Number CCDC 2116151 (3x), 2116149 (4k), 2116154 (3d), 2116152 (3e), and 2116153 (4d) contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.