Abstract
Polysomnography (PSG) studies of sleep changes in Alzheimer’s disease (AD) have reported but not fully established the relationship between sleep disturbances and AD. To better detail this relationship, we conducted a systematic review and meta-analysis of reported PSG differences between AD patients and healthy controls. An electronic literature search was conducted in EMBASE, MEDLINE, All EBM databases, CINAHL, and PsycINFO inception to Mar 2021. Twenty-eight studies were identified for systematic review, 24 of which were used for meta-analysis. Meta-analyses revealed significant reductions in total sleep time, sleep efficiency, and percentage of slow-wave sleep (SWS) and rapid eye movement (REM) sleep, and increases in sleep latency, wake time after sleep onset, number of awakenings, and REM latency in AD compared to controls. Importantly, both decreased SWS and REM were significantly associated with the severity of cognitive impairment in AD patients. Alterations in electroencephalogram (EEG) frequency components and sleep spindles were also observed in AD, although the supporting evidence for these changes was limited. Sleep in AD is compromised with increased measures of wake and decreased TST, SWS, and REM sleep relative to controls. AD-related reductions in SWS and REM sleep correlate with the degree of cognitive impairment. Alterations in sleep EEG frequency components such as sleep spindles may be possible biomarkers with relevance for diagnosing AD although their sensitivity and specificity remain to be clearly delineated. AD-related sleep changes are potential targets for early therapeutic intervention aimed at improving sleep and slowing cognitive decline.
Subject terms: Learning and memory, Psychiatric disorders, Biomarkers
Introduction
Alzheimer’s disease (AD), a neurodegenerative disorder characterized by an impairment in global cognition and progressive memory loss, has been the main cause of dementia and is quickly becoming one of the most lethal, expensive, and burdening diseases in this century [1]. In 2018, AD International estimated a worldwide dementia prevalence of about 50 million people, estimated to triple in 2050, with two-thirds living in middle- and low-income countries [1–3].
Several years before the onset of cognitive impairment (preclinical AD), cerebrospinal fluid (CSF) biomarkers of AD, including amyloid β (Aβ) and tau, begin to pathologically accumulate in the brain [3, 4]. It is believed sleep changes can start in this preclinical stage of AD [5]. Patients at the preclinical stage have more rest-activity rhythm fragmentation, independent of age or sex [6]. More recently in a meta-analysis, including 27 studies, Bubu et al. showed individuals with sleep problems had a 3.78 (95% confidence interval (CI): 2.27–6.30) times higher risk of preclinical AD [7]. It has been suggested that sleep disturbances contribute to cognitive decline and increase the risk of AD by increasing the brain’s Aβ burden [8–10]. Indeed, accumulating evidence indicates that over 45% of AD patients have sleep disturbances [9, 11]. These findings suggest that assessments of sleep disturbances in AD patients may be helpful for identifying targets for preventive and therapeutic approaches to this disease [12–15].
Polysomnography (PSG) is the gold standard method for objectively assessing components of sleep. Evidence suggests that PSG-determined sleep alterations are highly important for understanding the etiology and neurobiology of AD. Recently Ju et al. [16]. found that disrupted slow-wave sleep (SWS) activity, as measured by the change in delta spectral power, significantly increased levels of Aβ, suggesting an important role of SWS in modulating levels of Aβ in the brain. Lucey et al. reported that slow-wave EEG activity (particularly at 1 to 2 Hz) might be helpful in discriminating tau pathology and cognitive decline before or at the earliest stages of AD [17]. Further, rapid eye movement (REM) sleep helps to maintain neuronal homeostasis of the brain; while disturbed REM sleep disrupts neurogenesis and synaptic pruning, and loss of REM sleep has been suggested to result in neurodegeneration [18]. In addition, Liguori et al. reported that disruption in REM sleep was associated with an increase in the levels of CSF orexin in individuals with mild cognitive impairment due to AD [19]. CSF orexin levels have been reported to be positively associated with the levels of tau protein levels in AD patients, suggesting that the dysregulation of the orexinergic system expressed as an increase in CSF orexin levels, is a reflection of more marked and faster tau-mediated neurodegeneration of AD [20].
Many previous reviews discuss sleep changes in AD, but they focus mainly on proposed mechanisms/hypothesis underlying the associations between sleep disturbances and AD (e.g., [21–23]). For PSG features, these reviews typically say that AD patients show significantly altered PSG measured sleep features (e.g., decreased SWS). However, there is a significant problem with the evidence these reviews cite to support these conclusions. These narrative reviews support their conclusions by unsystematically citing PSG studies which show statistically significant PSG parameter changes (e.g., decreased SWS, etc.), while studies with negative findings (e.g., no significant difference in SWS between AD and healthy controls) go unmentioned. (e.g., [24–26]). Thus, the conclusions regarding PSG changes in AD in these reviews are based on selective reporting (ignoring studies with negative findings). Variations in findings across different studies may involve heterogeneity in demographic characteristics (i.e., sex, age, and educational attainment), clinical variability (i.e., disease severity and medication status), and experimental methodology (i.e., PSG recording and scoring methods and the use of adaptation nights). Meta-analytic techniques are useful for resolving discrepancies across studies and for estimating the potential impact of moderators. To our knowledge, no meta-analytic study on PSG measured sleep in AD has been conducted to date. To fill this gap, we systematically reviewed previous case-control studies and used meta-analytic procedures to identify the pooled effect sizes for the differences in PSG measured sleep between AD patients and healthy controls where possible. We also explored potential moderators of the PSG changes in AD patients compared with healthy controls.
Methods
Protocol and registration
We registered the protocol for this study (PROSPERO ID: CRD42021240066) according to the preferred reporting criteria for systematic reviews and meta-analyses statements [27].
Inclusion and exclusion criteria
To explore the nighttime PSG differences between AD patients and healthy controls, we included only case-control studies that made comparisons between these groups. The included studies were selected to meet the following criteria:
The patients met diagnostic criteria for AD according to recognized criteria from the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association workgroup [28], the National Institute on Aging-Alzheimer’s Association workgroup [29], or the Diagnostic and Statistical Manual of Mental Disorders [30]. When the diagnostic criteria for AD were not specified, studies in which AD status was determined by physicians’ clinical assessments (i.e., patients having acquired global impairment of intellect, memory, and personality) and neuroimaging findings (i.e., presence of diffuse atrophy in the brain but without cerebrovascular accident or other focal intracranial pathologic changes.) were also included.
The included studies included a healthy control group.
The studies reported differences in some PSG measured nighttime sleep parameters between AD patients and healthy controls (the PSG parameters of interest are listed below in the section on “Data collection process”).
The studies were published in English in peer-reviewed journals.
If the same participants were used in more than one study, then only the dataset with the most relevant information was used to avoid data duplication.
By screening titles, abstracts, and full text, we excluded: (1) animal studies; (2) editorials, case reports, case series, guidelines, comments, statements, and review papers; (3) studies unrelated to AD; (4) studies not including healthy controls or not clearly clarifying whether their controls are healthy controls; (5) studies not reporting PSG data in AD patients or healthy controls; and (6) studies containing no information on outcomes of interest.
Information sources, search, and study selection
We searched MEDLINE via OVID; EMBASE via OVID; all EBM databases via OVID; CINAHL via EBSCO; and PsycINFO via EBSCO. The following terms were searched for in the abstract or title: (“Alzheimer Disease” OR “Alzheimer’s disease”) AND (“polysomnogra*” OR “PSG” OR “sleep architect*” OR “sleep monit*” OR “sleep stage*” OR “electroencephalogra*” OR “EEG”). The detailed search strategies used in each literature database are provided in Tables S1–S5. The reference lists of all primary studies were also screened for additional references. We performed the literature search on March 6, 2021. Two reviewers (RR and YZ) independently selected relevant papers. Discrepancies were resolved by discussion with the senior author (XT). If the PSG data of multiple AD groups (e.g., mild and moderate AD patients) were reported and separately compared with controls in a study, this approach was carried over into the current meta-analysis.
Data collection process and quality assessment of included studies
RR and YZ independently extracted the data from the reviewed studies using a pre-designed form. The extracted data were entered by YZ and verified by RR and YZ. Discrepancies were resolved by discussion with XT. The PSG variables examined in our systematic review include sleep macrostructure: total sleep time (TST), sleep efficiency (SE), wake time after sleep onset (WASO), sleep latency (SL), and percentage of sleep stages N1, N2, N3 and REM sleep, REM latency (REML), and REM density (REMD). In the American Academy of Sleep Medicine (AASM) scoring rules, N3 represents SWS and replaces sleep stages 3 and 4 in the Rechtschaffen and Kales (R&K) nomenclature [31]. Thus, the data for stages 3 and 4 in the included studies were also extracted for estimating SWS. Other PSG variables include periodic limb movement during sleep (PLMS) index and sleep microstructure parameters: cyclic alternating pattern (CAP) parameters, power spectral analysis (PSA) data (i.e., alpha, beta, delta, theta, and gamma frequency activity), and sleep spindle data. We also extracted demographic, clinical, and methodological variables, including the number of participants and their mean age, sex (male percentage), educational attainment (years), Mini-Mental State Examination (MMSE) scores, whether the patients were free of medications impacting sleep (Yes vs. No), PSG scoring methods (R&K vs. AASM), and use of an adaptation night (Yes vs. No). RR and YZ independently assessed the risk of bias of the included studies by using the adapted version of the National Institute for Health and Care Excellence (NICE) checklist [32], with discrepancies resolved in discussion with XT.
Statistical analysis
To calculate the pooled effect sizes (standardized mean difference (SMD)) for the PSG changes in AD patients compared with controls, the sample size, mean, and standard deviation for the two groups were entered. For the estimation of global effect-size for each PSG parameter, the I2 and Q statistics were calculated to examine the presence and magnitude of heterogeneity and to inform on the degree of overlap between the 95% CIs of included studies. The random-effects model was applied to obtain relatively conservative meta-analysis findings. The Egger regression method [33] was used to examine publication bias, with p values of <0.05 suggesting the presence of bias. Duval and Tweedie’s trim and fill test was conducted to get the adjusted effect sizes when publication bias was present [34]. A meta-regression or subgroup analysis (depending on whether the potential moderators were continuous or categorical variables) was conducted to determine the potential factors that could moderate heterogeneity between studies. All analysis in our meta-analysis was done using Comprehensive Meta-Analysis software.
Results
Study selection
Our search yielded 7533 publications (Fig. 1). After removing the duplicates, the title/abstract of the remaining 4465 articles were screened. A total of 143 studies were selected for full-text review. Of these, 28 articles [24–26, 35–59] were found to meet inclusion criteria for the systematic review (Table 1), and 24 of the 28 studies were included in our meta-analysis. The excluded studies with reasons for their exclusion are shown in Table S6.
Fig. 1.
Flow chart used for the identification of eligible studies.
Table 1.
Study characteristics.
| Study | Sample size | Percentage male | Mean age | Education (years) | MMSE | Free of medications impacting sleep | Adaptation | PSG scoring methods |
|---|---|---|---|---|---|---|---|---|
| Bonakis et al. [24] | 17 AD | 52.9% | 69.0 ± 9.9 | NR | 17.9 ± 5.63 | Yes | Yes | AASM |
| 20 controls | 60% | 70.2 ± 12.5 | NR | Yes | AASM | |||
| Bonanni et al. [35] | 11 AD (mild) | 45.5% | 65.6 ± 7.4 | NR | 22.1 ± 1.4 | Yes | Yes | R&K |
| 9 AD (moderate) | 44.4% | 64 ± 8.7 | NR | 13.7 ± 3.3 | Yes | Yes | R&K | |
| 12 controls | 58.3% | 61.1 ± 5.1 | NR | 28.4 ± 1.4 | Yes | R&K | ||
| Brunetti et al. [36] | 47 AD | 40% | 73.57 ± 6.71 | 8.89 ± 4.91 | 18.38 ± 4.70 | Yes | No | R&K |
| 44 controls | 62% | 70.64 ± 6.73 | 11.37 ± 4.64 | 27.54 ± 1.65 | No | R&K | ||
| Chen et al. [37] | 22 AD (mild) | 50% | 70.8 ± 10.7 | 12.3 ± 3.4 | 22.0 ± 2.3 | Yes | Yes | R&K |
| 21 AD (moderate) | 42.9% | 69.2 ± 11.5 | 11.0 ± 3.2 | 15.5 ± 4.4 | Yes | Yes | R&K | |
| 22 controls | 50% | 66.9 ± 6.7 | 10.3 ± 3.2 | 30 ± 0 | Yes | R&K | ||
| Dykierek et al. [38] | 35 AD | 45.7% | 62.1 ± 8.9 | NR | 19.5 ± 5.2 | Yes | Yes | R&K |
| 42 controls | 52.4% | 64.4 ± 7.5 | NR | 29.2 ± 1.0 | Yes | R&K | ||
| Gagnon et al. [39] | 15 AD | 46.7% | 70.2 ± 5.6 | NR | NR | Yes | No | R&K |
| 15 controls | 73.3% | 67.9 ± 5.4 | NR | NR | No | R&K | ||
| Gorgoni et al. [40] | 15 AD | 33.3% | 70.80 ± 9.30 | 9.4 ± 5.77 | 16.07 ± 4.26 | Yes | No | R&K |
| 15 controls | 66.7% | 70.80 ± 6.31 | 11.8 ± 4.80 | 29.07 ± 1.05 | No | R&K | ||
| Liguori et al. [43] | 20 AD | 35% | 66.3 ± 4.18 | NR | 21.4 ± 1.93 | Yes | Yes | AASM |
| 15 controls | 53.3% | 63.8 ± 8.46 | NR | 29.6 ± 1.47 | Yes | AASM | ||
| Liguori et al. [44] | 56 AD (mild) | 37.5% | 69.93 ± 7.27 | NR | 24.45 ± 1.85 | Yes | Yes | AASM |
| 48 AD (moderate-severe) | 39.6% | 71.71 ± 7.19 | NR | 15.40 ± 3.21 | Yes | Yes | AASM | |
| 41 controls | 48% | 67.17 ± 9.83 | NR | 29.20 ± 0.90 | Yes | AASM | ||
| Liu et al. [45] | 30 AD | 20% | 75.77 ± 5.69 | 4.63 ± 3.99 | 21.23 ± 1.65 | Yes | Yes | AASM |
| 30 controls | 26.7% | 75.13 ± 6.32 | 5.27 ± 4.43 | 29.03 ± 1.16 | Yes | AASM | ||
| Rauchs et al. [25] | 14 AD | 35.7% | 76.9 ± 4.1 | NR | 24.9 ± 2 | Yes | Yes | R&K |
| 14 controls | 35.7% | 75.1 ± 4.6 | NR | 29.4 ± 0.9 | Yes | R&K | ||
| Reda et al. [51] | 20 AD | 35% | 72 ± 8.59 | 9.65 ± 5.23 | 16.40 ± 4.70 | Yes | No | R&K |
| 20 controls | 60% | 70.35 ± 6.26 | 11.60 ± 5 | 28.75 ± 1.30 | No | R&K | ||
| Yin et al. [58] | 123 AD | 36.6% | 72.1 ± 7.2 | 11.4 ± 3.3 | 19.7 ± 5.4 | Yes | Yes | R&K |
| 120 controls | 34.2% | 70.9 ± 7.3 | 11.6 ± 2.8 | 28.3 ± 1.5 | Yes | R&K | ||
| Tseng et al. [55] | 5 AD | 40% | 76.40 ± 3.51 | NR | 19.80 ± 1.79 | Yes | No | R&K |
| 9 controls | 66.7% | 76.89 ± 7.51 | NR | 30 | No | R&K | ||
| Reynolds et al. [54] | 25 AD | 28% | 70.4 ± 8.3 | 11.4 ± 4.0 | NR | Yes | Yes | R&K |
| 25 controls | 32% | 69.0 ± 5.0 | 14.5 ± 4.1 | NR | Yes | R&K | ||
| Reynolds et al. [53] | 49 AD | 20.4% | 72.8 ± 8.0 | NR | 16.5 ± 6.0 | Yes | Yes | R&K |
| 77 controls | 44.2% | 69.3 ± 6.4 | NR | 29.4 ± 0.8 | Yes | R&K | ||
| Reynolds et al. [52] | 22 AD | 31.8% | 70.9 ± 8.1 | NR | NR | Yes | Yes | R&K |
| 24 controls | 33.3% | 69.5 ± 4.5 | NR | NR | Yes | R&K | ||
| Prinz et al. [50] | 18 AD (Mild) | 50% | 67.8 ± 9.46 | 15.2 ± 3.52 | 16.7 ± 5.52 | NR | Yes | R&K |
| 16 AD (Moderate) | 63% | 70.2 ± 6.16 | 15. 1 ± 3.16 | 5.4 ± 3.6 | NR | Yes | R&K | |
| 10 AD (Severe) | 100% | 72.8 ± 10.97 | 12.6 ± 2.45 | 1.3 ± 1.83 | NR | Yes | R&K | |
| 22 controls | 50% | 69 ± 6.43 | 14.2 ± 5.47 | 29.6 ± 0.86 | Yes | R&K | ||
| Prinz et al. [49] | 10 AD | 100% | 73.30 ± 11.4 | NR | 3.30 ± 6.52 | Yes | Yes | R&K |
| 11 controls | 100% | 72.18 ± 10.5 | NR | NR | Yes | R&K | ||
| Petit et al. [48] | 8 AD | 50% | 60.6 | NR | NR | Yes | Yes | R&K |
| 8 controls | 50% | 58.3 | NR | NR | Yes | R&K | ||
| Vitiello et al. [57] | 44 AD | 45% | 70.7 ± 7.5 | 13.8 ± 3.1 | 22.7 ± 2.9 | Yes | Yes | R&K |
| 45 controls | 44% | 66.8 ± 6.7 | 14.3 ± 3.5 | 29.7 ± 0.6 | Yes | R&K | ||
| Vitiello et al. [56] | 9 AD (Mild) | 44.4% | 65.7 ± 3.1 | NR | 18.9 ± 1.5 | Yes | Yes | R&K |
| 9 AD (Moderate) | 55.6% | 70.0 ± 2.0 | NR | 6.2 ± 1.4 | Yes | Yes | R&K | |
| 9 AD (Severe) | 100% | 73.0 ± 3.9 | NR | 3.3 ± 2.3 | Yes | Yes | R&K | |
| 9 controls | 22.2% | 65.6 ± 1.9 | NR | 29.7 ± 0.3 | Yes | R&K | ||
| Martin et al. [26] | 8 AD | 75% | NR | NR | NR | Yes | Yes | R&K |
| 9 controls | 66.7% | NR | NR | NR | Yes | R&K | ||
| Maestri et al. [46] | 11 AD | 36.4% | 72.7 ± 5.9 | NR | 21.2 ± 0.8 | Yes | No | AASM |
| 11 controls | 54.5% | 69.2 ± 12.6 | NR | 29.3 ± 1.0 | No | AASM | ||
| Hot et al. [42] | 14 AD | 50% | 76.7 ± 3.8 | NR | 24.8 ± 2.4 | Yes | Yes | R&K |
| 14 controls | 42.9% | 76.7 ± 4.1 | NR | 29.4 ± 1 | Yes | R&K | ||
| Hassainia et al. [41] | 27 AD | 44.4% | 70.1 | NR | 20.2 | Yes | No | R&K |
| 25 controls | 52% | 67.8 | NR | 28.6 | No | R&K | ||
| Montplaisir et al. [47] | 10 AD | NR | 60.6 | NR | 20.6 ± 5.3 | NR | Yes | R&K |
| 10 controls | NR | 58.3 | NR | 29.3 ± 1.0 | Yes | R&K | ||
| Prinz et al. [59] | 20 AD (male) | 100% | 70 ± 7 | 14 ± 3 | 23 ± 3 | Yes | Yes | R&K |
| 19 AD (female) | 0 | 72 ± 8 | 14 ± 3 | 22 ± 3 | Yes | Yes | R&K | |
| 17 controls (male) | 100% | 66 ± 5 | 15 ± 4 | 30 ± 0 | Yes | R&K | ||
| 26 controls (female) | 0 | 68 ± 8 | 14 ± 3 | 30 ± 1 | Yes | R&K |
AASM American Academy of Sleep Medicine, AD Alzheimer’s disease, MMSE mini-mental state examination, NR not reported, R&K Rechtschaffen and Kales.
Description of the included studies
As shown in Table 1, the sample sizes of the 28 studies ranged from 16 participants (eight AD patients and eight controls) [48] to 243 participants (123 AD patients and 120 controls) [58]. The mean age of AD patients and controls ranged from 58.3 to 75.8 y (reported in 27 studies). Males as percentages of AD patients and controls ranged from 0–100% (reported in 27 studies). Five studies [24, 43–46] used AASM PSG criteria and the other 23 studies used R&K rules. For PSG adaptation nights, seven studies [36, 39–41, 46, 51, 55] did not include an adaptation night and the other 21 studies included an adaptation night. Among the 28 studies, two studies [47, 50] did not report whether they excluded AD patients who used medication impacting sleep, the other 26 studies clearly stated that their AD patients were drug naïve or had a washout period for medications impacting sleep before PSG examinations. For quality assessments of these studies (Table S7), no study addressed all ten aspects of the NICE checklist. For instance, no study reported a participation rate nor compared participants vs. non-participants. Furthermore, 15 studies did not report whether the same exclusion criteria were used for both cases and controls.
Meta-analysis
In the whole sample, the meta-analysis revealed significantly decreased TST, SE, SWS, and REM sleep percentage, and REMD, and significantly increased N1 percentage, SL, WASO, number of awakenings, and REML in AD patients compared with controls (Table 2). There were no significant differences in N2 percentage and PLMS index between AD patients and controls (p > 0.05).
Table 2.
Summary of meta-analysis comparing AD patients and controls.
| No. of comparisons | No. of AD/controls | Means of AD | Means of controls | SMD (95%CI) | Q | I2 | |
|---|---|---|---|---|---|---|---|
| N1% | 19 | 504/495 | 18.410 | 12.707 | 0.820 (0.369 to 1.271)*** | 186.036*** | 90.324 |
| N2% | 20 | 551/539 | 57.707 | 58.310 | 0.092 (−0.233 to 0.416) | 118.626*** | 83.983 |
| Number of awakenings | 15 | 383/415 | 16.147 | 13.547 | 0.551 (0.246 to 0.855)*** | 53.118*** | 73.644 |
| PLMS index | 5 | 80/88 | 2.422 | 4.088 | −0.158 (−0.501 to 0.185) | 4.929 | 18.855 |
| REM% | 25 | 668/711 | 13.965 | 17.673 | −0.767 (−1.142 to −0.391)*** | 241.492*** | 90.062 |
| REMD | 7 | 129/165 | – | – | −0.286 (−0.545 to −0.028)* | 6.644 | 9.698 |
| REML | 23 | 609/646 | 111.404 | 92.814 | 0.352 (0.127 to 0.578)** | 74.566*** | 70.496 |
| SE | 20 | 579/594 | 71.331 | 80.643 | −0.962 (−1.358 to −0.567)*** | 174.611*** | 89.119 |
| SL | 20 | 588/610 | 22.629 | 14.894 | 0.451 (0.292 to 0.609)*** | 30.177* | 37.038 |
| SWS% | 25 | 644/659 | 4.722 | 9.956 | −0.861 (−1.142 to −0.580)*** | 125.750*** | 80.915 |
| TST | 23 | 644/660 | 330.904 | 369.504 | −0.596 (−0.856 to −0.335)*** | 102.455*** | 78.527 |
| WASO | 16 | 540/541 | 104.419 | 73.799 | 0.739 (0.375 to 1.103)*** | 112.309*** | 86.644 |
Means for REMD were not calculated because definitions and algorithms of REMD varied across studies.
% percentage, AD Alzheimer’s disease, Q Cochran’s Q statistic, HCs healthy controls, PLMS periodic limb movement during sleep, REM rapid eye movement sleep, REMD REM density, REML REM latency, SWS slow-wave sleep, SE sleep efficiency, SL sleep latency, SMD standardized mean difference, TST total sleep time, WASO wake time after sleep onset.
*p < 0.05, **p < 0.01, ***p < 0.001.
For these findings, the Egger test failed to detect any publication bias (Figs. S1–S11), although it should be noted that the Egger test for the differences in PLMS index and REMD could not be performed because of limited available data.
Moderator analysis
As shown in Table 3, a meta-regression analysis revealed that decreased percentage of male AD patients (p = 0.01) and increased age of AD patients (p = 0.008) across different studies were significantly associated with increased SL in AD patients compared with controls.
Table 3.
Moderator analyses.
| Moderators | TST | WASO | Number of awakenings | SE | SL | N1% | N2% | SWS% | REM% | REML | REMD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (male%) | No. of comparisons | 22 | 16 | 15 | 20 | 19 | 18 | 19 | 24 | 24 | 22 | 6 |
| No. of AD/Controls | 634/650 | 540/541 | 383/415 | 579/594 | 578/600 | 494/485 | 541/529 | 634/649 | 658/701 | 599/636 | 119/155 | |
| Point estimate | 0.477 | 0.793 | 1.354 | 1.651 | −1.290 | −3.561 | −1.189 | 0.378 | −0.557 | 0.202 | −1.077 | |
| SE | 1.319 | 2.410 | 0.991 | 1.836 | 0.502 | 2.168 | 1.626 | 0.989 | 1.270 | 0.743 | 0.550 | |
| P | 0.718 | 0.742 | 0.172 | 0.369 | 0.010 | 0.100 | 0.464 | 0.702 | 0.661 | 0.786 | 0.0503 | |
| Mean age | No. of comparisons | 22 | 16 | 15 | 19 | 19 | 18 | 19 | 24 | 24 | 22 | 6 |
| No. of AD/Controls | 636/651 | 540/541 | 383/415 | 571/585 | 580/601 | 496/486 | 543/530 | 636/650 | 660/702 | 601/637 | 121/156 | |
| Point estimate | −0.019 | −0.071 | −0.023 | −0.001 | 0.048 | 0.041 | 0.004 | −0.002 | 0.028 | 0.004 | 0.036 | |
| SE | 0.033 | 0.056 | 0.041 | 0.053 | 0.018 | 0.057 | 0.041 | 0.037 | 0.049 | 0.027 | 0.024 | |
| P | 0.567 | 0.206 | 0.575 | 0.983 | 0.008 | 0.470 | 0.922 | 0.952 | 0.570 | 0.882 | 0.133 | |
| Education (years) | No. of comparisons | 8 | 8 | 9 | 7 | 6 | 7 | 8 | 12 | 11 | 8 | – |
| No. of AD/Controls | 322/318 | 322/318 | 289/310 | 278/273 | 287283 | 256/254 | 303/298 | 391/409 | 366/384 | 287/304 | – | |
| Point estimate | 0.053 | 0.019 | 0.151 | 0.030 | −0.035 | −0.473 | −0.200 | 0.080 | 0.023 | 0.032 | – | |
| SE | 0.088 | 0.079 | 0.086 | 0.098 | 0.038 | 0.059 | 0.119 | 0.054 | 0.078 | 0.046 | – | |
| P | 0.542 | 0.811 | 0.079 | 0.762 | 0.359 | <0.001 | 0.094 | 0.138 | 0.768 | 0.492 | – | |
| MMSE score | No. of comparisons | 21 | 16 | 15 | 18 | 18 | 16 | 17 | 22 | 23 | 21 | 6 |
| No. of AD/Controls | 621/636 | 540/541 | 383/415 | 556/570 | 565/586 | 456/446 | 503/490 | 596/610 | 645/687 | 586/622 | 121/156 | |
| Point estimate | 0.044 | 0.023 | −0.022 | −0.027 | 0.027 | −0.011 | 0.011 | 0.049 | 0.067 | −0.026 | 0.011 | |
| SE | 0.045 | 0.057 | 0.025 | 0.067 | 0.021 | 0.080 | 0.054 | 0.028 | 0.037 | 0.022 | 0.030 | |
| P | 0.330 | 0.681 | 0.370 | 0.693 | 0.202 | 0.888 | 0.842 | 0.075 | 0.071 | 0.229 | 0.714 | |
| Medication free | ||||||||||||
| Not reported | No. of comparisons | 1 | – | 3 | – | 1 | 1 | 1 | 4 | 4 | 4 | 1 |
| No. of AD/Controls | 10/10 | – | 44/66 | – | 10/10 | 10/10 | 10/10 | 54/76 | 54/76 | 54/76 | 10/10 | |
| SMD | −0.062 | – | 0.895*** | – | −0.240 | 0.601 | 0.277 | −1.100*** | −1.375*** | 0.713*** | −0.749 | |
| Q | 0 | – | 0.010 | – | 0 | 0 | 0 | 3.198 | 10.498* | 2.654 | 0 | |
| I2 | 0 | – | 0 | – | 0 | 0 | 0 | 6.184 | 71.424 | 0 | 0 | |
| Yes | No. of comparisons | 22 | 16 | 12 | 20 | 19 | 18 | 19 | 21 | 21 | 19 | 6 |
| No. of AD/Controls | 634/650 | 540/541 | 339/349 | 579/594 | 578/600 | 494/485 | 541/529 | 590/583 | 614/635 | 555/570 | 119/155 | |
| SMD | −0.616*** | 0.739*** | 0.471** | −0.962*** | 0.471*** | 0.831** | 0.084 | −0.818*** | −0.654** | 0.290* | −0.245 | |
| Q | 101.331*** | 112.309*** | 47.307*** | 174.611*** | 27.648 | 186.036*** | 118.443*** | 120.355*** | 216.640*** | 66.595*** | 5.472 | |
| I2 | 79.216 | 86.644 | 76.748 | 89.119 | 34.895 | 90.862 | 84.803 | 83.383 | 90.768 | 72.971 | 8.633 | |
| Between-group difference | Q | 1.404 | – | 2.415 | – | 2.431 | 0.199 | 0.162 | 1.196 | 2.737 | 3.531 | 1.094 |
| P | 0.236 | – | 0.120 | – | 0.119 | 0.656 | 0.688 | 0.274 | 0.098 | 0.060 | 0.296 | |
| Adaptation night | ||||||||||||
| No | No. of comparisons | 6 | 4 | 3 | 5 | 3 | 4 | 5 | 6 | 6 | 4 | – |
| No. of AD/Controls | 113/114 | 93/90 | 46/46 | 102/103 | 67/68 | 61/61 | 108/105 | 113/114 | 113/114 | 78/79 | – | |
| SMD | −0.526** | 0.018 | 0.054 | −0.495** | 0.657*** | 0.870*** | −0.006 | −0.635** | −0.415 | 0.164 | – | |
| Q | 7.131 | 0.034 | 3.998 | 1.017 | 0.777 | 4.303 | 15.965** | 12.638* | 17.452** | 5.657 | – | |
| I2 | 29.880 | 0 | 49.979 | 0 | 0 | 30.280 | 74.946 | 60.438 | 71.350 | 46.972 | – | |
| Yes | No. of comparisons | 17 | 12 | 12 | 15 | 17 | 15 | 15 | 19 | 19 | 19 | 7 |
| No. of AD/Controls | 531/546 | 447/451 | 337/369 | 477/491 | 521/542 | 443/434 | 443/434 | 531/545 | 555/597 | 531/567 | 129/165 | |
| SMD | −0.599*** | 0.974*** | 0.663*** | −1.091*** | 0.413*** | 0.789** | 0.119 | −0.925*** | −0.862*** | 0.385** | −0.286* | |
| Q | 95.029*** | 93.838*** | 44.358*** | 168.870*** | 28.124* | 179.947*** | 98.831*** | 109.629*** | 218.276*** | 67.292*** | 6.644 | |
| I2 | 83.163 | 88.278 | 75.202 | 91.710 | 43.110 | 92.220 | 85.834 | 83.581 | 91.754 | 73.251 | 9.698 | |
| Between-group difference | Q | 0.092 | 12.830 | 3.045 | 4.037 | 1.491 | 0.051 | 0.121 | 0.976 | 1.524 | 0.603 | – |
| P | 0.792 | <0.001 | 0.081 | 0.045 | 0.222 | 0.822 | 0.728 | 0.323 | 0.217 | 0.438 | – | |
| PSG scoring methods | ||||||||||||
| AASM | No. of comparisons | 6 | 5 | 1 | 5 | 4 | 5 | 5 | 5 | 5 | 6 | |
| No. of AD/Controls | 182/158 | 165/138 | 11/11 | 171/147 | 151/132 | 162/143 | 162/143 | 162/143 | 162/143 | 182/158 | – | |
| SMD | −1.108*** | 1.386*** | 0.774 | −1.996*** | 0.488** | 2.156*** | 0.456 | −1.699** | −2.170*** | 0.587* | – | |
| Q | 26.862*** | 26.104*** | 0 | 59.169*** | 4.833 | 14.677** | 65.047*** | 49.543*** | 35.402*** | 18.508** | – | |
| I2 | 81.386 | 84.677 | 0 | 93.240 | 37.924 | 72.747 | 93.851 | 91.926 | 88.701 | 72.984 | – | |
| R&K | No. of comparisons | 17 | 11 | 14 | 15 | 16 | 14 | 15 | 20 | 20 | 17 | 7 |
| No. of AD/Controls | 462/502 | 375/403 | 372/404 | 408/447 | 437478 | 342/352 | 389/396 | 482/516 | 506/568 | 427/488 | 129/165 | |
| SMD | −0.398** | 0.435** | 0.539** | −0.597*** | 0.437*** | 0.301* | −0.044 | −0.650*** | −0.385** | 0.250* | −0.286* | |
| Q | 41.806*** | 31.063** | 52.508*** | 32.323** | 25.181* | 25.200* | 44.029*** | 39.866** | 62.067*** | 39.630** | 6.644 | |
| I2 | 61.728 | 67.808 | 75.242 | 56.687 | 40.430 | 48.412 | 68.203 | 52.340 | 69.388 | 59.626 | 9.698 | |
| Between-group difference | Q | 5.190 | 6.250 | 0.249 | 5.964 | 0.074 | 34.444 | 0.843 | 4.129 | 14.221 | 1.719 | – |
| P | 0.023 | 0.012 | 0.618 | 0.015 | 0.786 | <0.001 | 0.358 | 0.042 | <0.001 | 0.190 | – | |
A meta-regression or subgroup analysis (depending on whether the potential moderators were continuous or categorical variables) was conducted to determine the potential factors that could moderate heterogeneity between studies. Of which, the effects of sex, age, education, and MMSE score were explored by using a meta-regression analysis, while the effects of whether taking medication impacts sleep (Yes vs. No), adaptation night (Yes vs. No), and PSG scoring methods (AASM vs. R&K) were explored by using subgroup analysis.
AASM American Academy of Sleep Medicine, AD Alzheimer’s disease, AHI apnea-hypopnea index, Q Cochran’s Q statistic, MMSE mini-mental state examination, PSG polysomnography, REM rapid eye movement sleep, REMD REM density, REML REM latency, R&K Rechtschaffen and Kales, SMD standardized mean difference, SWS slow-wave sleep, SE sleep efficiency, SL sleep latency, TST total sleep time, WASO wake time after sleep onset.
*p < 0.05, **p < 0 .01, ***p < 0.001.
To exclude the potential effects of medication status, the meta-analysis was rerun using only the studies which clearly reported that AD patients were drug naïve or had a washout period before PSG examination. The results were unchanged compared to the full sample analysis.
A subgroup analysis revealed that having an adaptation night or not across different studies was a significant source of heterogeneity between AD patients and controls for differences in WASO and SE. Specifically, significantly increased WASO and decreased SE in AD patients compared with controls were only found in studies using an adaptation night (p < 0.05).
Another subgroup analysis revealed that the PSG scoring method (AASM vs. R&K) was a significant source of heterogeneity between AD patients and controls for differences in TST, WASO, SE, and percentage of N1, SWS, and REM sleep (p < 0.05). Significantly decreased TST, SE, SWS, and REM sleep percentage, and increased N1 percentage and WASO were observed regardless of scoring criteria used. However, the magnitude of changes in these parameters in AD patients compared with controls were greater in studies using AASM criteria compared with those in studies using R&K criteria (p < 0.05).
Associations of PSG measured sleep with cognitive decline in AD patients
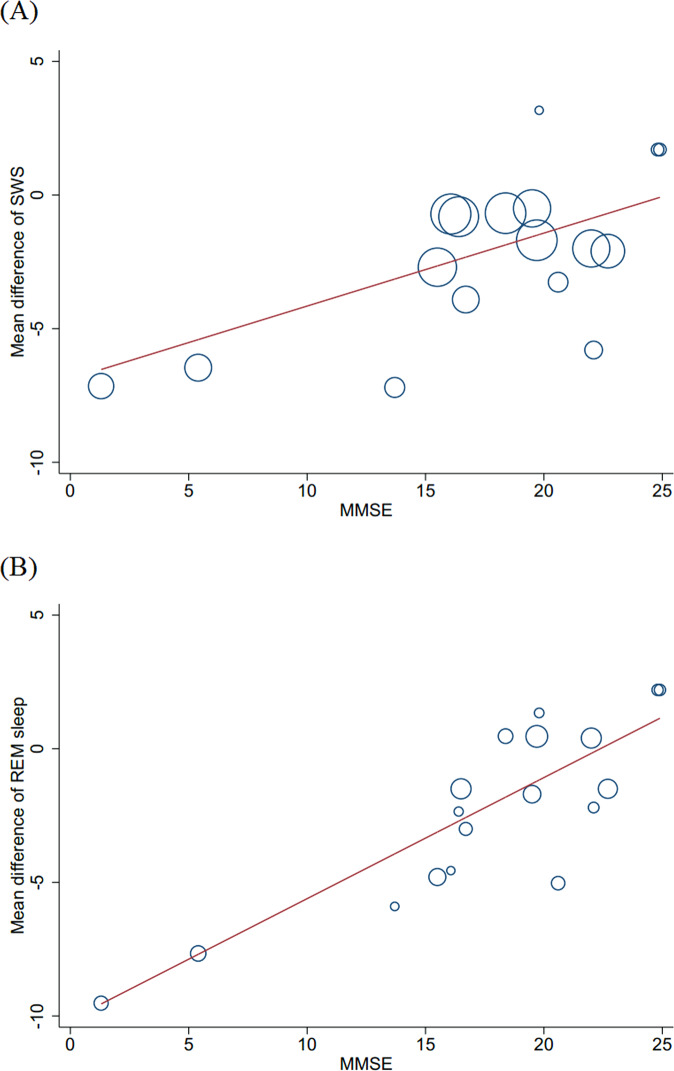
Meta-regression analysis did not reveal any significant associations between sleep measures and MMSE score in AD patients compared to controls; although associations between decreased SWS and REM sleep percentage and decreased MMSE score in AD patients approached statistical significance, p = 0.075 and p = 0.071, respectively. Given that PSG scoring rules (AASM vs. R&K) were found to be a significant source of heterogeneity for SWS and REM sleep differences, a second analysis was conducted with studies using R&K criteria, which revealed statistically significant associations between decreased MMSE with decreased SWS (p = 0.004) and REM sleep (p < 0.001) percentage in AD patients (Fig. 2). A comparable analysis for studies using AASM criteria was not possible because of limited available data.
Fig. 2. Significant associations between sleep changes and MMSE score in patients with Alzheimer’s disease.
A Associations of the changes in slow wave sleep (coefficient = 0.273; p = 0.004) with MMSE score in patients with Alzheimer’s disease in studies using R&K criteria; B Associations of the changes in rapid eye movement sleep (coefficient = 0.453; p < 0.001) with MMSE score in patients with Alzheimer’s disease in studies using R&K criteria. MMSE mini-mental state examination.
Sleep parameters which could not be meta-analyzed
PSA data [41, 42, 48, 49, 52, 59], CAP parameters [46], and sleep spindles [25, 40, 45, 47, 49] were also explored for possible differences between AD patients and controls (Table S8). However meta-analytic evaluation of these parameters was not possible due to the limited number of available studies and methodological differences across studies.
Discussion
Summary of findings
To our knowledge, this is the first systematic review and meta-analysis to explore how the PSG changes in AD patients. The results showed decreased TST, SE, SWS and REM sleep percentage, and REMD, and increased number of awakenings, WASO, N1 percentage, SL, and REML in AD patients compared with controls. Importantly, decreased SWS and REM sleep percentage were significantly associated with decreased MMSE scores in AD patients, although these associations were found only in studies using R&K scoring criteria. Sophisticated analyses of sleep microstructure (i.e., PSA, CAP, etc.), while studied in AD, could not be meta-analyzed because of the limited number of studies that have reported on these parameters.
Sleep changes in AD
Our systematic review showed that sleep continuity and architecture (decreased TST, SWS, and REM sleep) are disturbed in AD patients. Accumulating evidence shows that sleep disturbance contributes to cognitive decline and may increase the risk of AD dementia [8, 10, 60–62]. A meta-analysis of 18 longitudinal studies revealed that sleep disturbances predict the development of AD dementia [10]. Supporting this hypothesis are reports that similar alterations in PSG measured sleep parameters including increased N1 sleep, WASO, SL, REML, and decreased TST, SE, and REM sleep are found in individuals with mild cognitive impairment, a prodromal stage of AD [63]. Taken together, these findings suggest a bidirectional relationship between sleep and AD [8].
Circadian dysrhythmia is commonly observed in AD patients and has been considered to be a major cause of their sleep problems [64, 65]. For instance, a 24-h PSG study in AD patients revealed obvious fragmentation in the sleep and waking rhythm with nighttime wakefulness periods and frequent daytime napping [49]. Circadian dysrhythmia in AD patients is likely the result of progressive neuropathological changes in brain regions that play an important role in circadian regulation, such as the suprachiasmatic nucleus [61].
Furthermore, sleep disturbance has been demonstrated to be associated with increased inflammation activation [66]. Studies have also suggested that inflammation, which may promote Aβ accumulation, might be a biological risk factor for mild cognitive impairment preceding the AD onset [67, 68]. Thus, inflammation is hypothesized to be a biologically plausible pathway linking sleep disturbance and increased risk of AD [69, 70]. It has also been speculated that treating sleep disturbances may mitigate inflammatory processes potentially ameliorating the development of AD (see Irwin and Vitiello [8] for more details on inflammation as an underlying mechanism of the sleep/AD relationship).
Associations of changes in sleep macrostructure with AD severity
Clinically, it is important to ask which PSG parameter changes are associated with progressive memory loss and decline in global cognition, the core symptoms of AD. Our meta-regression analysis revealed significant associations of decreased SWS and REM sleep with decreased MMSE scores. Previous studies have indicated that SWS benefits the consolidation of declarative memories [71–73]. Disruptions in slow-wave activity increase Aβ levels and are associated with impairments of learning, memory, attention, and executive processes [16, 74]. REM sleep benefits non-declarative (emotional and procedural) memory [71, 72, 75], and loss of REM sleep impacts non-declarative memory consolidation [18, 76, 77]. These findings indicate potential contributions of disturbed SWS and REM sleep to the cognitive impairments seen in AD and suggest that developing sleep therapies improving SWS and REM sleep may have potential for slowing cognitive decline in AD if begun early enough in the disease trajectory. Furthermore, given that MMSE is one of the most widely used tools to reflect the severity of cognitive decline or stage/progression of dementia, decreased SWS and REM sleep are also useful indicators to reflect the severity of cognitive decline or stage/progression of dementia. It should be noted that the associations of SWS and REM sleep with MMSE performance were found only in studies using R&K scoring rules and that studies using AASM scoring rules could not be separately examined because of limited available data. Although the differences between the AASM and R&K scoring rules are minor, SWS and REM sleep in the same participants could vary with the application of different scoring rules [78]. Thus, new studies using AASM scoring rules are needed to confirm our findings.
Effects of sex, age, and education level on sleep in AD
It has been demonstrated that being female and having advanced age are highly significant risk factors for AD [79]. By comparison, high education level is a protective factor against AD, and previous findings suggest that the risk of dementia may be decreased by 7% for each year of additional education [80]. Our findings revealed that being female and of advanced age were associated with increased SL in AD patients compared with controls and that higher education level was associated with less N1 sleep in AD patients compared with controls. Together, these findings suggest that shorter SL and less N1 sleep might be beneficial for AD patients, again indicating the potential usefulness of strategies to improve sleep that might be beneficial for those at risk for or at an early stage of AD.
The mechanisms underlying the relationships of sex, age, and education to sleep changes in AD are unclear. We speculate that they may impact sleep by moderating AD pathology. Regarding the sex difference, Nebel et al. suggested that the increased risk of AD in women may be attributed to a relative lack of some protective factors, such as estrogen deficiency of post-menopausal women, with increased vulnerability to AD pathology [81]. Regarding age, AD pathology including intracellular neurofibrillary tangles and extracellular senile plaques is worse with advanced age in AD patients [1, 82]. Regarding education, neuroimaging studies revealed that education level is positively associated with brain reserves including regional cortical thickness [83] and white and gray matter volume [84]. In addition, high education level may be associated with protection against developing AD pathology, including tau [85] and Aβ [86].
Sleep spindle and PSA
Sleep spindles, a key EEG feature of N2 sleep are generated by a complex interaction between thalamic, limbic, and cortical regions [87], and can be compromised by disruptions in the structural and functional integrity of these regions from neurodegenerative diseases [88]. These factors suggest that altered sleep spindles may be a potential biomarker of neurodegenerative disease [88]. Liu et al. reported that AD patients had poorer spindle activity compared with controls, and this alteration was associated with decreased MMSE and Montreal Cognitive Assessment scores [45]. Rauchs et al. reported that sleep spindles were globally reduced in aging and AD, while AD patients also exhibited a further decrease in fast spindles compared with normally aging individuals [25]. Furthermore, the mean intensity of fast spindles was positively associated with immediate recall performance in AD patients [25]. These findings suggest that monitoring sleep spindles could be of clinical and biomarker relevance for diagnosing AD. However, both the sensitivity and specificity of sleep spindles for diagnosing AD need to be fully delineated.
Previous studies suggested that quantifying changes in EEG frequency components could provide important neurobiological insight into the disease and its clinical relevance. For instance, previous studies suggested that EEG slowing during REM sleep, which is related to the degeneration of the cholinergic structures in the brain stem and forebrain [89], may be a biological marker of AD [41, 49] although its sensitivity and specificity for diagnosing AD are also unclear. Additional evidence showed that increases in the faster theta frequency band in SWS is significantly linked to better delayed episodic recall in AD patients, suggesting a potential compensatory mechanism during SWS against the impact of AD pathology [42]. However, it is difficult to make unequivocal conclusions regarding the relevance of EEG frequency components in AD, because of limited available studies and methodological differences across studies.
Limitations
This review has limitations. First, some factors, such as variations in bedtime schedule across sleep labs and discomfort from wearing PSG devices, which may potentially impact the pooled effect sizes of sleep changes, could not be accounted for in our meta-analysis. Second, some factors, such as daytime sleepiness, depression, and anxiety which also potentially influence PSG changes in AD could not be explored due to limited available data for these variables. Third, it should be noted that obstructive sleep apnea (OSA) may significantly impact sleep [90–92]. In the 24 studies included in our meta-analysis, the majority of studies did not report whether they excluded OSA, while the eight studies that stated that they excluded the OSA used a variety of exclusion criteria. Thus, the potential effects of OSA on our findings are unknown. Fourth, although a majority of our included studies reported that their participants were mild to moderate AD patients, the detailed disease progression/stage (when the PSG examination was performed) was still unclear, which may be associated with the effect sizes of PSG changes in AD. These limitations suggest that our findings should be interpreted with caution.
Conclusions
The present study reports the first in-depth exploration of the existing literature on PSG measured sleep changes in AD. Although there was methodological heterogeneity across the included studies, this systematic review clearly identified disrupted objective sleep as a significant problem in AD. Importantly, decreased SWS and REM sleep are associated with impairments in cognitive function and are potential targets for therapeutic intervention aimed at improving both sleep and cognitive function in mild cognitive impairment and mild stage AD. Furthermore, alterations in sleep spindles and EEG frequency activity may be clinically useful for diagnosing AD but further studies are needed to confirm this possibility.
Supplementary information
Acknowledgements
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (2021ZD0201900) and the National Natural Science Foundation of China (82120108002, 82170099, and 82170100).
Author contributions
XT and MVV designed the study. YZ drafted the manuscript. YZ, RR, YS, and HZ contributed to database preparation. MVV, LDS, HRO, and LY provided important suggestions for improving the manuscript and critically revised the manuscript. All authors commented on and approved drafts and the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rong Ren, Email: 498880651@qq.com.
Xiangdong Tang, Email: 2372564613@qq.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-022-01897-y.
References
- 1.Scheltens P, De Strooper B, Kivipelto M, Holstege H, Chételat G, Teunissen CE, et al. Alzheimer’s disease. Lancet. 2021;397:1577–90. doi: 10.1016/S0140-6736(20)32205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer’s Disease International. World Alzheimer Report 2018. The state of the art of dementia research: new frontiers. 2018. https://www.alzint.org/u/WorldAlzheimerReport2018.pdf. Accessed 9 Sept 2020.
- 3.Graff-Radford J, Yong KXX, Apostolova LG, Bouwman FH, Carrillo M, Dickerson BC, et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021;20:222–34. doi: 10.1016/S1474-4422(20)30440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jack CR, Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol. 2014;10:115–9. doi: 10.1038/nrneurol.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju YS. Circadian rest-activity pattern changes in aging and preclinical alzheimer disease. JAMA Neurol. 2018;75:582–90. doi: 10.1001/jamaneurol.2017.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bubu OM, Brannick M, Mortimer J, Umasabor-Bubu O, Sebastiao YV, Wen Y, et al. Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep. 2017;40:zsw032. [DOI] [PubMed]
- 8.Irwin MR, Vitiello MV. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 2019;18:296–306. doi: 10.1016/S1474-4422(18)30450-2. [DOI] [PubMed] [Google Scholar]
- 9.McCurry SM, Reynolds CF, Ancoli-Israel S, Teri L, Vitiello MV. Treatment of sleep disturbance in Alzheimer’s disease. Sleep Med Rev. 2000;4:603–28. doi: 10.1053/smrv.2000.0127. [DOI] [PubMed] [Google Scholar]
- 10.Shi L, Chen SJ, Ma MY, Bao YP, Han Y, Wang YM, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. doi: 10.1016/j.smrv.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 11.Moran M, Lynch CA, Walsh C, Coen R, Coakley D, Lawlor BA. Sleep disturbance in mild to moderate Alzheimer’s disease. Sleep Med. 2005;6:347–52. doi: 10.1016/j.sleep.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 12.McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Nighttime insomnia treatment and education for Alzheimer’s disease: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:793–802. doi: 10.1111/j.1532-5415.2005.53252.x. [DOI] [PubMed] [Google Scholar]
- 13.Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: epidemiology, pathophysiology and treatment. CNS Drugs. 2001;15:777–96. doi: 10.2165/00023210-200115100-00004. [DOI] [PubMed] [Google Scholar]
- 14.Vitiello MV, Poceta JS, Prinz PN. Sleep in Alzheimer’s disease and other dementing disorders. Can J Psychol. 1991;45:221–39. doi: 10.1037/h0084283. [DOI] [PubMed] [Google Scholar]
- 15.Vitiello MV, Prinz PN. Alzheimer’s disease. Sleep and sleep/wake patterns. Clin Geriatr Med. 1989;5:289–99. [PubMed] [Google Scholar]
- 16.Ju Y-ES, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. 2017;140:2104–11. doi: 10.1093/brain/awx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lucey BP, McCullough A, Landsness EC, Toedebusch CD, McLeland JS, Zaza AM, et al. Reduced non-rapid eye movement sleep is associated with tau pathology in early Alzheimer’s disease. Sci Transl Med. 2019;11:eaau6550. doi: 10.1126/scitranslmed.aau6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chauhan AK, Mallick BN. Association between autophagy and rapid eye movement sleep loss-associated neurodegenerative and patho-physio-behavioral changes. Sleep Med. 2019;63:29–37. doi: 10.1016/j.sleep.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Liguori C, Nuccetelli M, Izzi F, Sancesario G, Romigi A, Martorana A, et al. Rapid eye movement sleep disruption and sleep fragmentation are associated with increased orexin-a cerebrospinal-fluid levels in mild cognitive impairment due to Alzheimer’s disease. Neurobiol Aging. 2016;40:120–6. doi: 10.1016/j.neurobiolaging.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol. 2014;71:1498–505. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 21.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13:1017–28. doi: 10.1016/S1474-4422(14)70172-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. 2020;45:104–20. doi: 10.1038/s41386-019-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag. 2014;4:351–62. doi: 10.2217/nmt.14.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonakis A, Economoua NT, Paparrigopoulos T, Bonanni E, Maestri M, Carnicelli L, et al. Sleep in frontotemporal dementia is equally or possibly more disrupted, and at an earlier stage, when compared to sleep in Alzheimer’s disease. J Alzheimers Dis. 2014;38:85–91. doi: 10.3233/JAD-122014. [DOI] [PubMed] [Google Scholar]
- 25.Rauchs G, Schabus M, Parapatics S, Bertran F, Clochon P, Hot P, et al. Is there a link between sleep changes and memory in Alzheimer’s disease? NeuroReport. 2008;19:1159–62. doi: 10.1097/WNR.0b013e32830867c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin PR, Loewenstein RJ, Kaye WH. Sleep EEG in Korsakoff’s psychosis and Alzheimer’s disease. Neurology. 1986;36:411–4. doi: 10.1212/wnl.36.3.411. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 29.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Washington, DC: American Psychiatric Association 1980.
- 31.Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine 2007.
- 32.ExcellenceNIfHaC. Appendix E: methodology checklist: case econtrol studies. NICE article [PMG6B] 2012.
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Publication bias. In: Borenstein M, Hedges LV, Higgins JPT, Rothstein HR, editors. Introduction to meta-analysis. 1st ed. New Jersey: John Wiley & Sons, Ltd. 2009.
- 35.Bonanni E, Maestri M, Tognoni G, Fabbrini M, Nucciarone B, Manca ML, et al. Daytime sleepiness in mild and moderate Alzheimer’s disease and its relationship with cognitive impairment. J Sleep Res. 2005;14:311–7. doi: 10.1111/j.1365-2869.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 36.Brunetti V, D’Atri A, Della Marca G, Vollono C, Marra C, Vita MG, et al. Subclinical epileptiform activity during sleep in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol. 2020;131:1011–8. doi: 10.1016/j.clinph.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Chen R, Yin Y, Zhao Z, Huang L, Huang S, Zhuang J, et al. Elevation of serum TNF-α levels in mild and moderate Alzheimer patients with daytime sleepiness. J Neuroimmunol. 2012;244:97–102. doi: 10.1016/j.jneuroim.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 38.Dykierek P, Stadtmüller G, Schramm P, Bahro M, van Calker D, Braus DF, et al. The value of REM sleep parameters in differentiating Alzheimer’s disease from old-age depression and normal aging. J Psychiatr Res. 1998;32:1–9. doi: 10.1016/s0022-3956(97)00049-6. [DOI] [PubMed] [Google Scholar]
- 39.Gagnon J-F, Petit D, Fantini ML, Rompré S, Gauthier S, Panisset M, et al. REM sleep behavior disorder and REM sleep without atonia in probable Alzheimer disease. Sleep. 2006;29:1321–5. doi: 10.1093/sleep/29.10.1321. [DOI] [PubMed] [Google Scholar]
- 40.Gorgoni M, Lauri G, Truglia I, Cordone S, Sarasso S, Scarpelli S, et al. Parietal fast sleep spindle density decrease in Alzheimer’s disease and amnesic mild cognitive impairment. Neural Plasticity. 2016;2016:8376108. doi: 10.1155/2016/8376108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hassainia F, Petit D, Nielsen T, Gauthier S, Montplaisir J. Quantitative EEG and statistical mapping of wakefulness and REM sleep in the evaluation of mild to moderate Alzheimer’s disease. Eur Neurol. 1997;37:219–24. doi: 10.1159/000117446. [DOI] [PubMed] [Google Scholar]
- 42.Hot P, Rauchs G, Bertran F, Denise P, Desgranges B, Clochon P, et al. Changes in sleep theta rhythm are related to episodic memory impairment in early Alzheimer’s disease. Biol Psychol. 2011;87:334–9. doi: 10.1016/j.biopsycho.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Liguori C, Mercuri NB, Nuccetelli M, Izzi F, Cordella A, Bernardini S, et al. Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation: Is it a further proof for Alzheimer’s disease risk? Sleep Med. 2019;56:171–6. doi: 10.1016/j.sleep.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Liguori C, Placidi F, Izzi F, Spanetta M, Mercuri NB, Di Pucchio A. Sleep dysregulation, memory impairment, and CSF biomarkers during different levels of neurocognitive functioning in Alzheimer’s disease course. Alzheimers Res Ther. 2020;12:5. doi: 10.1186/s13195-019-0571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Pan J, Tang K, Lei Q, He L, Meng Y, et al. Sleep spindles, K-complexes, limb movements and sleep stage proportions may be biomarkers for amnestic mild cognitive impairment and Alzheimer’s disease. Sleep Breath. 2020;24:637–51. doi: 10.1007/s11325-019-01970-9. [DOI] [PubMed] [Google Scholar]
- 46.Maestri M, Carnicelli L, Tognoni G, Di Coscio E, Giorgi FS, Volpi L, et al. Non-rapid eye movement sleep instability in mild cognitive impairment: a pilot study. Sleep Med. 2015;16:1139–45. doi: 10.1016/j.sleep.2015.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Montplaisir J, Petit D, Lorrain D, Gauthier S, Nielsen T. Sleep in Alzheimer’s disease: further considerations on the role of brainstem and forebrain cholinergic populations in sleep-wake mechanisms. Sleep. 1995;18:145–8. doi: 10.1093/sleep/18.3.145. [DOI] [PubMed] [Google Scholar]
- 48.Petit D, Montplaisir J, Lorrain D, Gauthier S. Spectral analysis of the rapid eye movement sleep electroencephalogram in right and left temporal regions: A biological marker of Alzheimer’s disease. Ann Neurol. 1992;32:172–6. doi: 10.1002/ana.410320208. [DOI] [PubMed] [Google Scholar]
- 49.Prinz PN, Peskind ER, Vitaliano PP. Changes in the sleep and waking EEGs of nondemented and demented elderly subjects. J Am Geriatr Soc. 1982;30:86–93. doi: 10.1111/j.1532-5415.1982.tb01279.x. [DOI] [PubMed] [Google Scholar]
- 50.Prinz PN, Vitaliano PP, Vitiello MV, Bokan J, Raskind M, Peskind E, et al. Sleep, EEG and mental function changes in senile dementia of the Alzheimer’s type. Neurobiol Aging. 1982;3:361–70. doi: 10.1016/0197-4580(82)90024-0. [DOI] [PubMed] [Google Scholar]
- 51.Reda F, Gorgoni M, Lauri G, Truglia I, Cordone S, Scarpelli S, et al. In search of sleep biomarkers of Alzheimer’s disease: K-complexes do not discriminate between patients with mild cognitive impairment and healthy controls. Brain Sci. 2017;7:51. doi: 10.3390/brainsci7050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reynolds CF, Kupfer DJ, Taska LS, Hoch CH, Sewitch DE, Grochocinski VJ. Slow wave sleep in elderly depressed, demented, and healthy subjects. Sleep. 1985;8:155–9. doi: 10.1093/sleep/8.2.155. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds CF, Kupfer DJ, Houck PR, Hoch CC, Stack JA, Berman SR, et al. Reliable discrimination of elderly depressed and demented patients by electroencephalographic sleep data. Arch Gen Psychiatry. 1988;45:258–64. doi: 10.1001/archpsyc.1988.01800270076009. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds CF, Kupfer DJ, Taska LS, Hoch CC, Spiker DG, Sewitch DE, et al. EEG sleep in elderly depressed, demented, and healthy subjects. Biol Psychiatry. 1985;20:431–42. doi: 10.1016/0006-3223(85)90045-9. [DOI] [PubMed] [Google Scholar]
- 55.Tseng IJ, Liu HC, Yuan RY, Sheu JJ, Yu JM, Hu CJ. Expression of inducible nitric oxide synthase (iNOS) and period 1 (PER1) clock gene products in different sleep stages of patients with cognitive impairment. J Clin Neurosci. 2010;17:1140–3. doi: 10.1016/j.jocn.2010.01.035. [DOI] [PubMed] [Google Scholar]
- 56.Vitiello MV, Bokan JA, Kukull WA, Muniz RL, Smallwood RG, Prinz PN. Rapid eye movement sleep measures of Alzheimer’s-type dementia patients and optimally healthy aged individuals. Biol Psychiatry. 1984;19:721–34. [PubMed] [Google Scholar]
- 57.Vitiello MV, Prinz PN, Williams DE, Frommlet MS, Ries RK. Sleep disturbances in patients with mild-stage Alzheimer’s disease. J Gerontol. 1990;45:M131–8. doi: 10.1093/geronj/45.4.m131. [DOI] [PubMed] [Google Scholar]
- 58.Yin Y, Liu Y, Pan X, Chen R, Li P, Wu HJ, et al. Interleukin-1beta promoter polymorphism enhances the risk of sleep disturbance in Alzheimer’s disease. PLoS ONE. 2016;11:e0149945. doi: 10.1371/journal.pone.0149945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prinz PN, Larsen LH, Moe KE, Vitiello MV. EEG markers of early Alzheimer’s disease in computer selected tonic REM sleep. Electroencephalogr Clin Neurophysiol. 1992;83:36–43. doi: 10.1016/0013-4694(92)90130-a. [DOI] [PubMed] [Google Scholar]
- 60.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev. 2015;19:29–38. doi: 10.1016/j.smrv.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 61.Van Erum J, Van Dam D, De Deyn PP. Sleep and Alzheimer’s disease: a pivotal role for the suprachiasmatic nucleus. Sleep Med Rev. 2018;40:17–27. doi: 10.1016/j.smrv.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 62.Cedernaes J, Osorio RS, Varga AW, Kam K, Schioth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev. 2017;31:102–11. doi: 10.1016/j.smrv.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Rozario AL, Chapman JL, Phillips CL, Palmer JR, Hoyos CM, Mowszowski L, et al. Objective measurement of sleep in mild cognitive impairment: a systematic review and meta-analysis. Sleep Med Rev. 2020;52:101308. doi: 10.1016/j.smrv.2020.101308. [DOI] [PubMed] [Google Scholar]
- 64.McCurry SM, Logsdon RG, Vitiello MV, Teri L. Treatment of sleep and nighttime disturbances in Alzheimer’s disease: a behavior management approach. Sleep Med. 2004;5:373–7. doi: 10.1016/j.sleep.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Vitiello MV, Bliwise DL, Prinz PN. Sleep in Alzheimer’s disease and the sundown syndrome. Neurology. 1992;42:83–93. [PubMed] [Google Scholar]
- 66.Irwin MR, Olmstead R, Carroll JE. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry. 2016;80:40–52. doi: 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wood H. Dementia: peripheral inflammation could be a prodromal indicator of dementia. Nat Rev Neurol. 2018;14:127. doi: 10.1038/nrneurol.2018.8. [DOI] [PubMed] [Google Scholar]
- 68.Lo JC, Groeger JA, Cheng GH, Dijk DJ, Chee MW. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med. 2016;17:87–98. doi: 10.1016/j.sleep.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 69.Spira AP, Chen-Edinboro LP, Wu MN, Yaffe K. Impact of sleep on the risk of cognitive decline and dementia. Curr Opin Psychiatry. 2014;27:478–83. doi: 10.1097/YCO.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahnaou A, Drinkenburg W. Sleep, neuronal hyperexcitability, inflammation and neurodegeneration: does early chronic short sleep trigger and is it the key to overcoming Alzheimer’s disease? Neurosci Biobehav Rev. 2021;129:157–79. doi: 10.1016/j.neubiorev.2021.06.039. [DOI] [PubMed] [Google Scholar]
- 71.Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. doi: 10.1162/jocn.1997.9.4.534. [DOI] [PubMed] [Google Scholar]
- 72.Plihal W, Born J. Effects of early and late nocturnal sleep on priming and spatial memory. Psychophysiology. 1999;36:571–82. [PubMed] [Google Scholar]
- 73.Barrett TR.Ekstrand BR, Effect of sleep on memory. 3. Controlling time-of-day effects. J Exp Psychol. 1972;96:321–7. [DOI] [PubMed]
- 74.Leger D, Debellemaniere E, Rabat A, Bayon V, Benchenane K, Chennaoui M. Slow-wave sleep: from the cell to the clinic. Sleep Med Rev. 2018;41:113–32. doi: 10.1016/j.smrv.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 75.Wagner U, Gais S, Born J. Emotional memory formation is enhanced across sleep intervals with high amounts of rapid eye movement sleep. Learn Mem. 2001;8:112–9. doi: 10.1101/lm.36801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacDonald KJ, Cote KA. Contributions of post-learning REM and NREM sleep to memory retrieval. Sleep Med Rev. 2021;59:101453. doi: 10.1016/j.smrv.2021.101453. [DOI] [PubMed] [Google Scholar]
- 77.Schafer SK, Wirth BE, Staginnus M, Becker N, Michael T, Sopp MR. Sleep’s impact on emotional recognition memory: a meta-analysis of whole-night, nap, and REM sleep effects. Sleep Med Rev. 2020;51:101280. doi: 10.1016/j.smrv.2020.101280. [DOI] [PubMed] [Google Scholar]
- 78.Moser D, Anderer P, Gruber G, Parapatics S, Loretz E, Boeck M, et al. Sleep classification according to AASM and Rechtschaffen & Kales: effects on sleep scoring parameters. Sleep. 2009;32:139–49. doi: 10.1093/sleep/32.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang MY, Katzman R, Salmon D, Jin H, Cai GJ, Wang ZY, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27:428–37. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 80.Xu W, Tan L, Wang HF, Tan MS, Tan L, Li JQ, et al. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol Neurobiol. 2016;53:3113–23. doi: 10.1007/s12035-015-9211-5. [DOI] [PubMed] [Google Scholar]
- 81.Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K, et al. Understanding the impact of sex and gender in Alzheimer’s disease: a call to action. Alzheimers Dement. 2018;14:1171–83. doi: 10.1016/j.jalz.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park JE, Lee YJ, Byun MS, Yi D, Lee JH, Jeon SY, et al. Differential associations of age and Alzheimer’s disease with sleep and rest-activity rhythms across the adult lifespan. Neurobiol Aging. 2021;101:141–9. doi: 10.1016/j.neurobiolaging.2021.01.006. [DOI] [PubMed] [Google Scholar]
- 83.Liu Y, Julkunen V, Paajanen T, Westman E, Wahlund LO, Aitken A, et al. Education increases reserve against Alzheimer’s disease–evidence from structural MRI analysis. Neuroradiology. 2012;54:929–38. doi: 10.1007/s00234-012-1005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Foubert-Samier A, Catheline G, Amieva H, Dilharreguy B, Helmer C, Allard M, et al. Education, occupation, leisure activities, and brain reserve: a population-based study. Neurobiol Aging. 2012;33:423 e15–25. doi: 10.1016/j.neurobiolaging.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 85.Rolstad S, Nordlund A, Eckerstrom C, Gustavsson MH, Blennow K, Olesen PJ, et al. High education may offer protection against tauopathy in patients with mild cognitive impairment. J Alzheimers Dis. 2010;21:221–8. doi: 10.3233/JAD-2010-091012. [DOI] [PubMed] [Google Scholar]
- 86.Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Education modifies the association of amyloid but not tangles with cognitive function. Neurology. 2005;65:953–5. doi: 10.1212/01.wnl.0000176286.17192.69. [DOI] [PubMed] [Google Scholar]
- 87.Caporro M, Haneef Z, Yeh HJ, Lenartowicz A, Buttinelli C, Parvizi J, et al. Functional MRI of sleep spindles and K-complexes. Clin Neurophysiol. 2012;123:303–9. doi: 10.1016/j.clinph.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ktonas PY, Golemati S, Xanthopoulos P, Sakkalis V, Ortigueira MD, Tsekou H, et al. Time-frequency analysis methods to quantify the time-varying microstructure of sleep EEG spindles: possibility for dementia biomarkers? J Neurosci Methods. 2009;185:133–42. doi: 10.1016/j.jneumeth.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 89.Montplaisir J, Petit D, Gauthier S, Gaudreau H, Decary A. Sleep disturbances and eeg slowing in alzheimer’s disease. Sleep Res Online. 1998;1:147–51. [PubMed] [Google Scholar]
- 90.Smallwood RG, Vitiello MV, Giblin EC, Prinz PN. Sleep apnea: relationship to age, sex, and Alzheimer’s dementia. Sleep. 1983;6:16–22. doi: 10.1093/sleep/6.1.16. [DOI] [PubMed] [Google Scholar]
- 91.Bubu OM, Andrade AG, Umasabor-Bubu OQ, Hogan MM, Turner AD, de Leon MJ, et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med Rev. 2020;50:101250. doi: 10.1016/j.smrv.2019.101250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liguori C, Maestri M, Spanetta M, Placidi F, Bonanni E, Mercuri NB, et al. Sleep-disordered breathing and the risk of Alzheimer’s disease. Sleep Med Rev. 2021;55:101375. doi: 10.1016/j.smrv.2020.101375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.