Abstract
Sulforaphane (SFN) is an organic isothiocyanate and an NF-E2-related factor-2 (Nrf2) inducer that exerts prophylactic effects on depression-like behavior in mice. However, the underlying mechanisms remain poorly understood. Brain-derived neurotrophic factor (BDNF), a neurotrophin, is widely accepted for its antidepressant effects and role in stress resilience. Here, we show that SFN confers stress resilience via BDNF upregulation and changes in abnormal dendritic spine morphology in stressed mice, which is accompanied by rectifying the irregular levels of inflammatory cytokines. Mechanistic studies demonstrated that SFN activated Nrf2 to promote BDNF transcription by binding to the exon I promoter, which is associated with increased Nrf2, and decreased methyl-CpG binding protein-2 (MeCP2), a transcriptional suppressor of BDNF, in BV2 microglial cells. Furthermore, SFN inhibited the pro-inflammatory phenotype and activated the anti-inflammatory phenotype of microglia, which was associated with increased Nrf2 and decreased MeCP2 expression in microglia of stressed mice. Hence, our findings support that Nrf2 induces BDNF transcription via upregulation of Nrf2 and downregulation of MeCP2 in microglia, which is associated with changes in the morphology of damaged dendritic spines in stressed mice. Meanwhile, the data presented here provide evidence for the application of SFN as a candidate for the prevention and intervention of depression.
Keywords: sulforaphane, Nrf2, BDNF, microglia, stress resilience
Introduction
Sulforaphane (SFN, 1-isothiocyanato-4-methylsulfinylbutane, Fig. 1a), an organosulfur compound found in cruciferous vegetables, such as broccoli, brussels sprouts, and cabbage, is an NF-E2-related factor-2 (Nrf2) inducer. It has been demonstrated that SFN exerts antioxidant and anti-inflammatory effects by upregulating Nrf2-induced transcription of phase II detoxification enzymes and antioxidant proteins of the Nrf2 [1–3]. Brain-derived neurotrophic factor (BDNF) is a neurotrophin that plays a crucial role in neuronal survival and growth. Convergent evidence indicates that altered BDNF levels and function are correlated with the pathogenesis of depression [4–6]. Our previous studies demonstrated that intraperitoneal injection of SFN exerts antidepressant effects in lipopolysaccharide (LPS) and chronic social defeat stress (CSDS) models of depression by activating Nrf2 and BDNF signaling pathways [7]. Moreover, dietary intake of glucoraphanin (a precursor of SFN) also displays antidepressant effects in the CSDS mouse model by activating the BDNF signaling pathway [8]. Recently, our group revealed that Nrf2 activates BDNF, contributing to antidepressant-like action in rodents [9]. However, the mechanisms by which SFN regulates BDNF resulting in its antidepressant effects have not been well defined.
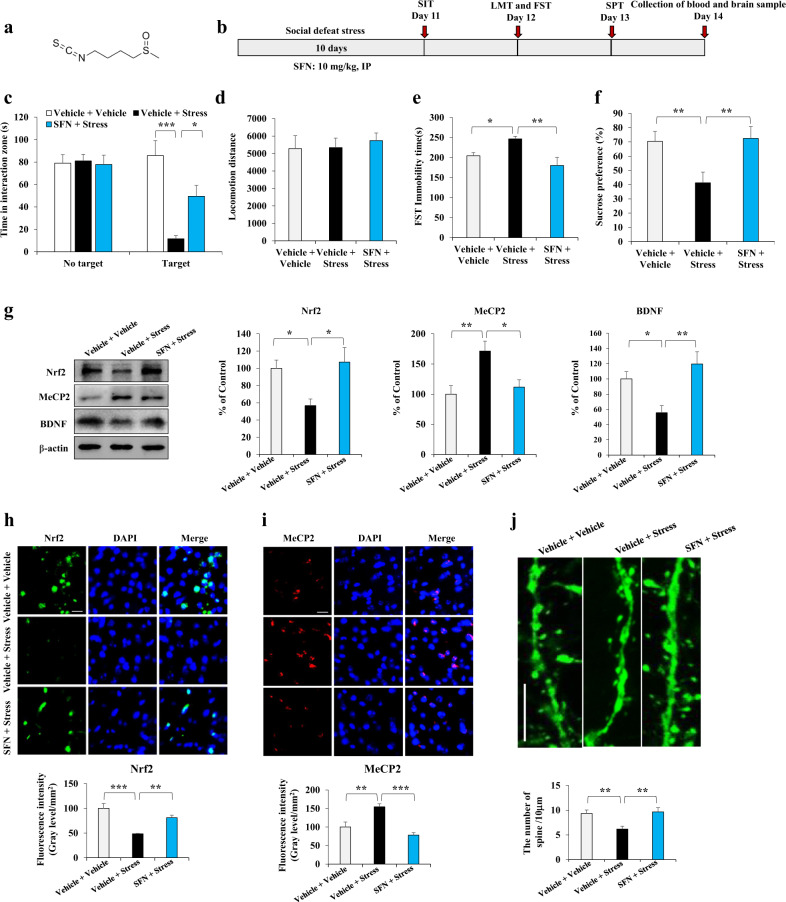
Fig. 1. SFN increases Nrf2 and decreases MeCP2 expression, changing abnormal BDNF expression in stressed mice.
a Chemical structure of Sulforaphane (SFN). b Schedule of behavior test and treatment. c The social interaction test for no target and target time (Mean ± SEM, n = 8 per group, one-way ANOVA, *P < 0.05, ***P < 0.001). d Locomotion test (Mean ± SEM, n = 9 per group, one-way ANOVA, *P < 0.05 and **P < 0.01). e Forced swimming test (Mean ± SEM, n = 9 per group, one-way ANOVA, **P < 0.01). f The sucrose preference test (Mean ± SEM, n = 8 per group, one-way ANOVA, **P < 0.01). g Western blot analysis of Nrf2, MeCP2, and BDNF in the mPFC (Mean ± SEM, n = 4 per group, one-way ANOVA, *P < 0.05, **P < 0.01). h Representative images and quantification of Nrf2 staining in the mPFC. Scale bar, 50 μm. (Mean ± SEM, n = 4 per group, one-way ANOVA, **P < 0.01, ***P < 0.001). i Representative images and quantification of MeCP2 staining in the mPFC. Scale bar, 50 μm. (Mean ± SEM, n = 4 per group, one-way ANOVA, **P < 0.01, ***P < 0.001). j SFN changes synaptic plasticity impairments in the mPFC of stressed Thy1-YFP mice. Representative photomicrographs for high magnification of spine density in the mPFC (Mean ± SEM, n = 6 per group, one-way ANOVA, **P < 0.01). Scale bar, 10 μm.
Microglia are immune cells in the central nervous system that play an important role in maintaining normal brain function [10]. Accumulating evidence has demonstrated that microglia are associated with a variety of neuropsychiatric diseases, including depression, through the release of inflammatory cytokines, regulation of cell apoptosis via phagocytosis, and synaptic plasticity [11, 12]. It has been established that microglia have a dual state of pro-inflammatory and anti-inflammatory phenotypes depending on the microenvironment [13–15]. Microglia with an anti-inflammatory phenotype regulate various anti-inflammatory factors, leading to neuronal protection [13, 16–18]. Nrf2 is a key transcription factor that regulates antioxidant and anti-inflammatory responses. Therefore, it is interesting to investigate the relationship between the antidepressant effects of SFN and microglial function.
Here, we reveal that SFN stimulates BDNF transcription through Nrf2 binding to its exon I promoter, which is associated with increased Nrf2 and decreased methyl-CpG binding protein-2 (MeCP2, a transcription suppressor of BDNF) in BV2 cells. Further studies demonstrated that mice with depression-like behavior displayed downregulated Nrf2 and enhanced MeCP2 in microglia, which can be reversed by SFN treatment, endowing stress resilience. SFN inhibits the pro-inflammatory phenotype and activates the anti-inflammatory phenotype of microglia in stressed mice. Collectively, our findings support that SFN confers stress resilience via induction of BDNF in stressed mice, which is associated with the upregulation of Nrf2 and downregulation of MeCP2 in microglia, providing compelling evidence for SFN application as a candidate for the treatment of depression.
Materials and methods
Mice, cell lines, antibody, and drug treatment
Male adult C57BL/6 mice (8 weeks old, 20–25 g each, Guangdong Experimental Animal Center), CD1 mice (14 weeks old, 40–45 g each, Guangdong Experimental Animal Center), and male adult Thy1-Yellow fluorescent protein (YFP) mice were used in the experiments. The animals were housed under a controlled temperature and kept on a 12-h light/dark cycle (lights on between 07:00 and 19:00), with ad libitum access to food and water. The study protocol was approved by the Institutional Animal Care and Use Committee of Jinan University. All experiments were carried out in accordance with the Guide for Animal Experimentation of Jinan University. BV2 microglial cells were cultured in high-glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Excell Bio., Taicang, China) and penicillin (100 units/mL)-streptomycin (100 μg/mL) (Hyclone). The cells were incubated at 37 °C in a humidified incubator containing 5% CO2. The following antibodies were used for the experiments: Nrf2 (ab137550), MeCP2 (ab2828), BDNF (ab108319), IBA1 (GTX632426), iNOS (Invitrogen PA3-030A), and Arginase1 (cst 93668S). Beta-actin antibody was purchased from EarthOx. LPS was purchased from Sigma-Aldrich (LPS, 1 μg/mL for BV2 cells; L-4130, serotype 0111:B4, Sigma-Aldrich). SFN was purchased from MedChemExpress (Shanghai, China). SFN (10 mg/kg, dissolved in distilled water containing 10% corn oil) was administered intraperitoneally (i.p.) to mice before exposure to social defeat stress for 30 min over 10 days. The doses of LPS and SFN were selected according to previous studies [8, 19].
Chronic social defeat stress (CSDS) modeling
The chronic social defeat procedure was performed as previously reported [20]. C57BL/6 mice or Thy1-YFP mice were defeated by different CD1 mice for 10 min for a total of 10 days. Following the social defeat session, CD1 mice and C57BL/6 mice or Thy1-YFP mice were housed in half of the cage for 24 h using a perforated Plexiglas divider, which allowed visual, olfactory, and auditory contacts over 24 h. C57BL/6 mice or Thy1-YFP mice were raised separately after the end of the last session. The social interaction test (SIT) was performed to examine the mice that were accordingly susceptible and resistant.
For the SIT, an open box (42 cm × 42 cm) with an interaction zone that included a mesh-plastic target box (10 cm × 4.5 cm) and two opposing corner zones was employed. The two parts were used for this test (no social or social targets). For the no social target, the test mouse was placed into an open field arena for 2.5 min with no social target (no CD1 mouse) in the mesh-plastic target box. After the no social target test, the mouse was placed into the open field arena again in the second 2.5 min with a social target (a novel CD1 mouse) in the mesh-plastic target box. The residence time in the interaction zone was counted using the stopwatch, and the time of ratio for social target, and no social target was calculated accordingly. Approximately 70% of mice were susceptible to social defeat stress.
Behavioral tests
The behavioral tests included the locomotion test, forced swimming test (FST), and 1% sucrose preference test (SPT). The locomotion test, FST, and SPT were performed as previously reported [21]. The locomotor activities of the mice were analyzed using Ethovision XT 14.0 software (Noldus). The cumulative exercise was recorded for 60 min. For the FST, the mice were placed individually in a cylinder (diameter: 23 cm; height: 31 cm) containing 15 cm of water and maintained at 23 ± 1 °C. The mice were monitored using a video tracking system (EthoVision XT 14.0) for 6 min. For the SPT, mice were habituated to a 1% sucrose solution for 48 h before the test day, and then the mice were deprived of water and food for 4 h followed by a preference test with water and 1% sucrose for 1 h. Bottles containing water and sucrose were weighed before and at the end of this period to calculate the sucrose preference (%).
Luciferase reporter assay
The luciferase reporter assay was performed as described previously [22, 23]. BV2 cells were transfected with pRL-TK Renilla luciferase plasmid and BDNF exon I, II, or IV luciferase reporter plasmid in 6-wells plates, followed by treatment with SFN or siRNA-Nrf2. Following transfection for 24 h, the cells were collected and subjected to analysis using a dual-luciferase reporter assay kit (Promega, Madison, USA) according to the manufacturer’s instructions.
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed as described previously [22, 23]. Cells were subjected to ChIP assay according to the manual of the SimpleChIP® Enzymatic Chromatin IP Kit (Cell Signaling). Specifically, 7.5 μg of Nrf2 antibody (Abcam) was added to the homogenate of the cell lysates. The mixture was incubated overnight at 4 °C. The washing, elution, and reverse cross-linking of free DNA were performed according to the manufacturer’s protocol. BDNF exon I-specific primers were used to amplify the promoter region. The primer sequences were: forward 5′-GGCTTCTGTGTGCGTGAATTTGC-3′; reverse 5′-AAAGTGGGTGGGAGTCCACGAG-3′. The PCR amplicons were separated on a 2% agarose gel after 35 cycles of PCR (denaturation at 95 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 30 s).
Immunofluorescence staining
Mice were anesthetized with sodium pentobarbital and perfused transcardially with 10 mL of isotonic saline, followed by 40 mL of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brain samples were collected after perfusion and postfixed overnight at 4 °C. Serial coronal sections (50 μm) of brain tissue were cut in ice-cold, 0.01 M phosphate-buffered saline (pH 7.5) using a vibrating blade microtome (VT1000S, Leica Microsystems AG, Wetzlar, Germany). For staining, the cells or mouse brain sections were incubated with 3% hydrogen peroxide at room temperature for 10 min after fixation with 4% paraformaldehyde. Then, the sections were blocked with blocking solution for 1 h and incubated with primary antibodies overnight. The next day, Alexa Fluor 488- or 568- conjugated isotype-specific secondary antibodies were incubated for 1 h at room temperature. Images were collected using an Olympus fluorescence microscope (Olympus BX53, Tokyo, Japan). IBA1, iNOS, and Arginine1 immunoreactivity or Nrf2 and MeCP2 fluorescence intensity were quantified in the anterior regions (0.018 mm2) of each brain section using Image J.
Enzyme-linked immunosorbent assay
Blood samples were obtained via cardiac puncture 14 days after the CSDS. Serum samples were obtained from blood by centrifugation at 2000 × g for 20 min. The samples were diluted ten-fold with an enzyme-linked immunosorbent assay (ELISA) diluent solution. Levels of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-10 (IL-10), and interleukin-4 (IL-4) were measured using a Ready-SET-Go ELISA kit (eBioscience, San Diego, USA) according to the manufacturer’s instructions.
Western blotting assay
Cells and mouse brain samples were lysed in RIPA buffer (20 mM pH 7.5 Tris-HCl, 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride) on ice for 30 min. The cell lysates or brain lysates were then centrifuged at 13,000 × g for 30 min at 4 °C. The supernatant was collected, and protein concentrations were determined using a Coomassie Brilliant Blue protein assay kit (Bio-Rad). The same amount of supernatant was boiled in SDS loading buffer. Protein samples (20 µg) were resolved on 7.5%, 10%, or 15% polyacrylamide gels, which were selected according to the molecular weights of the target proteins. After SDS-PAGE, the proteins were transferred to a polyvinylidene difluoride membrane, blocked with 2% BSA for 1 h at room temperature, and then incubated with primary antibody (the concentration was chosen according to the manufacturer’s instructions) at 4 °C overnight. The next day, the blots were incubated with an anti-mouse (1:5000) or anti-rabbit (1:5000) secondary antibody. Images were captured with a Tanon-5200CE imaging system (Tanon, Shanghai, China), and immunoreactive bands were quantified using the Tanon-5200CE system analysis software.
Dendritic spine analysis
For the dendritic spine analysis, we employed Thy1-YFP mice, which express spectral variants of GFP (yellow-YFP) in motor and sensory neurons, as well as subsets of central neurons under the thy-1 promoter. Axons are brightly fluorescent throughout the terminals [24]. After the SIT, Thy1-YFP mice were deeply anesthetized with sodium pentobarbital and perfused transcardially with 10 mL of isotonic saline, followed by 40 mL of ice-cold 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed from the skulls and postfixed overnight at 4 °C with the same fixative. For dendritic spine analysis, 50-μm-thick serial coronal sections of brain tissue were cut in ice-cold 0.01 M phosphate-buffered saline (pH 7.5) using a vibrating blade microtome (VT1000S, Leica Microsystems AG, Wetzlar, Germany). The sections were mounted on gelatinized slides, dehydrated, cleared, and coverslipped using Permount® (Fisher Scientific, Fair Lawn, NJ, USA). Next, the sections were observed under a fluorescence microscope (Olympus BX53, Japan). For spine density measurements, all clearly evaluable areas containing secondary dendrites (50–100 μm) from each imaged neuron were used. To determine relative spine density, spines on multiple dendritic branches from a single neuron were counted to obtain an average spine number per 10 μm. For spine number measurements, only spines that emerged perpendicular to the dendritic shaft were counted. Three neurons per section, three sections per animal, and four animals were analyzed. The average value for each region was calculated for each individual. These individual averages were then combined to yield a grand average for each region.
Statistical analysis
Data are shown as mean ± standard error of the mean. The data were analyzed using PASW Statistics 20 (formerly SPSS Statistics, SPSS). The behavior data, quantification of Western blot data, immunofluorescence staining data, luciferase reporter assay data, dendritic spine analysis data, and ELISA data were analyzed using a one-way analysis of variance (ANOVA), followed by the post hoc Fisher LSD test. Statistical significance was set at P < 0.05. Chromatin immunoprecipitation assay data were analyzed using the Student’s t test.
Results
SFN confers stress resilience associated with increased Nrf2 and decreased MeCP2 expressions in stressed mice
To explore the mechanisms by which SFN regulates BDNF resulting in stress resilience, a CSDS mouse model was employed (Fig. 1b). Data showed that pretreatment with SFN endowed stress resilience in the social defeat stress mice model assayed by the SIT, locomotion test, FST, and SPT. In the SIT without a target, the social interaction times were similar among groups, while in the SIT with target, SFN increased social avoidance time in stressed mice. One-way ANOVA revealed significant effects (F2, 23 = 14.651, P < 0.001) (Fig. 1c). For the locomotion test, no changes in locomotion were observed among the three groups (Fig. 1d). In the FST, SFN attenuated the increased immobility time in the stressed mice (Fig. 1e). In the SPT, SFN enhanced sucrose water intake in stressed mice (Fig. 1f). One-way ANOVA revealed significant effects (F2, 26 = 6.545, P = 0.005 for FST; F2, 23 = 6.3, P = 0.007 for SPT). Further data showed that CSDS was associated with decreased Nrf2 and BDNF expression and increased MeCP2 expression in the medial prefrontal cortex (mPFC), which could be reversed by SFN treatment (Fig. 1g). One-way ANOVA revealed significant effects (F2, 11 = 5.165, P = 0.032 for Nrf2; F2, 11 = 7.444, P = 0.012 for BDNF; F2, 11 = 7.178, P = 0.014 for MeCP2). In addition, immunofluorescence staining indicated that CSDS led to decreased Nrf2 fluorescence intensity and increased MeCP2 fluorescence intensity in the mPFC, which could also be reversed by SFN (Fig. 1h, i). One-way ANOVA revealed significant effects (F2, 11 = 16.618, P = 0.001 for Nrf2; F2, 11 = 15.623, P = 0.001 for MeCP2). Since BDNF is a crucial neurotrophic factor mediating neural plasticity, we further examined the effect of SNF on the density of dendritic spines in the mPFC. In Thy1-YFP and spine-labeled model mice [24], we found that dendritic spine density significantly decreased in the mPFC of stressed mice, which could be notably reversed by pretreatment with SFN (Fig. 1j and Supplementary Fig. S1). One-way ANOVA revealed significant effects (F2, 17 = 7.07, P = 0.007 for spine density). Taken together, these data indicate that increased Nrf2 and decreased MeCP2 expression are associated with SFN treatment, which changes abnormal BDNF expression and repairs dendritic spine impairment in stressed mice, inducing stress resilience.
Anti-inflammatory effects of SFN in stressed mice
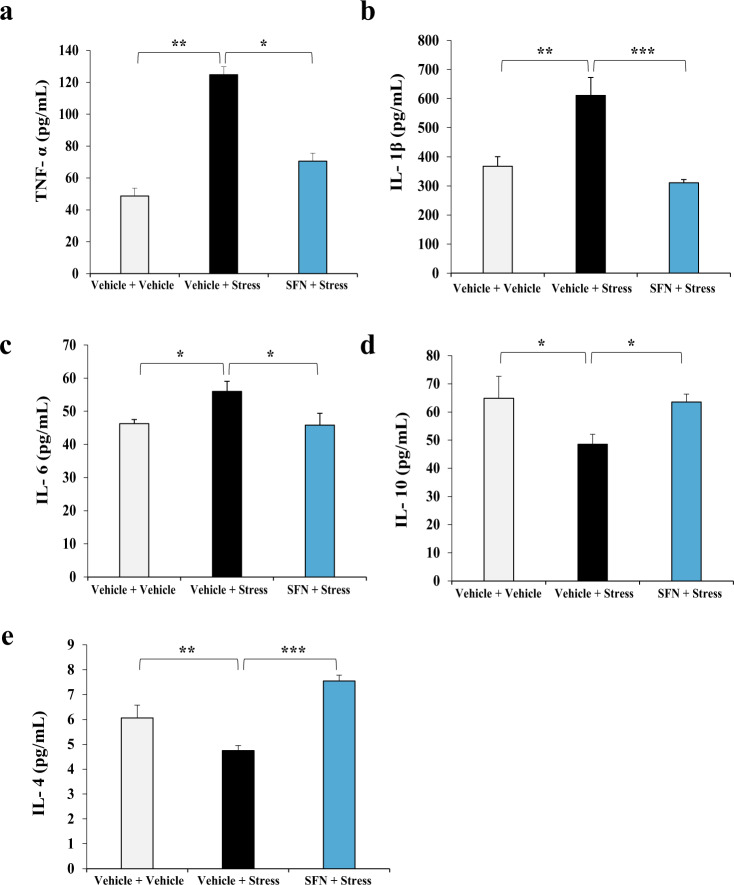
Inflammatory activation has been demonstrated to play a key role in the pathogenesis of depression [25, 26]. Patients with major depressive disorder (MDD) or stress vulnerability exhibit higher levels of circulating pro-inflammatory cytokines and low levels of anti-inflammatory cytokines [27, 28]. In this study, we examined the effects of SFN on pro- and anti-inflammatory cytokine release in CSDS mice. Data showed that pro-inflammatory cytokine levels, including those of TNF-α, IL-1β, and IL-6, were significantly higher in stressed mice than in vehicle-treated mice, which can be rectified by pretreatment with SFN (Fig. 2a–c). One-way ANOVA revealed significant effects (F2, 20 = 5.637, P = 0.013 for TNF-α; F2, 20 = 13.415, P < 0.001 for IL-1β; F2, 23 = 4.237, P = 0.028 for IL-6). In addition, the decreased levels of two anti-inflammatory cytokines, namely IL-10 and IL-4, in the stressed mice were reversed by SNF treatment (Fig. 2d, e). One-way ANOVA revealed significant effects (F2, 17 = 4.548, P = 0.029 for IL-10; F2, 20 = 28.581, P < 0.001 for IL-4). These data suggest that rectifying abnormal inflammation may play an important role in SFN-conferred stress resilience.
Fig. 2. Anti-inflammatory effects of SFN in stressed mice.
a SFN decreases stress-induced upregulation of TNF-α. Serum levels of TNF-α on day 14 of SFN treatment (Mean ± SEM, n = 7 per group, one-way ANOVA, *P < 0.05, **P < 0.01). b SFN suppresses stress-induced upregulation of IL-1β. Serum levels of IL-1β on day 14 of SFN treatment (Mean ± SEM, n = 7 per group, one-way ANOVA, **P < 0.01, ***P < 0.001). c Serum levels of IL-6 on day 14 of SFN treatment (Mean ± SEM, n = 7 per group, one-way ANOVA, *P < 0.05). d SFN increases stress-induced downregulation of IL-10. Serum levels of IL-10 on day 14 of SFN treatment (Mean ± SEM, n = 7 per group, one-way ANOVA, *P < 0.05). e SFN upregulates stress-induced decrease of IL-4. Serum levels of IL-4 on day 14 of SFN treatment (Mean ± SEM, n = 7 per group, one-way ANOVA, **P < 0.01, ***P < 0.001).
SFN activates BDNF transcription associated with upregulation of Nrf2 and downregulation of MeCP2 in BV2 cells
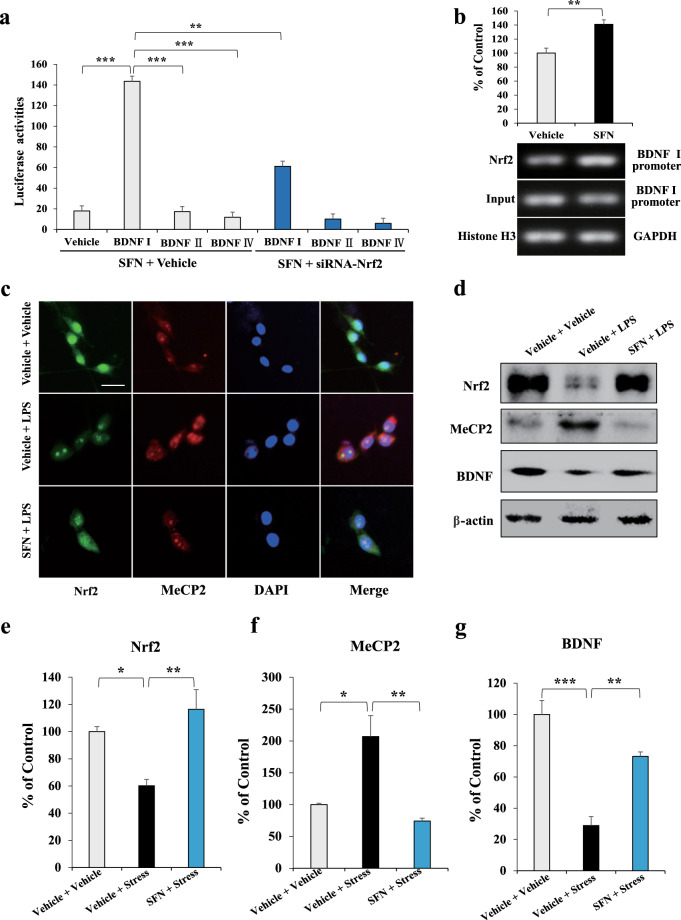
Microglia play important roles in the maintenance of normal brain functions, and those with an anti-inflammatory phenotype regulate various anti-inflammatory and neurotrophic factors, playing a role neuronal protection [10, 13]. Thus, we further explored whether SFN promotes BDNF expression by affecting bdnf transcription in microglia. First, we analyzed the activation effects of SFN on bdnf exons I, II, and IV promoter regions using a luciferase reporter assay in BV2 cells. Data showed that SFN prominently activated the bdnf exon I promoter, which could be blocked by siRNA-Nrf2 (Fig. 3a). One-way ANOVA revealed significant effects (F6, 55 = 506.479, P < 0.001). To further verify the regulatory effect of SFN on Nrf2-induced BDNF transcription, we carried out a ChIP analysis on BV2 cells treated with SFN using an Nrf2-specific antibody. The data indicated that SFN promoted Nrf2 interaction with the bdnf exon I promoter (Fig. 3b). Student’s t test revealed significant effects (P = 0.002). Since localization status could reflect the action of transcription regulators in cells [29], we carried out immunofluorescence staining in BV2 cells treated with SFN and/or LPS. The data showed that LPS treatment led to more MeCP2 within the nucleus and diffused nuclear Nrf2 became punctate, which could be reversed by SFN (Fig. 3c). In addition, we observed that LPS decreased BDNF and Nrf2 expression and increased MeCP2 in BV2 cells, which could also be reversed by SFN (Fig. 3d–g). These results suggest that Nrf2 is a transcriptional activator of BDNF, and activation of Nrf2 promotes BDNF expression by binding to the bdnf exon I promoter in BV2 cells.
Fig. 3. Activation of Nrf2 by SFN results in BDNF transcription in BV2 cell.
a The luciferase reporter assay for the activation of BDNF I, II, and IV promoter. BV2 cells are treated with SFN or siRNA-Nrf2 for 24 h followed by luciferase examination. Data of the pcDNA, BDNF II, and IV promoters are compared with those of BDNF I promoter (mean ± SEM, n = 8 per group, one-way ANOVA, **P < 0.01, ***P < 0.001). b ChIP assay for the BDNF I promoter. The Nrf2 protein–DNA cross-linking samples are obtained from BV2 cells treated with SFN or vehicle via co-immunoprecipitation with anti-Nrf2 antibody. PCR is carried out with the BDNF exon I promoter primers (mean ± SEM, n = 4 per group, Student’s t test, **P < 0.01). c Immunofluorescence staining for Nrf2 and MeCP2. BV2 cells are treated with SFN or LPS for 24 h followed by immunofluorescence staining. Scale bar, 50 μm. d Representative images of the Western blot analysis for Nrf2, MeCP2, and BDNF. Quantifications of Nrf2 (e), MeCP2 (f), and BDNF (g) in Western blot analysis. (Mean ± SEM, n = 4 per group, one-way ANOVA, *P < 0.05, **P < 0.01, and ***P < 0.01).
SFN reverses the abnormal Nrf2 and MeCP2 expressions in microglia of stressed mice
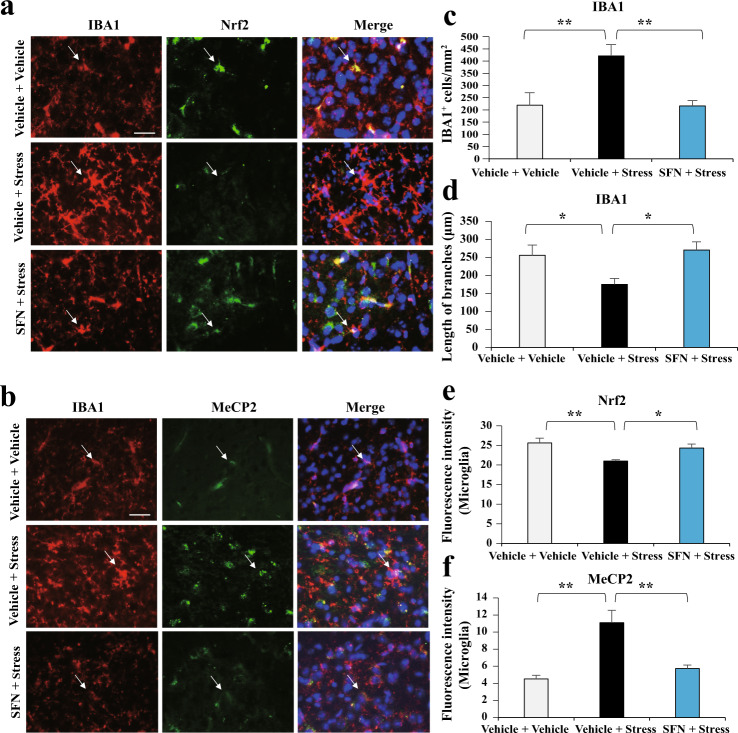
To verify the results of the in vitro study, we further explored the effects of SFN on the regulation of Nrf2 and MeCP2 in microglia. The results showed that stress leads to an increase in IBA1 (microglia marker) immunoreactivity in the mPFC (Fig. 4a–d), indicating the induction of abnormal inflammation in the CSDS mice. Pretreatment with SNF rectified the irregular inflammatory status demonstrated by downregulation of IBA1 staining (Fig. 4a–d). One-way ANOVA revealed significant effects (F2, 11 = 17.333, P = 0.001 for IBA1+ cells/mm2; F2, 11 = 4.937, P = 0.036 for the length of branches of IBA1). Moreover, the decreased levels of Nrf2 and increased levels of MeCP2 were caused by stress, which could be prominently reversed by SFN treatment (Fig. 4a, b, e, f). One-way ANOVA revealed significant effects (F2, 11 = 6.629, P = 0.017 for Nrf2; F2, 11 = 15.148, P = 0.001 for MeCP2). These results indicate that SFN induces Nrf2 expression, decreases MeCP2 expression, and prevents stress-induced microglial activation.
Fig. 4. SFN administration is associated with changed Nrf2 and MeCP2 abnormal expression in microglia of stressed mice.
a SFN suppresses stress-induced upregulation of IBA1 positive microglia, which is associated with increased Nrf2 in IBA1 positive microglia. Representative images of Nrf2 expression in IBA1 positive microglia in the mPFC assayed by immunofluorescence staining. b SFN suppresses stress-induced upregulation of IBA1 positive microglia, which is associated with decreased MeCP2 expression in IBA1 positive microglia. Representative images of MeCP2 expression in IBA1 positive microglia in the mPFC assayed by immunofluorescence staining. Scale bar, 50 μm. Quantification of IBA1 (c and d) immunoreactivity, Nrf2 (e) and MeCP2 (f) fluorescence intensity (Mean ± SEM, n = 4 per group, one-way ANOVA, *P < 0.05, **P < 0.01, and ***P < 0.001).
SFN inhibits pro-inflammation and activates anti-inflammation phenotype in microglia, associating with changed Nrf2 and MeCP2 abnormal expression in stressed mice
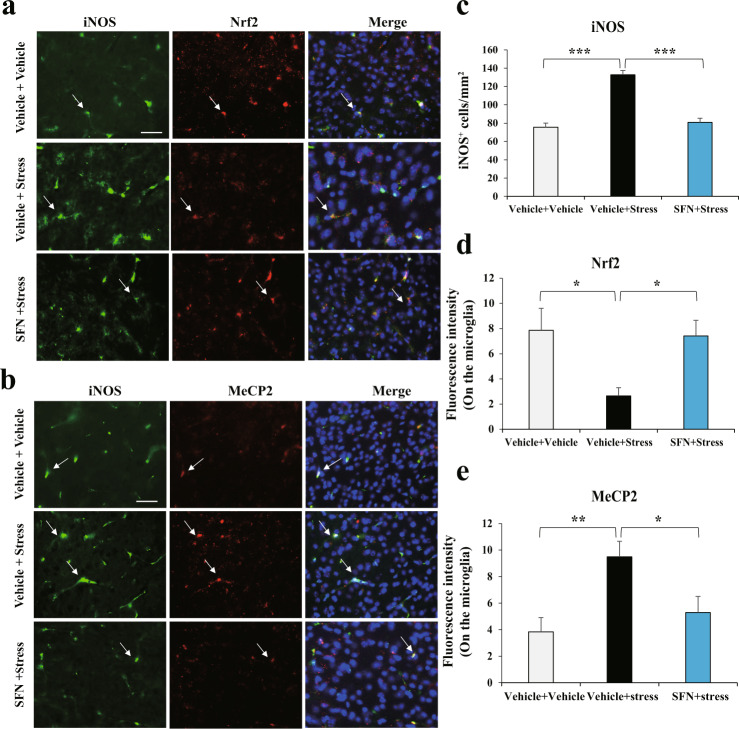
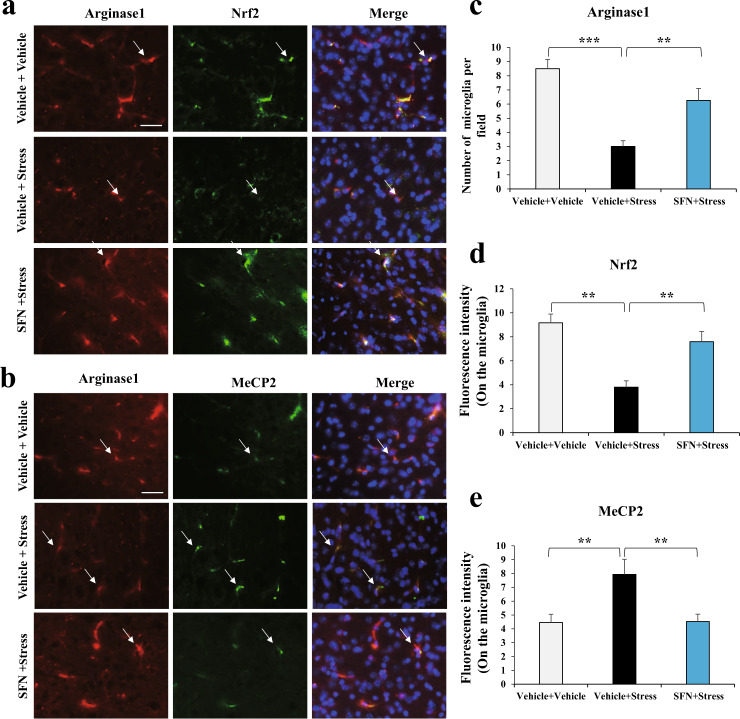
In the central nervous system, microglial activation is heterogeneous and functions with pro-inflammatory and anti-inflammatory effects associated with cytotoxic or neuroprotective effects, respectively [13]. BDNF can be released from active microglia, leading to neurogenesis [30, 31]. Hence, we further explored the specificity of SFN in changing Nrf2 and MeCP2 abnormal expression in the two phenotypes of microglia in stressed mice. First, to distinguish the two microglia, the staining of iNOS—a marker for the pro-inflammatory Phenotypes—and Arginase1—a marker for the anti-inflammatory Phenotypes—were employed [14, 15]. Co-localization of iNOS or Arginase1 with IBA1 was prominently observed in the mPFC (Supplementary Fig. S2). With these staining systems, our data showed that CSDS led to an increase in iNOS immunoreactivity (Fig. 5a–c) in the microglia of stressed mice, indicating higher pro-inflammatory effects of microglia. However, pretreatment with SFN notably reversed the enhanced pro-inflammatory microglia, represented by downregulated iNOS-positive cells (Fig. 5a–c). One-way ANOVA revealed significant effects (F2, 11 = 48.7, P < 0.001). In addition, the immunoreactivity of Nrf2 was decreased (Fig. 5a, d) with upregulated MeCP2 (Fig. 5b, e) in iNOS-positive cells of stressed mice, which was reversed by SFN treatment. One-way ANOVA revealed significant effects (F2, 11 = 5.06, P = 0.034 for Nrf2; F2, 11 = 6.458, P = 0.018 for MeCP2). Strikingly, we found that Arginase1 positive microglia had evidently decreased in stressed mice, which was also reversed by pretreatment with SFN (Fig. 6a–c). A one-way ANOVA revealed significant effects (F2, 11 = 4.984, P = 0.035). The regulation of Nrf2 (Fig. 6a, d) and MeCP2 (Fig. 6b, e) was similar to that in the iNOS-positive microglia. One-way ANOVA revealed significant effects (F2, 11 = 7.245, P = 0.013 for Nrf2; F2, 11 = 7.059, P = 0.014 for MeCP2). Collectively, these data demonstrate that SFN inhibits pro-inflammation and activates anti-inflammation phenotypes of microglia, changes Nrf2 and MeCP2 abnormal expression in microglia of stressed mice, and confers stress resilience.
Fig. 5. SFN suppresses stress-induced upregulation of iNOS-positive microglia, which is associated with changed Nrf2 and MeCP2 abnormal expression in microglia with pro-inflammatory phenotype in stressed mice.
a SFN suppresses stress-induced upregulation of iNOS-positive microglia, which is associated with increased Nrf2 expression in iNOS-positive microglia. Representative images of iNOS and Nrf2 in the mPFC of stressed mice. Representative positive stainings are indicated by arrows. Scale bar, 50 μm. b SFN suppresses stress-induced upregulation of iNOS-positive microglia, which is associated with decreased MeCP2 expression in iNOS-positive microglia. Representative images of iNOS and MeCP2 in the mPFC of stressed mice. Representative positive stainings are indicated by arrows. Scale bar, 50 μm. Quantification of iNOS (c), Nrf2 (d), and MeCP2 (e) fluorescent intensity in the iNOS-positive microglia (Mean ± SEM, n = 4 per group, one-way ANOVA, *P < 0.05, **P < 0.01, and ***P < 0.001).
Fig. 6. SFN stimulates stress-induced downregulation of Arginase1 positive microglia, associated with changed Nrf2 and MeCP2 abnormal expression in microglia with anti-inflammatory phenotype in stressed mice.
a SFN enhances stress-induced downregulation of Arginase1 positive microglia, which is associated with increased Nrf2 expression in Arginase1 positive microglia. Representative images of Arginase1 and Nrf2 in the mPFC of stressed mice. Representative positive stainings are indicated by arrows. Scale bar, 50 μm. b SFN enhances stress-induced downregulation of Arginase1 positive microglia, which is associated with decreased MeCP2 expression in Arginase1 positive microglia. Representative images of Arginase1 and MeCP2 in the mPFC of stressed mice. Representative positive stainings are indicated by arrows. Scale bar, 50 μm. Quantification of Arginase1 (c), Nrf2 (d), and MeCP2 (e) fluorescent intensity in the Arginase1 positive microglia (Mean ± SEM, n = 4 per group, one-way ANOVA, **P < 0.01, ***P < 0.001).
Discussion
In the current study, a natural compound called SFN, confers stress resilience by activating BDNF transcription in microglia and reversing abnormal dendritic spine morphology in stressed mice. Recent studies have shown that SFN exerts antioxidant and anti-inflammatory effects, which are thought to occur due to the activation of Nrf2 to promote phase II detoxification enzymes and antioxidant protein transcription [2, 3, 32, 33]. Our group has revealed that SFN exerts antipsychotic effects, such as depression and schizophrenia, via BDNF activation in the mouse brain [7, 34, 35]. However, the precise molecular and cellular mechanisms underlying the antipsychotic effects of SFN remain unclear. Here, using luciferase reporter and ChIP assays, we found that SFN promotes Nrf2 binding to the bdnf exon I promoter. Using immunofluorescence staining, we observed that SFN induced the redistribution of Nrf2 and MeCP2 in the nucleus of BV2 cells. Western blot analysis indicated that SFN increased Nrf2 and BDNF levels and decreased MeCP2 expression in LPS-treated BV2 cells. These data suggest that SFN induces bdnf transcription, resulting in BDNF protein expression by activating Nrf2 and inhibiting MeCP2 expression in microglia in vitro.
BDNF plays a key role in the pathophysiology of depression and the therapeutic mechanisms of antidepressants [36–44]. Several studies have shown that decreased BDNF levels and polymorphisms in the BDNF gene are associated with MDD. Low levels of plasma BDNF have been linked to suicidal behaviors in patients with major depression [45]. In addition, reduced BDNF levels were detected in the parietal cortex, mPFC, and hippocampus of postmortem brains of patients with psychiatric disorders, including MDD [46, 47]. In another study, decreased mRNA and protein expression of BDNF and TRKB were found in the hippocampus of patients who committed suicide [48]. In contrast, elevated levels of BDNF have been detected in the parietal cortex of postmortem patients with MDD who received antidepressant treatment compared with MDD-untreated patients [49]. Therefore, decreased levels of BDNF in the brain may contribute to the pathophysiology of depression. Recently, we reported that in the learned helplessness (LH) paradigm, the levels of BDNF and Nrf2 were both decreased in LH rats (susceptible) compared with the non-LH (resilient) rats and control rats, suggesting that regional differences in BDNF and Nrf2 levels in the rat brain may promote resilience to inescapable electric stress [50, 51]. Collectively, abnormalities in Nrf2 and BDNF crosstalk in the brain may play a role in depression-like phenotypes. In our current study, SFN induced BDNF expression was associated with the upregulation of the transcription activator Nrf2 and the downregulation of the transcription repressor MeCP2. These results suggest that SFN-induced antidepressant effects may be mediated by alterations in BDNF transcriptional activity in stressed mice. In contrast, it has been reported that reduced levels of BDNF in mice with depression-like behavior can prevent Nrf2 translocation and the activation of detoxifying/antioxidant enzymes, and BDNF can upregulate Nrf2 expression in neurons [52, 53]. Therefore, it may represent an interesting positive feedback loop for Nrf2 and BDNF.
In addition, for dendritic spine morphology, SFN attenuated the CSDS-induced reduction of dendritic spine density in the mPFC. BDNF protein synthesis is crucial to the structural plasticity of single dendritic spines [54–56], suggesting that changes in BDNF transcription in the mPFC with altered dendritic spine density in these regions by the administration of SFN is very important. Synaptogenesis is a key regulator of the mechanism of antidepressants [54–56]. Therefore, the activation of BDNF transcription by SFN and the resulting alteration in dendritic spine morphology after CSDS are important aspects of the mechanisms of SFN-induced antidepressant action. The association between CSDS and BDNF transcription in the mPFC and the role of SFN in the activation of BDNF transcription in the mPFC of stressed mice will be examined in the future.
Microglia have both pro-inflammatory and anti-inflammatory phenotypes depending on the microenvironment [13–15]. The pro-inflammatory phenotype of microglia induces iNOS and NF-κB signaling pathways, leading to the production of pro-inflammatory cytokines [13, 16, 17]. The anti-inflammatory phenotype of microglia regulates various anti-inflammatory factors and enhances neurotrophic factors, leading to neuronal protection [13, 16, 17]. We noticed that CSDS is associated with increased numbers of pro-inflammatory and decreased numbers of anti-inflammatory phenotypes of microglia activation. Pretreatment with SFN suppressed the pro-inflammatory phenotype of microglia activation and induced the anti-inflammatory phenotype of microglia in stressed mice. Moreover, it has been reported that SFN can pass the blood brain barrier and decrease the release of pro-inflammatory cytokines in the striatum of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated mice [57]. Therefore, these findings suggest that SFN-induced antidepressant effects are associated with the balance of microglial dysfunction. Moreover, immunofluorescence staining indicated that Nrf2, MeCP2, and IBA1 shared common localization. In addition, Nrf2, MeCP2, iNOS, and Arginase1 also shared a common localization, and CSDS was associated with decreased Nrf2 expression and increased MeCP2 expression in microglia. The anti-inflammatory phenotype of microglia can coordinate the regulation of various anti-inflammatory factors and enhance neurotrophic factors, resulting in neuron protection [13, 16–18]. Therefore, our findings suggest that SFN may promote BDNF transcription in the anti-inflammatory phenotype of microglia, showing antidepressant effects in stressed mice. A deeper understanding of the interaction between microglia and BDNF release is necessary to stimulate the development of future strategies for the treatment of depression.
In summary, our data demonstrate that CSDS is associated with increased expression of MeCP2 and decreased Nrf2, which in turn is associated with inhibition of BDNF transcription in microglia. SFN administration is associated with increased expression of Nrf2 and decreased MeCP2 expression in microglia, resulting in stress resilience from the activation of BDNF transcription (Fig. 7). The dynamic changes in the relationship between microglia and BDNF release may contribute to the antidepressant effects of SFN and may aid in the development of new strategies for the treatment of depression.
Fig. 7. A working model of SNF-conferred stress resilience.
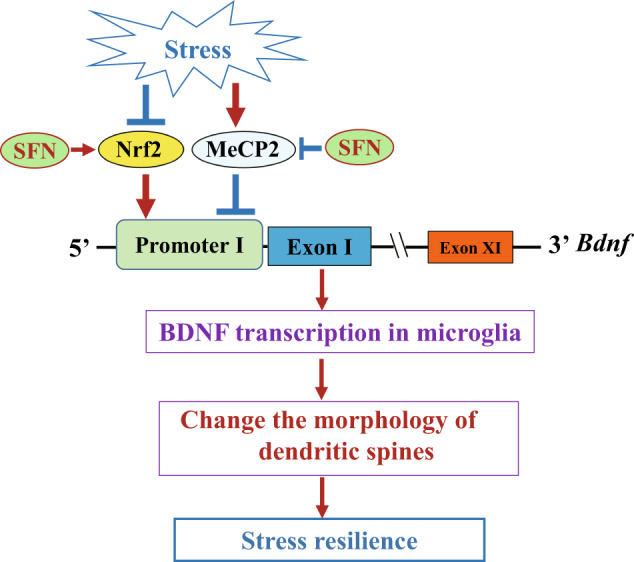
Nrf2 induces BDNF transcription via upregulation of Nrf2 and downregulation of MeCP2 in microglia, which is associated with the change of the damaged dendritic spines in stressed mice.
Supplementary information
Acknowledgements
The authors are thankful to Dr. Li Zhang (Joint International Research Laboratory of CNS Regeneration, Guangdong-Hong Kong-Macau Institute of CNS Regeneration, Jinan University) for providing the Thy1-YFP mice. We would like to thank Editage (www.editage.com) for English language editing. This work was supported by the National Natural Science Foundation of China (81973341 to QQ), the Fundamental Research Funds for the Central Universities (11620425 to JCZ; 21620426 to QQ), the Science and Technology Program of Guangzhou (202002030010 to QQ), Science and Technology Jiaxing (Grant No: 2017AY33030 to PFD), and the Natural Science Foundation of Guangdong Province (No. 2019A1515010936 to FX).
Author contributions
JCZ, QQ, and YRS conceived the project, designed the experiments, analyzed the data, and wrote the manuscript. RT, SWH, and QQC performed the social defeat stress model, behavior study, Western blot, immunofluorescence, and ELSA for the in vivo study. LJH and PFD performed the luciferase assay, ChIP assay, Western blot, and immunofluorescence for the in vitro study. GC, RF, and FX assisted with data analysis and interpretation, and critically read the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Rui Tang, Qian-qian Cao, Sheng-wei Hu
Contributor Information
Yi-rong Sun, Email: sun_yirong@gibh.ac.cn.
Ji-chun Zhang, Email: jczhang@jnu.edu.cn.
Qi Qi, Email: qiqikc@jnu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-021-00727-z.
References
- 1.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobayashi E, Suzuki T, Yamamoto M. Roles Nrf2 plays in myeloid cells and related disorders. Oxid Med Cell Longev. 2013;2013:529219. doi: 10.1155/2013/529219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suzuki T, Yamamoto M. Molecular basis of the Keap1-Nrf2 system. Free Radic Biol Med. 2015;88:93–100. doi: 10.1016/j.freeradbiomed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Phillips C. Brain-derived neurotrophic factor, depression, and physical activity: making the neuroplastic connection. Neural Plast. 2017;2017:7260130. doi: 10.1155/2017/7260130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rana T, Behl T, Sehgal A, Srivastava P, Bungau S. Unfolding the role of BDNF as a biomarker for treatment of depression. J Mol Neurosci. 2020. 10.1007/s12031-020-01754-x. [DOI] [PubMed]
- 6.Rosa PB, Bettio LEB, Neis VB, Moretti M, Kaufmann FN, Tavares MK, et al. Antidepressant-like effect of guanosine involves activation of AMPA receptor and BDNF/TrkB signaling. Purinergic Signal. 2021;17:285–301. doi: 10.1007/s11302-021-09779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. Prophylactic effects of sulforaphane on depression-like behavior and dendritic changes in mice after inflammation. J Nutr Biochem. 2017;39:134–44. doi: 10.1016/j.jnutbio.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Yao W, Zhang JC, Ishima T, Dong C, Yang C, Ren Q, et al. Role of Keap1-Nrf2 signaling in depression and dietary intake of glucoraphanin confers stress resilience in mice. Sci Rep. 2016;6:30659. doi: 10.1038/srep30659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao W, Lin S, Su J, Cao Q, Chen Y, Chen J, et al. Activation of BDNF by transcription factor Nrf2 contributes to antidepressant-like actions in rodents. Transl Psychiatry. 2021;11:140. doi: 10.1038/s41398-021-01261-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yirmiya R, Rimmerman N, Reshef R. Depression as a microglial disease. Trends Neurosci. 2015;38:637–58. doi: 10.1016/j.tins.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Deng SL, Chen JG, Wang F. Microglia: a central player in depression. Curr Med Sci. 2020;40:391–400. doi: 10.1007/s11596-020-2193-1. [DOI] [PubMed] [Google Scholar]
- 12.Santos LE, Beckman D, Ferreira ST. Microglial dysfunction connects depression and Alzheimer’s disease. Brain Behav Immun. 2016;55:151–65. doi: 10.1016/j.bbi.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, Le W. Differential roles of M1 and M2 microglia in eurodegenerative diseases. Mol Neurobiol. 2016;53:1181–94. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 14.Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–8. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–44. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou XL, Spittau B, Krieglstein K. TGF beta signalling plays an important role in IL4-induced alternative activation of microglia. J Neuroinflamm. 2012;9:210. doi: 10.1186/1742-2094-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 18.Han Y, Zhang LJ, Wang QZ, Zhang DD, Zhao QY, Zhang JQ, et al. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology. 2019;107:37–45. doi: 10.1016/j.psyneuen.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Zhao B, Lin C, Gong Z, An X. TREM2 inhibits inflammatory responses in mouse microglia by suppressing the PI3K/NF-kappaB signaling. Cell Biol Int. 2019;43:360–72. doi: 10.1002/cbin.10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden SA, Covington HE, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–91. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang JC, Yao W, Dong C, Yang C, Ren Q, Ma M, et al. Comparison of ketamine, 7,8-dihydroxyflavone, and ANA-12 antidepressant effects in the social defeat stress model of depression. Psychopharmacology (Berl) 2015;232:4325–35. doi: 10.1007/s00213-015-4062-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang ZH, Gong K, Liu X, Zhang Z, Sun X, Wei ZZ, et al. C/EBPbeta regulates delta-secretase expression and mediates pathogenesis in mouse models of Alzheimer’s disease. Nat Commun. 2018;9:1784. doi: 10.1038/s41467-018-04120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Xia Y, Wang Z, Su Kang S, Lei K, Liu X, et al. C/EBPbeta/delta-secretase signaling mediates Parkinson’s disease pathogenesis via regulating transcription and proteolytic cleavage of alpha-synuclein and MAOB. Mol Psychiatry. 2020;26:568–85. doi: 10.1038/s41380-020-0687-7. [DOI] [PubMed] [Google Scholar]
- 24.Popescu IR, Le KQ, Palenzuela R, Voglewede R, Mostany R. Marked bias towards spontaneous synaptic inhibition distinguishes non-adapting from adapting layer 5 pyramidal neurons in the barrel cortex. Sci Rep. 2017;7:14959. doi: 10.1038/s41598-017-14971-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vogelzangs N, Duivis HE, Beekman AT, Kluft C, Neuteboom J, Hoogendijk W, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haroon E, Chen X, Li Z, Patel T, Woolwine BJ, Hu XP, et al. Increased inflammation and brain glutamate define a subtype of depression with decreased regional homogeneity, impaired network integrity, and anhedonia. Transl Psychiatry. 2018;8:189. doi: 10.1038/s41398-018-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfau ML, Russo SJ. Peripheral and central mechanisms of stress resilience. Neurobiol Stress Neurobiol Stress. 2015;1:66–79. doi: 10.1016/j.ynstr.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menard C, Pfau ML, Hodes GE, Russo SJ. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology. 2017;42:62–80. doi: 10.1038/npp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–3. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Rong P, Zhang L, He H, Zhou T, Fan Y, et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci Adv. 2021;7. 10.1126/sciadv.abb9888. [DOI] [PMC free article] [PubMed]
- 31.Mohammadi M, Manaheji H, Maghsoudi N, Danyali S, Baniasadi M, Zaringhalam J. Microglia dependent BDNF and proBDNF can impair spatial memory performance during persistent inflammatory pain. Behav Brain Res. 2020;390:112683. doi: 10.1016/j.bbr.2020.112683. [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi A, Kang MI, Watai Y, Tong KI, Shibata T, Uchida K, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26:221–9. doi: 10.1128/MCB.26.1.221-229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kometsi L, Govender K, Mofo Mato EP, Hurchund R, Owira PMO. By reducing oxidative stress, naringenin mitigates hyperglycaemia-induced upregulation of hepatic nuclear factor erythroid 2-related factor 2 protein. J Pharm Pharmacol. 2020;72:1394–404. doi: 10.1111/jphp.13319. [DOI] [PubMed] [Google Scholar]
- 34.Shirai Y, Fujita Y, Hashimoto K. Effects of the antioxidant sulforaphane on hyperlocomotion and prepulse inhibition deficits in mice after phencyclidine administration. Clin Psychopharmacol Neurosci. 2012;10:94–8. doi: 10.9758/cpn.2012.10.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirai Y, Fujita Y, Hashimoto R, Ohi K, Yamamori H, Yasuda Y, et al. Dietary Intake of sulforaphane-rich broccoli sprout extracts during juvenile and adolescence can prevent phencyclidine-induced cognitive deficits at adulthood. PLoS ONE. 2015;10:e0127244. doi: 10.1371/journal.pone.0127244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjorkholm C, Monteggia LM. BDNF–a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–9. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castrén E. Neurotrophins and psychiatric disorders. Handb Exp Pharmacol. 2014;220:461–79. doi: 10.1007/978-3-642-45106-5_17. [DOI] [PubMed] [Google Scholar]
- 38.Hashimoto K. Brain‐derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci. 2010;64:341–57. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto K. Rapid-acting antidepressant ketamine, its metabolites and other candidates: a historical overview and future perspective. Psychiatry Clin Neurosci. 2019;73:613–27. doi: 10.1111/pcn.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto K. Molecular mechanisms of the rapid-acting and long-lasting antidepressant actions of (R)-ketamine. Biochem Pharmacol. 2020;177:113935. doi: 10.1016/j.bcp.2020.113935. [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev. 2004;45:104–14. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Lindholm JS, Castrén E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci. 2014;8:143. doi: 10.3389/fnbeh.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 44.Zhang JC, Yao W, Hashimoto K. Brain-derived neurotrophic factor (BDNF)-TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol. 2016;14:721–31. doi: 10.2174/1570159X14666160119094646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, et al. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 2007;62:423–8. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 46.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005;136:29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 47.Yang B, Ren Q, Zhang JC, Chen QX, Hashimoto K. Altered expression of BDNF, BDNF pro-peptide and their precursor proBDNF in brain and liver tissues from psychiatric disorders: rethinking the brain-liver axis. Transl Psychiatry. 2017;7:e1128. doi: 10.1038/tp.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banerjee R, Ghosh AK, Ghosh B, Bhattacharyya S, Mondal AC. Decreased mRNA and protein expression of BDNF, NGF, and their receptors in the hippocampus from suicide: an analysis in human postmortem brain. Clin Med Insights Pathol. 2013;6:1–11. doi: 10.4137/CPath.S12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheldrick A, Camara S, Ilieva M, Riederer P, Michel TM. Brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) levels in post-mortem brain tissue from patients with depression compared to healthy individuals—a proof of concept study. Eur Psychiatry. 2017;46:65–71. doi: 10.1016/j.eurpsy.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Yang B, Yang C, Ren Q, Zhang JC, Chen QX, Shirayama Y, et al. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur Arch Psychiatry Clin Neurosci. 2016;266:765–9. doi: 10.1007/s00406-016-0693-6. [DOI] [PubMed] [Google Scholar]
- 51.Zhang JC, Yao W, Dong C, Han M, Shirayama Y, Hashimoto K. Keap1-Nrf2 signaling pathway confers resilience versus susceptibility to inescapable electric stress. Eur Arch Psychiatry Clin Neurosci. 2018;268:865–70. doi: 10.1007/s00406-017-0848-0. [DOI] [PubMed] [Google Scholar]
- 52.Ishii T, Mann GE. When and how does brain-derived neurotrophic factor activate Nrf2 in astrocytes and neurons? Neural Regen Res. 2018;13:803–4. doi: 10.4103/1673-5374.232468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, et al. Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry. 2017;22:1701–13. doi: 10.1038/mp.2016.144. [DOI] [PubMed] [Google Scholar]
- 54.Zhang JC, Wu J, Fujita Y, Yao W, Ren Q, Yang C, et al. Antidepressant effects of TrkB ligands on depression-like behavior and dendritic changes in mice after inflammation. Int J Neuropsychopharmacol. 2014;18:pyu077. doi: 10.1093/ijnp/pyu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magarinos AM, Li CJ, Gal Toth J, Bath KG, Jing D, Lee FS, et al. Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus. 2011;21:253–64. doi: 10.1002/hipo.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Govindarajan A, Rao BSS, Nair D, Trinh M, Mawjee N, Tonegawa S, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci USA. 2006;103:13208–13. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jazwa A, Rojo AI, Innamorato NG, Hesse M, Fernandez-Ruiz J, Cuadrado A. Pharmacological targeting of the transcription factor Nrf2 at the basal ganglia provides disease modifying therapy for experimental parkinsonism. Antioxid Redox Signal. 2011;14:2347–60. doi: 10.1089/ars.2010.3731. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.