Abstract
Three tetracyclines (tetracycline, doxycycline, and minocycline) were found to possess iron-chelating activity in a colorimetric siderophore assay. Determination of MICs indicated that the activity of doxycycline against the periodontopathogen Actinobacillus actinomycetemcomitans was only slightly influenced by the presence of an excess of iron that likely saturates the antibiotic. On the other hand, the MICs of doxycycline and minocycline were significantly lower for A. actinomycetemcomitans cultivated under iron-poor conditions than under iron-rich conditions.
Actinobacillus actinomycetemcomitans is a gram-negative bacterial species which has been associated with periodontal diseases, especially with localized juvenile periodontitis (10, 12). Several studies have shown that tetracyclines are active against A. actinomycetemcomitans (15, 17, 23). Tetracyclines are extensively used as adjuncts in the treatment of periodontitis, a disease affecting the tooth-supporting tissues (gingiva, periodontal ligament, and alveolar bone) and resulting in tooth loss (21, 23). The beneficial effect of tetracyclines relates to three distinctive characteristics: (i) efficiency in suppressing growth of periodontopathogenic gram-negative anaerobic bacteria, (ii) capacity to reach high concentrations in the gingival crevicular fluid, and (iii) capacity to extend their antimicrobial effect by binding to the tooth surface and being slowly released in the periodontal pocket in an active form (23). However, the effectiveness of this group of antibiotics in periodontal therapy may be due not only to its bacteriostatic nature but also to additional nonantimicrobial properties. Indeed, previous studies have shown the capacity of tetracyclines to inhibit matrix metalloproteinase activity as well as bone resorption and to stimulate fibroblast attachment to the radicular surface (6, 7, 23).
Iron is an essential nutrient for most bacteria and therefore is an important factor in the establishment of infections (2, 18, 29). In order to have sufficient iron to survive and to multiply, pathogenic bacteria have developed several strategies to obtain this element (18, 27, 29). While screening a number of molecules for the presence of iron-chelating (siderophore) activity, we found that members of the tetracycline family could strongly chelate iron. The presence of this activity in tetracyclines suggests that these drugs may complex iron in the bacterial environment and create bacteriostatic conditions. This property may thus contribute to the antimicrobial activity of the molecule. The aims of the study were to (i) characterize the iron-chelating activity of three tetracyclines (tetracycline, doxycycline, and minocycline), (ii) evaluate the antibacterial activity of doxycycline under a condition of iron excess that likely saturates the antibiotic molecule, and (iii) determine the MICs of doxycycline and minocycline for A. actinomycetemcomitans cultivated under either poor or rich iron conditions.
The following antibiotics were used: tetracycline hydrochloride, doxycycline hydrochloride, minocycline hydrochloride, penicillin G, gentamicin, clindamycin, spiramycin, and metronidazole (Sigma Chemical Co., St. Louis, Mo.). A. actinomycetemcomitans Y4 and ATCC 29522 were routinely cultivated in an anaerobic chamber (N2-H2-CO2, 80:10:10) at 37°C in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) containing 1% yeast extract (THB-YE).
The universal siderophore assay of Schwyn and Neilands (22) was used to measure the iron-chelating activity of the antibiotics. Ferrichrome (Sigma Chemical Co.), a siderophore produced by Ustilago sphaerogena (4), was used as the positive control. The ability of tetracyclines, penicillin G, and metronidazole to remove iron from human holotransferrin was determined by urea-borate-EDTA–polyacrylamide gel electrophoresis (PAGE) analysis (11). This electrophoretic procedure allows the distinction of transferrin as apotransferrin (iron-free form), holotransferrin (diferric form), or the monomeric form, in which the iron is associated with either the N- or C-domain binding site. Two colorimetric assays were performed to detect chemical groups known to possess the iron-chelating property in microbial siderophores. The Arnow assay (1) was used to detect the presence of catechol-phenolate-like groups in the three tetracyclines, and 2,3-dihydroxybenzoic acid was used as a positive control. The presence of hydroxamate-like groups was determined by the Csaky procedure (3), and hydroxylamine hydrochloride served as a positive control. Both assays were performed in triplicate and the mean ± standard deviation was calculated.
To evaluate the effect of an excess of iron on the antibacterial activity of doxycycline, the MICs for A. actinomycetemcomitans Y4 and ATCC 29522 were determined in three different media: (i) THB-YE, (ii) THB-YE supplemented with 100 μM FeSO4 (Fe3+ form), and (iii) THB-YE supplemented with 100 μM FeCl3 (Fe2+ form). Doxycycline was dissolved in each medium and twofold serially diluted in order to obtain concentrations ranging from 50 to 0.098 μg/ml. These media were inoculated with a 24-h culture of A. actinomycetemcomitans in THB-YE and incubated at 37°C for 48 h in an anaerobic chamber, and optical densities were measured at 660 nm. The MIC was defined as the lowest concentration of doxycycline at which no bacterial growth occurred. All experiments were performed in duplicate.
The susceptibility to doxycycline and minocycline of A. actinomycetemcomitans ATCC 29522 cultivated under either poor or rich iron conditions was determined using the Epsilometer test (E-test) (AB Biodisk, Solna, Sweden). FeCl3 and FeSO4 were used as iron sources. The tests were performed on THB-YE solid media containing 250 μM 2,2′-dipyridyl and supplemented with either 1, 5, or 50 μM FeCl3 or FeSO4. 2,2′-Dipyridyl was added as an iron-chelating agent to scavenge the iron initially present in the medium. Agar plates were inoculated with a sterile cotton-tipped swab dipped into a 24-h culture of A. actinomycetemcomitans in THB-YE adjusted to the turbidity standard of McFarland 1. One E-test strip, either doxycycline or minocycline, was applied in the center of each inoculated plate so as to visualize the interpretation scale. The E-test plates were incubated until visible bacterial growth was observed (3 to 5 days). The MIC was read at the intersection of the bottom of the elliptic inhibition zone with the E-test strip. All experiments were performed in duplicate.
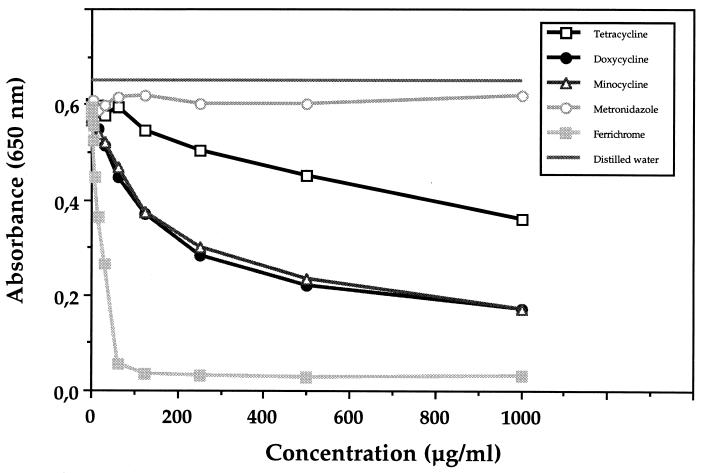
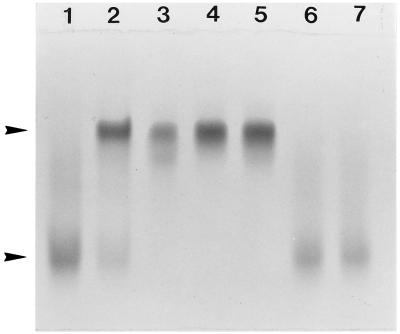
A strong iron-chelating activity for doxycycline and minocycline and a less pronounced one for tetracycline were detected using a siderophore colorimetric assay (Fig. 1). Ferrichrome, the positive control, reduced the initial absorbance by approximately 50% when used at a concentration of 25 μg/ml, while doxycycline and minocycline reached a comparable decrease at 250 μg/ml and tetracycline reached a comparable decrease at a concentration of >1,000 μg/ml. Penicillin G, gentamicin, clindamycin, spiramycin, and metronidazole did not show any iron-chelating activity (Fig. 1 and data not shown). Incubation of tetracycline, doxycycline, or minocycline with holotransferrin resulted in a complete removal of iron from the molecule, as determined by urea-borate-EDTA–PAGE analysis (Fig. 2). No such phenomenon was observed with metronidazole or penicillin. This electrophoretic procedure confirmed the iron-chelating activity of tetracyclines determined by the colorimetric assay. Tetracycline, doxycycline, and minocycline were further analyzed in assays aimed at determining the presence of either hydroxamate-like or catechol-phenolate-like groups, which are known to chelate iron and are usually found in microbial siderophores (Table 1). Results suggested the presence of hydroxamate-like groups in all three tetracyclines and catechol-phenolate-like groups in minocycline.
FIG. 1.
Iron-chelating activity of ferrichrome and different antibiotics as determined by the chrome azurol sulfate colorimetric assay. Reduction of A650 was measured for various concentrations of the compounds. This reduction occurs when a strong chelator removes the iron from the dye chrome azurol sulfate.
FIG. 2.
Urea-borate-EDTA–PAGE analysis of human holotransferrin incubated with different antibiotics. Lane 1, control holotransferrin; lane 2, control apotransferrin; lane 3, holotransferrin and tetracycline; lane 4, holotransferrin and doxycycline; lane 5, holotransferrin and minocycline; lane 6, holotransferrin and penicillin G; lane 7, holotransferrin and metronidazole. The upper and lower arrowheads indicate the apotransferrin (iron-free form) and the holotransferrin (iron-saturated form), respectively. Assay mixtures consisted of antibiotics (50 μg/ml; 50 μl) incubated at room temperature with holotransferrin (1 mg/ml in 100 mM phosphate-buffered saline pH 7.2; 50 μl).
TABLE 1.
Detection in tetracycline, doxycycline, and minocycline of catechol-phenolate-like and hydroxamate-like groups
| Antibiotica | A510 in Arnow assay for catechol-phenolate | A526 in Csaky assay for hydroxamate |
|---|---|---|
| Tetracycline | 0.07 ± 0.02 | 0.54 ± 0.09 |
| Doxycycline | 0.04 ± 0.03 | 0.25 ± 0.04 |
| Minocycline | 1.23 ± 0.09 | 1.44 ± 0.11 |
| Positive controlb | 2.82 ± 0.14 | 2.71 ± 0.09 |
| Negative controlc | 0 | 0.04 ± 0.02 |
Antibiotics were used at 1 mg/ml.
2,3-Dihydroxybenzoic acid (1 mg/ml) for the Arnow assay and hydroxylamine hydrochloride (1 mg/ml) for the Csaky assay.
Distilled water.
The broth dilution method was used to determine the MICs of doxycycline for A. actinomycetemcomitans in media containing iron in excess (100 μM FeSO4 or 100 μM FeCl3) and in a control medium not supplemented with iron. The MICs of doxycycline for A. actinomycetemcomitans Y4 in THB supplemented with either FeSO4 or FeCl3 were twofold higher (0.78 μg/ml instead of 0.39 μg/ml) than the ones obtained in THB-YE with no iron supplement. However, this phenomenon was not observed with the strain ATCC 29522. Similar data were obtained in two independent experiments.
Table 2 reports the susceptibility to doxycycline and minocycline of A. actinomycetemcomitans ATCC 29522 cultivated under either poor or rich iron conditions as determined by the E-test. When no iron was added to THB-YE containing 2,2′-dipyridyl, no bacterial growth occurred. The MICs of both doxycycline and minocycline were found to be the lowest when A. actinomycetemcomitans was cultivated in a medium with an iron concentration of 1 μM. The MICs became higher as the concentration of iron was increased. For instance, the MIC of doxycycline for A. actinomycetemcomitans grown in the presence of 1 μM FeSO4 was 0.094 μg/ml, whereas it was 0.5 μg/ml in the presence of 50 μM FeSO4. A similar tendency was observed with FeCl3 as the iron supplement. These observations were reproducible.
TABLE 2.
Susceptibility to doxycycline and minocycline of A. actinomycetemcomitans ATCC 29522 cultivated under either poor or rich iron conditions
| Antibiotic and iron source (μM) | MIC (μg/ml)
|
|
|---|---|---|
| Assay 1 | Assay 2 | |
| Doxycycline | ||
| FeSO4 | ||
| 1 | 0.094 | 0.094 |
| 5 | 0.25 | 0.125 |
| 50 | 0.50 | 0.38 |
| FeCl3 | ||
| 1 | 0.032 | 0.032 |
| 5 | 0.25 | 0.125 |
| 50 | 0.75 | 0.38 |
| Minocycline | ||
| FeSO4 | ||
| 1 | 0.032 | 0.064 |
| 5 | 0.047 | 0.064 |
| 50 | 0.19 | 0.25 |
| FeCl3 | ||
| 1 | 0.032 | 0.047 |
| 5 | 0.047 | 0.047 |
| 50 | 0.19 | 0.19 |
In this study, we showed that tetracyclines possess a strong iron-chelating activity. This property appears to be unique, since none of the other antibiotics tested showed this capacity to chelate iron. Previous reports have indicated that tetracyclines form complexes with metallic cations, including iron (20, 25, 26). A number of studies have also revealed that tetracyclines possess a strong capacity to inhibit the activity of matrix metalloproteinases (MMPs) (5, 8, 9). This inhibition appeared to be related to a chelating property, since it could be reversed by the presence of an excess of calcium. It is possible that the same portion of the tetracycline molecule could be responsible for the binding of both calcium and iron. The iron-chelating activity of tetracyclines could also regulate MMP activity by chelating iron and other transition metals present in trace amounts in inflamed periodontal tissues. In fact, the generation of reactive oxygen species, which activate MMPs and induce tissue breakdown, may be prevented by this chelating activity (19, 28).
The low concentration of free iron in the human body constitutes a limiting factor for invading pathogenic bacteria by creating bacteriostatic conditions (2, 18). As for iron in the periodontal pocket, little is known about its exact sources and concentration during periodontitis. However, it has been reported that the concentration of total iron in human gingival crevicular fluid is often higher than in serum (16). The concentration of iron in gingival crevicular fluid is increased significantly in periodontally diseased sites and has been reported to be in the range of 26 to 170 μM (16, 24). In this study, it was found that the activity of doxycycline against A. actinomycetemcomitans Y4 was only slightly reduced in the presence of an excess of iron, whereas no differences were noted with the second strain (ATCC 29522). Thus, the interaction between iron and tetracycline appears not to affect strongly the antibacterial activity of the molecule. This finding is in agreement with the results obtained in an animal model by Miles and Maskell (13, 14), who concluded that the complexing of tetracycline with iron did not affect the efficacy of tetracycline but that the efficacy of iron as an enhancer of infection was substantially diminished. Lastly, our study indicated that a much lower concentration of doxycycline or minocycline is required to inhibit growth of A. actinomycetemcomitans in an iron-restricted environment than in an iron-rich environment. It is proposed that the antibiotic could bind either Fe2+ or Fe3+ and make less iron available to bacteria. Under this iron-limiting condition, a lower concentration of the antibiotic would be required to inhibit cell growth. This suggests that the iron-chelating activity of tetracyclines may participate in their antimicrobial action.
Acknowledgments
This work was supported by the Fonds Émile-Beaulieu. M.-P. Huot was a recipient of a Burroughs Wellcome studentship.
REFERENCES
- 1.Arnow L E. Colorimetric determination of the compounds of 3,4-dihydroxyphenylalanine-tyrosine mixture. J Biol Chem. 1937;118:531–537. [Google Scholar]
- 2.Boelaert J R. Iron and infection. Acta Clin Belg. 1996;51:213–220. doi: 10.1080/22953337.1996.11718513. [DOI] [PubMed] [Google Scholar]
- 3.Csaky T Z. On the estimation of bound hydroxylamine in biological materials. Acta Chem Scand. 1948;2:450–454. [Google Scholar]
- 4.Emery T. Role of ferrichrome as a ferric ionophore in Ustilago sphaerogena. Biochemistry. 1971;10:1483–1488. doi: 10.1021/bi00784a033. [DOI] [PubMed] [Google Scholar]
- 5.Golub L M, Lee H M, Lehrer G, Nemiroff A, McNamara T F, Kaplan R, Ramamurthy N S. Minocycline reduces gingival collagenolytic activity during diabetes: preliminary observations and a proposed new mechanism of action. J Periodontal Res. 1983;18:516–526. doi: 10.1111/j.1600-0765.1983.tb00388.x. [DOI] [PubMed] [Google Scholar]
- 6.Golub L M, Ramamurthy N S, McNamara T F, Greenwald R A, Rifkin B R. Tetracyclines inhibit connective tissue breakdown: new therapeutic implications for an old family of drugs. Crit Rev Oral Biol Med. 1991;2:297–322. doi: 10.1177/10454411910020030201. [DOI] [PubMed] [Google Scholar]
- 7.Golub L M, Wolff M, Roberts S, Lee H M, Leung M, Payonk G S. Treating periodontal diseases by blocking tissue destructive enzymes. J Am Dent Assoc. 1994;125:163–171. doi: 10.14219/jada.archive.1994.0261. [DOI] [PubMed] [Google Scholar]
- 8.Golub L M, Sorsa T, Lee H M, Ciancio S, Sorbi D, Ramamurthy N. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis gingiva. J Clin Periodontol. 1995;21:1–9. doi: 10.1111/j.1600-051x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- 9.Greenwald R A, Golub L M, Lavietes B, Ramamurthy N S, Guber B, Laskin R S. Tetracyclines inhibit human synovial collagenase in vivo and in vitro. J Rheumatol. 1987;14:28–32. [PubMed] [Google Scholar]
- 10.Haffajee A D, Socransky S S. Microbial etiological agents of destructive periodontal diseases. Periodontol 2000. 1994;5:78–111. doi: 10.1111/j.1600-0757.1994.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 11.Makey D G, Seal U S. The detection of four molecular forms of human transferring during the iron binding process. Biochim Biophys Acta. 1976;453:250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- 12.Meyer D H, Fives-Taylor P M. The role of Actinobacillus actinomycetemcomitans in the pathogenesis of periodontal disease. Trends Microbiol. 1997;5:224–228. doi: 10.1016/S0966-842X(97)01055-X. [DOI] [PubMed] [Google Scholar]
- 13.Miles A A, Maskell J P. The antagonism of tetracyclines and ferric iron in vivo. J Med Microbiol. 1985;20:17–26. doi: 10.1099/00222615-20-1-17. [DOI] [PubMed] [Google Scholar]
- 14.Miles A A, Maskell J P. The neutralization of antibiotic action by metallic cations and iron chelators. J Antimicrob Chemother. 1986;17:481–487. doi: 10.1093/jac/17.4.481. [DOI] [PubMed] [Google Scholar]
- 15.Miyake Y, Tsuruda K, Okuda K, Widomati, Iwamoto Y, Suginaka H. In vitro activity of tetracyclines, macrolides, quinolones, clindamycin and metronidazole against periodontopathic bacteria. J Periodontal Res. 1995;30:290–293. doi: 10.1111/j.1600-0765.1995.tb02136.x. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee S. The role of crevicular fluid iron in periodontal disease. J Periodontol. 1985;56:22–27. doi: 10.1902/jop.1985.56.11s.22. [DOI] [PubMed] [Google Scholar]
- 17.Olsvik B, Hansen B F, Tenover F C, Olsen I. Tetracycline-resistant microorganisms recovered from patients with refractory periodontal disease. J Clin Periodontol. 1995;22:391–396. doi: 10.1111/j.1600-051x.1995.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 18.Otto B R, Verweij-van Vught A M J J, MacLaren D M. Transferrins and heme-compounds as iron sources for pathogenic bacteria. Crit Rev Microbiol. 1992;18:217–233. doi: 10.3109/10408419209114559. [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan S, Mang X P, Ramasamy S, Harrison D G, Galis Z S. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. J Clin Investig. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riaz M, Pilpel N. Complexation of tetracyclines with metal ions in relation to photosensitization. J Pharm Pharmacol. 1984;36:153–156. doi: 10.1111/j.2042-7158.1984.tb06929.x. [DOI] [PubMed] [Google Scholar]
- 21.Rifkin B R, Vernillo A T, Golub L M. Blocking periodontal disease progression by inhibiting tissue-destructive enzymes: a potential therapeutic role for tetracyclines and their chemically-modified analogs. J Periodontol. 1993;64:819–827. doi: 10.1902/jop.1993.64.8s.819. [DOI] [PubMed] [Google Scholar]
- 22.Schwyn B, Neilands J B. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 23.Seymour R A, Heasman P A. Tetracyclines in the management of periodontal diseases. J Clin Periodontol. 1995;22:22–35. doi: 10.1111/j.1600-051x.1995.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang H-L, Greenwell H, Bissada N F. Crevicular fluid iron changes in treated and untreated periodontically diseased sites. Oral Surg Oral Med Oral Pathol. 1990;69:450–456. doi: 10.1016/0030-4220(90)90378-6. [DOI] [PubMed] [Google Scholar]
- 25.Weinberg E D. The mutual effects of antimicrobial compounds and metallic cations. Bacteriol Rev. 1957;21:46–68. doi: 10.1128/br.21.1.46-68.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinberg E D. Roles of iron in host-parasite interactions. J Infect Dis. 1971;24:401–410. doi: 10.1093/infdis/124.4.401. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg E D. Acquisition of iron and other nutrients in vivo. In: Roth J A, et al., editors. Virulence mechanisms of bacterial pathogens. 2nd ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 79–93. [Google Scholar]
- 28.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 29.Wooldridge K G, Williams P H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol Rev. 1993;12:325–348. doi: 10.1111/j.1574-6976.1993.tb00026.x. [DOI] [PubMed] [Google Scholar]