Abstract
Background
Since the emergence of COVID-19 vaccinations, many women around the world are reporting abnormalities in their menstrual periods post-vaccination. The aim of this study is to investigate the prevalence and impact of menstrual abnormalities after the COVID-19 vaccine among females residing within the Middle East and North Africa (MENA).
Methods
The study utilized a cross-sectional online self-administered survey from July 2021 to August 2021 targeting females living in the MENA region above the age of menarche who had received vaccine and were not pregnant or lactating, and do not have a history of primary ovarian insufficiency, hypothalamic menopause, or have undergone a hysterectomy. The survey was distributed regionally via social media.
Results
A total of 2269 females were included in our study, with a mean age of 34.3 ± 8.5 years. About 66.3% of participants reported menstrual symptoms post-vaccination, of which 46.7% experienced them after their first dose. However, in 93.6% of participants, the symptoms resolved within 2 months. Vaccine type did not significantly influence the incidence of abnormalities (p > 0.05). Participants who had confirmed previous COVID-19 infection had a very similar percentage of menstrual abnormalities compared to people who did not have COVID-19 infection or symptoms suspected of COVID-19 infection and did not test (67.5%, 66.8%, respectively); nevertheless, those who had experienced the COVID-19 vaccine general side effects had significantly more abnormalities (p < 0.001). Compared to their pandemic status, females reported significantly more abnormalities post-vaccination.
Conclusion
The study showed a possible link between the COVID-19 vaccine and menstrual abnormalities that have impacted their quality of life.
Keywords: COVID-19, MENA, menstrual abnormalities, menstrual cycle, vaccine
Introduction
Over the past year and a half, the COVID-19 pandemic has taken the world by storm, affecting every aspect of human life. As a response, numerous vaccines were developed and approved in less than a year from when the virus was first identified.1 By mid-2021, three billion doses had been administered around the world.2 Nevertheless, this rapid worldwide use of the vaccines led the Centers for Disease Control and Prevention (CDC) to utilize a real-time Vaccine Adverse Event Reporting System called V-Safe to track potential side effects of the vaccine.3 In addition, many studies reported a variety of vaccine-related side effects, ranging from mild symptoms like fever, chills, headache, fatigue, and arm pain to severe side effects such as thrombosis and anaphylaxis.4–7 Moreover, a recent study reported several menstrual abnormalities following COVID-19 vaccination, including increased cycle length, pain, and bleeding.8
The menstrual cycle reflects women’s general health status, as women with irregular and longer menstrual cycles are at a higher risk of death before the age of 70.9 This is partially attributed to women with irregular menstrual cycles being more prone to metabolic disorders such as diabetes mellitus and dyslipidemia.10 Therefore, menstrual abnormalities pose a significant challenge for the healthcare system, particularly when accounting for its impact on women’s daily tasks.10 In light of the prevalence of menstrual abnormalities,11,12 the American Academy of Pediatrics recommended adding the menstrual cycle to the vital signs of adolescent females.13
The Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom reported 41,919 cases of menstrual problems, including heavier than usual periods, delayed periods, and unexpected vaginal bleeding, up to their latest update on 17/11/2021.14 This emphasizes the importance of investigating menstrual abnormalities post COVID-19 vaccination.15 Therefore, this study attempts to investigate the menstrual cycle abnormalities and their relation to the different types of COVID-19 vaccines, which will help us provide better medical care to achieve a satisfactory outcome for these patients by alleviating their concerns and helping them deal with their symptoms, thus improving their quality of life.
Materials and Methods
Study Design
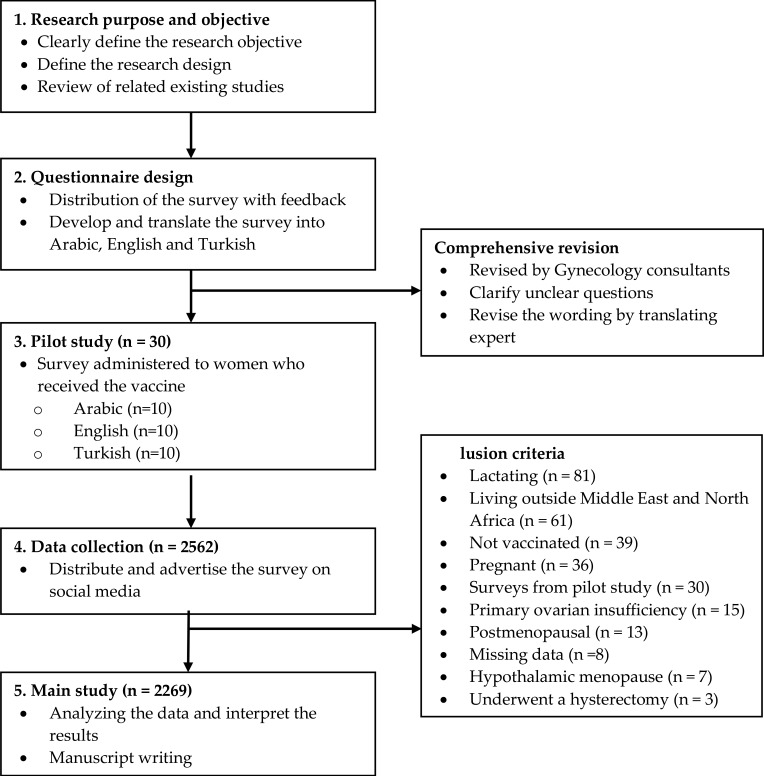
This study utilized a descriptive cross-sectional design that took place between July 2021 and August 2021. The study aimed to explore the impact of COVID-19 vaccines on the menstrual cycle. It involved an anonymous questionnaire distributed via social media platforms that included Facebook, Twitter, Instagram, and WhatsApp across the Middle East and North Africa (MENA) region, including Jordan, UAE, KSA, Kuwait, Qatar, Turkey, Palestine, Iraq, Lebanon, Egypt, Oman, Morocco, Al-Bahrain, Tunisia, Sudan, and Syria. Data collection did not involve direct contact with participants to prevent the spread of COVID-19. Figure 1 shows a summary of the study design.
Figure 1.
Summary of the study design.
The study investigated three main associations. Firstly, the association between the COVID-19 vaccine and menstrual abnormalities and the associated factors. Secondly, the association between the COCID-19 infection and menstrual abnormalities. Lastly, the lifestyle and mental health change after the COVID-19 vaccination.
Study Population
The study included female participants over the age of menarche who had any type of COVID-19 vaccine. The exclusion criteria were those women who did not receive the vaccine, living outside the Middle East and North Africa, were premenopausal, postmenopausal, pregnant, lactating, had primary ovarian insufficiency, hypothalamic menopause, underwent a hysterectomy or had failed to complete at least 80% of the questionnaire.
Study Tool
The study used an online-based questionnaire created through Google Forms® online survey development software. The questionnaire was first developed in Arabic and then translated into English and Turkish, and back-translated into Arabic to ensure comprehensibility and content validity by translating expert.
The questionnaire (available in the Supplementary materials) consists of 28 detailed self-report questions covering six integral domains. The first of which is demographics, including age, country of residence, number of children, and marital status (4 questions). The second section contains questions about menstruation and menstrual cycle, method of contraception used in the previous year, pre-existing medical diagnosis, and smoking (8 questions). The third category deals with the number of doses, the type of vaccine received, and objective scores for the severity of the COVID-19 vaccination side effects (3 questions). The fourth category is about previous COVID-19 infection, the severity of the symptoms of the infection according to NIH, and menstrual abnormalities during the COVID-19 pandemic (3 questions). The fifth category includes questions about menstrual abnormalities after vaccination, the duration of symptoms, their relationship to the dose, and their impact on life (6 questions). The sixth category consists of an open-ended question about the change in the menstruation and menstrual cycle length between baseline and after vaccination (4 questions).
A pilot study was conducted that included 30 participants to find any faults that might exist and ensure the validity and reliability of the overall questionnaire. Accordingly, the survey was reviewed again by a group of consultants from the Department of Gynecology at Jordan University Hospital (JUH) to ensure the validity of the construction prior to distribution. The 30 participants in the pilot study were excluded from the main study and subsequent analyses (Figure 1).
Data Collection Procedure
An anonymous free online survey was used to collect responses over six weeks directed at women who have received at least one dose of the COVID-19 vaccine and live in the MENA region. The questionnaire was distributed and advertised across several social media groups, and participants were encouraged to share it with their peers and social circles in order to maximize the sample size. It took 7 minutes to complete all the questions.
Ethical approval was granted by The University of Jordan and this study was conducted in accordance with the Declaration of Helsinki. This study was created to ensure that the ethical standards of voluntary participation were followed, that it was not emotionally distressing, and that the participants’ rights to privacy, anonymity, and self-determination were protected. An informed consent was obtained from all participants on the first page of the study’s questionnaire, it explained the aims of the study and emphasized on the confidentiality of the data collected.
Statistical Analysis
SPSS version 26.0 (Chicago, USA) was used in our analysis. The data was described using variability analysis in the form of means (standard deviation). The sociodemographic factors were calculated and provided as frequencies (percentages) using standard descriptive statistical parameters. The relationship between study factors were examined using the Chi-square test. A paired parametric t-test was used to compare the mean change in the length of the menstruation and of the menstrual cycle between baseline and after vaccination. Statistical significance was defined as a p-value of less than 0.05.
Results
Demographics
Out of the 2562 participants who completed the questionnaire, 293 were excluded because they did not meet the eligibility criteria. Eventually, 2269 female respondents were included in this analysis. Among the participants, the age ranged from 14 to 54 years, with a mean of 34.32 (± 8.53). The participants were from 16 different countries, and the majority of the participants were from Jordan, United Arab Emirates, Saudi Arabia, Kuwait, and Qatar, accounting for 64.7%, 10.5%, 7.9%, 3.5%, and 2.4%, respectively.
Most respondents (62.4%) were married, and 59.9% had a child or more, with a mean of 1.74 (± 1.74). The majority of participants received Pfizer-BioNTech, Sinopharm, and AstraZeneca (48.4%, 35.3%, and 13.4%, respectively), and the majority (85.4%) received two doses. Concerning COVID-19 infection, 22% of participants had confirmed infection, and 11.7% of participants reported having COVID-19 like symptoms; however, they were not confirmed through laboratory testing. Table S1 demonstrates the country of residence of the participants and information on the COVID-19 immunization.
Clinical Characteristics
Among the participants, 75.1% had regular menstrual cycles before taking the vaccine for the last year, and 24.9% had irregular menstrual cycles. About a third (29.7%) of the participants were smokers, and only 6.7% had a history of coagulation disorders (including bleeding, blood clots, thrombocytopenia, or taking coagulation medication). Additionally, 20.8% of the participants reported having a history of asthma, drug allergy, or food allergy.
The vast majority (81.2%) of participants were disease-free. However, PCOS, thyroid disorders, uterine fibroids, endometriosis, and adenomyosis were present in 10.1%, 6.7%, 2.9%, 1.8%, and 0% of participants, respectively. Over the last calendar year, 19.2% of the participants began using a contraceptive, predominantly combined oral contraceptive pill (COCP) (8.4%). Moreover, 8.4% of women discontinued using a contraceptive within the last calendar year, with COCP (5.8%) being the most often discontinued.
Menstrual Cycle Abnormalities and COVID-19 Infection
Among patients who complained of post-vaccination menstrual abnormalities, 77.6% had no previous COVID-19 infection. When comparing menstrual abnormalities among those with a previous history of infection and those without a history of infection there was no significant associations with post-vaccination menstrual abnormalities (p = 0.136); it was found that 66.8% had menstrual abnormalities among those who did not have previous COVID-19 infection or symptoms suspected of COVID-19 infection and did not test. Similarly, 67.5% of those with confirmed previous COVID-19 infection had menstrual abnormalities. Nevertheless, the post-vaccination menstrual abnormalities were significantly associated with the severity of COVID-19 infection (p = 0.006).
Menstrual Cycle Abnormalities After COVID-19 Vaccination
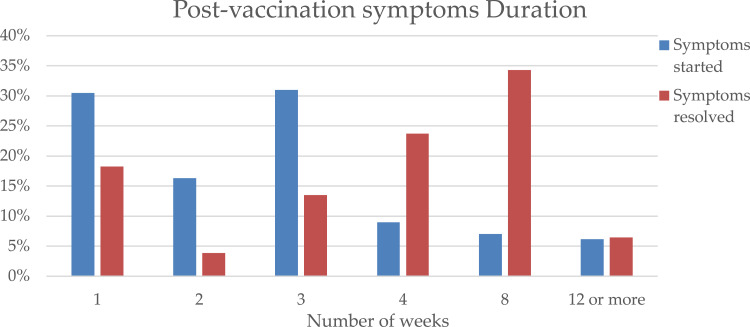
Overall, 66.3% of women experienced menstrual abnormalities after vaccination. Of those, symptoms appeared after a week in 30.5%, and within a month in 86.8% (Figure 2). Furthermore, in 93.6% the symptoms resolved within 2 months (Figure 2). The majority (46.7%) had the symptoms after the first dose, while 32.4% after the second dose and 20.9% after both doses. When comparing AstraZeneca, Sinopharm and Pfizer, differences in the incidence of menstrual abnormalities were statistically insignificant, 68.4%, 66.2%, 65.4%, respectively (p > 0.05). Moreover, post-vaccination menstrual abnormalities symptoms and PCOS, thyroid disorders, uterine fibroids, endometriosis, and adenomyosis were not associated (p > 0.05).
Figure 2.
Timeline for the onset and resolution of post-vaccination symptoms.
There was a significantly higher prevalence of menstrual abnormalities among those who also experienced other adverse effects associated with the COVID-19 vaccination, including fever, fatigue, headache, nausea, and arm pain (17.6% vs 82.4%) (p < 0.001). In addition, there was a statistically significant association with the objective severity grade for vaccine general side effects (p < 0.001).
Almost one-third (35.3%) of the participants experienced menstrual abnormalities during the COVID-19 pandemic before vaccination. In addition, there is a significant difference between the menstrual abnormalities during the COVID-19 pandemic and menstrual abnormalities after the vaccination (35.3% vs 66.3%) (p < 0.001). Table 1 summarizes the symptoms experienced by the participants post-vaccination after each dose in detail and during the COVID-19 pandemic.
Table 1.
Menstrual Symptoms Post-Vaccination and During COVID-19 Pandemic n (%)
| Post-Vaccination | During COVID-19 Pandemic | p value | |||
|---|---|---|---|---|---|
| First Dose Only | Second Dose Only | Both Doses | |||
| Irregular menstruation | 319 (14.1) | 212 (9.3) | 149 (6.6) | 278 (12.3) | <0.001 |
| Menstrual cramps | 214 (9.4) | 151 (6.7) | 98 (4.3) | 444 (19.6) | <0.001 |
| Increased period frequency | 197 (8.7) | 146 (6.4) | 103 (4.5) | 172 (7.6) | <0.001 |
| Menorrhagia | 201 (8.9) | 145 (5.4) | 78 (3.4) | 170 (7.5) | <0.001 |
| Increase duration of the menstruation | 193 (8.5) | 133 (5.9) | 70 (3.1) | 158 (6.8) | <0.001 |
| Menstruation has stopped | 78 (3.4) | 41 (1.8) | 32 (1.4) | 39 (1.7) | <0.001 |
| Worsening of premenstrual symptoms | 38 (1.7) | 30 (1.3) | 21 (0.9) | 115 (5.1) | <0.001 |
| Intermenstrual bleeding | 26 (1.1) | 9 (0.4) | 13 (0.6) | 62 (2.7) | <0.001 |
Notes: p value was calculated using a chi-squared test and indicates the associations between the menstrual symptoms post-vaccination in total (after the first dose only, second dose only, and after both doses) and menstrual symptoms during the COVID-19 pandemic.
Factors Associated with Menstrual Abnormalities After COVID-19 Vaccine
Our results showed that there were no significant associated with age, number of children, marital status, vaccination type, previous history of COVID-19 infection, previous diagnosis including PCOS, thyroid disorders, uterine fibroids, endometriosis, and adenomyosis, stopping or starting any king of contraceptive method, history of coagulation disorders (including bleeding, blood clots, thrombocytopenia, or taking coagulation medication), menstrual cycle length, and duration of menstruation (p > 0.05). However, there was a significant relationship with country of residence (p < 0.001), irregular cycles (p < 0.001), smoking (p < 0.001), menstrual abnormalities during the COVID-19 pandemic (p < 0.001), negative impact on quality of life (p < 0.001), symptoms of COIVD-19 vaccine general symptoms (p < 0.001), objective severity grade for vaccine general side effects (p < 0.001), and severity of COVID-19 infection (p = 0.006).
Duration of Menstruation and Duration of the Menstrual Cycle After COVID-19 Vaccination
Mean duration of menstruation as reported by respondents had significantly increased from 6 ± 0.03 days pre-vaccine to 6.5 ± 0.1 post-vaccine (p < 0.001). Moreover, participants mean menstrual cycle length had significantly increased from 27 ± 6 days prior to taking the vaccine to 28.1 ± 10 days after being vaccinated (p < 0.001).
Lifestyle and Mental Health Change After COVID-19 Vaccination
After receiving the COVID-19 vaccination, 78.3% reported side effects, including fever, fatigue, headache, nausea, and arm pain, which in 14.4% were described as severe. In addition to common post-vaccination side effects, 56.2% of participants reported that the gynaecological abnormalities they experienced after vaccination significantly impacted their quality of life. This impact led 15.2% of those who experienced any menstrual abnormalities to visit a gynaecologist for regular check-up. Table 2 demonstrates the different ways participants coped with post-vaccine menstrual abnormalities.
Table 2.
Participants Reaction to the Menstrual Abnormalities After Vaccination
| n (%) | |
|---|---|
| I did not do anything | 983 (65.4) |
| I went to the gynecologist | 229 (15.2) |
| I searched online for my symptoms | 226 (15) |
| I asked my relatives and acquaintances | 172 (11.4) |
| Self-treatment | 122 (8.1) |
| I went to the family doctor or the general practitioner | 26 (1.7) |
Notes: 1504 (66.3%) had menstrual abnormalities; the sum of responses is greater than 100% because participants could select more than one response.
Discussion
Our study demonstrated that 66.3% of our sample in the MENA region had complained of menstrual abnormalities after being vaccinated against COVID-19, particularly after the first dose (46.7%). Vaccine type did not influence the incidence of menstrual abnormalities among these women. However, females that were subjected to pronounced COVID-19 vaccine side effects had significantly higher rates of menstrual abnormalities. After taking the vaccine, women reported a significantly longer mean duration of menstruation and menstrual cycle length compared to their pre-vaccine status. These symptoms were alleviated in less than a month of taking the vaccine for 86.8% of respondents. Lastly, 56.2% of the participants indicated that post-vaccine menstrual abnormalities had negatively impacted their quality of life.
The menstrual bleeding pattern is an important indicator of reproductive health.16 However, menstrual symptoms, such as perimenstrual mood disorders, menstrual cramps, and heavy menstrual bleeding, are considered common gynecological problems.11 One nationwide study conducted by Schoep et al, including 42,879 healthy premenopausal Dutch women, reported that 53.7% of respondents complained of heavy bleeding, 77.3% complained of perimenstrual psychological complaints, and 85.4% complained of menstrual cramps.11 This can be attributed to the female menstrual cycle being affected by several factors that can be transient such as infections, weight gain, anxiety, hormonal changes, and periods of psychological stress, or long term and require treatment such as endocrinopathies, polycystic ovary syndrome,17–19 Stressors may activate the hypothalamic-pituitary-gonadal axis, leading to a disruption of the regularity of hormone release. These menstrual changes can impact women’s quality of life, leading to work and school limitations, hindering achievements, and affecting social and professional activities, which can further cause stress.20,21 One source of stress that has taken the world by storm was the COVID-19 pandemic, such that several studies showed an increase in menstrual cycle abnormalities during the pandemic compared to before.17,18,22,23 In our study, nearly one-third (35.3%) of the participants experienced menstrual changes during the COVID-19 pandemic before vaccination. However, 66.3% of women experienced abnormal periods after vaccination. Even after accounting for changes in menstrual bleeding during the COVID-19 pandemic, there is a significant difference between the menstrual changes during the COVID-19 pandemic and menstrual abnormalities after the vaccination. Our results are consistent with a recent preprint study of 39,129 participants in the USA, where 42% reported heavier bleeding after vaccination.24 In another preprint of a retrospective study of 4989 premenopausal vaccinated participants in the UK, only 20% did not report any menstrual cycle abnormalities up to four months after their first COVID-19 vaccine injection.25
Irregular menstruation incidence in the literature ranges from 5% to 35.6%, varying with occupation, age, and area of residence.26–31 In our study, the percentage of females with menstrual irregularities prior to vaccination lies within that range, with 24.9% having irregular menstrual cycles. According to our findings, the vaccine altered the duration of menstruation and length of the menstrual cycle in the women who received it, resulting in a significant difference between before and after vaccination. Although some participants reported a reduction in the duration of menstruation and length of menstrual cycle while others reported a prolongation, the mean duration significantly increased from 6 ± 0.03 days pre-vaccine to 6.5 ± 0.1 post-vaccine (p < 0.001). While the mean length of the menstrual cycle significantly increased from 27 ± 6 days prior to taking the vaccine to 28.1 ± 10 days after being vaccinated (p < 0.001).
In our study, 65.4% of those with symptoms after their vaccine reported not resorting to any modality to alleviate these symptoms. This percentage is comparable to a study surveying 383 women between 17 and 25 years of age, where 73.9% reported not seeking medical help for their menstrual symptoms.32 In another study, only 43.7% of respondents consulted a physician in their lifetime regarding any menstruation-related symptoms.11 Although 65.4% of the participants did not seek treatment, 56.2% reported that their gynecological symptoms had negatively impacted their quality of life. Various reasons can be attributed to this observed lack of help-seeking behavior. Firstly, some may have experienced mild symptoms that did not warrant intervention, in their opinion. Additionally, the state of emergency caused by COVID-19 may have created a psychological or physical barrier that further prevented individuals from consulting about complaints not associated with COVID-19. On the organizational level, health care facilities may have resorted to tending to patients with serious conditions only, particularly in low-resource settings where medical supplies were scarce. Secondly, some individuals may lack awareness of the possible risks associated with irregular menstrual cycles. Thirdly, participants may have wanted to avoid a stressful situation out of fear that their complaint is serious.33 Lastly, the negative attitude towards menstrual symptoms may discourage help-seeking behavior, particularly in the younger age group of a conservative society such as the MENA region.34 In a qualitative study done by Flynn, the results showed that the social pressure to avoid discussing menstruation strongly influenced women’s health-related behavior.35 This social pressure may also explain why only 16.9% of those with menstrual symptoms visited a gynecologist; meanwhile, 34.1% decided to seek help through relatives, online searches, or self-treatment. Fortunately, these symptoms were alleviated in less than a month of taking the vaccine for 86.8% of respondents. Similarly, in a preprint done by Alvergne et al, menstrual abnormalities resolved within two months of the vaccine in 93.6% of the cases, suggesting that such vaccine side-effects are self-limiting.25
The mechanism by which the vaccine may cause menstrual symptoms is unknown. However, it is plausible that it is the result of immune-mediated vaccine-induced thrombocytopenia.36 This speculation results from many other vaccines, including measles-mumps-rubella (MMR), hepatitis A and B, diphtheria-tetanus-acellular pertussis (DTaP), varicella, and even influenza, being previously linked to vaccine-induced thrombocytopenia causing menstrual irregularities.37 In support of this speculation, our study shows trends and factors that may suggest the symptoms are a result of an immunologic response. Firstly, the symptoms were delayed appearing after a week in 30.5% and 15.1% appearing after one month of taking the vaccine. Secondly, there was a significantly higher prevalence of menstrual irregularities among those who experienced other adverse effects associated with COVID-19 vaccination (p < 0.001). Further research is needed concerning the mechanism by which COVID-19 vaccination causes menstrual abnormalities.
The main strength of our study is that it is one of the first articles to tackle in-depth the phenomenon of post-vaccination menstrual abnormalities, despite the various reports on social media platforms and news outlets.26,38–40 In our study, vaccinated women from different MENA countries were recruited, which allowed us to estimate the prevalence of post-vaccination menstrual abnormalities in a conservative society, which is considered a sensitive topic. Additionally, it provides a baseline for assessing the impact of COVID-19 vaccines on the female menstrual cycle. Another strength lies in the data collection instrument, which has been meticulously developed and validated to encapsulate the problem accurately and comprehensively. Accordingly, we believe that our data accurately represents the menstrual abnormalities that women experience post-vaccination.
However, the study falls prey to some limitations. The study’s cross-sectional design limited our ability to determine causal relationships. Moreover, self-reported data extraction has an increased likelihood of recall bias or self-selection as those with menstrual disorders might be more interested in participating in the study. In addition, the use of an internet-based survey might have underrepresented or overrepresented certain target groups, especially older populations with limited internet access or technological awareness. Nevertheless, online questionnaires are the most effective and safest data extraction method in light of the current epidemiological status. To add to this, due to the rapid addition of papers in the COVID-19 literature, limitations to the addition of the most up-to-date papers are present. Finally, while our study reports the menstrual changes of a large, sampled population, the sample might not be representative of each country’s cluster.
Conclusions
This study provides preliminary evidence that females subjected to COVID-19 vaccines may experience menstrual abnormalities including, but not limited to longer menstruation duration and increased menstrual cycle lengths. Such abnormalities may impact females’ daily life activities and ultimately impair their overall quality of life. Our study also provides preliminary evidence that these symptoms may be self-limited and transient. Nevertheless, due to the altered prioritization influenced by the COVID-19 pandemic, women are less likely to adopt health-seeking behaviors. Therefore, it is imperative to alert healthcare professionals and women about menstrual abnormalities after vaccination. Further prospective cohort studies are needed to identify the temporal link between menstrual cycle changes and the different types of COVID-19 vaccines.
Acknowledgments
The authors cordially thank Alaa Albandi and Minolia A. Al-Kubaisy for their proofreading and comments on the manuscript. We would also like to thank all the participants for their contributions to this study.
Funding Statement
No funding was provided for this study.
Data Sharing Statement
The data from the present research that were utilized and analyzed are accessible from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Jordan University Hospital (reference number 10/2021/20524). Informed consent was obtained from all participants involved in the study.
Author Contributions
NM, MAA, MIA and MWN conceived and designed the study. NM, MAA, MIA, and MWN contributed to the methodology. MAA, MIA, AMK and MWN performed literature review. NM, MAA, MIA, AMK and MWN contributed to data collection and cleaned the raw data. MAA conducted the data analysis with support from AA. NM supervision. MAA, MIA, AMK, MWN and AA drafted the manuscript, with extensive comments from AA. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no known competing financial or personal interests that could have influenced the work reported in this paper.
References
- 1.Bok K, Sitar S, Graham BS, Mascola JR. Accelerated COVID-19 vaccine development: milestones, lessons, and prospects. Immunity. 2021;54(8):1636–1651. doi: 10.1016/J.IMMUNI.2021.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ndwandwe D, Wiysonge CS. COVID-19 vaccines. Curr Opin Immunol. 2021;71:111–116. doi: 10.1016/j.coi.2021.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. V-safe after vaccination health checker. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html. Accessed August 27, 2021.
- 4.Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locht C. Vaccines against COVID-19. Anaesthesia Crit Care Pain Med. 2020;39(6):703–705. doi: 10.1016/j.accpm.2020.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimabukuro TT, Cole M, Su JR. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325(11):1101–1102. doi: 10.1001/jama.2021.1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath PT, Galiza EP, Baxter DN, et al. Safety and efficacy of NVX-CoV2373 COVID-19 vaccine. N Engl J Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alghamdi AN, Alotaibi MI, Alqahtani AS, Al Aboud D, Abdel-Moneim AS. BNT162b2 and ChAdOx1 SARS-CoV-2 post-vaccination side-effects among Saudi vaccinees. Front Med. 2021;8:1796. doi: 10.3389/fmed.2021.760047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y-X, Arvizu M, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ. 2020;371:m3464. doi: 10.1136/BMJ.M3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rostami Dovom M, Ramezani Tehrani F, Djalalinia S, Cheraghi L, Behboudi Gandavani S, Azizi F. Menstrual cycle irregularity and metabolic disorders: a Population-Based Prospective Study. PLoS One. 2016;11(12):e0168402. doi: 10.1371/journal.pone.0168402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoep ME, Nieboer TE, van der Zanden M, Braat DDM, Nap AW. The impact of menstrual symptoms on everyday life: a survey among 42,879 women. Am J Obstet Gynecol. 2019;220(6):569.e1–569.e7. doi: 10.1016/j.ajog.2019.02.048 [DOI] [PubMed] [Google Scholar]
- 12.Ansong E, Arhin SK, Cai Y, Xu X, Wu X. Menstrual characteristics, disorders and associated risk factors among female international students in Zhejiang Province, China: a cross-sectional survey. BMC Women’s Heal. 2019;19(1):1–10. doi: 10.1186/S12905-019-0730-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Pediatrics. 2006;118(5):2245–2250. doi: 10.1542/PEDS.2006-2481 [DOI] [PubMed] [Google Scholar]
- 14.Coronavirus vaccine - weekly summary of yellow card reporting. GOV.UK; 2021. [Google Scholar]
- 15.Male V. Menstrual changes after covid-19 vaccination. BMJ. 2021;374:n2211. doi: 10.1136/bmj.n2211 [DOI] [PubMed] [Google Scholar]
- 16.Dasharathy SS, Mumford SL, Pollack AZ, et al. Menstrual bleeding patterns among regularly menstruating women. Am J Epidemiol. 2012;175(6):536–545. doi: 10.1093/aje/kwr356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurdoğlu Z. Do the COVID-19 vaccines cause menstrual irregularities? Inte J Women’s Health Reprod Sci. 2021;9(3):158–159. doi: 10.15296/ijwhr.2021.29 [DOI] [Google Scholar]
- 18.Demir O, Sal H, Comba C. Triangle of COVID, anxiety and menstrual cycle. J Obstet Gynaecol. 2021;41(8):1257–1261. doi: 10.1080/01443615.2021.1907562 [DOI] [PubMed] [Google Scholar]
- 19.Foster C, Al-Zubeidi H. Menstrual irregularities. Pediatr Ann. 2018;47(1):e23–e28. doi: 10.3928/19382359-20171219-01 [DOI] [PubMed] [Google Scholar]
- 20.Kadir RA, Edlund M, Von Mackensen S. The impact of menstrual disorders on quality of life in women with inherited bleeding disorders. Haemophilia. 2010;16(5):832–839. doi: 10.1111/j.1365-2516.2010.02269.x [DOI] [PubMed] [Google Scholar]
- 21.Karlsson TS, Marions LB, Edlund MG. Heavy menstrual bleeding significantly affects quality of life. Acta Obstet Gynecol Scand. 2014;93(1):52–57. doi: 10.1111/aogs.12292 [DOI] [PubMed] [Google Scholar]
- 22.Phelan N, Behan LA, Owens L. The impact of the COVID-19 pandemic on women’s reproductive health. Front Endocrinol. 2021;2021:191. doi: 10.3389/FENDO.2021.642755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozimek N, Velez K, Anvari H, Butler L, Goldman KN, Woitowich NC. Impact of stress on menstrual cyclicity during the coronavirus disease 2019 pandemic: a survey study. J Women's Health. 2022;31(1):84–90. doi: 10.1089/jwh.2021.0158 [DOI] [PubMed] [Google Scholar]
- 24.Lee KMN, Junkins EJ, Fatima UA, Cox ML, Clancy KBH. Characterizing menstrual bleeding changes occurring after SARS-CoV-2 vaccination. medRxiv. 2021;2021. doi: 10.1101/2021.10.11.21264863 [DOI] [Google Scholar]
- 25.Alvergne A, Kountourides G, Argentieri MA, et al. COVID-19 vaccination and menstrual cycle changes: a United Kingdom (UK) retrospective case-control study. medRxiv. 2021;2021. doi: 10.1101/2021.11.23.21266709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BBC News. Covid vaccine: period changes could be a short-term side effect. Available from: https://www.bbc.com/news/health-56901353. Accessed August 27, 2021.
- 27.Sakai H, Ohashi K. Association of menstrual phase with smoking behavior, mood and menstrual phase-associated symptoms among young Japanese women smokers. BMC Women's Health. 2013;13:10. doi: 10.1186/1472-6874-13-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toffol E, Koponen P, Luoto R, Partonen T. Pubertal timing, menstrual irregularity, and mental health: results of a population-based study. Arch Women's Ment Health. 2014;17(2):127–135. doi: 10.1007/s00737-013-0399-y [DOI] [PubMed] [Google Scholar]
- 29.Nohara M, Momoeda M, Kubota T, Nakabayashi M. Menstrual cycle and menstrual pain problems and related risk factors among Japanese female workers. Ind Health. 2011;49(2):228–234. doi: 10.2486/indhealth.ms1047 [DOI] [PubMed] [Google Scholar]
- 30.Zhou M, Wege N, Gu H, Shang L, Li J, Siegrist J. Work and family stress is associated with menstrual disorders but not with fibrocystic changes: cross-sectional findings in Chinese working women. J Occup Health. 2010;52(6):361–366. doi: 10.1539/joh.l10057 [DOI] [PubMed] [Google Scholar]
- 31.Kwak Y, Kim Y, Baek KA, Erbil N. Prevalence of irregular menstruation according to socioeconomic status: a population-based nationwide cross-sectional study. PLoS One. 2019;14(3):e0214071–e0214071. doi: 10.1371/journal.pone.0214071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White PA, Wildman BG. Factors related to medical help-seeking in women with menstrual discomfort. Behav Res Ther. 1986;24(4):471–474. doi: 10.1016/0005-7967(86)90012-4 [DOI] [PubMed] [Google Scholar]
- 33.Ristvedt SL, Trinkaus KM. Psychological factors related to delay in consultation for cancer symptoms. Psychooncology. 2005;14(5):339–350. doi: 10.1002/pon.850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farotimi AA, Esike J, Nwozichi CU, Ojediran TD, Ojewole FO. Knowledge, attitude, and healthcare-seeking behavior towards dysmenorrhea among female students of a private university in Ogun state, Nigeria. J Basic Clin Reprod Sci. 2015;4(1).33–38. [Google Scholar]
- 35.O’Flynn N. Menstrual symptoms: the importance of social factors in women’s experiences. Br J Gen Pract. 2006;56(533):950–957. [PMC free article] [PubMed] [Google Scholar]
- 36.Hunter PR. Thrombosis after covid-19 vaccination. BMJ. 2021;373. doi: 10.1136/BMJ.N958 [DOI] [PubMed] [Google Scholar]
- 37.Perricone C, Ceccarelli F, Nesher G, et al. Immune thrombocytopenic purpura (ITP) associated with vaccinations: a review of reported cases. Immunol Res. 2014;60(2–3):226–235. doi: 10.1007/s12026-014-8597-x [DOI] [PubMed] [Google Scholar]
- 38.Cleveland Clinic. Will a COVID-19 vaccine throw your period off?. Available from: https://health.clevelandclinic.org/will-a-covid-19-vaccine-throw-your-period-off/. Accessed August 27, 2021.
- 39.COVID-19 vaccines and periods: what do we know so far? Available from: https://www.medicalnewstoday.com/articles/can-covid-19-vaccines-affect-periods. Accessed August 27, 2021.
- 40.NPR. Can COVID vaccines cause temporary menstrual changes? Research aims to find out: shots health news. Available from: https://www.npr.org/sections/health-shots/2021/08/09/1024190379/covid-vaccine-period-menstrual-cycle-research. Accessed August 27, 2021.