Abstract
Background
Cognitive impairment has a great negative impact on quality of life for breast cancer survivors. Emerging evidence suggested that physical exercise can improve cognitive function in order adults with Alzheimer's disease. However, less is known about the effects of physical exercise on cognitive function for breast cancer survivors. The purpose of this meta-analysis was to evaluate the effect of physical exercise on cognitive function in breast cancer survivors.
Methods
EMBASE, the Cochrane Library, Web of Science and PubMed were searched from the establishment of the databases to June 2021. Randomized controlled trials were included. All analysis were conducted using the Revman 5.3.
Results
12 studies (936 participants) indicated that exercise improved self-reported cognitive function (MD 10.12, 95% CI [5.49,14.76], p < 0.0001), cognitive fatigue (MD -5.41, 95% CI [-10.31,-0.51], p = 0.03) and executive function (MD -13.63, 95% CI [-21.86,-5.39], p = 0.0001).
Conclusion
Physical exercise can improve cognitive function for breast cancer survivors, particularly in self-reported cognitive function, and executive function. Future studies need to explore the effect of exercise on cognitive function from the frequency and duration of exercise.
Keywords: Physical exercise, Cognitive function, Breast cancer, Executive function, Meta-analysis
Highlights
-
•
Physical exercise can improve cognitive function among breast cancer survivors.
-
•
Aerobic exercise and combined exercise intervention were more effective than other exercise.
-
•
Physical exercise can improve self-reported cognitive function, cognitive fatigue and executive function in breast cancer patients.
1. Introduction
Breast cancer has become the most common type of cancer and the leading cause of cancer-related death among women around the world. Breast cancer worldwide constituted approximately 2.26 million new cases and 680,000 deaths annually [1]. Largely due to improved treatments [2], such as neoadjuvant chemotherapy, radiotherapy, hormone therapy, there are increasing survival rate for breast cancer in many high-income countries [3], in spite of the decreasing mortality of breast cancer recently [2,4]. However, previous studies have reported that breast cancer survivors can experience a variety of side effects and symptoms of long-term treatment [[5], [6], [7]], of which reduced cognitive function is a common one [7,8].
Cognitive function is the information processing aspect of multiple behavior, including attention, learning, memory, visual memory, visuospatial ability, verbal learning and executive function. Cancer-related cognitive impairment (CRCI) is reported by 75% of breast cancer survivors [9], especially for those receiving chemotherapy. Among CRCI, attention, processing speed, memory and executive function are the most common areas of impairment after chemotherapy [8,10,11]. Currently, studies have found that chemotherapy, radiation and hormone therapy can cause CRCI in cancer survivors, while cognitive decline in breast cancer survivors is mainly due to chemotherapy [12]. On the one hand, chemotherapeutic drugs can cause structural and functional changes in certain brain regions through the blood-brain barrier, thus leading to cognitive decline [13,14]; on the other hand, chemotherapy drugs may induce the damage of normal cells, cause acute elevation inflammatory cells, accelerate cell aging, and further aggravate the cognitive function of breast cancer survivors [15]. Hence, there is an expression of “Chemobrain”, this meta-analysis included breast cancer survivors with CRCI caused by chemotherapy. About 1 in 5 of breast cancer survivors received therapy have difficulties in memory and executive function, generally have worse attention than their peers and experienced more cognitive complaints than before treatment [16]. Although this cognitive decline is usually mild to moderate for breast cancer survivors, it can last for months to years after treatment and negatively impact survivors' daily life, mental health, social relationships and work [8,[17], [18], [19], [20]]. At the same time, CRCI can also affect the survivor's ability to make decisions [21,22]. Therefore, effective intervention strategies are urgently needed to improve survivors' cognitive function and quality of life.
There are many factors leading to cognitive dysfunction in breast cancer survivors, including age, hormone levels, social economy, sleep disorders and stress, although these mechanisms are not clear [8,23]. While the optimal intervention that can improve cognitive function of breast cancer survivors is unknown, recent studies have demonstrated that physical exercise may help to maintain or even improve cognitive function [[24], [25], [26]]. There is evidence that, on the one hand, physical exercise can induce the release of neurotrophic factors, neurotransmitters and enzymes, therefore, promoting the nerve growth and development and contributing to the growth of the prefrontal cortex and hippocampus [[27], [28], [29], [30]]. On the other hand, exercise indirectly affects cognitive function by mitigating symptoms of depression and anxiety. Dopaminergic, serotonergic substances caused by depression and anxiety are associated with cognitive impairment, but exercise can relieve depression and anxiety and prevent cognitive deterioration. In recent years, there has been an increasing number of studies on the effects of exercise on cognitive function. However, different types, intensity and duration of exercise have different effects on cognitive function, which has not yet reached a consensus. A meta-analysis showed that moderate-to-strong physical exercise can reduce the risk of cognitive impairment in patients with dementia [30]. Groot's meta-results showed that both combined exercise and aerobic-only exercise had positive effects on patients' cognition, while non-aerobic exercise alone did not [31]. Law's study found that aerobic exercise at moderate-to-high intensity with a total duration of >24 h had pronounced effects on the overall cognition [32]. Práxedes’ experiments demonstrated that passing action showed significant differences in decision making and execution between the intervention and experimental groups, but dribbling did not show such differences [33]. At the same time, other studies have shown that dance can improve executive function and episodic memory of Parkinson's disease patients, but the effect on attention language fluency and visuospatial ability was not significant [34]. In contrast, the effects of different types of physical exercise for improving the cognitive function in breast cancer survivors have not been determined.
Some studies have shown that individual skills can be transferred and applied to different settings, suggesting that physical exercise therapy for cognitive function in patients with Alzheimer's disease could also be adapted to breast cancer survivors [35]. Previous studies exploring the effect of physical exercise on cognitive function in breast cancer survivors produced conflicting results. Hartman's RCT of moderate-intensity aerobic exercise interventions for 12 weeks in 87 breast cancer survivors, who achieved 150 min of moderate-to-vigorous aerobic exercise per week, found that physical exercise improved processing speed but had no effect on self-reported cognitive function [36]. While, Galiano-Castillo conducted a resistance combined aerobic exercise intervention in 76 breast cancer patients for 8 weeks, asking patients to exercise 90 min per day (3 sessions per week), and found physical exercise improves self-reported cognitive function [6,37]. The conflicting results of previous studies may be due to different modes of physical exercise and duration of intervention [38]. Furthermore, a large number of exercise intervention trials in breast cancer survivors demonstrated that physical exercise has a positive impact on upper limb edema and muscle strength, vascular function, health-related quality of life, fatigue, depression, anxiety, sleep, etc. [18,[39], [40], [41], [42]]. However, few studies focus on the effects of physical exercise and cognitive function on breast cancer survivors, and most studies use self-report and lack objective, multidimensional measures of cognitive function.
Therefore, the purpose of this meta-analysis randomized controlled trail is to evaluate the effect of physical exercise on cognitive function in breast cancer survivors, and examine the effect of physical exercise interventions on the cognitive domains of processing speed, executive function, and verbal memory.
2. Methods
This review followed the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA), and was registered with the International Prospective Register of Systematic Reviews (PROSPERO), and the registration number is CRD42021230372.
2.1. Data sources and searches
This systemic review searched five electronic bibliographic databases, including EMBASE (from 1974 to June 2021), the Cochrane Library (the Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials; from the inception to June 2021), Web of Science (from 1900 to June 2021), PubMed (from 1950 to June 2021). MeSH terms and keywords included those relating to breast (eg, mammary), cancer (eg, neoplasm, cancer, carcinoma, tumor, or malignancy), physical exercise (eg, exercise, physical activity, training, sport, aerobic, strength, resistance, endurance, weight, running, walk, cycling, biking, bicycling, yoga, Tai Chi, Qigong, pilate), and cognition (eg, cognitive function, cognitive effect, cognition disorders, cognitive dysfunction neuropsychological tests, learning, memory, attention, executive function) and were combined with an “AND” term. Search terms were modified according to suggestions from the different databases and are reported in full in Appendix A. Only articles published in English were accepted for language restriction. The original published articles of all references, which including relevant articles and additional articles, suitable for inclusion were retrieved for further analysis.
2.2. Eligibility criteria and study selection
Population: Adults with breast cancer.
Intervention: Intervention protocols based on physical exercise for adults with breast cancer.
Comparison: Usual care.
Outcome: Cognitive effects from physical exercise.
Study design chosen: Randomized clinical trials.
The inclusion criteria were: 1) Randomized clinical trials; 2) adults, ≥18 years old; 3) diagnosed with stage I–III breast cancer, stage Ⅳ breast cancer survivors who may not be able to participate due to their condition and physical condition were not included; 4) receive exercise intervention for at last 6 weeks or more; 5) exercise interventions included high- or moderate-intensity continuous or interval resistance (eg, body weight, machine and free weights), aerobic (eg, walking, cycling, strength training), or mind-body exercise (eg, yoga, Tai Chi, Qigong, Pilates); 6) the intervention could take place in any setting (supervised or homebased); 7) the outcomes of trials must evaluate the cognitive function of breast cancer survivors, the measurement tools include self-reported [[43], [44], [45]] or subjective measures. The exclusion criteria were: 1) co-morbid conditions that could alter cognitive testing results, such as a psychiatric conditions, history of substance use disorder, or other neurological disorder; 2) survivors with other cancer diagnosis; 3) other therapeutic interventions through changing behavior or cognition. The effects of other diseases or interventions on cognitive function were excluded to ensure homogeneity of the study. For studies not reporting relevant data, such as standard deviation and/or mean, the first/corresponding authors of these studies were asked to provide additional information. The studies from authors unable to provide this missing information and grey literature were excluded from this analysis.
2.3. Data extraction and quality assessment
Two reviewers screened independently the information of identified studies (title and abstract) in the research and assessed the full texts of potentially eligible articles to determine whether they met the inclusion criteria. Eligible articles were then extracted data by 2 independent reviewers using a unified table to make a last list of identified studies. If the original data were not reported in the eligible studies, contact the author to obtain data or exclude the study. When there is a disagreement, a third reviewer was consulted.
The risk of bias of the RCTs was independently evaluated by 2 reviewers, the Cochrane Handbook for Systematic Reviews of Interventions Version 5.3.0 of the Cochrane Collaboration was used [46]. The following criteria of the Cochrane Handbook was assessed: 1) Random sequence generation, 2) Allocation concealment, 3) Blinding of participants and researchers, 4) Blinding of outcome assessment, 5) Incomplete outcome data, 6) Selective reporting, and 7) Other bias. The results of assessment could be rated as ‘high risk’ (+), ‘low risk’ (−) or ‘unclear risk’ (?). The disagreements were adjudicated by the third reviewers. The methodological quality of the included studies in this meta-analysis was assessed using the 8 criteria from Cuijpers [47], the eight criteria and results are shown in Appendices B and C. In general, when no or insufficient information was provided concerning a quality criterion, we rated it as negative.
For each study included in this meta-analysis, the following data were recorded: first author's information and publication year, country, sample size, participants (age and number of breast cancer survivors in the control and intervention groups), treatment type, exercise mode, duration, frequency, supervised versus home-based, exercise prescription, type of outcome, outcome measures.
2.4. Data synthesis and analysis
A quantitative synthesis for the effect size of each study was calculated as the outcomes were continuous data. The mean difference (MD) was calculated as an effect method when the included studies used the same scale, and the standardized mean difference (SMD) was calculated when the included studies used different scales to evaluate intervention effects. This study adopted random effect model as the main analysis method. I2 statistic were used to assess statistical heterogeneity, with I2 values of 0%, 25%, 50% and 75% represented no, low, moderate and high heterogeneity, respectively [48,49]. Subgroup analysis was performed if significant heterogeneity was found, and subgroup analysis was conducted based on type of training (resistance or aerobic) or training time of each exercise. The stability of the results was tested by sensitivity analysis. Publication bias were presented by funnel plots. All analysis was conducted using the Review Manager Software (Revman 5.3).
3. Results
3.1. Search results and study selection
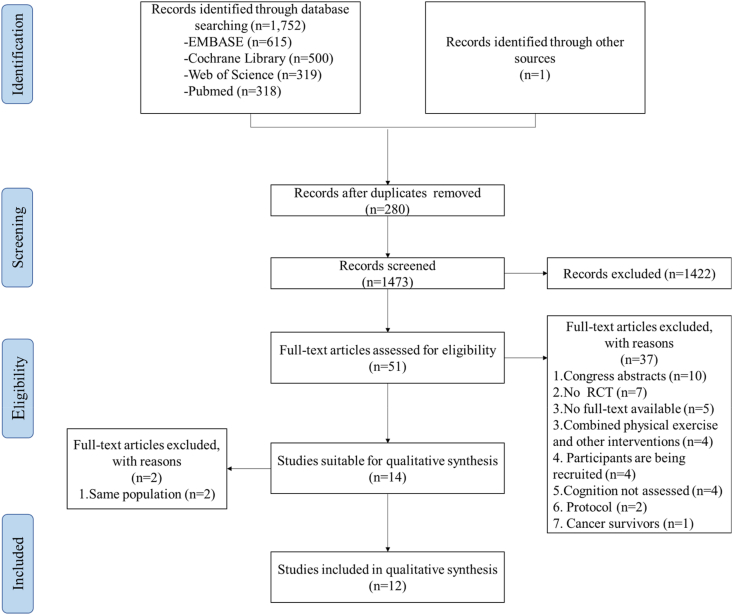
The initially research retrieved 1752 references, along with one additional reference identified from other sources. First, 280 duplicates were removed. Subsequently, 1422 articles were excluded due to irrelevant interventions and the population after screening the abstracts and titles. Then, the full text of 51 articles were received for review and 37 were excluded. After further review of the remaining 14 articles, we found that four articles actually reported the results of two studies at different follow-up time points. Finally, 12 studies were included in this study [6,36,37,39,[50], [51], [52], [53], [54], [55], [56], [57], [58], [59]]. The results of the search progress and study selection are depicted in Fig. 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the literature search used.
3.2. Characterization of the included studies
The characteristics of the 12 RCTs are showed in Table 1. Included trials were distributed in the United States (n = 2) [36,54], England (n = 2) [52,53], Sweden (n = 1) [50,51], Germany (n = 2) [56,58], Australia (n = 1) [55], Ireland (n = 1) [57], Spain (n = 1) [6,37], Iran (n = 1) [39] and India (n = 1) [59] during the period of 2009–2021. Cognitive function data were available for 523 survivors who participated in the physical exercise intervention and 402 survivors who served as a control group. The breast cancer survivors all received chemotherapy, and some also received other treatments such as surgery, radiation and hormone therapy.
Table 1.
Characteristics of included studies.
| Author, year | Country | Sample Size | Participants | Treatment type | Mode | Duration | Frequency | Supervised versus home-based | Exercise prescription | Type of outcome | Outcome measures |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sara et al., [50,51] | Sweden | 206 | UC = 60, age = 52.6 ± 10.2 RT-HIIT = 74, age = 52.7 ± 10.3 AT-HIIT = 72, age = 54.4 ± 10.3 |
Chemotherapy | Aerobic exercise, resistance exercise | 16 weeks | 2/week | Supervised | 60min; RT-HIIT: 2 sets of 8–12 reps at 70–80% HRR AT-HIIT: 20 min + 2 sets of 8–12 reps at 70–80% HRR |
Self-report | EORTC-QLQ; PFS |
| Campbell et al. [52] | England | 19 | Con = 9, age = 51.4 ± 5.1 Ex = 10, age = 53.2 ± 7.0 |
Chemotherapy; radiation | Walking, machine-based, aerobic exercise | 24 weeks | 4/week | Supervised, home-based | 150 min/week; 20–45min at 60–80% HRR & 4 sets of HIIT (5–10 min) by week 12 |
Self-report | FACT-Cog |
| Objective; | TMT; Verbal learning |
||||||||||
| Noelia et al. [6,37] | Spain | 81 | Con = 41, age = 49.2 ± 7.9 Ex = 40, age = 47.4 ± 9.6 |
Chemotherapy surgery; radiation; | Aerobic exercise, resistance exercise |
8 weeks | 3/week | Home-based | AER: 90min | Self-report | EORTC-QLQ |
| Objective | TMT | ||||||||||
| Kajal et al. [53] | England | 50 | Con = 25, age = 52.36 ± 8.9 Ex = 25, age = 52.1 ± 11.7 |
Chemotherapy | Walking | 12 weeks | 3/week | Home-based | AER: 30 min; | Self-report | CFQ |
| Objective | Executive function; | ||||||||||
| Sheri et al. [36] | America | 87 | Con = 44, age = 56.2 ± 9.3 Ex = 43, age = 58.2 ± 11.37 |
Chemotherapy surgery; | Aerobic exercise | 12 weeks | 3-5/week | Supervised, home-based | 30–45 min; Walking at 65%–75% HRR |
Self-report | PROMISE |
| Objective | Verbal learning | ||||||||||
| Jamie et al. [54] | America | 50 | Con = 11, age = 56.2 ± 11.3 GE = 20, age = 53.1 ± 10.7 Qigong = 19, age = 52.9 ± 11.9 |
Chemotherapy radiation; ; hormonal-therapy |
Qigong; gentle exercise; |
8 weeks | 7/week | Home-based | QG: 30min | Self-report | FACT-Cog; PROMIS |
| Objective | TMT; Verbal learning |
||||||||||
| Joseph et al. [55] | Australia | 17 | Con = 6, age = 61.5 ± 7.8 MOD = 5, age = 67.8 ± 7.0 HIIT = 6, age = 60.3 ± 8.1 |
Chemotherapy surgery; radiation; hormonal-therapy; |
Cycling, aerobic exercise |
12 weeks | 3/week | Supervised | MOD: 20–30 min at 50% HRR; HIIT: 20–30 min at 105% HRR |
Objective | Executive function, Verbal learning |
| Nilofar et al. [39] | Iran | 40 | Con = 20, age = 51.8 ± 11.4 Ex = 20, age = 51.6 ± 10.5 | Chemotherapy radiation; | Yoga | 8 weeks | 3/week | Supervised, home-based | Unclear | Self-report | EORTC QLQ_C30 |
| Martina et al. [56] | Germany | 101 | Con = 46, age = 53.3 ± 10.2 RE = 49, |
Chemotherapy; surgery; hormonal- |
Machine-based, resistance exercises | 12 weeks | 2/week | Supervised | 60 min: 3 sets of 8–12 repetitions at 60–80% HRR | Self-report | FAQ; EORTC QLQ-C30 |
| Objective | TMT | ||||||||||
| Patricia et al. [57] | Ireland | 37 | Con = 18, age = 56.3 ± 2.0 Ex = 19, age = 53.9 ± 2.3 |
Chemotherapy surgery; radiation; | Exercise | 10 weeks | 1-2/week | Supervised, home-based | 1–5 weeks: 2/week at 65%–85% HRR, 6–10 weeks: 1/week at 65%–85% HRR |
Self-report | EORTC QLQ-C30 |
| Steindorf et al. [58] | Germany | 160 | Con = 78, age = 56.4 ± 8.7 RE = 77, age = 55.2 ± 9.5 |
Chemotherapy; surgery; radiation; | Machine-based, resistance exercise | 12 weeks | 2/week | Supervised | 60 min: 3 sets, 8–12 reps at 60%–80% HRR | Self-report | FAQ; EORTC QLQ-C30 |
| Objective | TMT | ||||||||||
| Vadiraja et al. [59] | India | 88 | Con = 44, age = 48.5 ± 10.2 Yoga = 44, age = 46.7 ± 9.3 |
Chemotherapy; surgery; radiation; |
Yoga | 6 weeks | 7/week | Supervised, home-based | YOGA:60 min: | Self-report | EORTC QLQ-C30 |
Con, control; CFQ, Cognitive Failures Questionnaire; Ex, exercise; EORTC-QLQ, The European Organisation for Research and Treatment of Cancer quality of life questionnaire; FACT-Cog, Functional Assessment of Cancer Therapy-Cognitive Function; FAQ, Fatigue Assessment Questionnaire; HIIT, High-intensity interval training; HRR, heart rate reserve; MOD, moderate; PFS, Piper fatigue scale; PROMIS, Patient-Reported Outcomes Measurement Information System scales; QG/TCE, Qigong/Tai Chi Easy intervention; REP, repetitions; SQG, Sham Qigong Control; TMT, Trail Making A/B test; UC, usual care.
One trial may include 1 or 2 interventions. Kate conducted a study with two interventions: the AT-HIIT (Concurrent aerobic high-intensity interval training and continuous moderate-intensity aerobic exercise training) and RT-HIIT (Concurrent aerobic high-intensity interval training and resistance exercise training) [50,51]. Jamie's trail consisted of two interventions, Qigong and gentle exercise, while Joseph's interventions were moderate and high intensity exercise [54]. Overall, the most common physical exercise was aerobic exercise (n = 6), followed by yoga (n = 2), qigong (n = 1) and combination exercise (aerobic and resistance exercise, n = 2), and resistance exercise (n = 1). The total duration of intervention ranged from 6 to 24 weeks, with 8–12 weeks selected for most trials. The exercise frequency of the majority of trials were 12 weeks, with 2–3 times per week as the most frequent, and some experiments also required exercise every day. Each exercise duration ranged from 20 min to 90 min, 30–60 min was the most popular. Some trials stipulated that the intensity of exercise was 60–80% (n = 6) [36,[50], [51], [52],[56], [57], [58]], and some experiments were not reported in detail (n = 5) [6,37,39,53,54,59]. More than half of the trials had permanent staff supervising survivors to complete the exercise at a fixed location (n = 9) [36,39,[50], [51], [52],[55], [56], [57], [58], [59]], and others were supervised with a home-based (n = 3) [6,37,53,54].
3.3. Quality assessment
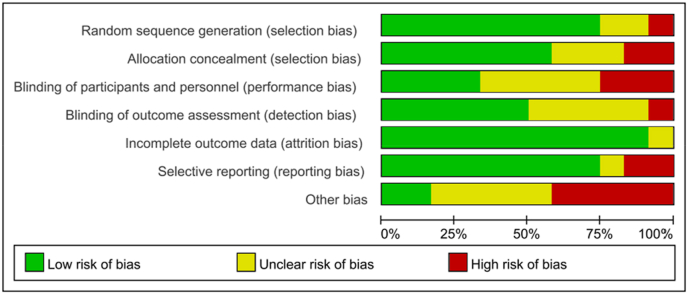
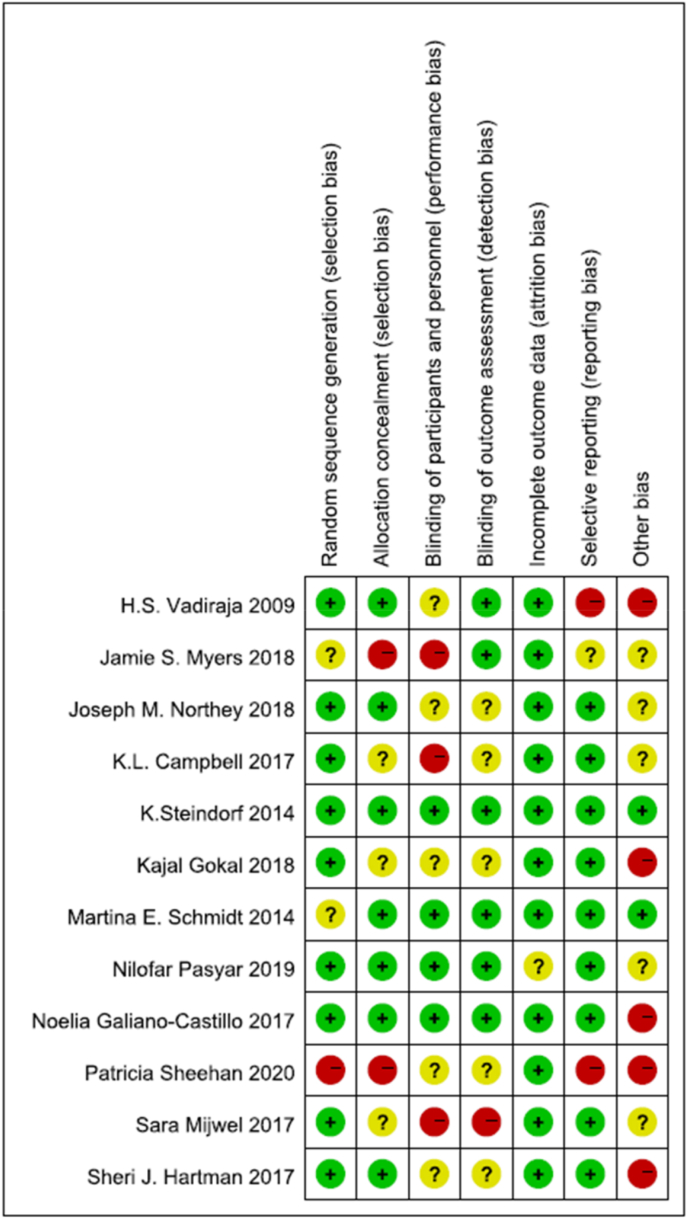
Risk of bias are shown for all included studies in Fig. 2 and for each study in Fig. 3. In general, the risk of random sequence generation (n = 9), full reporting of results (n = 11), and selection offset (n = 9) for most trials were low. 10 trials described the generation of random sequences in detail, used the computer automatic sequence generation method (n = 5), permutated blocks (n = 3) and random number table (n = 1). 8 trials were allocated in appropriate ways, those random numbers were grouped in sealed opaque envelopes (n = 2), or randomly grouped to ensure participants were comparable by block randomization method (n = 5) or random list numbers (n = 1). Due to the specific nature of exercise interventions, some studies did not blind to investigators or participants. Only 4 trials blinded survivors and participants and 6 of the trials also blinded the outcome measurement. 4 trials did not lose participants and 2 did not report the reasons for the loss of follow-up, 6 trials had the following reasons: 1) death, 2) cancer recurrence, 3) changes in treatment, 4) side effects of chemotherapy, 5) personal scheduling conflicts. And there were 4 trials performed the intent-to-treat analysis. 5 of the trials may have high risk of other bias, possibly caused by the higher educational level of the study population, the participants’ preference for sports, and the fact that the sports data were not obtained through objective means.
Fig. 2.
Risk of bias graph.
Fig. 3.
Risk of bias summary.
Overall, the included studies were of moderate quality with an average score of 5.33 (SD = 0.42),the results are shown in Appendices C. and the distribution of the quality scores was generally normal. No studies had a quality score of zero, one or two, two trials had a low score of three, three had a medium score, seven had above average scores, most of the articles had a quality score of 6 (n = 6), and only one trial met all eight quality criteria. Therefore, we determined that this meta-analysis was of moderate quality.
3.4. Effects of physical exercise on cognitive function
3.4.1. Self-report cognitive function
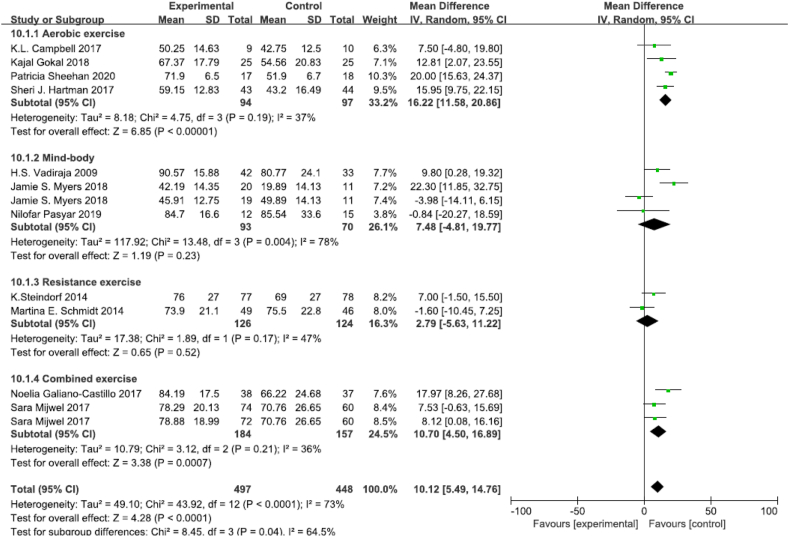
A total of 12 RCTs of self-reported cognitive function, which involving 497 participants in physical exercise group and 448 participants in control group, including 6 aerobic exercise, 3 mind-body exercise, 2 combined aerobic exercise and resistance exercise, and 1 resistance exercise (Fig. 4) [6,36,37,39,50,51,54,[56], [57], [58], [59]]. 8 trials used the self-reported EORTC QLQ-C30 (The European Organisation for Research and Treatment of Cancer quality of life questionnaire) cognitive function subscale [6,37,39,50,51,[57], [58], [59]], 2 trials used the FACT-Cog (Functional Assessment of Cancer Therapy-Cognitive Function) subscale [52,54], and 2 trials used other self-reported scales [36,54]. The meta-analysis showed that exercise intervention improved cognitive function sores in breast cancer survivors (MD 10.12, 95% CI [5.49,14.76], p < 0.0001). Subgroup analysis was conducted to reduce clinical heterogeneity, and the results showed that aerobic exercise could improve cognitive function (MD 16.22, 95% CI [11.58,20.86], p < 0.00001), but body-mind exercise, resistance exercise and combined exercise did not significantly improve cognitive function (MD 7.48, 95% CI [−4.81,19.77], p = 0.23; MD 2.76, 95% CI [−5.63,11.22], p = 0.11; MD 10.70, 95% CI [4.50,16.89], p = 0.0007). Sensitivity analysis showed that the results were stable and reliable.
Fig. 4.
Effects of physical exercise training on self-reported cognition.
3.4.2. Self-report cognitive fatigue
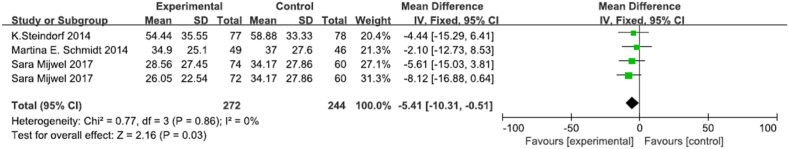
A total of 4 RCTs of self-reported cognitive fatigue, which involving 272 participants in exercise group and 244 participants in controll group (Fig. 5) [50,51,56,58]. Of the 2 trials used the PFS (Piper Fatigue Scale) [50,51], 2 trials used the FAQ (Fatigue Assessment Questionaire) [56,58]. The meta-analysis showed that there was low heterogeneity among studies for cognitive fatigue (I2 = 0%, p = 0.86), and exercise significantly improve cognitive fatigue in breast cancer survivors (MD -5.41, 95% [CI -10.31,-0.51], p = 0.03). Sensitivity analysis showed that the results were stable and reliable.
Fig. 5.
Effects of physical exercise training on self-report cognitive fatigue.
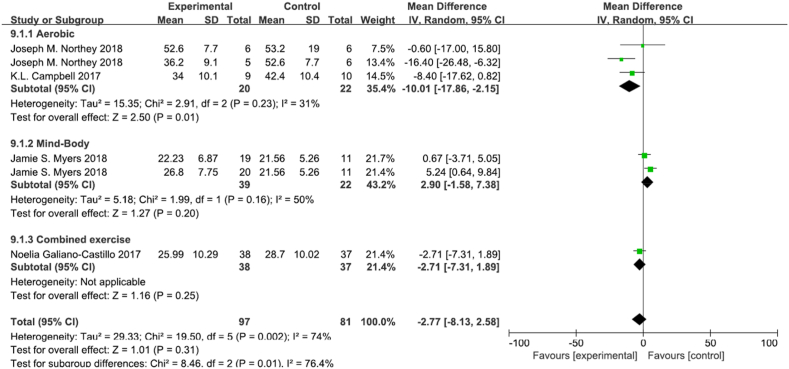
3.4.3. Processing speed
In 6 trials, there were 97 participants in the experimental group and 81 participants in the control group in which TMT-A was objectively neuropsychological (Fig. 6, Fig. 7) [6,37,52,54,55]. The results showed that the exercise intervention did not improve the overall processing speed of breast cancer survivors (MD -2.77, 95% CI [−8.13,2.58], p = 0.31). However, subgroup analysis was performed and showed that aerobic exercise improved cognitive function (MD -10.01, 95% CI [−17.86,-2.15], p = 0.01), but both mind-body exercise and combined exercise did not significantly improve cognitive function (MD 2.90, 95% CI [−1.58,7.38], p = 0.20; MD -2.71, 95% CI [−7.31,1.89], p = 0.25). Sensitivity analysis showed that the results were stable and reliable.
Fig. 6.
Effects of physical exercise training on processing speed.
Fig. 7.
Effects of physical exercise training on executive function.
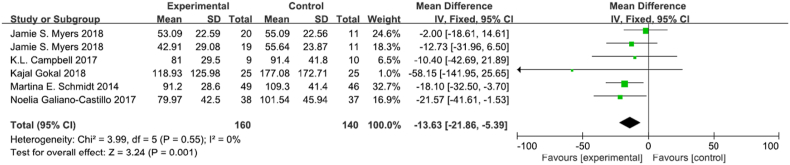
3.4.4. Executive function
TMT-B was tested in 6 trials, there were 160 participants in the experimental group and 140 participants in the control group (Fig. 8) [6,37,[50], [51], [52],54,56]. There was no heterogeneity in executive function among studies (I2 = 0%, p = 0.55) Meta-analysis showed that exercise intervention significantly improved cognitive function in breast cancer survivors (MD -13.63, 95% CI [−21.86,-5.39], p = 0.0001). Sensitivity analysis showed that the results were stable and reliable.
Fig. 8.
Effects of physical exercise training on verbal memory.
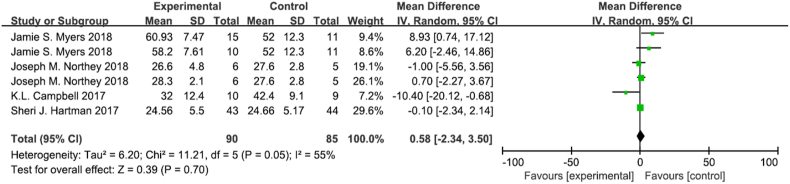
3.4.5. Verbal memory
Verbal memory was tested in 6 trials, there were 90 participants in the experimental group and 85 participants in the control group (Fig. 8) [36,52,54,55]. There was moderate heterogeneity among verbal memory studies (I2 = 55%, p = 0.05) Meta-analysis showed that exercise intervention did not significantly improve cognitive function in breast cancer survivors (MD 0.58, 95% CI [−2.34,3.50], p = 0.70). Sensitivity analysis showed that the results were stable and reliable.
4. Discussion
This meta-analysis evaluated the effects of physical exercise on overall self-reported cognitive function in breast cancer survivors, and then explored the effects of self-reported cognition, cognitive fatigue, processing speed, executive function, and verbal memory. A total of 12 RCTs published between 2009 and 2021 were included in the meta-analysis.
The results of this meta-analysis highlight the potential of physical exercise to positively affect overall self-reported cognitive function, cognitive fatigue and executive function in breast cancer survivors. The significant effects of aerobic exercise and combined exercise intervention on self-cognition report was found. These results suggested that physical exercise, especially moderate to high-intensity aerobic exercise and combined exercise, can improve overall cognitive function. However, the current results did not show significant effects of exercise interventions on specific cognitive areas of processing speed and verbal memory.
4.1. Interpretation of results and comparison with previous research
To our knowledge, this is the first meta-analysis to examine the impact of physical exercise intervention on cognitive function in breast cancer survivors. Campbell et al. evaluated the benefits of physical exercise on cognitive function in cancer survivors in a systematic review [60]. Twelve of the 29 trials reported a significant effect of physical exercise on self-reported cognitive function. Importantly, only three of the 10 trials that used neurocardiological tests to assess cognitive function showed a significant effect of physical exercise, and only the three trials used cognitive function as the primary outcome. This meta-analysis confirmed the results of Campbell's study, which used self-report and objective tests to evaluate the cognitive function of breast cancer survivors and determine the efficacy of exercise intervention on the cognitive function. The underlying mechanisms between physical exercise and cognitive function are as follows: firstly, exercise can induce the increase of neurotrophic factors, neurotransmitters and enzymes (such as brain-derived neurotrophic factors, dopamine, tyrosine hydroxylase) to promote nerve growth and development [27]. Secondly, both animal studies and human studies have shown that exercise contributes to the growth of the prefrontal cortex and hippocampus, and promotes cerebrovascular generation by enhancing cerebral perfusion, especially when physical exercise is combined with an enriched environment [29]. Thirdly, physical exercise is effective in enhancing cognitive function by regulating blood sugar, inflammation and hormone levels [30]. Although there are many mechanisms between physical exercise and cognitive function, further studies are needed to explore regulatory pathways among them. Future studies should use objective tests or other more accurate methods to detect subtle changes in cognitive function in breast cancer survivors, and explore the regulation of physical exercise and cognitive functions.
The current results are comparable to a recent systematic review by Erickson et al. [30]. They investigated the effects of physical exercise interventions investigated the effects of physical exercise intervention on cognitive function throughout the life cycle and found that moderate-to-vigorous physical exercise improved cognitive function in healthy individuals and even patients with cognitive impairment. Studies in their specific field have shown that exercise interventions can improve processing speed, memory and executive function, which are partly similar to the results of the current study. Our study did not show any significant effects on processing speed and verbal memory, but aerobic exercise improved processing speed in breast cancer patients, which consistent with Smith's findings [61]. Some studies suggested that combined exercise improves memory in patients more than exercise alone, explained by changes in brain structure, brain function and brain connectivity [62]. The reason for the different results may be that included studies that involved different intensities and different modes of exercise [38]. This emphasizes the need for more detailed and accurate research into specific cognitive domains.
A meta-analysis by Northey suggested that physical exercise improves cognitive function in adults older than 50 [38]. They found that interventions of aerobic exercise, resistance training and tai chi, all had significant effects on cognitive function, with each exercise lasting 45–60 min and at least moderate intensity having cognitive benefits, which similar to those observed in the current study. And the American Heart Association recommends 70% HRR exercise of more than 30 min three times a week, or 150 min of moderate-intensity exercise and 75 min of high-intensity exercise per week to prevent cognitive decline. Participants in seven of the 12 RCTS included in the current study participated in 30–60 min of resistance/aerobic exercise at 50–80% HRR, However, our results need to be interpreted with caution, since the results of tai Chi and resistance training were based on two trials. Assessing more modes of physical exercise should be a the focus of future research.
4.2. Strengths and limitations
There are several advantages in this study. This meta-analysis is the first systematic review to evaluate the effect of physical exercise on cognitive function in breast cancer survivors, and found that physical exercise can improve cognitive function in breast cancer survivors. Second, only RCT studies were included in this meta-analysis. Third, this study adopted a combination of subjective and objective measurements, provided stronger evidence of beneficial effects of physical exercise on cognitive function in breast cancer survivors. Nevertheless, this study has some limitations. Firstly, the meta-analysis only searched studies published in English, which may lead to incomplete literature review. Secondly, this study included 12 studies, and some of which had varying degrees of heterogeneity. In addition, due to publication bias, this study may be biased towards positive results. Therefore, the results of study need to be cautiously interpreted. Thirdly, this study lacks the exploration of exercise parameters, such as duration and frequency. Furthermore, this meta-analysis didn't provide evidence that physical exercise affects other cognitive functions, such as attention, working memory, visuospatial ability and so on, further studies need explore other areas of cognitive function.
4.3. Implications for future research
Future studies are needed to investigate different patterns and durations of physical exercise. We recommend a multi-arm design that includes single exercise training, combined exercise training, and a control group to distinguish the contributions of different exercise types. In addition, future research should focus on using objective measures such as neuropsychological tests to examine the positive effects of physical exercise. Finally, it is important to detect exercise modes in different cognitive domains. And different physical exercises can provide different kinds of stimulation, including duration, frequency, intensity and type or type of physical exercise. Moreover, Den Heijer's study has shown that physical exercise at a young age can better promote the proliferation and positive response of nerve cells [28], and Fabel's animal experiments have shown that physical activity combined with a rich environment can induce hippocampal neurogenesis [63], suggesting that the timing and combination of physical exercise interventions should also be the focus of future research. In conclusion, more objective measures are needed to confirm the effects of physical exercise on cognitive function in the future.
5. Conclusion
This meta-analysis found that physical exercise can improve cognitive function in breast cancer survivors, particularly in self-reported cognitive function, cognitive fatigue and executive function, but did not seem to improve processing speed and verbal memory. There was methodological heterogeneity in included study samples, thus the results of the present study need to be cautiously interpreted. Meanwhile, this meta-analysis showed that aerobic exercise and combined exercise were more effective than resistance exercise, mind-body exercise. Physical therapists can start exercise interventions earlier, and combine exercise with other measures to be more effective in reducing cognitive decline in breast cancer survivors. Future studies need to focus on the effect of physical exercise on cognitive function, which can be explored from the frequency and duration of physical exercise.
Author contributions
Xiaohan Ren: contributed to conception, design of the work, the acquisition and analysis of data, writing the original draft, and review and editing of the paper. Xiaoqin Wang: contributed to writing the original draft, and review and editing of the paper. Jiaru Sun, Zhaozhao Hui, Shuangyan Lei, Caihua Wang: contributed to the acquisition and analysis of data, review the paper. Mingxu Wang: contributed to review and editing of the paper. All authors read and approved the final manuscript.
Funding statement
No external funding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.03.014.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Narod S.A., Iqbal J., Miller A.B. Why have breast cancer mortality rates declined? J Cancer Pol. 2015;5:8–17. [Google Scholar]
- 3.Britt K.L., Cuzick J., Phillips K.A. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20(8):417–436. doi: 10.1038/s41568-020-0266-x. [DOI] [PubMed] [Google Scholar]
- 4.Hu K., Ding P., Wu Y., Tian W., Pan T., Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open. 2019;9(10) doi: 10.1136/bmjopen-2018-028461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenlee H., DuPont-Reyes M.J., Balneaves L.G., Carlson L.E., Cohen M.R., Deng G., Johnson J.A., Mumber M., Seely D., Zick S.M., et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA A Cancer J Clin. 2017;67(3):194–232. doi: 10.3322/caac.21397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galiano-Castillo N., Cantarero-Villanueva I., Fernández-Lao C., Ariza-García A., Díaz-Rodríguez L., Del-Moral-Ávila R., Arroyo-Morales M. Telehealth system: a randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer. 2016;122(20):3166–3174. doi: 10.1002/cncr.30172. [DOI] [PubMed] [Google Scholar]
- 7.Lovelace D.L., McDaniel L.R., Golden D. Long-term effects of breast cancer surgery, treatment, and survivor care. J Midwifery Wom Health. 2019;64(6):713–724. doi: 10.1111/jmwh.13012. [DOI] [PubMed] [Google Scholar]
- 8.Lange M., Joly F., Vardy J., Ahles T., Dubois M., Tron L., Winocur G., De Ruiter M.B., Castel H. Cancer-related cognitive impairment: an update on state of the art, detection, and management strategies in cancer survivors. Ann Oncol. 2019;30(12):1925–1940. doi: 10.1093/annonc/mdz410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohler C., Chang M., Allemann-Su Y.Y., Vetter M., Jung M., Jung M., Conley Y., Paul S., Kober K.M., Cooper B.A., et al. Changes in attentional function in patients from before through 12 Months after breast cancer surgery. J Pain Symptom Manag. 2020;59(6):1172–1185. doi: 10.1016/j.jpainsymman.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wefel J.S., Vardy J., Ahles T., Schagen S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703–708. doi: 10.1016/S1470-2045(10)70294-1. [DOI] [PubMed] [Google Scholar]
- 11.Joly F., Giffard B., Rigal O., De Ruiter M.B., Small B.J., Dubois M., LeFel J., Schagen S.B., Ahles T.A., Wefel J.S., et al. Impact of cancer and its treatments on cognitive function: advances in research from the Paris international cognition and cancer task force symposium and update since 2012. J Pain Symptom Manag. 2015;50(6):830–841. doi: 10.1016/j.jpainsymman.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 12.Oberste M., Schaffrath N., Schmidt K., Bloch W., Jäger E., Steindorf K., Hartig P., Joisten N., Zimmer P. Protocol for the "Chemobrain in Motion - study" (CIM - study): a randomized placebo-controlled trial of the impact of a high-intensity interval endurance training on cancer related cognitive impairments in women with breast cancer receiving first-line chemotherapy. BMC Cancer. 2018;18(1):1071. doi: 10.1186/s12885-018-4992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vichaya E.G., Chiu G.S., Krukowski K., Lacourt T.E., Kavelaars A., Dantzer R., Heijnen C.J., Walker A.K. Mechanisms of chemotherapy-induced behavioral toxicities. Front Neurosci. 2015;9:131. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yabroff K.R., Lawrence W.F., Clauser S., Davis W.W., Brown M.L. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96(17):1322–1330. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 15.Carroll J.E., Van Dyk K., Bower J.E., Scuric Z., Petersen L., Schiestl R., Irwin M.R., Ganz P.A. Cognitive performance in survivors of breast cancer and markers of biological aging. Cancer. 2019;125(2):298–306. doi: 10.1002/cncr.31777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ganz P.A., Kwan L., Castellon S.A., Oppenheim A., Bower J.E., Silverman D.H., Cole S.W., Irwin M.R., Ancoli-Israel S., Belin T.R. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahles T.A., Root J.C., Ryan E.L. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joly F., Lange M., Dos Santos M., Vaz-Luis I., Di Meglio A. Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers. 2019;11(12) doi: 10.3390/cancers11121896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahles T.A., Root J.C. Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol. 2018;14:425–451. doi: 10.1146/annurev-clinpsy-050817-084903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boykoff N., Moieni M., Subramanian S.K. Confronting chemobrain: an in-depth look at survivors' reports of impact on work, social networks, and health care response. J Cancer Survivorship Res Pract. 2009;3(4):223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spronk I., Burgers J.S., Schellevis F.G., van Vliet L.M., Korevaar J.C. The availability and effectiveness of tools supporting shared decision making in metastatic breast cancer care: a review. BMC Palliat Care. 2018;17(1):74. doi: 10.1186/s12904-018-0330-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholas Z., Butow P., Tesson S., Boyle F. A systematic review of decision aids for patients making a decision about treatment for early breast cancer. Breast. 2016;26:31–45. doi: 10.1016/j.breast.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Campbell K.L., Zadravec K., Bland K.A., Chesley E., Wolf F., Janelsins M.C. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys Ther. 2020;100(3):523–542. doi: 10.1093/ptj/pzz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellens A., Roelant E., Sabbe B., Peeters M., van Dam P.A. A video-game based cognitive training for breast cancer survivors with cognitive impairment: a prospective randomized pilot trial. Breast. 2020;53:23–32. doi: 10.1016/j.breast.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boscher C., Joly F., Clarisse B., Humbert X., Grellard J.M., Binarelli G., Tron L., Licaj I., Lange M. Perceived cognitive impairment in breast cancer survivors and its relationships with psychological factors. Cancers. 2020;12(10) doi: 10.3390/cancers12103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erickson K.I., Hillman C.H., Kramer A.F. Physical activity, brain, and cognition. Curr Opin Behav Sci. 2015;4:27–32. [Google Scholar]
- 27.Wheeler M.J., Green D.J., Ellis K.A., Cerin E., Heinonen I., Naylor L.H., Larsen R., Wennberg P., Boraxbekk C.J., Lewis J., et al. Distinct effects of acute exercise and breaks in sitting on working memory and executive function in older adults: a three-arm, randomised cross-over trial to evaluate the effects of exercise with and without breaks in sitting on cognition. Br J Sports Med. 2020;54(13):776–781. doi: 10.1136/bjsports-2018-100168. [DOI] [PubMed] [Google Scholar]
- 28.Den Heijer A.E., Groen Y., Tucha L., Fuermaier A.B., Koerts J., Lange K.W., Thome J., Tucha O. Sweat it out? The effects of physical exercise on cognition and behavior in children and adults with ADHD: a systematic literature review. J Neural Trans (Vienna, Austria: 1996. 2017;124(Suppl 1):3–26. doi: 10.1007/s00702-016-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karssemeijer E.G.A., Aaronson J.A., Bossers W.J., Smits T., Olde Rikkert M.G.M., Kessels R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: a meta-analysis. Ageing Res Rev. 2017;40:75–83. doi: 10.1016/j.arr.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Erickson K.I., Hillman C., Stillman C.M., Ballard R.M., Bloodgood B., Conroy D.E., Macko R., Marquez D.X., Petruzzello S.J., Powell K.E., et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. 2019;51(6):1242–1251. doi: 10.1249/MSS.0000000000001936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groot C., Hooghiemstra A.M., Raijmakers P.G., van Berckel B.N., Scheltens P., Scherder E.J., van der Flier W.M., Ossenkoppele R. The effect of physical activity on cognitive function in patients with dementia: a meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Law C.K., Lam F.M., Chung R.C., Pang M.Y. Physical exercise attenuates cognitive decline and reduces behavioural problems in people with mild cognitive impairment and dementia: a systematic review. J Physiother. 2020;66(1):9–18. doi: 10.1016/j.jphys.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 33.Práxedes A., Del Villar F., Pizarro D., Moreno A. The impact of nonlinear pedagogy on decision-making and execution in youth soccer players according to game actions. J Hum Kinet. 2018;62:185–198. doi: 10.1515/hukin-2017-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kalyani H.H.N., Sullivan K.A., Moyle G., Brauer S., Jeffrey E.R., Kerr G.K. Impacts of dance on cognition, psychological symptoms and quality of life in Parkinson's disease. NeuroRehabilitation. 2019;45(2):273–283. doi: 10.3233/NRE-192788. [DOI] [PubMed] [Google Scholar]
- 35.Camiré M., Trudel P., Forneris T. Examining how model youth sport coaches learn to facilitate positive youth development. Phys Educ Sport Pedagog. 2014;19(1):1–17. [Google Scholar]
- 36.Hartman S.J., Nelson S.H., Myers E., Natarajan L., Sears D.D., Palmer B.W., Weiner L.S., Parker B.A., Patterson R.E. Randomized controlled trial of increasing physical activity on objectively measured and self-reported cognitive functioning among breast cancer survivors: the memory & motion study. Cancer. 2018;124(1):192–202. doi: 10.1002/cncr.30987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galiano-Castillo N., Arroyo-Morales M., Lozano-Lozano M., Fernández-Lao C., Martín-Martín L., Del-Moral-Ávila R., Cantarero-Villanueva I. Effect of an Internet-based telehealth system on functional capacity and cognition in breast cancer survivors: a secondary analysis of a randomized controlled trial. Support Care Cancer. 2017;25(11):3551–3559. doi: 10.1007/s00520-017-3782-9. [DOI] [PubMed] [Google Scholar]
- 38.Northey J.M., Cherbuin N., Pumpa K.L., Smee D.J., Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–160. doi: 10.1136/bjsports-2016-096587. [DOI] [PubMed] [Google Scholar]
- 39.Pasyar N., Barshan Tashnizi N., Mansouri P., Tahmasebi S. Effect of yoga exercise on the quality of life and upper extremity volume among women with breast cancer related lymphedema: a pilot study. Eur J Oncol Nurs. 2019;42:103–109. doi: 10.1016/j.ejon.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 40.Soriano-Maldonado A., Carrera-Ruiz A., Diez-Fernandez D.M., Esteban-Simon A., Maldonado-Quesada M., Moreno-Poza N., Garcia-Martinez M.D.M., Alcaraz-Garcia C., Vazquez-Sousa R., Moreno-Martos H., et al. Effects of a 12-week resistance and aerobic exercise program on muscular strength and quality of life in breast cancer survivors: study protocol for the EFICAN randomized controlled trial. Medicine. 2019;98(44) doi: 10.1097/MD.0000000000017625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee K., Kang I., Mack W.J., Mortimer J., Sattler F., Salem G., Lu J., Dieli-Conwright C.M. Effects of high-intensity interval training on vascular endothelial function and vascular wall thickness in breast cancer patients receiving anthracycline-based chemotherapy: a randomized pilot study. Breast Cancer Res Treat. 2019;177(2):477–485. doi: 10.1007/s10549-019-05332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.A M., K E., O I., Mb B., Z A D.D. The effect of exercise on life quality and depression levels of breast cancer patients. Asian Pac J Cancer Prev APJCP. 2021;8(3):725–732. doi: 10.31557/APJCP.2021.22.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aaronson N.K.A.S., Bergman B., Bullinger M., Cull A., Duez N.J., Filiberti A., Flechtner H., Fleishman S.B., de Haes J.C., et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(85):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 44.Lai J.S., Butt Z., Wagner L., Sweet J.J., Beaumont J.L., Vardy J., Jacobsen P.B., Shapiro P.J., Jacobs S.R., Cella D. Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manag. 2009;37(6):982–995. doi: 10.1016/j.jpainsymman.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai J.S., Wagner L.I., Jacobsen P.B., Cella D. Self-reported cognitive concerns and abilities: two sides of one coin? Psycho Oncol. 2014;23(10):1133–1141. doi: 10.1002/pon.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cumpston M., Li T., Page M.J., Chandler J., Welch V.A., Higgins J.P., Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cuijpers P., van Straten A., Bohlmeijer E., Hollon S.D., Andersson G. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol Med. 2010;40(2):211–223. doi: 10.1017/S0033291709006114. [DOI] [PubMed] [Google Scholar]
- 48.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 49.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ (Online) 2003;327(7414):7557–7560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolam K.A., Mijwel S., Rundqvist H., Wengström Y. Two-year follow-up of the OptiTrain randomised controlled exercise trial. Breast Cancer Res Treat. 2019;175(3):637–648. doi: 10.1007/s10549-019-05204-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mijwel S., Backman M., Bolam K.A., Jervaeus A., Sundberg C.J., Margolin S., Browall M., Rundqvist H., Wengström Y. Adding high-intensity interval training to conventional training modalities: optimizing health-related outcomes during chemotherapy for breast cancer: the OptiTrain randomized controlled trial. Breast Cancer Res Treat. 2018;168(1):79–93. doi: 10.1007/s10549-017-4571-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campbell K.L., Kam J.W.Y., Neil-Sztramko S.E., Liu Ambrose T., Handy T.C., Lim H.J., Hayden S., Hsu L., Kirkham A.A., Gotay C.C., et al. Effect of aerobic exercise on cancer-associated cognitive impairment: a proof-of-concept RCT. Psycho Oncol. 2018;27(1):53–60. doi: 10.1002/pon.4370. [DOI] [PubMed] [Google Scholar]
- 53.Gokal K., Munir F., Ahmed S., Kancherla K., Wallis D. Does walking protect against decline in cognitive functioning among breast cancer patients undergoing chemotherapy? Results from a small randomised controlled trial. PLoS One. 2018;13(11) doi: 10.1371/journal.pone.0206874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Myers J.S., Mitchell M., Krigel S., Steinhoff A., Boyce-White A., Van Goethem K., Valla M., Dai J., He J., Liu W., et al. Qigong intervention for breast cancer survivors with complaints of decreased cognitive function. Support Care Cancer. 2019;27(4):1395–1403. doi: 10.1007/s00520-018-4430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Northey J.M., Pumpa K.L., Quinlan C., Ikin A., Toohey K., Smee D.J., Rattray B. Cognition in breast cancer survivors: a pilot study of interval and continuous exercise. J Sci Med Sport. 2019;22(5):580–585. doi: 10.1016/j.jsams.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt M.E., Wiskemann J., Armbrust P., Schneeweiss A., Ulrich C.M., Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer. 2015;137(2):471–480. doi: 10.1002/ijc.29383. [DOI] [PubMed] [Google Scholar]
- 57.Sheehan P., Denieffe S., Murphy N.M., Harrison M. Exercise is more effective than health education in reducing fatigue in fatigued cancer survivors. Support Care Cancer. 2020;28(10):4953–4962. doi: 10.1007/s00520-020-05328-w. [DOI] [PubMed] [Google Scholar]
- 58.Steindorf K., Schmidt M.E., Klassen O., Ulrich C.M., Oelmann J., Habermann N., Beckhove P., Owen R., Debus J., Wiskemann J., et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer-related fatigue and quality of life. Ann Oncol. 2014;25(11):2237–2243. doi: 10.1093/annonc/mdu374. [DOI] [PubMed] [Google Scholar]
- 59.Vadiraja H.S., Rao M.R., Nagarathna R., Nagendra H.R., Rekha M., Vanitha N., Gopinath K.S., Srinath B.S., Vishweshwara M.S., Madhavi Y.S., et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Compl Ther Med. 2009;17(5–6):274–280. doi: 10.1016/j.ctim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Campbell K.L., Zadravec K., Bland K.A., Chesley E., Wolf F., Janelsins M.C. The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: systematic review of randomized controlled trials. Phys Ther. 2020;100(3):523–542. doi: 10.1093/ptj/pzz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smith P.J., Blumenthal J.A., Hoffman B.M., Cooper H., Strauman T.A., Welsh-Bohmer K., Browndyke J.N., Sherwood A. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosom Med. 2010;72(3):239–252. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nouchi R., Taki Y., Takeuchi H., Sekiguchi A., Hashizume H., Nozawa T., Nouchi H., Kawashima R. Four weeks of combination exercise training improved executive functions, episodic memory, and processing speed in healthy elderly people: evidence from a randomized controlled trial. Age (Dordr) 2014;36(2):787–799. doi: 10.1007/s11357-013-9588-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fabel K., Wolf S.A., Ehninger D., Babu H., Leal-Galicia P., Kempermann G. Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front Neurosci. 2009;3:50. doi: 10.3389/neuro.22.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.