Abstract
Background
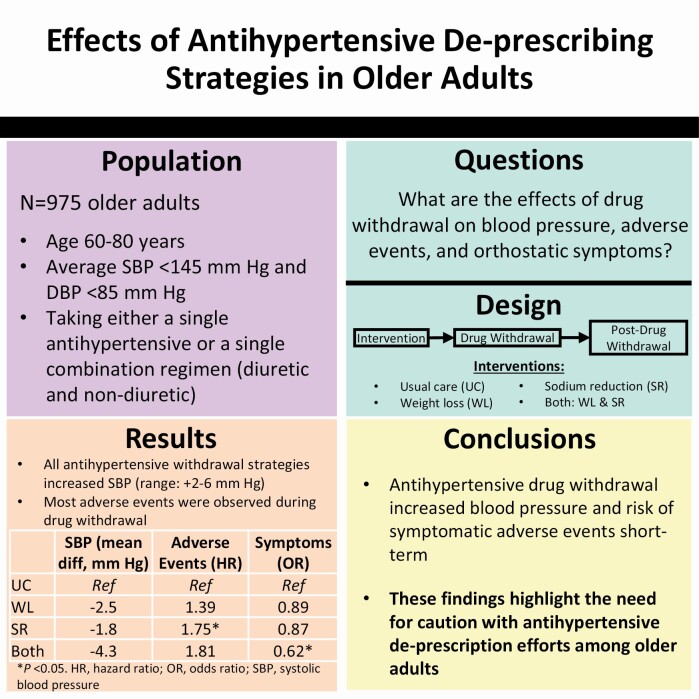
The Trial of Nonpharmacologic Interventions in the Elderly (TONE) demonstrated the efficacy of weight loss and sodium reduction to reduce hypertension medication use in older adults. However, the longer-term effects of drug withdrawal (DW) on blood pressure (BP), adverse events, and orthostatic symptoms were not reported.
Methods
TONE enrolled adults, ages 60–80 years, receiving treatment with a single antihypertensive and systolic BP (SBP)/diastolic BP <145/<85 mm Hg. Participants were randomized to weight loss, sodium reduction, both, or neither (usual care) and followed up to 36 months; ~3 months postrandomization, the antihypertensive was withdrawn and only restored if needed for uncontrolled hypertension. BP and orthostatic symptoms (lightheadedness, feeling faint, imbalance) were assessed at randomization and throughout the study. Two physicians independently adjudicated adverse events, masked to intervention, classifying symptomatic (lightheadedness, dizziness, vertigo), or clinical events (fall, fracture, syncope).
Results
Among the 975 participants (mean age 66 years, 48% women, 24% black), mean (±SD) BP was 128 ± 9/71 ± 7 mm Hg. Independent of assignment, DW increased SBP by 4.59 mm Hg (95% confidence interval [CI]: 3.89, 5.28) compared with baseline. There were 113 adverse events (84 symptomatic, 29 clinical), primarily during DW. Compared with usual care, combined weight loss and sodium reduction mitigated the effects of DW on BP (β = −4.33 mm Hg; 95% CI: −6.48, −2.17) and reduced orthostatic symptoms long term (odds ratio = 0.62; 95% CI: 0.41, 0.92), without affecting adverse events (hazard ratio = 1.81; 95% CI: 0.90, 3.65). In contrast, sodium reduction alone increased risk of adverse events (hazard ratio = 1.75; 95% CI: 1.04, 2.95), mainly during DW.
Conclusions
In older adults, antihypertensive DW may increase risk of symptomatic adverse events, highlighting the need for caution in withdrawing their antihypertensive medications.
Clinical trials registration
Trial Number NCT00000535.
Keywords: blood pressure, deprescription, falls, hypertension, sodium reduction, weight loss
Graphical Abstract
Hypertension and its treatment are common among older adults1,2 and have been thought to contribute to adverse events such as falls.3 Effective blood pressure (BP) treatment often requires the combination of multiple classes of BP agents,4 an approach recommended by hypertension management guidelines.5 Nevertheless, emerging evidence has promoted the practice of deprescribing medications as advantageous to avoid adverse drug events and financial and logistic burdens of polypharmacy among older adults.6,7 However, the impact and safety of withdrawing antihypertensive medications in older adults are understudied.8
The Trial of Nonpharmacologic Interventions in the Elderly (TONE) was a randomized controlled trial of hypertension medication withdrawal that tested distinct nonpharmacologic replacement strategies (weight loss and sodium reduction) in community-dwelling older adults prescribed a single antihypertensive medication.9,10 Ultimately, TONE demonstrated that weight loss and sodium reduction, alone or combined, were effective in controlling hypertension compared with usual care over 3 years of follow-up.10 Moreover, withdrawal of antihypertensive medication did not increase cardiovascular disease (CVD) events.11 However, the impact of drug withdrawal (DW) on BP control and the safety of DW were not fully examined.12
Our objectives were to determine the impact of antihypertensive deprescription on (i) BP, (ii) the composite outcome of ad hoc reported adverse clinical and symptomatic events, and (iii) monitored participant-reported orthostatic symptoms. We hypothesized that during medication withdrawal, sodium reduction and weight loss, alone or combined, would maintain BP control without adversely impacting adverse events or participant-reported symptoms.
METHODS
The data underlying this study will be shared upon reasonable request to the corresponding author.
TONE was an investigator-initiated study conducted between August 1992 and June 1994 at 4 academic health centers in Maryland, New Jersey, North Carolina, and Tennessee and supported by the National Institute on Aging and National Heart, Lung, and Blood Institute.9,10 In brief, TONE examined weight loss or sodium reduction as nonpharmacologic replacement strategies for pharmacologic hypertension treatment in older adults. Institutional Review Boards at each institution approved the original study design and protocol. All participants provided written, informed consent.
Participants
TONE enrolled 975 older adults, aged 60–80 years with an average systolic BP (SBP) <145 mm Hg and an average diastolic BP (DBP) <85 mm Hg while taking either a single antihypertensive medication or a single combination regimen (diuretic and nondiuretic). Adults with a heart attack or stroke in the preceding 6 months, congestive heart failure, insulin-dependent diabetes, or a serum creatinine >2.0 mg/dl were excluded.9,10
In the original trial, participants were followed up to 36 months or until they experienced a primary end point, namely, (i) high BP at 1 or more TONE study visits following attempted withdrawal of the antihypertensive medication, (ii) treatment with an antihypertensive medication, or (iii) occurrence of a clinical CVD complication during follow-up (myocardial infarction, angina, congestive heart failure, stroke, coronary artery bypass surgery, or coronary artery angioplasty).9,10 The high BP criteria used to define the primary endpoint of the trial were (i) a mean SBP of ≥190 mm Hg or DBP of ≥110 mm Hg at a single visit (based on 3 BP measurements), (ii) a mean SBP of ≥170 mm Hg or DBP of ≥100 mm Hg over 2 sequential visits (6 BP measurements), or (iii) a mean SBP of ≥150 mm Hg or DBP ≥90 mm Hg or greater over 3 sequential visits (9 BP measurements). Determination of study endpoints was based on an independent committee masked to intervention assignment.
Nonpharmacologic drug replacement strategies
Following a factorial design, TONE investigators randomized overweight or obese participants to combined sodium reduction and weight loss, weight loss alone, sodium reduction alone, or usual care (neither intervention), while nonoverweight participants were randomized to sodium reduction or usual care. The interventions were delivered through behavioral counseling administered in small group and individual meetings with nutrition and exercise counselors. The weight loss strategy focused on achieving and maintaining 4.5 kg of weight loss throughout the study period, and focused on both Calorie restriction and increased physical activity. The sodium reduction strategy provided education on low-sodium food patterns to reduce intake to less than 1,800 mg of sodium per day. Those assigned usual care received no lifestyle counseling. Interventions were delivered in 3 phases: intensive (initial 4 months; focused on skill acquisition), extended (next 4 months; focused on problem solving), and maintenance (up to 28 weeks; focused on longitudinal engagement). Interventions were customized to participant needs over time.
Approximately 3 months after the first group session (range 76–104 days), all participants underwent an attempt at antihypertensive medication withdrawal using drug-specific tapering regimens. During this time, participants were evaluated weekly and then biweekly to ensure SBP was <150 mm Hg and DBP <90 mm Hg.
BP measurement
BP was measured during the randomization visit (prior to intervention initiation) and every 3 months throughout the study. During the DW phase (months 3–6), BP was measured weekly and then biweekly. BP could also be measured ad hoc as part of monitoring visits to follow-up on concerning measurements. These ad hoc visits were grouped with the nearest scheduled visit. BP was measured 3 times in the seated position after a 5-minute wait by trained observers using Hawksley random-zero sphygmomanometers defined by the first (for SBP) and fifth (for DBP) Korotkoff sounds. We used the following groupings of visits to indicate study phase: randomization visits (the visit at or just before the randomization date), visits after randomization and up through the first DW visit, visits after the first DW visit up through the 9-month visit, the 12- to 18-month visits, and the 21- through 36-month visits.
Adverse symptomatic or clinical events
During the trial, adverse symptomatic or clinical events were reported either during scheduled visits or ad hoc (i.e., by contacting clinical sites between scheduled visits at the discretion of the participant) and documented by study physicians in event logs. The date of each event was recorded. For this analysis, 2 physicians (S.P.J. and J.L.C.) blinded to randomized assignment separately reviewed the event logs, classifying events as the composite of symptomatic events (dizziness, lightheadedness, or blacking out/presyncope) or clinical events (fall, fracture, or syncope). If both symptoms and clinical events were present, these events were classified as clinical events. Discrepancies were adjudicated by a third independent physician (K.J.M.). Our study focused on the time-to-event after the date the nonpharmacologic replacement strategies were initiated and included recurrent events. The number of events was tabulated according to study phase, as described previously.
Scheduled participant-reported orthostatic symptoms
Distinct from event adjudication, participants underwent scheduled assessments where they were asked about orthostatic symptoms. This occurred during the randomization visit, DW visits (up to as many as 3 visits), and the 6-, 9-, 18-, and 30-month visits. Participants were asked about feeling lightheaded when standing up, faintness or dizziness at rest, and balance problems (never, mild, moderate, severe). These symptoms were treated as dichotomous variables (present or absent) as well as a composite variable (any of the 3 vs. none of the 3). We included monitoring visits or additional DW visits as available provided they occurred after randomization. These ad hoc visits were grouped with the nearest occurring visit, as previously described.
Other covariates
Participants reported age, sex, and race (black, non-black). Body mass index (kg/m2) was derived from measured height and weight, and obesity was defined as a body mass index ≥30 kg/m2. Diabetes history (yes or no) and smoking status (current, yes or no) were self-reported.
Statistical analysis
We described baseline population characteristics by DW assignment using means (SD) and proportions.
Blood pressure
We used generalized estimating equations (GEE) to estimate change in BP over time (GEE using a normal family and identity link). These models were adjusted for baseline obesity status and field center and used a treatment-by-study phase interaction term to determine change in BP compared with baseline and to compare across randomized assignment at specific study phases. Values of the study phase were based on the mean follow-up time for each grouping of visits (0 for the randomization visit, 3.8 for pre-DW, 5.9 for post-DW through the 9-month visits, 15.2 for the 12- to 18-month visits, and 25.7 for the 21- to 36-month visits). Overall effects between assignments were determined using models with a follow-up variable (0 for the randomization visit or 1 for all visits after randomization) in place of a study phase variable. Comparisons were performed relative to baseline or the usual care assignment. The interventions were compared individually (weight loss, sodium reduction, or weight loss and sodium reduction) and according to TONE’s factorial design (weight loss [yes/no] or sodium reduction [yes/no]).
Adverse events
We examined incident and recurrent adverse events reported after randomization. Cumulative incidence over time was visualized via a cumulative incidence curve according to randomized assignment. We determined the incidence rate per 10 person-years of a composite of adverse events (symptomatic events and falls or fracture or syncope) according to randomized assignment (individual and factorial) using Poisson regression, adjusted for baseline obesity status and field center. We determined the hazard ratio of adverse events according to randomized assignment (individual and factorial) using Cox proportional hazards models, following the Andersen–Gill approach for multiple failure survival analysis.13,14 The proportionality assumption for the primary outcome was assessed using Schoenfeld residuals (estat phtest command). Poisson and Cox models were repeated for symptomatic events, clinical events, or falls alone.
Monitored participant-reported orthostatic symptoms
We used GEE to estimate the proportion feeling lightheaded when standing up, faintness or dizziness at rest, and balance problems, or any of these 3 symptoms over time by determining the mean of each symptom as a binary outcome (GEE with normal family, identity link). These models were adjusted for baseline obesity status and field center and used a treatment-by-study phase interaction term to determine change compared with baseline across randomized assignment at each study phase. Values of the study phase were based on the mean follow-up time for each grouping of visits (0 for the randomization visit, 3.9 for pre-DW, 7.2 for post-DW through the 9-month visits, 18.3 for the 18-month visit, and 30.3 for the 30-month visit). We also examined the effect of the interventions on symptoms postrandomization using GEE (binomial family, logit link) adjusted for baseline obesity status, field center, and a treatment-by-follow-up variable (0 for randomization visit or 1 for all visits after randomization) in place of a study phase variable, using the usual care assignment as the reference group. The interventions were compared with the usual care group individually (i.e., weight loss vs. usual care, sodium reduction vs. usual care, or weight loss and sodium reduction vs. usual care) and according to TONE’s factorial design (i.e., weight loss vs. usual care or sodium reduction vs. usual care).
All analyses were performed with Stata version 15.1 (Stata Corporation, College Station, TX). A 2-tailed P value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Baseline characteristics of the participants of TONE are shown in Table 1. Differences in weight status were expected, given the trial design. Otherwise, there were minimal differences across the randomized groups.
Table 1.
Baseline characteristics of trial participants overall and according to randomized assignment
| Overall, N = 975 | Usual care, N = 341 | Weight loss, N = 147 | Sodium reduction, N = 340 | Combination, N = 147 | |
|---|---|---|---|---|---|
| Age, mean (SD), y | 65.8 (4.6) | 65.9 (4.5) | 65.8 (4.7) | 65.8 (4.6) | 65.7 (4.8) |
| Women, % | 47.8 | 46.9 | 51.0 | 49.1 | 43.5 |
| Black race, % | 23.6 | 24.0 | 26.5 | 21.8 | 23.8 |
| Systolic blood pressure, mm Hg | 128.2 (9.3) | 127.6 (9.4) | 129.1 (9.1) | 128.3 (9.4) | 128.4 (9.4) |
| Diastolic blood pressure, mm Hg | 71.4 (7.3) | 71.3 (7.2) | 71.5 (7.8) | 71.3 (7.4) | 71.5 (6.8) |
| Normal blood pressurea, % | 17.1 | 18.2 | 15.0 | 17.4 | 16.3 |
| Obesity, % | 60.0 | 43.1 | 100.0 | 42.4 | 100.0 |
| Diabetes, % | 4.2 | 3.6 | 4.1 | 5.4 | 2.7 |
| Current smoking, % | 5.3 | 5.9 | 2.7 | 5.3 | 6.8 |
aSystolic blood pressure <120 mm Hg and diastolic blood pressure <80 mm Hg. Only 963 participants provided baseline diabetes information; thus, the denominators for diabetes across randomized assignments were 334 for usual care, 146 for weight loss, 336 for sodium reduction, and 147 for the combination of weight loss and sodium reduction.
Effects of study phase on BP
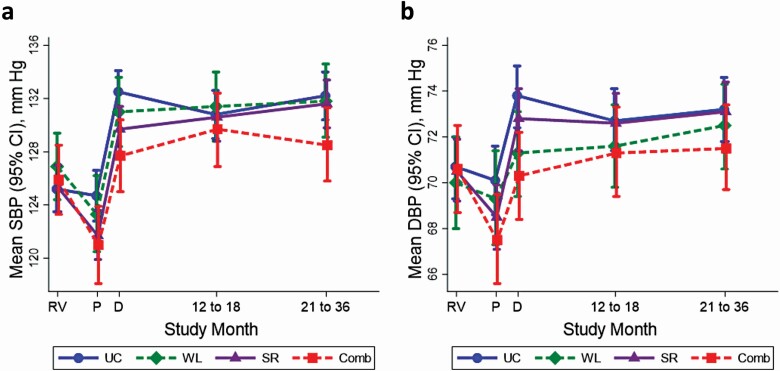
With the exception of usual care, BP decreased after randomization prior to DW, but then increased during DW regardless of assignment and remained elevated for the duration of the study (Figure 1a,b; Supplementary Table ST1 online). After randomization and before DW, usual care had no effect on SBP (−0.5; 95% confidence interval [CI]: −1.9, 0.9 mm Hg) (Supplementary Tables ST2–ST4 online). In contrast, prior to DW, mean SBP was reduced by weight loss (−3.6; 95% CI: −5.9, −1.3 mm Hg), sodium reduction (−3.6; 95% CI: −4.9, −2.2 mm Hg), and the combination of weight loss and sodium reduction (−4.9; 95% CI: −7.1, −2.6 mm Hg). During DW compared with baseline, the greatest increase in mean SBP was observed among those assigned usual care (7.3; 95% CI: 6.0, 8.5 mm Hg), while the lowest increase in mean SBP was observed among those assigned the combined weight loss and sodium reduction intervention (1.8; 95% CI: −2.0, 3.8 mm Hg). However, over time, increases in SBP ranging from 3 to 7 mm Hg were observed among all groups compared with baseline. Effects on DBP were similar (Supplementary Table ST3 online).
Figure 1.
Mean mm Hg (95% CI) for (a) systolic blood pressure (SBP) and (b) diastolic blood pressure (DBP) according to randomized intervention throughout the TONE trial: usual care (UC, circle), weight loss (WL, diamond), sodium reduction (SR, triangle), and both weight loss and sodium reduction (Comb, square). Point estimates are shown by study visit: RV (randomization visit), P (visits prior to drug withdrawal), D (drug withdrawal visits, 6-month visits, and 9-month visits), 12–18 (12-, 15-, and 18-month visits), and 21–36 (21-, 24-, 27-, 30-, 33-, and 36-month visits). Point estimates are positioned according to the median follow-time corresponding to each cluster of visits. Number of visits contributing to each point estimate is found in Supplement Table ST3. Note that participants were censored if they experienced the primary outcome (which included high blood pressure). All means are adjusted for obesity status and clinic center. Abbreviations: CI, confidence interval; TONE, Trial of Nonpharmacologic Interventions in the Elderly.
Effects of nonpharmacologic replacement strategies on BP
Compared with usual care, sodium reduction mitigated the effects of DW on SBP by 1.79 mm Hg (95% CI: −3.44, −0.14). The combination of weight loss and sodium reduction mitigated the effects of DW on SBP by 4.33 mm Hg (95% CI: −6.48, −2.17) (Table 2) and on DBP by 2.47 (95% CI: −3.88, −1.06), compared with usual care.
Table 2.
Effects of interventions on mean blood pressure across the whole trial, N = 975 participants (13,925 study visits for systolic blood pressure and 12,924 visits for diastolic blood pressure)
| Change in systolic blood pressure, mm Hg | Change in diastolic blood pressure, mm Hg | |||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
| All groups | ||||
| Usual care | Reference | — | Reference | — |
| Weight loss | −2.46 (−4.55, −0.37) | 0.021 | −0.98 (−2.50, 0.54) | 0.21 |
| Sodium reduction | −1.79 (−3.44, −0.14) | 0.034 | −0.50 (−1.50, 0.50) | 0.33 |
| Weight loss and sodium reduction | −4.33 (−6.48, −2.17) | <0.001 | −2.47 (−3.88, −1.06) | 0.001 |
| Factorial design—weight loss | ||||
| No weight lossa | Reference | — | Reference | — |
| Weight loss | −2.49 (−4.00, −0.98) | 0.001 | −1.48 (−2.51, −0.44) | 0.005 |
| Factorial design—sodium reduction | ||||
| No sodium reductiona | Reference | — | Reference | — |
| Sodium reduction | −1.81 (−3.20, −0.42) | 0.011 | −0.79 (−1.68, 0.09) | 0.08 |
Change in follow-up blood pressure was modeled using a generalized estimating equation adjusted for baseline obesity status, field center, and baseline systolic or diastolic blood pressure. Interaction terms between weight loss and sodium reduction were nonsignificant. Abbreviation: CI, confidence interval.
aNo weight loss includes usual care and sodium reduction. No sodium reduction includes usual care and weight loss.
Effects of nonpharmacologic replacement strategies on adverse events
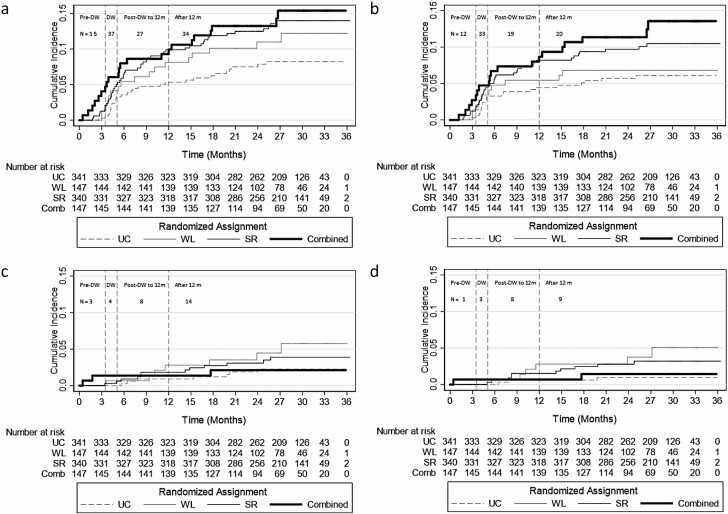
There were 113 adverse events (affecting 95 distinct participants): 84 symptomatic events (affecting 72 participants) and 29 clinical events (affecting 26 participants); of the clinical events, 21 were falls (affecting 19 participants). There were 5 fractures, all among participants with falls, and 12 syncopal events with 6 among participants with falls. The majority of events (36 of 113) occurred during the DW phase (Figure 2). Across the usual care, weight loss, sodium reduction, and combined weight loss and sodium reduction groups, incidence rates were 5.2, 5.4, 5.4, and 5.7 per 10 person-years (Table 3). Compared with usual care, sodium reduction significantly increased the risk of adverse events (hazard ratio 1.76; 95% CI: 1.04, 2.95), which was predominantly driven by symptomatic events when subcomponents were examined. None of the assignments were associated with clinical events or specifically just falls.
Figure 2.
Cumulative incidence curves portraying risk of (a) any adverse events, (b) orthostatic symptoms, (c) falls, fracture, or syncope, or (d) falls with the number at risk according to the 4 randomized assignments over the 36-month trial surveillance period: usual care (UC), weight loss (WL), sodium reduction (SR), or combined weight loss and sodium reduction (Combined). Participants were censored if they reported an event or administratively (as a result of developing the primary outcome in the study or at 36 months). N is the number of recurrent events reported during each study phase. Pre-drug withdrawal (DW) is the study period after randomization and up to and including the visit initiating DW. DW includes all DW visits after drug withdrawal began up to and including the 12-month visit. In the event that study phases were not clearly labeled (6 orthostatic symptoms or adverse events, 2 orthostatic symptoms, 4 adverse events, 3 falls), we relied on the mean follow-time for first DW (3.5 months) and last DW (5.1 months).
Table 3.
Effects of randomized intervention on symptomatic events (dizziness, lightheadedness, or blacking out/presyncope) and clinical events (fall, fracture, or syncope), N = 975
| Incidence rate (per 10 person-years)a | Hazard ratio (95% CI) | P | |
|---|---|---|---|
| Any adverse event, N = 113 | |||
| All groups | |||
| Usual care | 5.2 (4.4, 6.0) | Reference | — |
| Weight loss | 5.4 (4.3, 6.6) | 1.39 (0.66, 2.91) | 0.38 |
| Sodium reduction | 5.4 (4.6, 6.1) | 1.75 (1.04, 2.95) | 0.035 |
| Weight loss and sodium reduction | 5.7 (4.3, 7.0) | 1.81 (0.90, 3.65) | 0.10 |
| Factorial design—weight loss | |||
| No weight lossb | 5.3 (4.7, 5.8) | Reference | — |
| Weight loss | 5.6 (4.6, 6.5) | 1.17 (0.70, 1.95) | 0.55 |
| Factorial design—sodium reduction | |||
| No sodium reductionb | 5.3 (4.6, 5.9) | Reference | |
| Sodium reduction | 5.5 (4.8, 6.1) | 1.58 (1.04, 2.41) | 0.032 |
| Symptomatic events, N = 84 | |||
| All groups | |||
| Usual care | 3.7 (2.7, 4.8) | Reference | — |
| Weight loss | 3.2 (2.1, 4.2) | 1.13 (0.48, 2.66) | 0.78 |
| Sodium reduction | 3.9 (3.0, 4.7) | 1.77 (0.97, 3.23) | 0.065 |
| Weight loss and sodium reduction | 4.9 (3.2, 6.5) | 2.16 (0.96, 4.87) | 0.062 |
| Factorial design—weight loss | |||
| No weight lossb | 3.8 (3.1, 4.5) | Reference | — |
| Weight loss | 4.1 (3.1, 5.2) | 1.20 (0.65, 2.21) | 0.57 |
| Factorial design—sodium reduction | |||
| No sodium reductionb | 3.5 (2.7, 4.3) | Reference | — |
| Sodium reduction | 4.2 (3.4, 4.9) | 1.82 (1.12, 2.94) | 0.015 |
| Fall, fracture, or syncope, N = 29 | |||
| All groups | |||
| Usual care | 1.1 (0.3, 2.0) | Reference | — |
| Weight loss | 1.8 (0.5, 3.1) | 2.06 (0.54, 7.91) | 0.29 |
| Sodium reduction | 1.1 (0.6, 1.7) | 1.71 (0.67, 4.39) | 0.26 |
| Weight loss and sodium reduction | 0.7 (−0.1, 1.5) | 0.89 (0.21, 3.80) | 0.87 |
| Factorial design—weight loss | |||
| No weight lossb | 1.1 (0.6, 1.6) | Reference | — |
| Weight loss | 1.2 (0.4, 1.9) | 1.09 (0.40, 2.99) | 0.87 |
| Factorial design—sodium reduction | |||
| No sodium reductionb | 1.4 (0.6, 2.1) | Reference | — |
| Sodium reduction | 1.0 (0.5, 1.5) | 1.07 (0.48, 2.41) | 0.87 |
| Falls, N = 21 | |||
| All groups | |||
| Usual care | 0.5 (−0.2, 1.1) | Reference | — |
| Weight loss | 1.6 (0.2, 3.0) | 4.23 (0.73, 24.59) | 0.11 |
| Sodium reduction | 1.0 (0.5, 1.5) | 3.32 (0.93, 11.90) | 0.065 |
| Weight loss and sodium reduction | 0.5 (−0.2, 1.1) | 1.40 (0.20, 9.76) | 0.73 |
| Factorial design—weight loss | |||
| No weight lossb | 0.8 (0.3, 1.2) | Reference | — |
| Weight loss | 1.0 (0.2, 1.7) | 1.31 (0.39, 4.39) | 0.66 |
| Factorial design—sodium reduction | |||
| No sodium reductionb | 0.8 (0.2, 1.5) | Reference | — |
| Sodium reduction | 0.8 (0.4, 1.2) | 1.33 (0.49, 3.63) | 0.58 |
Incidence rates were modeled using Poisson regression adjusted for baseline obesity status and field center. Relative risk of adverse events was modeled using Cox proportional hazards models adjusted for baseline obesity status and field center. Abbreviation: CI, confidence interval.
aNote while mathematically the confidence interval is negative, this should be interpreted as zero as an incidence rate cannot be negative.
bNo weight loss includes usual care and sodium reduction. No sodium reduction includes usual care and weight loss.
Monitored participant-reported orthostatic symptoms
Compared with baseline, weight loss combined with sodium reduction tended to decrease the proportion of participants reporting lightheadedness or any of the 3 symptoms (Supplementary Figure SF1 online). Compared with usual care, weight loss combined with sodium reduction lowered the odds of lightheadedness (odds ratio 0.59; 95% CI: 0.37, 0.93), feeling faint (odds ratio 0.22; 95% CI: 0.09, 0.58), or any symptom (odds ratio 0.62; 95% CI: 0.41, 0.92) (Table 4). Sodium reduction was also associated with a lower odds of feeling faint (odds ratio 0.42; 95% CI: 0.20, 0.87); however this effect was primarily observed prior to DW (Supplementary Tables ST5 and ST6 online).
Table 4.
Effect of randomized intervention on self-reported orthostatic symptomsa
| Lightheadedness, N = 556 instances | Faint, N = 186 instances | Imbalance, N = 513 instances | Combination, N = 843 instances | |
|---|---|---|---|---|
| OR (95% CI)b | OR (95% CI)b | OR (95% CI)b | OR (95% CI)b | |
| All groups | ||||
| Usual care | Reference | Reference | Reference | Reference |
| Weight loss | 1.23 (0.70, 2.16) | 0.62 (0.22, 1.74) | 0.88 (0.56, 1.40) | 0.89 (0.59, 1.33) |
| Sodium reduction | 1.07 (0.72, 1.58) | 0.42 (0.20, 0.87) | 0.84 (0.58, 1.21) | 0.87 (0.62, 1.21) |
| Weight loss and sodium reduction | 0.59 (0.37, 0.93) | 0.22 (0.09, 0.58) | 0.70 (0.43, 1.14) | 0.62 (0.41, 0.92) |
| Factorial design—weight loss | ||||
| No weight lossc | Reference | Reference | Reference | Reference |
| Weight loss | 0.78 (0.55, 1.12) | 0.58 (0.31, 1.09) | 0.86 (0.62, 1.20) | 0.79 (0.60, 1.04) |
| Factorial design—sodium reduction | ||||
| No sodium reductionc | Reference | Reference | Reference | Reference |
| Sodium reduction | 0.86 (0.62, 1.20) | 0.41 (0.23, 0.75) | 0.83 (0.61, 1.12) | 0.81 (0.62, 1.06) |
Interaction terms between weight loss and sodium reduction were nonsignificant. Abbreviations: CI, confidence interval; OR, odds ratio.
a N = 975 participants with 4,842 visits for lightheadedness, 4,841 visits for faint, 4,833 visits for imbalance, and 4,843 visits for any combination of symptoms.
bOdds of symptoms were modeled using generalized estimating equations, adjusted for baseline obesity status, field center, and baseline symptom corresponding to the outcome (i.e., lightheadedness, faint, imbalance, or the combination).
cNo weight loss includes usual care and sodium reduction. No sodium reduction includes usual care and weight loss.
DISCUSSION
In this trial of nonpharmacologic strategies to replace antihypertensive medication use in older adults, weight loss and sodium reduction, alone and combined, lowered BP compared with usual care prior to DW. However, BP rose during DW and was higher than baseline in all randomized groups. The adverse effects of DW on BP were mitigated by sodium reduction, alone or combined with weight loss; however, sodium reduction alone was associated with a higher risk of symptomatic adverse events (i.e., dizziness, lightheadedness, blacking out/presyncope), mostly in the context of DW. In contrast with respect to longer-term, monitored symptoms, weight loss combined with sodium reduction was associated with fewer self-reported orthostatic symptoms during scheduled assessments without increasing the risk of clinical events (i.e., falls, fractures, or syncope). These findings highlight the short-term risks associated with DW in older adults.
Polypharmacy has been cited as an independent risk factor for adverse events in older adults.15,16 Prior work in TONE demonstrated the ability of lifestyle interventions to maintain BP <150 mm Hg without increasing risk for CVD events.11 However, TONE was not designed to test for differences in CVD events across interventions. Given the poorer long-term BP control demonstrated by our study and seen by others6 as well as new evidence of the value of more aggressive BP lowering in older adults17,18 and small population-wide reductions in BP,19 whether deprescription of antihypertensive medications is safe is an important question raised by the present study.
Our study showed an increased risk of symptomatic events among older adults that were assigned to the sodium reduction strategy. However, sodium reduction was also associated with a lower odds of feeling faint prior to DW. Moreover, the majority of adverse events occurred during the DW period. Together, these findings reinforce the observations of others that the immediate time surrounding drug changes are among the highest risk periods for adverse events in older adults.20–22 Moreover, it suggests that substitution of BP medications with lifestyle interventions, particularly sodium reduction, is not without risks. As the decision to deprescribe is complex and ideally linked with shared patient values, our study reinforces the importance of vigilance when antihypertensive medications are being withdrawn (or changed).
Participant-reported orthostatic symptoms assessed during scheduled BP assessments did not reflect population risks for adverse events. Similar discordance has been observed for BP measured during monitoring visits and outside of the clinic in other studies.23 In some respects, this demonstrates the challenge of clinic-based assessments for predicting clinic events as scheduled clinic visits may inherently select for less symptomatic times in a person’s life. In other words, an individual may cancel or reschedule their visit if they are not feeling well. This also highlights the limitations of episodic clinic-based assessments and the importance of more comprehensive monitoring of health in the home environment.24
Our study has limitations. First, adverse events like falls were not a primary focus of TONE. As a result, some descriptions were incomplete, and it is possible events were missed during the study. This could conservatively bias our findings toward the null. With only 21 fall events, we had limited power to detect associations between the interventions and falls. Moreover, orthostatic hypotension was not measured, which can differ from symptoms with regard to their associations with adverse outcomes.25 Given the ad hoc reporting of these events, mechanistic information about the cause of the events (e.g., phlebotomy or physical assessments) were not available. Subsequent studies should focus on mechanisms for falls, particularly through formal assessments of orthostatic hypotension. Second, participants were censored when they restarted their antihypertensive medication. As a result, the impacts of DW on BP control are likely underestimated. Moreover, whether restarting medications contributed to orthostatic symptoms and adverse events cannot be determined in our study. Third, there was no direct comparison of individuals undergoing similar lifestyle interventions for whom DW was not attempted. However, this is mitigated to some extent by the within-person pre- vs. postwithdrawal comparisons. Fourth, although each intervention assignment generally consisted of a similar number of scheduled clinical contacts, the trial allowed for some degree of customization of visits to meet the needs of individual participants. Thus, it is possible that there were a greater number of scheduled visits for some participants than others, which could have influenced the reporting of adverse events. Fifth, interactions with participants differed by phase of the study with the highest frequency occurring during the DW phase. This design feature could contribute to increased surveillance and event reporting during the DW phase. Thus, temporal inferences about the direct effects of DW on events should be interpreted cautiously, although our results may suggest that the increased surveillance during this period was clinically warranted. Finally, generalizability to more frail or institutionalized older adults as well as adults with diabetes, heart failure, chronic kidney disease, or recent CVD events, who were excluded from TONE is unclear.
Our study has strengths. First, TONE is one of the largest trials of nonpharmacologic drug replacement strategies in older adults with excellent follow-up and adherence rates. Second, TONE randomized participants to 2 of the most evidenced-based lifestyle interventions for BP reduction: weight loss and sodium reduction,26 allowing us to examine the causal effects of these interventions on adverse events. Finally, repeated measurements up to 36 months allowed us to determine within-person changes in BP control and symptoms over nearly 3 years.
Our study has clinical implications. Hypertension affects over 75% of adults over age 65 years and 60% of older adults are actively treated for hypertension.1,27 Given the independent association of polypharmacy with adverse events in older adults, deprescription of antihypertensive medications has been a natural focus in aging populations.28,29 Moreover, antihypertensive agents have been associated with falls leading many to advocate strongly for more lenient BP goals in this population.30 However, our study demonstrates that deprescribing not only worsened BP control, but is not itself without risks, likely due to short-term changes in BP regulation. Given the challenge of maintaining consistency in some lifestyle interventions like sodium reduction, due to the pervasive and often unrecognized availability of high sodium-dense foods,31 it is possible that deprescribing could result in greater BP variability, increasing the occurrence of symptomatic events associated with fall risk. This combined with recent observations from the Salt Substitute and Stroke Study (SSaSS) that reductions in SBP of 3–4 mm Hg were associated with reduced risk of stroke and all-cause mortality,19 should raise questions about the safety of antihypertensive deprescription in older adults.
In conclusion, antihypertensive DW increases BP and may increase risks of symptomatic adverse events in older adults short term. These findings highlight a need for caution when withdrawing antihypertensive medications in older adults.
SUPPLEMENTARY MATERIAL
Supplementary data are available at American Journal of Hypertension online.
Supplementary Figure SF1. Percentage reporting lightheadedness, feeling faint, or imbalance over time.
Supplementary Table ST1. Effects of drug withdrawal on mean blood pressure compared with baseline, by randomized assignment, factorial assignments, and overall.
Supplementary Table ST2. Effects of interventions on systolic blood pressure over time.
Supplementary Table ST3. Effects of interventions on diastolic blood pressure over time.
Supplementary Table ST4. Number of visits contributing to systolic or diastolic blood pressure means over the course of the study.
Supplementary Table ST5. Effects of interventions on monitored participant-reported orthostatic symptoms over time.
Supplementary Table ST6. Number of visits contributing to prevalence estimates of monitored participant-reported orthostatic symptoms over the course of the study.
ACKNOWLEDGMENTS
We are indebted to the study participants for their sustained commitment to the TONE trial.
Previous presentation of the whole or part of this work: This work was presented at the 2021 AHA Epidemiology/Lifestyle Scientific Sessions as a moderated poster presentation.
FUNDING
S.P.J. is supported by a NIH/NHLBI K23HL135273. K.J.M. is supported by NIH/NIA K24AG065525.
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1. Muntner P, Einhorn PT, Cushman WC, Whelton PK, Bello NA, Drawz PE, Green BB, Jones DW, Juraschek SP, Margolis KL, Miller ER III, Navar AM, Ostchega Y, Rakotz MK, Rosner B, Schwartz JE, Shimbo D, Stergiou GS, Townsend RR, Williamson JD, Wright JT Jr, Appel LJ; 2017 National Heart, Lung, and Blood Institute Working Group . Blood pressure assessment in adults in clinical practice and clinic-based research: JACC scientific expert panel. J Am Coll Cardiol 2019; 73:317–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sheppard JP, Singh S, Fletcher K, McManus RJ, Mant J. Impact of age and sex on primary preventive treatment for cardiovascular disease in the West Midlands, UK: cross sectional study. BMJ 2012; 345:e4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP, Zhou B, Lin H. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med 2014; 174:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med 2009; 122:290–300. [DOI] [PubMed] [Google Scholar]
- 5. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018; 71:e13–e115. [DOI] [PubMed] [Google Scholar]
- 6. Sheppard JP, Burt J, Lown M, Temple E, Lowe R, Fraser R, Allen J, Ford GA, Heneghan C, Hobbs FDR, Jowett S, Kodabuckus S, Little P, Mant J, Mollison J, Payne RA, Williams M, Yu LM, McManus RJ; OPTIMISE Investigators . Effect of antihypertensive medication reduction vs usual care on short-term blood pressure control in patients with hypertension aged 80 years and older: the OPTIMISE randomized clinical trial. JAMA 2020; 323:2039–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, Gnjidic D, Del Mar CB, Roughead EE, Page A, Jansen J, Martin JH. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med 2015; 175:827–834. [DOI] [PubMed] [Google Scholar]
- 8. Reeve E, Jordan V, Thompson W, Sawan M, Todd A, Gammie TM, Hopper I, Hilmer SN, Gnjidic D. Withdrawal of antihypertensive drugs in older people. Cochrane Database Syst Rev 2020; 6:CD012572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Appel LJ, Espeland M, Whelton PK, Dolecek T, Kumanyika S, Applegate WB, Ettinger WH Jr, Kostis JB, Wilson AC, Lacy C. Trial of Nonpharmacologic Intervention in the Elderly (TONE). Design and rationale of a blood pressure control trial. Ann Epidemiol 1995; 5:119–129. [DOI] [PubMed] [Google Scholar]
- 10. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998; 279:839–846. [DOI] [PubMed] [Google Scholar]
- 11. Kostis JB, Espeland MA, Appel L, Johnson KC, Pierce J, Wofford JL. Does withdrawal of antihypertensive medication increase the risk of cardiovascular events? Trial of Nonpharmacologic Interventions in the Elderly (TONE) Cooperative Research Group. Am J Cardiol 1998; 82:1501–1508. [DOI] [PubMed] [Google Scholar]
- 12. Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med 2001; 161:685–693. [DOI] [PubMed] [Google Scholar]
- 13. FAQ: Analysis of Multiple Failure-Time Survival Data | Stata. https://www.stata.com/support/faqs/statistics/multiple-failure-time-data/. Accessed 9 September 2021.
- 14. Andersen PK, Gill RD. Cox’s regression model for counting processes: a large sample study. Ann Stat 1982; 10:1100–1120. [Google Scholar]
- 15. Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc 2014; 62:2261–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dagli RJ, Sharma A. Polypharmacy: a global risk factor for elderly people. J Int Oral Health 2014; 6:i–ii. [PMC free article] [PubMed] [Google Scholar]
- 17. Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, Cutler JA, Davatzikos C, Desiderio L, Erus G, Fine LJ, Gaussoin SA, Harris D, Hsieh MK, Johnson KC, Kimmel PL, Tamura MK, Launer LJ, Lerner AJ, Lewis CE, Martindale-Adams J, Moy CS, Nasrallah IM, Nichols LO, Oparil S, Ogrocki PK, Rahman M, Rapp SR, Reboussin DM, Rocco MV, Sachs BC, Sink KM, Still CH, Supiano MA, Snyder JK, Wadley VG, Walker J, Weiner DE, Whelton PK, Wilson VM, Woolard N, Wright JT Jr, Wright CB; SPRINT MIND Investigators for the SPRINT Research Group . Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, Kitzman DW, Kostis JB, Krousel-Wood MA, Launer LJ, Oparil S, Rodriguez CJ, Roumie CL, Shorr RI, Sink KM, Wadley VG, Whelton PK, Whittle J, Woolard NF, Wright JT Jr, Pajewski NM; SPRINT Research Group . Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA 2016; 315:2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Neal B, Wu Y, Feng X, Zhang R, Zhang Y, Shi J, Zhang J, Tian M, Huang L, Li Z, Yu Y, Zhao Y, Zhou B, Sun J, Liu Y, Yin X, Hao Z, Yu J, Li KC, Zhang X, Duan P, Wang F, Ma B, Shi W, Di Tanna GL, Stepien S, Shan S, Pearson SA, Li N, Yan LL, Labarthe D, Elliott P. Effect of salt substitution on cardiovascular events and death. N Engl J Med 2021; 385:1067–1077. [DOI] [PubMed] [Google Scholar]
- 20. Shimbo D, Barrett Bowling C, Levitan EB, Deng L, Sim JJ, Huang L, Reynolds K, Muntner P. Short-term risk of serious fall injuries in older adults initiating and intensifying treatment with antihypertensive medication. Circ Cardiovasc Qual Outcomes 2016; 9:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shuto H, Imakyure O, Matsumoto J, Egawa T, Jiang Y, Hirakawa M, Kataoka Y, Yanagawa T. Medication use as a risk factor for inpatient falls in an acute care hospital: a case-crossover study. Br J Clin Pharmacol 2010; 69:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butt DA, Mamdani M, Austin PC, Tu K, Gomes T, Glazier RH. The risk of hip fracture after initiating antihypertensive drugs in the elderly. Arch Intern Med 2012; 172:1739–1744. [DOI] [PubMed] [Google Scholar]
- 23. Ghazi L, Pajewski NM, Rifkin DE, Bates JT, Chang TI, Cushman WC, Glasser SP, Haley WE, Johnson KC, Kostis WJ, Papademetriou V, Rahman M, Simmons DL, Taylor A, Whelton PK, Wright JT, Bhatt UY, Drawz PE. Effect of intensive and standard clinic-based hypertension management on the concordance between clinic and ambulatory blood pressure and blood pressure variability in SPRINT. J Am Heart Assoc 2019; 8:e011706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kario K. Management of hypertension in the digital era: small wearable monitoring devices for remote blood pressure monitoring. Hypertension 2020; 76:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Juraschek SP, Longstreth WT Jr, Lopez OL, Gottdiener JS, Lipsitz LA, Kuller LH, Mukamal KJ. Orthostatic hypotension, dizziness, neurology outcomes, and death in older adults. Neurology 2020; 95:e1941–e1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Whelton PK, Appel L, Charleston J, Dalcin AT, Ewart C, Fried L, Kaidy D, Klag MJ, Kumanyika S, Steffen L, Walker WG, Oberman A, Counts K, Hataway H, Raczynski J, Rappaport N, Weinsier R, Borhani NO, Bernauer E, Borhani P, de la Cruz C, Ertl A, Heustis D, Lee M, Lovelace W, O’Connor E, Peel L, Sugars C, Taylor JO, Corkery BW, Evans DA, Keough ME, Morris MC, Pistorino E, Sacks F, Cameron M, Corrigan S, Wright NK, Applegate WB, Brewer A, Goodwin L, Miller S, Murphy J, Randle J, Sullivan J, Lasser NL, Batey DM, Dolan L, Hamill S, Kennedy P, Lasser VI, Kuller LH, Caggiula AW, Milas NC, Yamamoto ME, Vogt TM, Greenlick MR, Hollis J, Stevens V, Cohen JD, Mattfeldt-Beman M, Brinkmann C, Roth K, Shepek L, Hennekens CH, Buring J, Cook N, Danielson E, Eberlein K, Gordon D, Hebert P, MacFadyen J, Mayrent S, Rosner B, Satterfield S, Tosteson H, Van Denburgh M, Cutler JA, Brittain E, Farrand M, Kaufmann P, Lakatos E, Obarzanek E, Belcher J, Dommeyer A, Mills I, Neibling P, Woods M, Goldman BJK, Blethen E. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the trials of hypertension prevention, phase I. JAMA 1992; 267:1213–1220. [DOI] [PubMed] [Google Scholar]
- 27. Samanic CM, Barbour KE, Liu Y, Wang Y, Fang J, Lu H, Schieb L, Greenlund KJ. Prevalence of self-reported hypertension and antihypertensive medication use by county and rural-urban classification—United States, 2017. MMWR Morb Mortal Wkly Rep 2020; 69:533–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gulla C, Flo E, Kjome RL, Husebo BS. Deprescribing antihypertensive treatment in nursing home patients and the effect on blood pressure. J Geriatr Cardiol 2018; 15:275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Middelaar T, Ivens SD, van Peet PG, Poortvliet RKE, Richard E, Pols AJ, Moll van Charante EP. Prescribing and deprescribing antihypertensive medication in older people by Dutch general practitioners: a qualitative study. BMJ Open 2018; 8:e020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 31. Hu J-R, Sahni S, Mukamal KJ, Millar CL, Wu Y, Appel LJ, Juraschek SP. Dietary sodium intake and sodium density in the United States: estimates from NHANES 2005–2006 and 2015–2016. Am J Hypertens 2020; 33:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.