Version Changes
Revised. Amendments from Version 1
The new manuscript version has been revised based on reviewer comments. Addition of text has been made to Introduction and Methods sections to rephrase statements which initially were not explicitly explained. In the ‘Introduction’ section, a paragraph and a reference were added to provide evidence of impaired transplacental transfer of antibodies among malnourished women. Two other references were added to support definition of efficiency of transplacental transfer and analysis of efficiency of transplacental transfer of RSV antibodies using cord to maternal antibody titre ratio. In 'Methods' section, a paragraph was added to explain how sampling was done from the two cohorts of pregnant women. The plaque reduction neutralisation test procedure was added and described in detail. A sentence on how logistic regression model was conducted was added in statistical analysis section. In the 'Results' section, Table 2 and Figure 2 were removed from the main manuscript and deposited as supplementary files at Havard Dataverse. Table 3 was edited and changed to Table 2 while Table 4 was edited and changed to Table 3 in version two(V2) of the manuscript. In the 'Discussion' section, paragraph 3 which described levels of RSV-specific antibodies and protection against RSV disease was deleted. A total of seven references were added to the revised manuscript.
Abstract
Background: Maternal immunisation to boost respiratory syncytial virus (RSV) antibodies in pregnant women, is a strategy being considered to enhance infant protection from severe RSV associated disease. However, little is known about the efficiency of transplacental transfer of RSV-specific antibodies in a setting with a high burden of malaria and HIV, to guide the implementation of such a vaccination program.
Methods: Using a plaque reduction neutralization assay, we screened 400 pairs of cord and maternal serum specimens from pregnant women for RSV-specific antibodies. Participants were pregnant women of two surveillance cohorts: 200 participants from a hospital cohort in Kilifi, Coastal Kenya and 200 participants from a surveillance cohort in Siaya, Western Kenya. Transplacental transfer efficiency was determined by the cord to maternal titre ratio (CMTR). Logistic regression was used to determine independent predictors of impaired transplacental transfer of RSV-specific antibodies.
Results: A total of 800 samples were screened from the 400 participants. At enrollment the median age was 25 years (Interquartile range (IQR): 21-31). Overall, transplacental transfer was efficient and did not differ between Kilifi and Siaya cohort (1.02 vs. 1.02; p=0.946) but was significantly reduced among HIV-infected mothers compared to HIV-uninfected mothers (mean CMTR: 0.98 vs 1.03; p=0.015). Prematurity <33 weeks gestation (Odds ratio [OR]: 0.23, 95% confidence interval [CI] 0.06–0.85; p=0.028), low birth weight <2.5 kgs (OR: 0.25, 95% CI: 0.07–0.94; p=0.041) and HIV infection (OR: 0.47, 95% CI:0.23-0.98; p=0.045) reduced efficiency of transplacental transfer among these women.
Conclusions: Transplacental transfer of RSV-specific antibodies among pregnant women in Kenya is efficient. A consideration to integrate other preventive interventions with maternal RSV vaccination targeting infants born premature (<33 weeks gestation), with low birth weight <2.5 kgs, or HIV-infected mothers is likely to improve vaccine outcomes in this setting.
Keywords: Pregnant women; transplacental transfer efficiency; Respiratory Syncytial Virus; Neutralising antibody, Maternal vaccine, Effectiveness
Abbreviations
RSV Respiratory syncytial virus
KHDSS Kilifi Health and Demographic Surveillance System
KEMRI Kenya Medical Research Institute
KWTRP KEMRI Wellcome Trust Research Programme
KCH Kilifi County Hospital
LMICs Low- and Middle -Income Countries
SD Standard deviation
95% CI 95% Confidence interval
CMTR Cord to Maternal Transfer Ratio
Introduction
Globally, respiratory syncytial virus (RSV) is a significant cause of acute lower respiratory tract infection (LRTI) among infants leading to hospital admissions and in-hospital deaths, with 99% of these deaths occurring in developing countries 1 . In sub-Saharan Africa and Asia, RSV has been observed to be responsible for about 40% of all hospital admissions with severe or very severe pneumonia among infants under 1 year 2 . Severe RSV-associated LRTI is most common among infants under six months of age 3, 4 , resulting in about 32% of hospitalised infants in the rural coast of Kenya, during epidemics 3 .
Maternal immunisation is currently being considered as a strategy to protect infants from severe RSV-associated disease because of the lack of licenced RSV vaccines targeting infants 5, 6 . Additionally, the frequency of cases and the peak hospitalization occurs at 2 months of age which is less than the timing for first scheduled infant immunisations and during this neonatal period, the infant may not mount strong immune response against RSV infection due to immature immune system and/or interference from maternal antibodies 7– 11 . Efforts to advance maternal immunisation for RSV have shown promise and several candidate maternal RSV vaccines are in the late stages of clinical trials 6, 12, 13 . However, despite the advancement in development of maternal RSV vaccines, the success of this program will depend on how efficiently vaccine-induced RSV-specific antibodies can be transferred in utero to the infant.
Previous studies have shown that transplacental transfer of RSV-specific antibody to the infant is usually efficient with a cord blood to maternal blood antibody titre ratio of ≥ 1 14– 16 . This is because IgG antibody is actively transported across the syncytiotrophoblast cells rather than a passive diffusion process which is why transplacental transfer is expected be more than one 17 . Transplacental transfer efficiency has been defined as either normal or impaired in other studies 16, 18 . Where the cord to maternal titre ratio (CMTR) is one or greater than one, this is considered as a normal or efficient transfer, while a CMTR of less than one is considered as an impaired transfer 16 .
Transplacental transfer of IgG antibodies begins during the 28 th week of gestation which is coincident with the timing for expression of Fc gamma RII (FcgII) receptor responsible for the materno-foetal transfer of antibodies 19 . Thus, infants born preterm, shortly after or before initiation of transplacental transfer of antibodies may be less likely to benefit from a maternal RSV vaccine program. In general, placental malaria, hypergammaglobinaemia (total IgG >15g/L), HIV infection and possible illness episodes or infection occurring during the third trimester of pregnancy have been known to influence the level of antibodies transferred to the infant 20– 22 . Moreover, passive transfer of Haemophilus influenzae type b (Hib) vaccine antibodies was found to be 14% lower in malnourished pregnant group compared to control group among Brazilian women, suggesting less efficient transplacental transfer of antibody among malnourished women 23 . However, there is limited data on the efficiency of transplacental transfer of RSV-specific antibodies in settings where the maternal population experiences comorbidities such as malaria, HIV and undernutrition as well as premature deliveries, which could negatively impact the effectiveness of a maternal RSV vaccine.
In this study, we describe the efficiency of transplacental transfer of RSV-specific antibodies measured as CMTR among pregnant women in Kenya using cord-maternal blood sample pairs collected from pregnant women in the counties of Kilifi (coast region) and Siaya (western region) where population-level prevalence of malaria and HIV, respectively, have been documented and we explore factors that could influence transplacental transfer of RSV-specific antibodies to support the successful implementation of a maternal RSV vaccine program in Kenya.
Methods
Study sites and population
We utilized existing data and serum samples from two independent cohorts of pregnant women in Kilifi and Siaya County, Kenya. One cohort was a hospital-based surveillance investigating risk factors for severe morbidity and mortality in mothers and their infants in Kilifi, coastal Kenya, and the other, a cohort in Siaya County, Western Kenya, for surveillance of influenza disease among pregnant women and their infants.
The surveillance to investigate risk factors for severe morbidity and mortality in mothers and their infants was set up by KEMRI-Wellcome Trust Research Programme (KWTRP) at the maternity ward of Kilifi County Hospital (KCH) and the Kilifi Health and Demographic Surveillance System (KHDSS) area in Coastal Kenya in 2011 24 . This surveillance was designed to observe 4600 births with approximately 2300 being residents of KHDSS. All mothers presenting for delivery at the maternity ward of KCH were invited to enroll. Routine clinical data were collected using a standardized questionnaire at admission to the maternity department and following delivery. During this surveillance, consent was sought from pregnant women presenting at KCH maternity ward to collect cord and maternal blood samples after delivery. These samples were securely stored at -80˚C at KEMRI-Wellcome Trust laboratories in Kilifi for molecular and serological testing for viral and bacterial pathogens. The KIPMAT surveillance enrolled 4047 pregnant women in 2018 and 2019 who had a cord and maternal blood samples collected at delivery and stored for future use.
From the Western part of Kenya, in Siaya County, a cohort of pregnant women was set up through a collaboration between KEMRI-Centre for Global Health Research and the U.S. Centers for Disease Control and Prevention (CDC) Kenya in 2015. This surveillance included pregnant women recruited either from their homes or when they visited for antenatal care at Bondo sub-County or Siaya County Referral Hospital. Participants were enrolled at gestational age <20 weeks. These pregnant women were followed up weekly through a phone call or home visit to record any occurrence of influenza-like illness episodes. Blood samples were collected at enrolment and a maternal and cord blood at birth. If a pregnant woman was identified with cough or fever during follow up, a respiratory specimen was collected and screened for influenza virus type A and B and for RSV using molecular methods 25 . All participants were requested to deliver their children in the hospital where birth outcomes were recorded; thereafter, both the baby and the mother were followed up weekly for up to six months post-delivery to assess infection from respiratory viruses by testing nasal and throat swabs from symptomatic cases by RT-PCR.
During the RSV RT-PCR procedure, nucleic acids were extracted from 100ul of the combination of nasopharyngeal and oropharyngeal specimens using the MagMAX™ Viral RNA Isolation Kit on the Kingfisher mL platform (Life Technologies, New York). A 5ul of the Nucleic acid extract was then tested for RSV in a 1-step real-time reverse-transcription polymerase chain reaction (rRT-PCR) assay, using the AgPath-ID One-step RT-PCR kit (Applied Biosystems, Foster City, California) using CDC’s primers and fluorescent-labelled hydrolysis probes. The assay was considered positive for RSV when exponential fluorescence curves crossed the assigned threshold at a cycle threshold value of <37.0.
The influenza surveillance had initially proposed to recruit 2250 pregnant women in a period of 3–5 years from 2015, based on assumptions that, each participant was to be followed up for a period of six months during pregnancy and 15% of participants were expected to have at least one influenza-like illness (ILI) episode. The study changed to a surveillance in 2018 and continued to enrol participants beyond the proposed sample size. From 2018 to 2019, the influenza surveillance had recruited 1458 pregnant women from whom, 795 participants had a cord and maternal blood samples at delivery, and these were used as a sampling frame for this study.
For Kilifi cohort, 200 women were randomly selected from the cohort registers based on the availability of meta-data and paired cord and maternal blood samples for births (including preterm births) that occurred in 2018 and 2019. For Siaya cohort, where we had additional meta data, we selected all women (n=106) with lab-confirmed RSV infection, HIV, malaria, or anaemia, and randomly selected the other 94 women with available blood (maternal and cord) sample. A total of 400 participants were selected (200 from each region). The sample size was estimated using Edgar C. Fieller methods of calculating confidence intervals for the ratio of two means. This sample size method used CMTRs of 1.03 (0.88–1.19) observed in women in rural Nepal 18 . Assuming that CMTR of RSV-specific antibodies among women in Kenya were similar to those of Nepalese women and both the cord and maternal antibody levels followed a Gaussian distribution, a sample size of 200 mother-infant pair was sufficient to detect a CMTR of 1.03 with a 95% confidence interval of 1.01–1.06.
Laboratory procedures to identify RSV specific-antibodies
All blood samples were screened for RSV specific antibodies using an inhouse plaque reduction neutralization titre (PRNT) assay 26, 27 at KWTRP laboratories, Kilifi, Kenya. The PRNT procedure determines the concentration of functional antibodies from a human serum sample (or antibody preparation) required to induce 50% neutralization of a known titration of RSV virus using the Spearman Karber method 28 . The assay has two stages, the neutralization step and a detection step. In this assay, mother-cord pairs of blood samples were assayed in one plate without use of complement sera. In the neutralization step, each serum sample was repeatedly diluted 2-fold over ten consecutive dilutions and mixed with an equal volume of 50 plaque forming units (pfu) of RSV A2 virus. The virus-serum mixture (50μl per well) was dispensed over a confluent monolayer of Hep2 cells in a 96 well culture plate, incubated at 37°C for 48 hours.
In the detection step, fixation of cells was done by addition of 100μl/well of fixation reagent (30% methanol+70% acetone). This was followed by addition of a primary antibody (RSV F protein mouse monoclonal-BIO-RAD, Catalogue# MCA490) solution diluted 1:500 in PBS with 2 hours incubation at 37°C, and an addition of a 100μl/well of an anti-mouse HRP-conjugated secondary antibody (170-5047 Immun-Star Goat Anti-Mouse (GAM)- IgG (H/L) polyclonal antibody HRP–BIORAD). Micro RSV plaques were stained brown by 100μl/well detection reagent consisting of 16 μl of hydrogen peroxide and 0.6ml of 3-amino-9-ethlycarbazole 3.3mg/ml solution and counted with an ELISPOT reader.
Plaque counts generated by the ELISpot reader were copied and pasted onto an excel analysis template containing Spearman-Karber formula to generate the neutralizing antibody titre for each sample. The PRNT titre was calculated as a neutralizing dose (ND50) value as follows: log10ND50 = m - Δ (Σp – 0.5). Where m was the log10 dilution of the highest dilution of serum (i.e. log10 (1/10,240) = -4.01) Δ was the constant interval between dilutions expressed as log10 (i.e. log10 (2) = 0.3010). The reciprocal was considered as the final antibody titre. This assay procedure has been described elsewhere in detail 27 .
An RSV group A human reference standard (RSV IS 16/284) 29 obtained from National Institute for Biological Standards and Control (NIBSC), Potters Bar, UK, and an inhouse pooled adult sera were incorporated into each assay run to check for antibody deterioration, standardization of sample titres and quality control.
Ethical considerations
Written informed consent to collect samples and data for storage and use in other studies was obtained from all participants through the parent studies, i.e., the influenza cohort surveillance (SERU #2880; CDC IRB number 6709) and the surveillance for risk factors cohort (SERU #1778). Ethical approval to screen samples for RSV-specific antibodies and use of data from the parent studies for this study was granted by the KEMRI Scientific and Ethical Review Unit Committee (SERU #3716). All methods were carried out in accordance with relevant guidelines and regulations.
Statistical analyses
Separate analysis was done for each cohort and with combined data from both cohorts. All PRNT titres were log normalised (log base2) before analysis. The efficiency of transplacental transfer of RSV-specific antibodies was calculated for each mother-infant pair of blood samples. A CMTR (i.e., PRNT titre cord/PRNT titre maternal blood) of ≥1 was considered normal or efficient, CMTR <1 but ≥0.8 as moderately impaired and <0.8 as severely impaired or poor. Duration of transplacental transfer was calculated as gestational age at delivery minus 28; where 28 th week was estimated as the gestational age when a transplacental transfer of IgG antibodies begins during pregnancy. For this analysis, preterm birth (PTB) was defined as baby born alive before 37 weeks of pregnancy are completed and very early PTB as baby born <33 weeks of gestation. The difference in CMTR, cord or maternal RSV PRNT titres between HIV-infected versus HIV-uninfected mothers and RSV-infected infants vs. uninfected infants were analysed using a two-sample paired t-test. The Chi-square test was used to compare characteristics of women between Kilifi and Siaya cohort; and was also applied to determine the association between maternal/infant characteristics (HIV infection, malaria infection, RSV infection, anaemia, education level, occupation, gestational age at delivery and birth weight) and efficiency of transplacental transfer of RSV-specific antibodies. Logistic regression adjusted for each variable category (HIV infection, malaria infection, gestational age at delivery, gravida, birthweight and RSV infection during pregnancy) was used to determine independent predictors of an impaired transplacental transfer of RSV specific antibodies. In the multivariable logistic regression model, transplacental transfer efficiency was used as a binary outcome (normal [CTMR ≥1] vs impaired [CMTR <1] transfer). All data analysis was conducted using STATA version 15.0 (Stata Corp, College Station, Texas). However, a R Statistical software version 4.1.1 which is on open-access, can perform the equivalent analysis. To replicate the same analysis in R, we advise the user to import the CSV version of the data and follow the steps provided in the STATA do-file.
Results
Characteristics of study participants
A total of 800 cord and maternal blood samples from 400 participants selected from the two cohorts were screened for RSV-specific neutralizing antibodies 30 . The median age of the women at enrollment was 25 years (Interquartile range (IQR): 21–31 years). About 95% of these women reported being married, 6% had no formal education, 57% were housewives and 21% had experienced more than six live births ( Table 1).
Table 1. Characteristics of study participants from Kilifi and Siaya.
| Characteristic | Kilifi (n) | % | Siaya (n) | % | Total (n) | % | P * value |
|---|---|---|---|---|---|---|---|
| 200 | 50 | 200 | 50 | 400 | 100 | ||
| Maternal age | |||||||
| 15-19 | 31 | 15.50 | 4 | 2.00 | 35 | 8.75 | |
| 20-24 | 65 | 32.50 | 81 | 40.50 | 146 | 36.50 | |
| 25-29 | 43 | 21.50 | 60 | 30.00 | 103 | 25.75 | <0.001 |
| 30-34 | 30 | 15.00 | 32 | 16.00 | 62 | 15.50 | |
| 35-39 | 16 | 8.00 | 19 | 9.50 | 35 | 8.75 | |
| 40-44 | 15 | 7.50 | 2 | 1.00 | 17 | 4.25 | |
| 45-49 | 0 | 0.00 | 2 | 1.00 | 2 | 0.50 | |
| Marital status | |||||||
| Married | 191 | 95.50 | 190 | 95.00 | 381 | 95.25 | |
| Single | 9 | 4.50 | 10 | 5.00 | 19 | 4.75 | 0.814 |
| Gestational age at delivery | |||||||
| <33 weeks | 10 | 5.00 | 3 | 1.50 | 13 | 3.25 | |
| 33-37 weeks | 59 | 29.50 | 44 | 22.00 | 103 | 25.75 | 0.022 |
| 38-42 weeks | 131 | 65.50 | 153 | 76.50 | 284 | 71.00 | |
| Education level | |||||||
| None | 23 | 11.50 | 2 | 1.00 | 25 | 6.25 | |
| Primary | 124 | 62.00 | 108 | 54.00 | 232 | 58.00 | <0.001 |
| Secondary | 37 | 18.50 | 74 | 37.00 | 111 | 27.75 | |
| Tertiary-College/University | 16 | 8.00 | 16 | 8.00 | 32 | 8.00 | |
| Gravida | |||||||
| 1-2 | 112 | 56.00 | 41 | 20.50 | 153 | 38.25 | |
| 3-5 | 65 | 32.50 | 127 | 63.50 | 192 | 48.00 | <0.001 |
| 6-9 | 18 | 9.00 | 31 | 15.50 | 49 | 12.25 | |
| 10-15 | 5 | 2.50 | 1 | 0.50 | 6 | 1.50 | |
| Occupation | |||||||
| Farmer | 1 | 0.50 | 13 | 6.50 | 14 | 3.50 | |
| Business woman | 23 | 11.50 | 59 | 29.50 | 82 | 20.50 | |
| House wife | 136 | 68.00 | 92 | 46.00 | 228 | 57.00 | <0.001 |
| Salaried worker | 16 | 8.00 | 18 | 9.00 | 34 | 8.50 | |
| Other | 24 | 12.00 | 18 | 9.00 | 42 | 10.50 | |
| Number of ANC visits | |||||||
| 1 | 9 | 4.52 | 146 | 73.00 | 155 | 38.85 | |
| 2 | 22 | 11.06 | 45 | 22.50 | 67 | 16.79 | |
| 3 | 45 | 22.61 | 7 | 3.50 | 52 | 13.03 | <0.000 |
| 4 | 57 | 28.64 | 2 | 1.00 | 59 | 14.79 | |
| 5 | 38 | 19.10 | 0 | 0.00 | 38 | 9.52 | |
| 6 | 28 | 14.07 | 0 | 0.00 | 28 | 7.02 | |
| Sex of child | |||||||
| Female | 94 | 47.00 | 96 | 48.00 | 190 | 47.50 | 0.841 |
| Birthweight | |||||||
| Underweight (<2.5kgs) | 43 | 21.50 | 12 | 6.00 | 55 | 13.75 | <0.000 |
|
Transplacental transfer
Efficiency |
|||||||
| Impaired | 77 | 38.50 | 89 | 44.50 | 166 | 41.50 | 0.223 |
(*P-Chi squared value)
The overall mean (standard deviation [SD]) birth weight (from both cohorts combined) of infants was 3.03 kgs (0.56), and 55 (14%) of the infants were born with low birth weight <2.5 kilograms. The mean (SD) gestational age at delivery was 38.3 weeks (2.62). There were 11 infants out of 200 infants born from women sampled from the Siaya cohort who got RSV infection under 6 months of age. Additionally, among women from the Siaya cohort, 37 (19%) were HIV infected, 52 (26%) had malaria infection, 5 (3%) had RSV infection and 12 (6%) had severe anaemia during pregnancy. These additional data were not available for women from the Kilifi cohort.
Analysis of the difference in characteristics of participants from the two cohorts showed these women were significantly different in most characteristics ( Table 1). Compared to the Siaya sample, Kilifi pregnant women had more premature births with gestational age <33 weeks (5% vs. 2%; P=0.022), more babies were born with low birthweight <2.5 kgs (21.5% vs. 6%; P<0.001), more women had lower than the secondary level of formal education (73.5% vs. 55%; P<0.001), more were younger, i.e.19 years and below (16% vs. 2%; P<0.001), and more women were housewives (68% vs. 46%; P<0.001). However, these women were similar in proportion of those married (95.5% vs. 95%; P= 0.814) and female sex of the infant (47% vs. 48%; P=0.841).
Distribution of Cord and Maternal RSV-specific antibodies among pregnant women in Kenya
Overall, the mean cord PRNT titres from both cohorts was 10.69 log 2PRNT (SD: 1.17), with a median titre of 10.85 log 2PRNT (IQR 10.00–11.59), while the mean maternal log 2PRNT RSV antibodies was 10.53 log 2PRNT (SD:1.19), with a median of 10.62 log 2PRNT (IQR 9.92–11.40). The mean titres of cord RSV-specific antibodies from the Kilifi cohort was 10.64 log 2PRNT (SD:1.25), with a median titre of 10.91 log 2PRNT (IQR 9.92 -11.62). In the Siaya cohort, the mean cord RSV antibody titre was 10.74 log 2PRNT (SD:1.08), and median 10.79 log 2PRNT (IQR 10.09–11.55) respectively ( Figure 1). Both mean cord (10.64 vs. 10.74; p=0.374) and mean maternal (10.47 vs. 10.59; p=0.319) log 2PRNT titres of RSV-specific antibodies between Kilifi and Siaya cohorts were not significantly different.
Figure 1. Frequency distribution of cord and maternal RSV specific antibodies (log2 transformed PRNT titres) at birth from 400 women from Kilifi and Siaya, Kenya.
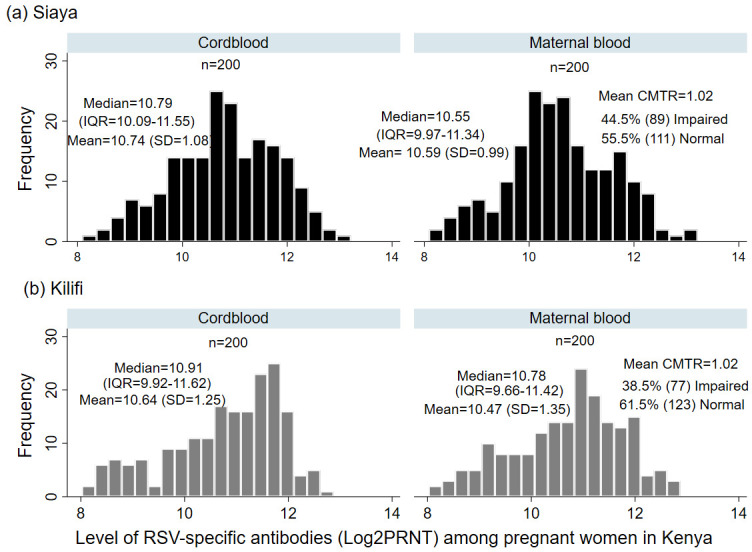
The mean (standard deviation), median (Inter quartile range) and the mean cord to maternal transfer ratio for each study cohort is shown.
For the Siaya cohort, we found no difference in the mean log 2PRNT titres in cord blood for infants with RSV infection compared to infants without RSV infection in the first 6 months of life (10.50 log 2PRNT (SD: 0.99) vs. 10.74 (SD: 1.07) respectively, p=0.186). The mean (SD) cord PRNT titres transferred to infants of 37 (19%) HIV-infected mothers of 10.41 (SD: 1.14) log 2PRNT was significantly lower (p=0.039) than that of infants from 163 HIV-uninfected mothers 10.81 (SD: 1.05) log 2PRNT. The cord log 2PRNT RSV antibody levels of infants from mothers with and without severe anaemia (10.87 vs. 10.73: p=0.202) and mothers with and without malaria (10.90 vs. 10.68: p=0.666) were not significantly different.
Efficiency of Transplacental Transfer of RSV-specific antibodies
The transplacental transfer of RSV-specific antibodies among these women was efficient with a mean CMTR of 1.02 (SD=0.09) and median 1.01 (IQR 0.97–1.06). The mean CMTR values were similar for pregnant women from Kilifi and Siaya (1.02 vs. 1.02; p=0.946; t=0.067).
The transplacental transfer of RSV specific antibodies for women from Kilifi and Siaya cohort was severely impaired in 1 (0.5%) and 6 (3.0%), moderately impaired in 76 (38.0%) and 83 (41.5%), and normal in 123 (61.5%) and 111 (55.5%), respectively. The overall proportion of women from the two cohorts with impaired transplacental transfer was 41.5% (166/400; 77 Kilifi vs. 89 Siaya).
Analysis of the trend in efficiency of transplacental transfer and characteristics of these women (Supplementary File 1 30 ), showed a significantly lower CMTR value among women who were HIV-infected (mean CMTR: 0.98 vs. 1.03; p=0.015) and women who reported their occupation as farming (mean CMTR: 0.96 vs.1.02; p=0.012). The CMTR value among women who got RSV infection during pregnancy (mean CMTR: 0.98 vs. 1.02; p=0.416) and that of mothers whom infants got RSV disease under 6 months of age (1.01 vs. 1.02; p=0.489) was not different.
Illness episodes during pregnancy and transplacental transfer of RSV-specific antibodies
Assessment of illness episodes from the 200 women sampled from the Siaya cohort showed 120 (60%) pregnant women had sick outpatient visits captured during weekly follow-ups and 6 of them required hospitalisation. Data on illness episodes during pregnancy among the Kilifi women was not available for this study because the surveillance was not designed to follow up participants prior to delivery.
The most common complaints about the outpatient visits among Siaya women were cough 67 (55.8%), abdominal pain 67 (55.8%), other acute respiratory illness (runny nose, shortness in breathing and chest pain while breathing 35 (29.2%)), joint pain 27 (22.5%), vomiting or diarrhoea 22 (18.3%), urinary tract infection 18 (15.0%), fever 17 (14.2%) and sore throat 12 (10.0%). One participant had premature labour. Experiencing cough episodes during pregnancy was found to be associated with impaired transplacental transfer of RSV-specific antibodies (67.3% vs. 32.7%: Chi 2 p=0.027).
Multiple illness episodes during the third trimester of pregnancy occurred in 79/120 (66%) of the sick participants. There were none of the following illness episodes reported during pregnancy in this sample of women: gestational diabetes, hypertension or pre-eclampsia. No illness episode during the third trimester of pregnancy was found to be associated with the efficiency of transplacental transfer of RSV-specific antibodies.
Gestational age at delivery and transplacental transfer of RSV-specific antibodies
The effect of gestational age in influencing transplacental transfer of RSV antibodies is demonstrated in a scatterplot of CMTR by duration in weeks of transplacental transfer (Supplementary File 2 30 ). The graph shows the level of CMTR is less than 1 or impaired within 4 weeks after onset of transplacental transfer (onset period estimated at 28 th week of gestation); CMTR gradually increases above one in most participants in the next 8 weeks and starts to decline to low levels 12 weeks after onset of transplacental transfer.
Factors influencing transplacental transfer of RSV-specific antibodies among pregnant women in Kenya
Among pregnant women in Kilifi, gestational age at delivery of <33 weeks was found to be significantly associated with reduced transplacental transfer of RSV-specific antibodies (p=0.034). In a univariate logistic analysis, transplacental transfer was likely to be increased 5.8 times more in births occurring between 34–37 weeks compared to births in less than 33 weeks of gestation (Odds ratio (OR): 5.8, 95% confidence interval (CI)1.33–24.95) ( Table 2).
Table 2. Predictors of an Impaired transplacental transfer among pregnant women from Kilifi cohort (univariant analysis).
| Efficiency of transplacental
transfer (Kilifi) |
Chi2 | Odds
ratio |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Category | Normal | Impaired | P value | OR | Odds Ratio
(95% CI) |
P value | |||
| n(123) | % | n(77) | % | LCL | UCL | |||||
|
Gestational age
delivery |
||||||||||
| <33 weeks | 3 | 2.4 | 7 | 9.1 | Ref | |||||
| 34–37 weeks | 42 | 34.2 | 17 | 22.1 | 0.034 | 5.8 | 1.33 | 24.95 | 0.019 | |
| 38–42 weeks | 78 | 63.4 | 53 | 68.8 | 3.4 | 0.84 | 13.88 | 0.083 | ||
| Birthweight | ||||||||||
| Underweight (<2.5kgs) | 25 | 20.3 | 18 | 23.4 | ||||||
| Normal | 98 | 79.7 | 59 | 76.6 | 0.609 | |||||
|
Maternal
age(yrs) |
||||||||||
| 16–19 | 18 | 14.6 | 13 | 16.9 | ||||||
| 20–29 | 65 | 52.9 | 43 | 55.8 | 0.589 | |||||
| 30–39 | 32 | 26.0 | 14 | 18.8 | ||||||
| 40–49 | 8 | 6.5 | 7 | 9.1 | ||||||
| Gravida | ||||||||||
| 1–2 | 72 | 58.54 | 40 | 51.0 | ||||||
| 3–5 | 41 | 33.33 | 24 | 31.2 | 0.305 | |||||
| 6–9 | 8 | 6.5 | 10 | 13.0 | ||||||
| 10–15 | 2 | 1.63 | 3 | 3.9 | ||||||
In the Siaya cohort, transplacental transfer of RSV-specific antibodies was found to be significantly impaired among women with; gravida of more than 6 (OR: 0.56, 95% CI:0.35–0.91; p=0.02), occupation as farming (OR: 0.13, 95% CI:0.03–0.60; p=0.009), HIV infection (OR: 0.47, 95% CI:0.23–0.98; p=0.045) and infants with low birth weight <2.5 kilograms (kgs) (OR: 0.25, 95% CI:0.065–0.94; p=0.041). In a multivariate analysis including gravida, HIV infection, occupation and birthweight to the model for Siaya women, only low birth weight <2.5 kgs was strongly associated with reduced efficiency of transplacental transfer of RSV specific antibodies (OR: 0.21, 95% CI:0.05–0.85; p= 0.029). Malaria infection was significantly associated with increased transplacental transfer of RSV-specific antibodies (OR: 1.95, 95% CI:1.01–3.78; p=0.048) ( Table 3). The majority of pregnancies (76%) among Siaya women were delivered at term, i.e., ≥37 weeks and there was no significant association found between efficiency of transplacental transfer and gestational age at delivery (p=0.186).
Table 3. Predictors of an Impaired transplacental transfer among pregnant women from Siaya cohort (univariant analysis).
| Transplacental transfer efficiency
(Siaya) |
Chi2 | Odds
ratio |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Category | Normal | Impaired | P value | OR | Odds Ratio
(95%CI) |
P value | |||
| n(111) | % | n(89) | % | LCL | UCL | |||||
| Maternal age (yrs) | 15-19 | 2 | 1.82 | 1 | 1.12 | |||||
| 20-29 | 83 | 74.77 | 58 | 65.17 | ||||||
| 30-39 | 25 | 22.52 | 26 | 29.21 | 0.068 | |||||
| 40-49 | 0 | 0.00 | 4 | 4.49 | ||||||
|
Gestational age at
delivery |
<33 weeks | 0 | 0.00 | 2 | 2.25 | |||||
| 34-37 weeks | 22 | 19.82 | 22 | 24.72 | 0.186 | |||||
| 38-42 weeks | 88 | 80.18 | 65 | 73.03 | ||||||
| Birthweight | ||||||||||
| Underweight (<2.5kgs) | 3 | 2.70 | 9 | 10.11 | ||||||
| Normal | 108 | 97.30 | 80 | 89.89 | 0.028 | 4.05 | 1.06 | 15.40 | 0.041 | |
| Gravida | ||||||||||
| 1-2 | 27 | 24.32 | 14 | 15.73 | Ref | |||||
| 3-5 | 72 | 64.86 | 55 | 61.80 | 0.049 | 0.67 | 0.33 | 1.42 | 0.301 | |
| 6-9 | 12 | 10.81 | 20 | 22.47 | 0.31 | 0.12 | 0.82 | 0.018 | ||
| Occupation | ||||||||||
| Farmer | 2 | 1.80 | 11 | 12.36 | Ref | |||||
| Business woman | 37 | 33.33 | 22 | 24.72 | 9.25 | 1.87 | 45.65 | 0.006 | ||
| Housewife | 50 | 45.05 | 42 | 47.19 | 0.031 | 6.55 | 1.37 | 31.21 | 0.018 | |
| Salaried worker | 10 | 9.01 | 8 | 8.99 | 6.87 | 1.17 | 40.38 | 0.033 | ||
| Other | 12 | 10.81 | 6 | 6.74 | 11.00 | 1.82 | 66.37 | 0.009 | ||
| HIV status | ||||||||||
| Negative | 96 | 86.49 | 67 | 75.28 | Ref | |||||
| Positive | 15 | 13.51 | 22 | 24.72 | 0.043 | 0.48 | 0.23 | 0.98 | 0.045 | |
| Sick_Cough | ||||||||||
| No | 36 | 52.94 | 17 | 32.69 | Ref | |||||
| Yes | 32 | 47.06 | 35 | 67.31 | 0.027 | 2.30 | 1.09 | 4.90 | 0.028 | |
| Malaria | ||||||||||
| No | 76 | 68.47 | 72 | 80.90 | Ref | |||||
| Yes | 35 | 31.53 | 17 | 19.10 | 0.046 | 1.95 | 1.00 | 3.78 | 0.048 | |
In a multivariate logistic analysis of combined data from the Kilifi and Siaya cohorts, occupation as a farmer (OR: 0.16, 95% CI:0.03–0.73; p=0.018), gravida>6 (OR: 0.70, 95% CI:0.52–0.94; p=0.023) and gestational age at delivery <33 weeks (OR: 0.22, 95% CI:0.06–0.84; p=0.027) were significantly associated with reduced transplacental transfer of RSV-specific antibodies. Adjusting for the study site did not have any effect on these factors.
Discussion
In this study, we found Kenyan women from the two geographical regions of Siaya and Kilifi differing in many characteristics, but these differences did not affect the mean levels of RSV-specific antibodies transferred to infants or the overall efficiency of transplacental transfer. We also found multiple factors, including gestational age less than 33 weeks, having had multiple pregnancies and farming as an occupation to be associated with reduced transplacental transfer of RSV specific antibodies among pregnant women from the two cohorts.
The concentration of RSV antibody transferred to infants by HIV-infected mothers were significantly reduced. Similarly, the trend of cord to maternal antibody titre ratio showed a decrease with HIV infection. These findings are in line with previous studies 31 which together raise concerns involving the effectiveness of a maternal RSV vaccine introduction to low-and middle-income countries (LMICs), which is thought might be negatively impacted by the existing comorbidities. Ongoing clinical trials (NCT04424316; NCT04605159) 6 of maternal RSV vaccines are not taking into account HIV-diverse populations, or populations with high malaria prevalence. Therefore, investigating differences in transplacental transfer in these populations could be important in validating vaccine response in the future.
In this study, gestational age at delivery <33 weeks showed reduced transplacental transfer of RSV-specific antibodies. Gestational age has been known to influence the transplacental transfer of IgG antibodies in the Gambia 20 and in Sri Lanka 32 where materno-foetal transfer of RSV-specific antibodies was impaired in premature babies. Furthermore, in estimating the duration of transplacental transfer by gestational age using the 28 th week of pregnancy as the onset for transplacental transfer, we found babies born shortly (<4 weeks) after the beginning of this transfer had an impaired transplacental transfer. Similarly, babies born more than 3 months after onset of transplacental transfer showed decreased CMTR which was as a result of antibody decay 33 . Studies have also shown that, before the 26 th week of gestation, IgG transfer is blocked by a barrier of cytotrophoblasts under the syncytiotrophoblast layer 34 and Fc gamma RII (FcgII) receptors responsible for mediation of materno-foetal transfer of antibodies are not well expressed 19 . Our results, therefore, demonstrate the phenomenon of accumulation of antibody concentration among infants with the time of transfer, waning of RSV-specific antibodies occurring with wild type RSV infection and confirm the influence of gestational age on timing for vaccination, likely to be observed during the implementation of a maternal RSV vaccine in this setting.
We also found having had more than 6 pregnancies, HIV infection, and low birth weight <2.5 kgs was associated with impaired transplacental transfer of RSV-specific antibodies. These results are similar to findings in the Gambia, where low birthweight <2.5 kgs was found to influence the transplacental transfer of RSV-specific antibodies 20 . The role of HIV infection in impairing transplacental transfer of RSV antibodies has been found in studies conducted in Botswana 35 and Malawi 36 . HIV-infected pregnant women have shown reduced immunogenicity to vaccines and this is thought to be related to immune activation leading to the production of inflammatory cytokines at the materno-foetal interface 37, 38 . Women with multiple births or pregnancies are usually older and due to repeated exposure to RSV infection, they are thought to have an accumulation of higher levels of antibodies which causes saturation of Fc transport receptors 39 leading to reduced transfer and thereby low levels of antibodies observed in neonates.
Women diagnosed with malaria during pregnancy had an efficient transplacental transfer in this study contrary to what has been observed in other studies in Papua New Guinea and Malawi 36, 40 where malaria was associated with a decrease in the transplacental transfer of IgG antibodies of 81%. The process of materno-foetal transfer of pathogen specific antibodies is likely to vary between different populations due to impairment caused by saturation of these receptors with infection-related hypergammaglobineamia 37 . We would therefore like to argue that, since antimalarial prophylaxis uptake is mandatory for all pregnant women in Kenya during antenatal visits, this might have played an important role in reducing malaria related hypergammaglobineamia. Thus, 67% of the women diagnosed with malaria infection were found to have a normal transplacental transfer of RSV-specific antibodies. However, further screening of samples for evidence of placental malaria is warranted among this sample of women.
This study has some limitations. First, we did not screen for total immuno-globulin G levels and thus are not able to confirm any infection related hypergammaglobulinemia to the impaired placental transfer of RSV antibody seen in HIV-infected women, women with illness episodes during pregnancy and those diagnosed with malaria in this study. Second, by the time this analysis was conducted, results for placental malaria among women from Siaya were not yet available, we could not, therefore, ascertain the positive effect of transplacental transfer in the presence of malaria infection. In addition, we could not get data for HIV antiviral therapy, adherence, or viral load among these women, although they were all under comprehensive care programme. We also used data from cohorts not necessarily designed for this study outcome. For instance, Kilifi cohort women did not have data on follow ups during pregnancy. The sample size was small for women with premature births, HIV infection, RSV infection, leading to wider confidence intervals and not so strong positive effect on predictors. However, we have provided important baseline data on the efficiency of transplacental transfer of RSV-specific antibodies in a setting where the maternal population experiences a high prevalence of malaria and HIV infections, and we have outlined some of the factors which would require mitigation or use of alternative prevention strategies during the introduction of a maternal RSV vaccine program for optimal vaccine outcome among infants.
Conclusions
Transplacental transfer of RSV-specific antibodies among pregnant women in Kenya is efficient. Maternal characteristics differed between women from the two different geographical regions, but this did not have a significant effect on the overall transplacental transfer of RSV antibodies. Maternal immunisation in the third trimester of pregnancy as a strategy to prevent infants from severe RSV disease should be considered with other interventions which will help protect infants born prematurely<33 weeks gestation, infants with low birth weight and infants from HIV infected mothers.
Data availability
The data is stored under restricted access and available from the authors upon request through our Data Governance Committee ( dgc@kemri-wellcome.org).
The dataset used and analysis scripts generated for this manuscript are available at Harvard Dataverse: Replication Data for: Efficiency of transplacental transfer of respiratory syncytial virus (RSV) specific antibodies among pregnant women in Kenya, https://doi.org/10.7910/DVN/XOKFFK 30 .
This project contains the following underlying data:
-
-
JNyiro_RSV_Antibody_Efficiency_Data_Codebook.pdf
-
-
JNyiro_RSV_Antibody_Efficiency_Data_Readme.txt
-
-
RSV_antibody_efficiency analysis script_04042021.do
-
-
RSV_antibody_Efficiency_KIPMAT_MATFLU_dataset_04062021–1.tab
-
-
RSV_antibody_Efficiency_KIPMAT_MATFLU_dataset_04062021.tab
-
-
Supplementary File 1: Transplacental transfer of RSV specific antibodies provided as cord to maternal transfer ratio (CMTR) with characteristics of women from Kilifi and Siaya, Kenya
-
-
Supplementary File 2: A scatter plot for cord to maternal transfer ratio by duration of transplacental transfer among pregnant women from Kilifi and Siaya, Kenya. The line for efficiency in each cohort is shown.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Disclaimer
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
Acknowledgements
We thank all pregnant women from Siaya and Kilifi for availing themselves and willingly provided data which answered the questions in this study. We are very grateful to the field study teams both CGHR-Kisumu and KWTRP-Kilifi who collected data from the surveillance cohorts. We appreciate the funding support from CDC (Grant# 5U01GH002133–02) for the influenza surveillance cohort and that of Wellcome Trust for surveillance of factors for mortality and morbidity in mothers and infants (Grant # 093804 and 077092). James Berkley was supported by MRC/DFiD/Wellcome Trust Joint Global Health Trials scheme grant# MR/M007367/1. This work was made possible in part by the funding support from Pfizer (Grant #52863073_CRG) and James Nokes’ Wellcome Trust senior investigator award (Grant # 102975). This paper is published with the permission of the Director of KEMRI.
Funding Statement
This work was supported by Pfizer (Grant # 52863073_CRG), CDC (Grant# 5U01GH002133-02) and Wellcome (Grant # 093804, 102975 and 077092).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Nair H, Nokes DJ, Gessner BD, et al. : Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375(9725):1545–1555. 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pneumonia Etiology Research for Child Health (PERCH) Study Group: Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet. 2019;394(10200):757–779. 10.1016/S0140-6736(19)30721-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nokes DJ, Ngama M, Bett A, et al. : Incidence and severity of respiratory syncytial virus pneumonia in rural Kenyan children identified through hospital surveillance. Clin Infect Dis. 2009;49(9):1341–1349. 10.1086/606055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shi T, McAllister DA, O'Brien KL, et al. : Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet. 2017;390(10098):946–958. 10.1016/S0140-6736(17)30938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Englund J, Glezen WP, Piedra PA: Maternal immunization against viral disease. Vaccine. 1998;16(14–15):1456–1463. 10.1016/s0264-410x(98)00108-x [DOI] [PubMed] [Google Scholar]
- 6. Madhi SA, Polack FP, Piedra PA, et al. : Respiratory Syncytial Virus Vaccination during Pregnancy and Effects in Infants. N Engl J Med. 2020;383(5):426–439. 10.1056/NEJMoa1908380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Glezen WP, Paredes A, Allison JE, et al. : Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98(5):708–715. 10.1016/s0022-3476(81)80829-3 [DOI] [PubMed] [Google Scholar]
- 8. Roca A, Abacassamo F, Loscertales MP, et al. : Prevalence of respiratory syncytial virus IgG antibodies in infants living in a rural area of Mozambique. J Med Virol. 2002;67(4):616–623. 10.1002/jmv.10148 [DOI] [PubMed] [Google Scholar]
- 9. Sande CJ, Cane PA, Nokes DJ: The association between age and the development of respiratory syncytial virus neutralising antibody responses following natural infection in infants. Vaccine. 2014;32(37):4726–4729. 10.1016/j.vaccine.2014.05.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Esposito S, Scarselli E, Lelii M, et al. : Antibody response to respiratory syncytial virus infection in children < 18 months old. Hum Vaccin Immunother. 2016;12(7):1700–1706. 10.1080/21645515.2016.1145847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jounai N, Yoshioka M, Tozuka M, et al. : Age-Specific Profiles of Antibody Responses against Respiratory Syncytial Virus Infection. EBioMedicine. 2017;16:124–135. 10.1016/j.ebiom.2017.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins D, Trujillo C, Keech C: Advances in RSV vaccine research and development - A global agenda. Vaccine. 2016;34(26):2870–2875. 10.1016/j.vaccine.2016.03.109 [DOI] [PubMed] [Google Scholar]
- 13. PATH: RSV vaccine and mAb snapshot.2020; (updated 26 March 2020). Reference Source [Google Scholar]
- 14. Suara RO, Piedra PA, Glezen WP, et al. : Prevalence of neutralizing antibody to respiratory syncytial virus in sera from mothers and newborns residing in the Gambia and in The United States. Clin Diagn Lab Immunol. 1996;3(4):477–479. 10.1128/cdli.3.4.477-479.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cox MJ, Azevedo RS, Cane PA, et al. : Seroepidemiological study of respiratory syncytial virus in Sao Paulo state, Brazil. J Med Virol. 1998;55(3):234–239. [DOI] [PubMed] [Google Scholar]
- 16. Homaira N, Binks M, Walker G, et al. : Transplacental transfer of RSV antibody in Australian First Nations infants. J Med Virol. 2022;94(2):782–786. 10.1002/jmv.27383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palmeira P, Quinello C, Silveira-Lessa AL, et al. : IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol. 2012;2012:985646. 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chu HY, Tielsch J, Katz J, et al. : Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J Clin Virol. 2017;95:90–95. 10.1016/j.jcv.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kameda T, Koyama M, Matsuzaki N, et al. : Localization of three subtypes of Fc gamma receptors in human placenta by immunohistochemical analysis. Placenta. 1991;12(1):15–26. 10.1016/0143-4004(91)90506-b [DOI] [PubMed] [Google Scholar]
- 20. Okoko JB, Wesumperuma HL, Hart CA: The influence of prematurity and low birthweight on transplacental antibody transfer in a rural West African population. Trop Med Int Health. 2001;6(7):529–534. 10.1046/j.1365-3156.2001.00741.x [DOI] [PubMed] [Google Scholar]
- 21. Okoko BJ, Wesumperuma LH, Ota MO, et al. : The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J Infect Dis. 2001;184(5):627–632. 10.1086/322808 [DOI] [PubMed] [Google Scholar]
- 22. Hartter HK, Oyedele OI, Dietz K, et al. : Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J. 2000;19(7):635–641. 10.1097/00006454-200007000-00010 [DOI] [PubMed] [Google Scholar]
- 23. Cavalcante RS, Kopelman BI, Costa-Carvalho BT: Placental transfer of Haemophilus influenzae type b antibodies in malnourished pregnant women. Braz J Infect Dis. 2008;12(1):47–51. 10.1590/s1413-86702008000100011 [DOI] [PubMed] [Google Scholar]
- 24. Seale AC, Barsosio HC, Koech AC, et al. : Embedding surveillance into clinical care to detect serious adverse events in pregnancy. Vaccine. 2015;33(47):6466–6468. 10.1016/j.vaccine.2015.07.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nyawanda BO, Otieno NA, Otieno MO, et al. : The impact of maternal HIV infection on the burden of respiratory syncytial virus among pregnant women and their infants, western Kenya. J Infect Dis. 2020;jiaa490. 10.1093/infdis/jiaa490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sande CJ, Mutunga MN, Okiro EA, et al. : Kinetics of the neutralizing antibody response to respiratory syncytial virus infections in a birth cohort. J Med Virol. 2013;85(11):2020–2025. 10.1002/jmv.23696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nyiro JU, Kiyuka PK, Mutunga MN, et al. : Agreement between ELISA and plaque reduction neutralisation assay in Detection of respiratory syncytial virus specific antibodies in a birth Cohort from Kilifi, coastal Kenya [version 1; peer review: 2 approved, 2 approved with reservations]. Wellcome Open Res. 2019;4:33. 10.12688/wellcomeopenres.15108.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sande CJ, Mutunga MN, Medley GF, et al. : Group- and genotype-specific neutralizing antibody responses against respiratory syncytial virus in infants and young children with severe pneumonia. J Infect Dis. 2013;207(3):489–492. 10.1093/infdis/jis700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McDonald JU, Rigsby P, Dougall T, et al. : Establishment of the first WHO International Standard for antiserum to Respiratory Syncytial Virus: Report of an international collaborative study. Vaccine. 2018;36(50):7641–7649. 10.1016/j.vaccine.2018.10.087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nyiro JU, Bukusi E, Mwaengo D, et al. : Replication Data for: Efficiency of transplacental transfer of respiratory syncytial virus (RSV) specific antibodies among pregnant women in Kenya.Harvard Dataverse, V1, UNF:6:aOIBDpFDzoTNDQaRw/DH0w== [fileUNF].2021. 10.7910/DVN/XOKFFK [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alonso S, Vidal M, Ruiz-Olalla G, et al. : Reduced Placental Transfer of Antibodies Against a Wide Range of Microbial and Vaccine Antigens in HIV-Infected Women in Mozambique. Front Immunol. 2021;12:614246. 10.3389/fimmu.2021.614246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wesumperuma HL, Perera AJ, Pharoah PO, et al. : The influence of prematurity and low birthweight on transplacental antibody transfer in Sri Lanka. Ann Trop Med Parasitol. 1999;93(2):169–177. 10.1080/00034989958654 [DOI] [PubMed] [Google Scholar]
- 33. Swamy GK, Garcia-Putnam R: Maternal immunization to benefit the mother, fetus, and infant. Obstet Gynecol Clin North Am. 2014;41(4):521–534. 10.1016/j.ogc.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 34. Kristoffersen EK: Placental Fc receptors and the transfer of maternal IgG. Transfus Med Rev. 2000;14(3):234–243. 10.1053/tm.2000.7393 [DOI] [PubMed] [Google Scholar]
- 35. Patel SM, Jallow S, Boiditswe S, et al. : Placental Transfer of Respiratory Syncytial Virus Antibody Among HIV-Exposed, Uninfected Infants. J Pediatric Infect Dis Soc. 2020;9(3):349–356. 10.1093/jpids/piz056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. : Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79(3):F202–205. 10.1136/fn.79.3.f202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilcox CR, Holder B, Jones CE: Factors Affecting the FcRn-Mediated Transplacental Transfer of Antibodies and Implications for Vaccination in Pregnancy. Front Immunol. 2017;8:1294. 10.3389/fimmu.2017.01294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abu-Raya B, Smolen KK, Willems F, et al. : Transfer of Maternal Antimicrobial Immunity to HIV-Exposed Uninfected Newborns. Front Immunol. 2016;7:338. 10.3389/fimmu.2016.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clements T, Rice TF, Vamvakas G, et al. : Update on Transplacental Transfer of IgG Subclasses: Impact of Maternal and Fetal Factors. Front Immunol. 2020;11:1920. 10.3389/fimmu.2020.01920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Atwell JE, Thumar B, Formica MA, et al. : Hypergammaglobulinemia and Impaired Transplacental Transfer of Respiratory Syncytial Virus Antibody in Papua New Guinea. Pediatr Infect Dis J. 2019;38(9):e199–e202. 10.1097/INF.0000000000002364 [DOI] [PubMed] [Google Scholar]


