Abstract
Purpose of Review
Patients diagnosed with CLL have an increased susceptibility to infections. Over the years, there has been a shift of the treatment arsenal to an increasing use of chemotherapy-free regimens, particularly small molecule inhibitors. These therapies have proven to be effective and have a favorable toxicity profile. Infections continue to represent a significant complication in the era of novel therapies.
Recent Findings
Recent studies continue to bring new insights into the effects of modern therapies on the immune system. Evidence supporting infection prevention strategies is scarce. We will review the available recommendations to prevent infections in patients with CLL treated with novel therapies.
Summary
New CLL therapies are broadly adopted in routine practice, requiring optimization of their side effects. Timely prevention, recognition, and treatment of infections should remain an important aspect of the standard management of a patient with CLL.
Keywords: Chronic lymphocytic leukemia, Treatment of CLL, Infections in CLL, COVID-19 and CLL
Introduction
Chronic lymphocytic leukemia (CLL) is the most prevalent leukemia in adults in the Western world [1]. At the time of diagnosis, the median age is 72 years, with an incidence of 4–5/100,000 population per year, which can go up to 30/100,000 in people older than 80 years [1–3]. CLL is a clonal B-cell lymphoproliferative neoplasm that is diagnosed by the detection of sustained lymphocytosis for at least 3 months (> 5 × 109/L) with the accumulation of small mature lymphocytes in the peripheral blood, bone marrow, spleen, and other lymphoid tissues. These lymphocytes are characterized by CD5 + CD19 + aberrant co-expression and immunoglobulin light chain restriction detected through flow cytometry [4]. This disease can have a heterogeneous clinical course, with most cases being indolent. Several disease-associated factors bear a negative prognostic impact; these include IGHV unmutated, chromosome 11q and 17p deletions, complex karyotype, CD49 expression, and mutations in TP53 [5–7]. Patients with CLL have various degrees of immunosuppression due to T-cell deregulation and a shift toward an increased number of exhausted T-cells, abnormal differentiation of macrophages toward a suppressive phenotype, and often marked and prolonged hypogammaglobulinemia resulting in an increased risk for infections [8].
In the last 20 years, major therapeutic discoveries have reshaped the treatment landscape in CLL. Small molecule inhibitors targeting BTK, PI3K, and BCL-2 have been approved for CLL treatment and are being increasingly used both as initial therapy and in treating recurrent disease. These therapies are less immunosuppressive and myelosuppressive compared to traditional chemoimmunotherapy regimens. However, they are not free of infectious complications.
Review
Risk of Infection Associated with Chronic Lymphocytic Leukemia
CLL precipitates the dysfunction of the innate and adaptive immune system. T-cells can exhibit malfunction activity leading to reduced cell-to-cell interaction blocked by CLL cells. Dendritic cells show incomplete maturation, lacking the maturation marker CD83 and costimulatory molecule CD80 generating ineffective stimulation of T-cells due to reduced IL-12 release. Of note, dendritic cell deficits are reversible once CLL achieves remission [9, 10]. Again, monocyte phagocytic capacity is impaired; for this reason, the production of reactive oxygen species is halted with poor reaction to bacterial lipopolysaccharide. Moreover, natural killer cells have reduced cytotoxic capabilities due to the defective expression of the NKG2D coreceptor [11, 12]. In like manner, complement dysfunction of the classical and alternate pathways has been reported, which can be worsened with CLL progression, further reducing its levels, and the inability to coat bacteria with C3b. Comparatively, hypogammaglobulinemia has been observed in up to 85% of patients. Progressive CLL is associated with a reduction of IgG and IgA. Patients are more susceptible to have recurrent infections when there is a deficiency of IG subtypes such as IgG3 and IgG4. Additionally, previous reports have observed that IgG subclass deficiency has been associated with shorter treatment-free survival [13, 14]. Neutropenia can result from CLL infiltration in the bone marrow in advanced cases. Additionally, functional deficiencies of the neutrophils can lead to defective bactericidal activity coupled with the decline of C5a-induced chemotaxis [15].
Risk of Infection Associated with BTK Inhibitor-Based Regimen
Bruton’s tyrosine kinase inhibitors (BTKi) block the B-cell receptor signaling pathway resulting in an effective anti-tumor activity with good overall tolerability. Ibrutinib is a first-generation irreversible BTKi; its effect is achieved by binding covalently to the cysteine 481 in the ATP binding site. Ibrutinib also has an affinity to other kinases such as interleukin-2-inducible T-cell kinase (ITK) and three epidermal growth factor receptor family kinases (EGFR): EGFR, ErbB2/HER2, and ErbB4/HER4, thereupon causing off-target effects [16•]. Ibrutinib’s safety and efficacy have been evaluated in several clinical trials. The RESONATE-2 and iLLUMINATE trials evaluated first-line ibrutinib single agent and in combination with obinutuzumab, respectively, demonstrating superior overall survival (OS) over 80% and progression-free survival (PFS) between 70 and 80% with high overall response rates (ORRs) of about 90%. In like manner, in patients with relapsed/refractory (R/R) CLL, ibrutinib was evaluated in the RESONATE and RESONATE-17 trials, demonstrating improved OS of over 70%, with a PFS of about 60%, and ORR of 90% (Table 1) [17•, 18–20].
Table 1.
Managing the risk of infection in chronic lymphocytic leukemia in the era of new therapies
| Clinical trial | Agent | PFS (%) | OS (%) | ORR/MRD (%) | All-grade infection (%) |
|---|---|---|---|---|---|
| RESONATE-2 [17•] | Ibrutinib vs. chlorambucil | 5 years: 70 vs. 12 | 5 years: 83 vs. 68 | 92 vs. 37 |
URI 26 PNA 12 |
| iLLUMINATE [18] | Ibrutinib + obunutuzumab vs. chlorambucil + obinutuzumab | 30 months: 79 vs. 31 | 88 vs. 73 |
URI 20 PNA 20 UTI 19 FN 13 |
|
| RESONATE [19] | Ibrutinib vs. ofatumumab | 3 years: 59 vs. 3 | 3 years: 74 vs. 65 | 91 |
PNA 17 Infections after 3 years 10% |
| RESONATE-17 [20] | Ibrutinib single-arm | 24 months: 63 | 24 months: 75 | 83 |
PNA 24 UTI 21 URI 17 |
| ELEVATE TN [21] | Acalabrutinib + obinutuzumab vs. acalabrutinib single-agent vs. chlorambucil + obinutuzumab | 24 months: 93 vs. 87 vs. 47 | 24 months: 95 vs. 95 vs. 92 | 94 vs. 86 vs. 79 |
URI 21; 18 UTI 12; 12 PNA 10;7 |
| ASCEND [22] | Acalabrutinib single-agent vs. I-R or BR | 16 months: 83 vs. 56 | 12 months: 94 vs. 91 | 81 vs. 75 |
URI 14 RTI 11 PNA 10 |
| NCT02343120 [23] | Zanubrutinib | 12 months: 100 | 96 |
URI 33 UTI 10.6 PNA 7.4 Cellulitis 5.3 |
|
| BRUIN [24•] | Pirtobrutinib | 63 | URI 7 | ||
| CLL-14 [41•] | Venetoclax + vs. obinutuzumab chlorambucil + obinutuzumab | 3 years: 81 vs. 49 | NR vs. NR | PB MRD: 75 vs. 35 |
PNA 10 FN 6 Sepsis 5 |
| MURANO [43] | Venetoclax + rituximab vs. BR | 4 years: 57 vs. 4.6 | 4 years: 85 vs. 66 | 92 vs. 72 |
Infections 17 FN 3.6 PNA 5.2 |
| NCT01539512 [49] | Idelalisib + rituximab vs. placebo + rituximab | 24 weeks: 93 vs. 46 | 12 months: 92 vs. 80 | 81 vs. 13 |
PNA 6 FN 5 Sepsis 4 PJP 3 Neutropenic sepsis 3 Cellulitis 1 |
| IDELA [50] | Idelalisib + rituximab vs. placebo + rituximab, with open-label idelalisib extension | 48 weeks: 76 | 24 months: 69 vs. 51 | 85 vs. 47 |
FN 4.5 PJP 3.6 CMV 0.9 PNA 2.7 URI 2.7 |
| DUO [52] | Duvelisib vs. ofatumumab | 12 months: 60 vs. 39 | 12 months: 86 vs. 86 | 73 vs. 45 |
Infections 69 PNA 18 URI 16 |
| DYNAMO [51] | Duvelisib single-agent | 6 months: 62 | 12 months: 77 | 67 |
FN 9 PNA 7 |
PFS progression-free survival, OS overall survival, ORR overall response rate, MRD measurable residual disease, VS versus, URI upper respiratory tract infection, RTI respiratory tract infection, PNA pneumonia, UTI urinary tract infection, FN febrile neutropenia, PJP Pneumocystis jirovecii
The second-generation BTKi acalabrutinib has shown the capacity to covalently bind BTK with less off-target effects. The ELEVATE-TN trial evaluated acalabrutinib in untreated CLL, while the ASCEND trial studied acalabrutinib in patients with R/R CLL, demonstrating an OS of over 90% with and PFS that ranges between 80 and 93%. Additionally, the ORR ranged from 80 to 94% (Table1). [21, 22] More recently, the safety and efficacy of zanubrutinib have been reported in a phase I trial showing a PFS at 1 year of 100% with an ORR over 95%. Preliminary data on pirtobrutinib shows an ORR of over 60%, and the adverse events grade 3 or higher associated with these BTKi were uncommon [23, 24•].
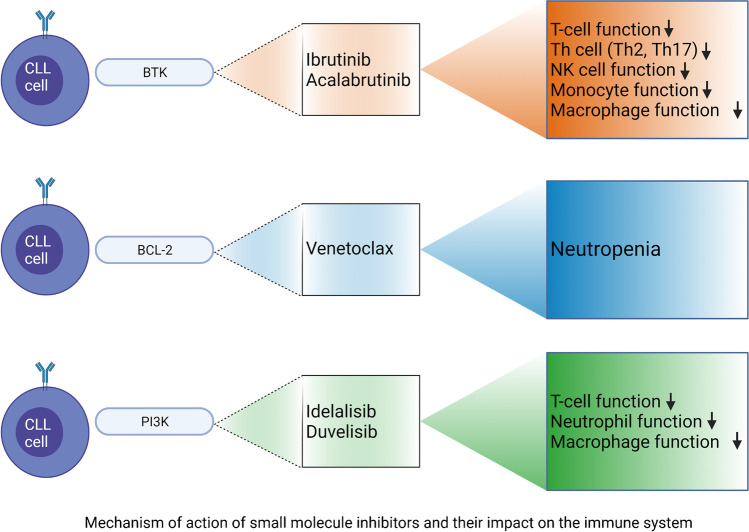
The immune system has several critical functions that can be affected by inhibiting BTK (Fig. 1). Preclinical studies have shown that ibrutinib can impair NK cell function, halting the capacity of antibody-dependent cellular cytotoxicity [25, 26]. Fioraci et al. demonstrated that ibrutinib and acalabrutinib could reduce the production of pro-inflammatory cytokines such as TNFα and IL-1β and phagocytic function of monocytes and macrophages. Moreover, the immune response of nurse-like cells (NLCs) and the level of IκBα and AKT phosphorylation levels are markedly inhibited during fungal infection [27]. Multer and coll. showed that treatment with ibrutinib decreases CD4 + and CD8 + T-cells and CD3-CD56 + NK cells, with possible T-cell recovery after 6 months of therapy and reducing the disease tumor burden. However, CD4 + and CD8 + effector memory cells can decrease after 12 months of treatment. Similarly, a reduction in CTLA-4 and PD-1 expressing T-cells has also been observed [28]. Yin, et al. reported that ibrutinib carries a selective effect reducing Th1, Th2, and Th17 type cytokines, in addition to an increased ratio of INF-γ/IL-4 after 12 months of therapy, suggesting that IFN-γ-producing Th1 cells became more prevalent during ibrutinib treatment. [29] The incidence of all-grade infections reported in clinical trials with ibrutinib is about 56%. Upper respiratory tract infection (URI) and urinary tract infection (UTI) were the most common source of infection [30]. Grade ≥ 3 infections occurred in 26% of the patients, with pneumonia being the most frequent infection in 13%. In clinical studies with acalabrutinib, grade ≥ 3 pneumonia was seen in 8% of the patients [16•, 31].
Fig. 1.
Managing the risk of infection in chronic lymphocytic leukemia in the era of new therapies
In terms of fungal infections, the first 6 months of treatment with ibrutinib carry the highest risk for acquiring this type of infection [32]. Aspergillus species were the most frequent type of fungus causing infection in 61% of the patients, whereas Cryptococcus species caused 25% of the fungal infections [32–34]. Another opportunistic infection to remember is Pneumocystis jirovecii pneumonia (PJP), previously reported in clinical trials and case series. The possibility of acquiring this opportunistic infection supports the idea of ibrutinib-associated T-cell dysfunction. [35–37]
Risk of Infection Associated with BCL-2 Inhibitor-Based Regimen
Venetoclax is an oral BH-3 mimetic and selective BCL-2 inhibitor; it also interacts weakly with BCL-XL and BCL-W. In CLL, BCL-2 is overexpressed, and its inhibition leads to the initiation of apoptosis mediated by BAX and BAK [38, 39]. For untreated CLL, the BCL-2 containing arm of the CLL-14 trial evaluated the combination of venetoclax plus obinutuzumab, showing a PFS of 81%, while the OS was non-reached in the venetoclax arm. Additionally, higher rates of undetectable measurable residual disease (U-MRD) were observed with venetoclax. The MURANO study in patients with R/R CLL also randomized patients to a BCL-2 containing arm venetoclax plus rituximab showing a PFS of 57%, with an OS of 85%, and the ORR was 92% (Table 1) [40, 41•, 42, 43]. Cytopenia seems to be the primary immunosuppressive effect of venetoclax (Fig. 1). In the CLL-14 trial, neutropenia was the most common grade ≥ 3 adverse event in 53% of patients, of which 13% required dose-reduction and venetoclax discontinuation in 2% of patients. Grade ≥ 3 infection occurred in 18% of patients in the venetoclax arm, and pneumonia represented the most frequent type of grade ≥ 3 infection in 5% of patients. Infection was the most common cause of death in 1% of the patients in the venetoclax arm, which did not differ from the chlorambucil arm [41•]. In the MURANO trial, 60% of patients in the venetoclax plus rituximab arm experienced any grade of neutropenia. Neutropenia was the most common grade ≥ 3 adverse event in the venetoclax plus rituximab (57%) with a median duration of 8 days. The incidence of grade ≥ 3 febrile neutropenia was about 3% in the venetoclax plus rituximab arm. Grade ≥ 3 infections occurred in 17%, with pneumonia being the most frequent type of infection in 5% of the patients [42].
Davids et al. reported that all-grade infections could occur in up to 72% of patients, with upper respiratory tract infections being the most frequent in 25% of patients followed by pneumonia in 11%, nasopharyngitis in 10%, and urinary tract infection in 10%. They also identified patients with prior exposure to fludarabine to have a higher risk of infections when subsequently treated with venetoclax. The incidence of opportunistic infections is 3.1%, including Aspergillus pneumonia, PJP, ocular toxoplasmosis, nocardiosis, herpes pharyngitis, multidermatomal herpes zoster, and candida esophagitis. The median time to opportunistic infection was 4.5 months; there were no deaths related to opportunistic infections in patients treated with venetoclax [44].
Risk of Infection Associated with PI3K Inhibitor-Based Regimen
Phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT/PKB)/mammalian target of rapamycin (mTOR) pathways is an important signaling pathway promoting B-cell development and function. CLL cells rely on signaling through BCR, which activates the PI3K-dependent pathway resulting in increased survival, proliferation, adhesion to stromal cells via VLA-4, and chemokine secretion (CCL3 and CCL4) [45]. PI3K inhibitors such as idelalisib bind the pocket of the catalytic subunit of PI3K, nullifying the PI3K/AKT/mTOR cascade resulting in apoptosis of CLL cells [46, 47]. There are four different isoforms of the catalytic subunits (p110α, p110β, p110γ, p110δ). The expression of the isoforms p110α and p110β is present in various tissues, while p110γ and p110δ are restricted to leukocytes [48]. Idelalisib has been evaluated in clinical trials in R/R CLL. The PFS ranges between 76 and 93%, with an OS of 90% at 1 year and 70% at 2 years. The ORR is over 80% [49, 50]. Duvelisib is another PI3K inhibitor with dual PI3Kγ/δ activity approved in 2018 for R/R CLL after ≥ 2 prior therapies. The DUO and DYNAMO trials reported a PFS of about 60%, with an OS between 77 and 86%. The ORR was about 70% (Table 1) [51, 52]. Studies have shown that the knockout of p110γ leads to altered T-cell, neutrophil, and macrophage function; moreover, the dual blockade of p110γ, p110δ resulted in survival impairment of B-cells (Fig. 1) [53]. PI3K inhibition has been associated with a decrease in Treg and surface levels of PD-1 and CTLA-4. Furthermore, Maus et al. have demonstrated in preclinical studies that inhibition of PI3Kγ leads to disruption of the innate immune response of the lung to the challenge by S. pneumoniae [54]. Similarly, PI3Kγ-deficient neutrophils exhibit defects in migration and oxidative burst [55]. In the multicenter, randomized, double-blind placebo-controlled phase 3 study with idelalisib plus rituximab, the reported serious adverse events included pneumonia 6% and febrile neutropenia 5%. Additionally, sepsis was observed in 4% of the patients. In the multicenter, randomized, double-blind placebo-controlled phase 3 study in patients with R/R CLL with idelalisib versus bendamustine-rituximab (BR), the most common grade ≥ 3 adverse events in the idelalisib arm were febrile neutropenia in 23% and neutropenia in 60% of the cases. Sixty-nine percent of patients in the idelalisib arm developed infections. Furthermore, in this group of patients, the causes of death included pneumonia in three patients, sepsis in three patients, and septic shock in two patients [49, 56]. In the DUO trial, a global phase 3 randomized to oral duvelisib or ofatumumab. In the duvelisib arm, infections occurred in 69% of the cases, with pneumonia in 18% (grade ≥ 3 in 14%) and upper respiratory tract infection in 16% of the patients. In the open-label global phase 2 DYNAMO trial, patients treated with duvelisib acquired CMV pneumonia and bronchopulmonary aspergillosis in one patient, respectively. Duvelisib was discontinued due to pneumonia in three patients, and three patients with febrile neutropenia required dose reduction. In this study, one patient died from a suspected fatal viral infection, one died from grade 4 neutropenia with septic shock, and one died from pneumonia [51, 52].
How Do We Manage the Risk of Infections in the Era of New CLL Therapies?
When taking care of a patient with CLL, the risk for infection complications should always be carefully evaluated as part of a comprehensive assessment. Several factors contribute to the risk of infections; these include older age; comorbidities such as chronic kidney disease, type 2 diabetes mellitus, or chronic pulmonary disease; and the type and number of prior therapies. Once the patient has started a small molecule inhibitor for CLL, it is crucial to monitor blood counts regularly. If the patient is being treated with venetoclax or a PI3K inhibitor, either monotherapy or in combination grade ≥ 3, neutropenia can develop in up to 41% of the cases. In these patients, the incidence of febrile neutropenia remains low likely because of its short duration since it responds to dose adjustment and/or granulocyte colony-stimulating factor (G-CSF) if necessary [44, 51, 56] and because these regimens are not associated with mucositis. Patients treated with BTK inhibitors less frequently develop neutropenia. However, among BTKi, grade ≥ 3 neutropenia was observed with acalabrutinib in 16%, going up to 40% when combined with rituximab. A phase 1 study with zanubrutinib reported grade ≥ 3 neutropenia in 6.4% of patients. Recent data on the phase 1/2 study with pirtobrutinib reported grade ≥ 3 neutropenia in 5% of the patients [22, 23, 24•].
Prevention of Bacterial Infections
There is no standard recommended antibacterial prophylaxis in patients with CLL treated with modern therapies. The clinicians need to be familiar with the most common sites of infection that have been observed in patients treated with these therapies, which include the respiratory tract, the lungs, and the urinary tract. Moreover, hypogammaglobulinemia can be present before therapy or can develop with these treatments, which can be associated with an increased risk of infections often caused by Streptococcus pneumoniae and Haemophilus influenza [57]. The use of immunoglobulin (Ig) replacement therapy can be helpful in reducing the frequency of infections. However, it should only be administered to patients with severe hypogammaglobulinemia (< 400 mg/dL) and/or recurrent or severe infections (Table 2) [2].
Table 2.
Available strategies for infection prevention
| Bacterial infection |
1. No routine antibiotic prophylaxis is recommended 2. Monitor ANC, especially with BCL-2i or PI3Ki 3. Ig replacement considered if IgG levels < 400 mg/dL 400–600 mg/dL: if severe recurrent infections |
| Fungal infection |
1. Suggested: a. Elderly patients with comorbidities b. Prolonged neutropenia (> 6 months) c. R/R CLL d. Chronic concomitant steroid therapy 2. Recommended: a. Pneumocystis jirovecii prophylaxis in patients treated with PI3Ki |
| Viral infection |
1. Pre-treatment of HBV, HCB, HIV, HSV 1/2, VZV, and CMV 2. Treatment with PI3ki, monitor CMV viral load monthly 3. If HBV reactivation is detected, prophylaxis with entecavir or tenofovir is recommended |
| Vaccinations |
1. Recommended vaccines: a. Seasonal influenza vaccine, preferably the high dose quadrivalent b. Pneumococcal vaccine: Pneumovax (PPSV23) followed by Prevnar (PCV13) c. Recombinant zoster vaccine two doses d. Recombinant hepatitis B vaccine three doses |
| SARS-COVID-2 |
1. Encourage patients to get vaccinated in the US with Pfizer-BioNtech or Moderna 2. If severe COVID-19 infection: Hold CLL treatment until the patient has been asymptomatic for 48 h, 14 days have elapsed from the start of the infection, and two consecutive negative RT-PCR tests |
ANC absolute neutrophil count, Ig immunoglobulin, IgG immunoglobulin G, BCL-2i BCL-2 inhibitor, PI3Ki PI3K inhibitor, MG milligrams, dL deciliter, R/R CLL relapsed/refractory chronic lymphocytic leukemia, HBV hepatitis B virus, HCV hepatitis C virus, HIV human immunodeficiency virus, HSV1/2 herpes simplex virus 1 and 2, VZV varicella-zoster virus, CMV cytomegalovirus, PPSV23 pneumococcal polysaccharide vaccine, PCV13 pneumococcal 13-valent conjugate vaccine, SARS-COVID-2/COVID19 severe acute respiratory syndrome coronavirus 2
Prevention of Fungal Infections
The evidence on the incidence of invasive fungal infections (IFI) is scarce; according to a multicenter Italian study, the incidence rate of IFI is 0.5% [58]. There are no guidelines regarding which patients will benefit from prophylaxis. However, it has been suggested that prophylaxis should be considered in frail older patients (> 75 years) with R/R CLL and/or prolonged neutropenia (> 6 months) [59]. For patients receiving BTK inhibitors or BCL-2 inhibitors along with chronic steroid therapy, i.e., to treat autoimmune cytopenias or with a previous history of fungal infections, prophylaxis may be advised [60]. If prophylaxis with an azole is started, it is important to remember that these drugs are potent CYP3A inhibitors that interact with small molecule inhibitors, temporary discontinuation, or dose adjustment, along with close monitoring for toxicities will be needed. The risk of opportunistic infections has decreased with new therapies. However, PJP infections have been reported in patients treated with PI3Ki. In patients with CLL not receiving PJP prophylaxis, the incidence of PJP pneumonia is 3.5%. The incidence is lower compared to 9.1% in those treated with fludarabine-cyclophosphamide and rituximab; however, it prompted the development of specific recommendations for patients with CLL treated with PI3Ki [61, 62]. It is recommended that CLL patients treated with idelalisib or duvelisib start prophylaxis at the beginning of the CLL therapy, maintaining it for as long as 2 to 6 months after the end of the PI3K inhibitor treatment. Clinical trials with idelalisib reported an incidence of 2 to 3.6% of patients with PJP. All patients that contracted PJP infection were not taking prophylaxis. In the Idelalisib vs. placebo ± BR study, one patient acquired PJP while on prophylaxis. In the DUO trial, three patients treated with duvelisib who were not on prophylaxis developed PJP despite the protocol requiring the patients to be on prophylaxis. Moreover, in the DYNAMO trial, one patient who was prescribed prophylaxis acquired PJP [56, 63, 64•]. TMP-SMX has been the drug of choice for prophylaxis due to its high effectiveness, which uniquely offers protection against toxoplasmosis, nocardiosis, and actinomycosis. Patients with intolerance or severe adverse events associated with TMP-SMX can use atovaquone, dapsone, or pentamidine (aerosolized or intravenous) as alternative agents. Of these, pentamidine has a more favorable profile given the absence of hematologic toxicity or drug-to-drug interactions in addition to a dosing interval of 28 days [63]. TMP-SMX dosing options include one single-strength (80/400 mg) tablet daily or one double-strength (160/800 mg) tablet thrice a week. These dose regimens have shown no difference in terms of efficacy (Table 2) [65].
Prevention of Viral Infections
The current practice to monitor for viral infections recommends reviewing the serological evidence of prior exposure or laboratory evidence of chronic infection with viruses such as hepatitis B and C viruses, cytomegalovirus (CMV), and human immunodeficiency virus (HIV), Herpes simplex virus 1/2, and Varicella zoster virus before starting any therapy for CLL [2, 7, 64•]. Hepatitis B virus (HBV) reactivation is defined by the elevation of serum alanine aminotransferase (ALT) and HBV-DNA increased with or without HBsAg recurrence (reverse seroconversion) in anti-HBc positive patients [66]. With this in mind, if a patient is found to have HBV reactivation, pre-emptive therapy with entecavir or tenofovir has been recommended because of their high potency and low resistance rate, in addition to superior prevention of further reactivation episodes and reduction of HBV-related mortality [67]. Important to note is that if an anti-CD20 monoclonal antibody is part of the CLL regimen, HBV therapy should be maintained for 12 to 18 months after the last dose of the monoclonal antibody [68]. In the study with idelalisib plus rituximab in the IDELA/R-to-IDELA arm, two patients had grade1-2 non-fatal CMV infection. In the idelalisib vs. placebo ± BR trial, 13 (6%) patients in the idelalisib arm had CMV infection compared to three (1%) in the placebo group [56, 69]. For this reason, seronegative patients should receive CMV negative or filtered blood products, and CMV serology should be tested before starting the treatment, and monthly follow-up of the viral load through quantitative polymerase chain reaction (qPCR) testing is required. For patients with positive CMV PCR and/or increasing viral load or symptoms suggestive of CMV infection, idelalisib should be discontinued, and ganciclovir or valganciclovir should be initiated [70, 71]. Furthermore, anti-viral prophylaxis against the Herpes simplex virus in patients treated with idelalisib or duvelisib is also recommended [64•]. The impact of latent viral infections has not been described in patients treated with BTKi or BCL-2i. Hence, these patients should be managed in a case-to-case manner (Table 2) [60].
Vaccine Recommendations
Vaccines represent an essential tool to prevent infections in healthy individuals. In patients with CLL, response to vaccination is blunted by the immunosuppression that accompanies this disease. Recent reports have shown that the immune system’s capacity to respond to the vaccine in patients treated with modern therapies is limited. Douglas et al. reported that only 26% of patients treated with BTKi showed seroconversion with the high-dose trivalent influenza vaccine [72]. Similarly, the level of seroconversion after receiving the recombinant hepatitis B vaccine was diminished in patients with CLL under treatment with BTK inhibitors (28% vs. 3.8%). Conversely, seroconversion to the recombinant zoster vaccine did not differ between treatment-naïve CLL and those treated with BTK inhibitors [73]. Pneumococcal vaccine should also be encouraged in patients with CLL. Ideally, like all the other vaccinations, it should be given when CLL is at an early stage and/or stable disease without requiring treatment to obtain a better immune response. Factors associated with lower pneumococcal vaccination response are patients aged > 60 years, IgG levels < 400 mg/L, prior treatment, and progressive disease [74•].
Current Recommendations for SARS-COVID-2
The occurrence of COVID-19 cases among patients with CLL and the outcome of the infection is a topic of particular interest during the ongoing COVID pandemic. Early data were reported in an Italian study conducted between February and December 2020. The incidence of severe acute respiratory syndrome coronavirus 2 (SARS-COVID-2) in patients with CLL was 3437.7 COVID-19 cases per 100,000; 23.3% of them occurred in people 65 years or older [75]. The mortality rate was high during the early phases of the pandemic; according to the European Research Initiative on CLL (ERIC) and Campus CLL, it was 32.5% in hospitalized patients [76•]. In the worldwide study of patients with CLL hospitalized with COVID-19, the median age was 72 in cohort 1 and 68 in cohort 2. A multivariate analysis of this patient population identified advanced age at COVID-19 diagnosis as an independent predictor for overall survival. For patients with CLL and COVID-19 requiring hospitalization, the case-fatality rate was between 30 and 34%. On the other hand, individual case reports have hypothesized that ibrutinib can abrogate pulmonary inflammatory cytokines and lung injury. However, this evidence is conflicting. Roeker LE et al. concluded that CLL-directed therapies were not conclusive for predicting COVID-19-related survival nor played a protective role for COVID-19 outcome [77•, 78]. In the GAIA/CLL13 multicenter phase 3 investigator-initiated trial in treatment-naive patients in the venetoclax-based regimen arm, six patients developed COVID-19, and the mortality rate of those diagnosed with COVID-19 was 28.6% [79]. If a patient requires treatment for his CLL, the clinician should weigh risks and benefits in addition to contemplating the risk of contracting COVID-19 infection. The addition of an anti-CD20 monoclonal antibody should also be delayed because it compromises humoral immunity, which is an important mechanism to support recovery from COVID-19. Currently, there is a debate on making definitive recommendations for the use of anti-CD20 monoclonal antibodies in this context [80, 81]. For patients who present with moderate to severe COVID-19-related symptoms, it is recommended to hold the CLL treatment, in particular, anti_CD20 monoclonal antibodies, venetoclax, or PI3K inhibitors due to their myelosuppressive effect. The CLL treatment can be resumed if the patient has been asymptomatic for 48 h, 14 days have elapsed from the start of the infection, and has two consecutive negative RT-PCR tests [75, 80].
Vaccination against COVID-19 is recommended for patients with CLL. Several reports have estimated a low overall antibody response of about 40% to COVID-19 mRNA vaccines in patients with CLL [82]. With this in mind, younger age, female gender, untreated CLL, and normal levels of IgG and IgM have been considered predictors of positive seroconversion in response to the COVID-19 vaccine. In patients undergoing treatment regimens involving BTK inhibitors or BCL-2 inhibitors ± anti-CD20 monoclonal antibody, the response rate ranged between 13 and 16%. If an anti-CD20 monoclonal antibody was given less than 12 months before vaccination, these patients did not exhibit seroconversion [75]. In our routine practice, we continue to encourage patients to receive the vaccine, including the third booster dose; at present, there is a need to elucidate the role of T-cell populations in supporting the immune response after vaccination beyond the detection of antibodies through serological testing. We also continue to reinforce maintaining general safety precautions (Table 2).
Upcoming Targeted Therapies:
In the upcoming years, additional targeted therapies are likely to become available to patients with CLL based on the preliminary efficacy and safety shown by ongoing studies. The third-generation BTKi pirtobrutinib (LOXO-305), vecabrutinib (SNS-062), and ARQ-531 have a non-covalent inhibitory capacity that does not require a cysteine C481S binding site. These agents are in late-phase clinical trials that will provide more information about safety profiles and efficacy in the near future [83]. The next-generation PI3Ki umbralisib (TGR-1202) has a dual inhibitory effect on PI3K δ and casein kinase-1. A phase 2 study showed a 24-month PFS of 46%, and the median OS was not reached with a median follow-up of 23 months. The ORR was 44%. All grade URI incidence was 14% and PNA in 14% of patients [84]
Cellular therapies are also likely to join the treatment armamentarium in patients with recurrent disease. In clinical trials, chimeric antigen receptor T (CART) therapy has shown activity in patients with R/R CLL. Fey and coll, conducted a prospective study in 38 patients treated with anti-CD19 CART cells (autologous T-cells were collected, and clinical-grade CD19 TCR-ζ/4-1BB lentiviral vector was manufactured). The ORR at 4 weeks was 44%. The OS at 36 months was around 60% in patients treated with both dose levels (low [5 × 107] and high [5 × 108]) [85•]. Patients undergoing CART therapy should always be screened for hepatitis virus B and C in addition to human immunodeficiency virus. Furthermore, screening for other pathogens is recommended, including herpes simplex virus 1/2, varicella-zoster virus, CMV, human T-cell lymphotropic virus type 1, Treponema pallidum, and Toxoplasma gondii. It is also important to be aware that Mycobacterium tuberculosis can be reactivated in those patients with prolonged steroid exposure or tocilizumab (IL-6 receptor antagonist). For patients who have spent time in a tropical or subtropical region, Strongyloides stercolaris should also be considered. In our center, we implement fluoroquinolone prophylaxis during any prolonged neutropenic period (< 0.5 × 103/µL), although it is not routinely used. Anti-viral prophylaxis is recommended with acyclovir 400 mg twice daily or valacyclovir 500 mg once daily (others may consider twice daily), starting at the time of lymphodepletion and continuing it for 6 months post-CART therapy [86]. Patients with detectable HBV DNA in blood should be treated with entecavir 0.5 mg daily starting pre-CART therapy and continuing it for at least 6 months. If the patient is positive for HBc antibodies with HBsAg and HBV DNA negative, alanine aminotransferase and viral DNA could be monitored every 1 to 3 months as an alternative to entecavir prophylaxis [87]. Antifungal prophylaxis is also recommended with an azole or echinocandins until the neutrophil count recovers. PJP prophylaxis with TMP-SMX should be started after the neutrophil count is > 0.5 × 103/µL or at day 28 after CART infusion and continued for at least 6 months [88•]. Ig replacement is recommended if serum IgG is < 400 mg/dL. If IgG is between 400 and 600 mg/dL, consider Ig replacement if a patient has severe recurrent infections [89]. Before CART therapy, patients should be advised to be vaccinated against COVID-19. The influenza vaccine can be given 2 weeks before lymphodepletion during flu season. Six months after CART therapy, patients should receive the pneumococcal vaccine, Clostridium tetani, Corynebacterium diphtheriae, Bordetella pertussis, and HBV vaccines (if required). [90]
Conclusions
Patients diagnosed with CLL and treated with novel therapies achieve better outcomes in terms of progression-free survival, overall survival, and responses. At the same time, the risk of infection continues to represent a significant cause of morbidity and, if not adequately addressed, can cause mortality. As we continue to incorporate these new therapies more broadly and become familiar with their effects on the immune system, the management of infection prevention will continue to be optimized. With more research on this matter, new recommended practices will need to be adopted to optimize supportive care measures as we continue to shift toward a chemotherapy-free era.
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical collection on Leukemia
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
- 1.DeSantis CE, Miller KD, Dale W, Mohile SG, Cohen HJ, Leach CR, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69(6):452–467. doi: 10.3322/caac.21577. [DOI] [PubMed] [Google Scholar]
- 2.Eichhorst B, Robak T, Montserrat E, Ghia P, Niemann CU, Kater AP, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(1):23–33. doi: 10.1016/j.annonc.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Hallek M, Shanafelt TD, Eichhorst B. Chronic lymphocytic leukaemia. Lancet. 2018;391(10129):1524–1537. doi: 10.1016/s0140-6736(18)30422-7. [DOI] [PubMed] [Google Scholar]
- 4.Kipps TJ, Stevenson FK, Wu CJ, Croce CM, Packham G, Wierda WG, et al. Chronic lymphocytic leukaemia. Nat Rev Dis Primers. 2017;3:16096. doi: 10.1038/nrdp.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Döhner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi: 10.1056/nejm200012283432602. [DOI] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–2760. doi: 10.1182/blood-2017-09-806398. [DOI] [PubMed] [Google Scholar]
- 8.Williams AM, Baran AM, Meacham PJ, Feldman MM, Valencia HE, Newsom-Stewart C, et al. Analysis of the risk of infection in patients with chronic lymphocytic leukemia in the era of novel therapies. Leuk Lymphoma. 2018;59(3):625–632. doi: 10.1080/10428194.2017.1347931. [DOI] [PubMed] [Google Scholar]
- 9.Orsini E, Guarini A, Chiaretti S, Mauro FR, Foa R. The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 2003;63(15):4497–4506. [PubMed] [Google Scholar]
- 10.Orsini E, Pasquale A, Maggio R, Calabrese E, Mauro FR, Giammartini E, et al. Phenotypic and functional characterization of monocyte-derived dendritic cells in chronic lymphocytic leukaemia patients: influence of neoplastic CD19 cells in vivo and in vitro. Br J Haematol. 2004;125(6):720–728. doi: 10.1111/j.1365-2141.2004.04971.x. [DOI] [PubMed] [Google Scholar]
- 11.Maffei R, Bulgarelli J, Fiorcari S, Bertoncelli L, Martinelli S, Guarnotta C, et al. The monocytic population in chronic lymphocytic leukemia shows altered composition and deregulation of genes involved in phagocytosis and inflammation. Haematologica. 2013;98(7):1115–1123. doi: 10.3324/haematol.2012.073080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huergo-Zapico L, Acebes-Huerta A, Gonzalez-Rodriguez AP, Contesti J, Gonzalez-García E, Payer AR, et al. Expansion of NK cells and reduction of NKG2D expression in chronic lymphocytic leukemia. Correlation with progressive disease PLoS One. 2014;9(10):e108326. doi: 10.1371/journal.pone.0108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman JA, Crassini KR, Best OG, Forsyth CJ, Mackinlay NJ, Han P, et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2013;54(1):99–104. doi: 10.3109/10428194.2012.706285. [DOI] [PubMed] [Google Scholar]
- 14.Crassini KR, Zhang E, Balendran S, Freeman JA, Best OG, Forsyth CJ, et al. Humoral immune failure defined by immunoglobulin class and immunoglobulin G subclass deficiency is associated with shorter treatment-free and overall survival in Chronic Lymphocytic Leukaemia. Br J Haematol. 2018;181(1):97–101. doi: 10.1111/bjh.15146. [DOI] [PubMed] [Google Scholar]
- 15.Manukyan G, Papajik T, Gajdos P, Mikulkova Z, Urbanova R, Gabcova G, et al. Neutrophils in chronic lymphocytic leukemia are permanently activated and have functional defects. Oncotarget. 2017;8(49):84889–84901. doi: 10.18632/oncotarget.20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.•.Palma M, Mulder TA, Österborg A. BTK inhibitors in chronic lymphocytic leukemia: biological activity and immune effects. Front Immunol. 2021;12:686768. 10.3389/fimmu.2021.686768. This study provides insights into the off-target effects of BTK inhibitors in the innate and adaptive immune systems. This information is helpful to optimize infection prevention strategies. [DOI] [PMC free article] [PubMed]
- 17.•.Burger JA, Barr PM, Robak T, Owen C, Ghia P, Tedeschi A, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia. 2020;34(3):787-98. 10.1038/s41375-019-0602-x. This study continues to show the excellent efficacy of ibrutinib. However, neutropenia and pneumonia are among the most common grade ≥ 3 AEs, highlighting the importance of infection prevention in these patients. [DOI] [PMC free article] [PubMed]
- 18.Moreno C, Greil R, Demirkan F, Tedeschi A, Anz B, Larratt L, et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(1):43–56. doi: 10.1016/s1470-2045(18)30788-5. [DOI] [PubMed] [Google Scholar]
- 19.Byrd JC, Hillmen P, O'Brien S, Barrientos JC, Reddy NM, Coutre S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133(19):2031–2042. doi: 10.1182/blood-2018-08-870238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Brien S, Jones JA, Coutre SE, Mato AR, Hillmen P, Tam C, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409–1418. doi: 10.1016/s1470-2045(16)30212-1. [DOI] [PubMed] [Google Scholar]
- 21.Sharman JP, Egyed M, Jurczak W, Skarbnik A, Pagel JM, Flinn IW, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial. Lancet. 2020;395(10232):1278–1291. doi: 10.1016/s0140-6736(20)30262-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2849–2861. doi: 10.1200/jco.19.03355. [DOI] [PubMed] [Google Scholar]
- 23.Tam CS, Trotman J, Opat S, Burger JA, Cull G, Gottlieb D, et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood. 2019;134(11):851–859. doi: 10.1182/blood.2019001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.•.Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892-901. 10.1016/s0140-6736(21)00224-5. This study demonstrates the significant efficacy of pirtobrutinib, with an encouraging safety profile. Thus, it could become an important treatment strategy from the risk of infection standpoint. [DOI] [PubMed]
- 25.Bojarczuk K, Siernicka M, Dwojak M, Bobrowicz M, Pyrzynska B, Gaj P, et al. B-cell receptor pathway inhibitors affect CD20 levels and impair anti-tumor activity of anti-CD20 monoclonal antibodies. Leukemia. 2014;28(5):1163–1167. doi: 10.1038/leu.2014.12. [DOI] [PubMed] [Google Scholar]
- 26.Kohrt HE, Sagiv-Barfi I, Rafiq S, Herman SE, Butchar JP, Cheney C, et al. Ibrutinib antagonizes rituximab-dependent NK cell-mediated cytotoxicity. Blood. 2014;123(12):1957–1960. doi: 10.1182/blood-2014-01-547869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fiorcari S, Maffei R, Vallerini D, Scarfò L, Barozzi P, Maccaferri M, et al. BTK inhibition impairs the innate response against fungal infection in patients with chronic lymphocytic leukemia. Front Immunol. 2020;11:2158. doi: 10.3389/fimmu.2020.02158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulder TA, Peña-Pérez L, Berglöf A, Meinke S, Estupiñán HY, Heimersson K, et al. Ibrutinib has time-dependent on- and off-target effects on plasma biomarkers and immune cells in chronic lymphocytic leukemia. Hemasphere. 2021;5(5):e564. doi: 10.1097/hs9.0000000000000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin Q, Sivina M, Robins H, Yusko E, Vignali M, O'Brien S, et al. Ibrutinib therapy increases T cell repertoire diversity in patients with chronic lymphocytic leukemia. J Immunol. 2017;198(4):1740–1747. doi: 10.4049/jimmunol.1601190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tillman BF, Pauff JM, Satyanarayana G, Talbott M, Warner JL. Systematic review of infectious events with the Bruton tyrosine kinase inhibitor ibrutinib in the treatment of hematologic malignancies. Eur J Haematol. 2018;100(4):325–334. doi: 10.1111/ejh.13020. [DOI] [PubMed] [Google Scholar]
- 31.Byrd JC, Harrington B, O'Brien S, Jones JA, Schuh A, Devereux S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323–332. doi: 10.1056/NEJMoa1509981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruchlemer R, Ben-Ami R, Bar-Meir M, Brown JR, Malphettes M, Mous R, et al. Ibrutinib-associated invasive fungal diseases in patients with chronic lymphocytic leukaemia and non-Hodgkin lymphoma: an observational study. Mycoses. 2019;62(12):1140–1147. doi: 10.1111/myc.13001. [DOI] [PubMed] [Google Scholar]
- 33.Frei M, Aitken SL, Jain N, Thompson P, Wierda W, Kontoyiannis DP, et al. Incidence and characterization of fungal infections in chronic lymphocytic leukemia patients receiving ibrutinib. Leuk Lymphoma. 2020;61(10):2488–2491. doi: 10.1080/10428194.2020.1775215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghez D, Calleja A, Protin C, Baron M, Ledoux MP, Damaj G, et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood. 2018;131(17):1955–1959. doi: 10.1182/blood-2017-11-818286. [DOI] [PubMed] [Google Scholar]
- 35.Reynolds G, Slavin M, Teh BW. Ibrutinib and invasive fungal infections: the known, the unknown and the known unknowns. Leuk Lymphoma. 2020;61(10):2292–2294. doi: 10.1080/10428194.2020.1797017. [DOI] [PubMed] [Google Scholar]
- 36.Fraser GAM, Chanan-Khan A, Demirkan F, Santucci Silva R, Grosicki S, Janssens A, et al. Final 5-year findings from the phase 3 HELIOS study of ibrutinib plus bendamustine and rituximab in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 2020;61(13):3188–3197. doi: 10.1080/10428194.2020.1795159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn IE, Jerussi T, Farooqui M, Tian X, Wiestner A, Gea-Banacloche J. Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016;128(15):1940–1943. doi: 10.1182/blood-2016-06-722991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perini GF, Feres CCP, Teixeira LLC, Hamerschlak N. BCL-2 Inhibition as treatment for chronic lymphocytic leukemia. Curr Treat Options Oncol. 2021;22(8):66. doi: 10.1007/s11864-021-00862-z. [DOI] [PubMed] [Google Scholar]
- 39.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves anti-tumor activity while sparing platelets. Nat Med. 2013;19(2):202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 40.Anderson MA, Deng J, Seymour JF, Tam C, Kim SY, Fein J, et al. The BCL2 selective inhibitor venetoclax induces rapid onset apoptosis of CLL cells in patients via a TP53-independent mechanism. Blood. 2016;127(25):3215–3224. doi: 10.1182/blood-2016-01-688796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.•.Al-Sawaf O, Zhang C, Tandon M, Sinha A, Fink AM, Robrecht S, et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for previously untreated chronic lymphocytic leukaemia (CLL14): follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020;21(9):1188-200. 10.1016/s1470-2045(20)30443-5. This study describes the efficacy of the combination of venetoclax plus obinutuzumab. However, it also shows AEs that clinicians need to be aware of to provide optimal infection prevention in patients treated with this regimen. [DOI] [PubMed]
- 42.Seymour JF, Kipps TJ, Eichhorst B, Hillmen P, D'Rozario J, Assouline S, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107–1120. doi: 10.1056/NEJMoa1713976. [DOI] [PubMed] [Google Scholar]
- 43.Kater AP, Wu JQ, Kipps T, Eichhorst B, Hillmen P, D'Rozario J, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study. J Clin Oncol. 2020;38(34):4042–4054. doi: 10.1200/jco.20.00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davids MS, Hallek M, Wierda W, Roberts AW, Stilgenbauer S, Jones JA, et al. Comprehensive safety analysis of venetoclax monotherapy for patients with relapsed/refractory chronic lymphocytic leukemia. Clin Cancer Res. 2018;24(18):4371–4379. doi: 10.1158/1078-0432.Ccr-17-3761. [DOI] [PubMed] [Google Scholar]
- 45.Guarente V, Sportoletti P. Lessons, Challenges and future therapeutic opportunities for PI3K inhibition in CLL. Cancers (Basel). 2021;13(6). doi: 10.3390/cancers13061280. [DOI] [PMC free article] [PubMed]
- 46.Pauls SD, Lafarge ST, Landego I, Zhang T, Marshall AJ. The phosphoinositide 3-kinase signaling pathway in normal and malignant B cells: activation mechanisms, regulation and impact on cellular functions. Front Immunol. 2012;3:224. doi: 10.3389/fimmu.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somoza JR, Koditek D, Villaseñor AG, Novikov N, Wong MH, Liclican A, et al. Structural, biochemical, and biophysical characterization of idelalisib binding to phosphoinositide 3-kinase δ. J Biol Chem. 2015;290(13):8439–8446. doi: 10.1074/jbc.M114.634683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okkenhaug K. Rules of engagement: distinct functions for the four class I PI3K catalytic isoforms in immunity. Ann N Y Acad Sci. 2013;1280:24–26. doi: 10.1111/nyas.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final results of a randomized, phase III study of rituximab with or without idelalisib followed by open-label idelalisib in patients with relapsed chronic lymphocytic leukemia. J Clin Oncol. 2019;37(16):1391–1402. doi: 10.1200/jco.18.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flinn IW, Miller CB, Ardeshna KM, Tetreault S, Assouline SE, Mayer J, et al. DYNAMO: a phase II study of duvelisib (IPI-145) in patients with refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37(11):912–922. doi: 10.1200/jco.18.00915. [DOI] [PubMed] [Google Scholar]
- 52.Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–2455. doi: 10.1182/blood-2018-05-850461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539(7629):437–442. doi: 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maus UA, Backi M, Winter C, Srivastava M, Schwarz MK, Rückle T, et al. Importance of phosphoinositide 3-kinase gamma in the host defense against pneumococcal infection. Am J Respir Crit Care Med. 2007;175(9):958–966. doi: 10.1164/rccm.200610-1533OC. [DOI] [PubMed] [Google Scholar]
- 55.Sasaki T, Irie-Sasaki J, Jones RG, Oliveira-dos-Santos AJ, Stanford WL, Bolon B, et al. Function of PI3Kgamma in thymocyte development, T cell activation, and neutrophil migration. Science. 2000;287(5455):1040–1046. doi: 10.1126/science.287.5455.1040. [DOI] [PubMed] [Google Scholar]
- 56.Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18(3):297–311. doi: 10.1016/s1470-2045(16)30671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinot M, Oswald L, Parisi E, Etienne E, Argy N, Grawey I, et al. Immunoglobulin deficiency in patients with Streptococcus pneumoniae or Haemophilus influenzae invasive infections. Int J Infect Dis. 2014;19:79–84. doi: 10.1016/j.ijid.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 58.Pagano L, Caira M, Candoni A, Offidani M, Fianchi L, Martino B, et al. The epidemiology of fungal infections in patients with hematologic malignancies: the SEIFEM-2004 study. Haematologica. 2006;91(8):1068–1075. [PubMed] [Google Scholar]
- 59.Maertens JA, Girmenia C, Brüggemann RJ, Duarte RF, Kibbler CC, Ljungman P, et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother. 2018;73(12):3221–30. doi: 10.1093/jac/dky286. [DOI] [PubMed]
- 60.Ruiz-Camps I, Aguilar-Company J. Risk of infection associated with targeted therapies for solid organ and hematological malignancies. Ther Adv Infect Dis. 2021;8:2049936121989548. doi: 10.1177/2049936121989548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sehn LH, Hallek M, Jurczak W, Brown JR, Barr PM, Catalano J, et al. A retrospective analysis of Pneumocystis jirovecii pneumonia infection in patients receiving idelalisib in clinical trials. Blood. 2016;128(22):3705-. doi: 10.1182/blood.V128.22.3705.3705.
- 62.Haeusler GM, Slavin MA, Seymour JF, Lingaratnam S, Teh BW, Tam CS, et al. Late-onset Pneumocystis jirovecii pneumonia post–fludarabine, cyclophosphamide and rituximab: implications for prophylaxis. Eur J Haematol. 2013;91(2):157–163. doi: 10.1111/ejh.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Classen AY, Henze L, von Lilienfeld-Toal M, Maschmeyer G, Sandherr M, Graeff LD, et al. Primary prophylaxis of bacterial infections and Pneumocystis jirovecii pneumonia in patients with hematologic malignancies and solid tumors: 2020 updated guidelines of the Infectious Diseases Working Party of the German Society of Hematology and Medical Oncology (AGIHO/DGHO) Ann Hematol. 2021;100(6):1603–1620. doi: 10.1007/s00277-021-04452-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.•.Wierda WG, Byrd JC, Abramson JS, Bilgrami SF, Bociek G, Brander D, et al. Chronic lymphocytic leukemia/small lymphocytic lymphoma, Version 4.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18(2):185–217. 10.6004/jnccn.2020.0006. These guidelines are an essential source of information to review current treatment strategies and recommendations for managing AEs. However, it shows that infection prevention strategies are still an unmet need..
- 65.Di Cocco P, Orlando G, Bonanni L, D'Angelo M, Clemente K, Greco S, et al. A systematic review of two different trimetoprim-sulfamethoxazole regimens used to prevent Pneumocystis jirovecii and no prophylaxis at all in transplant recipients: appraising the evidence. Transplant Proc. 2009;41(4):1201–1203. doi: 10.1016/j.transproceed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 66.Innocenti I, Morelli F, Autore F, Corbingi A, Pasquale R, Sorà F, et al. HBV reactivation in CLL patients with occult HBV infection treated with ibrutinib without viral prophylaxis. Leuk Lymphoma. 2019;60(5):1340–1342. doi: 10.1080/10428194.2018.1523401. [DOI] [PubMed] [Google Scholar]
- 67.Zhang MY, Zhu GQ, Shi KQ, Zheng JN, Cheng Z, Zou ZL, et al. Systematic review with network meta-analysis: comparative efficacy of oral nucleos(t)ide analogues for the prevention of chemotherapy-induced hepatitis B virus reactivation. Oncotarget. 2016;7(21):30642–30658. doi: 10.18632/oncotarget.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakaya A, Fujita S, Satake A, Nakanishi T, Azuma Y, Tsubokura Y, et al. Delayed HBV reactivation in rituximab-containing chemotherapy: how long should we continue anti-virus prophylaxis or monitoring HBV-DNA? Leuk Res. 2016;50:46–49. doi: 10.1016/j.leukres.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 69.Jones JA, Robak T, Brown JR, Awan FT, Badoux X, Coutre S, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017;4(3):e114–e126. doi: 10.1016/s2352-3026(17)30019-4. [DOI] [PubMed] [Google Scholar]
- 70.Maschmeyer G, De Greef J, Mellinghoff SC, Nosari A, Thiebaut-Bertrand A, Bergeron A, et al. Infections associated with immunotherapeutic and molecular targeted agents in hematology and oncology. A position paper by the European Conference on Infections in Leukemia (ECIL). Leukemia. 2019;33(4):844–62. doi: 10.1038/s41375-019-0388-x. [DOI] [PMC free article] [PubMed]
- 71.Cheah CY, Fowler NH. Idelalisib in the management of lymphoma. Blood. 2016;128(3):331–336. doi: 10.1182/blood-2016-02-702761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Douglas AP, Trubiano JA, Barr I, Leung V, Slavin MA, Tam CS. Ibrutinib may impair serological responses to influenza vaccination. Haematologica. 2017;102(10):e397–e399. doi: 10.3324/haematol.2017.164285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pleyer C, Ali MA, Cohen JI, Tian X, Soto S, Ahn IE, et al. Effect of Bruton tyrosine kinase inhibitor on efficacy of adjuvanted recombinant hepatitis B and zoster vaccines. Blood. 2021;137(2):185–189. doi: 10.1182/blood.2020008758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.•.Mauro FR, Giannarelli D, Galluzzo CM, Vitale C, Visentin A, Riemma C, et al. Response to the conjugate pneumococcal vaccine (PCV13) in patients with chronic lymphocytic leukemia (CLL). Leukemia. 2021;35(3):737-46. 10.1038/s41375-020-0884-z. This study highlights important factors and challenges with immunizations in patients with CLL that need to be considered in clinical practice. [DOI] [PubMed]
- 75.Chatzikonstantinou T, Herishanu Y, Montserrat E, Ghia P, Cuneo A, Foà R, et al. COVID-19 and chronic lymphocytic leukemia: where we stand now. Cancer J. 2021;27(4):328–333. doi: 10.1097/ppo.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 76.•.Scarfò L, Chatzikonstantinou T, Rigolin GM, Quaresmini G, Motta M, Vitale C, et al. COVID-19 severity and mortality in patients with chronic lymphocytic leukemia: a joint study by ERIC, the European Research Initiative on CLL, and CLL Campus. Leukemia. 2020;34(9):2354-63. 10.1038/s41375-020-0959-x. This study describes the negative impact of COVID-19 in patients with CLL and factors that further worsen the outcomes on these patients. [DOI] [PMC free article] [PubMed]
- 77.•.Roeker LE, Scarfo L, Chatzikonstantinou T, Abrisqueta P, Eyre TA, Cordoba R, et al. Worldwide examination of patients with CLL hospitalized for COVID-19. Blood. 2020;136(Supplement 1):45-9. 10.1182/blood-2020-136408. This provides information about the challenges of hospitalized patients with COVID-19 and their adverse outcomes. Additionally, the impact of modern CLL therapies is still controversial in this setting.
- 78.Treon SP, Castillo JJ, Skarbnik AP, Soumerai JD, Ghobrial IM, Guerrera ML, et al. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19–infected patients. Blood. 2020;135(21):1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fürstenau M, Langerbeins P, De Silva N, Fink AM, Robrecht S, von Tresckow J, et al. COVID-19 among fit patients with CLL treated with venetoclax-based combinations. Leukemia. 2020;34(8):2225–2229. doi: 10.1038/s41375-020-0941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rossi D, Shadman M, Condoluci A, Brown JR, Byrd JC, Gaidano G, et al. How we manage patients with chronic lymphocytic leukemia during the SARS-CoV-2 pandemic. Hemasphere. 2020;4(4):e432. doi: 10.1097/hs9.0000000000000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sehn LH, Kuruvilla P, Christofides A, Stakiw J. Management of chronic lymphocytic leukemia in Canada during the coronavirus pandemic. Curr Oncol. 2020;27(3):e332–e335. doi: 10.3747/co.27.6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iovino L, Shadman M. Novel therapies in chronic lymphocytic leukemia: a rapidly changing landscape. Curr Treat Options Oncol. 2020;21(4):24. doi: 10.1007/s11864-020-0715-5. [DOI] [PubMed] [Google Scholar]
- 84.Mato AR, Ghosh N, Schuster SJ, Lamanna N, Pagel JM, Flinn IW, et al. Phase 2 study of the safety and efficacy of umbralisib in patients with CLL who are intolerant to BTK or PI3Kδ inhibitor therapy. Blood. 2021;137(20):2817–2826. doi: 10.1182/blood.2020007376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.•.Frey NV, Gill S, Hexner EO, Schuster S, Nasta S, Loren A, et al. Long-term outcomes from a randomized dose optimization study of chimeric antigen receptor modified T cells in relapsed chronic lymphocytic leukemia. J Clin Oncol. 2020;38(25):2862-71. 10.1200/jco.19.03237. CART therapy has become part of the therapeutic arsenal of patients with relapsed CLL. It is important to be familiar with the impact on the immune system that needs to be addressed to prevent infections. [DOI] [PMC free article] [PubMed]
- 86.Hill JA, Li D, Hay KA, Green ML, Cherian S, Chen X, et al. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood. 2018;131(1):121–130. doi: 10.1182/blood-2017-07-793760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hwang JP, Torres HA. Hepatitis B virus and hepatitis C virus infection in immunocompromised patients. Curr Opin Infect Dis. 2018;31(6):535–541. doi: 10.1097/qco.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 88.•.Hill JA, Seo SK. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood. 2020;136(8):925-35. 10.1182/blood.2019004000. This study brings important insight on how to approach the risk of infection in patients with CLL who are treated with CART therapies. [DOI] [PMC free article] [PubMed]
- 89.Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T - and a side order of IgG, to go? - Immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev. 2019;38:100596. doi: 10.1016/j.blre.2019.100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cordonnier C, Einarsdottir S, Cesaro S, Di Blasi R, Mikulska M, Rieger C, et al. Vaccination of haemopoietic stem cell transplant recipients: guidelines of the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis. 2019;19(6):e200-e12. doi: 10.1016/s1473-3099(18)30600-5. [DOI] [PubMed]