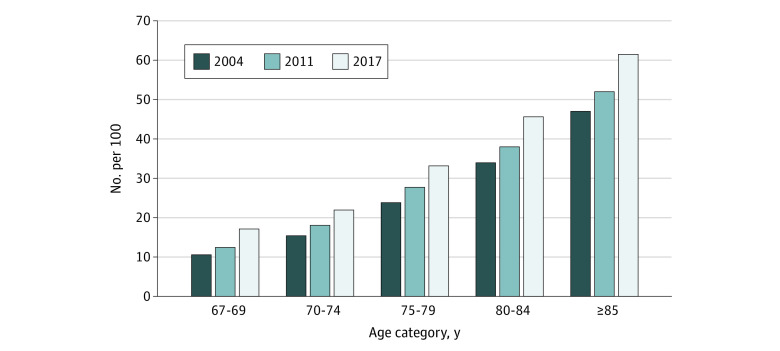This cross-sectional study assesses trends in frequency of diagnosis of Alzheimer disease and related dementias in the last 2 years of life and any associated changes in billing practices, characteristics of the diagnosed population, and intensity of end-of-life care.
Key Points
Question
To what degree did the diagnosis of Alzheimer disease and related dementias (ADRD) change at the end of life between 2004 and 2017?
Findings
Among 3 515 329 Medicare fee-for-service decedents, the percentage who received an ADRD diagnosis within 2 years of death increased from 34.7% in 2004 to 47.2% in 2017. The likelihood of receiving an ADRD diagnosis particularly increased in the inpatient, hospice, and home health settings; individual characteristics and service use were stable over time, while the intensity of end-of-life care declined on most measures.
Meaning
Dying with an ADRD diagnosis has become more common among older US decedents, potentially owing to increased awareness and temporal changes in billing.
Abstract
Importance
Alzheimer disease and related dementias (ADRD) have received considerable attention among clinicians, researchers, and policy makers in recent years. Despite increased awareness, few studies have documented temporal changes in the documentation of ADRD diagnoses despite its new importance for risk adjustment for health plans in Medicare.
Objective
To assess trends in frequency of ADRD diagnosis in the last 2 years of life from 2004 to 2017, as well as any associated changes in billing practices, characteristics of the population with diagnosed ADRD, and intensity of end-of-life care.
Design, Setting, and Participants
This is a serial cross-sectional study of older adult decedents (67 years or older) from 2004 to 2017 using a 20% sample of fee-for-service Medicare decedents. An ADRD diagnosis within the last 2 years of life was identified using diagnosis codes from inpatient, professional service, home health, or hospice claims, requiring the standard claims algorithm that required at least 1 claim and a more stringent algorithm that required at least 2 claims. Trends in ADRD diagnosis among decedents were used to lessen influence of new diagnostic technologies for early stage disease. Demographic characteristics, selected comorbidities, place of death, and health service use at the end-of-life were also examined. Data were analyzed from July 9, 2020, to May 3, 2021.
Exposures
Calendar year 2004 to 2017.
Main Outcome and Measure
An ADRD diagnosis within 2 years of death.
Results
Among the included 3 515 329 Medicare fee-for-service decedents, when adjusted for age and sex, the percentage of older decedents with an ADRD diagnosis increased from 34.7% in 2004 to 47.2% in 2017. The trend was attenuated (25.2% to 39.2%) using a stringent ADRD definition. There was an inflection in the curve from 2011 to 2013, the time at which additional diagnoses were added to Medicare claims and the National Alzheimer Care Act was enacted. The ADRD diagnosis frequency increased considerably in inpatient (49.0% to 67.3%), hospice (12.2% to 42.0%), and home health (10.1% to 28.7%) claims. However, individual characteristics, number of visits, and hospitalizations were similar across the study period, and the intensity of end-of-life care declined on most measures.
Conclusions and Relevance
In this cross-sectional study, nearly half of older Medicare decedents had a diagnosis of ADRD at the time of death. From 2004 to 2017, the percentage of older adult decedents who received an ADRD diagnosis increased substantially prior to announcement of the addition of ADRD to Medicare risk adjustment strategies.
Introduction
Alzheimer disease and related dementias (ADRD) for which we have no effective disease-modifying treatment are estimated to cost the US as much as $215 billion annually.1 As the population ages, the importance of ADRD as a cause of or contributor to death continues to increase.2 The recognition of dementia and the burden to affected individuals and their families has increased efforts to address the needs of this population. In addition to the human toll of the disease, there are also high costs associated with both medical care and long-term care as people with dementia progress from mild impairment toward death. The increased attention has led to reassessments of how to account for the presence of dementia in the processes leading up to death.
Recently, contributing factors such as ADRD have been taken into consideration when determining causes of death,2,3 and ADRD has increased from the eighth leading cause of death in the US in 2000 to the sixth in 20184,5 (and the fifth among older adults6). This change reflects changing attitudes and practices for documenting the presence of this disease, which is also likely happening in clinical practice. Dementia has long been noted to be underdiagnosed,7 but higher levels of public education and more diagnostic and treatment options may be changing how often clinicians document and bill for ADRD. Moreover, several external factors could affect ADRD coding, such as the adoption of electronic health records, the influence of the National Alzheimer Project Act8 on disease awareness and increased research funding, and changes in the allowable number and coding systems used for diagnoses on Medicare claims.
Understanding whether ADRD identification has increased is important because it may suggest greater opportunity to address burdensome end-of-life care. But it also has health policy importance. Dementia was added into the risk adjustment strategy Hierarchical Condition Categories (HCC), constructed from algorithms of claims, and used by the Centers for Medicare & Medicaid Services for compensating health plans.9,10 In addition, ADRD coding is important for economic evaluations of how dementia contributes to the costs of care.
Therefore, the objective of this study was to determine whether the likelihood of dying with an ADRD diagnosis, identified from billing data, has changed over time. We examined diagnosis of ADRD among decedents because end-stage disease should be less influenced by temporal changes in diagnostic technologies used in early disease stages.11,12 The hypothesis was that ADRD diagnosis frequency would increase over time coincident with changes in diagnostic billing behavior. We examined whether the potential change in ADRD diagnosis frequency was associated with changes in the characteristics of the ADRD-identified population. Lastly, we examined whether trends toward greater diagnosis frequency were associated with changes in the intensity of care at the end of life.
Methods
Study Design
A serial cross-sectional study of Medicare decedents using a 20% national sample of fee-for-service enrollees from 2004 to 2017 was conducted to examine changes in the percentage of older adults receiving an ADRD diagnosis within 2 years of death. This study received approval from the institutional review board of Dartmouth College, waiving informed consent for the use of deidentified data, and abides by Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Study Population
Using the 20% Master Beneficiary Summary Files, we identified all individuals who died between 2004 and 2017. Among decedents, we restricted to those who were continually enrolled in both Medicare Parts A and B, and not enrolled in Medicare Advantage for 24 months before the date of death. With a 2-year look-back period for diagnosis identification, decedents must have been 67 years or older at the time of death to be included. We chose a 2-year look-back period because the majority of decedents having an ADRD diagnosis will receive at least 1 ADRD diagnosis on a claim within 2 years of death.13 The final sample consisted of 3 515 329 older decedents.
Identification of Decedents With ADRD Diagnosis
The presence of an ADRD diagnosis was identified from inpatient records (Medicare Provider Analysis and Review [MedPAR]), professional service claims (carrier file and selected outpatient hospital claims for clinician visits in underserved settings), home health records, and hospice claims. An established list of International Classification of Diseases, Ninth Revision, Clinical Modification and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes were used (eTable in the Supplement).
We applied a standard, previously validated claims algorithm that requires the presence of a single claim with an ADRD diagnosis14,15 rather than 2 claims, as used for other diseases, owing to concerns for underdiagnosis. We also applied a more stringent approach by requiring 2 or more claims with an ADRD diagnosis for professional services at least 7 days apart. The latter approach reduces potential misclassification by removal of those who receive a spurious ADRD clinical diagnosis as in the case of rule-out diagnoses.16,17,18
Changes in clinical practice or volume of care delivered in each setting could influence frequency of diagnosis documentation or setting where diagnosis occurred across the inpatient, professional service, home health, or hospice settings. In addition to identifying how many decedents had received an ADRD diagnosis, we examined whether changes had occurred in the setting where the diagnosis was billed. Trends were examined in the occurrence of an ADRD diagnosis in each claim type (ie, nonmutually exclusive), as well as when the diagnosis appeared in only 1 claim type. For descriptive purposes, we superimposed the external events (adoption of electronic health records,19,20 billing rule changes, and enactment of the National Alzheimer Project Act) that we hypothesized could have been associated with the likelihood of ADRD diagnosis.
Demographic Characteristics
Changes in the likelihood of receiving an ADRD diagnosis could lead to differences in the composition of the ADRD decedent population. We examined demographic characteristics and selected comorbidities among ADRD decedents relative to changes in decedents who did not receive an ADRD diagnosis. We examined characteristics in 2004 vs 2017 and in 2011 when the expansion in the number of diagnoses on health care claims went into effect.21 Demographic characteristics at the time of death included age, sex, and race and ethnicity. Race and ethnicity was categorized as Black vs non-Black because the percentage of Black and non-Black individuals was stable over time, unlike categorization of White race, which declined as coding of Hispanic ethnicity increased.22 Non-Black race and ethnicity included American Indian or Alaska Native, Asian, Hispanic, White, and those of other races and ethnicities. The Elixhauser Comorbidity Index was used to identify clinically significant comorbidities in the last year of life,23 including cancer, diabetes, congestive heart failure, chronic pulmonary disease, and kidney failure.
Volume of Health Service Use
We also determined whether health service use temporally increased because previous research shows that more services would lead to a higher likelihood of diagnosis.24 Number of outpatient services (evaluation and management visits,25 emergency department visits, and home health days) and inpatient service use (inpatient evaluation and management visits, intensive care unit [ICU] stays, inpatient days, and skilled nursing facility days) were assessed 1 year before death.
End-of-Life Treatments
Based on the MedPAR file, we report place of death as occurred in a (1) hospital without an ICU stay, (2) hospital with an ICU stay, or (3) some other type of Medicare-reimbursed inpatient stay (primarily a skilled nursing facility). We classified all other decedents with no MedPAR services on the date of death as unknown owing to limits of Medicare data, but they were most likely to have died in a community setting or long-term care nursing home. Any claim for hospice services in the last 6 months of life was used to determine the percentage of decedents who used hospice. Lastly, life-prolonging treatment used in the last 6 months of life included gastrostomy feeding tubes, mechanical ventilation, and hemodialysis.26
Statistical Analysis
Overall trends from 2004 to 2017 in the percentage of decedents who received an ADRD diagnosis were age- and sex-adjusted using direct rate adjustment (with 2011 as the reference year). We used univariate statistical tests to compare the characteristics of decedents with an ADRD diagnosis over time (2004 vs 2017) and with decedents who did not receive an ADRD diagnosis in 2017. Statistical significance for trends in ADRD diagnosis across time was assessed by linear regression using calendar year as a continuous variable. All analyses were completed using SAS, version 9.4 (SAS Institute Inc). We set the critical α level to a 2-sided .05. Owing to the large sample size, even small differences can reach statistical significance.
Results
Trends in ADRD Diagnosis Among Decedents
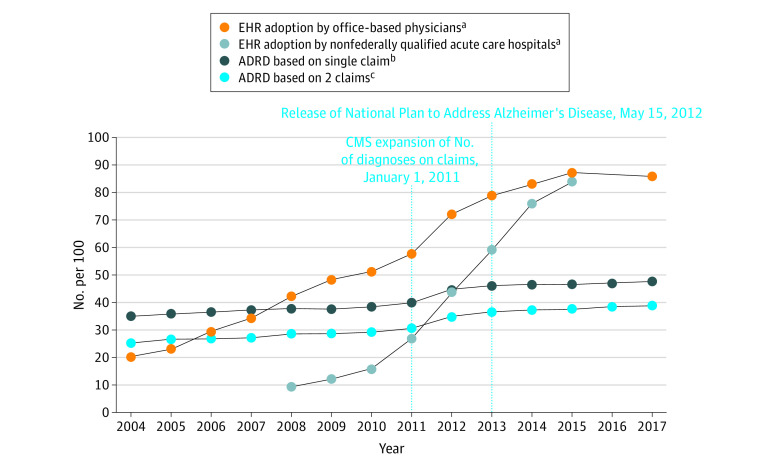
Among the 20% sample of Medicare fee-for-service decedents from 2004 to 2017, when adjusted for age and sex the percentage of decedents who received an ADRD diagnosis increased from 34.7% in 2004 to 47.2% in 2017, which equates to a 36.0% increase over the 14 years (P < .001; Figure 119,20). Use of the more stringent definition of ADRD attenuated the estimates but not the percentage increase: the age- and sex-adjusted percentage of decedents who received an ADRD diagnosis increased from 25.2% to 39.2% in 2017 (P < .001) for a 55.6% increase over time. While overall trends increased, the largest increase occurred from 2011 to 2013, when the Centers for Medicare & Medicaid Services expanded diagnosis codes on the claims and the National Plan to Address Alzheimer’s Disease was enacted.
Figure 1. Age- and Sex-Adjusted Trends in the Percentage of Older Adult Medicare Decedents With an ADRD Diagnosis Within the Last 2 Years of Life, 2004-2017.
ADRD indicates Alzheimer disease and related dementias; CMS, Centers for Medicare & Medicaid Services; EHR, electronic health record.
aData on EHR adoption for office-based physicians and nonfederally qualified acute care hospitals are from the Office of the National Coordinator for Health Information Technology.19,20
bADRD identified based on a single claim within the last 2 years of life.
cADRD identified based on 2 diagnoses on claims occurring more than 1 week apart within the last 2 years of life.
By age category, decedents who were 85 years or older experienced the largest increase in ADRD diagnosis from 2004 to 2017. The percentage diagnosed with ADRD increased from 46.9% (55 074 of 117 409 decedents) in 2004 to 61.6% (71 162 of 115 605 decedents) in 2017 (Figure 2). Decedents aged 67 to 69 years also experienced a large 61.3% increase, albeit at lower absolute frequency, rising from 10.6% (1533 of 14 524 decedents) in 2004 to 17.1% (2429 of 14 180 decedents) in 2017.
Figure 2. ADRD Diagnosis Among Older Adult Medicare Decedents by Age Category in 2004, 2011, and 2017.
Alzheimer disease and related dementias (ADRD) were identified based on a single claim within the last 2 years of life.
ADRD Diagnosis by Service Setting
Trends in the service types for which an ADRD diagnosis was billed have changed since 2004 in 2 main ways. The likelihood of receiving an ADRD diagnosis has increased considerably among the inpatient, hospice, and home health settings (Figure 3A). The percentage who received an ADRD diagnosis from an inpatient service increased from 49.0% (45 759 of 93 386 decedents) in 2004 to 67.3% (74 277 of 110 368 decedents) in 2017 (P < .001), with all of the increase occurring between 2010 and 2013. Likewise, the percentage of ADRD decedents receiving a diagnosis from a hospice service increased from 12.2% (11 393 of 93 386 decedents) in 2004 to 42.0% (46 354 of 110 368 decedents) in 2017 (P < .001). The second change is that the likelihood of receiving an ADRD diagnosis from only one service type statistically significantly decreased (51.7% to 25.8%), driven by a drop in receiving only claims for professional services, from 40.6% (37 914 of 93 386 decedents) in 2004 to 15.3% (16 886 of 110 368 decedents) in 2017 (P < .001; Figure 3B).
Figure 3. Trends in Older Adult Medicare Decedents Who Received an ADRD Diagnosis by Health Care Service and Single Health Care Service in the Last 2 Years of Life, 2004-2017.
ADRD indicates Alzheimer disease and related dementias.
Characteristics and Service Use Trends Among Decedents
In comparison with decedents without ADRD, the changes in demographics and comorbidities over time for the decedents with ADRD were very similar (Table 1). The age, sex, and race and ethnicity changes were small among decedents with ADRD and paralleled the changes in the non-ADRD group. The percentage-point change in each comorbidity was the same for decedents with ADRD compared with decedents without ADRD, although the level of comorbidities differed between the groups in expected ways owing to the nature of ADRD.
Table 1. Characteristics of Medicare Decedents With ADRD Diagnosis vs Those Without a Diagnosis in 2004, 2011, and 2017.
| Characteristic | 2004 | 2011 | 2017 | P valueb | ||||
|---|---|---|---|---|---|---|---|---|
| ADRD diagnosisa | No ADRD diagnosis | ADRD diagnosisa | No ADRD diagnosis | ADRD diagnosisa | No ADRD diagnosis | 2004 vs 2017 | ADRD vs no ADRD in 2017 | |
| Sample size, No. | 93 386 | 179 615 | 98 401 | 148 452 | 110 368 | 124 946 | NA | NA |
| Sociodemographic characteristics | ||||||||
| Age, mean (SD), y | 85.9 (7.3) | 81.3 (8.0) | 86.6 (7.3) | 81.7 (8.4) | 86.7 (7.8) | 80.9 (8.6) | <.001 | <.001 |
| Age category, No. (%), y | ||||||||
| 67-69 | 1533 (1.6) | 12 991 (7.2) | 1702 (1.7) | 11 912 (8.0) | 2429 (2.2) | 11 751 (9.4) | <.001 | <.001 |
| 70-74 | 5182 (5.5) | 28 630 (15.9) | 5158 (5.2) | 23 256 (15.7) | 6645 (6.0) | 23 473 (18.8) | ||
| 75-79 | 11 351 (12.2) | 36 226 (20.2) | 9792 (10.0) | 25 503 (17.2) | 11 489 (10.4) | 23 102 (18.5) | ||
| 80-85 | 20 246 (21.7) | 39 433 (22.0) | 18 346 (18.6) | 29 634 (20.0) | 18 643 (16.9) | 22 177 (17.8) | ||
| >85 | 55 074 (59.0) | 62 335 (34.7) | 63 403 (64.4) | 58 147 (39.2) | 71 162 (64.5) | 44 443 (35.6) | ||
| Sex, No. (%) | ||||||||
| Female | 60 063 (64.3) | 94 255 (52.5) | 62 293 (63.3) | 75 975 (51.2) | 67 333 (61.0) | 60 607 (48.5) | <.001 | <.001 |
| Male | 33 323 (35.7) | 85 360 (47.5) | 36 108 (36.7) | 72 477 (48.8) | 43 035 (39.0) | 64 339 (51.5) | ||
| Race, No. (%)c | ||||||||
| Black | 8655 (9.3) | 14 433 (8.0) | 8423 (8.6) | 10 377 (7.0) | 9115 (8.3) | 8684 (7.0) | <.001 | <.001 |
| Non-Black | 84 731 (90.7) | 165 182 (92.0) | 89 978 (91.4) | 138 075 (93.0) | 101 253 (91.7) | 116 262 (93.0) | ||
| Health conditions, No. (%)d | ||||||||
| Cancer | 17 817 (19.1) | 89 955 (50.1) | 17 073 (17.4) | 73 759 (49.7) | 20 390 (18.5) | 66 272 (53.0) | <.001 | <.001 |
| Diabetes | 32 719 (35.0) | 68 681 (38.2) | 38 082 (38.7) | 63 990 (43.1) | 60 090 (54.4) | 70 869 (56.7) | <.001 | <.001 |
| Congestive heart failure | 41 083 (44.0) | 81 672 (45.5) | 37 722 (38.3) | 59 640 (40.2) | 43 063 (39.0) | 50 475 (40.4) | <.001 | <.001 |
| Chronic pulmonary disease | 29 643 (31.7) | 71 858 (40.0) | 29 820 (30.3) | 57 225 (38.5) | 32 617 (29.6) | 47 053 (37.7) | <.001 | <.001 |
| Kidney failure | 11 109 (11.9) | 25 906 (14.4) | 25 399 (25.8) | 42 308 (28.5) | 34 509 (31.3) | 40 480 (32.4) | <.001 | <.001 |
| Health service associated with ADRD claim, % | ||||||||
| Any inpatient service | 49.0 | NA | 51.8 | NA | 67.3 | NA | NA | NA |
| Any professional servicee | 87.7 | NA | 86.3 | NA | 87.1 | NA | NA | NA |
| Any hospice service | 12.2 | NA | 21.5 | NA | 42.0 | NA | NA | NA |
| Any home health service | 10.1 | NA | 19.6 | NA | 28.7 | NA | NA | NA |
| Only from inpatient service | 8.4 | NA | 7.6 | NA | 7.5 | NA | NA | NA |
| Only from professional servicee | 40.6 | NA | 31.3 | NA | 15.3 | NA | NA | NA |
| Only from hospice service | 1.5 | NA | 2.5 | NA | 2.2 | NA | NA | NA |
| Only from home health service | 1.2 | NA | 1.7 | NA | 0.8 | NA | NA | NA |
Abbreviations: ADRD, Alzheimer disease and related dementias; NA, not applicable.
Diagnosis of ADRD among decedents based on at least 1 ADRD claim within 2 years of death.
t Test was used for continuous measures and χ2 test for proportions.
Race and ethnicity was categorized as Black vs non-Black because the percentage of Black and non-Black individuals was stable over time. Non-Black race and ethnicity includes American Indian or Alaska Native, Asian, Hispanic, White, and those of other races and ethnicities.
Based on Elixhauser Comorbidity Index using diagnoses within 1 year of death.
Professional service identified using claims from outpatient and carrier files.
Between 2004 and 2017, there were changes in demographic characteristics within the ADRD decedent population worth noting (Table 1). While there were increases in both the oldest and youngest age, the average age of decedents with ADRD increased from 85.9 years in 2004 to 86.7 years in 2017 owing to a greater number of oldest old identified in claims. Other changes were consistent with the non-ADRD population change but are important to note for their potential influence on future ADRD studies that use longitudinal data. Among decedents with ADRD, the percentage of female decedents decreased from 64.3% in 2004 to 61.0% in 2017. The percentage of decedents with ADRD who were Black also decreased from 9.3% in 2004 to 8.3% in 2017. There were notable increases in the percentage of decedents with ADRD with diabetes (35.0% in 2004 vs 54.4% in 2017) and kidney failure (11.9% in 2004 vs 31.3% in 2017).
We found that the number and type of health service contacts increased for some types of care but not for the services from which most ADRD diagnosis claims are occurring. The number of outpatient evaluation and management visits, which are face-to-face visits with a clinician, increased slightly from 14.3 visits per decedent in the last year of life in 2004 to 15.9 visits in 2017 (P < .001; Table 2). The number of emergency department visits was low but increased from 0.8 per decedent in 2004 to 1.2 in 2017.
Table 2. Use of Health Care Services in the Last Year of Life Among Medicare Decedents With an ADRD Diagnosis in 2004, 2011, and 2017.
| Characteristic | Year | P value (2004 vs 2017)a | ||
|---|---|---|---|---|
| 2004 | 2011 | 2017 | ||
| Sample size with ADRD diagnosis, No. | 93 386 | 98 401 | 110 368 | NA |
| Health service use in last year | ||||
| Outpatient services, mean No. | ||||
| Outpatient EM visits | 14.3 | 14.8 | 15.9 | <.001 |
| ED visits | 0.8 | 1.0 | 1.2 | <.001 |
| Home health days | 25.0 | 38.7 | 40.6 | <.001 |
| Inpatient services, mean No. | ||||
| Inpatient EM visits | 12.1 | 11.4 | 10.9 | <.001 |
| ICU stays | 3.2 | 3.8 | 3.8 | <.001 |
| Inpatient days | 15.3 | 13.6 | 11.8 | <.001 |
| SNF days | 21.6 | 27.2 | 22.9 | <.001 |
| Intensity of end-of-life care | ||||
| Place of death, % | ||||
| Hospital without ICU stay | 12.3 | 7.8 | 5.4 | <.001 |
| Hospital with ICU stay | 11.3 | 10.6 | 9.6 | |
| Other inpatient | 10.9 | 8.5 | 6.1 | |
| Unknown | 65.6 | 73.2 | 78.9 | |
| Hospice use in last 6 mo, % | ||||
| Yes | 36.4 | 54.6 | 62.7 | <.001 |
| No | 63.6 | 45.4 | 37.3 | |
| Life-prolonging treatment in last 6 mo, % | ||||
| On ventilator | 6.8 | 7.2 | 7.6 | <.001 |
| With feeding tube | 5.7 | 4.5 | 3.7 | <.001 |
| Received hemodialysisb | 2.2 | 2.4 | 2.7 | <.001 |
Abbreviations: ADRD, Alzheimer disease and related dementias; ED, emergency department; EM, evaluation and management; ICU, intensive care unit; SNF, skilled nursing facility.
t Test was used for continuous measures and χ2 test for proportions.
International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes for dialysis not used for 2016 Medicare Provider Analysis and Review data.
Intensity of Care at the End of Life
From 2004 to 2017, there was a shift toward lower ICU use during a terminal hospitalization among decedents with ADRD (Table 2). Those who died in the hospital without an ICU stay also decreased from 12.3% in 2004 to 5.4% in 2017, whereas death in the community or other non-Medicare reimbursed setting (ie, the unknown category) increased from 65.6% to 78.9% during the same time period. Hospice use increased considerably from 36.4% in 2004 to 62.7% in 2017. Changes from 2004 to 2017 in life-prolonging treatment in the last 6 months of life were mixed: use of ventilation increased from 6.8% to 7.6%, whereas the use of feeding tubes decreased from 5.7% to 3.7%.
Discussion
This study documents that ADRD diagnosis at the end of life is common and has increased from 2004 to 2017 among Medicare beneficiaries such that nearly half of fee-for-service Medicare beneficiaries die with a documented ADRD diagnosis. This is despite few changes in the characteristics of decedents with an ADRD diagnosis relative to the changes in the non-ADRD population, suggesting greater identification of previously undiagnosed cases. Most of the increase in ADRD diagnoses occurred in 2011 through 2013 when there was a change in the number of diagnoses allowed on Medicare claims. Of note, the transition from International Classification of Diseases, Ninth Revision, Clinical Modification to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision diagnostic codes in 2015 was not associated with much change in frequency of ADRD diagnosis. There is some evidence that the settings in which ADRD was diagnosed have changed. In more recent years, decedents were more likely to receive an ADRD diagnosis from an inpatient, hospice, or home health service. As a corollary, we found a drop in diagnosis only in professional services claims, decreasing from 40.6% to 15.3%. Despite greater recognition of dementia in the last years of life, the use of life-prolonging services such as mechanical ventilation and dialysis have not declined even while more people with dementia are receiving hospice and fewer dying in hospitals or with feeding tubes.
To our knowledge, these analyses are among the first to examine trends in ADRD diagnosis among Medicare decedents. Prior cross-sectional studies estimate 40% of decedents with ADRD using Medicare claims,13 37.7% with ADRD using claims and survey report,27 and 21.7% with dementia and 43.1% with cognitive impairment without dementia using cognitive testing.28 We find higher rates—47.2% of decedents with ADRD when using the standard single-claim algorithm and still 39.2% when we increase stringency by requiring 2 claims. The high and increasing rate of ADRD diagnosis, particularly for services often used in late-stage disease (ie, hospice, home health, hospital) and without the identified ADRD population characteristics changing differentially from the general population suggest that rates of underdiagnosis for late-stage disease may be declining. That said, identification within the last 2 years of life is a minor improvement considering the disease course for ADRD spans 10 years.29
One potential benefit of greater disease identification in late-stage disease is the opportunity to engage in end-of-life care discussions. The present results do not support an association between higher ADRD diagnosis frequency and less aggressive end-of-life care. Instead, we find that the secular trends identified by other studies are evident for the ADRD decedent populations, including the general population toward more hospice use,30 lower use of feeding tubes among non–community dwelling decedents with ADRD,31 and lower likelihood of dying in hospital albeit with high rates of between-facility transfers.32,33,34
From a health policy perspective, the implications of changes in ADRD diagnosis leading up to death extend beyond the implications related to quality of care. Diagnoses of ADRD were added to the HCC used for risk adjustment by the Centers for Medicare & Medicaid in 2020, though initially announced in December 2018.35 This study demonstrates that coding was increasing in fee-for-service (which is not subject to HCC adjustment) prior to this announcement. Further studies need to address the degree that these secular trends, along with new incentives to code ADRD by health plans, will have on costs to Medicare. Medicare claims have recently been shown to have a positive predictive value between 60% and 70%, which may make it challenging to disentangle upcoding, correction of historical underdiagnosis, and misdiagnosis.36 Additionally, 2 recent articles36,37 and the present analysis demonstrate that underdiagnosis may no longer warrant using 1 claim as opposed to the standard approach of using 2 in ADRD-diagnostic criteria. Thus, changes in ADRD diagnosis over time among Medicare decedents could affect payment and comparison of quality-of-care outcomes. Changes in the identification of ADRD at the end of life also likely affects both epidemiological measures of disease burden (eg, years of potential life lost) and estimates of the cost burden of ADRD to the US economy.
Limitations
Several limitations must be acknowledged. First, while the present analyses demonstrate changes in ADRD diagnosis at the end of life among Medicare fee-for-service beneficiaries, these results may not extend to other groups (eg, beneficiaries enrolled in Medicare Advantage plans) or early stage disease. Second, inaccuracy of ADRD diagnoses in clinical practice and by extension in claims data likely leads to misclassification; nevertheless, these are the data used in most policy studies. Third, as an observational study we cannot rule out potential residual confounding affecting the results. Lastly, the analyses were not designed to determine whether the changes we observed are related to underlying population disease prevalence or lead to differences in clinical outcomes.
Conclusions
In summary, in this serial cross-sectional study of Medicare decedents, the number of decedents dying with an ADRD diagnosis in the US increased by 36.0% between 2004 and 2017. Inpatient, hospice, and home health settings had the most statistically significant increases in ADRD diagnosis documentation. Individual characteristics and use of services, however, were relatively stable over time. These findings indicate that diagnosis of ADRD has become more common among older US decedents. Although these analyses are unable to fully explain such increases, it may be owing to increased awareness among patients, families, and/or health care professionals, as well as temporal changes in billing practices.
eTable. International Classification of Disease diagnosis codes used to identify ADRD
References
- 1.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334. doi: 10.1056/NEJMsa1204629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. doi: 10.1002/alz.12328 [DOI] [PubMed] [Google Scholar]
- 3.James BD, Leurgans SE, Hebert LE, Scherr PA, Yaffe K, Bennett DA. Contribution of Alzheimer disease to mortality in the United States. Neurology. 2014;82(12):1045-1050. doi: 10.1212/WNL.0000000000000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson RN. Deaths: leading causes for 2000. Natl Vital Stat Rep. 2002;50(16):1-85. [PubMed] [Google Scholar]
- 5.Xu J, Murphy SL, Kockanek KD, Arias E. Mortality in the United States, 2018. NCHS Data Brief. 2020;(355):1-8. [PubMed] [Google Scholar]
- 6.About Underlying Cause of Death, 1999-2020. Centers for Disease Control and Prevention . Accessed March 3, 2022. https://wonder.cdc.gov/ucd-icd10.html
- 7.Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: prevalence and contributing factors. Alzheimer Dis Assoc Disord. 2009;23(4):306-314. doi: 10.1097/WAD.0b013e3181a6bebc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Plan to Address Alzheimer’s Disease. Office of the Assistant Secretary for Planning and Evaluation . Accessed March 3, 2022. https://aspe.hhs.gov/napa-documents
- 9.Advance notice of methodological changes to calendar year (CY) 2020. for the Medicare Advantage (MA) CMS-HCC risk adjustment model. Centers for Medicare & Medicaid Services . December 20, 2018. Accessed March 3, 2022. https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/Downloads/Advance2020Part1.pdf
- 10.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119-141. [PMC free article] [PubMed] [Google Scholar]
- 11.Langa KM, Burke JF. Preclinical Alzheimer disease—early diagnosis or overdiagnosis? JAMA Intern Med. 2019;179(9):1161-1162. doi: 10.1001/jamainternmed.2019.2629 [DOI] [PubMed] [Google Scholar]
- 12.Wennberg JE, Staiger DO, Sharp SM, et al. Observational intensity bias associated with illness adjustment: cross sectional analysis of insurance claims. BMJ. 2013;346:f549. doi: 10.1136/bmj.f549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pyenson B, Sawhney TG, Steffens C, et al. The real-world Medicare costs of Alzheimer disease: considerations for policy and care. J Manag Care Spec Pharm. 2019;25(7):800-809. doi: 10.18553/jmcp.2019.25.7.800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chronic Conditions Data Warehouse. Centers for Medicare & Medicaid Services . Accessed March 3, 2022. https://www2.ccwdata.org/
- 15.Taylor DH Jr, Fillenbaum GG, Ezell ME. The accuracy of Medicare claims data in identifying Alzheimer’s disease. J Clin Epidemiol. 2002;55(9):929-937. doi: 10.1016/S0895-4356(02)00452-3 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: insights from linked survey and administrative claims data. Alzheimers Dement (N Y). 2019;5:197-207. doi: 10.1016/j.trci.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258-1267. doi: 10.1016/S0895-4356(00)00256-0 [DOI] [PubMed] [Google Scholar]
- 18.Zhu Y, Chen Y, Crimmins EM, Zissimopoulos JM. Sex, race, and age differences in prevalence of dementia in Medicare claims and survey data. J Gerontol B Psychol Sci Soc Sci. 2021;76(3):596-606. doi: 10.1093/geronb/gbaa083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Non-federal acute care hospital electronic health record adoption. Office of the National Coordinator for Health Information Technology . September 2017. Accessed March 3, 2022. https://www.healthit.gov/data/quickstats/non-federal-acute-care-hospital-electronic-health-record-adoption
- 20.Office-based physician electronic health record adoption. Office of the National Coordinator for Health Information Technology . January 2019. Accessed March 3, 2022. https://dashboard.healthit.gov/quickstats/pages/physician-ehr-adoption-trends.php
- 21.5010 Implementation—processing additional International Classification of Diseases, 9th Revision-Clinical Modification (ICD-9-CM) diagnosis and procedure codes in pricer, grouper, and the Medicare Code Editor (MCE). Centers for Medicare & Medicaid Services . August 13, 2010. Accessed March 3, 2022. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2010-Transmittals-Items/CMS1238530
- 22.Arday SL, Arday DR, Monroe S, Zhang J. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21(4):107-116. [PMC free article] [PubMed] [Google Scholar]
- 23.Elixhauser Comorbidity Software, Version 3.7. Agency for Healthcare Research and Quality . June 2017. Accessed March 3, 2022. http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp
- 24.Welch HG, Sharp SM, Gottlieb DJ, Skinner JS, Wennberg JE. Geographic variation in diagnosis frequency and risk of death among Medicare beneficiaries. JAMA. 2011;305(11):1113-1118. doi: 10.1001/jama.2011.307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berenson-Eggers Type of Service (BETOS). Centers for Medicare & Medicaid Services . Updated December 1, 2021. Accessed March 3, 2022. https://www.cms.gov/Medicare/Coding/HCPCSReleaseCodeSets/BETOS
- 26.Davis MA, Nallamothu BK, Banerjee M, Bynum JP. Identification of four unique spending patterns among older adults in the last year of life challenges standard assumptions. Health Aff (Millwood). 2016;35(7):1316-1323. doi: 10.1377/hlthaff.2015.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amjad H, Snyder SH, Wolff JL, Oh E, Samus QM. Before hospice: symptom burden, dementia, and social participation in the last year of life. J Palliat Med. 2019;22(9):1106-1114. doi: 10.1089/jpm.2018.0479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicholas LH, Bynum JP, Iwashyna TJ, Weir DR, Langa KM. Advance directives and nursing home stays associated with less aggressive end-of-life care for patients with severe dementia. Health Aff (Millwood). 2014;33(4):667-674. doi: 10.1377/hlthaff.2013.1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldman H, Gracon S. Symptomatic treatments for Alzheimer’s disease. In: Gauthier S, ed. Clinical Diagnosis and Management of Alzheimer’s Disease. CRC Press; 1996:239-253. [Google Scholar]
- 30.Aldridge MD, Ornstein KA, McKendrick K, Moreno J, Reckrey JM, Li L. Trends in residential setting and hospice use at the end of life for Medicare decedents. Health Aff (Millwood). 2020;39(6):1060-1064. doi: 10.1377/hlthaff.2019.01549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell SL, Mor V, Gozalo PL, Servadio JL, Teno JM. Tube feeding in US nursing home residents with advanced dementia, 2000-2014. JAMA. 2016;316(7):769-770. doi: 10.1001/jama.2016.9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callahan CM, Tu W, Unroe KT, LaMantia MA, Stump TE, Clark DO. Transitions in care in a nationally representative sample of older Americans with dementia. J Am Geriatr Soc. 2015;63(8):1495-1502. doi: 10.1111/jgs.13540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross SH, Kaufman BG, Taylor DH Jr, Kamal AH, Warraich HJ. Trends and factors associated with place of death for individuals with dementia in the United States. J Am Geriatr Soc. 2020;68(2):250-255. doi: 10.1111/jgs.16200 [DOI] [PubMed] [Google Scholar]
- 34.Wang SY, Aldridge MD, Gross CP, Canavan M, Cherlin E, Bradley E. End-of-life care transition patterns of Medicare beneficiaries. J Am Geriatr Soc. 2017;65(7):1406-1413. doi: 10.1111/jgs.14891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin PJ, Maciejewski ML, Paul JE, Biddle AK. Risk adjustment for Medicare beneficiaries with Alzheimer’s disease and related dementias. Am J Manag Care. 2010;16(3):191-198. [PubMed] [Google Scholar]
- 36.McCarthy EP, Chang CH, Tilton N, Kabeto MU, Langa KM, Bynum JPW. Validation of claims algorithms to identify Alzheimer’s disease and related dementias. J Gerontol A Biol Sci Med Sci. 2021;glab373. doi: 10.1093/gerona/glab373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grodstein F, Chang CH, Capuano AW, et al. Identification of dementia in recent Medicare claims data, compared to rigorous clinical assessments. J Gerontol A Biol Sci Med Sci. 2021;glab377. doi: 10.1093/gerona/glab377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. International Classification of Disease diagnosis codes used to identify ADRD