Abstract
Background
Patients with post-acute sequela of COVID-19 (PASC) often report symptoms of orthostatic intolerance and autonomic dysfunction. Numerous case reports link postural orthostatic tachycardia syndrome (POTS) to PASC. No prospective analysis has been performed.
Objectives
This study performed head-up tilt table (HUTT) testing in symptomatic patients with PASC to evaluate for orthostatic intolerance suggestive of autonomic dysfunction.
Methods
We performed a prospective, observational evaluation of patients with PASC complaining of poor exertional tolerance, tachycardia with minimal activity or positional change, and palpitations. Exclusion criteria included pregnancy, pre-PASC autonomic dysfunction or syncope, or another potential explanation of PASC symptoms. All subjects underwent HUTT.
Results
Twenty-four patients with the described PASC symptoms were included. HUTT was performed a mean of 5.8 ± 3.5 months after symptom onset. Twenty-three of the 24 had orthostatic intolerance on HUTT, with 4 demonstrating POTS, 15 provoked orthostatic intolerance (POI) after nitroglycerin, 3 neurocardiogenic syncope, and 1 orthostatic hypotension. Compared with those with POTS, patients with POI described significantly earlier improvement of symptoms.
Conclusions
This prospective evaluation of HUTT in patients with PASC revealed orthostatic intolerance on HUTT suggestive of autonomic dysfunction in nearly all subjects. Those with POI may be further along the path of clinical recovery than those demonstrating POTS.
Key Words: head-up tilt table (HUTT), neurocardiogenic syncope, orthostatic hypotension, orthostatic intolerance, postural orthostatic tachycardic syndrome (POTS)
Central Illustration
Relatively little is known about the potential lasting impact of COVID-19. There is recognition of a debilitating post COVID-19 syndrome referred to as post-acute sequela of COVID-19 (PASC), defined as the presence of at least 1 clinical sequela at least 4 weeks after acute COVID-19 infection.1, 2, 3 Although current PASC literature describes fatigue as a primary symptom, palpitations and orthostatic intolerance (OI) have also been reported, albeit less frequently.4 , 5 The etiology of these symptoms is unknown, and a purported mechanism is dysregulation of the autonomic nervous system. Autonomic dysfunction associated with OI, including new-onset postural orthostatic tachycardia syndrome (POTS) in patients with PASC, has been described in case reports.6, 7, 8, 9, 10 We identified patients with specific PASC symptoms following COVID-19 infection and report a prospective evaluation of OI in these patients.
Methods
The study is a prospective, longitudinal, observational evaluation of patients between the ages of 18 and 70 with PASC symptoms of poor exertional tolerance, tachycardia with minimal activity or positional change, and palpitations. Diagnosis of acute COVID-19 illness required a positive polymerase chain reaction test. The period of acute infection was defined as the time between onset of COVID-19 symptoms, regardless of severity or need for medical attention, and 3 weeks after initial acute symptom onset. PASC symptoms were defined as those that were present a minimum of 3 months after recovery from acute COVID-19 illness. For study enrollment, patients required testing for an alternate etiology of symptoms after PASC diagnosis and were excluded for any of the following: abnormal chest x-ray, left ventricular ejection fraction <50% by transthoracic echocardiography, abnormal hemoglobin/hematocrit, or abnormal thyroid-stimulating hormone levels. Patients were also excluded for pregnancy, pre-COVID-19 history of syncope, or pre-COVID-19 autonomic dysfunction by symptoms or diagnosis. Patients taking beta-blockers initiated for PASC symptoms were not excluded from the study.
After enrollment, demographic data were collected and patients underwent head-up tilt table (HUTT) testing. HUTT was performed as per the Italian protocol on all patients after an overnight fast.11 After a minimum 5-minute supine position, subjects were tilted to 70° with an initial 21-minute pharmacology-free passive component and, if a normal response was demonstrated, administration of a 0.4-mg sublingual nitroglycerin (NTG) tablet with continuation of a 15-minute observation component. Blood pressure was recorded in the right arm with an automated BP cuff at 3-minute intervals, and additional recordings were performed as guided by development of patient symptoms. Heart rate, electrocardiogram, and pulse oximetry were continuously recorded. HUTT testing was performed by a single research team member (S.M.J.). Beta-blockers were not held before HUTT. Five response types were defined for the study and are delineated in Table 1 : POTS, provoked OI (POI) seen after NTG, neurocardiogenic syncope, orthostatic hypotension, and normal. The first 4 responses were evidence of OI. Both POTS and POI diagnoses required reproduction of a patient’s exact clinical symptoms during HUTT, with rapid improvement on return to a supine position. Dedicated follow-up of PASC symptoms was performed at 3 and 6 months after subject enrollment.
Table 1.
Response Categories of HUTT Testing
| POTS | Development of typical PASC symptoms with heart rate increase of ≥30 beats/min, or absolute heart rate >120 beats/min within 10 minutes of standing, with systolic blood pressure (SBP) decrease <20 mm Hg and diastolic blood pressure (DBP) decrease <10 mm Hg. |
| POI | Development of typical PASC symptoms with heart rate increase of ≥30 beats/min with SBP decrease <20 mm Hg and DBP decrease <10 mm Hg occurring after administration of sublingual NTG. Heart rate and blood pressure changes within 10 minutes of NTG administration compared with the last set of vital signs obtained in the passive, nonpharmacological component (the 21st minute). |
| Neurocardiogenic syncope | Significant fall in heart rate or blood pressure with loss of consciousness or inability to maintain posture. |
| Orthostatic hypotension | Any heart rate increase with a SBP decrease >20 mm Hg or DBP decrease >10 mm Hg. |
| Normal | Absence of patterns noted above. |
HUTT = head-up tilt table; NTG = sublingual nitroglycerin; PASC = post-acute sequela of COVID-19; POI = provoked orthostatic intolerance; POTS = postural orthostatic tachycardia syndrome.
Statistical analyses
Descriptive statistics were calculated for the sample. The Shapiro-Wilk test and Q-Q plots were used in conjunction to assess the normality of continuous variables. Continuous variables were reported as mean ± SD, and categorical variables were reported as number (%). Comparisons between groups were performed using unpaired Student’s t-test for continuous variables and Fisher exact test for categorical variables due to small sample sizes. All tests were 2-sided and a P value <0.05 was used to indicate statistical significance. Analyses were performed in SAS version 9.4 (SAS Institute Inc).
Ethical approval
The study was approved by the Institutional Review Board at Hackensack University Medical Center at Hackensack Meridian School of Medicine.
Results
Thirty-two patients with the described PASC symptoms were referred for this study from a dedicated post COVID-19 recovery clinic or from other clinics within our hospital network. Three referred patients chose not to participate in the study, 2 were excluded for anemia, 1 was excluded for pregnancy, 1 was excluded for pre-existing syncope, and 1 did not wish to have HUTT performed. The remaining 24 patients underwent HUTT. Most of the subjects (83.3 %) were women with a mean age of 43.1 ± 11.3 years. Only 2 patients had required hospitalization for acute COVID-19 infection, one of whom was managed in the intensive care unit. All patients continued to describe PASC symptoms at the time of HUTT, which was performed 5.8 ± 3.5 months from PASC symptom onset. We performed initial follow-up of PASC symptoms on all patients at 9.1 ± 3.6 and final follow-up at 11.9 ± 3.7 months from PASC symptom onset. Table 2 describes patient characteristics of all enrolled subjects.
Table 2.
Patient Characteristics (N = 24)
| Age, y | 43.1 ± 11.3 |
| Sex | |
| Male | 4 |
| Female | 20 |
| Acute COVID-19 symptoms | |
| Fever | 23 |
| Cough | 22 |
| Shortness of breath | 20 |
| Loss of taste | 15 |
| Loss of smell | 14 |
| Hospitalized for acute COVID-19 infection | 2 |
| Required intensive care unit admission | 1 |
| PASC symptoms | |
| Palpitations | 24 |
| Exertional intolerance | 24 |
| Fatigue | 21 |
| Cognitive dysfunction | 16 |
| Headache | 11 |
| Chest pain | 6 |
| Beta-blocker use for PASC symptoms | 7 |
| Months from positive COVID-19 test to onset of PASC symptoms | 1.0 ± 0.6 |
| Months from onset of PASC symptoms to tilt table test | 5.8 ± 3.5 |
| PASC symptoms resolved/improving/unchanged at time of HUTT | 0/18/6 |
| Months from onset of PASC symptoms to first study follow-up | 9.1 ± 3.6 |
| PASC symptoms resolved/improving/unchanged at first study follow-up | 2/17/5 |
| Months from onset of PASC symptoms to final study follow-up | 11.9 ± 3.7 |
| PASC symptoms resolved/improving/unchanged at final study follow-up | 4/16/4 |
Values are mean ± SD, number of patients describing PASC symptoms resolved/improving/unchanged at various time points.
Abbreviations as in Table 1.
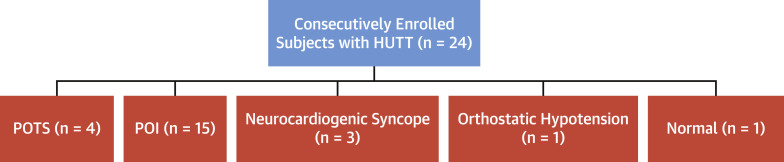
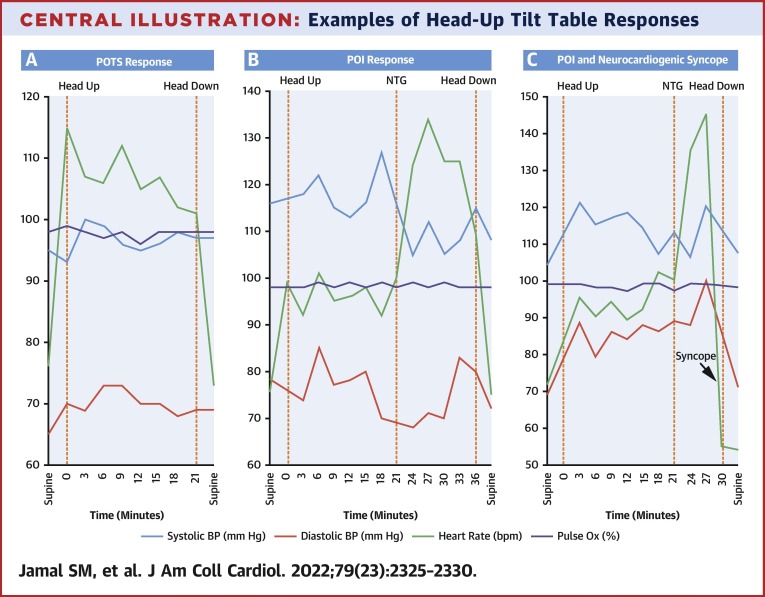
Figure 1 demonstrates subject responses to HUTT. Four patients demonstrated POTS, 15 demonstrated POI, 3 had neurocardiogenic syncope, 1 had orthostatic hypotension, and 1 response was normal. In addition to the 3 patients exhibiting neurocardiogenic syncope alone, 1 of 4 patients with POTS and 5 of 15 patients with POI also developed neurocardiogenic syncope during HUTT. Graphical examples of POTS, POI, and combination POI and neurocardiogenic syncope responses on HUTT are shown in the Central Illustration .
Figure 1.
Subject Responses to HUTT Testing
Twenty-four consecutively enrolled patients underwent HUTT with 4 demonstrating POTS, 15 exhibiting POI, 3 with neurocardiogenic syncope, 1 demonstrating orthostatic hypotension, and 1 with a normal response. HUTT = head-up tilt table; POI = provoked orthostatic intolerance; POTS = postural orthostatic tachycardia syndrome.
Central Illustration.
Examples of Head-Up Tilt Table Responses
(A) Example of POTS response. On standing, classic PASC symptoms develop and heart rate increases >30 beats/min with minimal change in blood pressure. The heart rate normalizes and symptoms resolve with resumption of supine position. (B) Example of POI response. During the initial, nonpharmacological phase, no PASC symptoms are described and heart rate increases, although <30 beats/min. After administration of NTG, there is reproduction of classic PASC symptoms and heart rate increases >30 beats/min with minimal change in blood pressure. The heart rate normalizes and symptoms resolve with resumption of supine position. (C) Example of combination POI and neurocardiogenic syncope. POI response develops with sudden and significant fall in heart rate with loss of consciousness denoted by arrow labeled “syncope.” HUTT = head-up tilt table; NTG = sublingual nitroglycerin; PASC = post-acute sequela of COVID-19; POI = provoked orthostatic intolerance; POTS = postural orthostatic tachycardia syndrome.
Compared to patients with POTS, those with POI trended older in age, had significantly higher body mass indexes, and were significantly more likely to describe improvement of their PASC symptoms by the time of HUTT testing and at initial follow-up (Table 3 ). None of the 4 patients diagnosed with POTS was taking beta-blockers.
Table 3.
Associations Between Patients Demonstrating POTS or POI Responses During HUTT
| POTS (n = 4) | POI (n = 15) | P Value | |
|---|---|---|---|
| Age, y | 31.8 ± 4.6 | 42.7 ± 10.7 | 0.067 |
| BMI, kg/m2 | 19.5 ± 3.6 | 26.0 ± 5.7 | 0.048 |
| Months from positive COVID-19 test to onset of PASC symptoms | 1.2 ± 0.9 | 0.9 ± 0.4 | 0.36 |
| Beta-blocker use for PASC symptoms | 0 | 5 | 0.53 |
| Months from onset of PASC symptoms to tilt table test | 5.1 ± 3.0 | 6.2 ± 3.8 | 0.58 |
| PASC symptoms present but improving at time of HUTT | 1 | 15 | 0.004 |
| PASC symptoms improving or resolved at first study follow-up | 1 | 14 | 0.016 |
| PASC symptoms improving or resolved at final study follow-up | 3 | 14 | 0.39 |
Values are mean ± SD or n, unless otherwise indicated. Bold indicates P value <0.05.
BMI = body mass index; other abbreviations as in Table 1.
Discussion
We performed a prospective evaluation of OI by HUTT in PASC. All but 1 of our patients exhibited OI on HUTT, suggestive of autonomic dysfunction. Given that only 2 of our patients required hospitalization, our study suggests that even mild cases of COVID-19 illness can lead to PASC symptoms. Although nearly one-third of our subjects demonstrated either POTS or neurocardiogenic syncope by classic definitions,12, 13, 14 nearly all others had a normal passive HUTT response but went on to exhibit OI with reproduction of their PASC symptoms provoked by NTG administration. The pattern of OI provoked by NTG is similar to the triad seen in classic POTS: reproduction of exact clinical symptoms, significant heart rate increase, and minimal blood pressure reduction. As POTS is traditionally diagnosed in the absence of provocative agents,15 we categorized this observation as POI. Previous investigation of HUTT response after sublingual NTG administration in control subjects demonstrated relatively little impact on blood pressure, heart rate, or symptom reproduction.16, 17, 18 We believe the POI response seen in these subjects is significant and may suggest underlying autonomic dysfunction after COVID. This dysfunction is presumably PASC related, as no patients reported history suggestive of OI or autonomic disease before their illness.
We investigated differences between those demonstrating POTS and POI responses, understanding the inherent limitation of small group sizes. Despite this, subjects with POI response trended older with significantly higher body mass index, and all reported significantly greater improvement in PASC symptoms by the time of HUTT performance with symptom improvement or resolution at first follow-up, an average of 9 months from the time of PASC symptom onset. One potential explanation of these findings is that compared with the older POI subjects, younger individuals afflicted with PASC may manifest more substantial forms of PASC-related OI that in turn takes longer to improve or resolve. Another possibility is that those with improvement in PASC symptoms require a greater degree of provocation to elicit the OI suggestive of autonomic dysfunction. We speculate that those with POI would potentially have demonstrated a true POTS pattern during the nonpharmacologic phase had HUTT been performed earlier in the course of disease and before PASC symptom improvement.
POTS is thought to be a disorder of the autonomic nervous system associated with OI, and clinically presents as orthostatic tachycardia with varied symptoms of fatigue, palpitations, dizziness, and cognitive dysfunction.15 The precise pathophysiology of POTS is unclear, and whether the purported mechanisms are similarly responsible for the OI seen in patients with PASC is an area in need of additional investigation. Regardless, the development of POTS after viral and bacterial illness is well established19 beginning with the earliest description of the disorder.20 With the overwhelming number of COVID-19 infections, it seems logical that case studies and retrospective analyses now demonstrate the development of POTS in those recovering from COVID-19.6, 7, 8, 9, 10 Our investigation adds to this emerging body of knowledge, suggesting in a prospective manner that nearly all individuals with the described PASC symptoms are impacted by OI that suggests autonomic dysfunction.
In our study, patients were excluded for history of syncope before PASC development. Although only 1 patient described syncope as a symptom of their PASC, neurocardiogenic syncope was observed on HUTT in 9 patients. Neurocardiogenic syncope is known to occur in 10%-38% of patients with POTS.21, 22, 23, 24 It is theorized that the characteristic findings in POTS are a result of increased sympathetic discharge that may dissipate, resulting in bradycardia, hypotension, and syncope from relative sympathetic withdrawal.25 Although the mechanism is unclear, we believe POI may have an overlap with neurocardiogenic syncope similar to POTS. Although neurocardiogenic syncope on HUTT may represent a paroxysmal event in other scenarios, we speculate that this finding may be part of a constellation of PASC-related disorders of autonomic tone.
Study limitations
Our investigation is limited by both the small overall population of patients and enrollment at a single site with an increased potential for type II error. We do not have an accurate assessment of the total PASC population from which our patients were selected, as they were referred from several clinics. The small size of the patient population and the heterogeneity of management did not lend themselves to analysis of the potential effects of therapy on symptom resolution. We evaluated patients by updated criteria of PASC and included those with specific symptoms, but recognize that the definition, symptoms, and timeline of PASC is evolving. Before HUTT, beta-blockers were not held for the subjects taking these medications. Our objective was to assess patients in their current PASC state, even if on beta-blocker therapy. Although beat-to-beat invasive or noninvasive arterial pressure monitoring can be used during HUTT, we performed fixed interval blood pressure monitoring. In this prospectively gathered but retrospectively analyzed database of patients with COVID-19, we did not systematically collect data regarding other autonomic dysfunction symptoms, hence we acknowledge that these patients had OI that was strongly suggestive but not necessarily diagnostic of autonomic dysfunction. Nevertheless, we feel that the OI is a potentially important, objective element of autonomic dysfunction with limited alternate explanations. Finally, the POI group of patients defined by OI after NTG had identical PASC symptoms and an HUTT response strikingly similar to POTS. Although we recognize that this pattern cannot be categorized as POTS and appreciate that the explanation of the POI pattern has not been clearly established, we believe it is highly suggestive of underlying autonomic dysfunction.
Conclusions
This is a prospective evaluation of HUTT in patients with PASC with specific symptoms. We found OI on HUTT in nearly all study patients, and neurocardiogenic syncope in 9 of 24. These findings suggest the presence of autonomic dysfunction. Compared with those with POTS response on HUTT, subjects demonstrating POI may be further along the path of clinical recovery and describe significantly earlier improvement of symptoms.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with exercise intolerance, tachycardia on minimal activity or positional change, and palpitations as post-acute sequelae of COVID-19 often exhibit abnormal orthostatic response to HUTT testing, suggesting autonomic dysfunction.
TRANSLATIONAL OUTLOOK: Additional studies are warranted to assess the impact of specific treatment modalities on autonomic function and symptoms in patients with these post-acute sequelae of COVID-19.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Nalbandian A., Sehgal K., Aakriti G., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lerner A.M., Robinson D.A., Yang L., et al. Toward understanding COVID-19 recovery: National Institutes of Health Workshop on Postacute COVID-19. Ann Intern Med. 2021;174(7):999–1003. doi: 10.7326/M21-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie Y., Bowe B., Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12:6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvalho-Schneider C., Laurent E., Lemaignen A., et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miglis M.G., Prieto T., Shaik R., Muppidi S., Sinn D., Jaradeh S. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30(5):449–451. doi: 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Umapathi T., Poh M.Q.W., Fan B.E., Li K.F.C., George J., Tan J.Y.L. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin Auton Res. 2020;30(6):571–573. doi: 10.1007/s10286-020-00733-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanjwal K., Jamal S., Kichloo A., Grubb B.P. New-onset postural orthostatic tachycardia syndrome following Coronavirus disease 2019 infection. J Innov Cardiac Rhythm Manage. 2020;11(11):4302–4304. doi: 10.19102/icrm.2020.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson M., Stahlberg M., Runold M., et al. Long-haul post-COVID-19 symptoms presenting as a variant of a postural orthostatic tachycardia syndrome: the Swedish experience. J Am Coll Cardiol Case Rep. 2021;3(4):573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blitshteyn S., Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69(2):205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartoletti A., Alboni P., Ammirati F., et al. 'The Italian Protocol': a simplified head-up tilt testing potentiated with oral nitroglycerin to assess patients with unexplained syncope. Europace. 2000;2(4):339–342. doi: 10.1053/eupc.2000.0125. [DOI] [PubMed] [Google Scholar]
- 12.Grubb B.P., Olshansky B. Chapter 7. Tilt Table Testing. Syncope: Mechanisms and Management. 2nd ed. Blackwell Publishing; 2008. p. 76. [Google Scholar]
- 13.Kanjwal K., Calkins H. Syncope in children and adolescents. CardiolClin. 2015;33:397–409. doi: 10.1016/j.ccl.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Grubb B.P., Kanjwal Y., Kosinski D.J. The postural tachycardia syndrome: as concise guide to diagnosis and management. J Cardiovasc Electrophysiol. 2006;17:1–5. doi: 10.1111/j.1540-8167.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 15.Bryarly M., Phillips L.T., Fu Q., Vernino S., Levine B.D. Postural orthostatic tachycardia syndrome. JACC Focus Seminar. J Am Coll Cardiol. 2019;73:1207–1228. doi: 10.1016/j.jacc.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Raviele A., Menozzi C., Brignole M., et al. Value of head-up testing potentiated with sublingual nitroglycerine to assess the origin of unexplained syncope. Am J Cardiol. 1995;76(4):267–272. doi: 10.1016/s0002-9149(99)80079-4. [DOI] [PubMed] [Google Scholar]
- 17.Gisolf J., Westerhof B.E., Van Dijk N., Wesseling K.H., Weiling W., Karemaker J.M. Sublingual nitroglycerine used in routine tilt testing provokes a cardiac output-mediated vasovagal response. J Am Coll Cardiol. 2004;44:588–593. doi: 10.1016/j.jacc.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Kurbaan A.S., Franzén A.C., Bowker T.J., et al. Usefulness of tilt test–induced patterns of heart rate and blood pressure using a two-stage protocol with glyceryl trinitrate provocation in patients with syncope of unknown origin. Am J Cardiol. 1999;84(6):665–670. doi: 10.1016/s0002-9149(99)00413-0. [DOI] [PubMed] [Google Scholar]
- 19.Thieben M.J., Sandroni P., Sletten D.M., et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 20.Schondorf R., Low P.A. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/wnl.43.1_part_1.132. [DOI] [PubMed] [Google Scholar]
- 21.Ojha A., McNeeley K., Heller E., Alshekhlee A., Chelimsky G., Chelimsky T.C. Orthostatic syndromes differ in syncope frequency. Am J Med. 2010;123:245–249. doi: 10.1016/j.amjmed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 22.Kanjwal K., Qadir R., Ruzieh M., Grubb B.P. Role of implantable loop recorders in patients with postural orthostatic tachycardia syndrome. Pacing Clin Electrophysiol. 2018;41:1201–1203. doi: 10.1111/pace.13441. [DOI] [PubMed] [Google Scholar]
- 23.Chouksey D., Rathi P., Sodani A., Jain R., Ishar H.S. Postural orthostatic tachycardia syndrome in patients of orthostatic intolerance symptoms: an ambispective study. AIMS Neurosci. 2020;8(1):74–85. doi: 10.3934/Neuroscience.2021004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raj S.R. The Postural Tachycardia Syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6(2):84–99. [PMC free article] [PubMed] [Google Scholar]
- 25.Kanjwal K., Sheikh M., Karabin B., Kanjwal Y., Grubb B.P. Neurocardiogenic syncope coexisting with postural orthostatic tachycardia syndrome in patients suffering from orthostatic intolerance: a combined form of autonomic dysfunction. Pacing Clin Electrophysiol. 2011;34(5):549–554. doi: 10.1111/j.1540-8159.2010.02994.x. [DOI] [PubMed] [Google Scholar]