Abstract
Purpose of Review
Lung cancer screening with low-dose CT (LDCT) scans has been widely accepted within the last decade. Our knowledge and ability to implement screening has greatly increased because of significant research efforts and guidelines from multiple professional societies. The purpose of this review is to summarize some of the significant findings pertaining to lung cancer screening.
Recent Findings
Screening with LDCT decreases lung cancer mortality in multiple studies. Use of validated risk prediction calculators can improve patient selection and screening efficiency. Shared decision making and smoking cessation counseling are essential screening components. Multidisciplinary involvement is required for the success of a screening program.
Summary
Lung cancer screening is complex, and implementation of a successful program requires multidisciplinary expertise. Further prospective studies are required to determine optimal patient selection, screening intervals, and strategies to maximize benefit while further decreasing harms.’
Keywords: Lung cancer, Cancer screening, Early detection of cancer, Low-dose computed tomography
Introduction
Lung cancer (LC) is the number one cause of cancer-related deaths in the United States and the world in both men and women [1, 2]. Worldwide, there are approximately 1.8 million new cases and 1.6 million deaths every year [3], and in the US alone, LC accounts for approximately 23% of cancer related mortality [1]. The overall 5-year survival rate for LC remains poor at approximately 19% [1]. The mortality rate for LCs is predictably much lower in early compared to late stages [4], when it is potentially curable by surgical resection. There has been a longstanding intense focus on the development of effective LC screening strategies, designed for early identification and intervention in patients who are well enough to benefit. However, unlike in breast, prostate, and colon cancers, there was no widely recommended and effective screening method for LC until this past decade.
The goal of this paper is to briefly review the background of LC screening, recent updates in guidelines and clinical practice, discuss recent challenges, and consider future directions.
Background and History
LC is strongly linked to tobacco smoking [4]. In fact, the rise in LC parallels the increase in tobacco smoking during the late 1800s and 1900s [5]. However, this association was only proved epidemiologically in 1950, and smoking cessation and abstinence was promoted as a public health effort by the US Surgeon General in 1964 [5]. Through widespread efforts, the rates of smoking cigarettes have been steadily decreasing [4].
Earlier studies in the 1980s [6–9] evaluating the role of chest X-rays (CXRs) and sputum cytology as screening tools suggested an overall survival advantage attributed to length or lead time bias and overdiagnosis [10], but failed to show a LC-specific mortality difference. The Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial was a randomized-controlled trial (RCT) evaluating 154,901 participants aged 55 to 74 years from 1993 to 2001 [11]. Annual screening with CXR for 4 years in the intervention group was compared to usual care without intervention in the control group, and follow-up was continued for up to 13 years. There was no significant LC mortality benefit detected with CXR screening (mortality RR, 0.99; 95% CI, 0.87–1.22).
Studies in the 2000s [12–15] evaluated low-dose computed tomography (LDCT) scans for LC screening, and while CT scans were able to detect more early stage cancers, there was no conclusive proof of a mortality benefit. Some of these studies had design flaws and either lacked controls, adequate power, or sufficient enrollment. The National Lung Screening Trial (NLST) was a landmark study published in 2011, showing for the first time that dedicated annual screening for LC in a high-risk population was effective in decreasing mortality by as much as 20%, when using LDCT compared to CXRs [16••]. The study showed that for every 320 patients screened with CT, one death was prevented. This finally provided the evidence required for wider acceptance of LC screening with LDCT, and set into motion the gradual implementation process of dedicated screening programs.
In 2013, various organizations started recommending LC screening in selected high-risk populations. The US Preventive Services Task Force (USPSTF) recommended it for high-risk smokers age 55–80 [17]. Compared to the NLST criteria, the age limit had been increased to 80 based on modeling results from the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET) for the Agency for Healthcare Research and Quality [18].
In 2015, the Centers for Medicare and Medicaid Services (CMS) announced their decision to approve LDCT for screening in high-risk individuals. However, CMS mandated that counseling with a shared decision-making (SDM) visit also be performed, in addition to ensuring eligibility criteria for the interpreting radiologist and imaging facility were met [19].
Lung Cancer Screening Trial Updates in the Last 5 Years
In the last 5 years, there were several RCT results published, including long-term follow-up of earlier trials [16••, 20–26, 27••]. These RCTs are summarized in Table 1, with NLST baseline data also included as a reference point.
Table 1.
Summary of randomized-controlled trials on LDCT screening with extended follow-up and lung cancer-specific mortality results published within last 5 years
| Name of study | Country | Year of publication/update | Inclusion criteria | Number of patients in randomized arm | Follow-up period (median years) | Comparison | Number of annual screens | Lung cancer mortality events | Conclusion | Comments | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LDCT | Control | ReR, RR, or HR | P value | ||||||||||
| NLST (baseline reference) [16••] | USA | 2011 |
▪ 55–74 years of age ▪ ≥ 30 pack years smoking ▪ Current smoker or quit < 15 years ▪ Males and females |
26,722 | 6.5 | CXR | 3 | 356 (1.3) | 443 (1.7) |
ReR 20% 95% CI, 6.8–26.7 |
0.004 | • Screening with LDCT decreased mortality by 20%, NNS 320 |
Conducted in centers with expertise, unsure if generalizable Only 3 rounds of screening |
| DANTE [20] | Italy | 2015 |
▪ 60–74 years of age ▪ ≥ 20 pack years smoking ▪ Current smoker or quit < 10 years ▪ Only males |
1264 | 8.35 | Clinical review | Baseline + 4 | 59 (4.7) | 55 (4.6) | HR 0.99 (95% CI 0.688–1.433) | NR |
• Unable to make conclusions re: efficacy of LDCT screening • No significant mortality difference |
Insufficient sample size, limited power, single site, low sensitivity of screening protocol |
| DLCST [21] | Denmark | 2016 |
▪ 50–70 years ▪ ≥ 20 pack years smoking ▪ Current smoker or quit < 10 years and after age of 50 ▪ FEV1 ≥ 30% ▪ Can climb 2 flights of stairs (36 steps) without pause ▪ Males and females |
2052 | 9.8 | Usual care | Baseline + 4 | 39 (1.9) | 38 (1.9) | HR 1.03 (95% CI 0.66–1.6) | 0.888 | • No significant all-cause or LC mortality difference noted with screening | Underpowered, single site |
| ITALUNG [22] | Italy | 2017 |
▪ 55–69 years of age ▪ ≥ 20 pack years smoking ▪ Current smoker or quit < 10 years ▪ Males and females |
1613 | 9.3 | Usual care | 4 | 43 (3) | 60 (3.8) | RR = 0.70 (95% CI: 0.47–1.03) | NR |
• 30% reduction in LC-specific and 17% reduction in all-cause mortality noted in LDCT group • This was not statistically significant • This trend suggests that screening with LDCT could decrease mortality |
Insufficient power |
| MILD [23, 24] | Italy | 2019 |
▪ 49–75 years of age ▪ ≥ 20 pack years smoking ▪ Current smoker or quit < 10 years ▪ No history of cancer in ≤ 5 years ▪ Males and females |
2376 1190 annual arm 1186 biennial arm |
10 | No intervention |
7 in annual 4 in biennial |
40 (1.7) | 40 (2.3) | HR 0.61 (95% CI: 0.39–0.95) | 0.14 |
• 39% decrease in 10-year risk of LC mortality with screening • No significant 10-year overall or LC-specific mortality difference between annual and biennial screening • Long-term screening with biennial screening is effective |
There was insufficient power in trial at 5 years of follow-up but adequate power was achieved after 10 years of screening follow-up |
| LUSI [25] | Germany | 2019 |
▪ 50–69 years of age ▪ ≥ 1/2 pack for ≥ 30 years ▪ ≥ 3/4 pack for ≥ 25 years ▪ Current smoker or quit < 10 years ▪ Males and females |
2029 | 8.8 | Usual care | Baseline + 4 | 29 (1.4) | 40 (2.0) | HR 0.74 (95% CI: 0.46–1.19) | 0.21 |
• No significant all-cause or LC mortality difference noted with screening • Significant decrease in LC mortality in subgroup of women as compared with men |
Insufficient sample size |
| NLST [26] | USA | 2019 |
▪ 55–74 years of age ▪ ≥ 30 pack years smoking ▪ Current smoker or quit < 15 years ▪ Males and females |
26,722 | 12.3 | CXR | 3 | 1147 (4.3) | 1236 (4.6) | 0.92 (95% CI: 0.85–1.00) | 0.06 | • Screening with LDCT decreased mortality by 8%, NNS 303 | |
| NELSON [27••] | Netherlands, Belgium | 2020 |
▪ 50–74 years of age ▪ ≥ 1/2 pack for ≥ 30 years ▪ ≥ 3/4 pack for ≥ 25 years ▪ Current smoker or quit < 10 years ▪ Males and females |
7900 | 10 | Usual care | Baseline + 3 (years 1, 3, and 5.5) | 186 (2.4) | 248 (3.2) | RR 0.76 (95 CI: 0.61–0.94) | 0.01 |
• LC screening with volume CT significantly decreased mortality • Significant decrease in LC-specific mortality in women compared to men |
|
DANTE Detection of Early Lung Cancer by Novel Imaging Technology and Molecular Essays, DLCST Danish Lung Cancer Screening Trial, ITALUNG Italian Lung Cancer Screening Trial, HR hazard ratio, LDCT low-dose computed tomography, LC lung cancer, LUSI German Lung Cancer Screening Intervention, MILD Multi-centric Italian Lung Detection Trial, NELSON Nederlands-Leuvens Longkanker Screenings Onderzoek Study, NLST National Lung Cancer Screening Trial, NR not reported, RR rate ratio, ReR relative reduction
The NELSON and the MILD trials showed mortality reduction after 10 years of follow-up and provided further evidence for LC screening with LDCT. The other studies did not show an overall mortality benefit, mostly related to small sample size and insufficient power, although some suggested a trend towards benefit. Interestingly, after 10 years of follow-up, both the ITALUNG and MILD studies showed that the benefit of LC screening was seen mainly after 5 years of initiating screening.
Both the LUSI and the NELSON trials [25, 27••, 28] showed a significant decrease in LC deaths among women more than men, with 69% decrease in women (HR = 0.31, 95% CI = 0.10–0.96, p = 0.04) compared to 6% in men (HR = 0.94, 95% CI = 0.54–1.61, p = 0.81) in the LUSI trial, and 33% decrease in women (RR = 0.67, 95% CI = 0.38–1.14) compared to 24% in men (RR = 0.76, 95% CI = 0.61–0.94, p = 0.01) in the NELSON trial [27••, 28]. The extended NLST follow-up data also confirmed a lower LC mortality for women compared to men [26].
Screening Guidelines
Appropriate patient selection is essential in order to balance the benefits of screening in at-risk patients, while minimizing the adverse effects.
Table 2 summarizes the current inclusion and exclusion criteria for screening as recommended by various groups [2, 17, 19, 28–32, 33••, 34, 35, 36••, 37, 38]. The ACCP guidelines were in turn endorsed by multiple other societies [33••]. Most groups have additional recommendations regarding the clinical setting for LC screening, use of risk calculators, use of shared decision making, and/or smoking cessation counseling.
Table 2.
Lung cancer screening criteria recommendations by specialty societies, institution, NLST, and CMS
| Age (years) | Current or former smoking (pack years) | Quit period for former smokers (years) | Additional criteria for inclusion | Who should not be screened (exclusion or discontinuation) | |
|---|---|---|---|---|---|
| NLST [16••] | 55–74 | ≥ 30 | < 15 | Asymptomatic |
Exclusion: -History of LC -Chest CT within 18 months -Hemoptysis -Unexplained weight loss of > 15 lb in last year |
| USPSTF [17, 39] | 50–80 | ≥ 20 | < 15 | Asymptomatic |
-Life-limiting health condition -Unable or unwilling to have curative surgery |
| CMS [19] | 55–77 | ≥ 30 | < 15 | Asymptomatic |
-Life-limiting health condition - Unable or unwilling to have screening/curative treatment |
| NCCN [2] |
Gp 1: 55–74 Gp 2: ≥ 50 |
Gp 1: ≥ 30 Gp 2: ≥ 20 |
< 15 |
Gp 1: Asymptomatic Gp 2: One of the following: personal history of cancer or certain chronic lung diseases (COPD, pulmonary fibrosis), family history of LC, radon/occupational exposures |
|
|
ATS IASCLC ACS ASCO |
55–74 | ≥ 30 | < 15 | Asymptomatic | |
| AATS [34] |
Gp 1: 55–79 Gp 3: 50–79 |
Gp 1: ≥ 30 Gp 3: ≥ 20 |
Gp 1: < 15 |
Gp 1: Asymptomatic Gp 2: Prior history of LC without recurrence × 4 years, starting 5 years post-treatment Gp 3: Comorbidities which confer ≥ 5% cumulative risk of LC within 5 years |
|
| ALA [35] | 55–80 | ≥ 30 | < 15 | ||
| AAFP [36••] | LC screening with LDCT not supported currently due to initial concerns about relying on one study alone | ||||
AATS American Association of Thoracic Surgery, AAFP American Academy of Family Physicians, ACS American Cancer Society, ACCP American College of Chest Physicians, ALA American Lung Association, ASCO American Society of Clinical Oncology, ATS American Thoracic Society, CMS Centers for Medicare and Medicaid Services, IASCLC International Association for the Study of Lung Cancer, NCCN National Comprehensive Cancer Network, NLST National Lung Screening Trial, USPSTF United States Preventive Services Task Force
The USPSTF has recently announced the finalized updates to its prior recommendations, by lowering the age to start screening to 50 years and decreasing the pack-year smoking eligibility to > 20 years [39], a grade “B” recommendation, based on evidence favoring moderate benefit by expanding the eligibility criteria. Further review by various professional societies and formal adoption of these recommendations by CMS and other healthcare payors, leading to more widespread implementation, is awaited.
Role of Prediction Models for Risk Assessment
The NLST study used fixed criteria (age, pack-years of smoking, and years since smoking cessation) to select high-risk patients for enrollment. However, there are additional risk factors that either individually or in combination increase LC risk, such as personal history of cancer, family history of LC, ethnicity, education, BMI, socioeconomic status, intensity of smoking (actual number of daily cigarettes instead of collective grouping as pack years), occupational/asbestos/radon exposures, and imaging in the past 3 years. Several risk prediction models have been developed to improve patient selection based on individual risk factors instead of subgroups based on risk factors (such as NLST criteria).
Tammemagi et al. compared PLCO and NLST criteria in development and validation cohorts, and the PLCO risk prediction model improved patient selection and LC detection compared to NLST criteria [40]. The PanCan model, a forerunner to the PLCOm2012 validated model, was studied prospectively in 2537 ever-smokers, and approximately 133 (77%) of detected LCs were early stage (stages I and II) and potentially curable [41]. Wider use of risk prediction models was recommended for improving patient selection.
Katki et al. compared absolute risk models with USPSTF recommendations to determine effective screening strategies [42]. In the risk-based fixed population size model, 36% of lower risk screen-eligible smokers were replaced by a similar number of high-risk smokers, either low-intensity longer term smokers or higher intensity smokers who quit > 15 years ago. There was improved screening efficacy, with decreased number needed to screen (NNS) to prevent one death, i.e., 162 (95% CI, 157–166) compared to 194 (95% CI, 128–137) and a decrease in false-positive CT examinations. In the risk-based fixed effectiveness strategy, the relative modeled preventable death was 34% higher. Overall, their study showed that application of risk-based models prevented more deaths at 5 years and improved effectiveness of screening by decreasing NNS to prevent one death.
Risk prediction models generally predict either LC incidence or mortality. Nine of these models with broad applicability [Bach model, Liverpool Lung Project (LLP) model, PLCOm2012 model, the Two-Stage Clonal Expansion (TSCE) model for incidence, two versions of TSCE model for death, Knoke model and simplified versions of PLCOm2012 and LLP models] were reviewed and validated by Ten Haaf et al. [43] and applied to both NLST and PLCO cohorts. They found that with risk thresholds specific to each model, all had a higher sensitivity and specificity than currently used NLST criteria, which had a specificity of 62.2% (95% CI: 61.7–62.7%) and sensitivity of 71.4% (95% CI: 68–74.6%). Overall, however, the models which performed the best, with sensitivities > 79.8% and specificities > 62.3%, were the PLCOm2012 model, followed by the Bach and TSCE incidence models.
When combined with LDCT results, the use of the PLCOm2012 validated model in NLST data was able to help stratify patients into high- and low-risk groups, and improve prediction of LC risk [44].
In summary, risk prediction models can help improve patient risk stratification and screening efficacy. Prospective studies are needed for comparative analysis of various models in different populations to help determine optimal patient selection. The ongoing Yorkshire Lung Cancer Screening Trial [45], designed to evaluate three selection methods (USPSTF criteria, the PLCOm2012 and LLP models), will help further our understanding.
At times, models can be tedious, and determination of the best risk threshold to use is not always clear. To combat this, online calculators have been developed, such as the Brock model (https://brocku.ca/lung-cancer-risk-calculator), which includes PLCOm2012 risk calculator and LDCT results, and are more user-friendly.
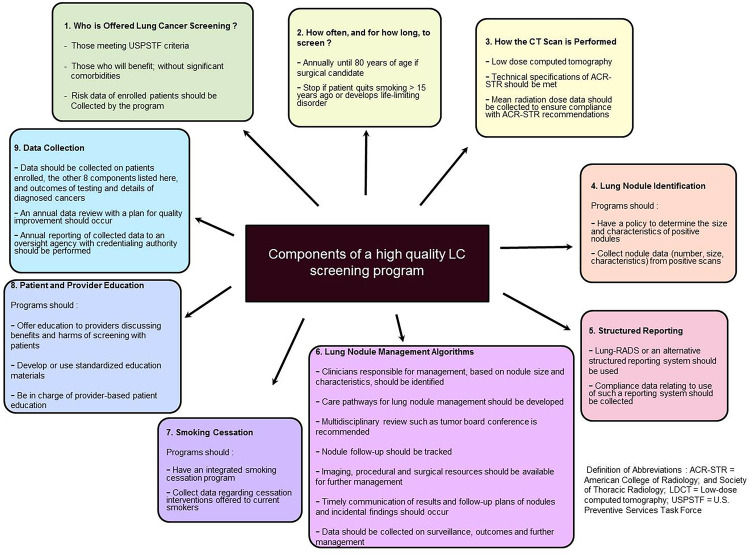
Components of a High-Quality Screening Program
Less than 5% eligible Americans are currently undergoing screening [46]. There is a need for high-quality programs to improve screening effectiveness.
A combined policy statement released by the ACCP and ATS in 2015 outlined the recommendations for screening programs to help implement LC screening in clinical practice [47]. Nine main components were identified, as summarized in Fig. 1.
Fig. 1.
Components of a high-quality lung cancer screening program: combined ACCP and ATS policy statement
Harms of Screening
False Positives In NLST, a 4-mm threshold was used to classify nodules as positive, and the LDCT group had a false positive rate of 96.4% [16••]. Increasing the threshold for nodule detection can decrease false positives at the risk of decreasing sensitivity. The rate of adverse events associated with a diagnostic procedure as a result of a false positive screening test was noted to be low at 0.4% [28].
False Negatives or “Missed” Diagnoses This accounts for approximately 90% versus 5% of the presumed errors in CXR and CT scan screening, respectively [48]. These occur due to observer error, characteristics of the lesion or technical error, and can have medicolegal consequences.
Overdiagnosis Overdiagnosis refers to detection of slow growing cancers that might not otherwise have caused symptoms or harm, and generally occur due to overdetection, i.e., decreasing the thresholds, or overdefinition, i.e., expanding the range of the definition for a diagnosis [49]. This can lead to unnecessary procedures, and contribute to morbidity, anxiety, and expense. There is significant variation in overdiagnosis reported in trials, and an extended follow-up period is helpful for estimation. The overdiagnosis rate in NLST was approximately 18.5% [50], although this decreased to 3% with increase in follow-up from 6.5 to 11 years [26]. Similarly, in the NELSON study, an overdiagnosis rate of 19.7% at 10 years decreased to 8.9% after extension of follow-up to 11 years [27••]. In the DLCST and ITALUNG trials, the rates of overdiagnoses were 67% and 0%, respectively. It is important that trials report overdiagnosis rates and that these are considered in clinical practice [51].
Invasive Procedures and Complications Screen-detected abnormalities often lead to additional diagnostic and therapeutic procedures. The invasive procedure rates are reported to be approximately 5.1% and 2.7% after screening with LDCT and CXR, respectively [34], with complication rates also higher in patients who were screen-positive by LDCT compared to CXR. In NLST, complications were higher in patients with malignancy (23.3%) versus false-positive benign disease (0.4%) [16••, 28]. Complications were higher after surgery compared to bronchoscopy in both malignant (32.4% vs 9.2%) and benign (15.9% vs 4.8%) diagnoses, with rates of complications of needle biopsies lying in between. The overall surgical perioperative mortality rate (within 60 days) was 1% for LDCT screened patients vs 0.2% for the control (CXR) patients [52].
Radiation Exposure The radiation exposure from a CXR is 0.1mSV and LDCT is 1.5 mSV. In contrast, the radiation from a full-dose CT scan and PET-CT are approximately 8 mSV and 14 mSV, respectively [52]. There is a cancer risk from exposure to ionizing radiation during scanning, which is cumulative throughout life [36••]. In NLST, the cumulative radiation dose per patient during 3 years of screening was approximately 8 mSV. It is predicted that for every 2500 patients undergoing LC screening, there would be 1 radiation-related cancer death [52]. For smokers undergoing LDCT screening from 50 to 75 years, annual screening is estimated to increase the baseline risk of cancer in males (15.8%) by 0.23% and females (16.9%) by 0.85%, representing a 1.5% and 5% increase in risk respectively [53]. In higher risk older patients, the benefits clearly outweigh the risks, but this is less certain in younger patients at lower risk who may face 20–30 years of annual LDCT screening. In such cases, spacing out of screening intervals may be important, and this warrants further prospective studies.
Psychosocial Impact from Screening Patients can develop anxiety, depression, and psychological distress over screening results, diagnosis of cancer, or related to complications. Distress briefly increases after an abnormal result, but returns back to baseline, without significant change in overall health-related quality of life (HRQOL) [54]. Distress is associated with smoking status, with ex-smokers reporting less worry compared to current smokers [55]. In NLST participants, there were no significant differences at 1 and 6 months in anxiety and HRQOL among patients with false positive or significant incidental findings (SIFs) compared to negative findings. However, physical and mental health scores were lower, and anxiety was higher, in those diagnosed with LC within 1 year of positive screens [56].
Other Screening Considerations
Significant Incidental Findings (SIF)
This refers to abnormal findings unrelated to lung nodules, but requiring follow-up, specialist referrals, and/or additional workup. In a retrospective analysis of 320 LDCT-screened patients, at least one incidental finding occurred in all patients [57]. Incidental findings were most frequently of pulmonary (69.6%), cardiovascular (67.5%), or gastrointestinal (25.9%) etiology. Approximately 46.2% of total reimbursement related to screening was associated with workup of SIFs. Some authors [58, 59] have noted inconsistent radiologic reporting of SIFs. Patients should be counseled on the probability of SIFs and screening programs should standardize reporting and evaluation.
Cost-Effectiveness
Approximately 8.6 million Americans are LC screen-eligible [60]. While this would significantly increase the costs of screening, studies suggest that the actual cost-effectiveness of screening was approximately $81,000 per quality adjusted life year (QALY) [61], and overall, there was economic benefit. A cost-effective analysis using 4 models studying annual LDCT using NLST, CMS, and USPSTF screening criteria found that incremental cost-effective ratios averaged $49,000, $68,600, and $96,7000 per QALY, respectively, and that it was cost-effective to use these criteria [62].
Shared Decision Making
Shared decision making (SDM) refers to an evidence-based risk–benefit discussion with the patient about LDCT screening, with decisions made taking into account the patient’s values and preferences. This is recommended by USPSTF and mandated with the use of decision aids by CMS as part of coverage requirements [19].
The goal of the SDM process is to promote patient-centered care [63]. Given risks, patients may opt not to proceed with LC screening. The success of the SDM encounter depends on the informed decision making process rather than the actual outcome of proceeding or not with screening [63].
Decision aids can be particularly helpful during SDM visits, and increase patients’ knowledge, especially related to screening risks [64]. They can be in the form of videos, pamphlets, or internet-based, and a variety of different media options may be required to cater to variations in literacy and comfort levels. There are several decision aids available online as links in websites of professional organizations [61].
SDM has not yet been widely adopted as intended [46, 65, 66]. Qualitative analyses of SDM conversations found that harms were not really explained, minimal time was spent on screening, and decision aids were likely not used [65, 67]. Several reasons for poor performance have been identified. Many patients and providers lack proper education about the nuances of the SDM process. Physician barriers include lack of time to integrate SDM with clinic visits, competing priorities, ambivalence towards screening, and concern over risks [67]. Patients were generally more accepting of screening, but often did not fully understand risks, and were guided by emotion, personal fears, and fatalism during decision making.
In summary, SDM is not optimally performed. Efforts at provider and patient education may help bridge barriers, and improve patient engagement, collaboration, and the overall quality of the encounter.
Smoking Cessation
Approximately 50% of patients enrolled in LC screening are current smokers [68]. There is a 20% mortality benefit after 7 years of smoking cessation, similar to that seen with LDCT screening in the NSLT trial [69]. There is even greater mortality benefit when smoking cessation is combined with lung cancer screening.
CMS has mandated that smoking cessation counseling services be integrated into LC screening programs, and the cost-effectiveness of screening may be improved by 20–45% with this integration [70]. However, there are knowledge gaps and challenges in the delivery of smoking cessation interventions in this setting [68].
LC screening is thought to represent a teachable moment for smoking cessation [71]. Enrolled patients are generally more interested in cessation and intervention [72]. During the screening process, patients have multiple scheduled interactions with the healthcare team, and each of these interactions represents an opportunity for counseling. Approximately 75% of patients enrolling in screening will have negative results [72], but there is no clear data suggesting that this provides false reassurance to continue smoking. However, patients with positive screening results have demonstrated higher quit rates at 1 year [73].
Only 12–20% of smokers are ready to quit within a month at any particular time [74]. All patients, regardless of motivation, should be offered intervention for smoking cessation, as quit rates are higher in those offered intervention [72]. Clinician training in motivational interviewing and smoking cessation counseling is important for success. Guidelines suggest using strategies such as the 5As (ask, advise, assess, assist, and arrange) to counsel patients motivated to quit, and the 5Rs (relevance, risks, rewards, roadblocks, and repetition) to improve future cessation in patients not yet ready to quit [75]. Counseling and medication are also recommended together, as the combination increases cessation success compared to either alone [75].
The Smoking Cessation within the Context of Lung Cancer Screening (SCALES) is an ongoing multi-institutional collaboration of 8 clinical trials [68]. Results generated from this group are awaited to further our understanding of design, implementation, practice, and outcomes of smoking cessation services within screening programs.
Radiology Considerations
A prerequisite of a successful screening program is the existence of structured reporting and standardized management algorithms [47].
Standardized Reporting: In NLST, a nodule threshold of ≥ 4 mm in largest transverse diameter was considered positive. The LUNG-RADS reporting system was developed by the American College of Radiology (ACR) to standardize classification and reporting of screen-detected lung nodules [76]. In LUNG-RADS, the positive threshold was increased to 6 mm as a transverse bi-dimensional average, and growth of pre-existing nodules was also considered [77]. The application of LUNG-RADS to the NLST cohort lowered the false positive rate at the expense of lower sensitivity [78]. The positive predictive value was improved by 2.5 [79]. There are some limitations with LUNG-RADS such as inconsistent reporting of certain significant abnormalities and increase in positive results due to rounding [79–81]. Prospective validation of LUNG-RADS and improvement in standardized reporting of significant abnormalities will be important for overall benefit.
Volumetric Analysis: The NELSON study [27••] focused on volumetric analysis of nodules instead of traditional two-dimensional measurements. Volumes and doubling times were used to determine positive, indeterminate, or negative results. A nodule size threshold of ≥ 27mm3 had a 95% sensitivity for detecting malignancy, while volume doubling time of > 600 days had a very low probability of malignancy [82]. Use of volumetric analysis decreased false positive rates and unnecessary diagnostic interventions. The British Thoracic Society Guidelines for the management of pulmonary nodules has incorporated volumetric analysis in their recommendations [83].
Disparities in Lung Cancer Screening
There are several disparities in screening. Current LC screening criteria do not consider the specific differences within a population with regards to race, ethnicity, socioeconomic status, gender, specific comorbidities, geography, and access to healthcare. For example, compared to white patients, African-Americans have a higher LC risk at an earlier age and despite a lower pack-year smoking history, would benefit from liberalizing eligibility criteria [84]. Even when referred to LC screening programs, they had lower screening rates as well as delayed follow-up [85]. Women are at higher risk for LC than men despite variations in smoking practices, and patients with HIV have a higher independent LC risk [86]. Patients with lower literacy levels or from different cultures may not equally benefit from current SDM tools, which are not catered specifically towards this population.
The ATS recently issued an official statement to address disparities, so that care and resources can be more equitably provided [86]. The committee has proposed several strategies and recommendations to improve eligibility and access to screening and overcome multiple barriers. Continued, committed efforts at the individual, local, and national levels are required to bridge barriers and minimize disparities.
Screening During COVID-19 Pandemic
The current COVID-19 pandemic has presented unique screening challenges. Most screening programs were modified or put on hold during active hospital surges due to cancellations in imaging services and non-emergent procedures. The ACCP Expert Panel group put forth recommendations, endorsed by ATS and ACR, to address the management of screening and lung nodules during this time [87]. Clinicians should refer to these guidelines for details, with clinical application recommended based on individual patient appropriateness and availability or constraints in local resources.
Biomarkers in Screening
Multiple biomarkers are being studied in LC screening, either to improve risk-based patient selection pre-screening or to improve risk-stratification after nodule detection. Some of these include blood-based biomarkers such as autoantibodies, complement fragments, circulating proteins, circulating DNA and microRNA signature profiles, and exhaled breath condensates, metabolomics, and image analysis of sputum [88]. Airway gene expression is currently being utilized in patients with lung nodules to assist with lung cancer risk stratification [88–90], and is covered by Medicare. At this time, there is no sole approved biomarker in lung cancer screening, and research efforts continue to be underway.
Conclusion: Challenges and Future Directions
In conclusion, LC screening with LDCT has a proven significant mortality benefit. There are still significant knowledge and communication gaps in patient and physician understanding of screening nuances [66], requiring further education. The United States is currently still in the infancy stages in implementation of screening programs, and European countries have been issued a call for action to set up screening [91]. Multiple professional societies and experts have worked together to put forth guidelines and recommendations to improve the efficacy of screening programs, standardize screening eligibility and reporting, bridge healthcare disparities, and weather unique challenges during the COVID-19 pandemic. Multiple trials have been completed or are ongoing to refine our understanding about patient selection, nodule assessment and reporting, benefits and harms of screening, and integration of smoking cessation interventions. It is commendable that so much national and international work has been done just within the last decade. However, much work is still left to be done.
Future research should focus on patient selection based on individual risk, optimizing screening practices such as frequency of screening, radiation exposure and reporting practices to enhance benefits and minimize harms, and improve local and national advocacy to minimize racial disparities and improve access to screening for all. Exciting advances in biomarkers and genetic testing, in combination with current screening practices, may help herald the next phase of personalized screening and lung cancer prediction.
Declarations
Conflict of Interest
Anuradha Ramaswamy declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Interventional Pulmonology
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as:
••Of major importance
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Wood DE, Kazerooni EA, Baum SL, Eapen GA, Ettinger DS, Hou L, et al. Lung cancer screening, version 3.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2018;16(4):412–41. 10.6004/jnccn. 2018.0020. PMID: 29632061; PMCID: PMC6476336. [DOI] [PMC free article] [PubMed]
- 3.Hirsch FR, Scagliotti GV, et al. Lung cancer: Current therapies and new targeted treatments. Lancet. 2017;389(10066):299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 4.Cancer facts & figures. 2020. Available at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf.
- 5.Vachani A, Sequist LV, Spira A. AJRCCM: 100-Year Anniversary. The shifting landscape for lung cancer: past, present, and future. Am J Respir Crit Care Med. 2017 May 1;195(9):1150–1160. 10.1164/rccm.201702-0433CI. [DOI] [PMC free article] [PubMed]
- 6.Melamed MR, Flehinger BJ, Zaman MB, Heelan RT, Perchick WA, Martini N. Screening for early lung cancer. Results of the Memorial Sloan-Kettering study in New York. Chest. 1984 Jul; 86(1):44–53. doi: 10.1378/chest.86.1.44. PMID: 6734291. [DOI] [PubMed]
- 7.Fontana RS, Sanderson DR, Taylor WF, Woolner LB, Miller WE, Muhm JR, Uhlenhopp MA. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis. 1984;130:561–565. doi: 10.1164/arrd.1984.130.4.561. [DOI] [PubMed] [Google Scholar]
- 8.Frost JK, Ball WC, Levin ML, Tockman MS, Baker RR, Carter D, Eggleston JC, Erozan YS, Gupta PK, Khouri NF. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns-Hopkins study. Am Rev Respir Dis. 1984;130:549–554. doi: 10.1164/arrd.1984.130.4.549. [DOI] [PubMed] [Google Scholar]
- 9.Kubik A, Polak J. Lung cancer detection: Results of a randomised prospective study in Czechoslovakia. Cancer. 1986;57(12):2427–2437. doi: 10.1002/1097-0142(19860615)57:12<2427::aid-cncr2820571230>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Bade BC, Brasher PB, Luna BW, Silvestri GA, Tanner NT. Reviewing lung cancer screening: The who, where, when, why, and how. Clin Chest Med. 2018;39(1):31–43. doi: 10.1016/j.ccm.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Oken MM, Hocking WG, Kvale PA, Andriole GL, Buys SS, Church TR, et al. Screening by chest radiograph and lung cancer mortality: The prostate, lung, colorectal, and ovarian (PLCO) randomized trial. JAMA 2011;306:1865–73. 10.1001/jama.2011.1591. Epub 2011 Oct 26. PMID: 22031728. [DOI] [PubMed]
- 12.Henschke CI, McCauley D, Yankelevitz DF, Naidich DP, McGuinness G, Miettinen OS, et al. Early lung cancer action project: Overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 13.Infante M, Cavuto S, Lutman FR, Brambilla G, Chiesa G, Ceresoli G, et al. A randomized study of lung cancer screening with spiral computed tomography. Three-year results from the DANTE trial. Am J Respir Crit Care Med. 2009;180(5):445–53. 10.1164/rccm.200901-0076OC. Epub 2009 Jun 11. PMID: 19520905. [DOI] [PubMed]
- 14.Pastorino U, Rossi M, Rosato V, Marchianò A, Sverzellati N, Morosi C, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308–15. 10.1097/CEJ.0b013e328351e1b6. PMID: 22465911. [DOI] [PubMed]
- 15.International Early Lung Cancer Action Program Investigators, Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–71. 10.1056/NEJMoa060476. Erratum in: N Engl J Med. 2008 Apr 24;358(17):1875. Erratum in: N Engl J Med. 2008 Aug 21;359(8):877. Erratum in: N Engl J Med. 2008 Apr 24;358(17): 1862. PMID: 17065637. [DOI] [PubMed]
- 16.••National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. 10.1056/NEJMoa1102873. Epub 2011 Jun 29. PMID: 21714641; PMCID: PMC4356534. Landmark study that proved for the first time that lung cancer screening with low dose computed tomography decreased mortality rate by 20%. [DOI] [PMC free article] [PubMed]
- 17.Moyer VA. U.S. Preventive Services Task Force: Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–8. [DOI] [PubMed]
- 18.de Koning HJ, Meza R, Plevritis SK, Ten Haaf K, Munshi VN, Jeon J, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the U.S. Preventive Services Task Force. Ann Intern Med. 2014;160(5):311–20. 10.7326/M13-2316. PMID: 24379002; PMCID: PMC4116741. [DOI] [PMC free article] [PubMed]
- 19.Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N). 2015. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- 20.Infante M, Cavuto S, Lutman FR, et al. Long-term follow-up results of the DANTE Trial, a randomized study of lung cancer screening with spiral computed tomography. Am J Respir Crit Care Med. 2015;191:1166–75. [DOI] [PubMed]
- 21.Wille MM, Dirksen A, Ashraf H, Saghir Z, Bach KS, Brooders J, Clementsen PF, Hansen H, Larsen KR, Mortensen J, Rasmussen JF, Seersholm N, Skov BG, Thomsen LH, Tønnesen P, Pedersen JH. Results of the randomized Danish lung cancer screening trial with focus on high-risk profiling. Am J Respir Crit Care Med. 2016;193(5):542–551. doi: 10.1164/rccm.201505-1040OC. [DOI] [PubMed] [Google Scholar]
- 22.Paci E, Puliti D, Lopes Pegna A, Carrozzi L, et al. Mortality, survival and incidence rates in the ITALUNG randomized lung cancer screening trial. Thorax. 2017;72:825–831. doi: 10.1136/thoraxjnl-2016-209825. [DOI] [PubMed] [Google Scholar]
- 23.Pastorino U, Silva M, Sestini S, et al. Prolonged lung cancer screening reduced 10-year mortality in the MILD trial: New confirmation of lung cancer screening efficacy. Ann Oncol. 2019;30:1162–1169. doi: 10.1093/annonc/mdz117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastorino U, Sverzellati N, Sestini S, Silva M, et al. Ten-year results of the multicentric Italian lung detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer. [DOI] [PMC free article] [PubMed]
- 25.Becker N, Motsch E, Trotter A, Heussel CP, et al. Lung cancer mortality reduction by LDCT screening—results from the randomized German LUSI trial. [DOI] [PubMed]
- 26.National Lung Screening Trial Research Team. Lung cancer incidence and mortality with extended follow-up in the national lung screening trial. J Thorac Oncol. 2019;(10):1732–42. 10.1016/j.jtho.2019.05.044. Epub 2019 Jun 28. PMID: 31260833; PMCID: PMC6764895. [DOI] [PMC free article] [PubMed]
- 27.••de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JJ, Weenink C, YousafKhan U, Horeweg N, van’t Westeinde S, Prokop M, Mali WP, Mohamed Hoesein FAA, van Ooijen PMA, Aerts JGJV, den Bakker MA, Thunnissen E, Verschakelen J, Vliegenthart R, Walter JE, Ten Haaf K, Groen HJM, Oudkerk M. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503–13. 10.1056/ NEJMoa1911793. Epub 2020 Jan 29. PMID: 31995683. Extended follow-up results from the NELSON study that also proved a significant lung-cancer specific mortality benefit with LDCT screening. [DOI] [PubMed]
- 28.Dezube AR, Jaklitsch MT. New evidence supporting lung cancer screening with low dose CT and surgical implications. Eur J Surg Oncol. 2020;46(6):982–990. doi: 10.1016/j.ejso.2020.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Detterbeck FC, Mazzone PJ, Naidich DP, Bach PB. Screening for lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e78S–e92S. doi: 10.1378/chest.12-2350.PMID:23649455;PMCID:PMC3749713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wiener RS, Gould MK, Arenberg DA, Au DH, Fennig K, Lamb CR, Mazzone PJ, Midthun DE, Napoli M, Ost DE, Powell CA, Rivera MP, Slatore CG, Tanner NT, Vachani A, Wisnivesky JP, Yoon SH. ATS/ACCP Committee on Low-Dose CT Lung Cancer Screening in Clinical Practice. An official American Thoracic Society/American College of Chest Physicians policy statement: implementation of low-dose computed tomography lung cancer screening programs in clinical practice. Am J Respir Crit Care Med. 2015;192(7):881–91. 10.1164/rccm.201508-1671ST. PMID: 26426785; PMCID: PMC4613898. [DOI] [PMC free article] [PubMed]
- 31.Roberts H, Walker-Dilks C, Sivjee K, Ung Y, Yasufuku K, Hey A, Lewis N. Lung cancer screening guideline development group. Screening high-risk populations for lung cancer: guideline recommendations. J Thorac Oncol. 2013;8(10):1232–7. 10.1097/JTO.0b013e31829fd3d5. PMID: 24457233. [DOI] [PubMed]
- 32.Wender R, Fontham ET, Barrera Jr E, Colditz GA, Church TR, Ettinger DS, Etzioni R, Flowers CR, Gazelle GS, Kelsey DK, LaMonte SJ, Michaelson JS, Oeffinger KC, Shih YC, Sullivan DC, Travis W, Walter L, Wolf AM, Brawley OW, Smith RA. American Cancer Society lung cancer screening guidelines. CA Cancer J Clin. 2013;63(2):107–17. 10.3322/caac.21172. Epub 2013 Jan 11. PMID: 23315954; PMCID: PMC3632634. [DOI] [PMC free article] [PubMed]
- 33.Tanoue LT, Tanner NT, Gould MK, Silvestri GA. Lung cancer screening. Am J Respir Crit Care Med. 2015;191(1):19–33. doi: 10.1164/rccm.201410-1777CI. [DOI] [PubMed] [Google Scholar]
- 34.Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, MacMahon H, Mulshine JL, Munden RF, Salgia R, Strauss GM, Swanson SJ, Travis WD, Sugarbaker DJ. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012;144(1):33–38. doi: 10.1016/j.jtcvs.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 35.American Lung Association screening guidelines. 2021. Available at: https://www.lung.org/getmedia/9f9c3528-c634-474d-a0ab-a2f011c92500/lung-cancer-screening-is-itright.pdf.
- 36.•• Mazzone PJ, Silvestri GA, Patel S, et. al. Screening for lung cancer: CHEST guideline and expert panel report. Chest 2018;153:954–85. Guidelines and expert panel recommendations from CHEST on lung cancer screening based on systematic review of literature, evaluating benefits, harms and implementation. Despite a delicate balance of risks and benefits, screening with LDCT had an overall favorable balance. [DOI] [PubMed]
- 37.American Academy of Family Physicians. Clinical preventive service recommendation: Lung cancer. https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/lung-cancer.html.
- 38.Thomas NA, Nichole TT. Lung cancer screening: Patient selection and implementation. Clin Chest Med. 2020;41(1):87–97. doi: 10.1016/j.ccm.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 39.U.S. Preventive Task Force. Final Recommendation Statement. Lung cancer: Screening. March 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening.
- 40.Tammemagi MC, Katki HA, Hocking WG, Church TR, Caproaso N, Kvale PA, Chaturvedi AK, Silvestri GA, Riley TL, Commins J, Berg CD. NEJM. 2013;368:728–736. doi: 10.1056/NEJMoa1211776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tammemagi MC, Schmidt H, Martel S, McWilliams A, Goffin JR, Johnston MR, Nicholas G, Tremblay A, Bhatia R, Liu G, Soghrati K, Yasufuku K, Hwang DM, Laberge F, Gingras M, Pasian S, Couture C, Mayo JR, Nasute Fauerbach PV, Atkar-Khattra S, Peacock SJ, Cressman S, Ionescu D, English JC, Finley RJ, Yee J, Puksa S, Stewart L, Tsai S, Haider E, Boylan C, Cutz JC, Manos D, Xu Z, Goss GD, Seely JM, Amjadi K, Sekhon HS, Burrowes P, MacEachern P, Urbanski S, Sin DD, Tan WC, Leighl NB, Shepherd FA, Evans WK, Tsao MS, Lam S, PanCan Study Team. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;18(11):1523–31. 10.1016/ S1470–2045(17)30597–1. Epub 2017 Oct 18. PMID: 29055736. [DOI] [PubMed]
- 42.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and validation of risk models to select ever-smokers for CT lung cancer screening. JAMA. 2016;315(21):2300–2311. doi: 10.1001/jama.2016.6255.PMID:27179989;PMCID:PMC4899131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ten Haaf K, Jeon J, Tammemägi MC, Han SS, Kong CY, Plevritis SK, Feuer EJ, de Koning HJ, Steyerberg EW, Meza R. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017;14(4):e1002277. doi: 10.1371/journal.pmed.1002277.Erratum.In:PLoSMed.2020Sep25;17(9):e1003403.PMID:28376113;PMCID:PMC5380315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tammemägi MC, Ten Haaf K, Toumazis I, Kong CY, Han SS, Jeon J, Commins J, Riley T, Meza R. Development and validation of a multivariable lung cancer risk prediction model that includes low-dose computed tomography screening results: a secondary analysis of data from the national lung screening trial. JAMA Netw Open. 2019;2(3):e190204. doi: 10.1001/jamanetworkopen.2019.0204.PMID:30821827;PMCID:PMC6484623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crosbie PA, Gabe R, Simmonds I, Kennedy M, Rogerson S, Ahmed N, Baldwin DR, Booton R, Cochrane A, Darby M, Franks K, Hinde S, Janes SM, Macleod U, Messenger M, Moller H, Murray RL, Neal RD, Quaife SL, Sculpher M, Tharmanathan P, Torgerson D, Callister ME. Yorkshire Lung Screening Trial (YLST): Protocol for a randomised controlled trial to evaluate invitation to community-based low-dose CT screening for lung cancer versus usual care in a targeted population at risk. BMJ Open. 2020;10(9):e037075. doi: 10.1136/bmjopen-2020-037075.PMID:32912947;PMCID:PMC7485242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodwin JS, Nishi S, Zhou J, Kuo YF. Use of the shared decision-making visit for lung cancer screening among Medicare enrollees. JAMA Intern Med. 2019;179(5):716–718. doi: 10.1001/jamainternmed.2018.6405.PMID:30640388;PMCID:PMC6503565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazzone P, Powell CA, Arenberg D, Bach P, Detterbeck F, Gould MK, Jaklitsch MT, Jett J, Naidich D, Vachani A, Wiener RS, Silvestri G. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy statement. Chest. 2015;147(2):295–303. doi: 10.1378/chest.14-2500.PMID:25356819;PMCID:PMC4502754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Del Ciello A, Franchi P, Contegiacomo A, Cicchetti G, Bonomo L, Larici AR. Missed lung cancer: when, where, and why? Diagn Interv Radiol. 2017;23(2):118–26. 10.5152/dir. 2016.16187. PMID: 28206951; PMCID: PMC5338577. [DOI] [PMC free article] [PubMed]
- 49.Brodersen J, Schwartz LM, Heneghan C, et al. Overdiagnosis: What it is and what it isn’t. BMJ Evid -Based Med. 2018;23:1–3. doi: 10.1136/ebmed-2017-110886. [DOI] [PubMed] [Google Scholar]
- 50.Patz EF, Pinksy P, Gatsonis C, et al. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174(2):269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heleno B, Siersma V, Brodersen J. Estimation of overdiagnosis of lung cancer in low-dose computed tomography screening. JAMA Intern Med. 2018;178:1420–1422. doi: 10.1001/jamainternmed.2018.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, Byers T, Colditz GA, Gould MK, Jett JR, Sabichi AL, SmithBindman R, Wood DE, Qaseem A, Detterbeck FC. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–2429. doi: 10.1001/jama.2012.5521.Erratum.In:JAMA.2012Oct3;308(13):1324.Erratumin:JAMA.2013Jun5;309(21):2212.PMID:22610500;PMCID:PMC3709596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brenner DJ. Radiation risks potentially associated with low dose CT screening of adult smokers for lung cancer. Radiology. 2004;231(2):440–445. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 54.Slatore CG, Sullivan DR, Pappas M, Humphrey LL. Patient centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol. 2014;9(7):927–934. doi: 10.1097/JTO.0000000000000210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chad-Friedman E, Coleman S, Traeger LN, Pirl WF, Goldman R, Atlas SJ, Park ER. Psychological distress associated with cancer screening: a systematic review. Cancer. 2017;123(20):3882–94. 10.1002/cncr.30904. Epub 2017 Aug 22. PMID: 28833054.3. [DOI] [PMC free article] [PubMed]
- 56.Gareen IF, Duan F, Greco EM, Snyder BS, Boiselle PM, Park ER, Fryback D, Gatsonis C. Impact of lung cancer screening results on participant health-related quality of life and state anxiety in the National Lung Screening Trial. Cancer. 2014;120(21):3401–9. 10.1002/cncr.28833. Epub 2014 Jul 25. PMID: 25065710; PMCID: PMC4205265. [DOI] [PMC free article] [PubMed]
- 57.Morgan L, Choi H, Reid M, Khawaja A, Mazzone PJ. Frequency of incidental findings and subsequent evaluation in low-dose computed tomographic scans for lung cancer screening. Ann Am Thorac Soc. 2017;14(9):1450–6. 10.1513/AnnalsATS.201612-1023OC. PMID: 28421812. [DOI] [PubMed]
- 58.Reiter MJ, Nemesure A, Madu E, Reagan L, Plank A. Frequency and distribution of incidental findings deemed appropriate for S modifier designation on low-dose CT in a lung cancer screening program. Lung Cancer. 2018;120:1–6. doi: 10.1016/j.lungcan.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Janssen K, Schertz K, Rubin N, Begnaud A. Incidental findings in a decentralized lung cancer screening program. Ann Am Thorac Soc. 2019;16(9):1198–1201. doi: 10.1513/AnnalsATS.201812-908RL.PMID:31251089;PMCID:PMC6812160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119(7):1381–1385. doi: 10.1002/cncr.27813. [DOI] [PubMed] [Google Scholar]
- 61.Hoffman RM, Sanchez R. Lung cancer screening. Med Clin North Am. 2017;101(4):769–785. doi: 10.1016/j.mcna.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Criss SD, Cao P, Bastani M, Ten Haaf K, Chen Y, Sheehan DF, Blom EF, Toumazis I, Jeon J, de Koning HJ, Plevritis SK, Meza R, Kong CY. Cost-effectiveness analysis of lung cancer screening in the United States: a comparative modeling study. Ann Intern Med. 2019;171(11):796–804. doi: 10.7326/M19-0322. [DOI] [PubMed] [Google Scholar]
- 63.Tanner NT, Silvestri GA. Shared decision-making and lung cancer screening: Let’s get the conversation started. Chest. 2019;155(1):21–24. doi: 10.1016/j.chest.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 64.Crothers K, Kross EK, Reisch LM, Shahrir S, Slatore C, Zeliadt SB, Triplette M, Meza R, Elmore JG. Patients’ attitudes regarding lung cancer screening and decision aids. A survey and focus group study. Ann Am Thor Soc. 2016;13(11):1992–2001. [DOI] [PMC free article] [PubMed]
- 65.Brenner AT, Malo TL, Margolis M, Lafata JE, James S, Vu MB, Reuland DS. Evaluating shared decision making for lung cancer screening. JAMA Intern Med. 2018;178(10):1311–1316. doi: 10.1001/jamainternmed.2018.3054.PMID:30105393;PMCID:PMC6233759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wiener RS, Koppelman E, Bolton R, Lasser KE, Borrelli B, Au DH, Slatore CG, Clark JA, Kathuria H. Patient and clinician perspectives on shared decision-making in early adopting lung cancer screening programs: a qualitative study. J Gen Intern Med. 2018;33(7):1035–42. 10.1007/s11606-018-4350-9. Epub 2018 Feb 21. PMID: 29468601; PMCID: PMC6025674. [DOI] [PMC free article] [PubMed]
- 67.Lowenstein M, Vijayaraghavan M, Burke NJ, Karliner L, Wang S, Peters M, Lozano A, Kaplan CP. Real-world lung cancer screening decision-making: Barriers and facilitators. Lung Cancer. 2019;133:32–37. doi: 10.1016/j.lungcan.2019.04.026. [DOI] [PubMed] [Google Scholar]
- 68.Joseph AM, Rothman AJ, Almirall D, Begnaud A, Chiles C, Cinciripini PM, Fu SS, Graham AL, Lindgren BR, Melzer AC, Ostroff JS, Seaman EL, Taylor KL, Toll BA, Zeliadt SB, Vock DM. Lung cancer screening and smoking cessation clinical trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) collaboration. Am J Respir Crit Care Med. 2018;197(2):172–82. 10.1164/rccm.201705-0909CI. PMID: 28977754; PMCID: PMC5768904. [DOI] [PMC free article] [PubMed]
- 69.Tanner NT, Kanodra NM, Gebregziabher M, Payne E, Halbert CH, Warren GW, Egede LE, Silvestri GA. The association between smoking abstinence and mortality in the national lung screening trial. Am J Respir Crit Care Med. 2016;193(5):534–541. doi: 10.1164/rccm.201507-1420OC. [DOI] [PubMed] [Google Scholar]
- 70.Villanti AC, Jiang Y, Abrams DB, Pyenson BS. A cost-utility analysis of lung cancer screening and the additional benefits of incorporating smoking cessation interventions. PLoS One. 2013;8(8):e71379. doi: 10.1371/journal.pone.0071379.PMID:23940744;PMCID:PMC3737088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deppen SA, Grogan EL, Aldrich MC, Massion PP. Lung cancer screening and smoking cessation: a teachable moment? J Natl Cancer Inst. 2014;106(6):dju122. 10.1093/jnci/dju122. Erratum in: J Natl Cancer Inst. 2014 Sep;106(9): 10.1093/jnci/dju280. PMID: 24872542; PMCID: PMC5073840. [DOI] [PMC free article] [PubMed]
- 72.Fucito LM, Czabafy S, Hendricks PS, Kotsen C, Richardson D, Toll BA. Association for the treatment of tobacco use and dependence/society for research on nicotine and tobacco synergy committee. Pairing smoking-cessation services with lung cancer screening: a clinical guideline from the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco. Cancer. 2016;122(8):1150–9. 10.1002/cncr.29926. Epub 2016 Feb 24. PMID: 26916412; PMCID: PMC4828323. [DOI] [PMC free article] [PubMed]
- 73.Styn MA, Land SR, Perkins KA, Wilson DO, Romkes M, Weissfeld JL. Smoking behavior 1 year after computed tomography screening for lung cancer: Effect of physician referral for abnormal CT findings. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3484–3489. doi: 10.1158/1055-9965.EPI-09-0895.PMID:19959699;PMCID:PMC2789354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richter KP, Ellerbeck EF. It’s time to change the default for tobacco treatment. Addiction. 2015;110(3):381–386. doi: 10.1111/add.12734. [DOI] [PubMed] [Google Scholar]
- 75.Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 200835(2):158–76. 10.1016/j.amepre.2008.04.009. PMID: 18617085; PMCID: PMC4465757. [DOI] [PMC free article] [PubMed]
- 76.American College of Radiology. Lung CT Screening Reporting and Data System (Lung-RADS). 2014. http://www.acr.org/QualitySafety/Resources/LungRADS.
- 77.van der Aalst CM, Ten Haaf K, De Koning HJ. Lung cancer screening: Latest developments and unanswered questions. Lancet Respir Med. 2016;4(9):749–61. [DOI] [PubMed]
- 78.Pinsky PF, Gierada DS, Black W, Munden R, Nath H, Aberle D, Kazerooni E. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–491. doi: 10.7326/M14-2086.PMID:25664444;PMCID:PMC4705835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mehta HJ, Mohammed TL, Jantz MA. The American college of radiology lung imaging reporting and data system: Potential drawbacks and need for revision. Chest. 2017;151(3):539–543. doi: 10.1016/j.chest.2016.07.028. [DOI] [PubMed] [Google Scholar]
- 80.Ravenel JG, Tanner NT, Silvestri GA. Viewing all the trees in the forest: the importance of reporting abnormal findings on CT scan when screening for lung cancer. Chest. 2017;151(3):525–526. doi: 10.1016/j.chest.2016.10.053. [DOI] [PubMed] [Google Scholar]
- 81.Li K, Yip R, Avila R, Henschke CI, Yankelevitz DF. Size and growth assessment of pulmonary nodules: Consequences of rounding. J Thorac Oncol. 2017;12(4):657–662. doi: 10.1016/j.jtho.2016.12/010. [DOI] [PubMed] [Google Scholar]
- 82.O’Dowd EL, Baldwin DR. Lung cancer screening-low dose CT for lung cancer screening: recent trial results and next steps. Br J Radiol. 2018;91(1090):20170460. 10.1259/bjr.20170460. Epub 2017 Oct 17. PMID: 28749712; PMCID: PMC6350493. [DOI] [PMC free article] [PubMed]
- 83.Callister ME, Baldwin DR, Akram AR, Barnard S, Cane P, Draffan J, Franks K, Gleeson F, Graham R, Malhotra P, Prokop M, Rodger K, Subesinghe M, Waller D, Woolhouse I. British Thoracic Society Pulmonary Nodule Guideline Development Group; British Thoracic Society Standards of Care Committee. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70(Suppl 2):ii1–54. 10.1136/thoraxjnl-2015-207168. Erratum in: Thorax. 2015 Dec; 70(12):1188. PMID: 26082159. [DOI] [PubMed]
- 84.Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Groagan EL, Blume JD. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318–24. 10.1001/jamaoncol.2019.1402. Epub ahead of print. Erratum in: JAMA Oncol. 2019 Aug 1; PMID: 31246249; PMCID: PMC6604090. [DOI] [PMC free article] [PubMed]
- 85.Lake M, Shusted CS, Juon H, et al. Black patients referred to a lung cancer screening program experience lower rates of screening and longer time to follow-up. BMC Cancer. 2020;20:561. doi: 10.1186/s12885-020-06923-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rivera MP, Katki HA, Tanner NT, Triplette M, Sakoda LC, Wiener RS, Cardarelli R, Carter-Harris L, Crothers K, Fathi JT, Ford ME, Smith R, Winn RA, Wisnivesky JP, Henderson LM, Aldrich MC. Addressing disparities in lung cancer screening eligibility and healthcare access. An official American Thoracic Society statement. Am J Respir Crit Care Med. 2020;202(7):e95–112. 10.1164/dccm.202008-3053ST. PMID: 33000953; PMCID: PMC7528802. [DOI] [PMC free article] [PubMed]
- 87.Mazzone PJ, Gould MK, Arenberg DA, Chen AC, Choi HK, Detterbeck FC, Farjah F, Fong KM, Iaccarino JM, Janes SM, Kanne JP, Kazerooni EA, MacMahon H, Naidich DP, Powell CA, Raoof S, Rivera MP, Tanner NT, Tanoue LK, Tremblay A, Vachani A, White CS, Wiener RS, Silvestri GA. Management of lung nodules and lung cancer screening during the COVID-19 pandemic: CHEST expert panel report. J Am Coll Radiol. 2020;17(7):845–54. 10.1016/j.jacr.2020.04.024. Epub 2020 Apr 23. PMID: 32485147; PMCID: PMC7177099. [DOI] [PMC free article] [PubMed]
- 88.Seijo LM, Peled N, Ajona D, Boeri M, Field JK, Sozzi G, Pio R, Zulueta JJ, Spira A, Massion PP, Mazzone PJ, Montuenga LM. Biomarkers in lung cancer screening: achievements, promises, and challenges. J Thorac Oncol. 2019;14(3):343–57. 10.1016/j.jtho.2018.11.023. Epub 2018 Dec 4. PMID: 30529598; PMCID: PMC6494979. [DOI] [PMC free article] [PubMed]
- 89.Silvestri GA, Vachani A, Whitney D, Elashoff M, Porta Smith K, Ferguson JS, et al. AEGIS study team. A bronchial genomic classifier for the diagnostic evaluation of lung cancer. N Engl J Med. 2015;373(3):243–51. 10.1056/NEJMoa1504601. Epub 2015 May 17. PMID: 25981554; PMCID: PMC4838273. [DOI] [PMC free article] [PubMed]
- 90.Vachani A, Whitney DH, Parsons EC, Lenburg M, Ferguson JS, Silvestri GA, Spira A. Clinical utility of a bronchial genomic classifier in patients with suspected lung cancer. Chest. 2016;150(1):210–218. doi: 10.1016/j.chest.2016.02.636. [DOI] [PubMed] [Google Scholar]
- 91.Oudkerk M, Devaraj A, Vliegenthart R, Henzler T, Prosch H, Heussel CP, Bastarrika G, Sverzellati N, Mascalchi M, Delorme S, Baldwin DR, Callister ME, Becker N, Heuvelmans MA, Rzyman W, Infante MV, Pastorino U, Pedersen JH, Paci E, Duffy SW, de Knoning H, Field JK. European position statement on lung cancer screening. Lancet Oncol. 2017;18(12):e754–e766. doi: 10.1016/S1470-2045(17)30861-6.PMID:29208441.OudkerkLancetOncol2018KnowingKoning. [DOI] [PubMed] [Google Scholar]