Abstract
Background
Glioblastomas (GBMs) are aggressive brain tumors despite radiation therapy (RT) to 60 Gy and temozolomide (TMZ). Spectroscopic magnetic resonance imaging (sMRI), which measures levels of specific brain metabolites, can delineate regions at high risk for GBM recurrence not visualized on contrast-enhanced (CE) MRI. We conducted a clinical trial to assess the feasibility, safety, and efficacy of sMRI-guided RT dose escalation to 75 Gy for newly diagnosed GBMs.
Methods
Our pilot trial (NCT03137888) enrolled patients at 3 institutions (Emory University, University of Miami, Johns Hopkins University) from September 2017 to June 2019. For RT, standard tumor volumes based on T2-FLAIR and T1w-CE MRIs with margins were treated in 30 fractions to 50.1 and 60 Gy, respectively. An additional high-risk volume based on residual CE tumor and Cho/NAA (on sMRI) ≥2× normal was treated to 75 Gy. Survival curves were generated by the Kaplan–Meier method. Toxicities were assessed according to CTCAE v4.0.
Results
Thirty patients were treated in the study. The median age was 59 years. 30% were MGMT promoter hypermethylated; 7% harbored IDH1 mutation. With a median follow-up of 21.4 months for censored patients, median overall survival (OS) and progression-free survival were 23.0 and 16.6 months, respectively. This regimen appeared well-tolerated with 70% of grade 3 or greater toxicity ascribed to TMZ and 23% occurring at least 1 year after RT.
Conclusion
Dose-escalated RT to 75 Gy guided by sMRI appears feasible and safe for patients with newly diagnosed GBMs. OS outcome is promising and warrants additional testing. Based on these results, a randomized phase II trial is in development.
Keywords: glioblastoma, MRSI, radiation dose-escalation, spectroscopic MRI
Key Points.
Infiltrative GBM not visualized on MRI can be delineated by sMRI.
RT to 75 Gy guided by sMRI is both feasible and tolerable for GBM patients.
GBM patients treated with sMRI-guided RT to 75 Gy in 30 fractions had a promising median OS of 23 months.
Importance of the Study.
Dose-escalated RT has not proven to be beneficial for GBM patients in previous studies. We hypothesized that better delineation of infiltrating GBM for RT planning could improve efficacy of dose escalation. We piloted this concept on a 3-institution trial using sMRI to guide RT dose escalation to 75 Gy for newly diagnosed GBM patients. Our cohort (N = 30) was not particularly favorable with a median age of 59 years and expected rates of MGMT hypermethylation (30%) and IDH mutation (7%). With a median follow-up of 21.4 months, median OS for our dose-escalated cohort was 23 months which compares quite favorably with an expected median OS of 16 months after standard dose RT. Our treatment regimen was well-tolerated with an acceptable rate of grade 3–4 toxicities that was largely attributable to TMZ. This study provides the foundation for broader testing of sMRI-guided RT dose escalation to potentially improve outcomes for GBM patients.
Glioblastomas (GBMs) are the most common malignant primary brain tumor in adults with an annual incidence of 3.19 per 100 000 in the United States.1 Standard-of-care treatment for patients with newly diagnosed GBM consists of maximally safe surgical resection followed by radiation therapy (RT) with concomitant and adjuvant temozolomide (TMZ). Despite advances in these treatment regimens over the last 2 decades, GBM continues to be an aggressive disease with a median overall survival (OS) of 15–16 months since the introduction of TMZ.2–5
Part of the challenge in treating GBMs is its infiltrative nature. To account for this, a 2-tier system of imaging is typically used to target disease. T1-weighted contrast-enhanced (T1w-CE) MRI utilizes intravenous injection of gadolinium contrast agent to identify leaky vasculatures accompanying high-grade gliomas and compromised blood–brain barrier, which is the hallmark of GBMs. Thus, T1w-CE is a reasonable diagnostic imaging method to differentiate GBMs from lower-grade gliomas. T2-weighted fluid-attenuated inversion recovery (FLAIR) MRI is less specific, but identifies not only tumor infiltration, but other pathologies such as edema, inflammation, and medication or radiation effects. To accommodate both imaging phenomena, high-dose RT (typically 60 Gy) is targeted to the T1w-CE region, including any surgical resection cavity, and a lower dose 45–54 Gy to the FLAIR hyperintensity, both delivered over 30 fractions.6 Given the infiltrative nature of GBMs, an imaging modality that could more completely identify the extent of tumor spread, even if the tumor has not yet disrupted local anatomy or induced leaky neovasculatures, would be beneficial in improving disease targeting to avoid marginal or distant failures. Furthermore, there has been ongoing work to determine the optimal radiation dosing to prevent in-field recurrence of disease, with higher doses up to 75 Gy being studied in trials such as the NRG-BN001 study.7
Magnetic resonance spectroscopic imaging (MRSI) can noninvasively identify the chemical composition of tissue based on the nuclear magnetic resonance phenomenon.8 Tumor cells have altered metabolism which can be detected using MRSI. Choline-containing compounds (Cho) make up the phospholipid bilayer of the cell membrane and are found in increased concentration in cells that are rapidly proliferating. N-acetylaspartate (NAA) is a protein found in neurons and is diminished in tissue samples when there is destruction of the local neuronal environment. The ratio of choline to NAA (Cho/NAA) has been previously shown to be greatly elevated in patients with GBM and can serve as a biomarker to differentiate regions of tumor from healthy brain.8–10
Based on these results, there have been studies in the past assessing the potential of MRSI for radiation treatment planning in GBM patients.8,10–14 However, very few trials have managed to use MRSI for interventional treatment of brain tumors. SPECTRO-GLIO is one such trial where regions of the brain with a ratio of Cho/NAA >2.0 received an escalated radiation dose of 72 Gy.15 This study used a 3D CSI sequence on 1.5T scanners with slices as thick as 25 mm and a nominal voxel size close to 1 cc for high-dose radiation targets. Results of this trial have not yet been reported. We have concurrently been pioneering development of high spatial resolution, 3D echo-planar spectroscopic imaging (EPSI), MRSI sequences with a full field-of-view that includes cortical surface regions, which we have termed spectroscopic MRI (sMRI). With sMRI, we can acquire images with more than 3-fold improved resolution compared to the 3D CSI sequence used in SPECTRO-GLIO as well as improved signal to noise (SNR) due to the use of 3T scanners. Furthermore, we have developed clinical software tools and a web application to facilitate inspection of metabolite overlays and rapid generation of radiation treatment contours in a collaborative manner.
Use of sMRI aids in the more complete delineation of infiltrative GBMs than is possible with standard MRI sequences. Previously, we identified that regions of elevated Cho/NAA, specifically with greater than twice the mean value of Cho/NAA in normal-appearing white matter (NAWM) outside of the radiation treatment regions, were later correlated with regions of disease recurrence in 81% of patients.16 In the same study, samples of tissue with high Cho/NAA abnormality were acquired by surgical biopsies and were stained to measure SRY (sex-determining region Y)-box 2 (Sox2), a transcription factor highly correlated with infiltrative tumor. Higher Cho/NAA was strongly correlated to elevated Sox2 density (P < .0001), even in regions without conventional MRI changes. Thus, targeting high-dose radiation to regions of metabolic abnormality based on sMRI may delay disease recurrence and improve outcomes in patients with GBM.
Based on this premise, we initiated a multi-institutional clinical trial utilizing sMRI to guide radiation dose escalation for newly diagnosed GBM patients. In this report, we present the initial results of our single-arm pilot study assessing the feasibility, safety, and efficacy of this treatment approach with high-dose radiation to 75 Gy for this patient population.
Methods
This study was performed across 3 institutions—Emory University (Atlanta, GA), University of Miami (Miami, FL), and Johns Hopkins University (Baltimore, MD)—and was approved by the institutional review boards of all 3 institutions. The study was registered with the National Clinical Trials Network (NCT03137888).
Eligibility Criteria
Patients eligible for this study were at least 18 years old, with a newly diagnosed WHO grade IV glioblastoma confirmed pathologically by a board-certified neuropathologist, were able to undergo MRI scans, had a life expectancy ≥12 weeks, Karnofsky performance status ≥60,17 had a negative pregnancy test if they were women of childbearing potential, and met the following laboratory criteria within 14 days of registration: white blood cell count ≥3000/µL, absolute neutrophil count ≥1500/µL, platelet count ≥75 000/µL, hemoglobin ≥9.0 gm/dL, serum glutamic-oxaloacetic acid ≤2.0× the upper limit of normal, bilirubin ≤2× the upper limit of normal, and creatinine ≤1.5 mg/dL.
Exclusion criteria included having pacemakers or other implants that were MRI-incompatible, history of another invasive cancer that was not in complete remission for ≥3 years, active infection or other serious medical illness, receiving any other investigational agents, history of prior cranial radiation, or history of prior medical therapies for brain tumors. Once patients were enrolled, they underwent a series of MRI scans as described below. Two additional imaging-based exclusion criteria were implemented: a GBM located in regions known to have increased magnetic susceptibility and poor sMRI signal (mesial temporal lobe, orbitofrontal cortex, brainstem, or cerebellum), and those with a dose-escalated radiation target (defined by Cho/NAA ≥2× normal) greater than 65 cc, in conformity with the NRG Oncology guidelines where dose-escalated targets with maximal dimensions greater than 5 cm (corresponding to ~65 cc sphere) were not permitted on the BN001 study.7
Image Acquisition
Enrolled patients underwent standard brain tumor imaging protocols at their treatment institution including T1w-CE and FLAIR sequences. Study-specific sMRI scans were obtained either in the same sessions as the standard sequences or at a separate scheduled session. An echo-planar sMRI pulse sequence with GRAPPA parallelization was performed on Siemens 3T scanners at each institution using a 32-channel or 20-channel head and neck coil (echo time [TE] = 50 ms, repetition time [TR] = 1551 ms, flip angle [FA] = 71°).18–21 The scan time was 15 min with a nominal voxel size of 314 μL, an FOV of 280 mm × 280 mm × 180 mm, and a matrix size of 50 × 50 × 18. After postprocessing, the matrix size is 64 × 64 × 32 leading to an interpolated resolution of 4.4 mm × 4.4 mm × 5.6 mm yielding a voxel size of 108 μL. In the same imaging session, a noncontrast T1w MRI with 1 mm isotropic voxels was obtained. Data from the sMRI and noncontrast T1w sequences were sent to a centralized location and converted into co-registered spatial-spectral data using the MIDAS software suite (University of Miami, Miami, FL).22
Target Generation
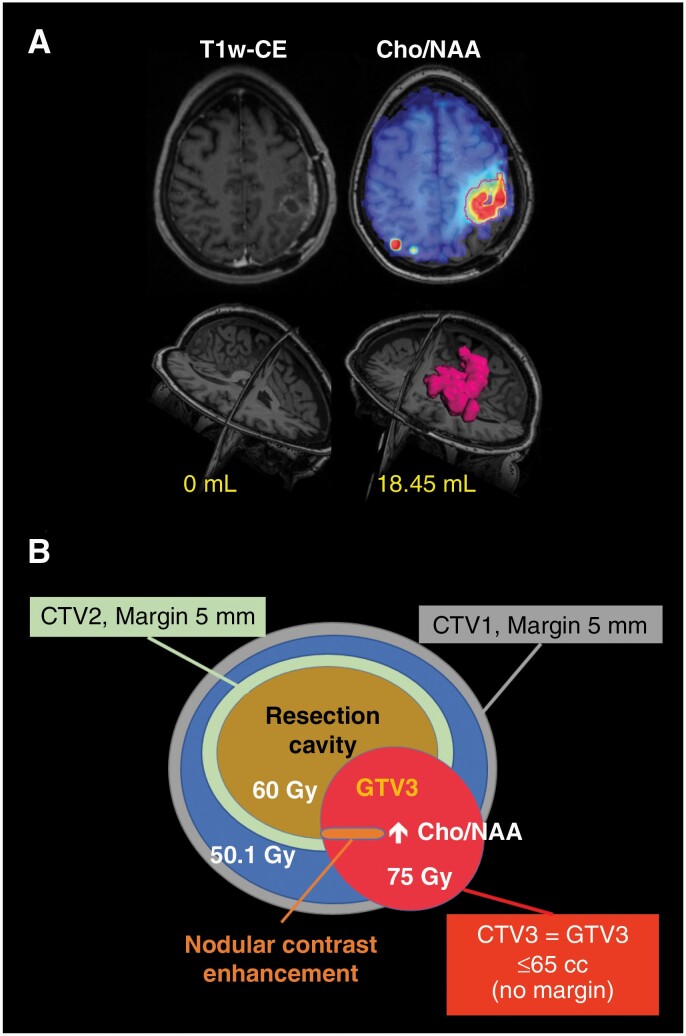
A centralized target generation process was implemented as previously described.23 Briefly, all imaging data obtained at each institution were sent in the DICOM format, and sMRI data sent in the internal format used by MIDAS, to a centralized HIPAA-compliant server hosted by Emory University. A custom-built web platform, the Brain Imaging Collaboration Suite (BrICS), coregistered all the images for each patient into a single coordinate system based on the isotropic T1w MRI space. Metabolite and ratio maps, including Cho/NAA, were interpolated into the high-resolution space and registered and overlaid on anatomic images. The Cho/NAA abnormality index, defined as the fold-increase in Cho/NAA in a voxel compared to the mean in contralateral NAWM, was automatically calculated as previously described to account for scanner and patient variations.16,23 The BrICS software was used to identify and contour the region with a Cho/NAA ≥2× normal (Figure 1A).
Figure 1.
(A) Example subject after gross total resection with only expected linear postoperative enhancement around the resection cavity on T1w-CE MRI (left). A contour was generated in BrICS where the Cho/NAA ≥2× normal results in a GTV3 volume of 18.45 mL (right). This GTV3 received 75 Gy. (B) Summary of the RT target volumes and doses used in this study. All doses were delivered in 30 fractions of concurrent dose-painted intensity-modulated RT.
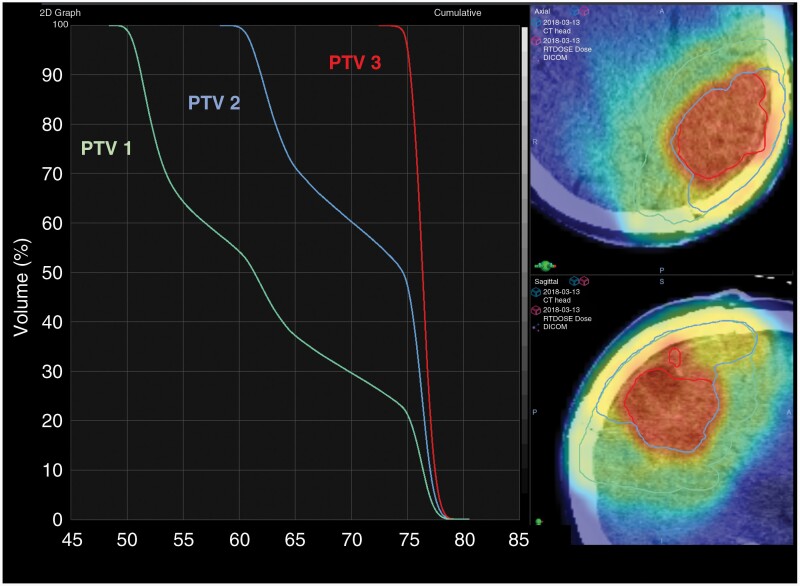
During the centralized review process, a board-certified neuroradiologist and 2 MRS experts and their trainees evaluated the contours and made manual adjustments based on spectral quality and anatomy. The neuroradiologist identified any residual enhancing disease based on T1w-CE imaging, excluding resection cavity or gross blood; the union of residual disease and the Cho/NAA ≥2× volume was computed and saved as a target known as gross tumor volume 3 (GTV3). Next, 2 board-certified radiation oncologists, including one from the patient’s local institution, reviewed GTV3 and made minor adjustments based on anatomy. GTV3 was then exported from BrICS in the DICOM-RT format and imported into the RT contouring/planning system at the local treating institution (Eclipse/VelocityAI, Varian Medical Systems; MIM, MIM Software, Inc.; and Pinnacle, Royal Phillips Electronics N.V.). Our certified medical physicist assessed the GTV3 overlay and ensured accurate registration. Standard GTV targets based on T1w-CE (GTV2) and FLAIR MRI (GTV1) were generated by the local radiation oncology team. A margin of 5 mm was added to create clinical tumor volumes (CTV2 and CTV1) to these 2 targets to account for microscopic invasion of tumor; no CTV margin was added to GTV3 (CTV3 = GTV3). Figure 1B illustrates the 3 CTV targets and prescribed doses. An additional margin of 3 mm was added to each CTV during RT planning to generate planning treatment volumes (PTVs) to which the RT would be delivered. Figure 1A shows an example patient on the study with a large difference in volume between residual contrast enhancement and the Cho/NAA ≥2× normal. The dose-volume histogram of each PTV volume and the dose cloud used to treat this patient and PTV contours overlaid on the patient’s treatment planning CT are also shown (Figure 2).
Figure 2.
For the patient in Figure 1, an RT dose-volume histogram is shown on the left for each PTV volume. Overlays of each PTV (PTV1 green line, PTV2 blue line, PTV3 red line) and radiation dose cloud over the patient’s treatment planning CT are shown.
Treatment Plan
Patients in this study received RT in 30 fractions using simultaneous integrated boost technique with 3 target volumes over 6 weeks (5 fractions per week), along with concurrent TMZ at standard-of-care dosing (75 mg/m2/day, 7 days/week) during RT. Following a 1-month rest period after RT, patients continued adjuvant cycles of TMZ (150–200 mg/m2/day days 1–5 every 28 days) per the standard of care as recommended by their treatment team.24 Due to institutional differences in administering adjuvant TMZ, a minimum of 6 cycles of adjuvant TMZ were administered by each site unless there were signs of tumor progression or toxicity. Neuro-oncologists at each site were given the discretion to treat up to 12 cycles of adjuvant TMZ as long as patients were tolerating the treatment.
Follow-up
Patients were followed at least every 3 months for 2 years from the start of RT. Standard MRIs were obtained every 2–3 months unless shorter intervals were clinically indicated. Toxicities were graded according to CTCAE v4.0 and reported to the central clinical trials office from all sites. Progression-free survival (PFS) was assessed through follow-up imaging uploaded to a module within BrICS called the Longitudinal Imaging Tracker and reviewed by a neuroradiologist and the patients’ oncology team.25 If patients received any follow-up surgeries or biopsies, the pathology reports from those procedures served as the ground truth for tumor progression. If the report indicates that over 20% of sampled specimens contained tumor or that the specimens were positive for tumor (without mention of a percentage), the follow-up date immediately preceding the re-resection/biopsy served as the tumor progression date. There were instances where we could not rely on pathology reports due to patients either not having re-resections or having surgeries much earlier in their follow-up period. In those cases, we would graph CE-T1w and FLAIR lesion volumes, as well as structured reporting scores from the Brain Tumor Reporting and Data System (BT-RADS). BT-RADS is a framework used in over 80% of neuro-radiology reports at Emory University and spreading to institutions such as Johns Hopkins, Duke University, and UCLA.26,27 By plotting these metrics over the patient’s entire posttreatment timeline, we can assess for sudden increases in lesion volumes and structured reporting scores. In cases where structured reporting scores increase to 3c (increasing tumor burden) or 4 (highly suspicious for tumor), then we would overlay radiation dose maps over the co-registered follow-up imaging. If enhancing lesions spread outside of the GTV3 radiation target, then those cases were marked as the disease progression date; otherwise, the enhancing lesion was attributed to mainly radiation necrosis. If patients underwent salvage therapies as dictated by the standard of care, these were reported to the central clinical trial office. OS was assessed through chart review and direct communication between the oncology team and patients or their families. For patients who left the study to seek care elsewhere, survival follow-up was obtained by communication with their new oncologist whenever possible.
Statistical Analysis
An as-treated analysis was performed for patients who completed the RT protocol. OS from the date of surgery was calculated using the Kaplan–Meier estimator28 with censoring for events not observed using the lifelines survival analysis library written in Python.29 For this analysis, patients were censored at the date of last clinical contact or date of admission to hospice care if the date of death was not observed. PFS from the surgery date was calculated in a similar manner with censoring for patients who had not yet progressed. Patients who died prior to confirmed tumor progression had their death date marked as their date of event. If there was a gap where interval MRIs were not available with a known date of death, these patients were marked as censored at their time of last MRI.
Results
Study Participants
The study enrolled patients from September 2017 to June 2019. Enrollment continued until a total of 30 subjects were deemed eligible and completed dose-escalated RT. A total of 45 patients initially provided informed consent for this study out of which 38 met initial laboratory and clinical eligibility criteria and underwent sMRI scans per the study protocol. Four patients did not meet imaging criteria due to poor-quality sMRI scans or having the GTV3 target volume greater than the upper limit of 65 cc. Two patients elected to drop out of the study after being deemed eligible but prior to beginning treatment. Of the 32 patients who began RT, 2 patients opted out of the study during the first 2 weeks of RT. A total of 30 patients successfully completed the dose-escalated RT according to protocol. The patient enrollment pipeline is shown as a diagram (Supplementary Figure 1). Demographics and clinical data of the 30 patients included in this analysis are given in Table 1. Mean/median age was 56.4/58.9 years and sex showed slight male preponderance (19 of 30 patients). 30% (9 of 30) were MGMT hypermethylated and 6.7% (2 of 30) harbored an IDH mutation (IDH1 R132H in both cases). These characteristics are typical of a random cohort of GBM patients and do not appear to skew our cohort toward a more favorable prognosis. If postsurgery T1w-CE lesion volumes were less than 1 cc, we marked the patient as having a gross total resection, otherwise, patients were considered to have subtotal resection. Of the 30 patients who completed treatment, 11 had a gross total resection while 19 had a subtotal resection prior to the start of RT. For the 30 treated patients, the median volume of residual enhancement was 1.6 cc (0.0–15.4 cc), while the median GTV3, which is the union of residual enhancement and Cho/NAA ≥2× normal volume was 19.6 cc (0.9–65.0 cc). In Supplementary Figure 2, we plotted residual enhancing volume versus GTV3 for every patient on the trial to illustrate just how much larger the treatment volumes were for each patient. Only 5 patients on the trial had residual enhancing volume after surgery that was larger than 6 cc. It was common to see patients with visible enhancing lesions less than 2 cc and their GTV3 volume be larger than 15 cc due to the larger amounts of visible tumor unearthed via the Cho/NAA maps. Finally, with respect to use of tumor-treating fields (TTFs), no study patients used TTF as part of their initial management of GBM. Two patients did opt to be treated with TTF at salvage 13–14 months after their initial diagnosis.
Table 1.
Subject Demographics and Clinical Data
| Numbers | |
|---|---|
| Demographics | |
| Age | |
| Mean/median | 56.4/58.9 years |
| Range | 20.8–71.6 years |
| <65 | 23 (77%) |
| ≥65 | 7 (23%) |
| Sex | |
| Female | 11 (37%) |
| Male | 19 (63%) |
| Molecular factors | |
| MGMT methylation status | |
| Not hypermethylated | 21 (70%) |
| Hypermethylated | 9 (30%) |
| IDH status | |
| Wild-type | 28 (93%) |
| Mutated | 2 (7%) |
| Pre-RT surgery | |
| Gross total resection | 11 (37%) |
| Subtotal resection | 19 (63%) |
Survival Analysis
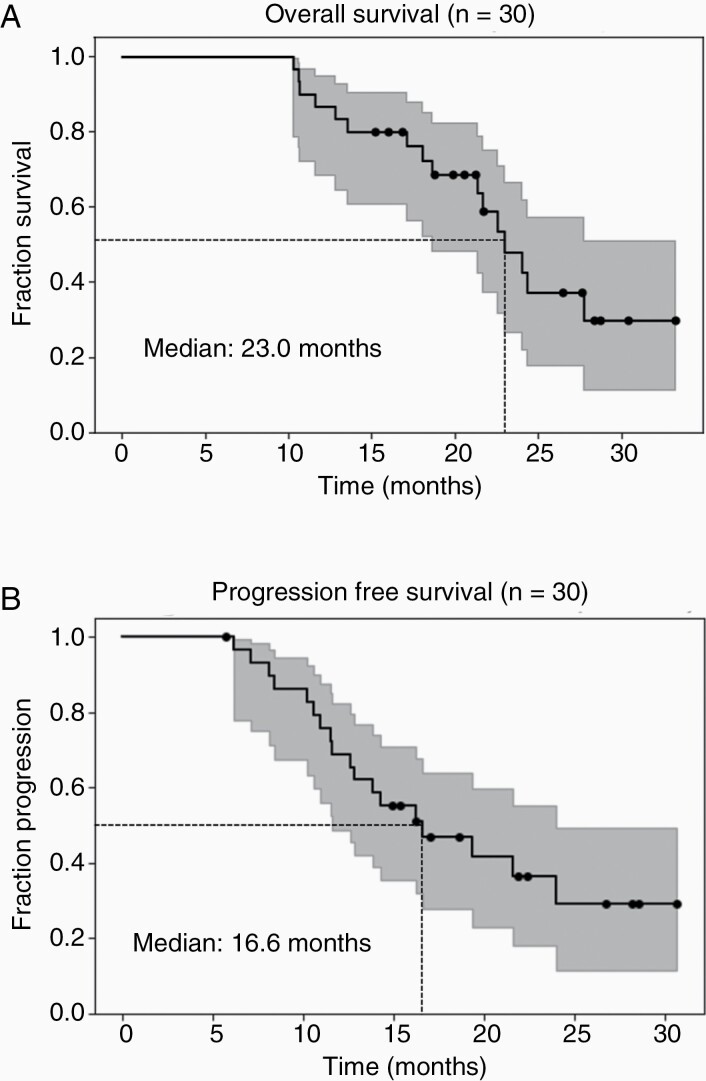
At the time of analysis, 16 patients had died. With a median time of 21.4 months to the last follow-up for censored patients, the median OS was 23.0 months for the 30 patients who completed our protocol (Figure 3A). The median PFS was 16.6 months, with 18 patients being marked as progressed and the remaining 12 patients censored due to either the patient not progressing yet or being lost to follow-up (Figure 3B).
Figure 3.
Kaplan–Meier estimator for (A) overall survival with a median of 23.0 months and (B) progression-free survival with a median of 16.6 months are shown. 95% confidence intervals are included (grayed out area).
Radiation Treatment Effects
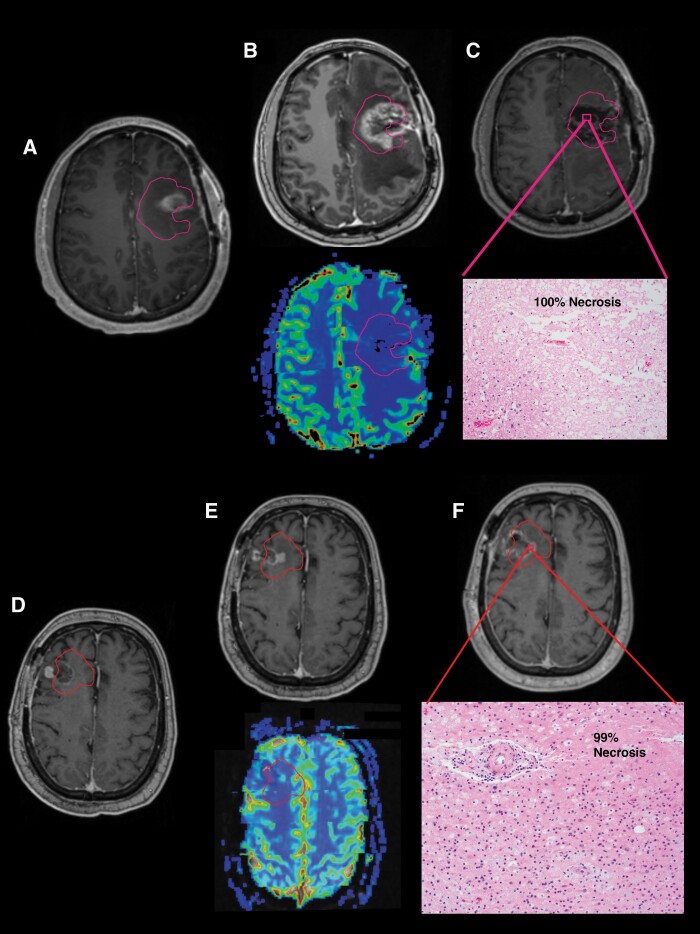
Pseudoprogression is a phenomenon where new or increasing enhancement on T1w-CE MRIs appears to indicate tumor progression, but in fact, represents treatment-related changes. This usually occurs early after RT (≤3 months) with the enhancing changes eventually subsiding with no alteration in treatment. Radiation necrosis is a phenomenon where tissue within and surrounding regions with malignant glioma experiences a severe reaction to radiation, generally occurring 3–12 months after RT, and is also visualized as a new or increasing enhancement on T1w-CE MRIs.30 In fact, this effect may be indistinguishable from pseudoprogression on MRI except by its timing and can also be termed “late pseudoprogression.” We show 2 examples of these phenomena where pathologic confirmation was obtained (Figure 4). In Case 1, residual enhancement was noted at 1-month post-RT (Figure 4A) with further increases over the ensuing 4 months (Figure 4B) indicating possible refractory tumor or pseudoprogression. Repeat resection was performed with histologic confirmation that the residual enhancement was, in fact, 100% necrosis, suggesting a treatment-induced response (Figure 4C). In Case 2, standard MRI appears to show recurrence 8 months after completion of RT (Figure 4D and E). However, a repeat resection showed that the enhancing tissue was nearly all (99%) necrosis (Figure 4F). Due to the longer time after completion of RT, the enhancing lesion in the second case was called radiation necrosis. Because pathologic confirmation was not obtained in all cases of possible pseudoprogression and/or radiation necrosis, accurate assessment of true progression and PFS was difficult to precisely determine. In this initial report, we do present PFS, but plan to report the methodology of determining progression in more depth in a later report based on more detailed dose–response and local control analyses.
Figure 4.
Examples of 2 cases with “late pseudoprogression” or radiation necrosis are shown. Case 1 (A) At 1-month post-RT, residual enhancement is seen on T1w MRI within the pink PTV3 contour. (B) At 5-month post-RT, residual enhancement has increased, but the relative cerebral blood volume (rCBV) map on dynamic susceptibility contrast (DSC) perfusion MRI shows minimal to no perfusion. (C) Repeat resection demonstrated 100% necrosis on H&E-stained sections. Case 2 (D) At 1-month post-RT, there is primarily linear, postoperative enhancement on T1w-CE MRI within the red PTV3 contour. (E) At 8-month post-RT, increasing enhancement is seen at the periphery of the cavity. The DSC perfusion MRI again shows no definitive hyperperfusion. (F) Repeat resection again found predominantly (99%) necrosis on H&E-stained sections.
Toxicity
Of the 30 patients who received dose-escalated RT, 11 patients experienced grade 3 or greater toxicity that was judged to be at least possibly attributed to their treatment. Of the 30 grade 3 or higher toxicities reported, 13 were thought to be at least possibly related to treatment. Five grade 3 toxicities occurred during adjuvant TMZ and were believed to be due mainly to TMZ rather than radiation. Three grade 3 toxicities occurred during the follow-up phase at least 1 year after RT. The 3 remaining grade 3 toxicities and 2 grade 4 toxicities occurred during RT/concurrent TMZ consisted of thrombocytopenia (2 cases), neutropenia, increased LFTs, and generalized weakness, with all except one case attributed to TMZ. As is evident by the listing of grade 3 or greater toxicities for our study cohort (Table 2), the majority of these toxicities were judged to be related to TMZ rather than dose-escalated RT. The complete list of toxicities for patients who were treated with the sMRI-guided RT dose escalation with attributions is given in Supplementary Table 1.
Table 2.
Grade 3 or Greater Toxicities At Least Possibly Due to Therapy
| Category | Grade 3 | Grade 4 | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Thrombocytopenia | 4 | 13.3 | 1 | 3.3 |
| Neutropenia | 0 | 1 | 3.3 | |
| Transaminitis | 1 | 3.3 | 0 | |
| Hypokalemia | 1 | 3.3 | 0 | |
| Edema | 1 | 3.3 | 0 | |
| Muscle weakness | 2 | 6.7 | 0 | |
| Fatigue | 1 | 3.3 | 0 | |
| Headaches | 1 | 3.3 | 0 | |
| Leg edema | 1 | 3.3 | 0 |
Discussion
GBM is a highly aggressive brain tumor with patients having a relatively poor median OS of only 16 months with standard therapies. While radiation can improve survival outcomes for patients with GBM, we hypothesize that dose-escalated radiation guided by Cho/NAA maps may further improve OS by treating a much larger extent of infiltrating, nonenhancing tumor. While other trials such as SPECTRO-GLIO attempt to similarly administer high-dose radiation based on the Cho/NAA ratio, we used an sMRI sequence that acquired images at a higher resolution, better SNR, and improved brain coverage. With that, our goal was to create a clinically translatable treatment pipeline from image acquisition to generation of high-dose radiation targets that could be inspected and assessed in a collaborative manner. Toward this goal, we demonstrate the clinical feasibility and early outcomes of sMRI-guided, dose-escalated radiation treatment for GBM. The volumetric sMRI acquisition had a scan time of 15 min and fit seamlessly with other clinical scans. Only 3 patients on our trial were excluded from starting treatment due to poor sMRI signal quality from patient motion. We are now developing the next version of the sequence with motion correction to overcome this issue. Raw data from the sMRI scan were transferred and processed before metabolite heat maps were generated in BrICS for visual assessment and contour generation. Clinicians and researchers from remote sites were easily able to login to BrICS and generate GTV3 treatment volumes collaboratively in real time, as well as manually edit the contours per physician’s discretion. With a turnaround time of 1.5 days from scan time to upload of GTV3 to standard RT planning systems,23 our workflow fit effortlessly into standard clinical procedures.
With a significantly larger volume of brain tissue also receiving a higher radiation dose, patient toxicity reports were carefully assessed for any negative responses to treatment. Of the 17 grade 3 or higher toxicities that occurred in 11 patients, all but one toxicity was attributed to TMZ, with the other toxicity being attributed to a combination of RT and TMZ. In the NRG-BN001 study, 229 patients were enrolled in the photon comparison randomized in a 1:2 fashion with treatment consisting of standard versus dose-escalated RT. The arm receiving dose-escalated RT had a 75 Gy radiation dose administered to residual enhancement (including the resection cavity), while our trial used the union of Cho/NAA ≥2× and residual enhancement as the GTV3 target volume receiving 75 Gy. Preliminary findings from the BN001 study found no significant difference in grade 3 toxicity between patients who received dose-escalated RT and those who received standard RT,7 supporting our assertion that overall toxicity was not excessive.
Not unexpectedly, we did identify instances of radiation necrosis that were confirmed pathologically. However, symptoms were well managed with steroids, when necessary, and did not result in serious toxicities prior to their repeat resections. An optimal treatment plan for patients will always be a balance between increasing tumor control while minimizing potential risks of toxicity. While we are likely to see a slightly higher incidence of radiation necrosis with a dose-escalated RT treatment regimen, both our current results and the preliminary results of NRG-BN001 suggest that a radiation dose of 75 Gy in 30 fractions to a select volume for GBM patients is tolerable overall despite an increased necrosis risk. We hypothesize that the escalated dose used on NRG-BN001 that focused only on the resection cavity and contrast-enhancing residual may have been of lesser benefit since it is less likely to target the full extent of tumor compared to our sMRI-guided contours.
Ultimately, our goal in this study was to demonstrate the clinical feasibility of utilizing Cho/NAA ≥2× normal to guide radiation dose escalation in newly diagnosed GBM patients. Through software suites and web applications like MIDAS and BrICS, we were able to generate GTV3 treatment volumes in a rapid manner with collaborative decision making among multisite investigators. Median OS in this study was 23.0 months with an appropriate toxicity profile, which compared favorably to 16 months for standard therapies.2,3,5 Preliminary results from the NRG-BN001 study show a median OS of 18.7 months for dose-escalated patients and 16.3 months for patients on standard treatment. The improved specificity at identifying infiltrative tumor by utilizing sMRI has led to a median OS that is potentially longer than even the high-dose treatment arm in NRG-BN001, albeit with a sample size that is smaller. We have also determined a median PFS of 16.6 months, which is significantly longer than previously reported and even approaches median OS with standard therapies.5 Based on these promising results, a randomized phase II trial is in development within ECOG-ACRIN (EAF211) that seeks to more definitively determine the efficacy of sMRI-guided, dose-escalated RT.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the amazing work and dedication by our multisite coordinator Latrisha Moore, our Institutional Review Board staff, as well as clinical coordinators at each of our 3 sites who helped with recruitment and management of patients on the trial and without whom, our efforts would have been immeasurably difficult. We would like to also thank all our patients and their caregivers who believed in our study.
Funding
This work is supported by the National Institutes of Health grant NCI R01 CA214557 (H.S., L.R.K., A.A.M., H.G.S.), BRP NIBIB U01 EB028145 (H.S., A.A.M.), and R01 EB016064 (A.A.M.). This work is also supported by predoctoral fellowships, F30CA206291 (S.S.G.) and F31CA247564-02 (K.R.).
Conflict of interest statement. None declared.
Ethics approval. The study was approved by the institutional review boards (IRBs) at each participating institution.
Consent to participate. Patients in this study provided informed consent for participation according to the IRB.
Authorship statement. E.A.M., S.S.G., A.A.M., L.R.K., H.S., and H.G.S. contributed to the study conception and design. Material preparation, data collection, and analysis were performed by all authors. The first draft of the manuscript and tables/figures was prepared by K.R., S.S.G., H.S., and H.G.S., and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1. Ostrom QT, Gittleman H, Farah P, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert MR, Dignam JJ, Armstrong TS, et al. . A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert MR, Wang M, Aldape KD, et al. . Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Hegi ME, Mason WP, et al. . Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 5. Stupp R, Taillibert S, Kanner A, et al. . Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wernicke AG, Smith AW, Taube S, Mehta MP. Glioblastoma: radiation treatment margins, how small is large enough? Pract Radiat Oncol. 2016;6(5):298–305. [DOI] [PubMed] [Google Scholar]
- 7. Gondi V, Pugh S, Tsien C, et al. . Radiotherapy (RT) dose-intensification (DI) using intensity-modulated RT (IMRT) versus standard-dose (SD) RT with temozolomide (TMZ) in newly diagnosed glioblastoma (GBM): preliminary results of NRG Oncology BN001. Int J Radiat Oncol Biol Phys. 2020;108(3):S22–S23. [Google Scholar]
- 8. Nelson SJ, Vigneron DB, Dillon WP. Serial evaluation of patients with brain tumors using volume MRI and 3D 1H MRSI. NMR Biomed. 1999;12(3):123–138. [DOI] [PubMed] [Google Scholar]
- 9. Narayana A, Chang J, Thakur S, et al. . Use of MR spectroscopy and functional imaging in the treatment planning of gliomas. Br J Radiol. 2007;80(953):347–354. [DOI] [PubMed] [Google Scholar]
- 10. Pirzkall A, Li X, Oh J, et al. . 3D MRSI for resected high-grade gliomas before RT: tumor extent according to metabolic activity in relation to MRI. Int J Radiat Oncol Biol Phys. 2004;59(1):126–137. [DOI] [PubMed] [Google Scholar]
- 11. Chang J, Thakur SB, Huang W, Narayana A. Magnetic resonance spectroscopy imaging (MRSI) and brain functional magnetic resonance imaging (fMRI) for radiotherapy treatment planning of glioma. Technol Cancer Res Treat. 2008;7(5):349–362. [PubMed] [Google Scholar]
- 12. Ken S, Vieillevigne L, Franceries X, et al. . Integration method of 3D MR spectroscopy into treatment planning system for glioblastoma IMRT dose painting with integrated simultaneous boost. Radiat Oncol. 2013;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nelson SJ, Graves E, Pirzkall A, et al. . In vivo molecular imaging for planning radiation therapy of gliomas: an application of 1H MRSI. J Magn Reson Imaging. 2002;16(4):464–476. [DOI] [PubMed] [Google Scholar]
- 14. Parra NA, Maudsley AA, Gupta RK, et al. . Volumetric spectroscopic imaging of glioblastoma multiforme radiation treatment volumes. Int J Radiat Oncol Biol Phys. 2014;90(2):376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laprie A, Ken S, Filleron T, et al. . Dose-painting multicenter phase III trial in newly diagnosed glioblastoma: the SPECTRO-GLIO trial comparing arm A standard radiochemotherapy to arm B radiochemotherapy with simultaneous integrated boost guided by MR spectroscopic imaging. BMC Cancer. 2019;19(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cordova JS, Shu HK, Liang Z, et al. . Whole-brain spectroscopic MRI biomarkers identify infiltrating margins in glioblastoma patients. Neuro Oncol. 2016;18(8):1180–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187–193. [DOI] [PubMed] [Google Scholar]
- 18. Goryawala M, Saraf-Lavi E, Nagornaya N, et al. . The association between whole-brain MR spectroscopy and IDH mutation status in gliomas. J Neuroimaging. 2020;30(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goryawala MZ, Sheriff S, Maudsley AA. Regional distributions of brain glutamate and glutamine in normal subjects. NMR Biomed. 2016;29(8):1108–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goryawala MZ, Sheriff S, Stoyanova R, Maudsley AA. Spectral decomposition for resolving partial volume effects in MRSI. Magn Reson Med. 2018;79(6):2886–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sabati M, Sheriff S, Gu M, et al. . Multivendor implementation and comparison of volumetric whole-brain echo-planar MR spectroscopic imaging. Magn Reson Med. 2015;74(5):1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maudsley AA, Darkazanli A, Alger JR, et al. . Comprehensive processing, display and analysis for in vivo MR spectroscopic imaging. NMR Biomed. 2006;19(4):492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gurbani S, Weinberg B, Cooper L, et al. . The Brain Imaging Collaboration Suite (BrICS): a cloud platform for integrating whole-brain spectroscopic MRI into the radiation therapy planning workflow. Tomography. 2019;5(1):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stupp R, Mason WP, van den Bent MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 25. Ramesh K, Gurbani SS, Mellon EA, et al. . The Longitudinal Imaging Tracker (BrICS-LIT): a cloud platform for monitoring treatment response in glioblastoma patients. Tomography. 2020;6(2):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gore A, Hoch MJ, Shu HG, Olson JJ, Voloschin AD, Weinberg BD. Institutional implementation of a structured reporting system: our experience with the brain tumor reporting and data system. Acad Radiol. 2019;26(7):974–980. [DOI] [PubMed] [Google Scholar]
- 27. Weinberg BD, Gore A, Shu HG, et al. . Management-based structured reporting of posttreatment glioma response with the brain tumor reporting and data system. J Am Coll Radiol. 2018;15(5):767–771. [DOI] [PubMed] [Google Scholar]
- 28. Bland JM, Altman DG. Survival probabilities (the Kaplan–Meier method). BMJ. 1998;317(7172):1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davidson-Pilon C. Lifelines: survival analysis in Python. J Open Source Softw. 2019;4(40):1317. [Google Scholar]
- 30. Zikou A, Sioka C, Alexiou GA, et al. . Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging. 2018;2018:6828396. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.