Abstract
Exposure to fine particulate matter (PM2.5) has been reported to increase the risks of chronic kidney disease. However, limited research has assessed the effect of PM2.5 and its constituents on renal function, and the underlying mechanism has not been well characterized. We aimed to evaluate the association of PM2.5 and its constituents with kidney indicators and to explore the roles of systematic oxidative stress and inflammation in the association. We conducted a longitudinal panel study among 35 healthy adults before-, intra- and after-the 2019 Wuhan Military World Games. We repeatedly measured 6 renal function parameters and 5 circulating biomarkers of oxidative stress and inflammation at 6 rounds of follow-ups. We monitored hourly personal PM2.5 concentrations with 3 consecutive days and measured 10 metals (metalloids) and 16 polycyclic aromatic hydrocarbons (PAHs) components. The linear mixed-effect models were applied to examine the association between PM2.5 and renal function parameters, and the mediation analysis was performed to explore potential bio-pathways. PM2.5 concentrations across Wuhan showed a slight decrease during the Military Games. We observed significant associations between elevated blood urea nitrogen (BUN) levels and PM2.5 and its several metals and PAHs components. For an interquartile range (IQR) increase of PM2.5, BUN increased 0.42 mmol/L (95% CI: 0.14 to 0.69). On average, an IQR higher of lead (Pb), cadmium (Cd), arsenic (As), selenium (Se), thallium (Tl) and Indeno (1,2,3-cd) pyrene (IPY) were associated with 0.90, 0.65, 0.29, 0.27, 0.26 and 0.90 mmol/L increment of BUN, respectively. Moreover, superoxide dismutase was positively associated with PM2.5 and mediated 18.24% association. Our research indicated that exposure to PM2.5 might affect renal function by activating oxidative stress pathways, in which the constituents of Pb, Cd, As, Se, Tl and IPY might contribute to the associations.
Keywords: PM2.5, Chemical components, Renal dysfunction, Oxidative stress, Mediation effect
Graphical abstract
Highlights
-
•
Short-term exposure to PM2.5 may affect renal function among healthy adults.
-
•
Ad, Pb, As, Se, Tl and IPY in PM2.5 might contribute to the association.
-
•
Oxidative stress might be a bio-pathway between PM2.5 and renal function.
1. Introduction
Renal dysfunction has gradually become a global public concern because of the increasing burden of chronic kidney disease (CKD) (Eknoyan et al., 2004; Couser et al., 2011). A study among 12 countries reported that the prevalence of CKD was 14.3% (Ene-Iordache et al., 2016), and it was estimated that 119.5 million CKD patients diagnosed in China (Zhang et al., 2012). Thus, it is urgent to identify the risk factors for reducing disease burden. Recent studies indicated that environment pollution, especially air pollution, may serve as an important risk factor of CKD (Xu et al., 2018; Al-Aly and Bowe, 2020; Stenvinkel et al., 2020). A modeling study estimated that 6.59 million disability-adjusted life years of CKD worldwide in 2017 were attributable to PM2.5 pollution (Bowe et al., 2020). It is of great public health significance to explore the adverse effects of PM2.5 on kidney and elucidate potential pathogenic mechanisms.
As a metabolic organ that maintains the fluid and acid-base balance, the filtration and concentration function of kidney make it vulnerable to environmental pollutants (Xu et al., 2018). Several pivotal blood renal function parameters were reported to be significantly associated with PM2.5 (Rahmani Sani et al., 2020; Wu et al., 2020; Zhao et al., 2020; Kuźma et al., 2021; Li et al., 2021). For example, a cross-sectional study among 2.5 million young adults reported that each 10 μg/m3 increment of PM2.5 in 3-years average exposure was associated with 0.85% decrease of eGFR (Li et al., 2021). Another research on rodent models found that sub-chronic exposure to PM2.5 could lead to elevated blood urea nitrogen (BUN) levels (Tavera Busso et al., 2018). However, most previous research focused on pregnant women (Zhao et al., 2020), children (Liu et al., 2020) or the elderly (Mehta et al., 2016; Fang et al., 2020), while the associations among healthy adults was not fully characterized. Additionally, PM2.5 is a heterogeneous mixture with nephrotoxic constituents, which may contribute to the major association between PM2.5 and renal dysfunction. Some studies reported that metals and polycyclic aromatic hydrocarbons (PAHs) in PM2.5 were related to oxidative damages and heart rate variability (Wei et al., 2009; Wu et al., 2011). However, limited evidence is available for the association between PM2.5-bound components and renal function.
The underlying bio-pathways for the association between PM2.5 and renal function remain uncertain. Previous reviews reported that exposure to PM2.5 may increase oxidative stress (Li et al., 2020) and inflammation (Tang et al., 2020), and these responses were found to be related with renal dysfunction in some populations (Yilmaz et al., 2006; Upadhyay et al., 2011; Correia-Costa et al., 2016). Since kidney is a highly vascularized organ and is susceptible to vascular dysfunction (Lue et al., 2013). Increased states of vasoconstriction and blood coagulation could decrease renal blood flow, further weakening the filtration function of the kidney. An experimental study in the rodent model reported that chronic exposure to PM2.5 could trigger inflammation and oxidative stress pathways which contributed to the PM2.5 induced kidney injury (Chenxu et al., 2018). Another research in rat model reported that PM2.5 may induce early kidney damage by activating systematic inflammation and oxidative stress response (Aztatzi-Aguilar et al., 2016). However, evidence about the underlying mechanisms remain scarce.
Therefore, we designed the current research with personal PM2.5 exposure and components measurements to explore their acute adverse effects on renal function parameters among healthy adults. In addition, we explored the possible mediation effect of circulating biomarkers on the aforementioned associations. The results of our research will contribute to the evidence of the association between PM2.5 and renal function and serve the potential bio-pathway.
2. Material and methods
2.1. Study design and participants
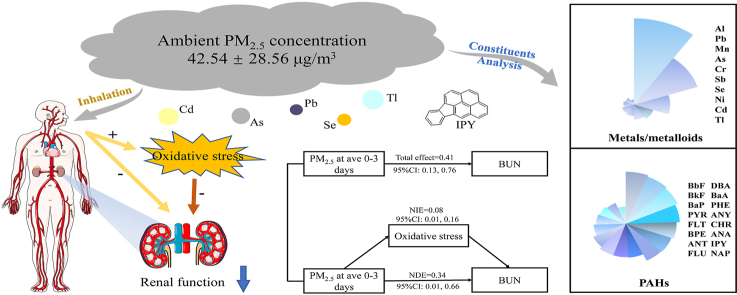
The 7th Military World Games were held in Wuhan, China, from October 18 to October 27, 2019. A series of policy measures were implemented to restrict the road traffic and control air pollution during the match. In our previous research (Peng et al., 2022), we recruited 70 college students for 8 rounds repeated measurements of blood samples collection, in-person investigation and physical examination to explore the adverse effects of PM2.5. Baseline demographic information, including sex, date of birth, weight, height, education was collected after signing the informed consent. We randomly selected a subset from the previous research for the current research. Briefly, 35 healthy adults were included with 6 rounds follow-up visits including twice in each of the three phases before (from Sep. 16th to Sep. 27th), during (from Oct. 17th to Oct. 28th) and after the Military games (from Dec. 5th to Dec. 16th). We measured individual-level hourly PM2.5 concentrations, and collected the venous blood at the 4th day. And participants were required to reported health status (Healthy or Sick), medication use (Yes or No), caffeine and alcohol consumption (Yes or No), exercise (Yes or No) and dietary intake frequency at each follow-up visit. The research design was approved by Wuhan University Medical Ethics Committee.
2.2. Exposure measurement
We conducted the personal hourly PM2.5 measurements for 3-consecutive day before each physical examination. We performed the HUAWEI individual PM2.5 monitor which was designed with low weight for portability based on Beta ray attenuation methods. And the DUSTTRAK™ DRX 8534 (TSI, USA) was used to calibrate personal exposure devices (Peng et al., 2022). Ambient PM2.5 samples were collected by a medium-volume sampler (TH-150C, Wuhan Tianhong Environment Protection Industry Co. Ltd., Wuhan, China) with Whatman quartz fiber filters (Whatman International Ltd., Maidstone, UK) and kept individually in a polystyrene box (SF-90BOX, Beijing Safelab Ltd, Beijing, China). The constituents of ambient PM2.5, including trace metals (metalloids) and the polycyclic aromatic hydrocarbons (PAHs) measured by Inductively Coupled Plasma-Mass Spectrum with iCAP-Q (Thermo Fisher Scientific, Waltham, MA, USA) and Gas Chromatography-Mass Spectrometry with Trace 1300-ISQ 7000 (Thermo Fisher Scientific, Waltham, MA, USA), respectively. All experimental manipulations were done at the laboratory of Wuhan Center for Disease Control and Prevention and details can be found in the previous research (Mao et al., 2020). Generally, 10 metals and 16 PAHs were measured from PM2.5, such as Aluminum (Al), Arsenic (As), Cadmium (Cd), Lead (Pb), Selenium (Se), Thallium (Tl), Indeno (1,2,3-cd) pyrene (IPY), etc. Additionally, we collected the city-level PM2.5 concentrations from 2018 to 2020 from Wuhan Municipal Ecological Environment Bureau (http://hbj.wuhan.gov.cn/) to evaluate the effect of restrictive measures during the Military World Games. The hourly concentrations of ozone (O3), nitrogen dioxide (NO2), sulfate dioxide (SO2) and carbon monoxide (CO) was also collected from the nearest air monitoring station (Wuhan Donghu Liyuan).
2.3. Blood collection and analysis
Fasting venous blood samples (20 mL in total, including 10 mL EDTA anticoagulated blood and 10 mL non-anticoagulated blood) were collected before 8:00 a.m. at each physical examination. And the centrifuged plasma and serum samples were stored at −80 °C before biomarkers measurement. Renal function indicators including BUN, sCr, and urea acid (UA) were detected by a full-automatic biochemical analyzer (Hitachi 7600, Hitachi Co., Tokyo, Japan). The blood urea nitrogen-to-creatinine ratio (BUN/sCr) was calculated as a commonly clinical renal function indicator. The eGFR was calculated according to the Chronic Kidney Disease Epidemiology Collaboration Equation (Levey et al., 2009), and the values of endogenous creatinine clearance rate (Ccr) were estimated following the Cockcroft-Gault Equation (Cockcroft and Gault, 1976, Winter et al., 2012). The fasting blood glucose concentrations were measured by an automatic biochemical analyzer (Cobas c701, Roche, Japan). The following 5 circulating biomarkers were measured as potential bio-mediators: (i) inflammation: Hypersensitive C-reactive protein (hsCRP), interleukin-6 (IL-6); (ii) coagulation: fibrinogen (FIB); (iii) oxidative stress: superoxide dismutase (SOD); (iv) vasoconstriction: Angiotensin-converting enzyme (ACE). The serum hsCRP and ACE were analyzed through the Beckman Coulter AU5800 (Beckman Coulter Inc., Brea CA, USA). SOD was analyzed using an automatic biochemical analyzer (Hitachi 7180, Hitachi, Tokyo, Japan) with Superoxide Dismutase Kit. The serum IL-6 was assayed by electrochemiluminescence on Roche cobas 8000 (Roche Diagnostics, Mannheim, Germany). The plasma FIB was analyzed by the Clauss method on SF-8200 coagulation analyzer (Beijing Succeeder Technology Inc., Beijing, China). All the biomarkers were analyzed in the laboratory of Wuhan Pulmonary Hospital (Wuhan, China).
2.4. Statistic analysis
Demographics, biological indicators and PM2.5 concentrations were described as mean ± standard deviation (SD) or frequency (%). The linear mixed-effect (LME) model with each participant as random intercept was performed to assess the relationship between PM2.5 exposure and renal function. We calculated the 1–3 days PM2.5 moving average (ave 0–1, ave 0–2 and ave 0–3) to identify the cumulative impact of exposure in the single-pollutant LME model. A group of priori covariates were adjusted in the LME model, including age, sex, body mass index, exercise, caffeine consumption, alcohol consumption, fasting blood glucose. Additionally, the temperature and relative humidity were adjusted in the form of natural splines, while the Akaike information criterion was applied to determine the degrees of freedom. Besides, as previous review reported that high-protein diets serve as a risk factor for renal dysfunction (Ko et al., 2020), we adjusted the high protein proportion food intake (i.e., the consumption frequency of meat, poultry, fish, and milk) in the association between PM2.5 and renal function. We also investigated the short-term association between 3-days moving average of PM2.5 components and renal function indicators in the single-constituent LME model. The effects were estimated as the changes of indicators and 95% confidence intervals (CIs) with an interquartile range (IQR) increment in PM2.5 or its constituents’ concentrations.
We hypothesized that associations of PM2.5 with renal function indicators might be mediated through inflammation, oxidative stress, or vasoconstriction. Therefore, we selected SOD, hsCRP, IL-6, FIB and ACE as potential mediators which were suggested to be associated with PM2.5 in the previous research. In this study, potential mediators were defined by the following criteria: (i) significantly associated with PM2.5 and (ii) significantly associated with renal function indicators (Valeri and Vanderweele, 2013). Two LME models were built for the mediation analysis (Bind et al., 2016), one fitting for the PM2.5-mediator association and the other one fitting for the mediator-renal function association (Equations [1], [2])).
| Mij = β0 + ui + βPM2.5PM2.5ij + β1X1ij + … + βpXpij + εij, | [1] |
| Yij = γ0 + gi + γPM2.5PM2.5ij + γMMij + γ1X1ij + … + γpXpij + ηij, | [2] |
In both of two equations, ꞵ0 and γ0 correspond to the intercept for the population mean; ui and gi correspond to the subject-specific random intercept. Mij correspond to the potential circulating biomarkers and Yij correspond to the renal function indicators measured for an individual i (i = 1, …, 35) at visit j (j = 1, …, 6). X1ij to Xpij represent the priori-selected covariates, and εij and ηij represent the within-subject error term. γPM2.5 represents the natural direct effect (NDE), and the natural indirect effect (NIE) could be given by βPM2.5 × γM. The proportion mediated, which means the percentage of NIE over the total effect, was calculated by (NIE/(NIE + NDE)).
To examine the robustness of our findings, we performed several sensitivity analyses. Firstly, outcomes of the previous visit might be potential confounding factors for subsequent visits and lead to bias in the longitudinal studies. Therefore, we built a LME model regression between Yij (BUNij) and Mij+1 (SODij+1) to examine the time-varying confounding assumption (Fig. S1). Then, we adjusted the other four gaseous pollutants (i.e., O3, SO2, NO2 and CO) into the two-pollutant models. Thirdly, we fitted a “constituent-PM2.5 joint model” and “constituent-residual model” to eliminate the extraneous variation of total PM2.5 and the collinearity between constituent and the PM2.5 mass concentrations (Liu et al., 2017). All the statistical analyses were conducted in the R software (4.0.5) with packages of “lmerTest”, “splines” and “mediation”, and the two-side p-value less than 0.05 was determined as statistical significance.
3. Results
3.1. Descriptive analysis
A total of 35 volunteers (28 females and 7 males) with an averaged age of 20.43 years and a mean BMI of 21.17 kg/m2 were recruited in this panel study. And all the participants were nonsmokers. However, 4 participants failed to complete 1 follow-up visit and 1 participant failed to complete 2 follow-up visits for various reasons and thus 6 observations were deleted. Eventually we examined a total number of 204 venous blood samples. Table 1 showed the 12 blood biomarkers levels, including the 6 renal function indicators (BUN, sCr, UA, eGFR, Ccr, BUN/sCr), the 5-potential bio-mediators (SOD, IL-6, hsCRP, ACE, FIB) and the fasting blood glucose. Fig. S2 showed the monthly averaged PM2.5 concentrations in Wuhan from September to December for the years of 2018–2020. We found an upward trend of ambient PM2.5 concentrations during the 4 months in 2018 and 2020, while the concentrations showed a slight decline in October (during the 7th Military World Games) in 2019. Table 2 summarized PM2.5 mass concentrations along with metals and PAHs constituents during the whole research periods. The average individual PM2.5 concentrations were 42.54 μg/m3, which exceed the Interim Target-2 standard of the WHO air quality guideline on PM2.5. Among the various constituents of the PM2.5, the metal/metalloid constituents had the higher proportion than PAHs and varied considerably, in which Al, Pb and Mn had large abundant while Ni, Cd and Tl were less.
Table 1.
Basic characteristics and the biological indicators of study participants.
| Mean ± SD or N (%) | ||
|---|---|---|
| Demographic characteristics | ||
| NO. | 35 | |
| Age, years | 20.43 ± 1.74 | |
| BMI, kg/m2 | 21.17 ± 2.59 | |
| Sex, female | 28 (80.00%) | |
| Serum and plasma biomarkers | ||
| FBG, mmol/L | 4.61 ± 0.37 | |
| SOD, U/mL | 146.79 ± 9.43 | |
| FIB, g/L | 2.44 ± 0.41 | |
| hsCRP, mg/dL | 0.99 ± 1.31 | |
| IL-6, pg/mL | 1.87 ± 1.75 | |
| ACE, U/L | 33.00 ± 10.00 | |
| Renal function indicators | ||
| BUN, mmol/L | 3.86 ± 1.21 | |
| sCr, μmol/L | 63.80 ± 9.50 | |
| UA, μmol/L | 341.00 ± 82.00 | |
| eGFR, mL/(min*1.73m2) | 123.79 ± 10.12 | |
| Ccr, mL/(min*1.73m2) | 118.63 ± 20.61 | |
| BUN/sCr | 15.52 ± 4.74 | |
Abbreviations: SD, standard deviation; BMI, body mass index; FBG, fasting blood glucose; SOD, superoxide dismutase; FIB, fibrinogen; hsCRP, hypersensitive C-reactive protein; ACE, angiotensin converting enzyme; IL-6, interleukin-6; BUN, blood urea nitrogen; sCr, serum creatinine; UA, blood urea acid; eGFR, estimated glomerular filtration rate; Ccr, endogenous creatinine clearance; BUN/sCr, the ratio of blood urea nitrogen to serum creatinine.
Table 2.
Descriptive statistics of 3-day average ambient PM2.5 and PM2.5 chemical components for the study participants over the study period.
| Mean | SD | Percentiles |
IQR | ||||
|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | |||||
| PM2.5 (μg/m3) | 42.54 | 28.56 | 23.45 | 30.25 | 56.38 | 32.94 | |
| Metals (ng/m3) | |||||||
| Sb | 2.60 | 1.24 | 1.77 | 2.72 | 3.58 | 1.82 | |
| Al | 148.77 | 61.89 | 114.12 | 131.36 | 174.55 | 60.42 | |
| As | 6.09 | 3.54 | 4.45 | 5.24 | 7.27 | 2.82 | |
| Cd | 1.30 | 0.66 | 0.87 | 1.20 | 1.88 | 1.02 | |
| Cr | 4.43 | 2.74 | 3.25 | 3.37 | 4.20 | 0.95 | |
| Pb | 65.98 | 32.55 | 45.93 | 65.66 | 93.66 | 47.72 | |
| Mn | 19.35 | 5.49 | 17.46 | 18.55 | 20.91 | 3.44 | |
| Ni | 1.67 | 0.57 | 1.39 | 1.82 | 2.02 | 0.63 | |
| Se | 2.26 | 0.95 | 1.62 | 2.11 | 2.38 | 0.76 | |
| Tl | 0.51 | 0.25 | 0.35 | 0.50 | 0.55 | 0.21 | |
| PAHs (ng/m3) | |||||||
| NAP | 0.15 | 0.06 | 0.10 | 0.13 | 0.17 | 0.06 | |
| ANY | 0.37 | 0.09 | 0.37 | 0.40 | 0.42 | 0.05 | |
| ANA | 0.29 | 0.08 | 0.29 | 0.30 | 0.30 | 0.01 | |
| FLU | 0.56 | 0.20 | 0.50 | 0.58 | 0.61 | 0.11 | |
| PHE | 0.47 | 0.12 | 0.45 | 0.49 | 0.52 | 0.07 | |
| ANT | 0.82 | 0.22 | 0.76 | 0.86 | 0.92 | 0.16 | |
| FLT | 0.93 | 0.29 | 0.75 | 0.93 | 1.12 | 0.37 | |
| PYR | 1.05 | 0.31 | 0.99 | 1.05 | 1.21 | 0.23 | |
| CHR | 0.31 | 0.22 | 0.18 | 0.21 | 0.35 | 0.17 | |
| BaA | 0.54 | 0.18 | 0.47 | 0.53 | 0.61 | 0.14 | |
| BbF | 1.32 | 0.34 | 1.23 | 1.39 | 1.49 | 0.26 | |
| BKF | 1.25 | 0.53 | 1.02 | 1.10 | 1.62 | 0.60 | |
| BaP | 1.17 | 0.33 | 1.05 | 1.26 | 1.43 | 0.39 | |
| DBA | 0.54 | 0.13 | 0.43 | 0.54 | 0.63 | 0.20 | |
| BPE | 0.85 | 0.60 | 0.48 | 0.62 | 1.04 | 0.55 | |
| IPY | 0.25 | 0.20 | 0.11 | 0.17 | 0.41 | 0.30 | |
Abbreviations: SD, standard deviation; IQR, interquartile range; Sb, Stibium; Al, Aluminum; As, Arsenic; Cd, Cadmium; Cr, Chromium; Pb, Lead; Mn, Manganese; Ni, Nickel; Se, Selenium; Tl, Thallium; PAHs, Polycyclic aromatic hydrocarbons; NAP, Naphthalene; ANA, Acenaphthene; ANY, Acenaphthylene; FLU, Fluorene; PHE, Phenanthrene; ANT, Anthracene; FLT, Fluoranthene; PYR, Pyrene; CHR, Chrysene; BaA, Benzo (a) pyrene; BbF, Benzo (b) fluoranthene; BkF, Benzo (k) fluoranthene; BaP, Benzo (a) pyrene; BPE, Benzo (g,h,i) perylene; DBA, Dibenzo (a,h) anthracene; IPY, Indeno (1,2,3-cd) pyrene.
3.2. Estimated association between PM2.5 and renal function
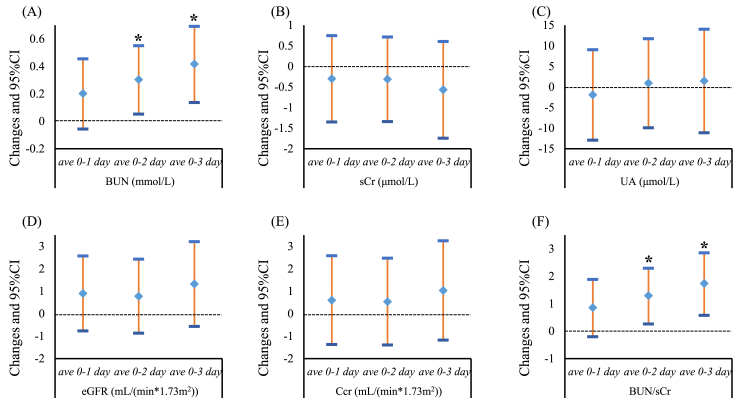
Fig. 1 presented the estimated associations of PM2.5 concentrations with renal function indicators (BUN, sCr, UA, eGFR, Ccr and BUN/sCr). Short-term exposure to PM2.5 were positively associated with BUN and BUN/sCr. An IQR increase in PM2.5 (32.94 μg/m3) was associated with 0.30 mmol/L (ave 0–2, 95% CI: 0.05 to 0.55) and 0.42 mmol/L (ave 0–3, 95% CI: 0.14 to 0.69) increment of BUN, respectively. For the BUN/sCr, an IQR increment in PM2.5 was associated with 1.29 (ave 0–2, 95% CI: 0.27 to 2.30) and 1.74 (ave 0–3, 95% CI: 0.58 to 2.86) elevated in BUN/sCr, respectively. However, the estimated effect of PM2.5 on other renal function indicators were not significant.
Fig. 1.
Changes in renal function indicators (mean and 95% confidence intervals) with an interquartile range increment of PM2.5 in different exposure windows. (A) BUN, blood urea nitrogen; (B) sCr, serum creatinine; (C) UA, urea acid; (D) eGFR, estimated glomerular filtration rate; (E) Ccr, endogenous creatinine clearance rate; (F) BUN/sCr, blood urea nitrogen-to-serum creatinine. *Estimated were statistically significant (p-value < 0.05).
3.3. Estimated relationship of PM2.5 constituents with renal function
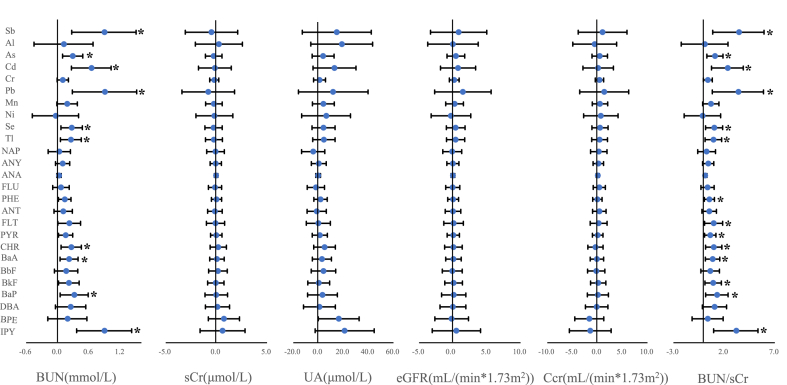
Fig. 2 illustrated the estimated changes in renal function indicators altered by ave 0–3 concentrations of trace metals and PAHs in PM2.5. Among the 10 metal (metalloid) constituents, short-term exposure to Sb, As, Cd, Pb, Se and Tl were related to increased BUN and BUN/sCr. For example, a IQR increment in Cd (1.02 ng/m3) was associated with 0.65 mmol/L increase in BUN (95% CI: 0.26 to 1.02) and 2.36 increase in BUN/sCr (95% CI: 0.75 to 3.89). The effects of each IQR increment in Pb (47.72 ng/m3) on BUN and BUN/sCr were 0.90 mmol/L (95% CI: 0.28 to 1.51) and 3.42 (95% CI: 0.84 to 5.89), respectively. Besides, PAHs of Chrysene, Benzo (a) anthracene, Benzo (a) pyrene and IPY in PM2.5 were also positively associated with BUN and BUN/sCr. For example, an IQR increment in IPY (0.30 ng/m3) were associated with 0.90 mmol/L (95% CI: 0.36 to 1.41) and 3.21 (95% CI: 0.97 to 5.34) higher of BUN and BUN/sCr, respectively. The relationships between the other 4 renal function indicators and the 26 PM2.5 constituents were insignificant.
Fig. 2.
The cumulative changes (mean and 95% confidence intervals) in renal function indicators associated with an interquartile range increment of 3-day moving average of PM2.5-bound components. Abbreviations same as in Table 2 and Fig. 1. *Estimated were statistically significant (p-value < 0.05).
3.4. Mediation analysis
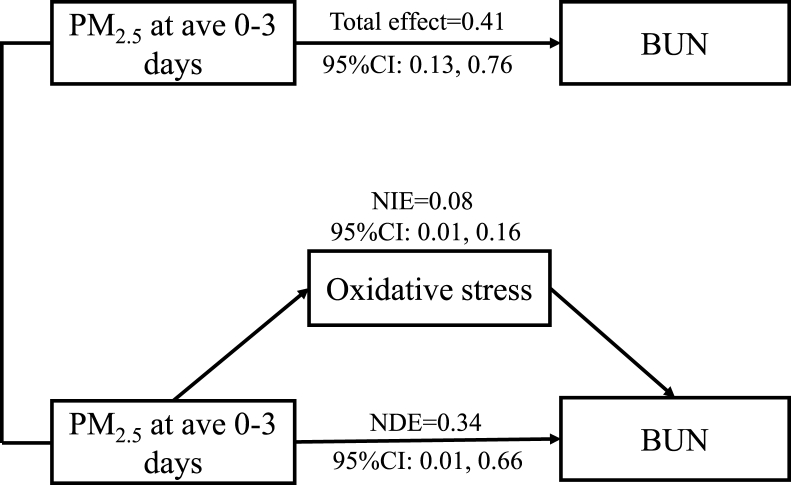
We explored the association between PM2.5 and potential mediators through LME models (Table 3). Each IQR increments in PM2.5 were associated with 1.64 U/mL (95% CI: 0.08 to 3.20) and 2.40 U/mL (95% CI: 0.37 to 4.42) increase in SOD at ave 0–2 and ave 0–3, respectively. The effects of PM2.5 exposure on IL-6, hsCRP, ACE and FIB were insignificant. Therefore, we further examined whether SOD could be a mediator of the associations between PM2.5 and renal function. It was estimated that SOD contributed to 18.24% of the associations between PM2.5 exposure and increased BUN at ave 0–3 (Fig. 3). Specifically, the NIE of PM2.5 (each 32.94 μg/m3 increment) on BUN was 0.08 mmol/L (95% CI: 0.01 to 0.16) at ave 0–3, while the NDE of PM2.5 was 0.34 mmol/L (95% CI: 0.01 to 0.66).
Table 3.
Changes in potential mediators (mean and 95% confidence intervals) associated with an interquartile range increment of PM2.5 in different exposure windows.
| ave 0–1 days | ave 0–2 days | ave 0–3 days | |
|---|---|---|---|
| SOD | 1.51 (−0.22, 3.24) | 1.64 (0.08, 3.20)* | 2.40 (0.37, 4.42)* |
| IL-6 | −0.05 (−0.33, 0.22) | −0.06 (−0.30, 0.19) | −0.09 (−0.37, 0.19) |
| hsCRP | −0.06 (−0.18, 0.07) | −0.05 (−0.16, 0.05) | −0.07 (−0.20, 0.06) |
| FIB | 0.00 (−0.05, 0.04) | −0.01 (−0.05, 0.03) | −0.01 (−0.05, 0.03) |
| ACE | 0.14 (−0.49, 0.75) | 0.14 (−0.40, 0.68) | 0.21 (−0.42, 0.84) |
*Estimated were statistically significant (p-value < 0.05). Abbreviation: SOD, superoxide dismutase; FIB, fibrinogen; hsCRP, hypersensitive C-reactive protein; ACE, angiotensin converting enzyme; IL-6, interleukin-6.
Fig. 3.
Mediation analysis of oxidative stress activation on blood urea nitrogen concentrations after PM2.5 exposure. Abbreviations: CI, confidence intervals; BUN, blood urea nitrogen; NIE, nature indirect effect; NDE, nature direct effect.
3.5. Sensitivity analysis
A series of sensitivity analyses were conducted to check the robustness of our results. Firstly, we examined the time-varying confounding assumption using LME models, and found that there was insignificant association between BUNij and SODij+1 (Table S1). Additionally, we adjusted the other gaseous air pollutants into the two-pollutant LME models (Fig. S3), and the positive associations of PM2.5 with BUN and BUN/sCr were significant. Moreover, we adjusted the PM2.5 in the “constituent-PM2.5 joint models” (Fig. S4A) and controlled the residual constituents of the total mass concentrations in the “constituent-PM2.5 residual models” (Fig. S4B). We found relatively robust association between As, Cd, Pb, Se, Tl and IPY on BUN levels in both of two models.
4. Discussion
The research evaluated the adverse effect of PM2.5 and its trace constituents on renal function parameters over a period of 3 days among 35 healthy young adults. After adjusting for several potential covariates, PM2.5 mass concentrations and its several metals (metalloids) and PAHs showed strong associations with increased BUN and BUN/sCr. Additionally, we found elevated SOD levels due to PM2.5 exposure may mediate the association between PM2.5 exposure and renal functions. Our findings indicate that short-term exposure to PM2.5 may increase the risks of renal dysfunction via systemic oxidative stress, in which the constituents of Pb, Cd, As, Se, Tl and IPY may play the leading roles.
Numerous studies provided epidemiological evidence that PM2.5 may serve as a risk factor of renal dysfunction (Mehta et al., 2016; Tavera Busso et al., 2018; Liu et al., 2020; Rahmani Sani et al., 2020; Zhao et al., 2020; Li et al., 2021). Most studies reported the significant associations between PM2.5 and the kidney indicators of sCr and eGFR among populations. A recent panel study on 135 children aged 4–13 years reported that a 10 μg/m3 increment of PM2.5 was related to −1.83% changes in eGFR (Liu et al., 2020). The VA Normative Aging Study reported that per 2.1 μg/m3 increment in annual average of PM2.5 was related with 1.87 mL/min/1.73 m2 declination of eGFR among 669 older adults with an average age of 73.5 (Mehta et al., 2016). Another research reported that exposure to PM2.5 has a negative impact on renal function with 0.03 mg/dL increase in sCr and 1.09 mL/min/1.73 m2 reduction in eGFR among 150 pregnant women (Rahmani Sani et al., 2020). However, the associations of sCr and eGFR with PM2.5 were insignificant in this current research. This might be explained that children, the elderly and pregnant women were more vulnerable to the acute effects of PM2.5 on kidney than the healthy young adults in this study (Peled, 2011; Mukherjee and Agrawal, 2018).
The BUN, an end product of protein metabolism, was synthesized from amino acid metabolites in the liver and excreted from kidney. A cross-sectional study on pregnant women reported that for per 3.9 μg/m3 increment of PM2.5 was associated with 0.05 mmol/L increase in BUN during the whole pregnancy (Zhao et al., 2020). Additionally, an experimental study on rodent models reported that sub-chronic exposure to PM2.5 was related to BUN elevation (Tavera Busso et al., 2018). Our results were consistent with the findings of the above two research. As well-recognized indicators to reflect renal function, the concentrations of BUN and sCr are determined by the balance of body generation and excretion by the kidneys (Kirtane et al., 2005). However, BUN is partly reabsorbed by proximal tubules with sodium and water under the influence of antidiuretic hormone (Conte et al., 1987), whereas sCr is not (Matsue et al., 2017). Our research indicated that exposure to PM2.5 may be related to the elevated BUN levels without directly affecting glomerular filtration, which in turn increases the burden of renal function. The results can be explained that the elevated BUN levels may be due to renal hypoperfusion or tubular dysfunction, which was independent on the changes of eGFR and sCr (Aronson et al., 2004). Moreover, previous research reported that oxidative stress and inflammatory responses might increase the catabolism of structural proteins and amino acids, which in turn caused increases of BUN generation (Macedo, 2011).
PM2.5 contains a mixture of metallic/metalloid elements, adsorbed organic compounds and trace amounts of biological components (Bell et al., 2007), which varies in different regions. The heterogeneous constituents may explain the inconsistent results on the health effects of PM2.5 from numerous research. Because previous studies have reported the nephrotoxicity of several metals, we focused on the metal components of PM2.5 to estimate their effects on kidney (Navas-Acien et al., 2009; Trzeciakowski et al., 2014; Tsai et al., 2021). Regarding the PM2.5-bound metals, a study among 76 old participants reported that exposure to copper, titanium, and Mn in PM2.5 within 3 days were related to reduced eGFR (Fang et al., 2020). Another panel study in 144 children demonstrated that Mg+, K+, Al+ and Li+ in size-fractionated particle number counts of 0.5 were linked to eGFR reduction (Liu et al., 2021). In this study, we found Sb, Cd, Pb, Tl and metalloids of As, Se in PM2.5 were significantly related to elevated levels of BUN. Our results could be supported by a published research reporting that co-exposure to As, Pb, Cd, and Hg measured in urine was associated with increased BUN (Sanders et al., 2019). Additionally, we found weak but robust associations between elevated BUN levels and several PM2.5-bound PAHs. Generally, Pb and Sb in PM2.5 are originated from vehicle emissions (Smichowski et al., 2007), and PAHs are mainly from the incomplete combustion of fossil fuels (Ravindra et al., 2008). Therefore, our research suggested that PM2.5 from traffic sources may have greater effects on renal function.
Currently, the underlying biological mechanisms of the association between PM2.5 and renal function were not well characterized. One mechanistic hypothesis suggested that inhaled PM2.5 could stimulate the systematic inflammatory response and those inflammatory cytokines may impair the kidney via blood circulation (Rückerl et al., 2014; Suárez-Álvarez et al., 2016). In addition, the ultrafine particles may traverse the alveolar space into bloodstream and causes fibrinolytic dysfunction and cellular responses to exacerbate the damage of remote organs (Bowe et al., 2017; Xie et al., 2021). In this study, we measured several biomarkers of inflammation, oxidative stress, and vasoconstriction to exam their bio-mediation effects between PM2.5 and renal function parameters. This study found that SOD might be a potential mediator of the association between 3-days exposure of PM2.5 and BUN. Our results were consistent with existing evidence. A panel study reported that elevated SOD concentrations as an adaptive response of the organism to the oxidative stress in response to PM2.5 exposure (Wu et al., 2016). The human nonmercaptalbumin, an oxidative stress biomarker, was also reported to be positively related with BUN and sCr (Masudo et al., 2017). Additionally, two published experimental studies on rat models reported that exposure to PM2.5 led to early kidney damage as a consequence of oxidative stress-antioxidant imbalance (Aztatzi-Aguilar et al., 2016, 2021). Our study provides population-based epidemiological evidence that exposure to PM2.5 may affect the renal function via systemic oxidative stress. Nevertheless, the results of the mediation analysis should be interpreted cautiously and further studies are needed to verify the causal relationship.
This study has several strengths. Firstly, the research used a quasi-experimental design of the air quality controls during the 7th CISM Military World Games, which was efficient for causal inference. In addition, since all the participants in this study were healthy young adults and had no history of chronic diseases, the potential confounding effects of medications and diseases could be excluded. Thirdly, we employed the mediation analysis to explore the potential bio-mechanisms between PM2.5 and renal function, which provided important epidemiological evidence for PM2.5 induced renal dysfunction.
There were also several limitations in this study. Firstly, participants in this study were only including healthy young adults and the sample size was small, therefore the extrapolation of the research findings may be limited. Further studies are needed to include larger sample size with general population from multiple cities. Secondly, measurement bias is inevitable as the measurements of PM2.5 constituents were based on measurements from the nearest ambient monitoring station. However, we do not expect the bias to be substantial as all participants lived and worked within Wuhan University School of Medicine (less than 0.2 km). Previous research suggested that the ambient measurement of PM2.5 constituents at fixed monitoring sites could adequately be used to predict the individual-level exposures (Lei et al., 2020). Thirdly, although the renal function parameters in our research were conventional biomarkers from blood which with commonly used in clinical diagnosis, more indicators of early impairment of renal function such as serum Cystatin C, Kidney injury molecule-1 and Neutrophil gelatinase-associated lipid carrier protein (van Veldhuisen et al., 2016) could be considered in the future research.
5. Conclusion
The current panel study provided the evidence that short-term exposure to PM2.5 may affect renal function among healthy young adults. Several metal (metalloid) and PAHs components of PM2.5, such as Pb, Cd, As, Se, Tl and IPY, might contribute to the observed association. Additionally, our findings suggested that oxidative stress may be a plausible pathway which mediate the association between PM2.5 and BUN. The adverse effect of PM2.5, especially traffic-related particulate matters on renal function should be given more attention. Further studies are needed to verify our findings and elucidate the underlying mechanisms.
Author contribution
Shouxin Peng: Data curation, Methodology, Formal analysis, Writing – original draft, Visualization. Tianjun Lu: Validation, Writing – review & editing. Yisi Liu: Validation, Writing – review & editing. Zhaoyuan Li: Methodology, Investigation, Software. Feifei Liu: Investigation, Data curation, Software. Jinhui Sun: Data curation, Software. Meijin Chen: Investigation. Huaiji Wang: Conceptualization, Investigation, Methodology. Hao Xiang: Conceptualization, Methodology, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Bill & Melinda Gates Foundation, Seattle, WA [grant number OOP1148464]. And we thank all the volunteers participating in the present study.
Handling Editor: Frederik-Jan van Schooten
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.chemosphere.2022.133570.
Contributor Information
Huaiji Wang, Email: hj@whcdc.org.
Hao Xiang, Email: xianghao@whu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Al-Aly Z., Bowe B. Air pollution and kidney disease. Clin. J. Am. Soc. Nephrol. 2020;15:301–303. doi: 10.2215/CJN.16031219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson D., Mittleman M.A., Burger A.J. Elevated blood urea nitrogen level as a predictor of mortality in patients admitted for decompensated heart failure. Am. J. Med. 2004;116:466–473. doi: 10.1016/j.amjmed.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Aztatzi-Aguilar O.G., Pardo-Osorio G.A., Uribe-Ramírez M., Narváez-Morales J., De Vizcaya-Ruiz A., Barbier O.C. Acute kidney damage by PM(2.5) exposure in a rat model. Environ. Toxicol. Pharmacol. 2021;83:103587. doi: 10.1016/j.etap.2021.103587. [DOI] [PubMed] [Google Scholar]
- Aztatzi-Aguilar O.G., Uribe-Ramirez M., Narvaez-Morales J., De Vizcaya-Ruiz A., Barbier O. Early kidney damage induced by subchronic exposure to PM2.5 in rats. Part. Fibre Toxicol. 2016;13:68. doi: 10.1186/s12989-016-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell M.L., Dominici F., Ebisu K., Zeger S.L., Samet J.M. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environ. Health Perspect. 2007;115:989–995. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind M.A., Vanderweele T.J., Coull B.A., Schwartz J.D. Causal mediation analysis for longitudinal data with exogenous exposure. Biostatistics. 2016;17:122–134. doi: 10.1093/biostatistics/kxv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Artimovich E., Xie Y., Yan Y., Cai M., Al-Aly Z. The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: a modelling study. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2019-002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowe B., Xie Y., Li T., Yan Y., Xian H., Al-Aly Z. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: a cohort study. The Lancet Planetary Health. 2017;1:e267–e276. doi: 10.1016/S2542-5196(17)30117-1. [DOI] [PubMed] [Google Scholar]
- Chenxu G., Minxuan X., Yuting Q., Tingting G., Jinxiao L., Mingxing W., Sujun W., Yongjie M., Deshuai L., Qiang L., Linfeng H., Jun T. iRhom2 loss alleviates renal injury in long-term PM2.5-exposed mice by suppression of inflammation and oxidative stress. Redox Biol. 2018;19:147–157. doi: 10.1016/j.redox.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft D.W., Gault M.H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Conte G., Dal Canton A., Terribile M., Cianciaruso B., Di Minno G., Pannain M., Russo D., Andreucci V.E. Renal handling of urea in subjects with persistent azotemia and normal renal function. Kidney Int. 1987;32:721–727. doi: 10.1038/ki.1987.266. [DOI] [PubMed] [Google Scholar]
- Correia-Costa L., Sousa T., Morato M., Cosme D., Afonso J., Areias J.C., Schaefer F., Guerra A., Afonso A.C., Azevedo A., Albino-Teixeira A. Oxidative stress and nitric oxide are increased in obese children and correlate with cardiometabolic risk and renal function. Br. J. Nutr. 2016;116:805–815. doi: 10.1017/S0007114516002804. [DOI] [PubMed] [Google Scholar]
- Couser W.G., Remuzzi G., Mendis S., Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011;80:1258–1270. doi: 10.1038/ki.2011.368. [DOI] [PubMed] [Google Scholar]
- Eknoyan G., Lameire N., Barsoum R., Eckardt K.U., Levin A., Levin N., Locatelli F., MacLeod A., Vanholder R., Walker R., Wang H. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66:1310–1314. doi: 10.1111/j.1523-1755.2004.00894.x. [DOI] [PubMed] [Google Scholar]
- Ene-Iordache B., Perico N., Bikbov B., Carminati S., Remuzzi A., Perna A., Islam N., Bravo R.F., Aleckovic-Halilovic M., Zou H., Zhang L., Gouda Z., Tchokhonelidze I., Abraham G., Mahdavi-Mazdeh M., Gallieni M., Codreanu I., Togtokh A., Sharma S.K., Koirala P., Uprety S., Ulasi I., Remuzzi G. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Global Health. 2016;4:e307–319. doi: 10.1016/S2214-109X(16)00071-1. [DOI] [PubMed] [Google Scholar]
- Fang J., Tang S., Zhou J., Zhou J., Cui L., Kong F., Gao Y., Shen Y., Deng F., Zhang Y., Liu Y., Dong H., Dong X., Dong L., Peng X., Cao M., Wang Y., Ding C., Du Y., Wang Q., Wang C., Zhang Y., Wang Y., Li T., Shi X. Associations between personal PM(2.5) elemental constituents and decline of kidney function in older individuals: the China BAPE study. Environ. Sci. Technol. 2020;54:13167–13174. doi: 10.1021/acs.est.0c04051. [DOI] [PubMed] [Google Scholar]
- Kirtane A.J., Leder D.M., Waikar S.S., Chertow G.M., Ray K.K., Pinto D.S., Karmpaliotis D., Burger A.J., Murphy S.A., Cannon C.P., Braunwald E., Gibson C.M., Group T.S. Serum blood urea nitrogen as an independent marker of subsequent mortality among patients with acute coronary syndromes and normal to mildly reduced glomerular filtration rates. J. Am. Coll. Cardiol. 2005;45:1781–1786. doi: 10.1016/j.jacc.2005.02.068. [DOI] [PubMed] [Google Scholar]
- Ko G.J., Rhee C.M., Kalantar-Zadeh K., Joshi S. The effects of high-protein diets on kidney health and longevity. J. Am. Soc. Nephrol. 2020;31:1667–1679. doi: 10.1681/ASN.2020010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuźma Ł., Małyszko J., Bachórzewska-Gajewska H., Kralisz P., Dobrzycki S. Exposure to air pollution and renal function. Sci. Rep. 2021;11:11419. doi: 10.1038/s41598-021-91000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X., Chen R., Wang C., Shi J., Zhao Z., Li W., Bachwenkizi J., Ge W., Sun L., Li S., Cai J., Kan H. Necessity of personal sampling for exposure assessment on specific constituents of PM(2.5): results of a panel study in Shanghai, China. Environ. Int. 2020;141:105786. doi: 10.1016/j.envint.2020.105786. [DOI] [PubMed] [Google Scholar]
- Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., Coresh J. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Wang Y.Y., Guo Y., Zhou H., Wang Q.M., Shen H.P., Zhang Y.P., Yan D.H., Li S., Chen G., Lin L., He Y., Yang Y., Peng Z.Q., Wang H.J., Ma X. Association between airborne particulate matter and renal function: an analysis of 2.5 million young adults. Environ. Int. 2021;147:106348. doi: 10.1016/j.envint.2020.106348. [DOI] [PubMed] [Google Scholar]
- Li Z., Liu Q., Xu Z., Guo X., Wu S. Association between short-term exposure to ambient particulate air pollution and biomarkers of oxidative stress: a meta-analysis. Environ. Res. 2020;191:110105. doi: 10.1016/j.envres.2020.110105. [DOI] [PubMed] [Google Scholar]
- Liu C., Cai J., Qiao L., Wang H., Xu W., Li H., Zhao Z., Chen R., Kan H. The acute effects of fine particulate matter constituents on blood inflammation and coagulation. Environ. Sci. Technol. 2017;51:8128–8137. doi: 10.1021/acs.est.7b00312. [DOI] [PubMed] [Google Scholar]
- Liu M., Guo W., Cai Y., Yang H., Li W., Yang L., Lai X., Fang Q., Ma L., Zhu R., Zhang X. Personal exposure to fine particulate matter and renal function in children: a panel study. Environ. Pollut. 2020;266:115129. doi: 10.1016/j.envpol.2020.115129. [DOI] [PubMed] [Google Scholar]
- Liu M., Guo W., Yang H., Zhao L., Fang Q., Li M., Shu J., Jiang Y., Lai X., Yang L., Zhang X. Short-term effects of size-fractionated particulate matters and their constituents on renal function in children: a panel study. Ecotoxicol. Environ. Saf. 2021;209:111809. doi: 10.1016/j.ecoenv.2020.111809. [DOI] [PubMed] [Google Scholar]
- Lue S.H., Wellenius G.A., Wilker E.H., Mostofsky E., Mittleman M.A. Residential proximity to major roadways and renal function. J. Epidemiol. Community Health. 2013;67:629–634. doi: 10.1136/jech-2012-202307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo E. Blood urea nitrogen beyond estimation of renal function. Crit. Care Med. 2011;39:405–406. doi: 10.1097/CCM.0b013e318205c33a. [DOI] [PubMed] [Google Scholar]
- Mao X., Hu X., Wang Y., Xia W., Zhao S., Wan Y. Temporal trend of arsenic in outdoor air PM2.5 in Wuhan, China, in 2015-2017 and the personal inhalation of PM-bound arsenic: implications for human exposure. Environ. Sci. Pollut. Res. Int. 2020;27:21654–21665. doi: 10.1007/s11356-020-08626-2. [DOI] [PubMed] [Google Scholar]
- Masudo R., Yasukawa K., Nojiri T., Yoshikawa N., Shimosaka H., Sone S., Oike Y., Ugawa A., Yamazaki T., Shimokado K., Yatomi Y., Ikeda H. Evaluation of human nonmercaptalbumin as a marker for oxidative stress and its association with various parameters in blood. J. Clin. Biochem. Nutr. 2017;61:79–84. doi: 10.3164/jcbn.17-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsue Y., van der Meer P., Damman K., Metra M., O'Connor C.M., Ponikowski P., Teerlink J.R., Cotter G., Davison B., Cleland J.G., Givertz M.M., Bloomfield D.M., Dittrich H.C., Gansevoort R.T., Bakker S.J., van der Harst P., Hillege H.L., van Veldhuisen D.J., Voors A.A. Blood urea nitrogen-to-creatinine ratio in the general population and in patients with acute heart failure. Heart. 2017;103:407–413. doi: 10.1136/heartjnl-2016-310112. [DOI] [PubMed] [Google Scholar]
- Mehta A.J., Zanobetti A., Bind M.A., Kloog I., Koutrakis P., Sparrow D., Vokonas P.S., Schwartz J.D. Long-term exposure to ambient fine particulate matter and renal function in older men: the veterans administration normative aging study. Environ. Health Perspect. 2016;124:1353–1360. doi: 10.1289/ehp.1510269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Agrawal M. A global perspective of fine particulate matter pollution and its health effects. Rev. Environ. Contam. Toxicol. 2018;244:5–51. doi: 10.1007/398_2017_3. [DOI] [PubMed] [Google Scholar]
- Navas-Acien A., Tellez-Plaza M., Guallar E., Muntner P., Silbergeld E., Jaar B., Weaver V. Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am. J. Epidemiol. 2009;170:1156–1164. doi: 10.1093/aje/kwp248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled R. Air pollution exposure: who is at high risk? Atmos. Environ. 2011;45:1781–1785. [Google Scholar]
- Peng S., Sun J., Liu F., Li Z., Wu C., Xiang H. The effect of short-term fine particulate matter exposure on glucose homeostasis: a panel study in healthy adults. Atmos. Environ. 2022;268 [Google Scholar]
- Rahmani Sani A., Abroudi M., Heydari H., Adli A., Miri M., Mehrabadi S., Pajohanfar N.S., Raoufinia R., Bazghandi M.S., Ghalenovi M., Rad A., Miri M., Dadvand P. Maternal exposure to ambient particulate matter and green spaces and fetal renal function. Environ. Res. 2020;184:109285. doi: 10.1016/j.envres.2020.109285. [DOI] [PubMed] [Google Scholar]
- Ravindra K., Sokhi R., Vangrieken R. Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos. Environ. 2008;42:2895–2921. [Google Scholar]
- Rückerl R., Hampel R., Breitner S., Cyrys J., Kraus U., Carter J., Dailey L., Devlin R.B., Diaz-Sanchez D., Koenig W., Phipps R., Silbajoris R., Soentgen J., Soukup J., Peters A., Schneider A. Associations between ambient air pollution and blood markers of inflammation and coagulation/fibrinolysis in susceptible populations. Environ. Int. 2014;70:32–49. doi: 10.1016/j.envint.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Sanders A.P., Mazzella M.J., Malin A.J., Hair G.M., Busgang S.A., Saland J.M., Curtin P. Combined exposure to lead, cadmium, mercury, and arsenic and kidney health in adolescents age 12-19 in NHANES 2009-2014. Environ. Int. 2019;131:104993. doi: 10.1016/j.envint.2019.104993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smichowski P., Gómez D., Frazzoli C., Caroli S. Traffic‐related elements in airborne particulate matter. Appl. Spectrosc. Rev. 2007;43:23–49. [Google Scholar]
- Stenvinkel P., Shiels P.G., Painer J., Miranda J.J., Natterson-Horowitz B., Johnson R.J. A planetary health perspective for kidney disease. Kidney Int. 2020;98:261–265. doi: 10.1016/j.kint.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez-Álvarez B., Liapis H., Anders H.J. Links between coagulation, inflammation, regeneration, and fibrosis in kidney pathology. Lab. Invest. 2016;96:378–390. doi: 10.1038/labinvest.2015.164. [DOI] [PubMed] [Google Scholar]
- Tang H., Cheng Z., Li N., Mao S., Ma R., He H., Niu Z., Chen X., Xiang H. The short- and long-term associations of particulate matter with inflammation and blood coagulation markers: a meta-analysis. Environ. Pollut. 2020;267:115630. doi: 10.1016/j.envpol.2020.115630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavera Busso I., Mateos A.C., Juncos L.I., Canals N., Carreras H.A. Kidney damage induced by sub-chronic fine particulate matter exposure. Environ. Int. 2018;121:635–642. doi: 10.1016/j.envint.2018.10.007. [DOI] [PubMed] [Google Scholar]
- Trzeciakowski J.P., Gardiner L., Parrish A.R. Effects of environmental levels of cadmium, lead and mercury on human renal function evaluated by structural equation modeling. Toxicol. Lett. 2014;228:34–41. doi: 10.1016/j.toxlet.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H.J., Hung C.H., Wang C.W., Tu H.P., Li C.H., Tsai C.C., Lin W.Y., Chen S.C., Kuo C.H. Associations among heavy metals and proteinuria and chronic kidney disease. Diagnostics. 2021;11 doi: 10.3390/diagnostics11020282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay A., Larson M.G., Guo C.Y., Vasan R.S., Lipinska I., O'Donnell C.J., Kathiresan S., Meigs J.B., Keaney J.F., Jr., Rong J., Benjamin E.J., Fox C.S. Inflammation, kidney function and albuminuria in the Framingham Offspring cohort. Nephrol. Dial. Transplant. 2011;26:920–926. doi: 10.1093/ndt/gfq471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L., Vanderweele T.J. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol. Methods. 2013;18:137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veldhuisen D.J., Ruilope L.M., Maisel A.S., Damman K. Biomarkers of renal injury and function: diagnostic, prognostic and therapeutic implications in heart failure. Eur. Heart J. 2016;37:2577–2585. doi: 10.1093/eurheartj/ehv588. [DOI] [PubMed] [Google Scholar]
- Wei Y., Han I.K., Shao M., Hu M., Zhang O.J., Tang X. PM2.5 constituents and oxidative DNA damage in humans. Environ. Sci. Technol. 2009;43:4757–4762. doi: 10.1021/es803337c. [DOI] [PubMed] [Google Scholar]
- Winter M.A., Guhr K.N., Berg G.M. Impact of various body weights and serum creatinine concentrations on the bias and accuracy of the Cockcroft-Gault equation. Pharmacotherapy. 2012;32:604–612. doi: 10.1002/j.1875-9114.2012.01098.x. [DOI] [PubMed] [Google Scholar]
- Wu M.Y., Lo W.C., Chao C.T., Wu M.S., Chiang C.K. Association between air pollutants and development of chronic kidney disease: a systematic review and meta-analysis. Sci. Total Environ. 2020;706:135522. doi: 10.1016/j.scitotenv.2019.135522. [DOI] [PubMed] [Google Scholar]
- Wu S., Deng F., Niu J., Huang Q., Liu Y., Guo X. Exposures to PM₂.₅ components and heart rate variability in taxi drivers around the Beijing 2008 Olympic Games. Sci. Total Environ. 2011;409:2478–2485. doi: 10.1016/j.scitotenv.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Wu S., Wang B., Yang D., Wei H., Li H., Pan L., Huang J., Wang X., Qin Y., Zheng C., Shima M., Deng F., Guo X. Ambient particulate air pollution and circulating antioxidant enzymes: a repeated-measure study in healthy adults in Beijing, China. Environ. Pollut. 2016;208:16–24. doi: 10.1016/j.envpol.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Xie W., You J., Zhi C., Li L. The toxicity of ambient fine particulate matter (PM2.5) to vascular endothelial cells. J. Appl. Toxicol. 2021;41:713–723. doi: 10.1002/jat.4138. [DOI] [PubMed] [Google Scholar]
- Xu X., Nie S., Ding H., Hou F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018;14:313–324. doi: 10.1038/nrneph.2018.11. [DOI] [PubMed] [Google Scholar]
- Yilmaz M.I., Saglam M., Caglar K., Cakir E., Sonmez A., Ozgurtas T., Aydin A., Eyileten T., Ozcan O., Acikel C., Tasar M., Genctoy G., Erbil K., Vural A., Zoccali C. The determinants of endothelial dysfunction in CKD: oxidative stress and asymmetric dimethylarginine. Am. J. Kidney Dis. 2006;47:42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- Zhang L., Wang F., Wang L., Wang W., Liu B., Liu J., Chen M., He Q., Liao Y., Yu X., Chen N., Zhang J.E., Hu Z., Liu F., Hong D., Ma L., Liu H., Zhou X., Chen J., Pan L., Chen W., Wang W., Li X., Wang H. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Cai J., Zhu X., van Donkelaar A., Martin R.V., Hua J., Kan H. Fine particulate matter exposure and renal function: a population-based study among pregnant women in China. Environ. Int. 2020;141:105805. doi: 10.1016/j.envint.2020.105805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.