Abstract
Background
Expectant management (EM) has been widely recommended for men with low-risk prostate cancers (PCa). We evaluated trends in EM and the sociodemographic and clinical factors associated with EM, initiating a National Comprehensive Cancer Network guideline-concordant active surveillance (AS) monitoring protocol, and switching from EM to active treatment (AT).
Methods
We used the SEER-Medicare database to identify men ages 66+ diagnosed with a low-risk PCa (PSA < 10 ng/mL, Gleason ≤ 6, stage ≤ T2a) in 2010–2013 with ≥1 year of follow-up. We used claims data to capture (1) PCa treatments, including surgical procedures, radiotherapy, and hormone therapy, and (2) AS monitoring procedures, including PSA tests and prostate biopsy. We defined EM as receiving no AT within 1 year of diagnosis. We used multivariable regression techniques to identify factors associated with EM, initiating AS monitoring, and switching to AT.
Results
During the study period, EM increased from 29.4% to 49.0%, p < 0.01. Age < 77, being married/partnered, non-Hispanic ethnicity, higher median ZIP code income, lower PSA levels, stage T1c, and more recent year of diagnosis were associated with EM. Nearly 39% of the EM cohort initiated AS monitoring; age <77, White race, being married/partnered, higher median ZIP code income, and lower PSA levels were associated with initiating AS. By three years after diagnosis, 21.3% of the EM cohort had switched to AT, usually after undergoing AS monitoring procedures.
Discussion
We found increasing uptake of EM over time, though over 50% still received AT. About 60% of EM patients did not initiate AS monitoring, even among those with life expectancy >10 years, implying that a substantial proportion was being managed by watchful waiting. AS monitoring was associated with switching to AT, suggesting that treatment decisions likely were based on cancer progression.
Introduction
The widespread use of PSA testing since the late 1980s raised concerns that a substantial number of men were being overtreated for indolent prostate cancers [1]. Consequently, active surveillance has emerged in the United States and Europe as a widely recommended initial therapy for healthy men with a low-risk prostate cancer and the preferred treatment for healthy men with a very low-risk cancer [2, 3]. By selecting active surveillance, men may reduce the like-lihood of undergoing unnecessary treatment without increasing their risk for prostate cancer mortality [4–7]. Watchful waiting, which offers only palliative treatment for symptoms of clinically progressive cancer, is recommended for men with a life expectancy less than 5–10 years, and typically involves less intensive surveillance testing [2, 3]. Numerous studies have shown that increasing proportions of men with a low-risk prostate cancer are initially being managed expectantly, i.e., not undergoing active treatment [8–15]. These studies, though, were unable to distinguish between active surveillance and watchful waiting because they lacked data on the monitoring procedures that define active surveillance.
Several studies of men who underwent initial surveillance monitoring suggest that longer-term adherence with recommended surveillance protocols has been poor. Loeb et al., using SEER-Medicare data from 2001 to 2009, reported that fewer than 13% of men on active surveillance underwent a biopsy beyond the first 2 years [16]. Luckenbaugh et al., using 2012–2013 data from a statewide registry in Michigan, found that only 30.6% of their active surveillance cohort underwent a guideline-concordant biopsy at a median follow-up of 2 years [17]. The European Prostate Cancer Research International Active Surveillance study, which followed men from 2006 through 2015, found substantially decreasing compliance with biopsies over time [18]. These findings are concerning because unmonitored men may have undiagnosed higher-grade prostate cancer or may progress to advanced-stage disease that is less likely to respond to treatment.
We used a cancer database of men over age 66 years with low-risk prostate cancer in combination with claims data to (1) quantify trends in the use of expectant management (EM), defined as not undergoing active treatment within 1 year of diagnosis, (2) to describe uptake of active surveillance monitoring procedures and switches to active treatment among the EM cohort, and (3) to determine sociodemographic and clinical factors associated with EM, initiating active surveillance monitoring concordant with the National Comprehensive Cancer Network (NCCN) guideline [2], and switching to active treatment.
Patients and methods
Data sources
We used the linked Surveillance, Epidemiology, and End Results (SEER)-Medicare database for the years 2010, when SEER first provided biopsy core data to define very low-risk cancers, through 2014 for our analyses. The SEER program is a population-based registry that collects cancer incidence and survival data in 19 US geographic areas representing about 35% of the US population [19]. SEER data include patient demographics, tumor characteristics, initial course of treatment, and vital status.
Study population
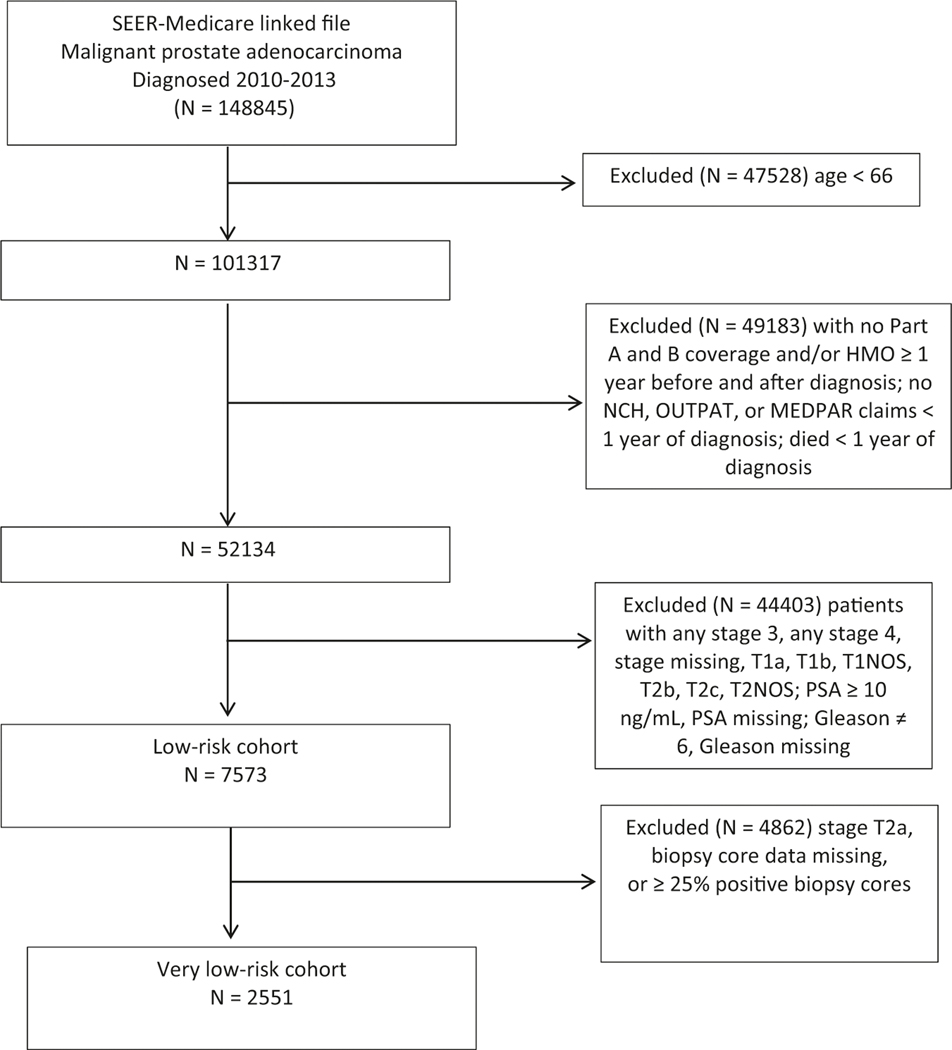
We used the SEER-Medicare Patient Entitlement and Diagnosis Summary File (PEDSF) file to identify men ages 66 and older who were diagnosed with malignant prostate adenocarcinoma (SEER ICD-O-3/WHO site recode of prostate and ICD-O-3 histology code 8140) between January 1, 2010, and December 31, 2013, with follow-up through December 31, 2014 (Fig. 1). Guidelines during this study period supported active surveillance as an option for men with a low-risk prostate cancer [20, 21]. We excluded men who did not survive at least 1 year after diagnosis, those who did not have at least 1 year of continuous Medicare Part A and Part B insurance without HMO coverage before and after diagnosis, and those who had no professional, hospital, or outpatient Medicare claims in the year following diagnosis.
Fig. 1.
Subject flow sheet, SEER-Medicare cohorts of men with low-risk and very low-risk prostate cancers, 2010–2013.
Risk groups
We used SEER collaborative stage variables and American Joint Committee on Cancer (AJCC) 7th edition T-stage data on disease characteristics (PSA, Gleason grade, and clinical stage) [22]. We defined low-risk prostate cancer as PSA < 10 ng/mL, Gleason = 6, and stage T1c or T2a. We defined very low risk as PSA < 10 ng/mL, Gleason = 6, stage T1c, and <25% positive biopsy cores. SEER staging is based on the best available data, which may include pathological staging data obtained following surgery.
Study variables
We used patient-level variables from the PEDSF file to determine sociodemographic characteristics, including age, race, ethnicity, marital status, and ZIP code level census data on population median income. We extracted data from the Medicare claims files (Medicare Provider and Analysis Review, Carrier Claims, and Outpatient facility claims) and used the NCI Combined Index to calculate a comorbidity score [23–25]. We used SEER data, ICD-9-CM codes, and HCPCS (Healthcare Common Procedure Coding System) codes to identify treatments and surveillance procedures (Supplementary Table 1). Prostate cancer treatments included radical prostatectomy, radiotherapy (external beam radiotherapy or brachytherapy), cryotherapy, and androgen deprivation therapy. We defined EM as no prostate cancer treatment for the first year following diagnosis. Among men initially managed expectantly, we determined whether they underwent surveillance monitoring, including PSA tests, prostate biopsies, and pelvic MRI imaging. In tracking surveillance monitoring, we avoided misclassifying diagnostic procedures as surveillance procedures by counting only those that took place at least 3 months after diagnosis and before starting treatment (among men who subsequently switched to active treatment). PSA tests and biopsies had to be separated by 30 days and MRIs had to be separated by 180 days to be considered a new procedure.
Statistical analyses
Cochran-Armitage tests were used to assess temporal trends in the proportion of patients receiving each management modality during the first year following diagnosis. The primary management modality was defined as the most aggressive treatment approach based on the following hierarchy from most to least aggressive: surgery, radiation, cryotherapy, hormone therapy, or no treatment. Because cryotherapy and hormone therapy were infrequently used as the primary management modality, we combined them under the category of “other” in the figures. Logistic regression models were applied to determine the effect of baseline sociodemographic and clinicopathologic variables on receiving no active treatment for at least 12 months post-diagnosis. For all multivariable analyses, we stratified age at 77, when actuarial tables estimate that life expectancy drops below 10 years and when guidelines preferentially recommend observation over active surveillance [2, 26].
Among patients who were managed expectantly, we estimated the cumulative incidences of surveillance procedure utilization and subsequent treatment using Fine and Gray’s method. In addition, Cox regression models were used to estimate the association of baseline sociodemographic and clinicopathologic variables on time to initiating NCCN-concordant active surveillance monitoring, which was operationally defined as ≥1 biopsy and ≥2 PSA tests within 2 years of diagnosis [2]. Time was calculated from the end of the 12-month no treatment period to the time by which the active surveillance monitoring criteria were met; treatment initiation before meeting the criteria and death were considered competing risks. Patients who did not meet the monitoring criteria were censored at change in insurance coverage or the end of the study period, whichever occurred first.
Cox regression models were also used to estimate the effect of baseline sociodemographic and clinicopathologic variables as well as receipt of surveillance monitoring procedures on time to initiating active treatment (switching from EM). We used time-dependent covariates for surveillance monitoring procedures (PSA, biopsy or MRI) occurring after the initial 12-month period of no treatment following diagnosis (deferment period). Time was calculated from the end of the deferment period to treatment initiation; death was considered a competing risk and patients who did not receive treatment were censored at change in insurance coverage or the end of the study period, whichever occurred first.
To account for the possible dependency between patients from each SEER registry, we used generalized estimating equations with a compound symmetry structure and a robust sandwich variance estimate for the logistic and Cox regression models, respectively. Estimated effects of predictors are reported as odds ratios or hazard ratios (HR) along with 95% confidence intervals (CIs). All tests were two-sided and assessed for significance at the 5% level using SAS v9.4 (SAS Institute, Cary, NC).
Results
We identified 7573 men from the SEER-Medicare database who were diagnosed with a low-risk prostate cancer in the years 2010–2013 and a subset of 2551 men with a very low-risk cancer. Table 1 shows baseline sociodemographic and clinical characteristics for the cohort of men with a low-risk prostate cancer, overall and stratified by whether they underwent any active treatment during the first year following diagnosis. The median age was 71 years, 87.3% were White, 5.7% were Hispanic, 80.3% were married/ partnered, about a third lived in ZIP code areas with a median income > $60000, and 58.7% had a comorbidity score of 0.
Table 1.
Patient characteristics, SEER-Medicare cohort diagnosed with low-risk prostate cancer 2010–2013, overall and by receipt of treatment within one year following diagnosis.
| Variable | Level | Overall | Active treatment during 1st year |
|
|---|---|---|---|---|
| N= 7573 | No (N= 2796) | Yes (N= 4777) | ||
| Age | <77 77+ |
6404 (84.6) 1169 (15.4) |
2262 (80.9) 534 (19.1) |
4142 (86.7) 635 (13.3) |
| Race | Black Other White Missing |
727 (9.8) 212 (2.9) 6446 (87.3) 188 |
237 (8.8) 88 (3.3) 2371 (87.9) 100 |
490 (10.4) 124 (2.6) 4075 (86.9) 88 |
| Ethnicity | Hispanic Non-Hispanic | 432 (5.7) 7141 (94.3) |
113 (4.0) 2683 (96.0) |
319 (6.7) 4458 (93.3) |
| Married/partner | No Yes Missing |
1276 (19.7) 5188 (80.3) 1109 |
489 (21.2) 1818 (78.8) 489 |
787 (18.9) 3370 (81.1) 620 |
| Income (ZIP code median) | <$40,000 $40–60,000 >$60,000 Missing |
2389 (33.5) 2458 (34.5) 2285 (32.0) 441 |
724 (27.6) 931 (35.5) 970 (37.0) 171 |
1665 (36.9) 1527 (33.9) 1315 (29.2) 270 |
| Charlson comorbidity index | 0 1 2+ Missing |
4396 (58.7) 1751 (23.4) 1340 (17.9) 86 |
1673 (60.9) 628 (22.9) 444 (16.2) 51 |
2723 (57.4) 1123 (23.7) 896 (18.9) 35 |
| PSA (ng/mL) | <4 4–9.9 |
1104 (14.6) 6469 (85.4) |
460 (16.5) 2336 (83.5) |
644 (13.5) 4133 (86.5) |
| Derived AJCC 7th Edition stage | T1c T2a |
6711 (88.6) 862 (11.4) |
2595 (92.8) 201 (7.2) |
4116 (86.2) 661 (13.8) |
| Year of diagnosis | 2010 2011 2012 2013 |
2265 (29.9) 2261 (29.9) 1620 (21.4) 1427 (18.8) |
666 (23.8) 786 (28.1) 645 (23.1) 699 (25.0) |
1599 (33.5) 1475 (30.9) 975 (20.4) 728 (15.2) |
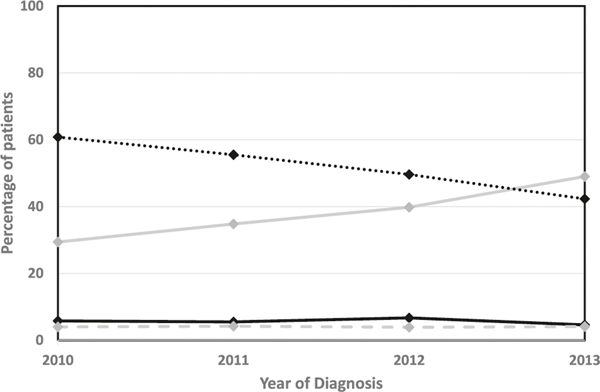
Figure 2 shows the treatment patterns for the most aggressive treatment received in the year following diagnosis of a low-risk prostate cancer in the years 2010–2013. The proportion initially selecting EM (not receiving active treatment for at least one year following diagnosis) increased over time from 29.4% to 49.0%, p < 0.01. The proportion undergoing radiation therapy decreased from 60.8% to 42.3%, p < 0.01, while the proportions undergoing surgery, cryotherapy, or hormone therapy remained consistently low. Among the cohort of men with very low-risk cancers, the proportion initially selecting EM increased from 36.4% to 60.1%, p < 0.01 (Supplementary Fig. 1).
Fig. 2.
Treatment patterns over time for men with a low-risk prostate cancer. No treatment  Radiation
Radiation  Surgery
Surgery  Other
Other  .
.
We evaluated factors associated with EM (Table 2).Multivariable analysis identified not being married/partnered, being of non-Hispanic ethnicity, having a ZIP code in the highest two tertiles of median income compared to the lowest tertile, lower stage cancer (T1c vs. T2a), lower PSA levels (<4 ng/mL vs 4–9.9 ng/mL), and a more recent year of diagnosis (2011–2013 vs. 2010) as significantly associated with EM.
Table 2.
Univariable and multivariable models evaluating expectant management for low-risk prostate cancer, n= 6001 (multivariable).
| Variable | Level | Univariable odds ratio (95% CI) |
p value | Multivariable odds ratio (95% CI) |
p value |
|---|---|---|---|---|---|
| Age | <77 77+ |
Reference 1.53 (1.30, 1.80) |
<0.01 | Reference 1.32 (1.19, 1.45) |
<0.01 |
| Race | White Black Other |
Reference 0.86 (0.66, 1.12) 1.07 (0.91, 1.25) |
0.46 | - | - |
| Ethnicity | Non-Hispanic Hispanic |
Reference 0.51 (0.40, 0.66) |
<0.01 | Reference 0.76 (0.67, 0.86) |
<0.01 |
| Married/partner | Yes No |
Reference 1.13 (0.99, 1.30) |
0.07 | Reference 1.09 (1.01, 1.16) |
0.02 |
| Income (ZIP code median) | <$40,000 $40–60,000 >$60,000 |
Reference 1.42 (1.24, 1.61) 1.79 (1.50, 2.12) |
<0.01 | Reference 1.26 (1.13, 1.40) 1.37 (1.20, 1.57) |
<0.01 |
| Comorbidity | 2+ 0 1 |
Reference 1.18 (1.05, 1.33) 1.10 (1.00, 1.21) |
0.02 | Reference 1.08 (1.01, 1.16) 1.08 (0.99, 1.16) |
0.06 |
| PSA (ng/mL) | 4–9.9 <4 |
Reference 1.27 (1.10, 1.48) |
<0.01 | Reference 1.25 (1.11, 1.41) |
<0.01 |
| Derived AJCC stage | T2a T1c |
Reference 2.19 (1.91, 2.53) |
<0.01 | Reference 1.77 (1.60, 1.96) |
<0.01 |
| Year of diagnosis | 2010 2011 2012 2013 |
Reference 1.28 (1.15, 1.42) 1.61 (1.49, 1.72) 2.33 (1.98, 2.74) |
<0.01 | Reference 1.15 (1.05, 1.27) 1.39 (1.30, 1.49) 1.61 (1.43, 1.82) |
<0.01 |
Among men being managed expectantly, the cumulative incidences for undergoing PSA testing, prostate biopsy, and pelvic MRI at 2 years after diagnosis were 91.8% (95% CI, 90.7–92.8%), 41.0% (95% CI, 39.1–42.9%), and 10.2% (95% CI, 9.0–11.3%), respectively. The cumulative incidence of men managed expectantly who initiated NCCN-concordant active surveillance monitoring, defined as undergoing ≥ one prostate biopsy and ≥ two PSA tests within 2 years of diagnosis, was 38.6% (95% CI, 36.7–40.4%). Men younger than 77 were more likely than older men to initiate active surveillance, 42.4% vs. 23.0%, p < 0.01. On multivariable analysis, the factors associated with initiating active surveillance monitoring were White race (compared to Black race), being younger than 77, being married/partnered, having a ZIP code in the highest two tertiles of median income compared to the lowest tertile, and having a PSA < 4 ng/mL vs 4–9.9 ng/mL (Table 3). We also found that 5.6% (95% CI, 4.7–6.5%) of the EM cohort underwent PSA testing and at least one MRI without repeating a prostate biopsy.
Table 3.
Univariable and multivariable models evaluating initial adherence with guideline-concordant active surveillance monitoring protocola n= 2067 (multivariable).
| Variable | Level | Univariable hazard ratio (95% CI) |
p value | Multivariable hazard ratio (95% CI) |
p value |
|---|---|---|---|---|---|
| Age | <77 77+ |
Reference 0.48 (0.42, 0.55) |
<0.01 | Reference 0.45 (0.39, 0.52) |
<0.01 |
| Race | White Black Other |
Reference 0.50 (0.36, 0.70) 0.81 (0.62, 1.06) |
<0.01 | Reference 0.58 (0.38, 0.87) 0.79 (0.59, 1.06) |
<0.01 |
| Ethnicity | Non-Hispanic Hispanic |
Reference 0.80 (0.50, 1.27) |
0.34 | - | - |
| Married/partner | Yes No |
Reference 0.69 (0.54, 0.88) |
<0.01 | Reference 0.75 (0.59, 0.96) |
0.02 |
| Income (ZIP code median) | <$40,000 $40–60,000 >$60,000 |
Reference 1.41 (1.15, 1.74) 1.97 (1.68, 2.31) |
<0.01 | Reference 1.47 (1.15, 1.86) 2.04 (1.71, 2.44) |
<0.01 |
| Comorbidity | 2+ 0 1 |
Reference 1.44 (1.04, 2.00) 1.33 (1.03, 1.72) |
0.08 | Reference 1.18 (0.87, 1.59) 1.26 (0.99, 1.62) |
0.08 |
| PSA (ng/mL) | 4–9.9 <4 |
Reference 1.21 (1.13, 1.29) |
<0.01 | Reference 1.11 (1.03, 1.20) |
<0.01 |
| Derived AJCC T stage | T2a T1c |
Reference 0.99 (0.63, 1.55) |
0.97 | - | - |
| Year of diagnosis | 2010 2011 2012 2013 |
Reference 0.94 (0.75, 1.17) 1.01 (0.87, 1.18) 1.14 (1.04, 1.24) |
0.03 | Reference 0.98 (0.81, 1.17) 0.98 (0.85, 1.13) 1.12 (1.00, 1.26) |
0.22 |
≥ one biopsy and ≥ two PSA tests within 24 months of diagnosis.
The cohort of men with a low-risk prostate cancer who were initially managed expectantly had a median 34 months of follow-up time. The cumulative incidence of switching to active treatment by 3 years following diagnosis was 21.3% (95% CI, 19.6–23.1%). The most frequent primary treatment modality was radiation therapy (17.1%, 95% CI 15.3–18.9%) followed by surgery (6.1%, 95% CI 5.1–7.3%) (Supplementary Fig. 2). In the multivariable analysis, the factors associated with switching were non-Hispanic ethnicity vs. Hispanic ethnicity (HR = 1.67, 95% CI, 1.25–2.22), initial PSA of <4.0 ng/mL vs. 4.0–9.9 ng/ mL (HR = 1.64, 95% CI, 1.28–2.05), and undergoing any surveillance monitoring procedure, particularly prostate biopsy (HR = 5.85, 95% CI, 4.45–7.50).
Discussion
Using SEER-Medicare linked data for prostate cancer diagnoses from 2010 through 2013, we found that the proportion of men with a low-risk prostate cancer who were initially managed expectantly (no active treatment within the first 12 months after diagnosis) significantly increased from 29.4% to 49.0%, p < 0.01. The proportion was even higher for men with a very low-risk prostate cancer, reaching 60.1% by 2013. Within 2 years of diagnosis, 38.6% in the EM cohort, including 42.4% of those <77, initiated NCCN-concordant active surveillance monitoring. Among men who deferred active treatment for 1 year, 21.3% subsequently received active treatment by 3 years following diagnosis; undergoing surveillance monitoring was the strongest factor associated with switching to active treatment.
Professional guidelines have been recommending EM, either active surveillance for healthy men or watchful waiting for men with a limited life expectancy, as a primary management option for men with a low-risk prostate cancer since 2007 [21]. Our finding that increasing proportions of men with a low-risk prostate cancer did not receive initial active treatment demonstrates a changing approach to management over time and expands on the results of previous studies by providing more recent SEER-Medicare management data, including for men with very low-risk prostate cancers [8–15]. We found that older age, not being married/partnered, living in a ZIP code area with higher median income, non-Hispanic ethnicity, more recent year of diagnosis, and having less aggressive disease characteristics were associated with being expectantly managed. Other studies have identified similar predictors of EM, particularly disease characteristics, as well as lower comorbidity and family history [10–13, 27].
Among men diagnosed with a low-risk prostate cancer, we found significantly decreased use of radiotherapy and consistently low levels of cryotherapy and primary ADT, which has been reported by others [8, 13–15, 28]. We also found consistent use of radical prostatectomy, but at a much lower level (<10%) than the 31–46% reported by studies using SEER data [12, 14, 29] and the ~50% reported by studies using the National Cancer Database (NCDB) [11, 14]. One explanation for this difference is that the SEER-Medicare cohort skews toward older men who are less likely to undergo surgery [30].
By using Medicare data, we were able to determine that 38.6% of men managed expectantly initiated monitoring concordant with the NCCN active surveillance guideline. We are unaware of any recent national-level reports similarly characterizing active surveillance uptake. The NCDB and SEER datasets have a treatment indicator variable for EM, but the variable does not distinguish active surveillance from watchful waiting. We found that being younger than 77, White race (compared to Black race), being married, living in a census tract with higher median income, and a lower PSA level, were associated with initiating active surveillance monitoring. The age stratification finding is notable because it corresponds to a 10-year actuarial life expectancy and aligns with guidelines considering active surveillance to be less appropriate for men with a shorter life expectancy. SEER-Medicare analyses of men with a localized prostate cancer diagnosed in the 2000s found younger age, higher socioeconomic status, lower comorbidity, and favorable disease characteristics associated with surveillance monitoring [16, 31].
Studies using the SEER Watchful Waiting database have found no racial differences in EM after adjusting for socioeconomic status [32–34]. However, the decreased initiation of surveillance monitoring among Black men, only 23.8% among those <77, is potentially concerning because observational data suggest that Black men are more likely than White men to be reclassified through surveillance biopsies with higher-grade disease [35]. Studies also suggest that Black men eligible for active surveillance who undergo initial prostatectomy are more likely than White men to have adverse pathological outcomes, including positive surgical margins or upstaging [36, 37]. A VA study did find that Black men on active surveillance were not at increased risk for metastatic disease or prostate cancer mortality [38]. However, all men underwent at least one surveillance biopsy. Lower adherence with surveillance monitoring protocols, particularly undergoing biopises, could lead to poorer clinical outcomes, heightening existing racial disparities for mortality [39].
We found that 5.6% of the EM cohort underwent repeat PSA testing and at least one MRI without repeating a prostate biopsy. Although some urologists may consider using MRI as a substitute for repeat biopsy for men on active surveillance, that practice is controversial and may not be strongly supported by evidence [40]. Professional society guidelines continue recommending a surveillance biopsy that can be augmented but not replaced by MRI [2, 41].
Overall, 21.3% of the cohort who did not undergo active treatment for 1 year following diagnosis switched to active treatment by the end of the subsequent two-year period. Most of these men underwent radiotherapy as their primary treatment modality. We found that undergoing surveillance testing was most strongly associated with switching. Test results are not available in the SEER-Medicare data, but the finding is consistent with data suggesting that the majority of men switching from AS do so because there is evidence of disease progression [42].
Our study had some inherent limitations. We were able to determine whether patients received active treatment during the first year following diagnosis, but we do not know whether the initial intent of the patient and physician was to actually manage the cancer expectantly, particularly with active surveillance. In 2010, SEER introduced a treatment variable documenting an initial intention for EM, but it was not available in the SEER-Medicare dataset that we received. However, the SEER Watchful Waiting Database does not distinguish between active surveillance and watchful waiting [34, 43]. While linking with Medicare provides data not captured by the population-based SEER registry, including comorbidity, receipt of primary androgen deprivation, surveillance procedures, and treatments occurring more than 1 year following diagnosis, the results are not necessarily generalizable to those younger than 66 years of age where management patterns may differ. Furthermore, the analyzed SEER-Medicare cohort is not population-based given the various eligibility requirements and our exclusion of men with incomplete staging information. With a median follow-up duration of 34 months, we were not able to assess long-term continuation of active surveillance monitoring or evaluate clinical outcomes.
We found increasing uptake of EM among men with a low-risk prostate cancer over time. However, by 2013 over 50% of patients were still receiving active treatment within the first year of diagnosis. Within 2 years of diagnosis, just over 40% of those age <77 managed expectantly met initial criteria for undergoing guideline-concordant active surveillance monitoring. Men who do not monitor their cancers could potentially have undiagnosed higher grade cancers that might progress to advanced stages that are more difficult to treat. Surveillance procedures, particularly biopsy, were associated with switching to active treatment suggesting that treatment decisions were likely based on cancer progression.
Data availability
The data that support the findings of this study are available from the National Cancer Institute, but restrictions apply to the availability of these data, which are under license for the study, and so are not publicly available.
Supplementary Material
Acknowledgements
This work was supported by the University of Iowa Holden Comprehensive Cancer Center Population Research and Biostatistics Cores and the National Cancer Institute (P30 CA086862; R50 CA243692). This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database. The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement # U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare no competing interests.
Ethical approval The research project was approved by the University of Iowa Human Subjects Review Board, IRB number 01. The study was performed in accordance with the Declaration of Helsinki.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41391-021-00393-6.
References
- 1.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. Prostate cancer. NCCNGuidelines Version 2.2021. 2021. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 3.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ ASTRO/SUO guideline. part i: risk stratification, shared decision making, and care options. J Urol. 2018;199:683–90. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L, Vesprini D, Sethukavalan P, Jethava V, Zhang L, Jain S,et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272–7. [DOI] [PubMed] [Google Scholar]
- 5.Tosoian JJ, Trock BJ, Landis P, Feng Z, Epstein JI, Partin AW,et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–90. [DOI] [PubMed] [Google Scholar]
- 6.Bul M, Zhu X, Valdagni R, Pickles T, Kakehi Y, Rannikko A,et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63:597–603. [DOI] [PubMed] [Google Scholar]
- 7.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375:1415–24. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA. 2015;314:80–2. [DOI] [PubMed] [Google Scholar]
- 9.Eggener SE, Mueller A, Berglund RK, Ayyathurai R, Soloway C, Soloway MS, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2013;189:S19–25. [DOI] [PubMed] [Google Scholar]
- 10.Loppenberg B, Friedlander DF, Krasnova A, Tam A, Leow JJ,Nguyen PL, et al. Variation in the use of active surveillance for low-risk prostate cancer. Cancer Cytopathol. 2018;124:55–64. [DOI] [PubMed] [Google Scholar]
- 11.Maurice MJ, Abouassaly R, Kim SP, Zhu H. Contemporary nationwide patterns of active surveillance use for prostate cancer. JAMA Intern Med. 2015;175:1569–71. [DOI] [PubMed] [Google Scholar]
- 12.Moschini M, Fossati N, Sood A, Lee JK, Sammon J, Sun M, et al. Contemporary management of prostate cancer patients suitable for active surveillance: a North American population-based study. Eur Urol Focus. 2018;4:68–74. [DOI] [PubMed] [Google Scholar]
- 13.Ritch CR, Graves AJ, Keegan KA, Ni S, Bassett JC, Chang SS,et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol. 2015;193:801–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner AB, Patel SG, Etzioni R, Eggener SE. National trends in the management of low and intermediate risk prostate cancer in the United States. J Urol 2015;193:95–102. [DOI] [PubMed] [Google Scholar]
- 15.Womble PR, Montie JE, Ye Z, Linsell SM, Lane BR, Miller DC, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol.2015;67:44–50. [DOI] [PubMed] [Google Scholar]
- 16.Loeb S, Walter D, Curnyn C, Gold HT, Lepor H, Makarov DV.How active is active surveillance? Intensity of followup during active surveillance for prostate cancer in the United States. J Urol. 2016;196:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luckenbaugh AN, Auffenberg GB, Hawken SR, Dhir A, Linsell S, Kaul S, et al. Variation in guideline concordant active surveillance followup in diverse urology practices. J Urol. 2017;197:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bokhorst LP, Alberts AR, Rannikko A, Valdagni R, Pickles T, Kakehi Y, et al. Compliance rates with the prostate cancer research international active surveillance (PRIAS) protocol and disease reclassification in noncompliers. Eur Urol. 2015;68:814–21. [DOI] [PubMed] [Google Scholar]
- 19.National Cancer Institute. Surveillance, epidemiology, and end results (SEER). U.S. Department of Health & Human Services; 2018. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf. [Google Scholar]
- 20.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Cancer Netw 2010;8:145. [DOI] [PubMed] [Google Scholar]
- 21.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–31. [DOI] [PubMed] [Google Scholar]
- 22.American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. Chicago, IL: Springer; 2017. [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 24.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 25.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. [DOI] [PubMed] [Google Scholar]
- 26.Social Security Administration. Actuarial life tables. 2021. https://www.ssa.gov/oact/STATS/table4c6.html.
- 27.Hoffman RM, Lobo T, Van Den Eeden SK, Davis KM, Luta G, Leimpeter AD, et al. Selecting active surveillance: decision making factors for men with a low-risk prostate cancer. Med Decis Mak. 2019;39:962–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loppenberg B, Sood A, Dalela D, Karabon P, Sammon JD, Vetterlein MW, et al. Variation in locoregional prostate cancer care and treatment trends at commission on cancer designated facilities: a national cancer data base analysis 2004 to 2013. Clin Genitourin Cancer. 2017;15:e955–68. [DOI] [PubMed] [Google Scholar]
- 29.Mahal BA, Butler S, Franco I, Spratt DE, Rebbeck TR, D’Amico AV, et al. Use of active surveillance or watchful waiting for low-risk prostate cancer and management trends across risk groups in the United States, 2010–2015. JAMA. 2019;321:704–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burt LM, Shrieve DC, Tward JD. Factors influencing prostate cancer patterns of care: an analysis of treatment variation using the SEER database. Adv Radiat Oncol. 2018;3:170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filson CP, Marks LS, Litwin MS. Expectant management for men with early stage prostate cancer. CA Cancer J Clin. 2015;65:265–82. [DOI] [PubMed] [Google Scholar]
- 32.Al Hussein Al Awamlh B, Ma X, Christos P, Hu JC, Shoag JE Active surveillance for black men with low-risk prostate cancer in the United States. N Engl J Med. 2019;381:2581–2. [DOI] [PubMed] [Google Scholar]
- 33.Butler S, Muralidhar V, Chavez J, Fullerton Z, Mahal A, Nezolosky M, et al. Active surveillance for low-risk prostate cancer in black patients. N Engl J Med. 2019;380:2070–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Washington SL 3rd, Jeong CW, Lonergan PE, Herlemann A, Gomez SL, Carroll PR, et al. Regional variation in active surveillance for low-risk prostate cancer in the US. JAMA Netw Open. 2020;3:e2031349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundi D, Faisal FA, Trock BJ, Landis PK, Feng Z, Ross AE, et al. Reclassification rates are higher among African American men than Caucasians on active surveillance. Urology. 2015;85:155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gokce MI, Sundi D, Schaeffer E, Pettaway C. Is active surveillance a suitable option for African American men with prostate cancer? A systemic literature review. Prostate Cancer Prostatic Dis. 2017;20:127–36. [DOI] [PubMed] [Google Scholar]
- 37.Deka R, Parsons JK, Simpson DR, Riviere P, Nalawade V, Vitzthum LK, et al. African-American men with low-risk prostate cancer treated with radical prostatectomy in an equal-access health care system: implications for active surveillance. Prostate Cancer Prostatic Dis. 2020;23:581–8. [DOI] [PubMed] [Google Scholar]
- 38.Deka R, Courtney PT, Parsons JK, Nelson TJ, Nalawade V, Luterstein E, et al. Association between African American race and clinical outcomes in men treated for low-risk prostate cancer with active surveillance. JAMA. 2020;324:1747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mahal BA, Chen YW, Muralidhar V, Mahal AR, Choueiri TK, Hoffman KE, et al. Racial disparities in prostate cancer outcome among prostate-specific antigen screening eligible populations in the United States. Ann Oncol. 2017;28:1098–104. [DOI] [PubMed] [Google Scholar]
- 40.Distler FA, Radtke JP, Bonekamp D, Kesch C, Schlemmer HP, Wieczorek K, et al. The value of PSA density in combination with PI-RADS for the accuracy of prostate cancer prediction. J Urol. 2017;198:575–82. [DOI] [PubMed] [Google Scholar]
- 41.Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, et al. Clinically localized prostate cancer: AUA/ ASTRO/SUO guideline. Part II: recommended approaches and details of specific care options. J Urol. 2018;199:990–7. [DOI] [PubMed] [Google Scholar]
- 42.Simpkin AJ, Tilling K, Martin RM, Lane JA, Hamdy FC, Holmberg L, et al. Systematic review and meta-analysis of factors determining change to radical treatment in active surveillance for localized prostate cancer. Eur Urol. 2015;67:993–1005. [DOI] [PubMed] [Google Scholar]
- 43.Jeong CW, Washington SL 3rd, Herlemann A, Gomez SL, CarrollPR, Cooperberg MR. The new surveillance, epidemiology, and end results prostate with watchful waiting database: opportunities and limitations. Eur Urol. 2020;78:335–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the National Cancer Institute, but restrictions apply to the availability of these data, which are under license for the study, and so are not publicly available.