Abstract
Escherichia coli biofilms on two polyethylene disks were implanted subcutaneously into rabbits receiving systemic gentamicin. Ultrasound was applied for 24 h to one disk. Both disks were removed, and viable bacteria were counted. Pulsed ultrasound significantly reduced bacterial viability below that of nontreated biofilms without damage to the skin.
Successful treatments of infections on implanted medical devices are rare, even with extensive antibiotic therapy (1, 3, 4). Recently our laboratory reported that the combination of systemic gentamicin and application of low-frequency continuous ultrasound to a simulated implant infection in a rabbit model significantly reduced the concentration of viable bacteria on the implant (6). However, the power density required to reduce the concentration (300 mW/cm2) also caused some damage to the skin. Herein, we report that using pulsed ultrasound and gentamicin significantly reduces biofilm viability without causing any apparent damage to the skin.
Seven New Zealand White female rabbits (2.0 to 2.5 kg) were treated in accordance with the regulations of the Institutional Animal Care and Use Committee of Brigham Young University and the U.S. Department of Agriculture. Procedures for rabbit maintenance, measurement of subcutaneous heating, implantation surgery, and evaluation of biofilm viability were reported previously (6) and are described briefly below.
Escherichia coli strain ATCC 10798 was grown in biofilms on sterile circular polyethylene disks with two sewing tabs. This strain produced a thick biofilm in 24 h of growth (2). After the rabbit was anesthetized and its back was denuded of fur, these disks were implanted subcutaneously and sewn into place, one on each side of the spine. The rabbit received gentamicin (8 mg/kg) by subcutaneous injection following surgery and every 24 h thereafter. Blood was sampled from the ear just prior to each gentamicin injection and prior to euthanization.
A 49-g ultrasound transducer (Tonpilz resonator, model 6147E; EDO Acoustics, Salt Lake City, Utah) operating at 28.48 kHz was fixed over one implant 24 h postsurgery. Unlike previous experiments with continuous ultrasound, the ultrasound was delivered in a pulse of 100 cycles with a 1:3 or 1:6 duty cycle wherein the temporal average intensity was 100 mW/cm2. For example, ultrasound at 300 mW/cm2 was pulsed in a 1:3 duty cycle in which the ultrasound was on for 3.51 ms (100 cycles) and off for 7.02 ms (average intensity of 100 mW/cm2). Likewise, ultrasound at 600 mW/cm2 was pulsed in a 1:6 duty cycle: on for 3.51 ms and off for 17.56 ms. This experimental design could show whether enhanced killing is a function of average intensity or peak intensity during the pulse.
Following 24 h of pulsed ultrasound, rabbits were euthanized, both disks were recovered, and the viable bacterial concentration was determined. Viable counts were analyzed using a one-tailed t test of the mean difference in paired observations of treated and nontreated sides. Skin and tissue from the implant site and heart, liver, spleen, and kidney samples were taken for histopathology as described previously (5, 6).
In an experiment without implantation of disks, pulsed ultrasound at duty cycles of 1:3 and 1:6 raised the subcutaneous skin temperature to 37.7 and 38.2°C, respectively, and no skin damage was observed.
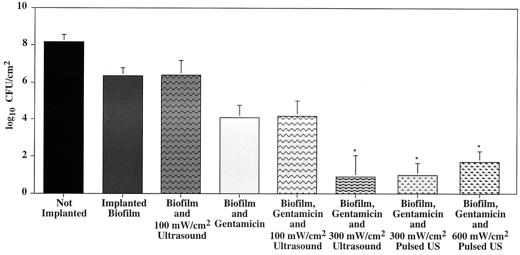
Our previously reported experiments showed that gentamicin combined with continuous ultrasound at 100 mW/cm2 did not reduce bacterial viability, whereas continuous ultrasound at 300 mW/cm2 reduced viability but caused skin damage (Fig. 1). Data from the 300-mW/cm2 pulsed-ultrasound (1:3 duty cycle) experiments also showed a significant decrease in viability (P = 0.017; n = 3), the average counts being reduced from 2.94 to 0.99 log10 CFU/cm2. More importantly, the skin showed no discoloration or damage.
FIG. 1.
Comparison of log10 numbers of viable CFU from biofilm-coated polyethylene disks. The second bar from the right represents data from experiments in which ultrasound (US) at 300 mW/cm2 was pulsed in a 1:3 duty cycle, producing a time-averaged intensity of 100 mW/cm2. Likewise, the far right bar represents experiments in which ultrasound at 600 mW/cm2 was pulsed in a 1:6 duty cycle, also producing a time-averaged intensity of 100 mW/cm2. The asterisks indicate sets of data that are significantly different (P < 0.05) from data for implants treated with gentamicin only.
Data from the 600-mW/cm2 pulsed-ultrasound (1:6 duty cycle) experiments also showed a significant viability reduction (P = 0.016; n = 3) in which average counts were reduced from 2.93 to 1.69 log10 CFU/cm2. Again, no skin damage was apparent.
Histopathology indicated necrosis, edema, a fibrous capsule, and inflammation in the skin and skeletal muscle tissues around both treated and nontreated implant sites. The histology of the skin receiving pulsed ultrasound was not unlike that of control skin or skin receiving continuous ultrasound at 100 mW/cm2. None of the blood samples taken before, during, or after ultrasound treatment showed the presence of E. coli bacteremia. The tissue around the implant site had some evidence of bacteria in both the ultrasound-treated and nontreated samples, but none of the other organs and tissues sampled showed any colonization by E. coli.
Comparison of all of the data from the pulsed-ultrasound and previous experiments shows that ultrasound enhances the action of gentamicin in killing E. coli when the pulse (or continuous) intensity is at least 300 mW/cm2. As Fig. 1 shows, viability is not reduced by continuous ultrasound at 100 mW/cm2 but it is reduced by continuous ultrasound at 300 mW/cm2 and by pulsed ultrasound at 300 or 600 mW/cm2. Enhancement does not appear to correlate with the temporal average intensity, since pulsed ultrasound at an average of 100 mW/cm2 enhanced killing while continuous ultrasound at the same average intensity did not. This suggests that the bacteria respond to the maximum or peak ultrasound intensity and not to the average intensity or the total amount of energy delivered.
Conversely, skin damage appears to correlate with average ultrasonic power and not maximum power during a pulse. An average intensity of 100 mW/cm2 produced no skin damage, whether pulsed or continuous, but an average intensity of 300 mW/cm2 produced significant damage. These results show that there is a window of operation in which one can use pulsed ultrasound of sufficiently high intensity (during the pulse) to enhance the action of the antibiotic while still maintaining the average intensity at a safe level that does not injure skin.
A final important observation about these higher-intensity ultrasonic pulses is that they do not appear to cause spread of bacteria from the infection site to other tissues. No viable E. coli cells were plated from blood, nor were any observed in histological sections of the internal organs.
Although we cannot report complete elimination of the infection, we are hopeful that higher pulse intensities and longer treatment times may lead to noninvasive treatment of implant infections.
Acknowledgments
This research was funded by NIH grant HL59923.
The transducers and technical expertise were donated by Gordon Snow of EDO Acoustics. We thank David O. Draper of BYU for assistance in measuring the subcutaneous temperature.
REFERENCES
- 1.Darouiche R O, Dhir A, Miller A J, Landon G C, Raad I I, Musher D M. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J Infect Dis. 1994;170:720–723. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 2.Johnson L, Peterson R V, Pitt W G. Treatment of bacterial biofilms on polymeric implants using antibiotics and ultrasound. J Biomater Sci Polym Ed. 1998;9:1177–1185. doi: 10.1163/156856298x00712. [DOI] [PubMed] [Google Scholar]
- 3.Khoury A E, Lam K, Ellis B, Costerton J W. Prevention and control of bacterial infections associated with medical devices. Am Soc Artif Intern Organs J. 1992;38:M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Potera C. Biofilms invade microbiology. Science. 1996;273:1795–1797. doi: 10.1126/science.273.5283.1795. [DOI] [PubMed] [Google Scholar]
- 5.Rediske A M. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. M.S. thesis. Provo, Utah: Brigham Young University; 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rediske A M, Roeder B L, Brown M K, Nelson J L, Robison R L, Draper D O, Schaalje G B, Robison R A, Pitt W G. Ultrasonic enhancement of antibiotic action on Escherichia coli biofilms: an in vivo model. Antimicrob Agents Chemother. 1999;43:1211–1214. doi: 10.1128/aac.43.5.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]