Abstract
Background
Campylobacter spp. are one of the most frequent causes of diarrhoeal disease in humans throughout the world. This study aimed at determining the prevalence and the genotypic distribution of Campylobacter spp. and their association with diarrhoea and child growth in children of less than the age of two in the Limpopo Province of South Africa.
Methods
A total of 4280 diarrheal and non-diarrheal stool samples were collected on a monthly basis from children recruited at birth and followed up to 24 months. All stool samples were screened for the presence Campylobacter antigen using ELISA technique after which CAH 16S primer was used on the positive samples to confirm the presence of Campylobacter. Subsequently, the PCR positive samples were further characterised using species specific primers for Campylobacter jejuni and Campylobacter coli.
Results
Campylobacter antigen was detected in 564/4280 (13.2%). Campylobacter was more commonly found in diarrheal stools (20.4%) compared to non-diarrheal stools (12.4%) with a statistically significant difference (χ2 = 7.345; p = 0.006). Throughout the year there were two main peaks of Campylobacter infection one in December- January and the second peak in June. The prevalence of Campylobacter increased with the age of the children up to 11 months after which the prevalence decreased. Out of 564 positive ELISA samples, 257 (45.6%) were confirmed to have 16S rRNA gene for Campylobacter spp. Furthermore, C. jejuni was found to be more prevalent (232/257) than C. coli (25/257) with a prevalence of 90.3% and 9.7%, respectively. Both C. jejuni and C. coli were significantly associated with diarrhea with statistical values of (χ2 = 22.224; p < 0.001) and (χ2 = 81.682; p < 0.001) respectively. Sequences generated from the analysis of hip gene confirmed the PCR positives samples were C. jejuni positive.
Conclusions
This study has delineated a high prevalence of Campylobacter spp. in the study cohort. Moreover, C. jejuni was found to be more prevalent than C. coli both of which were associated with diarrhea. These findings are of clinical and epidemiological significance.
Keywords: Campylobacter spp., Prevalence, ELISA, PCR, Young Children, South Africa
Background
The importance of Campylobacter as a cause of bacterial enteritis has grown tremendously for the past three decades. Moreover, it has been indicated that its prevalence is higher in developing nations than in developed nations even though studies focusing on detection of Campylobacter spp. have largely been performed in the developed world. In addition, Coker and co-authors [1] have highlighted that Campylobacter infection is mostly implicated as a cause of diarrhoea in the first six months of life in developing country settings with illness/infection ratios decreasing with age [2]. Nonetheless, there are few studies in Africa, which have reported on the prevalence of campylobacteriosis among young children. A study conducted by Randremanana and co-authors [3] in Moramanga, Madagascar reported 8.9% of Campylobacter isolation from diarrheic samples while that in non-diarrheic samples was 9.4%.It was estimated that approximately 90% of C. jejuni is responsible for human Campylobacter enteritis cases and C. coli responsible for most of the remaining cases [4].
One of the studies in Tanzania reported the presence of C. jejuni in 34.8% of children with gastroenteritis [5]. In addition, another study in Ile-Ife, Nigeria, found that C. jejuni is an important agent of diarrhoea in children of less than the age of five [6]. Another study in Cape Town, South Africa reported a prevalence of 40% of C. jejuni from children with diarrheal [7]. In Venda, stool samples were examined for the presence of Campylobacter species in adults and children with diarrheal, and the prevalence estimates of C. jejuni, C. coli and C. concisus were 6.2%, 3.1% and 2.8% respectively; however only 39 of the 255 samples analyzed were from children < 5 years of age [8]. Also, in Venda, South Africa, Campylobacter spp. was also isolated from 20% of stool samples tested from HIV-positive individuals [9].
Nonetheless, there is still limited information on the prevalence of Campylobacter spp. in healthy cohort children in a rural community of South Africa. Moreover, there still a dire need in developing countries to determine whether malnutrition is a risk factor of harboring Campylobacter spp. as most of the developing countries are underprivileged with no access to improved safe drinking water. This study therefore aimed at determining the prevalence of Campylobacter spp from stool samples of children in South Africa who were part of the MAL-ED study cohort using ELISA coupled with molecular technique and, to determine the prevalence of C. jejuni and C. coli and their association with diarrheal and child growth. The MAL-ED study has been previously described [10]. MAL-ED was a birth cohort study to investigate the interactions between enteric infections, gut function, physical growth, cognitive development, and vaccine response.
Methods
Ethical consideration
The study protocol was approved by the Safety, Health and Research Ethics Committee of the University of Venda, South Africa, and the Institutional Review Board of the University of Virginia, USA. The purpose of the study was explained to the mothers and/or their legal guardian and written informed consent was obtained before sample collection. Only participants who provided signed informed consent were enrolled for the study.
Study site and sample collection
The MAL-ED South Africa site has been described [11]. Briefly, the study community was rural and of low socio-economic status. Study participants were enrolled at birth or latest 17 days after birth. Children with birth weight less than 3.5 kg, or diagnosed with a congenital disease, or hospitalized for up to two weeks after birth were excluded from enrolment. Enrolment for the study began in November 2009 in the Dzimauli community of the Limpopo Province of South Africa. For the first 12 months of life, stool samples were collected monthly and thereafter every three months until 24 months of age. Additionally, a stool sample was collected whenever there was diarrhea. Diarrhea was defined as the passing of three loss stools in a 24-h period; and diarrhea episodes had to be separated by at least three days of no loss stools. We collected a total of 4280 diarrheal and non-diarrheal stool samples. Of the 4280 stool samples [2145 were from males and 2135 were from females]. Sample collection and transportation was done according to a standard common protocol for the MAL-ED Network [12]. Briefly, after production, stool samples were immediately placed in a sterile wide screwed cap container containing Cary-Blair medium, and transported in a cooler box with ice packs for subsequent laboratory processing within 4 h of collection. Demographic data such as, age and sex of each child were also collected.
Detection of Campylobacter species in stool samples by ELISA
All 4280 stool samples were screened for the presence of Campylobacter spp using a commercial enzyme-linked Immunosorbant assay (ELISA) kit, manufactured by ProSpecT (Oxoid Ltd, Wade Road, Basingstoke Hants, RG24 8PW UK),following the manufacturer’s instructions. Briefly, 200μL Campylobacter sample diluent was transferred into the 2 mL microcentrifuge tube (Thermo Fisher Scientific, Johannesburg, SA) containing 0.2 g of stool sample and vortexed for 15 s and 200μL of the diluted stool sample was transferred to the plastic microtiter wells coated with specific monoclonal antibodies. After 60 min of incubation at room temperature, the microwellplate was washed with washing buffer, 3 times and 4 drops (200μL) of enzyme conjugate was added to each microwell and incubated for 30 min at room temperature. The microwell plate was then washed 5 times before adding 2 drops (100μL) of substrate and incubated for 10 min at room temperature. One drop of stop solution was added to stop the reaction and the absorbance was read at 450–630 nm. All samples with an OD suspension greater than 0.170 were considered positive and those that are less than 0.170 were considered negative.
Molecular characterization of Campylobacter jejuni and Campylobacter coli
Extraction of Genomic DNA from stool samples
Genomic DNA was purified from 564/4280 stoolsamples which were positive for ELISA using protocol described by Mukherjee et al. [13] with some slight modification. Briefly, 0.2 g of stool sample, frozen at -70 °C, was diluted in 0.5 mL of lysis buffer (Tris–HCL, 0.5 M; EDTA, 20 mM; NaCl, 10 mM; SDS, %0.1; pH 9.0) in 2 mL microcentrifuge tube and vortexed for 15 s. After vortexing, samples were incubated at 65 °C for 2 h; 600µL of phenol–chloroform-isoamyl alcohol (25–24-1) was added to each sample and mixed thoroughly. Samples were centrifuged at 13,400 g for 15 min; the top layer was transferred in to a new 2 mL microcentrifuge tube and 600µL of chloroform was added and mixed well. The samples were centrifuged at the same speed and time, and the supernatant was collected into a new 2 mL microcentrifuge tube. To the supernatant, 50µL of 5 M NaCl and two volume of absolute ethanol was added and kept frozen overnight to allow DNA to precipitate. On the following day, the samples were centrifuged at 13,400 g for 15 min. To the pellet; 600µL of 70% ethanol was added, mixed and centrifuged once more at the same speed and time. The pellet was blot dried on a clean paper towel and 200µL of TE buffer (1 M Tris–HCl pH 8; 0.5 M EDTA) was added and incubated at 45 °C for 3 h. After incubation, the DNA was store at -20 °C for further analysis.
Detection of Campylobacter jejuni and Campylobacter coli by PCR
Prior to characterization to species level, CAH 16S primer (F5’-AATACATGCAAGTCGAACGA-3’ and R5’-TTAACCCAACATCTCACGAC-3’) was used to confirm the ELISA Campylobacter antigen positive samples. Subsequently, C. jejuni and C. coli were amplified from PCR confirmed samples using species specific primers as listed in (Table 1). Briefly, the reaction mixture consisted of 5.0μL of DNA, 25μL Dream Taq Green PCR master mix (Inqaba Biotech, Pretoria, South Africa), 0.1 μM primer and nuclease free water was added to make a final volume of 50μL. The PCR conditions were as follows; heat denaturation at 95 °C for 2 min, followed by 35 cycles at 95 °C for 30 s, annealing at 52 °C for 30 s and extension at 72 °C for 90 s, with final extension at 72 °C for 10 min. The PCR products were separated by electrophoresis in 1.8% agarose gel (w/v) containing 8µL of ethidium bromide at 100 V for 45 min in 1X Tris–acetate buffer, visualized by UV-transilluminator.
Table 1.
PCR primers used to detect C. jejuni and C. coli in stool samples
| Primer name | Organism detected | Gene name and size (bp) | Sequence (5’-3’) and annealing temperature | References |
|---|---|---|---|---|
|
Hip 1a Hip 2b |
C. jejuni | Hippuricase gene, 176 |
-ATG ATG GCT TCT TCG GAT AG- -GCT CCT ATG CTT ACA ACT GC- 58 °C |
Linton et al. [14] |
|
CC18F CC519R |
C. coli |
Aspartokinase gene and the downstream ORF of C. coli, 500 |
-GGT ATG ATT TCT ACA AAG CGA G- -ATA AAA GAC TAT CGT CGC GTG- 66 °C |
Linton et al. [14] |
Gene sequencing analysis
The PCR products of the hippuricase gene used for identification of C. jejuni were sequenced (Inqaba, Pretoria, South Africa). Sequences were assembled using Staden package. Sequences were edited using Bioedit package and aligned using Clustal W multiple aligner. A similarity plot was then done to determine points of mutation in line with the reference strain.
Statistical analysis
The results were analyzed using Statistical Package for the Social Sciences (SPSS version 22.0). The Chi square test was used to compare Campylobacter infection from children’s diarrheal and non-diarrheal stool samples, other characteristics of the stool and demographic information like gender. Differences were considered significant at P ≤ 0.05. Moreover, in order to indicate a child’s nutritional status, anthropometric indices (height-for-age, weight-for-height, and weight-for-age) were expressed as z-scores. Anthropometric data from reference populations published by the NCHS and United States Centers for Disease Control was used as standards. Anthropometric measurements were converted to z-scores using the EPINUT component of the EPI-INFO computer program version 6.
Results
Characteristics of study samples and Campylobacter infection
A total of 4280 samples were collected from 290 children over a period of 24 months. Of these samples, 2135 (49.9%) were from females, and 4084 samples were non-diarrheal (Table 2).
Table 2.
Characteristics of samples collected from the Dzimauli community of the Limpopo Province of South from 2009 to 2012
| Characteristics | Frequency | Percent (%) | |
|---|---|---|---|
| Gender | Male | 2145 | 50.1 |
| Female | 2135 | 49.9 | |
| Diarrhea | Non Diarrhea samples | 4084 | 95.4 |
| Diarrhea samples | 196 | 4.6 | |
| Mucus | Without mucus | 3605 | 87.42 |
| With mucus | 519 | 12.58 | |
| Blood | Without blood | 4123 | 99.975 |
| With blood | 1 | 0.02 | |
| Campylobacter infection | Negative | 3716 | 86.8 |
| Positive | 564 | 13.2 | |
Frequency of Campylobacter infection using ELISA method
Campylobacter infection was frequent in (564/4280) 13.2% of the stool samples evaluated in the present study. Frequency was higher in females 296/2135(13.9%) than in males 268/2145 (12.5%). Diarrheal samples that were found to be positive for Campylobacter were 40/196(20.4%) while 85(16.4%) stool samples with mucus were found to be positive for Campylobacter (Table 3).
Table 3.
Characteristics of the samples and Campylobacter infection from the Dzimauli community of the Limpopo Province of South from 2009 to 2012
| Sample characteristics | Campylobacter positive | Percentage (%) | Total | |
|---|---|---|---|---|
| Gender | Male | 268 | 12.5 | 2145 |
| Female | 296 | 13.9 | 2135 | |
| Sample type | Non diarrheal | 509 | 12.4 | 4084 |
| Diarrheal | 40 | 20.4 | 196 | |
| Sample consistency | Liquid | 24 | 17.3 | 139 |
| Watery | 4 | 22.2 | 18 | |
| Soft | 297 | 13.6 | 2182 | |
| Formed | 239 | 13.4 | 1785 | |
| Mucus | Non mucus | 479 | 12.7 | 3761 |
| Mucus | 85 | 16.4 | 519 | |
| Total positive Campylobacter samples by ELISA | 564 | 13.2 | 4280 | |
Distribution of Campylobacter species by age, season and years
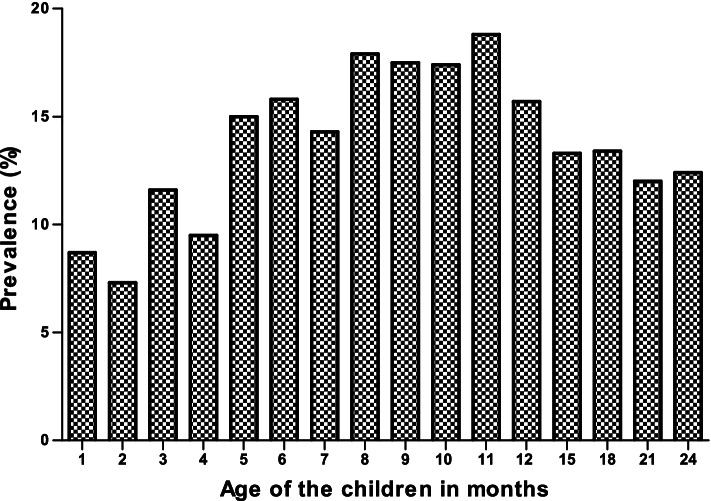
The samples were collected from month 1 to month 24. The results show that the Campylobacter infection increases as the child grows, with a high prevalence at month 9 and 11. The infection decreases from month 12 and start to increase again at 24 month (Fig. 1). Also, the results obtained showed that Campylobacter infection was more common in rainy months (58.1%) than in dry months (47.3%), autumn (31.5%) and spring (27.5%) (Fig. 2). The prevalence of Campylobacter infection was high in 2010 (14.7%) compared to 2011 (12.0%). In 2012, Campylobacter infection increased (13.9%) and decreased in 2013 (12.8%). This result confirms that Campylobacter infection is endemic in the study area as the prevalence remains almost at the same level (Fig. 3).
Fig. 1.
Overall distribution of Campylobacter spp. by age in the study population
Fig. 2.
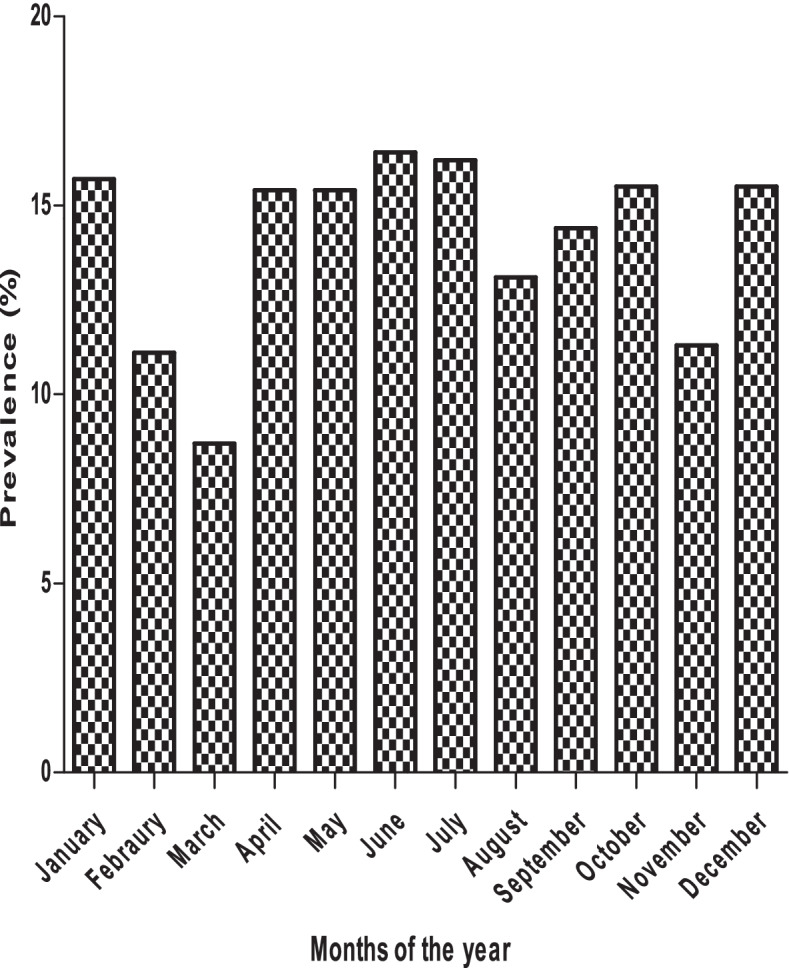
Seasonal distribution of Campylobacter spp. in the study population
Fig. 3.
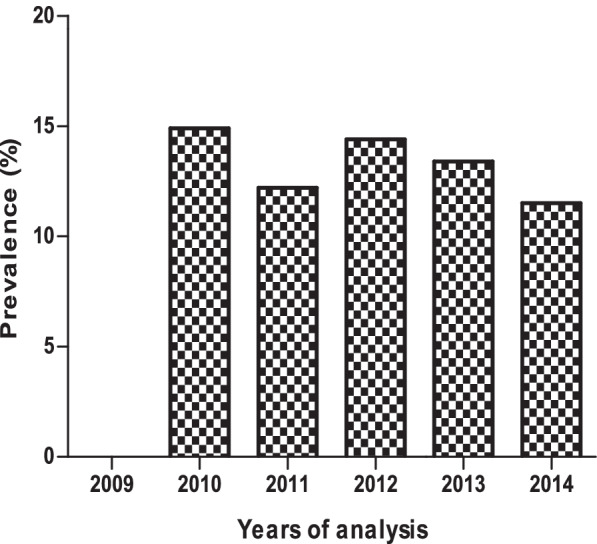
Yearly distribution of Campylobacter in the study
Prevalence of Campylobacter jejuni and Campylobacter coli and their distribution and child growth
All ELISA Campylobacter antigen positive samples, were confirmed by conventional PCR whereby 16S rRNA gene was detected in 257/564 (45.6%) leaving 307/564 (54.4%) to be negative for the PCR gene probe that is specific to only C. jejuni or coli, but not other Campylobacter strains, that may also be important [15]. For the detection of C. jejuni and C. coli from human stool samples, species specific primers were used and it was found that C. jejuni was more prevalent than C. coli with a prevalence of 90.3%(232/257) and 9.7% (25/257) respectively. Both C. jejuni and C. coli infection were more observed in children that had diarrhea than those without with a statistical significant difference of (χ2 = 22.224; p = 0.000) and (χ2 = 81.682; p = 0.000) respectively. C. jejuni was more common in females (15.2%) than in males (11.8%) and the difference was statistically significant (χ2 = 4.190; p = 0.041). For C. coli, the infection rate was similar between female (1.4%) and male (1.4%) children, and the difference was not statistically significant (χ2 = 0.000; p = 0.995) (Table 4).
Table 4.
Overall distribution of C. jejuni and C. coli with child growth from the Dzimauli community of the Limpopo Province of South from 2009 to 2012
| Anthropometric characteristics |
C. jejuni Positive |
Total | χ2 | P-Value | C. coli Positive | Total | χ2 | P-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | 106 (11.8%) | 898 | 4.190 | 0.041 | 13 (1.4%) | 898 | 0.000 | 0.995 |
| Female | 126 (15.1%) | 832 | 12 (1.4%) | 832 | |||||
| stunted | Not stunted | 164 (13.3%) | 1235 | 0.061 | 0.801 | 15 (1.2%) | 1235 | 1.610 | 0.204 |
| Stunted | 68 (13.7%) | 495 | 10 (2.0%) | 495 | |||||
| Underweight | Not underweight | 210 (13.2%) | 1585 | 0.423 | 0.515 | 23 (1.5%) | 1585 | 0.005 | 0.945 |
| Underweight | 22 (15.2%) | 145 | 2 (1.4%) | 145 | |||||
| Wasted | Not wasted | 225 (13.6%) | 1655 | 1.122 | 0.259 | 25(1.5%) | 1655 | 1.150 | 0.284 |
| Wasted | 7 (9.3%) | 75 | 0 (0.0%) | 75 | |||||
| Diarrhea | No diarrhea | 209 (12.6%) | 1658 | 22.224 | 0.000 | 15 (0.9%) | 1658 | 81.682 | 0.000 |
| Diarrhea | 23 (31.9%) | 72 | 10 (13.9) | 72 | |||||
| Total | 232 (13.4%) | 25(1.4%) | |||||||
Considering the occurrence of Campylobacter infection in relation to growth characteristics, children that were stunted (13.7%) had higher C. jejuni infection than those that were not stunted (13.3%), but the difference was not statistically significant (χ2 = 0.061; p = 0.801). C. coli infection was more common in stunted (2.0%) children compared to children that were not stunted (1.2%), but the difference was also not statistically significant (χ2 = 1.610; p = 0.204) (Table 4).
Sequence diversity of the hippuricase gene
The nucleotide sequences of the hippuricase gene (14 hip isolates) were edited using Bioedit package, and aligned with two other sequences (FJ655193) and (KF541296) obtained from GenBank using the Clustal W multiple aligner. Sequence analysis confirmed sequences as belonging to the species C. jejuni. Figure 4 shows the nucleotide sequences of Hip genes with some mutations on various positions. Changes were found at different positions and appeared like single nucleotide polymorphisms these included positions 50, 95, 96, 98, 99, 100,101 110, 116, 140. Based on the alignment and comparison using referral strains (FJ655193 and KF54196) there were no two strains that were identical to each other. This depicts a wide sequence variation that could be existing in Campylobacter jejuni strains in this environment.
Fig. 4.
Nucleotide sequence of Hip gene (FJ655193 and KF54196) are referral strains from gene Bank
Discussion
Campylobacter species are increasingly being recognised as agents of gastroenteritis worldwide with its prevalence varying from country to country [5, 6, 16, 17]. The current study aimed at determining the prevalence of Campylobacter infection in young children, and to ascertain the prevalence of C. jejuni and C. coli in the stool samples of cohort children in the Dzimuali community and the association of these pathogens with diarrheal and non-diarrheal samples and child growth.
Campylobacter spp were detected in 13.2% (564/4280) of the samples using ELISA. This is closely similar to reports generated from other countries. For example, in Algeria a prevalence of 17.7% was reported by Wren et al. [18], Nigeria (8.3%) [19], Tanzania (9.7%) [20], Bangladesh (19.3%) [21]. In Kenya, at a hospital in Kisii,5.8% of samples from patients with diarrheal were positive for Campylobacter species [22]. Also, in Egypt campylobacteriosis has been reported at (9%) [23]. These differences could be as a result of differences in geographical location, study population, study period and method employed for each study. Generally, the incidence of Campylobacter species (54.4%) infections was higher than that of Campylobacter jejuni /coli. This corroborates previous study which showed that promotion of, drinking treated water, improved latrines, and targeted antibiotic treatment may reduce the burden of Campylobacter species infection [16].
The higher prevalence of Campylobacter infection in children with diarrheal in this study may be due to unprotected water source and presence of domestic animals in almost all rural household. There was a high prevalence of Campylobacter in females than in male infants in this study. However, this is contrary to the reports of Coker et al. [1] who in their study among young children < 2yrs and young adults between the ages of 16-18yrs in Nigeria observed that Campylobacter infections are usually predominant in males. In Colorado, for example, males predominate in all age groups under 40 and the greatest difference between sexes was in infants where the male–female ratio was over 2:1 [1, 24].
Findings from this study showed higher Campylobacter infection in children between the age of five and eleven months (Fig. 1). Reports in developing countries show that isolation rates are higher among children less than one year of age with highest rates occurring in children between 6 to 12 months and do not increase in young adults [1, 25, 26]. The seasonal incidence of Campylobacter in this study is similar to that previously described by [27, 28] with a sharp rise in June and July. The period of June to July are early winter months in South Africa and the sharp rise could be in line with the explanation that milder winter temperatures may favour some transmission routes, as well as enhance the survival and multiplication of the bacteria [29]. However, is contrary with findings in most European countries where campylobacteriosis shows a marked seasonality peaks during the summer months [27] although it varies from country to country. Annually, consistent rise in incidence have been reported in the United Kingdom, Greece, the Netherlands, and Denmark [29]. A study in northwest England also indicated a consistent peak of human infection in March [28], which is the opposite of what has been observed in this study. The results obtained in this study showed that Campylobacter infection is endemic in the Vhembe district of South Africa since the prevalence remains almost at the same level throughout the 4 years.
In this study, more C. jejuni were detected than C. coli with a prevalence rate of 13.4% and 1.4%, respectively. Similar study in Venda region found that C. jejuni was higher (6.2%) than C. coli (3.1%) in children less than the age of five [8]. Again in Italy, Di Giannatale et al. [30], in their study, found that the prevalence of C. jejuni and C. coli was 62.75% and 37.25%, respectively. In a study on animals in Greater Washington, 53.6% of their isolates were C. jejuni, 41.3% were C. coli [31] which are in line with the findings of this study. Children that were stunted had higher C. jejuni and C. coli infection than those that were not. This corroborates previous reports which had it that, infection with specific enteric pathogens such as Campylobacter spp. can affect growth, even in the absence of overt diarrhea [16, 32, 33].
Several mutations were observed on the analysed hip gene (position 50, 95, 96, 98, 99, 100, 101, 110, 116, 140) which affected 12 participants out of 14. The mutation observed could be responsible for inability of some of the Campylobacter strains to induced clinical state of disease in some infants in this study. In this study, GGG was observed in children that showed no symptoms of Campylobacter enteritis. Phylogenetic analysis of the hip gene isolates from Venda region revealed that Campylobacter infection that had occurred among the children in this study were caused by non-related strains. From the distance matrix data, it is evident that hip genes that were sequenced show significant genetic differences.
Conclusions
The present study indicates that infection caused by Campylobacter species was very frequent in children under the age of two in our study environment. C. jejuni was more prevalent in this study than C. coli and both species were significantly associated with diarrheal and might impact on growth decline in these children. These findings are of public health significance as it delineates the high frequency of Campylobacter species which might be associated with growth shortfall in children within the study area.
Acknowledgements
Appreciation to all those who participated in this study.
Authors’ contributions
Bessong Pascal, Guerrant Richard and Dillingham Rebecca took part in study design, and contributed in drafting the manuscript. SamieAmidou took part in study design, carried out the data analysis and drafted the manuscript. Moropeng Resoketswe collected the data, managed it and contributed in the manuscript. Tanih Nicoline participated in data collection and in revising drafted the manuscript. All authors read and approved the final manuscript.
Funding
This study was a continuation of the MAL-ED study carried out as a collaborative project funded by Bill & Melinda Gates Foundation.
Availability of data and materials
Data generated or analysed during this study are included in this article. However, the absolute raw data are available from the corresponding author on request.
Declarations
Ethics approval and consent to participate
The Safety, Health and Research Ethics Committee of the University of Venda, South Africa, and the Institutional Review Board of the University of Virginia, USA approved the study protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Coker AO, Isokpehi RD, Thomas BN, Amisu KO, Obi CL. Human campylobacteriosis in developing countries. Emerg Infect Dis. 2002;18(3):237–243. doi: 10.3201/eid0803.010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global epidemiology of Campylobacter Infection. ClinMicrobiol Rev. 2015;28(3):687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Randremanana RV, Randrianirina F, Sabatier P, Rakotonirina HC, Randriamanantena A, Razanajatovo IM, Ratovoson R, Richard V. Campylobacter infection in a cohort of rural children in Moramanga. Madagascar BMC Infect Dis. 2014;14:372. doi: 10.1186/1471-2334-14-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dingle KE, Colles FM, Falush D, Maiden MC. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J ClinMicrobiol. 2005;43:340–347. doi: 10.1128/JCM.43.1.340-347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Platts-Mills JA, Liu J, Gratz J, et al. Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries. J ClinMicrobiol. 2014;52(4):1074–1080. doi: 10.1128/JCM.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aboderin AO, Smith SI, Oyelese AO, Onipede AO, Zailani SB, Coker AO. Role of Campylobacter jejuni/coli in diarrhoea in Ile-Ife. Nigeria East Afr Med J. 2002;79(8):423–426. [PubMed] [Google Scholar]
- 7.Lastovica A. Emerging Campylobacter spp.: the tip of the iceberg. Clin Microbiol Newsl. 2006;28:49–56. doi: 10.1016/j.clinmicnews.2006.03.004. [DOI] [Google Scholar]
- 8.Samie A, Obi CL, Barrett LJ, Powell SM, Guerrant RL. Prevalence of Campylobacter species, Helicobacter pylori and Arcobacter species in stool samples from the Venda region, Limpopo, South Africa: studies using molecular diagnostic methods. J Infect. 2007;54(6):558–566. doi: 10.1016/j.jinf.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 9.Obi CL, Bessong PO. Diarrheagenic bacterial pathogens in HIV-positive patients with diarrhoea in rural communities of Limpopo province. South Africa J Health Popul Nutr. 2002;20(3):230–234. [PubMed] [Google Scholar]
- 10.MAL-ED Network Investigators Childhood stunting in relation to the pre- and postnatal environment during the first 2 years of life: the MAL-ED longitudinal birth cohort study. PLoS Med. 2017;14(10):e1002408. doi: 10.1371/journal.pmed.1002408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bessong PO, Nyathi E, Mahopo TC, Netshandama V, MAL-ED South Africa Development of the Dzimauli community in Vhembe District, Limpopo province of South Africa, for the MAL-ED cohort study. Clin Infect Dis. 2014;1(59 Suppl 4):S317–24. doi: 10.1093/cid/ciu418. [DOI] [PubMed] [Google Scholar]
- 12.Houpt E, Gratz J, Kosek M, Zaidi AKM, Qureshi S, et al. Microbiologic methods utilized in the MAL-ED cohort study. Clin Infect Dis. 2014;59(Suppl 4):S225–S232. doi: 10.1093/cid/ciu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee P, Dutta S, Mukhopadhyay AK. Development and evaluation of a PCR assay for rapid detection of azithromycin resistant Campylobacter isolated from diarrhoeal patients in Kolkata. India Gut Pathog. 2017;9:37. doi: 10.1186/s13099-017-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linton D, Lawson AJ, Owen RJ, Stanley J. PCR detection, identification to species level, and fingerprinting of Campylobacter jejuni and Campylobacter coli direct from diarrheic samples. J Clin Microbiol. 1997;35(10):2568–2572. doi: 10.1128/jcm.35.10.2568-2572.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.François R, Yori PP, Rouhani S, et al. The other Campylobacters: Not innocent bystanders in endemic diarrhea and dysentery in children in low-income settings. PLoS Negl Trop Dis. 2018;12(2):e0006200. doi: 10.1371/journal.pntd.0006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haque MA, Platts-Mills JA, Mduma E, et al. Determinants of Campylobacter infection and association with growth and enteric inflammation in children under 2 years of age in low-resource settings. Sci Rep. 2019;9(1):17124. doi: 10.1038/s41598-019-53533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allos BM, Blaser MJ. Mandell, Douglas, and Bennett’s Principles and Practices of Infectious Diseases. 7th ed. New York; USA: Churchill Livingston; 2009.
- 18.Wren BW, Linton D, Dorrell N, Karlyshev AV. Post genome analysis of Campylobacter jejuni. SympSerSocApplMicrobiol. 2001;30:36S–44S. doi: 10.1046/j.1365-2672.2001.01352.x. [DOI] [PubMed] [Google Scholar]
- 19.Ohanu ME, Offune J. The prevalence of Campylobacter in childhood diarrhoea in Enugu state of Nigeria. J Commun Dis. 2009;41(2):117–120. [PubMed] [Google Scholar]
- 20.Deogratias AP, Mushi MF, Paterno L, et al. Prevalence and determinants of Campylobacter infection among under five children with acute watery diarrhea in Mwanza, North Tanzania. Arch Public Health. 2014;72(1):17. doi: 10.1186/2049-3258-72-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talukder KA, Aslam M, Islam Z, et al. Prevalence of virulence genes and cytolethal distending toxin production in Campylobacter jejuni isolates from diarrheal patients in Bangladesh. J ClinMicrobiol. 2008;46(4):1485–1488. doi: 10.1128/JCM.01912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swierczewski BE, Odundo EA, Koech MC, et al. Enteric pathogen surveillance in a case-control study of acute diarrhea in Kisii Town. Kenya J Med Microbiol. 2013;62:1774–1776. doi: 10.1099/jmm.0.059139-0. [DOI] [PubMed] [Google Scholar]
- 23.Rao MR, Naficy AB, Savarino SJ, et al. Pathogenicity and convalescent excretion of Campylobacter in rural Egyptian children. Am J Epidemiol. 2001;154(2):166–173. doi: 10.1093/aje/154.2.166. [DOI] [PubMed] [Google Scholar]
- 24.Tenkate TD, Stafford RJ. Risk factors for Campylobacter infection in infants and young children: a matched case-control study. Epidemiol Infect. 2001;127(3):399–404. doi: 10.1017/S0950268801006306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka. Bangladesh J ClinMicrobiol. 1999;37(11):3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prentice AM, Moore SE. Early programming of adult diseases in resource poor countries. Arch Dis Child. 2005;90(4):429–432. doi: 10.1136/adc.2004.059030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nylen G, Dunstan F, Palmer SR, Andersson Y, Bager F, Cowden J. The seasonal distribution of Campylobacter infection in nine European countries and New Zealand. Epidemiol Infect. 2002;128(3):383–390. doi: 10.1017/S0950268802006830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sopwith W, Ashton M, Frost JA, Tocque K, O’Brien S, Regan M. Enhanced. Surveillance of Campylobacter infection in the North West of England 1997–1999. J Infect. 2003;46(1):35–45. [DOI] [PubMed]
- 29.Kovats RS, Edwards SJ, Charron D, Cowden J, D’Souza RM, Ebi KL. Climate variability and Campylobacter infection: an international study. Int J Biometeorol. 2005;49(4):207–14. [DOI] [PubMed]
- 30.Di Giannatale E, Di Serafino G, Zilli K, Alessiani A, Sacchini L, Garofolo G, Aprea G, Marotta F. Characterization of antimicrobial resistance patterns and detection of virulence genes in Campylobacter Isolates in Italy. Sensors (Basel) 2014;14(2):3308–3322. doi: 10.3390/s140203308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao C, Ge B, De Villena J, et al. Prevalence of Campylobacter spp., Escherichia coli, and Salmonella serovars in retail chicken, turkey, pork, and beef from the Greater Washington, D.C., area. Appl Environ Microbiol. 2001;67(12):5431–5436. doi: 10.1128/AEM.67.12.5431-5436.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petri WA, Jr, Miller M, Binder HJ, Levine MM, Dillingham R, Guerrant RL. Enteric infections, diarrhea, and their impact on function and development. J Clin Invest. 2008;118(4):1277–1290. doi: 10.1172/JCI34005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giallourou N, Medlock GL, Bolick DT, et al. A novel mouse model of Campylobacter jejuni enteropathy and diarrhea. PLoS Pathog. 2018;14(3):e1007083. doi: 10.1371/journal.ppat.1007083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data generated or analysed during this study are included in this article. However, the absolute raw data are available from the corresponding author on request.