Abstract
The purpose of our study is to determine DDQ (diethyl (3,4-dihydroxyphenethylamino) (quinolin-4-yl) methylphosphonate)—a newly discovered molecule that has been shown to protect against phosphorylated tau (p-tau) in Alzheimer’s disease (AD) pathogenesis. We used a well-studied tau (P301L) transgenic mouse model to achieve our goal. We administered DDQ into 12-month-old Tau mice, at 20 mg/kg body weight intraperitoneally two times per week for 2 months. We also assessed DDQ levels in the blood, skeletal muscle and brain using biochemical and molecular techniques. We investigated the mRNA and protein levels of mitochondrial dynamics, biogenesis, synaptic, p-tau and longevity genes sirtuins in DDQ-treated tau mice using real-time quantitative PCR (q-RT-PCR), immunoblotting and immunofluorescence techniques. Our extensive pharmacodynamics investigations revealed that skeletal muscle had the greatest peak levels of DDQ, followed by serum and brain. Interestingly, DDQ-treated tau mice had higher levels of mitochondrial fusion, biogenesis, synaptic genes and sirtuins than DDQ-untreated tau mice. In addition, DDQ-treated tau mice had lower levels of mitochondrial fission and p-tau than untreated tau mice. The current findings, combined with our prior findings, firmly show that DDQ possesses anti-aging, anti-amyloid-beta and anti-p-tau properties, making it a promising molecule for reducing age-related, amyloid-beta and p-tau-induced synaptic and mitochondrial toxicities in AD.
Introduction
Alzheimer’s disease (AD) is a progressive nervous disorder that eventually destroys all mental and physical functions. AD affects 50 million people worldwide and currently there is no drug and/or agent that prevents and/or delays disease progression (1). AD is associated with phosphorylated tau (p-tau), neurofibrillary tangles (NFTs) and extracellular amyloid-beta (Aβ) plaques in regions of the brain that are responsible for learning and memory (2). In addition, AD is also associated with synaptic damage and loss of synapses, deregulation of microRNAs, hormonal imbalance, abnormal mitochondrial structural and functional alterations, and the proliferation of reactive astrocytes and microglia, and neuronal loss. Tau is the major microtubule-associated protein (MAP) of a normal mature neuron (3). The other two neuronal MAPs are MAP1 and MAP2. Tau has six molecular isoforms in the human brain. These isoforms are coded by a single gene on chromosome 17. Neurofibrillary degeneration of abnormally hyperphosphorylated tau is apparently required for the clinical expression of AD and other tauopathies (3).
Diethyl (3,4-dihydroxyphenethylamino) (quinolin-4-yl) methylphosphonate (DDQ) has a dopamine structure along with two phenolic groups, quinoline and phosphate groups in its structure. These structures in the DDQ molecule have anti-oxidant, anti-aging, anti-inflammatory, anti-amyloid and anti-tau properties. DDQ also inhibits abnormal protein–protein interactions, such as Aβ and Drp1 and p-tau and Drp1. Details of initial investigations were outlined in our earlier Human Molecular Genetics paper (4).
In AD neurons, overexpressed normal tau and/or hyperphosphorylated tau (p-tau) have been observed to impair mitochondrial axonal transport and aberrant mitochondrial distribution (5,6). Due to excessive mitochondrial fragmentation, especially in the presence of Aβ and p-tau, the mitochondria themselves tend to be more numerous and smaller in size. As mitochondrial dysfunction worsens, mitochondrial biogenesis slows, lowering the amount of ATP available for synaptic vesicles to deliver neurotransmitters to the synapse (7,8). This, in turn, also contributes to synaptic dysfunction. According to the current research, abnormal mitochondrial dynamics (increased fission and decreased fusion), mitochondrial biogenesis, defective mitophagy and synaptic function are all early and critical aspects in neurodegenerative disorders, including AD and Huntington’s disease (9,10).
Sirtuins (sirt1-sirt7) belong to the family of histone deacetylases. Sirtuins display complex cellular localization in the cytoplasm, nucleus and mitochondria. Sirtuins are conserved NAD + -dependent enzymes that display beneficial effects in many common neurodegenerative disorders such as Alzheimer’s, Parkinson’s and Huntington’s (11). Various substrates have been discovered for sirtuins, especially for sirt1, the most widely studied sirtuin in the brain (11). Sirtuins appear to be involved in the longevity-modulating role of insulin and insulin-like growth factor-I (12). In addition, sirtuins mediate the leptin-dependent inhibition of tau phosphorylation. Sirt1 also removes acetyl groups from tau, thus relieving the p300-mediated inhibition of p-tau degradation (13). Therefore, manipulation of sirtuin activity could influence age-related p-tau, and change the number of NFTs (12,13).
In our recent studies, we investigated the protective effects of DDQ against aging using in vitro mouse primary hippocampal neurons, in vivo in aged C57BL6/J mice (14) and anti-amyloid beta activity in APP mice (15). We determined the half-life period of DDQ in the blood and brain, and optimized the dose for mouse studies. Our initial analyses revealed that longevity genes, sirtuins, were upregulated in DDQ-treated HT22 cells, DDQ-treated aged wild-type mice and also in DDQ-treated APP mice. In addition, dendritic spines and the quality of mitochondria were significantly increased in DDQ-treated aged wild-type (14), DDQ-treated APP mice (15). However, it is unclear if DDQ has any protective role against mutant and/or p-tau-induced mitochondrial dynamics, biogenesis, synaptic and sirtuins in AD.
The purpose of our present study is to determine the protective effects of the newly developed molecule DDQ against p-tau in a transgenic Tau mouse model (P301L strain). We investigated pharmacokinetics, and anti-tau effects of DDQ—we (1) measured the levels of DDQ in blood, skeletal muscle and brain, (2) assessed mRNA and protein levels of mitochondrial dynamics, biogenesis, synaptic, sirtuins (longevity genes), and (3) appraised the total and phosphorylated tau.
Results
DDQ, serum, brain and skeletal muscle levels in the tau mice
In this study, DDQ was administered intraperitoneally twice a week for 2 months to 12-month-old Tau transgenic mice (n = 10). The mice were euthanized and harvested to isolate neuronal tissue found in DDQ-treated and untreated Tau mice. To determine DDQ levels, brain, serum and SM were collected in DDQ-treated mice. As shown in Figure 1, we were able to find sufficient levels of DDQ in serum, brain and SM in these mice, which indicates that DDQ successfully traversed the blood–brain barrier and entered the targeted neuronal mitochondria.
Figure 1.

Levels of DDQ in serum, brain and skeletal muscle of Tau mice. To determine whether DDQ crosses the blood–brain barrier and reaches mitochondria, using intraperitoneal injections, we administered DDQ (20 mg/kg body weight) twice a week for 2 months in 12-months-old Tau mice (n = 10). High peak values of DDQ in skeletal muscle, followed by serum and brain in 12-month-old Tau mice.
Gene expression differences between DDQ-treated tau and untreated tau mice
Several factors were measured to determine the effects of DDQ treatment on tau-induced mice including mRNA levels of mitochondrial dynamics, biogenesis, synaptic, total-tau and sirtuins genes using real-time reverse transcriptase-polymerase chain reaction (RT-PCR).
Mitochondrial dynamics genes
We found significantly decreased levels of mRNA expression levels of fission genes Drp1, by 0.80-fold (P = 0.006) and Fis1, by 0.58-fold (P = 0.0003) in DDQ-treated tau mice relative to untreated tau mice (Fig. 2A). In contrast, the levels of mRNA expression of the mitochondrial fusion genes Mfn1, by 5.65-fold (P = 0.002), Mfn2, by 2.68-fold (P = 0.005) and Opa1, by 19.14-fold (P = 0.0009) were increased in DDQ-treated tau mice compared to untreated tau mice (Fig. 2A). These findings indicate that DDQ decreases fission activity and increases fusion activity.
Figure 2.
mRNA level of mitochondrial dynamics, mitochondrial biogenesis, synaptic and Tau. 2A. represents mRNA expression of mitochondrial structural genes, 2B. represents mitochondrial biogenesis, 2C. represents synaptic (PSD95, Synaptophysin), tau and 2D. represents SIRTUIN genes in DDQ-treated tau mice compared to untreated tau mice. Fold change was calculated by the 2-ΔΔCT method. *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA followed by Holm–Sidak’s test for multiple comparisons. P < 0.05 is considered significant.
Mitochondrial biogenesis genes
Significantly increased mRNA levels were found in PGC1α, by 9.64-fold (P = 0.0002), NRF1, by 8.1-fold (P = 0.0004), NRF2, by 79.69-fold (P = 0.0002) and TFAM, by 11.16-fold (P = 0.0002) in DDQ-treated tau mice compared to untreated tau mice (Fig. 2B), suggesting that DDQ increases mitochondrial biogenesis.
Synaptic genes and tau
Significantly increased mRNA expression levels were found in synaptic genes, PSD95, by 28.76-fold (P = 0.0002), synaptophysin, by 15.75-fold (P = 0.0008), and decreased the expression of tau, by 0.57-fold (P = 0.04) in DDQ-treated tau mice compared to untreated tau mice (Fig. 2C), indicating that DDQ increases synaptic activities and decreases the total tau expression.
Sirtuins genes
mRNA levels of sirtuin genes were significantly increased in the DDQ-treated tau mice compared to untreated tau mice: sirt1, by 20.65-fold (P = 0.001), sirt2, by 1.76-fold (P = 0.012), sirt3, by 6.3-fold (P = 0.0004), sirt4, by 332.57-fold (P = 0.0011), sirt5, by 29.92-fold (P = 0.001), sirt6, by 1311.93-fold (P < 0.0001), sirt7, by 2.27-fold (P = 0.004) (Fig. 2D).
Immunoblotting analysis
To determine the beneficial effects of DDQ on protein levels, we performed an immunoblotting analysis of protein lysates prepared from DDQ-treated tau and untreated tau mice.
Mitochondrial dynamics proteins
Significantly decreased levels of fission proteins Drp1 (P = 0.03) and Fis1 (P = 0.0001) were found in DDQ-treated tau mice relative to DDQ untreated tau mice. On the contrary, mitochondrial fusion proteins Mfn1 (P = 0.001), Mfn2 (P = 0.003) and Opa1 (P = 0.003) were significantly increased in the DDQ-treated tau mice compared to untreated tau mice (Fig. 3).
Figure 3.
Immunoblotting analysis of mitochondrial dynamics in DDQ-treated tau and untreated tau mice. Representative immunoblots of DDQ-treated tau and untreated tau mice, quantitative densitometry analysis of mitochondrial dynamics-Drp1, Fis1 (fission) were significantly decreased, Mfn1, Mfn2 and Opa1 (fusion) were significantly increased in DDQ-treated tau mice and untreated tau mice. Each lane was loaded with 40 μg of total protein. Beta actin was used as loading control. N = 5 per group. Results were expressed as mean ± SEM. *p < 0.05, **p < 0.01, p < 0.001, unpaired student’s t-test.
Mitochondrial biogenesis proteins
As shown in Figure 4, significantly increased levels of biogenesis proteins PGC1α (P = 0.01), NRF1 (P = 0.03) and TFAM (P = 0.01) were found in DDQ-treated tau mice compared to untreated tau mice, indicating that mitochondrial biogenesis was increased in DDQ-treated tau mice.
Figure 4.

Immunoblotting analysis of mitochondrial biogenesis proteins in DDQ-treated tau and untreated tau mice. Representative immunoblots of DDQ-treated tau and untreated tau mice, quantitative densitometry analysis of biogenesis (PGC1α, NRF1, NRF2 and TFAM) proteins were significantly increased in DDQ-treated tau mice and untreated tau mice. Each lane was loaded with 40 μg of total protein. Beta actin was used as loading control. N = 5 per group. Results were expressed as mean ± SEM. *p < 0.05, **p < 0.01, p < 0.001, unpaired student’s t-test.
Synaptic proteins and p-tau
Significantly increased levels of synaptic proteins synaptophysin (P = 0.005) and PSD95 (P = 0.03) were found in DDQ-treated tau mice compared to untreated tau mice (Fig. 5). P-tau levels (P = 0.0001) were significantly reduced in the DDQ-treated tau mice.
Figure 5.
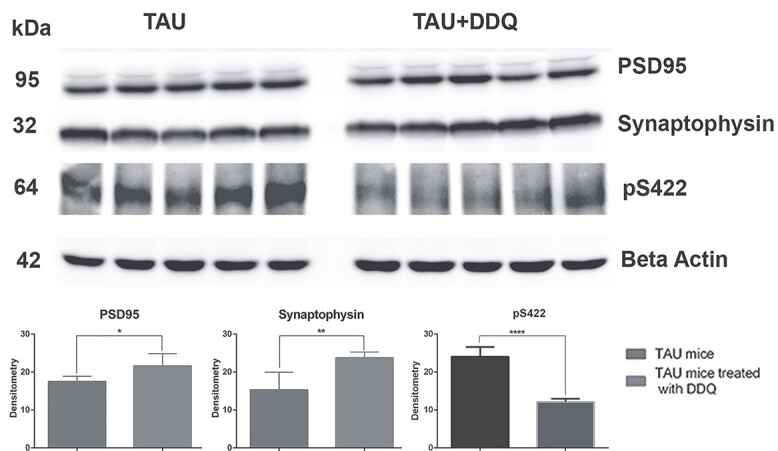
Immunoblotting analysis of synaptic, and p-tau proteins in DDQ-treated tau and untreated tau mice. Representative immunoblots of DDQ-treated tau and untreated tau mice, quantitative densitometry analysis of synaptic (PSD95, Synaptophysin) were significantly increased, and p-tau (pS422) proteins were significantly decreased in DDQ-treated tau mice and untreated tau mice (P = 0.0001).
These observations indicate that DDQ reduces mutant and/or p-tau. These observations, together with our earlier findings, strongly suggest DDQ has anti-aging [14], anti-amyloid [4,15] and anti-p-tau properties (current study).
Sirtuin proteins
We checked the quantity of Sirtuins (sirt2, sirt3, sirt5) proteins in DDQ-treated tau mice and untreated tau mice. Sirtuin proteins sirt2 (P = 0.035), sirt3 (P = 0.041) and sirt5 (P = 0.017) were significantly increased in the DDQ-treated tau mice relative to the untreated tau mice (Fig. 6).
Figure 6.

Immunoblotting analysis of SIRTUIN proteins in DDQ-treated tau and untreated tau mice. Representative immunoblotting analysis of DDQ-treated Tau mice and untreated Tau mice, quantitative densitometry analysis of Sirtuin proteins were significantly increased in DDQ-treated Tau mice and untreated Tau mice.
Immunofluorescence analysis
To determine the protective effects of DDQ on mitochondrial dynamics (Drp1, Fis1-fission; Mfn1, Mfn2 and Opa1-fusion), mitochondrial biogenesis (PGC1a, Nrf1, Nrf2 and TFAM), synaptic protein (synaptophysin and PSD95) and p-tau (AT270) levels and localizations, immunofluorescence analysis was performed in hippocampal sections of DDQ-treated tau and untreated tau mice.
Mitochondrial dynamics proteins
Significantly decreased fission proteins Drp1 (P = 0.001) and Fis1 (P = 0.003) were found in DDQ-treated tau mice relative to untreated tau mice. Mitochondrial fusion proteins Mfn1 (P = 0.04), Mfn2 (P = 0.01) and Opa1 (P = 0.001) were significantly increased in the DDQ-treated tau mice compared to untreated tau mice (Fig. 7A and B), indicating that DDQ decreases fission activity and increases fusion activity in tau mice.
Figure 7.

Immunofluorescence analysis of mitochondrial dynamics proteins in DDQ-treated tau and untreated tau mice. Representative images of immunofluorescence analysis, quantitative immunofluorescence analysis of mitochondrial dynamics-Drp1, Fis1 (fission), Mfn1, Mfn2 and Opa1 (fusion) proteins. Significantly decreased levels of Drp1 and Fis1, and significantly increased levels of Mfn1, Mfn2 and Opa1 were found in DDQ-treated tau mice compared to untreated tau mice. Results were expressed as mean ± SEM. *p < 0.05, **p < 0.01, p < 0.001, unpaired student’s t-test. N = 3 per group with 10–15 fields per mouse. Scale bars: 500 μm.
Mitochondrial biogenesis proteins
As shown in Figure 8A and B, significantly increased levels of biogenesis proteins, PGC1α (P = 0.01), NRF1 (P = 0.002) and TFAM (P = 0.009), were found in DDQ-treated tau mice compared to untreated tau mice.
Figure 8.

Immunofluorescence analysis of mitochondrial biogenesis proteins in DDQ-treated tau and untreated tau mice. Representative images of immunofluorescence analysis, quantitative immunofluorescence analysis of biogenesis (PGC1α, NRF1, NRF2 and TFAM) proteins. Significantly increased levels of PGC1α, MFN1, MFN2 and TFAM were found in DDQ-treated tau mice compared to untreated tau mice. Results were expressed as mean ± SEM. *p < 0.05, **p < 0.01, p < 0.001, unpaired student’s t-test. N = 3 per group with 10–15 fields per mouse. Scale bars: 500 μm.
Synaptic proteins and p-tau
Significantly increased levels of synaptic proteins synaptophysin (P = 0.002) and PSD95 (P = 0.008) were found in DDQ-treated tau mice compared to untreated tau mice (Fig. 9A and B). P-tau levels (P = 0.01) were significantly reduced in the DDQ-treated tau mice (Fig. 10A and B).
Figure 9.

Immunofluorescence analysis of synaptic proteins in DDQ-treated tau and untreated tau mice. Representative images of immunofluorescence analysis, quantitative immunofluorescence analysis of synaptic proteins. Significantly increased levels of synaptic proteins (PSD95, Synaptophysin) in DDQ-treated tau mice compared to untreated tau mice. N = 3 per group with 10–15 fields per mouse. Scale bars: 500 μm.
Figure 10.

Immunofluorescence analysis of phosphorylated-tau in DDQ-treated tau and untreated tau mice. Representative images of immunofluorescence analysis, quantitative immunofluorescence analysis of phosphorylated tau. Significantly decreased levels of p-tau in DDQ-treated tau mice compared to untreated tau mice. Images of phosphorylated tau were taken at 10X, 60X and 100X the original magnification. N = 3 per group with 10–15 fields per mouse. Scale bars: 500 μm (10X), 20 μm (60X) and 2 μm (100X).
Discussion
In the current study, we determined the protective effects of the newly discovered molecule DDQ against p-tau in AD. Our current blood–brain barrier studies indicate that DDQ reached brain. Our extensive pharmacokinetic analysis of DDQ revealed high DDQ peak values in skeletal muscle, followed by serum and brain in 12-month-old tau mice. In the preliminary investigation, using real-time RT-PCR, immunoblotting and immunofluorescence analysis, we measured mRNA and protein levels of mitochondrial dynamics, mitochondrial biogenesis and synaptic genes, p-tau, sirtuins in DDQ-treated tau and untreated tau mice. Decreased mRNA and protein levels of fission genes, p-tau and increased levels of mitochondrial fusion, biogenesis, synaptic, sirtuin genes were found in DDQ-treated tau relative to untreated tau mice. Phosphorylated Tau levels were significantly reduced in DDQ-treated tau mice relative to untreated tau mice. Current study findings strongly suggest that a DDQ decreases the levels of p-tau, maintains mitochondrial dynamics, and enhances mitochondrial biogenesis, synaptic activity and sirtuins in tau mice.
Our extensive pharmacokinetic analysis of DDQ revealed high DDQ peak values in skeletal muscle, followed by serum and brain in 12-month-old tau mice. Our tau mice pharmacokinetics findings concur with the 24-month wild-type (14), and APP mice (15). High DDQ levels in the brain, skeletal muscle and blood strongly suggest that DDQ may have strong anti-aging, anti-amyloid-beta and anti-p-tau properties.
To understand the impact of DDQ on mitochondrial dynamics, biogenesis and synaptic genes, we treated tau mice and assessed mRNA levels. As expected, mitochondrial biogenesis, mitochondrial fusion and synaptic genes were increased, and mitochondrial fission and p-tau were significantly decreased in DDQ-treated tau mice. Our current study observations concur with our previous findings in wild-type aged and APP mice (14,15). Recent mitochondrial studies revealed that abnormal mitochondrial dynamics (increased fission and decreased fusion) play a large role in the disease process, leading to mitochondrial dysfunction and neuronal damage in neurodegenerative diseases, including Alzheimer’s (16–18).
To determine if DDQ enhances longevity, we studied mRNA and protein levels of longevity genes sirtuins. As shown in the results section, both mRNA and protein levels of sirtuins were increased in DDQ-treated Tau mice relative to untreated Tau mice. Our current findings of sirtuins agree with observations of sirtuins from our previous study of aged WT and APP mice. These observations strongly indicate that DDQ has not only anti-aging and anti-amyloid but also anti-p-tau properties. It is known that sirtuins are reduced/declined with age and age-related neurological diseases (19–22). DDQ’s ability to enhance sirtuins has truly therapeutic value for aging and age-related diseases. We also found reduced levels of P-tau in DDQ-treated Tau mice relative to DDQ-untreated Tau mice.
DDQ protective mechanistic mechanisms
Dopamine structure was used as the base for making DDQ and two phenolic groups; quinoline and phosphate groups were added in its structure. These structures have anti-oxidant, anti-aging, anti-inflammatory, anti-amyloid and anti-tau properties. In addition, DDQ also inhibits abnormal protein–protein interactions, such as Aβ and Drp1 and p-tau and Drp1. Because of having multiple protective properties, DDQ was expected to reduce toxic insults of aging, oxidative damage, anti-amyloid and anti-p-tau and other toxicities generated due to abnormal protein–protein interactions. Current study findings of anti-p-Tau concur with our predictions of DDQ properties (4,14,15).
In summary, DDQ reached the brain, enhanced mitochondrial biogenesis, mitochondrial fusion, synaptic genes, and decreased fission and p-tau expressions. Based on these observations together with our studies of cell culture (4), aged wild-type mice (14) and transgenic APP mice (15), we cautiously propose that DDQ is a potential drug for further clinical studies and a promising molecule to treat patients with AD. Having high DDQ levels in the brain, skeletal muscle and blood firmly suggests that DDQ may have strong anti-aging, anti-amyloid beta and anti-p-tau properties, and could be a promising molecule to treat both early- and late-onset AD patients.
Materials and Methods
Chemicals and reagents
In our earlier studies, we designed and synthesized a DDQ molecule (molecular weight: 430.43 g/mol, with a purity >97%). To further investigate these properties, we requested Aurora Fine Chemical LLC to manufacture the DDQ molecule. DDQ was dissolved in 3% dimethyl sulfoxide (DMSO) and then diluted with normal saline to a detectable concentration; 1–5% DMSO and 95–99% saline were the final vehicle concentrations (14,15).
Animals
A total of 20 tau transgenic mice (P301L) (C57BL6) aged 12 months were purchased from The Jackson Laboratory (Bar Harbor, ME). The study had separated the animals into a treated or untreated group; 10 Tau animals received DMSO used as a control for this study. All animals were housed under air-conditioned rooms at a constant temperature of 22°C with a 12 h light/dark cycle and given access to water and food ad libitum. The animal study was approved by Texas Tech University Health Sciences Center—Institutional animal care and use committee (TTUHSC-IACUC).
Pharmacokinetic analysis
For the pharmacokinetic study, each of the 10 mice in the treated group received one dose of DDQ (20 mg/kg) twice a week for 2 months. Half-life and optimization had been previously determined and detailed in our previous publications (14,15). The serum was immediately isolated at 5000 rpm for 5 min at room temperature by centrifugation and stored frozen at −80°C before the study. The brain tissues and skeletal muscle were collected and preserved before an examination, frozen at −80°C. The examination of DDQ was performed with the developed quantitative method as described earlier (14,15). Protein precipitation extraction was carried out as described earlier (14,15).
Measurement of mRNA by qRT-PCR
Total RNA was isolated from the 50 mg of cerebral cortex tissues of untreated tau, and DDQ-treated tau mice as described earlier (23). Oligonucleotide primers were then designed using Primer-BLAST software for each of the genes (housekeeping genes b-actin and GAPDH; mitochondrial dynamics; mitochondrial biogenesis; synaptic genes; and sirtuin family genes). Using SYBR Green chemistry-based quantitative real-time RT-PCR, mRNA expression of the above-mentioned genes was also measured, as described earlier (6,23,24).
Immunoblotting analysis
Protein extracts from the cerebral cortex tissue were obtained by homogenization in RIPA lysis and extraction buffer as described earlier (14,15). Immunoblotting analysis was performed for mitochondrial dynamics, biogenesis, synaptic and sirtuin proteins. Details of antibody dilutions were given in Table 1. The blots were detected in chemiluminescent detection using ECL with ImageQuant LAS-4000 (GE Healthcare Life Sciences) as described earlier (14,15).
Table 1.
Summary of antibody dilutions used in the immunoblotting analysis of mitochondrial dynamics, mitochondrial biogenesis, synaptic, p-tau, sirtuin proteins in DDQ-treated tau and untreated tau mice
| Marker | Primary antibody-species and dilution | Purchased from company, city and state |
Secondary antibody, dilution |
|---|---|---|---|
| DRP1 | Rabbit Polyclonal 1:1000 | Protein Tech Group | Donkey anti-rabbit HRP 1:10000 |
| FIS1 | Rabbit Polyclonal 1:500 | Novus Biologicals | Donkey anti-rabbit HRP 1:10000 |
| MFN1 | Rabbit Polyclonal 1:500 | Protein Tech Group | Donkey anti-rabbit HRP 1:10000 |
| MFN2 | Rabbit Polyclonal 1:1000 | Novus Biologicals | Donkey anti-rabbit HRP 1:10000 |
| OPA1 | Rabbit Polyclonal 1:1000 | Novus Biologicals | Donkey anti-rabbit HRP 1:10000 |
| PGC1α | Rabbit Polyclonal 1:1000 | Novus Biologicals | Donkey anti-rabbit HRP 1:10000 |
| NRF1 | Rabbit Polyclonal 1:1000 | Cell Signaling Technology | Donkey anti-rabbit HRP 1:10000 |
| NRF2 | Rabbit Polyclonal 1:1000 | Novus Biologicals | Donkey anti-rabbit HRP 1:10000 |
| TFAM | Rabbit Polyclonal 1:2000 | Abcam | Donkey anti-rabbit HRP 1:10000 |
| PSD95 | Rabbit Polyclonal 1:1000 | Cell Signaling Technology | Donkey anti-rabbit HRP 1:10000 |
| SYNAPTOPHYSIN | Rabbit Polyclonal 1:3000 | Novus Biologicals | Donkey anti-rabbit HRP 1:10000 |
| P-TAU | Mouse Monoclonal 1:1000 | 4BioDx | Sheep anti-mouse HRP 1:10000 |
| SIRT-2 | Rabbit Polyclonal 1:1000 | Cell Signaling Technology | Donkey anti-rabbit HRP 1:10000 |
| SIRT-3 | Rabbit Polyclonal 1:1000 | Cell Signaling Technology | Donkey anti-rabbit HRP 1:10000 |
| SIRT-5 | Rabbit Polyclonal 1:1000 | Cell Signaling Technology | Donkey anti-rabbit HRP 1:10000 |
| B-Actin | Mouse Monoclonal 1:2000 | Millipore Sigma | Sheep anti-mouse HRP 1:10000 |
Immunofluorescence analysis
The brain sections that were stored in −80°C were first fixed with 1% paraformaldehyde in PBS for 10 min, and then washed three times with TBST. Next, the sections were blocked with SuperBlock™ (PBS) Blocking Buffer (Thermo Scientific) at room temperature for 1 h. Subsequently, the sections were then incubated with primary antibodies overnight (further outlined in Table 2). After incubation, the tissues were washed three times with TBST for 10 min each. The tissues were then ready to be incubated with a secondary antibody conjugated with Alexa Fluor 488 and Alexa Flour 594 (Thermo Scientific), at room temperature for 1 h. Once again, the tissues were washed three times with TBST and mounted on the slides. Microscopy of the sections was completed using an automated fluorescence microscope (Olympus IX83). Photographs were taken of 10–15 fields of each section at 10X, 60X and 100X magnifications; the relative immunoreactivities of these antibodies were quantified and statistical significance were assessed. A quantitative analysis using NIH ImageJ was performed on background-subtracted digital images (25).
Table 2.
Summary of antibody dilutions used in the immunofluorescence analysis of mitochondrial dynamics, mitochondrial biogenesis, synaptic, p-tau proteins in DDQ-treated tau and untreated tau mice
| Marker | Primary antibody-species and dilution | Purchased from company, city and state |
Secondary antibody, dilution |
|---|---|---|---|
| DRP1 | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| FIS1 | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| MFN1 | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| MFN2 | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| OPA1 | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| PGC1α | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| NRF1 | Rabbit Polyclonal 1:100 | Abcam | Goat anti-rabbit Alexa Flour 488 1:200 |
| NRF2 | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| TFAM | Rabbit Polyclonal 1:100 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| PSD95 | Mouse Monoclonal 1:250 | Invitrogen | Goat anti-mouse Alexa Flour 594 1:200 |
| SYNAPTOPHYSIN | Rabbit Polyclonal 1:250 | Novus Biologicals | Goat anti-rabbit Alexa Flour 488 1:200 |
| P-TAU | Mouse Monoclonal 1:100 | Thermo Scientific | Goat anti-mouse Alexa Flour 594 1:200 |
Statistical analysis
Both paired and unpaired t-tests were used for analyzing statistical significance between the two groups. For this study, a P-value less than 0.05 is considered as statistical significant. All analyses were performed by GraphPad Prism (version 6.0; GraphPad Software, La Zolla, CA).
Authors Contributions
P.H.R., M.V. and C.B. contributed to the conceptualization and formatting of the article. M.V, M.G. and L.E.B. performed experiments, and analyzed the data. M.V. and P.H.R. are responsible for writing, original draft preparation and finalization of the manuscript. P.H.R. is responsible for funding acquisition.
Contributor Information
Murali Vijayan, Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA.
Mathew George, Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA.
Lloyd E Bunquin, Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA.
Chhanda Bose, Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA.
P Hemachandra Reddy, Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA; Neuroscience & Pharmacology, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA; Neurology, Departments of School of Medicine, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA; Public Health Department of Graduate School of Biomedical Sciences, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA; Department of Speech, Language and Hearing Sciences, School Health Professions, Texas Tech University Health Sciences Center, Lubbock, TX 79430, USA.
Funding
The research presented in this article was supported by the National Institutes of Health (NIH) grants AG042178, AG047812, NS105473, AG060767, AG069333, AG066347 and R41 AG060836.
Declaration of Competing Interest. A patent is pending for the discovery of molecule, DDQ, and we have a minority financial interest with a small business company abSynapTex, LLC, based in Lubbock, TX.
References
- 1. Wingo, A.P., Liu, Y., Gerasimov, E.S., Gockley, J., Logsdon, B.A., Duong, D.M., Dammer, E.B., Robins, C., Beach, T.G., Reiman, E.M. et al. (2021) Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat. Genet., 53, 143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kandimalla, R., Manczak, M., Yin, X., Wang, R. and Reddy, P.H. (2018) Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Hum. Mol. Genet., 27, 30–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iqbal, K., Liu, F., Gong, C.X. and Grundke-Iqbal, I. (2010) Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res., 7, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuruva, C.S., Manczak, M., Yin, X., Ogunmokun, G., Reddy, A.P. and Reddy, P.H. (2017) Aqua-soluble DDQ reduces the levels of Drp1 and Aβ and inhibits abnormal interactions between Aβ and Drp1 and protects Alzheimer's disease neurons from Aβ- and Drp1-induced mitochondrial and synaptic toxicities. Hum. Mol. Genet., 26, 3375–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vossel, K.A., Zhang, K., Brodbeck, J., Daub, A.C., Sharma, P., Finkbeiner, S., Cui, B. and Mucke, L. (2010) Tau reduction prevents Abeta-induced defects in axonal transport. Science, 330, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Manczak, M., Mao, P., Calkins, M.J., Cornea, A., Reddy, A.P., Murphy, M.P., Szeto, H.H., Park, B. and Reddy, P.H. (2010) Mitochondria-targeted antioxidants protect against amyloid-beta toxicity in Alzheimer’s disease neurons. J. Alzheimers Dis., 20, S609–S631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reddy, P.H., Manczak, M., Yin, X., Grady, M.C., Mitchell, A., Tonk, S., Kuruva, C.S., Bhatti, J.S., Kandimalla, R., Vijayan, M. et al. (2018) Protective effects of Indian spice curcumin against amyloid Beta in Alzheimer’s disease. J. Alzheimers Dis., 61, 843–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reddy, P.H. and Oliver, D.M. (2019) Amyloid Beta and Phosphorylated tau-induced defective autophagy and Mitophagy in Alzheimer's disease. Cell, 8, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manczak, M., Calkins, M.J. and Reddy, P.H. (2011) Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: implications for neuronal damage. Hum. Mol. Genet., 20, 2495–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shirendeb, U., Reddy, A.P., Manczak, M., Calkins, M.J., Mao, P., Tagle, D.A. and Reddy, P.H. (2011) Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington’s disease: implications for selective neuronal damage. Hum. Mol. Genet., 20, 1438–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lalla, R. and Donmez, G. (2013) The role of sirtuins in Alzheimer's disease. Front. Aging Neurosci., 5, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jęśko, H., Wencel, P., Strosznajder, R.P. and Strosznajder, J.B. (2017) Sirtuins and their roles in brain aging and neurodegenerative disorders. Neurochem. Res., 42, 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Min, S.W., Cho, S.H., Zhou, Y., Schroeder, S., Haroutunian, V., Seeley, W.W., Huang, E.J., Shen, Y., Masliah, E., Mukherjee, C. et al. (2010) Acetylation of tau inhibits its degradation and contributes to tauopathy. Neuron, 67, 953–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vijayan, M., Bose, C. and Reddy, P.H. (2021) Anti-brain aging effects of small molecule inhibitor DDQ. Mol. Neurobiol., 58, 3588–3600. [DOI] [PubMed] [Google Scholar]
- 15. Vijayan, M., Bose, C. and Reddy, P.H. (2021) Protective effects of a small molecule inhibitor, DDQ against amyloid Beta in Alzheimer’s disease. Mitochondrion, 59, 17–29. [DOI] [PubMed] [Google Scholar]
- 16. Li, S., Yin, J., Nielsen, M., Beach, T.G., Guo, L. and Shi, J. (2019) Sirtuin 3 mediates tau Deacetylation. J. Alzheimers Dis., 69, 355–362. [DOI] [PubMed] [Google Scholar]
- 17. Wang, X., Su, B., Siedlak, S.L., Moreira, P.I., Fujioka, H., Wang, Y., Casadesus, G. and Zhu, X. (2008) Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proc. Natl. Acad. Sci. U. S. A., 105, 19318–19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calkins, M.J. and Reddy, P.H. (2011) Amyloid beta impairs mitochondrial anterograde transport and degenerates synapses in Alzheimer's disease neurons. Biochim. Biophys. Acta, 1812, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Julien, C., Tremblay, C., Emond, V., Lebbadi, M., Salem, N., Bennett, D.A. and Calon, F. (2009) Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J. Neuropathol. Exp. Neurol., 68, 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yin, J., Han, P., Song, M., Nielsen, M., Beach, T.G., Serrano, G.E., Liang, W.S., Caselli, R.J. and Shi, J. (2018) Amyloid-β increases tau by mediating Sirtuin 3 in Alzheimer's disease. Mol. Neurobiol., 55, 8592–8601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duan, W. (2013) Sirtuins: from metabolic regulation to brain aging. Front. Aging Neurosci., 5, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pugazhenthi, S. (2017) Metabolic syndrome and the cellular phase of Alzheimer's disease. Prog. Mol. Biol. Transl. Sci., 146, 243–258. [DOI] [PubMed] [Google Scholar]
- 23. Reddy, P.H., Yin, X., Manczak, M., Kumar, S., Pradeepkiran, J.A., Vijayan, M. and Reddy, A.P. (2018) Mutant APP and amyloid beta-induced defective autophagy, mitophagy, mitochondrial structural and functional changes and synaptic damage in hippocampal neurons from Alzheimer's disease. Hum. Mol. Genet., 27, 2502–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vijayan, M., Kumar, S., Yin, X., Zafer, D., Chanana, V., Cengiz, P. and Reddy, P.H. (2018) Identification of novel circulatory microRNA signatures linked to patients with ischemic stroke. Hum. Mol. Genet., 27, 2318–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hegde, V., Vijayan, M., Kumar, S., Akheruzzaman, M., Sawant, N., Dhurandhar, N.V. and Reddy, P.H. (2019) Adenovirus 36 improves glycemic control and markers of Alzheimer's disease pathogenesis. Biochim. Biophys. Acta Mol. basis Dis., 1865, 165531. [DOI] [PubMed] [Google Scholar]





