Abstract
Context
Research participants’ informed consent is integral to the protection of human subjects; studies exploring the enhancement of standard informed consent processes have had mixed success in increasing patients’ understanding of complex research protocols.
Objective
To determine the effect of a “study map,” a flow diagram of a research protocol, on research participants’ understanding of research purpose and procedures.
Design
This study was an experimental posttest-only design using 30 research participants enrolling in a study of decision making and recovery among living kidney donors. Participants were randomly assigned to the standard care group (verbal description with consent documents) or the experimental group (standard of care plus study map). An instrument measured perceived and objective understanding, and the differences between groups were determined by an independent t test.
Discussion
The high level of comprehension in the control group made detecting improvements in understanding difficult. Objective knowledge and perceived understanding were positively related, suggesting the importance of periodically confirming comprehension with research participants during the informed consent process. Future research should examine the effect of study maps in patients with lower educational levels.
Results
Knowledge levels were high in all participants (mean objective = 3.7 on a 5-point scale, SD = 1.02; mean subjective = 9.3 on a 10-point scale, SD = 1.29). There was a significant relationship between objective knowledge and perceived understanding (r = 0.56, P = .001); however, the study map itself had no significant effect on objective or perceived understanding.
There is a long history, dating back to the period after World War II, that emphasizes the importance of protection of human subjects and respect for the autonomy of those considering participation in research, balancing the tension between science and ethics.1 Three specific historical events, the 1946 Nuremberg Doctors Trial, the 1960s thalidomide tragedy, and the 1972 Tuskegee syphilis study exposé,2 have shaped the protections regulations that all researchers must follow. In their report, Integrity in Scientific Research: Creating an Environment That Promotes Responsible Conduct, the Institute of Medicine3 emphasizes that the responsibility for the protection of human subjects rests with both the institution and the individual researcher. In addition, the Code of Ethics for Nursing4 emphasizes that nurses have an ethical obligation to protect the health, safety, and rights of participants in research. The code4 mandates that all research participants be provided with the necessary information to make an informed decision to consent, including the nature of their participation, potential harms and benefits, alternatives to participation, and plans for data protection. These components of informed consent must be provided to lay public research volunteers in a clear understandable way if true consent is to be achieved. Despite widespread agreement on these ethical norms, however, the limitations of informed consent in research have been documented in several studies.5-11 One remedy for the limitations of research participants’ understanding of the information being presented has been the use of decision aids. These have included multi-media presentations, modified forms, extended discussion, and combinations of these methods.5 Investigators who use such aids must weigh their value in enhancing participants’ understanding against the added time required for the consent process.
Decision aids have been tested to support patient decisions about medical treatments6 and joining research studies.7-10 In a systematic review of decision aids to support medical treatments, Kennedy6 found that common outcomes examined were the decisions made, knowledge about the decision, and the decision-making process. He emphasized the need to measure those outcomes of importance to the patient, such as satisfaction with the decision process. He also noted the importance of ensuring that the aids were practical for use in large randomized clinical trials. Dunn and Jeste11 conducted a systematic review of decision aids in 34 studies that included 16 with research consent and 18 with treatment consent. They found that 11 of the 16 studies involving research consent increased participants’ understanding. However, the effect of the aids was difficult to measure because of the advanced educational level and vocabulary skills of some participants, as these characteristics were often correlated with greater understanding of the study protocol.
Decision aids in research studies alone were examined in a systematic review by Flory and Emanuel.5 In 30 studies of aids used in 42 trials, varied levels of success were reported, although the authors found that studies that included individual discussion with the potential research participant were most effective in enhancing understanding. We found no study that examined the use of icons in a study map to enhance the research consent process. Therefore, the purpose of the present study was to evaluate the effect of a study map on participants’ understanding of the purpose and procedures of the parent study. The purpose of the parent study was to describe how individuals decided to be a living kidney donor. Because persons who seek to donate a kidney may be anxious to present themselves as the ideal donor, they may quickly agree to participate in research without adequately reflecting on the requirements of their participation. A study map may have the effect of reinforcing what is being asked of him/her in the proposed research of the parent study.
Methods
Procedure
This experimental posttest-only control group design study and the parent study were approved by the institutional review board at Johns Hopkins University. Participants in the study map study were randomly assigned to a group that received a printed consent form and verbal explanation of the parent research study (usual care group) or that information in addition to a study map (Figure 1). The study map used icons to symbolize the components of the study, the sequence of events, and the compensation options. Components of the study included completing surveys, possibly being interviewed, allowing the researcher to view medical records, and returning surveys by mail. The sequence of events included activities at baseline, 3, 6, and 12 months. Compensation options included 3 different gift card choices for a grocery store, a coffee chain, or a retail store. The study map also indicated that both donors and those who ultimately did not donate would be included in the parent study.
Figure 1.
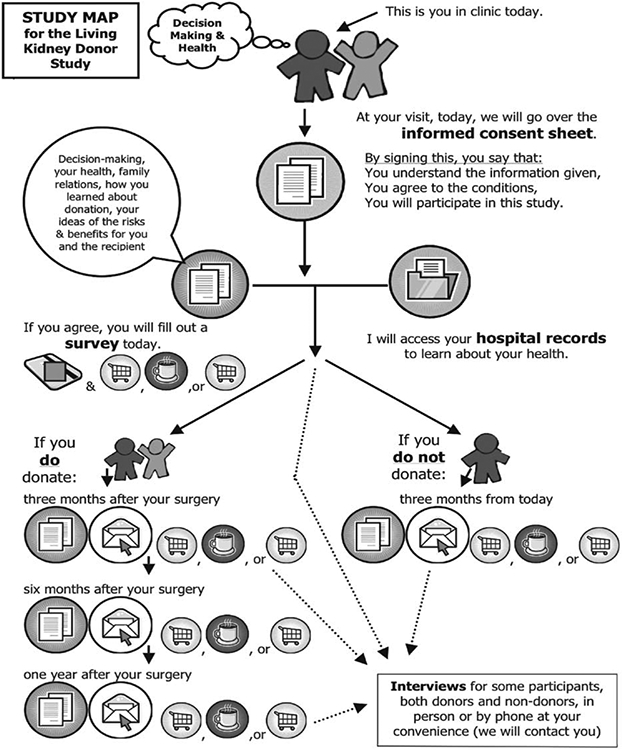
Study map used to supplement standard informed consent document and enrollment discussion.
The principal investigator of the present study to evaluate the study map is a master’s-prepared nurse who was also the project director of the parent study and was responsible for enrolling patients in the parent study.
The investigator designed a 7-item instrument that included 5 multiple-choice items measuring objective knowledge about aspects of the protocol (Figure 2). Multiple-choice items included questions about study purpose, informed consent, and compensation. Participants were given a score of 1 point for each of the 5 items answered correctly. Also included were 2 items that used a 10-point visual analogue scale (VAS) to measure perceived understanding and usefulness of the study map. The first VAS item asked, “How clear is it to you what people who join this study will do?” The available responses ranged from 0 (very confusing) to 10 (very clear). The second VAS item stated, “Was the study map helpful in understanding the study?” The response selection ranged from 0 (not at all helpful) to 10 (very helpful).
Figure 2.

One of 2 versions of a researcher-designed survey tool was given to all participants with the intent of quizzing each on the protocol of the parent study and evaluating the perceived clarity and helpfulness of the map. The version pictured here was for the study map (experimental) group.
Sample
The sample in the parent study was an accidental sample of all donor candidates being evaluated in the living kidney donor clinic of The Johns Hopkins Hospital. The sample for the present study map study was a convenience sample of a subset of 30 of these participants. Inclusion criteria included persons 18 and older who read and spoke English and were without severe visual impairment.
Data Analysis
Descriptive statistics described the sample, and an independent t test was used to determine the difference in both perceived understanding and objective knowledge between the usual care and experimental groups. Perceived usefulness of the study map was also described. An independent t test was performed to determine whether there were differences in objective knowledge and perceived understanding between participants with high school education or less and participants with some college or more education. Pearson correlation was used to determine whether the participants’ perceived understanding and objective knowledge were related. A Mann-Whitney U test was used to examine the difference between men and women in objective knowledge and perceived understanding.
Results
Thirty donor candidates were approached, and all agreed to participate in this study. Characteristics of the study sample are summarized in Table 1. There were no significant differences in education level, sex, or age between the 2 groups. Table 2 summarizes the results of independent t tests examining the difference in objective knowledge and perceived understanding between the usual care and study map groups. There were no differences between the groups. To determine whether the participant’s educational level had an impact, an independent t test was performed to measure both objective knowledge and perceived understanding in those with high school or less education and those with some college or more education. Neither objective knowledge (P = .53) nor perceived understanding (P = .45) differed significantly between the 2 educational levels.
Table 1.
Sample characteristics
| Age, y | ||
| Mean (SD) | 41.6 (11.0) | |
| Range | 21 - 63 | |
| Sex | ||
| Female | 20 | (67) |
| Male | 10 | (33) |
| Education | ||
| ≥Some college | 23 | (77) |
| ≤High school | 7 | (23) |
| Race | ||
| White | 24 | (80) |
| African American | 2 | (7) |
| Other/decline to answer | 4 | (13) |
Table 2.
Objective knowledge and perceived understanding for the usual care and study map groups
| Variable | Possible score | Usual care group | Study map group | t (df) | P |
|---|---|---|---|---|---|
| Mean (SD) |
Mean (SD) |
||||
| Objective knowledge | 0-5 | 3.57 (1.22) | 3.81 (0.83) | −0.638 (28) | .53 |
| Perceived understanding | 0-10 | 9.07 (1.59) | 9.44 (0.96) | −0.773 (28) | .45 |
| Usefulness of study map | 0-10 | 9.38 (1.36) |
A Pearson correlation revealed a significant relationship between objective knowledge and perceived understanding (r = 0.562, P = .001), indicating that participants’ perception of their understanding was accurate. A Mann-Whitney U test revealed no difference between men and women in their perceived understanding (P = .13). However, women had a significantly higher level of objective knowledge (mean, 17.88) than did men (mean, 10.75; P = .04).
The perceived usefulness of the study map, rated on another 0 (very confusing) to 10 (very clear) VAS, was generally high among all participants who were approached to join the parent study with this protocol. Of the 16 research participants who saw the map, most found it to be helpful in understanding the parent study. Twelve of the 16 rated it as helping the presentation be a “very clear” score of “10,” with 1 participant responding a “5,” 2 responding “8,” and 1 responding “9.”
Discussion and Implications
Participants in this study had a fairly high level of both objective knowledge and perceived understanding, and the lack of variation in these variables may have made it difficult to detect any impact of the study map. A possible reason for the high levels of both objective knowledge and perceived understanding of the protocol was the context of the study. Participants were all being evaluated for a possible surgery. Because this is a high-risk situation, they may have been particularly vigilant in their attention to information presented. The education level of the sample is another possible factor to be considered. The fact that 77% of the participants had some college education may have led to underestimation of the impact of the study map on understanding. In a systematic review, Dunn and Jeste11 reported that educational level and vocabulary skills were frequently correlated with better performance on measures of understanding. A systematic review by Flory and Emanuel5 also indicated that low educational level was associated with lower understanding scores.
Further study is needed to determine whether the finding that men are more likely than women to overreport their understanding occurs in other instances where informed consent is obtained for research participation.
From the perspective of the research project director, a study map ensures that all potential research candidates are given the same information in a format that is easy to understand. Because acquiring informed consent from new participants is so important, standardizing the study information given in addition to the informed consent document ensures complete regulation of the message given to research candidates, which may be especially important if multiple study staff members are charged with getting consent from new participants.
In addition to implications for research informed consent, future study may also explore the usefulness of the techniques described in this article and others in clinical decisions, such as the process by which living donors provide consent for surgery. Because of the complexity of the living donor experience, including testing, matching, preoperative laboratory tests, surgery, and follow-up care, research may explore the usefulness of an icon-based study map in the consent process.
As previously discussed, living donor candidates sometimes exhibit behaviors consistent with social desirability,12 presumably in an effort to be chosen as a donor for their intended recipient. A study map such as the one designed for this study may prove helpful in obtaining true informed consent from donor candidates, both for research and, potentially in the future, for surgery.
The correlation between objective knowledge and perceived understanding suggests that individuals can accurately self-report in a single question at the end of the consent process the extent to which they understand various aspects of the study. Measuring objective knowledge with multiple items may not be necessary. The Office of Human Subjects Research of the National Institutes of Health emphasized that respect for persons necessitates that participants in research be given the opportunity to choose what does or does not happen to them.13 Health professionals obtaining informed consent from potential research participants should continue to seek innovative ways to promote understanding of complex study protocols.
Acknowledgments
The authors express their gratitude to the Living Donor Study Team, especially Laura Taylor, rn, phd, and the health care team of the Johns Hopkins Hospital Comprehensive Transplant Center.
Financial Disclosures
This project was funded as a part of the National Institute of Nursing Research Grant R01 NR008727-01A1, Factors Related to Living Donor Decision Making and Outcomes, Nolan, MT (principal investigator).
References
- 1.Bankert EA, Amdur RJ. Institutional Review Board: Management and Function. Sudbury, MA: Jones and Bartlett Publishers; 2006. [Google Scholar]
- 2.Dunn CM, Chadwick GL. Protecting Study Volunteers in Research: A Manual for Investigative Sites. 3rd ed. Boston, MA: CenterWatch, Inc; 2004. [Google Scholar]
- 3.Committee on Assessing Integrity in Research Environments, National Research Council, Institute of Medicine. Integrity in Scientific Research: Creating an Environment That Promotes Responsible Conduct. Washington, DC: The National Academies Press; 2002. [PubMed] [Google Scholar]
- 4.American Nurses Association. Code of Ethics for Nurses With Interpretive Statements. 2001. http://nursingworld.org/MainMenuCategories/EthicsStandards/CodeofEthicsforNurses.aspx. Accessed August 2, 2011.
- 5.Flory J, Emanuel E. Interventions to improve research participants’ understanding in informed consent for research: a systematic review. JAMA. 2004;292(13):1593–1601. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy AD. On what basis should the effectiveness of decision aids be judged? Health Expect. 2003;6(3):255–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aaronson NK, Visser-Pol E, Leenhouts GH, et al. Telephone-based nursing intervention improves the effectiveness of the informed consent process in cancer clinical trials. J Clin Oncol. 1996;14(3):984–996. [DOI] [PubMed] [Google Scholar]
- 8.Campbell FA, Goldman BD, Boccia ML, Skinner M. The effect of format modifications and reading comprehension on recall of informed consent information by low-income parents: a comparison of print, video, and computer-based presentations. Patient Educ Couns. 2004;53(2):205–216. [DOI] [PubMed] [Google Scholar]
- 9.Coletti AS, Heagerty P, Sheon AR, et al. Randomized, controlled evaluation of a prototype informed consent process for HIV vaccine efficacy trials. J Acquir Immune Defic Syndr. 2003;32(2):161–169. [DOI] [PubMed] [Google Scholar]
- 10.Coyne CA, Xu R, Raich P, et al. Randomized, controlled trial of an easy-to-read informed consent statement for clinical trial participation: a study of the Eastern Cooperative Oncology Group. J Clin Oncol. 2003;21(5):836–842. [DOI] [PubMed] [Google Scholar]
- 11.Dunn LB, Jeste DV. Enhancing informed consent for research and treatment. Neuropsychopharmacology. 2001;24(6):595–607. [DOI] [PubMed] [Google Scholar]
- 12.Corley MC, Elswick RK, Sargeant CC, Scott S. Attitude, self-image, and quality of life of living kidney donors. Nephrol Nurs J. 2000;27(1):43–50, 51-52. [PubMed] [Google Scholar]
- 13.Office of Human Subjects Research, National Institutes of Health. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. 1979. http://ohsr.od.nih.gov/guidelines/belmont.html. Accessed July 29, 2011.


