Abstract
The right hemisphere is involved with the integrative processes necessary to achieve global coherence during reasoning and discourse processing. Specifically, the right temporal lobe has been proven to facilitate the processing of distant associate relationships, such as generating novel ideas. Previous studies showed a specific swing of alpha and gamma oscillatory activity over the right parieto-occipital lobe and the right anterior temporal lobe respectively, when people solve semantic problems with a specific strategy, i.e., insight problem-solving. In this study, we investigated the specificity of the right parietal and temporal lobes for semantic integration using transcranial Random Noise Stimulation (tRNS). We administered a set of pure semantics (i.e., Compound Remote Associates [CRA]) and visuo-semantic problems (i.e., Rebus Puzzles) to a sample of 31 healthy volunteers. Behavioral results showed that tRNS stimulation over the right temporal lobe enhances CRA accuracy (+12%), while stimulation on the right parietal lobe causes a decrease of response time on the same task (−2,100 ms). No effects were detected for Rebus Puzzles. Our findings corroborate the involvement of the right temporal and parietal lobes when solving purely semantic problems but not when they involve visuo-semantic material, also providing causal evidence for their postulated different roles in the semantic integration process and promoting tRNS as a candidate tool to boost verbal reasoning in humans.
Keywords: Creativity, Reasoning, Problem-solving, tRNS, Semantic integration, Neuromodulation
Introduction
Creativity is expressed by the recombination of existing knowledge to create new and meaningful associations (Beaty et al., 2020; Bendetowicz et al., 2018; Kenett et al., 2018; Kenett & Faust, 2019). Mednick was among the first to develop an associative theory of creativity postulating that the generation of novel ideas involves accessing previously unconnected remote concepts, or dissimilar thought elements (Mednick, 1962). Mednick theory was operationalized using the Remote Associates Test - RAT (Mednick, 1968) consisting of two sets of 30 triads of words, where each word can be associated with a fourth word by creating a compound word, via semantic association, or synonymy (Mednick, 1968). RAT problems, or its alternative version named Compound Remote Associate (CRA) (Bowden & Jung-Beeman, 2003), where the words can be exclusively combined forming a compound word, have been extensively used to study creativity, insight problem-solving, and overall convergent thinking (e.g., Jung-Beeman et al., 2004; Salvi et al., 2015a, 2020a, b; Sprugnoli et al., 2017; Santarnecchi et al. 2019).
Former research showed that creative people searching process is facilitated by a network characterized by a broader range of associations across their lexicon network (Gruszka & Necka, 2002) and by more associative links that can connect faster than less creative individuals (Rossmann & Fink, 2010). Specifically, as theorized by Mednick (1962), higher fluency and uncommonness of associations characterize creative people. However, the organization of associative memory is similar between highly creative and less creative subjects, with their creativity performance essentially relying on the speed of creating new associations and thus, uncommon responses (Benedek & Neubauer, 2013). Creatives, indeed, give lower estimates of the semantic distance between unrelated word pairs compared with less creative subjects (Rossmann & Fink, 2010). Recent work by Kenett and colleagues (Kenett et al., 2014, 2018) examined the difference in semantic network organization between low and high creative persons showing that the former has “steep,” modular, and less connected semantic memory network compared with a “flat,” more flexible semantic memory organization that characterizes creative people. A new corpus of research on semantic networks theoretically grounded the associative theory of creativity and robustly demonstrated that creative ability is related to flexible structures of semantic memory (Kenett & Faust, 2019).
Research on the neural bases of insight problem-solving upholds the importance of semantic integration of distant associate concepts when generating novel ideas. Insight is an unconscious method of problem-solving, also called “Aha!” moment, in which the subject reaches a solution in an unexpected, unpredictable, and sudden manner that could occur in perception, language comprehension, everyday problem-solving, and in many scientific discoveries as part of the creative cognition process (Kounios & Beeman, 2014; Peña et al., 2019; Salvi et al., 2020b). It contrasts with the analytical methods of problem-solving, which involves a systematic and voluntary research approach, often based on previous knowledge that can usually be explained by the subjects (Jung-Beeman et al., 2004; Kounios & Beeman, 2014).
One of the seminal experiments in the insight field demonstrated that people recognize solutions more quickly when the solution-related information is presented in the left-visual hemifield. The result suggested that information processes in the right hemisphere play an important role in insight problem-solving (Bowden & Beeman, 1998). Following experiments allowed to better localize increased neural activity over the right Anterior Temporal Lobe (rATL) a few seconds before participants solved CRA problems via insight (Jung-Beeman et al., 2004; Subramaniam et al., 2009). The specific sequence of activity seen was: an increase in alpha (α) activity on the right parieto-occipital cortex (rPOC) registered 900 ms before participants reported having an insight, followed by a burst of gamma (γ) activity over the rATL approximately 300 ms before the button press, i.e., presumably at the realization of the problem’s solution (Jung-Beeman et al., 2004). The authors suggested the α activity over the rPOC would be responsible for the selective gating of the visual inputs that allows to internally focusing on the problem, while the rATL would be responsible for the integration of distant semantic relations, leading to the final insight, thus correct solution (Jung-Beeman et al., 2004; Salvi et al., 2015a, 2016). Whereas several brain regions are involved in insight problem-solving (Sprugnoli et al., 2017; Subramaniam et al., 2009; Tik et al., 2018), rATL activation is interpreted as being a critical area for making connections between distantly related information during comprehension, allowing for the birth of new ideas (Kounios & Beeman, 2014; Shen et al., 2017; Salvi, et al., 2020a). Similarly, the rATL is involved in integrative processes necessary to achieve global coherence during reasoning and discourse elaboration (St George et al., 1999), understanding novel metaphoric expressions, implicit comprehension, and humor (Bartolo et al., 2006; Manfredi et al., 2017; Mashal et al., 2007). This explanation is grounded in the anatomical hemispheric asymmetry of neuronal networks, where the right hemisphere engages in relatively coarser semantic coding than the left hemisphere (Jung-Beeman, 2005) underlined by both structural neurobiological findings of asymmetric neuronal wiring and neuropsychological results of language deficits caused by right hemisphere brain damage (Joanette et al., 1990). For example, patients with injuries in the right hemisphere show problems in understanding jokes metaphors, or indirect requests (Joanette et al., 1990; St George et al., 1999). The idea is that when people meet a word or concept and information is processed in the right hemisphere, a broad but weak semantic field is activated (Jung-Beeman, 2005). This includes a field of properties and characteristics corollary to the word concept. Thus, each word’s semantic field is more likely to overlap with other words and concepts, facilitating drawing inferences, comprehending figurative language and metaphors (Mashal et al., 2007; Virtue et al., 2006). By contrast, when concepts are processed in the left hemisphere, smaller but stronger semantic fields are activated. This means that only few words and/or concepts closely related to the target are activated, thus limiting the possibility to explore distant semantic or conceptual relations (Chiarello et al., 1990).
Previous works explored the two oscillatory patterns singularly, deconstructing the alpha-gamma effects and independently studying the attention components or the singular involvement of the right temporal lobe (Cerruti & Schlaug, 2009; Luft et al., 2018; Salvi et al., 2020a; Santarnecchi et al., 2019) via transcranial Electrical Stimulation (tES), a noninvasive neuromodulatory technology that can causally probe the involvement of a brain region in a specific task by modulating its activity (Antal et al., 2017). To shed light on the relevance of both right parietal and temporal cortices in general semantic integration, i.e., regardless of the problems-solving strategy adopted (insight or analytical), we investigated the effects of a new neuromodulation technique—transcranial Random Noise Stimulation (tRNS), in participants solving a pure linguistic integration task and a visuo-semantic one.
Different tES techniques, each carrying different and complementary opportunities, have been used in the semantic integration field as well as in the broader field of creativity research (Luft et al., 2018; Lustenberger et al., 2015; Salvi et al., 2020a). Transcranial Alternating Current Stimulation (tACS) applies an alternating sinusoidal current that continuously changes its polarity at a frequency selected by the experimenter (e.g., 10 Hz), transcranially inducing an oscillatory pattern and therefore “entraining” (i.e., amplifying) ongoing spontaneous or evoked oscillatory brain activity (Antal et al., 2017; Santarnecchi et al., 2013). The postulated mechanism for the observed behavioral effects (e.g., entrainment of neuronal firing (Reed & Cohen Kadosh, 2018)) has been confirmed in nonhuman models: tACS applied on alert primates demonstrated entrainment of neuronal spiking to the frequency of stimulation at the target brain region (Krause et al., 2019). Following the theory of Kounios, Beeman and Bowden suggesting the involvement of right parietal and temporal areas with specific effects of oscillatory activity on insight problem-solving (Jung-Beeman et al., 2004), tACS has been successfully applied in participants solving CRA. In particular, γ tACS (e.g., 40 Hz) applied to the right temporal lobe improved CRA accuracy (Santarnecchi et al., 2019). On the other hand, transcranial Direct Current Stimulation (tDCS) delivers direct electrical current via at least two electrodes (i.e., one anode and one cathode), modulating the membrane potential—and thereby the excitability—of the underlying neuronal population in a polarity-specific manner: anodal currents generally provide an excitatory effect, whereas cathodal stimulation elicits inhibitory effects (Lefaucheur et al., 2017). Previous studies applying tDCS on temporal lobes to enhance semantic integration have reported mixed results. Salvi et al. found increased accuracy on CRA tasks (Salvi et al., 2020a), whereas Aihara et al. (2017) found no effects on both semantic and nonsemantic integration tasks, and Ruggiero et al. (2018) reported only a decrease in reaction times during RAT.
Transcranial Random Noise Stimulation (tRNS) delivers alternating current oscillating at random frequencies in the 100–500 Hz range, thus not requiring the selection of a specific frequency thought to be relevant for such a function (as in tACS) as well as eliminating the problem of concurrent inhibition effects related to the cathode (as with tDCS) (Terney et al., 2008). Behavioral effects observed in humans after/during tRNS delivered in the high-frequency ranges are supposedly due to the stochastic resonance mechanism derived from the injection of “random noise” on the stimulated neuronal populations, which increases their excitability (Pavan et al., 2019; Reed & Cohen Kadosh, 2018; van der Groen & Wenderoth, 2016). Studies have shown effects on attention, memory, perceptual learning, corticospinal excitability (Contemori et al., 2019; Shalev et al., 2018; Snowball et al., 2013; Terney et al., 2008), as well as perceptual and visual training in patients (Herpich et al., 2019; Moret et al., 2018), promoting tRNS as a potential tool for semantic integration modulation. In this regard, tRNS has been applied so far only over left DLPFC, reporting a significant improvement on RAT scores respect to the sham group (Peña et al., 2019); however to date, there have been no attempts to modulate the activity of right cortical regions.
Therefore, in the current study we applied tRNS in a double-blind, placebo-controlled design in 31 healthy participants during the performance of the CRA as well as a visuo-semantic integration task (Rebus Puzzles). Our hypotheses were: 1) tRNS will increase accuracy at CRA-semantic task when delivered over the right temporal lobe, given its role in semantic coding and creation of distant semantic relations independently from the problem-solving method adopted (i.e., insight or analytical process); 2) tRNS will not enhance performance when delivered on parietal cortex and when applied during the visuo-semantic task, giving the relevant role of these regions in pure semantic integration.
Materials & methods
Participants
Thirty-one healthy subjects (mean age 24.4 ± 3.8; 17 females) were enrolled in the study after giving written informed consent. The study, part of a comprehensive project including other types of noninvasive stimulation and methods (Santarnecchi et al., 2019), was approved by the Local Ethical Review Board in Siena (Italy). All participants were healthy, native Italian speakers. One was left-handed as assessed by the Oldfield Handedness questionnaire (Oldfield, 1971). Exclusion criteria included the use of medications or illicit substances acting on the central nervous system in the 30 days preceding the experiment or on the same day, alcohol consumption on the same day of the experiment or the preceding evening, abnormalities in the neurological or psychiatric examinations, and pregnancy. All participants were naïve regarding the neurostimulation techniques, and no participants reported previous practice with the type of insight tasks selected for the experiment. The same pool of participants also took part in a related experiment investigating the impact of tACS on insight capabilities (Santarnecchi et al., 2019).
Experimental procedures
Each participant performed an experimental session composed of 3 blocks of each task (i.e., CRA and Rebus Puzzles (Salvi et al., 2015)) while receiving tRNS (corresponding to T8 or P4 electrodes in the 10/20 EEG system) or Sham (i.e., placebo) stimulation in a within subjects design (Fig. 1). Stimuli and instructions were presented using E-prime 2.0 software (Psychology Software Tools, Inc., PA, USA). Participants were comfortably seated in a quiet room, positioned approximately 50 cm from an LCD screen, wearing insulating headphones to facilitate focus on the tasks. Subjects performed a training session consisting of solving a few examples of CRA and Rebus Puzzles before starting the actual experiment.
Fig. 1. Experimental design.
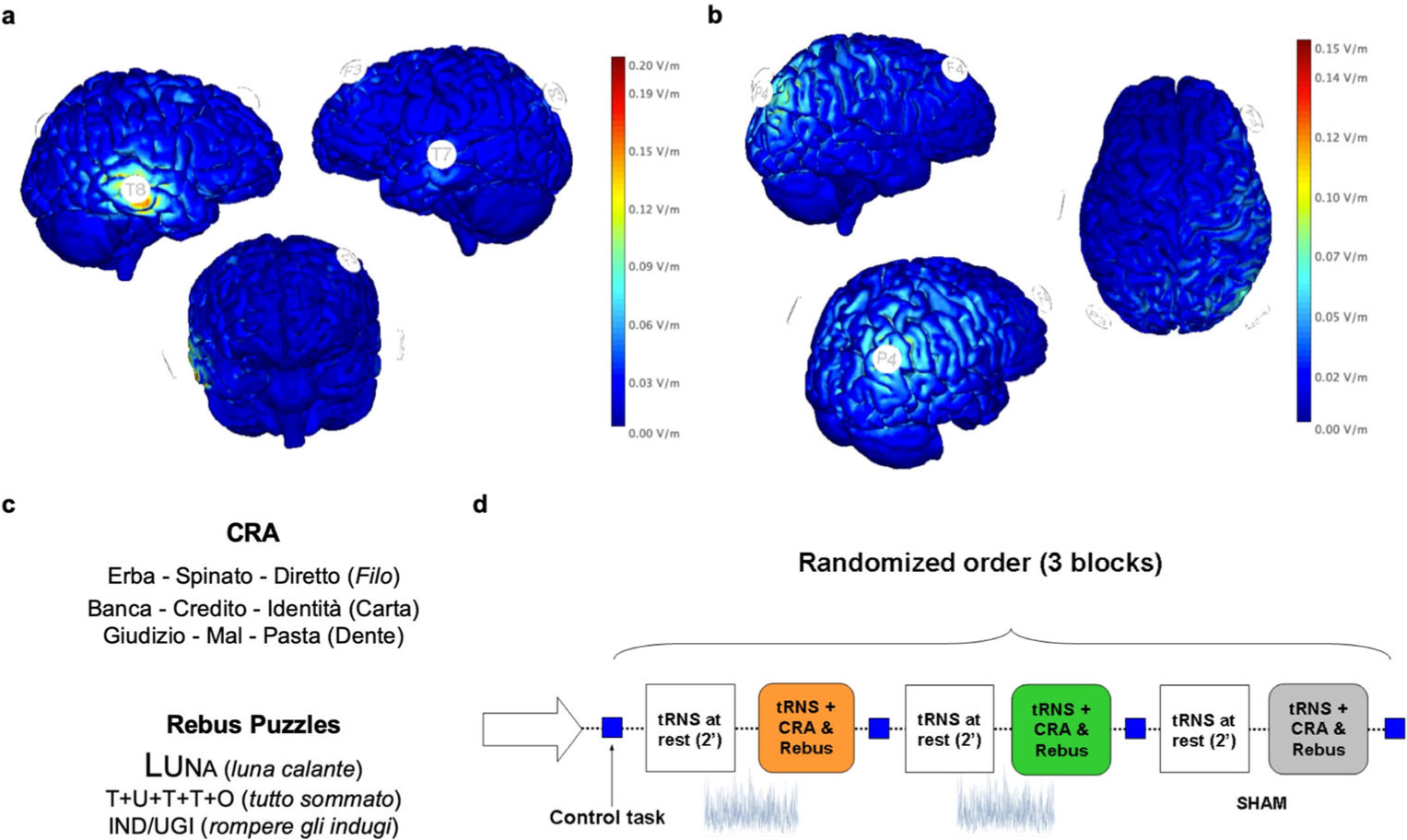
The theoretical model of semantic integration proposed by Jung-Beeman and colleagues (2004) suggests the specific involvement of right parietal and anterior temporal cortices in the insight process strictly associated with different brain oscillations. Giving our aim of testing the relevance of right temporal and parietal areas in semantic reasoning independently from the specific mechanism involved (i.e., insight or analytical process), tRNS was delivered (100–500 Hz) at these locations while participants solved CRA and Rebus Puzzles. We adopted a multifocal stimulation template (i.e., multiple return electrodes) to maximize the electric field on the target regions (i.e., stimulation electrode) corresponding to T8 in the 10/20 EEG system for the right anterior temporal lobe (a) and to P4 for the right parietal area (b). As shown by the normal electric fields of both stimulation templates in panels a and b, the multifocal approach allowed to minimize/abolish the current distribution under the return electrodes. c Examples’ trials of Italian semantic (CRA) and visuo-semantic (Rebus Puzzles) problems solved by participants. d Schematic representation of the experimental session composed by 3 stimulation blocks in randomized order (tRNS on P4, tRNS on T8 and Sham, mean duration = 7 minutes), interleaved by a 10-minutes pause (no stimulation) comprising also an odd-even control task assessing reaction times. CRA = Compound Remote Associate task; tRNS = transcranial Random Noise Stimulation; Sham = placebo stimulation
Stimuli were presented in the center of the LCD display for 10 seconds, and participants were instructed to provide answers as accurately and quickly as possible by pressing the spacebar with their preferred finger. After a button-press or 10 seconds—whichever occurred first—a text window appeared asking the participants to input their answer. After each stimulation block, subjects performed an odd-even reaction time task to assess vigilance levels.
tRNS
Transcranial Random Noise Stimulation (tRNS; 100–500 Hz) was delivered using a Starstim neurostimulator (Neuroelectrics, Barcelona) at an intensity equivalent to 2,000 μA peak-to-peak, separately over the right parietal lobe (P4) and right temporal lobe (T8) over different stimulation blocks. The theoretical model proposed by Jung-Beeman et al. (2004) suggests a right dominance for the insight process, specifically involving the parieto-occipital region and the temporal lobe. Therefore, to test for the functional relevance of such regions regardless the specific strategy adopted (i.e., insight or analytical method), stimulation of the right parietal lobe (roughly corresponding to electrode P4 in the 10–20 EEG system), anterior temporal lobe (electrode T8), and Sham (placebo) were delivered in randomized order to each participant (Fig. 1). To guarantee an adequate focality of each stimulation pattern, a multifocal approach was adopted (Ruffini et al., 2013), where one target electrode and multiple “return” ones were strategically placed on the scalp. In order to keep the intensity fixed on the target electrode (i.e., T8 and P4), 3 electrodes were positioned on the following locations and given one third of the stimulation intensity directed to T8/P4 each: for tRNS on T8, return electrodes on P3, T7, F3 (Fig. 1a); for tRNS on P4, return electrodes on F4, P3, T7 (Fig. 1b). This ensured the maximal current density on the target regions with very low-intensity stimulation being delivered on other sites, therefore theoretically reducing the efficacy of stimulation on the return electrodes. The induced electric fields for the 2 stimulation templates are reported in Fig. 1a–b. Stimulation intensity was ramped up for 30 seconds, then tRNS was delivered for 2 minutes while participants sat still staring at a crosshair on the LCD monitor and kept constant for the duration of a single tRNS block or ramped down after 30’ in Sham block (see Fig. 1 for a graphical depiction of the stimulation montages and information about experimental design and stimuli). The initial resting-state stimulation was included to potentially facilitate the stochastic resonance phenomenon between exogenous and endogenous oscillatory activity. Additionally, between each stimulation block, participants performed an odd-even reaction time task to assess vigilance levels. The experiment was performed on a single day, with a pause of 10 minutes between each stimulation block, including the odd-even reaction task. The total duration of stimulation for each block was approximately 7 minutes, including the 2 minutes of tRNS delivered “at rest.”
Tasks
Each experimental block was composed of 15 randomized semantic CRA problems followed by 11 visuo-semantic Rebus Puzzles problems in order of ascending difficulty.
Semantic - CRA problems
Participants first solved the Italian version of the recently validated CRA problems (Salvi et al., 2015b) (see Fig. 1c; for the original English version see (Bowden & Jung-Beeman, 2003)). These types of problems have been consistently used to study semantic integration (Bowden et al., 2005; Jung-Beeman et al., 2004; Kounios & Beeman, 2014). Each problem is formed by three words, and the solution is represented by a fourth word that can be associated with the others to form a compound word (i.e., manners, round, tennis; solution: table). We selected 105 items and divided them into 7 sets of 15 items each, balanced for difficulty and method of problem-solving usually applied (e.g., insight or analytical process) using the normative data reported in (Salvi et al., 2015a). Three random blocks (15 trials each) were selected for each participant. CRA was displayed in Times New Roman font size 34 on PowerPoint slides at the center of the screen.
Visuo-semantic rebus puzzles
Participants also solved the Italian version of the Rebus Puzzles (Salvi et al., 2015b). Unlike the CRA, this set of problems requires the integration of both visual and semantic information to find a common phrase fitting each item. As example, the “Cycle, Cycle, Cycle” Rebus Puzzle is solved with “Tricycle,” which requires merging the repetition of the word “cycle.” The Italian Rebus Puzzle “LUNA” (the Italian translation of “Moon”) indicates the “Descending moon” given the descending characters’ size (see Fig. 1c for other Italian examples; for the English version see (MacGregor & Cunningham, 2008)). We divided all trials into 7 sets of 11 trials each, balanced for difficulty and strategy usually adopted (Salvi et al., 2015b); 3 blocks (11 trials each) were randomly selected for each participant.
Statistical design and analysis
Accuracy and Reaction Times (RT) on correct responses were collected for both CRA and Rebus Puzzles tasks, regardless of the strategy adopted to solve them. Analyses were carried out using IBM SPSS Statistics (Version 21, release 21.0.0) and MATLAB (Mathworks, Massachusetts). Data were filtered for outliers (mean ± 2 SD of accuracy and RT values, respectively 7% and 13% of the overall trials). A repeated measures ANCOVA was used to investigate main effects and interactions of 1) Stimulation (Sham, tRNS-P4, tRNS-T8) and 2) Task (CRA, Rebus), with within-subject factors for both correct accuracy and correct reaction times. Gender, age, and the order of stimulation conditions were added as covariates. In the event of a significant effect of stimulation, further simple main effects were analyzed using a similarly structured ANCOVA to decompose the effect. In the event of interaction between stimulation and task type and a subsequent significant simple main effect of stimulation on a specific trial type, pairwise comparisons were performed to elucidate the nature of the effect. In the event of a violation of Mauchly’s test of sphericity, we employed multivariate measures. For all tests, the level of significance was set at p ≤ 0.05, and Bonferroni multiple comparisons correction was applied for the pairwise comparisons.
Results
CRA
Accuracy
tRNS over the right temporal area improved accuracy in CRA compared with sham stimulation. On average, during the Sham (= placebo) condition participants correctly solved 8.25 (SD = 2.8) CRA trials, equal to 54% of the presented problems. Statistical analysis revealed a significant effect of Stimulation [F(2,28) = 4.34, p < 0.001] and Task type [F(1,29) = 4.16, p < 0.001], as well as a significant Stimulation*Task type interaction [F(2,29) = 3.62, p < 0.01]. Regarding Stimulation effects, tRNS was significantly different from Sham [t(30) = 3.41, p < 0.008] (Fig. 2). In particular, the Stimulation*Task type interaction showed a significant effect for tRNS over the temporal lobe during CRA [t(30) = 4.85, p < 0.001; Cohen’s d = 0.85] (Fig. 2).
Fig. 2. Results for CRA.
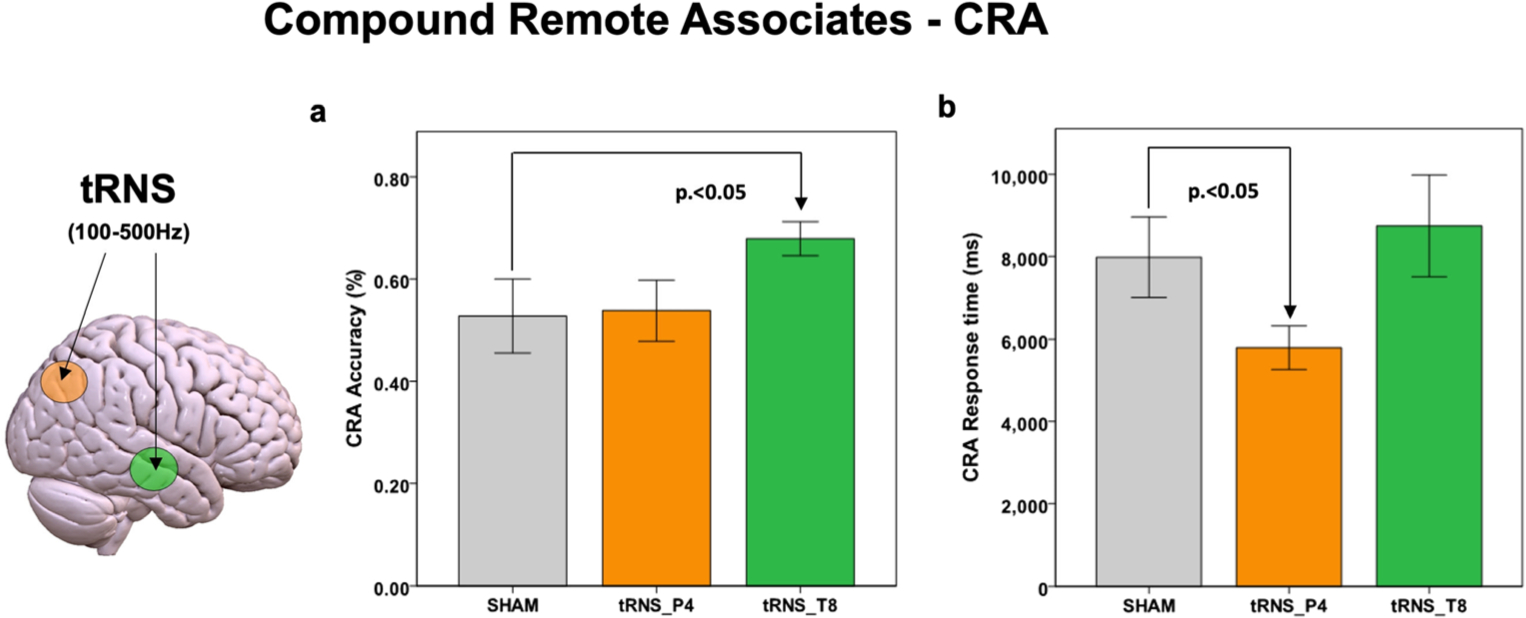
Accuracy (a) and response times for correct answers (b) at CRA (pure semantic integration tasks) are reported for both tRNS and Sham conditions. Statistical results refer to an ANCOVA model including age, gender and stimulation order as covariates (Bonferroni corrected). Lines represent standard errors of the mean. Arrow indicates the significant effect. CRA = Compound Remote Associates task; tRNS = transcranial Random Noise Stimulation; Sham = placebo stimulation.
tRNS on P4 was not significantly different than Sham [t(30) = 1.42, p = 0.396]. The average increase in accuracy during tRNS over T8 compared with Sham condition was 12%, whereas it was 2% when tRNS was delivered on P4 respect to Sham condition.
Reaction times
tRNS over the right parietal lobe improved reaction times for correct response at CRA compared with sham stimulation (Fig. 2). Statistical analysis revealed a significant effect for Stimulation [F(2,28) = 3.26, p = 0.008] but not for Task type [F(1,29) = 1.21, p = 0.545]. In particular, the Stimulation*Task type interaction showed a significant effect for tRNS over the parietal lobe (P4) for correct response at the CRA task [t(30) = 4.85, p < 0.001] (average decrease in reaction times vs. sham = 2,100 ms, Cohen’s d = 1.38), with no effect for tRNS over T8 [t(30) = 1.04, p = 0.582] (Fig. 2).
Rebus puzzles
Accuracy
For the Rebus Puzzles, participants correctly solved 8.68 (SD = 2.4) trials, equal to 78% of the presented problems. The Stimulation*Task type interaction showed no effect on Rebus Puzzles trials [t(30) = 1.21, p = 0.692] (Fig. 3).
Fig. 3. Results for Rebus Puzzles.
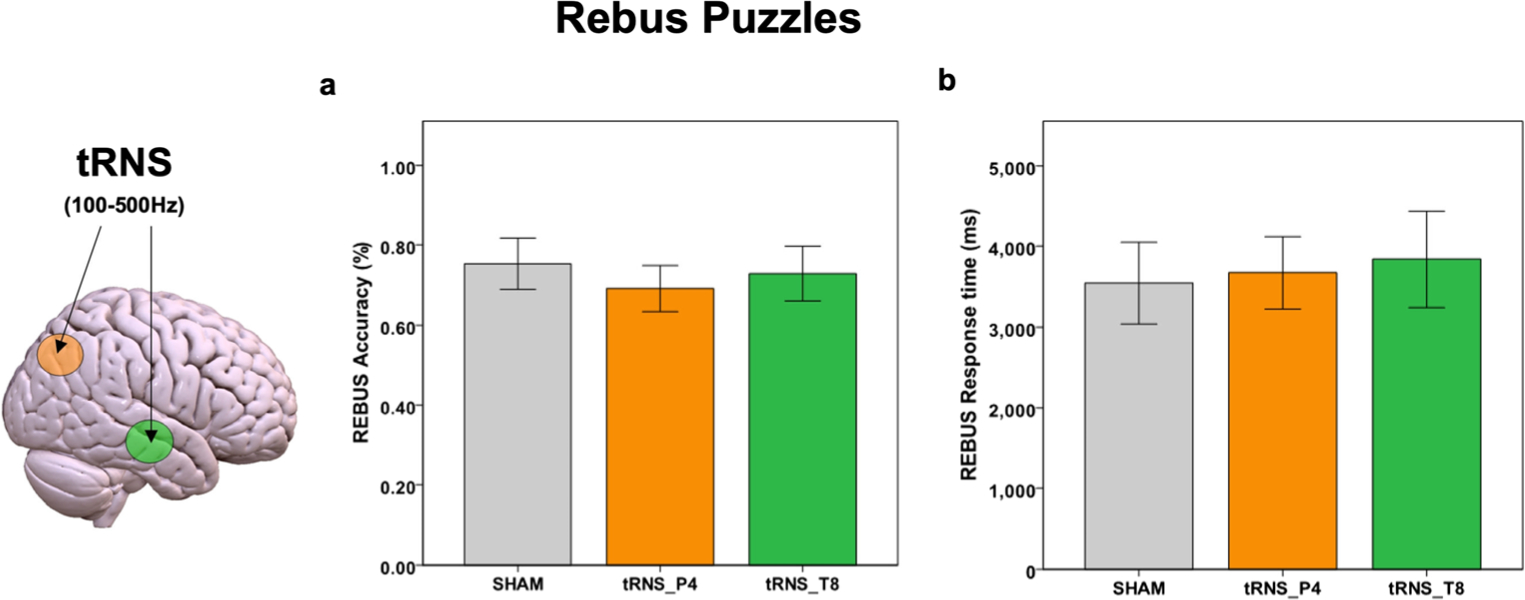
Accuracy (a) and response times for correct answers (b) for Rebus Puzzles (visuo-semantic integration task) are reported for both tRNS and Sham conditions. Statistical results refer to an ANCOVA model including age, gender and stimulation order as covariates (Bonferroni corrected). Lines represent standard errors of the mean. tRNS = transcranial Random Noise Stimulation; Sham = placebo stimulation.
Reaction times
The mean reaction times of correct responses are shown in Fig. 3. The Stimulation*Task type interaction did not showed effect on Rebus Puzzles in general [t(30) = 0.76, p = 0.745] (Fig. 3). Performances at CRA and Rebus Puzzles showed a weak correlation with each other across our participants (r = 0.24, p = 0.358).
Control task
Analyses of the odd/even task revealed significant main effects of the Order in which blocks were presented over RTs [F(3,81) = 3.15, p < 0.05]. Pairwise comparisons revealed that the only significantly different block was the first one [block 1 vs. block 2: t(23) = 3.47, p < 0.05; block 1 vs. block 3: t(23) = 2.19, p < 0.05; block 1 vs. block 4: t(23) = 2.10, p < 0.05, all other pairwise comparisons were not significant (p > 0.2)]. The same analyses were performed with blocks ordered by the stimulation type they followed, an important control that could detect whether any of the stimulation types had general after-effects on RT or accuracy levels. No significant differences were observed on RT and accuracy [RT: F(3,72) = 1.32, p > 0.325, Accuracy: F(3,72) = 1.53, p > 0.276].
Discussion
Prior fMRI and EEG research highlighted a crucial role for right parietal and temporal cortices during semantic integration in problem-solving (Sandkühler & Bhattacharya, 2008; Jung-Beeman et al., 2004). The oscillatory involvement of right temporal areas has been further recently demonstrated via tACS modulation in the gamma band for insight strategy (Santarnecchi et al., 2019). Albeit the specific role of such right hemisphere regions in the general semantic integration process remains unanswered, current findings demonstrate the causal involvement of the right parietal and temporal cortex on semantic-related processing, regardless of the problem-solving strategy adopted. On the other hand, the absence of behavioral effects of tRNS in these regions for the Rebus Puzzles task allows us to speculate on the existence of a specificity for semantic information in the right hemisphere.
The causal involvement of the right posterior parietal and temporal lobe in the semantic general process was postulated by correlational evidence (Jung-Beeman et al., 2004) and, moreover, by patients with brain lesions (Joanette et al., 1990; St George et al., 1999). The importance of the right temporal cortex for semantic integration aligns with its role in integrating distant or novel semantic relations during language comprehension (Bottini et al., 1994; Humphries et al., 2001), as in the integration of discourse processing (St George et al., 1999). These findings fit with the hypothesis (as well as with neuroanatomical evidence (Kounios & Beeman, 2014)) of a temporal role in coarse semantic field (Chiarello et al., 1990; Kounios & Beeman, 2014) that represents a crucial step for integrative processes necessary to achieve global coherence during reasoning and discourse processing (Kounios & Beeman, 2014; Salvi et al., 2020a). Finally, in a series of experiments applying 10 Hz-tACS (alpha frequency) on right temporal lobe in participants solving CRA, RAT, and an alternative uses task, Luft and colleagues showed an increase in unusual responses for alternate uses, further confirming the relevance of right temporal lobe for semantic processing, integration and associations (Luft et al., 2018).
The right parietal cortex seems to be involved in revealing false semantic relations on provided statements, suggesting its role in inference and inhibition processes necessary for determining semantic coherence (Raposo & Marques, 2013). Also, a role for the right parieto-occipital cortex in the suppression of visual inputs had been suggested and supported across studies, especially in relations to alpha activity (Kounios & Beeman, 2014; Salvi et al., 2015a, 2016; Salvi and Bowden 2016). Indeed, Luft and colleagues nicely showed that alpha oscillations in right hemisphere are responsible of suppressing dominant and common associations for both convergent and divergent reasoning (Luft et al., 2018). Notably, researchers applied tACS on T8 using large sponge electrodes (25 cm2) that reasonably have also affected the right parietal cortex, confirming previous literature and fitting to our findings that show an involvement of right parietal cortex in suppressing relevant information, irrespective to the specific frequency applied (Luft et al., 2018).
Our results show a reduction in reaction times for correct answers when tRNS is delivered on the right parietal cortex, aligning with its possible involvement in the initial phases of suppression of irrelevant information and representation that in turn speeds up the process (i.e., reduce response time) rather than increasing the number of correct answers. By delivering tRNS, we might have been able to increase the excitability of the right parietal cortex without forcing it in a specific oscillatory activity—and thus on a specific problem-solving strategy, leading to the observed decrement in response time.
The absence of concordant results in literature about the effects of stimulation of the temporal lobe in promoting CRA performance is probably related to the different protocols adopted among tDCS studies. The influence of cathodal stimulation (i.e., inhibition), which varied in positioning across the experiments with even an extracephalic montage (Aihara et al., 2017), needs to be considered. Additionally, there is discordance among the selected temporal target region (between F8 and T8 in (Ruggiero et al., 2018), 1.5 cm anterior to T4 in (Aihara et al., 2017), between T8 and FT8 in (Chi & Snyder, 2011), and over T8 in (Salvi et al., 2020a)). All of these variables, together with different stimulation parameters (i.e., intensity and duration), as well as with stimulating electrodes’ diameter could have significantly affected the current flow and thus caused varied and not comparable behavioral changes.
Finally, the absence of effects on performance on Rebus Puzzles is concordant with the previous tACS experiment in the same participants as the current study (Santarnecchi et al., 2019) and may indicate that different cortical networks are required for specific types of semantic/visuo-semantic tasks. Indeed, the CRA and Rebus Puzzles are profoundly different, with CRA requiring only a semantic (purely verbal) integration and Rebus Puzzles an integration of both verbal and visual information. Accordingly, performances at CRA and Rebus Puzzles showed only a weak correlation with each other in our sample, further corroborating the hypothesis that they do not activate the same neurophysiological substrates and likely do not measure the very same process. Unfortunately, no fMRI studies are testing the specific brain areas activations during Rebus Puzzles task, which could help to disentangle its neural substrates. On the other hand, ceiling effects could not be totally excluded in the light of the baseline performance obtained at Rebus Puzzles, both in terms of accuracy and RT.
tRNS is the most recently validated type of tES, introduced by Terney and colleagues in 2008 (Terney et al., 2008). In preclinical models, the mechanism of tRNS has been linked to the enhancement of synchronization among firing neurons via the amplification of subthreshold (i.e., noise) oscillatory activity (Reed & Cohen Kadosh, 2018). Even if the dominant physiological mechanism is still not completely understood, behavioral effects of tRNS have been demonstrated on multiple scales and domains (Antal et al., 2017; Santarnecchi et al., 2015), often exceeding the ones observed with other tES techniques (Inukai et al., 2016; Vanneste et al., 2013), especially if delivered with a full-band condition (100–700 Hz) (Moret et al., 2019). The current results suggest tRNS could be a promising technique to enhance semantic integration abilities, independently from the dominant cortical oscillatory activity and thus from the specific strategy adopted. The possibility of testing the involvement of a specific region without a priori selecting any frequency represents a fundamental advantage of random noise stimulation, especially compared with tACS, which requires a defined frequency (i.e., 10 Hz or 40 Hz) at which deliver alternating current stimulation. This restriction poses four main issues with tACS: 1) the accurate selection of a relevant oscillatory frequency crucial for the execution of a specific task in a target region, that may be not clearly obvious or previously defined in literature; 2) the individual variability of oscillatory activities, especially in higher ranges of oscillations, might reduce the effects if not perfectly tuned; 3) the necessity to test at least another control frequency to dissociate the nonspecific effect of stimulation relative to the frequency-specific ones; and 4) finally, the possible induction of phosphenes that can annoy the subjects as well as make them aware of stimulation condition (real vs. sham). In this context, tRNS is an advantageous cost-effective neuromodulation technique that can assess the relevance of a region’s activity in a single session paradigm, by simply applying all the frequencies in the 100–500 Hz range, ultimately enhancing brain cortical excitability (Reed & Cohen Kadosh, 2018). TRNS also avoids concurrent inhibitory effects of cathodal stimulation seen during tDCS. Specifically, differently than tDCS and tACS, tRNS delivers noise to each electrode, thus providing similar effects to all electrodes. During tDCS, the polarity of every electrode is defined (e.g., anodal or cathodal) and constantly maintained to assure that direct current flows from the anode to the cathode. The same process happens during tACS, with the difference that every electrode constantly alternates between the anodal and the cathodal polarity at the frequency of the alternating current (i.e., 40 Hz). This may cause either synchronization or desynchronization (depending on the specific electrodes montage) of the targeted areas, in addition to the “local entrainment” of neuronal populations stimulated by each electrode at the delivered frequency. On contrary, tRNS permits to singularly test the relevance of enhancing the excitability of a single region, eliminating the confounding factors of: 1) having the inhibition of the cathodal target (as for tDCS), or 2) the synchronization or desynchronization between brain regions, which theoretically could contribute to the observed behavioral effect. This is especially true when the selectivity of the induced electric field is obtained via multifocal approaches (e.g., adopting multiple return electrodes (Ruffini et al., 2013)), allowing to deliver a larger amount of current to the principal target region respect to the classical bifocal montage in which the amount of current is equally split between two electrodes.
Limitations of the study
Despite being the first study causally testing the involvement of both temporal and parietal lobes on semantic integration using tRNS, our study presents some limitations. First, we did not check for the specific strategy used to solve the semantic task (e.g., insight or analytical method), this should be investigated in the future to reveal a potential selective effect of tRNS on problem strategy (i.e., insight problem-solving). Second, several behavioral, cognitive, and demographics factors have been related to semantic integration (Kounios & Beeman, 2014), but we did not investigate individual cognitive profiles in determining individual response to tRNS, as well as for other potential factors, such as positive mood status, mindfulness scores, time of the day (Sprugnoli et al., 2017), and a more general gender effect on language areas’ activation (Yao et al., 2020). Future studies should address the role of these measures in explaining variability in the response to tRNS.
Third, previous investigations using tDCS, as well as met-analysis of fMRI studies, have suggested a role of bilateral temporal lobes (Chi & Snyder, 2011) and left prefrontal cortex (Cerruti & Schlaug, 2009; Metuki et al., 2012; Peña et al., 2019) in successful semantic problem-solving. Therefore, future experiments exploring bi-hemispheric stimulation montages could be performed. Additionally, fMRI studies revealing the activations during Rebus Puzzles tasks are needed to reveal task-specific activation and guide possible neuromodulatory interventions.
Conclusions
Our data support the involvement of both right parietal and temporal lobes in the generation of semantic integration in humans, suggesting tRNS as a suitable tool to boost such complex cognitive ability and verbal reasoning more in general.
Acknowledgments
The thank the participants to the study. E.S. and A.P.L. were supported by the Office of the Director of National Intelligence (ODNI), Intelligence Advanced Research Projects Activity (IARPA), via 2014-13121700007. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the ODNI, IARPA, or the U.S. Government. E.S. and A.P.L. are supported by the BROAD Institute at Harvard-MIT (Boston, MA) via 2016P000351. E.S. was supported by the Beth Israel Deaconess Medical Center (BIDMC) via the Chief Academic Officer (CAO) grant 2017, and the NIH (P01 AG031720-06A1, R01 MH117063-01, R01 AG060981-01). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University and its affiliated academic health care centers, or the National Institutes of Health.
Footnotes
Conflicts of interest All authors report no conflicts of interest.
Data statement
Data will be made available to all interested researchers upon request. The data and materials for all experiments will be made available upon reasonable request to the author.
References
- Aihara T, Ogawa T, Shimokawa T, & Yamashita O (2017). Anodal transcranial direct current stimulation of the right anterior temporal lobe did not significantly affect verbal insight. PLoS ONE, 12(9). 10.1371/journal.pone.0184749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A, Alekseichuk I, Bikson M, Brockmöller J, Brunoni AR, Chen R, Cohen LG, Dowthwaite G, Ellrich J, Flöel A, Fregni F, George MS, Hamilton R, Haueisen J, Herrmann CS, Hummel FC, Lefaucheur JP, Liebetanz D, Loo CK, … Paulus W (2017). Low intensity transcranial electric stimulation: Safety, ethical, legal regulatory and application guidelines. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 128(9), 1774–1809. 10.1016/j.clinph.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolo A, Benuzzi F, Nocetti L, Baraldi P, & Nichelli P (2006). Humor comprehension and appreciation: An FMRI study. Journal of Cognitive Neuroscience, 18(11), 1789–1798. 10.1162/jocn.2006.18.11.1789. [DOI] [PubMed] [Google Scholar]
- Beaty RE, Chen Q, Christensen AP, Kenett YN, Silvia PJ, Benedek M, & Schacter DL (2020). Default network contributions to episodic and semantic processing during divergent creative thinking: A representational similarity analysis. NeuroImage, 209, 116499. 10.1016/j.neuroimage.2019.116499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendetowicz D, Urbanski M, Garcin B, Foulon C, Levy R, Bréchemier M-L, Rosso C, Thiebaut de Schotten M, & Volle E (2018). Two critical brain networks for generation and combination of remote associations. Brain: A Journal of Neurology, 141(1), 217–233. 10.1093/brain/awx294. [DOI] [PubMed] [Google Scholar]
- Benedek M, & Neubauer AC (2013). Revisiting Mednick’s model on creativity-related differences in associative hierarchies. Evidence for a common path to uncommon thought. The Journal of Creative Behavior, 47(4), 273–289. 10.1002/jocb.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini G, Corcoran R, Sterzi R, Paulesu E, Schenone P, Scarpa P, Frackowiak RS, & Frith CD (1994). The role of the right hemisphere in the interpretation of figurative aspects of language. A positron emission tomography activation study. Brain: A Journal of Neurology, 117(Pt 6), 1241–1253. 10.1093/brain/117.6.1241. [DOI] [PubMed] [Google Scholar]
- Bowden EM, & Beeman MJ (1998). Getting the right idea: Semantic activation in the right hemisphere may help solve insight problems. Psychological Science, 9(6), 435–440. 10.1111/1467-9280.00082. [DOI] [Google Scholar]
- Bowden EM, & Jung-Beeman M (2003). Normative data for 144 compound remote associate problems. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc, 35(4), 634–639. 10.3758/bf03195543. [DOI] [PubMed] [Google Scholar]
- Bowden EM, Jung-Beeman M, Fleck J, & Kounios J (2005). New approaches to demystifying insight. Trends in Cognitive Sciences, 9(7), 322–328. 10.1016/j.tics.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Cerruti C, & Schlaug G (2009). Anodal transcranial direct current stimulation of the prefrontal cortex enhances complex verbal associative thought. Journal of Cognitive Neuroscience, 21(10), 1980–1987. 10.1162/jocn.2008.21143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi RP, & Snyder AW (2011). Facilitate insight by non-invasive brain stimulation. PloS One, 6(2), e16655. 10.1371/journal.pone.0016655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarello C, Burgess C, Richards L, & Pollock A (1990). Semantic and associative priming in the cerebral hemispheres: Some words do, some words don’t … sometimes, some places. Brain and Language, 38(1), 75–104. 10.1016/0093-934x(90)90103-n. [DOI] [PubMed] [Google Scholar]
- Contemori G, Trotter Y, Cottereau BR, & Maniglia M (2019). TRNS boosts perceptual learning in peripheral vision. Neuropsychologia, 125, 129–136. 10.1016/j.neuropsychologia.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Gruszka A, & Necka E (2002). Priming and Acceptance of Close and Remote Associations by Creative and Less Creative People. Creativity Research Journal, 14(2), 193–205. 10.1207/S15326934CRJ1402_6. [DOI] [Google Scholar]
- Herpich F, Melnick MD, Agosta S, Huxlin KR, Tadin D, & Battelli L (2019). Boosting Learning Efficacy with Noninvasive Brain Stimulation in Intact and Brain-Damaged Humans. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 39(28), 5551–5561. 10.1523/JNEUROSCI.3248-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Willard K, Buchsbaum B, & Hickok G (2001). Role of anterior temporal cortex in auditory sentence comprehension: An fMRI study. Neuroreport, 12(8), 1749–1752. 10.1097/00001756-200106130-00046. [DOI] [PubMed] [Google Scholar]
- Inukai Y, Saito K, Sasaki R, Tsuiki S, Miyaguchi S, Kojima S, Masaki M, Otsuru N, & Onishi H (2016). Comparison of three non-invasive transcranial electrical stimulation methods for increasing cortical excitability. Frontiers in Human Neuroscience, 10, 668. 10.3389/fnhum.2016.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanette Y, Goulet P, Hannequin D, & Boeglin J (1990). Right hemisphere and verbal communication. Springer-Verlag Publishing. 10.1007/978-1-4612-4460-8. [DOI] [Google Scholar]
- Jung-Beeman M (2005). Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences, 9(11), 512–518. 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M, Bowden EM, Haberman J, Frymiare JL, Arambel-Liu S, Greenblatt R, Reber PJ, & Kounios J (2004). Neural activity when people solve verbal problems with insight. PLoS Biology, 2(4), E97. 10.1371/journal.pbio.0020097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenett YN, Anaki D, & Faust M (2014). Investigating the structure of semantic networks in low and high creative persons. Frontiers in Human Neuroscience, 8. 10.3389/fnhum.2014.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenett YN, & Faust M (2019). A Semantic Network Cartography of the Creative Mind. Trends in Cognitive Sciences, 23(4), 271–274. 10.1016/j.tics.2019.01.007. [DOI] [PubMed] [Google Scholar]
- Kenett YN, Levy O, Kenett DY, Stanley HE, Faust M, & Havlin S (2018). Flexibility of thought in high creative individuals represented by percolation analysis. Proceedings of the National Academy of Sciences, 115(5), 867–872. 10.1073/pnas.1717362115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounios J, & Beeman M (2014). The cognitive neuroscience of insight. Annual Review of Psychology, 65, 71–93. 10.1146/annurev-psych-010213-115154. [DOI] [PubMed] [Google Scholar]
- Krause MR, Vieira PG, Csorba BA, Pilly PK, & Pack CC (2019). Transcranial alternating current stimulation entrains single-neuron activity in the primate brain. Proceedings of the National Academy of Sciences of the United States of America, 116(12), 5747–5755. 10.1073/pnas.1815958116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur J-P, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, Cotelli M, De Ridder D, Ferrucci R, Langguth B, Marangolo P, Mylius V, Nitsche MA, Padberg F, Palm U, Poulet E, Priori A, Rossi S, Schecklmann M, … Paulus W (2017). Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 128(1), 56–92. 10.1016/j.clinph.2016.10.087. [DOI] [PubMed] [Google Scholar]
- Luft CDB, Zioga I, Thompson NM, Banissy MJ, & Bhattacharya J (2018). Right temporal alpha oscillations as a neural mechanism for inhibiting obvious associations. Proceedings of the National Academy of Sciences, 115(52), E12144–E12152. 10.1073/pnas.1811465115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustenberger C, Boyle MR, Foulser AA, Mellin JM, & Fröhlich F (2015). Functional role of frontal alpha oscillations in creativity. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 67, 74–82. 10.1016/j.cortex.2015.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor JN, & Cunningham JB (2008). Rebus puzzles as insight problems. Behavior Research Methods, 40(1), 263–268. 10.3758/brm.40.1.263. [DOI] [PubMed] [Google Scholar]
- Manfredi M, Proverbio AM, Gonçalves Donate AP, Macarini Gonçalves Vieira S, Comfort WE, De Araújo Andreoli M, & Boggio PS (2017). TDCS application over the STG improves the ability to recognize and appreciate elements involved in humor processing. Experimental Brain Research, 235(6), 1843–1852. 10.1007/s00221-017-4932-5. [DOI] [PubMed] [Google Scholar]
- Mashal N, Faust M, Hendler T, & Jung-Beeman M (2007). An fMRI investigation of the neural correlates underlying the processing of novel metaphoric expressions. Brain and Language, 100(2), 115–126. 10.1016/j.bandl.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mednick S (1962). The associative basis of the creative process. Psychological Review, 69(3), 220–232. 10.1037/h0048850. [DOI] [PubMed] [Google Scholar]
- Mednick SA (1968). The Remote Associates Test*. The Journal of Creative Behavior, 2(3), 213–214. 10.1002/j.2162-6057.1968.tb00104.x. [DOI] [Google Scholar]
- Metuki N, Sela T, & Lavidor M (2012). Enhancing cognitive control components of insight problems solving by anodal tDCS of the left dorsolateral prefrontal cortex. Brain Stimulation, 5(2), 110–115. 10.1016/j.brs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Moret B, Camilleri R, Pavan A, Lo Giudice G, Veronese A, Rizzo R, & Campana G (2018). Differential effects of high-frequency transcranial random noise stimulation (hf-tRNS) on contrast sensitivity and visual acuity when combined with a short perceptual training in adults with amblyopia. Neuropsychologia, 114, 125–133. 10.1016/j.neuropsychologia.2018.04.017. [DOI] [PubMed] [Google Scholar]
- Moret B, Donato R, Nucci M, Cona G, & Campana G (2019). Transcranial random noise stimulation (tRNS): A wide range of frequencies is needed for increasing cortical excitability. Scientific Reports, 9(1), 15150. 10.1038/s41598-019-51553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113 [DOI] [PubMed] [Google Scholar]
- Pavan A, Ghin F, Contillo A, Milesi C, Campana G, & Mather G (2019). Modulatory mechanisms underlying high-frequency transcranial random noise stimulation (hf-tRNS): A combined stochastic resonance and equivalent noise approach. Brain Stimulation, 12(4), 967–977. 10.1016/j.brs.2019.02.018. [DOI] [PubMed] [Google Scholar]
- Peña J, Sampedro A, Ibarretxe-Bilbao N, Zubiaurre-Elorza L, & Ojeda N (2019). Improvement in creativity after transcranial random noise stimulation (tRNS) over the left dorsolateral prefrontal cortex. Scientific Reports, 9(1), 7116. 10.1038/s41598-019-43626-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo A, & Marques JF (2013). The contribution of fronto-parietal regions to sentence comprehension: Insights from the Moses illusion. NeuroImage, 83, 431–437. 10.1016/j.neuroimage.2013.06.052. [DOI] [PubMed] [Google Scholar]
- Reed T, & Cohen Kadosh R (2018). Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. Journal of Inherited Metabolic Disease. 10.1007/s10545-018-0181-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann E, & Fink A (2010). Do creative people use shorter associative pathways? Personality and Individual Differences, 49(8), 891–895. 10.1016/j.paid.2010.07.025. [DOI] [Google Scholar]
- Ruffini G, Wendling F, Merlet I, Molaee-Ardekani B, Mekonnen A, Salvador R, Soria-Frisch A, Grau C, Dunne S, & Miranda PC (2013). Transcranial current brain stimulation (tCS): Models and technologies. IEEE Transactions on Neural Systems and Rehabilitation Engineering: A Publication of the IEEE Engineering in Medicine and Biology Society, 21(3), 333–345. 10.1109/TNSRE.2012.2200046. [DOI] [PubMed] [Google Scholar]
- Ruggiero F, Lavazza A, Vergari M, Priori A, & Ferrucci R (2018). Transcranial direct current stimulation of the left temporal lobe modulates insight. Creativity Research Journal, 30(2), 143–151. 10.1080/10400419.2018.1446817. [DOI] [Google Scholar]
- Salvi C, & Bowden EM (2016). Looking for Creativity: Where Do We Look When We Look for New Ideas? Frontiers in Psychology. 10.3389/fpsyg.2016.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Bricolo E, Franconeri SL, Kounios J, & Beeman M (2015a). Sudden insight is associated with shutting out visual inputs. Psychonomic Bulletin & Review, 22(6), 1814–1819. 10.3758/s13423-015-0845-0. [DOI] [PubMed] [Google Scholar]
- Salvi C, Costantini G, Bricolo E, Perugini M, & Beeman M (2015b). Validation of Italian rebus puzzles and compound remote associate problems. Behavior Research Methods. 10.3758/s13428-015-0597-9. [DOI] [PubMed] [Google Scholar]
- Salvi C, Bricolo E, Kounios J, Bowden E, & Beeman M (2016). Insight solutions are correct more often than analytic solutions. Thinking & Reasoning, 22(4), 443–460. 10.1080/13546783.2016.1141798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Costantini G, Pace A, & Palmiero M (2018). Validation of the Italian remote associate test. The Journal of Creative Behavior, 54(1), 62–74. 10.1002/jocb.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Beeman M, Bikson M, McKinley R, & Grafman J (2020a). TDCS to the right anterior temporal lobe facilitates insight problem-solving. Scientific Reports, 10(1), 946. 10.1038/s41598-020-57724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi C, Simoncini C, Grafman J, & Beeman M (2020b). Oculometric signature of switch into awareness? Pupil size predicts sudden insight whereas microsaccades predict problem-solving via analysis. NeuroImage, 116933. 10.1016/j.neuroimage.2020.116933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler S, & Bhattacharya J (2008). Deconstructing insight: EEG correlates of insightful problem solving. PloS One, 3(1), e1459. 10.1371/journal.pone.0001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Brem A-K, Levenbaum E, Thompson T, Kadosh RC, & Pascual-Leone A (2015). Enhancing cognition using transcranial electrical stimulation. Current Opinion in Behavioral Sciences, 4, 171–178. 10.1016/j.cobeha.2015.06.003. [DOI] [Google Scholar]
- Santarnecchi E, Polizzotto NR, Godone M, Giovannelli F, Feurra M, Matzen L, Rossi A, & Rossi S (2013). Frequency-dependent enhancement of fluid intelligence induced by transcranial oscillatory potentials. Current Biology: CB, 23(15), 1449–1453. 10.1016/j.cub.2013.06.022. [DOI] [PubMed] [Google Scholar]
- Santarnecchi E, Sprugnoli G, Bricolo E, Costantini G, Liew S-L, Musaeus CS, Salvi C, Pascual-Leone A, Rossi A, & Rossi S (2019). Gamma tACS over the temporal lobe increases the occurrence of Eureka! Moments. Scientific Reports, 9(1), 5778. 10.1038/s41598-019-42192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev N, De Wandel L, Dockree P, Demeyere N, & Chechlacz M (2018). Beyond time and space: The effect of a lateralized sustained attention task and brain stimulation on spatial and selective attention. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior, 107, 131–147. 10.1016/j.cortex.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Shen W, Yuan Y, Liu C, & Luo J (2017). The roles of the temporal lobe in creative insight: An integrated review. Thinking & Reasoning, 23(4), 321–375. 10.1080/13546783.2017.1308885. [DOI] [Google Scholar]
- Snowball A, Tachtsidis I, Popescu T, Thompson J, Delazer M, Zamarian L, Zhu T, & Cohen Kadosh R (2013). Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Current Biology: CB, 23(11), 987–992. 10.1016/j.cub.2013.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprugnoli G, Rossi S, Emmendorfer A, Rossi A, Liew S-L, Tatti E, di Lorenzo G, Pascual-Leone A, & Santarnecchi E (2017). Intelligence Neural correlates of Eureka moment. Intelligence, 62, 99–118. 10.1016/j.intell.2017.03.004. [DOI] [Google Scholar]
- St George M, Kutas M, Martinez A, & Sereno MI (1999). Semantic integration in reading: Engagement of the right hemisphere during discourse processing. Brain: A Journal of Neurology, 122 (Pt 7), 1317–1325. 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- Subramaniam K, Kounios J, Parrish TB, & Jung-Beeman M (2009). A brain mechanism for facilitation of insight by positive affect. Journal of Cognitive Neuroscience, 21(3), 415–432. 10.1162/jocn.2009.21057. [DOI] [PubMed] [Google Scholar]
- Terney D, Chaieb L, Moliadze V, Antal A, & Paulus W (2008). Increasing human brain excitability by transcranial high-frequency random noise stimulation. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 28(52), 14147–14155. 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tik M, Sladky R, Luft CDB, Willinger D, Hoffmann A, Banissy MJ, Bhattacharya J, & Windischberger C (2018). Ultra-high-field fMRI insights on insight: Neural correlates of the Aha!-moment. Human Brain Mapping, 39(8), 3241–3252. 10.1002/hbm.24073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Groen O, & Wenderoth N (2016). Transcranial random noise stimulation of visual cortex: stochastic resonance enhances central mechanisms of perception. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(19), 5289–5298. 10.1523/JNEUROSCI.4519-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Fregni F, & De Ridder D (2013). Head-to-Head comparison of transcranial random noise stimulation, transcranial AC stimulation, and transcranial DC stimulation for tinnitus. Frontiers in Psychiatry, 4, 158. 10.3389/fpsyt.2013.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtue S, Haberman J, Clancy Z, Parrish T, & Jung Beeman M (2006). Neural activity of inferences during story comprehension. Brain Research, 1084(1), 104–114. 10.1016/j.brainres.2006.02.053. [DOI] [PubMed] [Google Scholar]
- Yao S, Liebenthal E, Juvekar P, Bunevicius A, Vera M, Rigolo L, Golby AJ, & Tie Y (2020). Sex Effect on Presurgical Language Mapping in Patients With a Brain Tumor. Frontiers in Neuroscience, 14. 10.3389/fnins.2020.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available to all interested researchers upon request. The data and materials for all experiments will be made available upon reasonable request to the author.


