Abstract
Objectives
Patients with giant cell arteritis (GCA) represent a fragile population with an increased infection risk. In a recent study, older age, a higher number of comorbidities, higher disease activity and prednisolone ≥ 10 mg/day were associated with worse COVID-19 outcome. We aimed to evaluate the frequency and severity of COVID-19 in a well-defined GCA cohort.
Methods
We reviewed medical records of histologically and/or by imaging-proven GCA patients diagnosed between September 2011 and February 2020 at our secondary/tertiary centre and followed during the COVID-19 pandemic between March 2020 and February 2022 (24 months). Descriptive statistics were used to explore the studied population.
Results
Of 314 patients with GCA diagnosed for the first time during a 102-month period, 49 patients died before March 2020. Of the remaining 265 patients, 55 (20.8%) patients suffered from a total of 57 SARS-CoV-2 infections. We observed 44 (77.2%) mild and 13 (22.8%) severe COVID-19 episodes (the latter defined as needing hospitalization, death or thrombotic complication). Patients with severe COVID-19 were more likely to have arterial hypertension (12 [92.3%] vs. 25 [56.8%]; p = 0.022), cardiovascular disease (7 [53.8%] vs. 10 [22.7%]; p = 0.043) or obesity (5 [38.5%] vs. 5 [11.4%]; p = 0.038). Neither prednisolone dose 1–5 mg/day (p = 0.483) nor leflunomide use (p = 1.000) was associated with COVID-19 course. There were no significant differences in sex, age, GCA type, GCA disease duration and other comorbidities in patients with mild and severe COVID-19 in our cohort.
Conclusion
More than a fifth of our GCA patients had severe COVID-19. Treatment with leflunomide or low doses of glucocorticoids were not associated with severe course in our cohort.
|
Key Points • Treatment with leflunomide or low doses of glucocorticoids were not associated with worse COVID-19 outcome. • Outcomes of COVID-19 improved as the COVID-19 pandemic, prevention and treatment options evolved. • Arterial hypertension, cardiovascular disease or obesity were associated with severe COVID-19. |
Keywords: COVID-19, Giant cell arteritis, Glucocorticoids, Leflunomide, Outcome assessment
Introduction
Giant cell arteritis (GCA) represents the most frequent primary systemic vasculitis of large- and medium-sized arteries in population aged > 50 years in Europe and North America [1]. It is a rheumatologic emergency, and as such requires rapid specialist assessment and treatment with glucocorticoids to prevent irreversible ischemic complications [2]. Fast-track pathways reduce the risk of permanent visual impairment in GCA [3]. During the coronavirus disease 2019 (COVID-19) pandemic, access to health-care services was greatly disrupted, and consequently, many GCA diagnoses were delayed [4]. Because of reduced medical personnel due to redeployments to treat COVID-19 cases or quarantine, temporal artery biopsies became less accessible [5]. Importance of imaging methods in early diagnosis of GCA became increasingly evident during the peak of the COVID-19 pandemic, colour Doppler sonography for cranial GCA and positron emission tomography/computed tomography (PET/CT) with the use of 18F-fluoro-2-deoxy-D-glucose (18F-FDG) in the setting of large vessel vasculitis presenting as fever of unknown origin when access to other extensive diagnostic modalities was limited [6–8]. Slovenia was one of the European countries considerably afflicted by COVID-19 pandemic in the first month of 2022, with one of the highest total numbers of new infections in the world with over 36,397 cases per 100,000 inhabitants by February 6, 2022 [9], which represented a significant public health problem with major impact especially in fragile population of patients with chronic diseases, such as patients with rheumatic diseases. Advanced age and pre-existing medical conditions are considered major risk factors for severe forms of COVID-19 [10–12]. In a recent study, high rates of severe COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica were reported and were associated with a higher number of comorbidities, higher disease activity and prednisolone ≥ 10 mg/day [13]. The high mortality rate observed in patients with GCA may reflect the impact of age on COVID-19 mortality, as these patients represent a fragile population at increased risk of infection [14, 15]. Data on the course of COVID-19 in patients with GCA remain scarce. In our study, we present a cohort of patients with GCA who contracted COVID-19 and describe their clinical characteristics, disease course and outcomes, with the aim to identify potential risk factors associated with severe COVID-19.
Materials and methods
Settings
This observational cohort study was conducted at the Department of Rheumatology, University Medical Centre Ljubljana, Slovenia, a secondary/tertiary level teaching hospital, where we treat most GCA cases from the region, representing approximately half of the Slovenian population.
Study design and GCA patient selection
We enrolled patients diagnosed with GCA between September 2011 and February 2020, which were alive in March 2020 (month when the first case of infection with severe acute respiratory syndrome 2 (SARS-CoV-2) in Slovenia was detected). GCA diagnosis was based on the corresponding clinical and laboratory features and the positive result of a temporal artery biopsy as defined by 1990 American College of Rheumatology criteria for the classification of GCA [16] and/or positive result of colour Doppler sonography (sonographic examination of 7 arterial territories — paired temporal, facial, occipital, carotid, vertebral, subclavian and axillary) or 18F-FDG PET/CT. Among the included patients, we identified those with a positive polymerase chain reaction (PCR) test result for SARS-CoV-2 via our electronically available National Central Patient Data Registry. From February 2022 onwards, we also identified patients with positive rapid antigen test (RAgT) for SARS-CoV-2, as this became sufficient to confirm SARS-CoV-2 infection in accordance with Slovenian government decree. All patients tested positive between March 2020 and February 2022 (24 months) were included (Fig. 1).
Fig. 1.
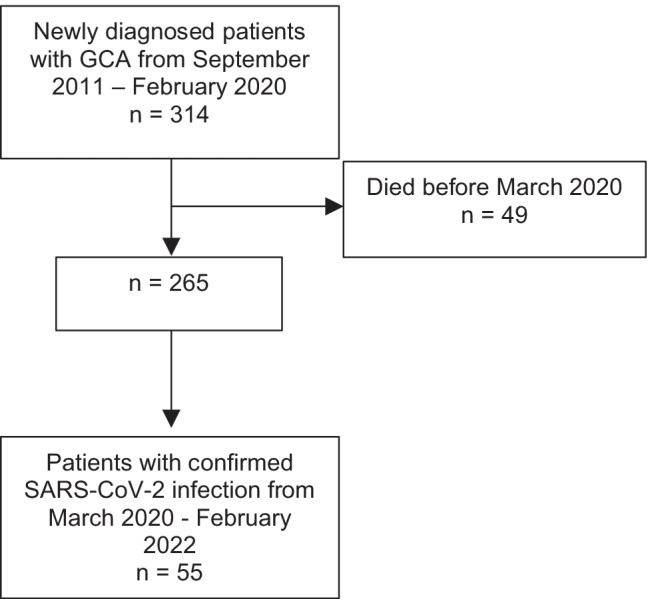
Patient selection flowchart
Data collection — COVID-19 characteristics
Among patients with a proven SARS-CoV-2 infection, we conducted a telephone survey with the patient or, in cases where the patient was unavailable, a close relative. When formulating the questionnaire, we leaned on COVID-19 Registry from European Alliance of Associations for Rheumatology (EULAR) regarding clinical symptoms of SARS-CoV-2 infection [17]. The survey consisted of the following questions: (1) during the SARS-CoV-2 infection, did you experience any of the following symptoms: fever, general fatigue, headache, arthralgia, myalgia, nasal discharge, throat pain, loss of smell or taste, cough, chest pain, dyspnoea, vomiting, abdominal pain and diarrhoea? (2) How many days did symptoms last? (3) Did you take any immunosuppressant medications like methylprednisolone, leflunomide, methotrexate, or biologic therapy before and during the SARS-CoV-2 infection and in what dose? (4) Did you require hospitalization because of the SARS-CoV-2 infection? (5) Did you require hospitalization in the intensive care unit because of the SARS-CoV-2 infection? For patients who required hospitalization, we searched for medical records and collected additional data on the treatment during hospitalization, the need for oxygen supplementation and, if applicable, the development of complications such as acute respiratory distress syndrome, cytokine storm, thrombocytopenia and the need for additional hospitalization for post-COVID-19 complications or death.
For the purpose of analysis, we defined COVID-19 outcome as mild or severe. Mild COVID-19 was defined as COVID-19 without admission to hospital and/or development of COVID-19-associated complications and/or death. Severe COVID-19 was defined as the need of admission to hospital and/or development of COVID-19-associated complications and/or death.
Furthermore, we stratified COVID-19 cases into three groups according to the time of proven COVID-19 diagnosis: (a) period between March 2020 and February 2021 (i.e. 12-month “pre-vaccination period” — prior to widely available vaccination in our country); (b) period between March 2021 and December 2021 (i.e. 10-month “vaccination period” with widely available vaccination in our country) and (c) period from January 2022 to February 2022 (i.e. 2-month “Omicron period” with the predominant Omicron SARS-CoV-2 variant in the population in our country [18]).
For all GCA patients enrolled in this study, we also collected data on SARS-CoV-2 vaccination status by reviewing data from the National Central Patient Data Registry.
GCA treatment and comorbidities
Immunomodulatory treatment for GCA at the time of SARS-CoV-2 infection was recorded and stratified into groups: no treatment, glucocorticoid treatment, conventional immunomodulatory drugs (leflunomide 10 mg/day, leflunomide 20 mg/day, methotrexate) and biologic immunomodulatory drugs (ustekinumab, tocilizumab). Glucocorticoids were further stratified by the prednisolone-equivalent dose (1–5 mg/day, 6–9 mg/day or ≥ 10 mg/day). Therapeutic schemes used for GCA treatment at the time of SARS-CoV-2 infection were also included and categorized into groups: glucocorticoid monotherapy, leflunomide monotherapy, glucocorticoid and leflunomide combination therapy and biologic therapy. Additionally, we searched for possible GCA relapse and comorbidities in each patient by closely reviewing available medical records during the pandemic period between March 2020 and February 2022. Covariates included age, sex, GCA disease duration, GCA type (large vessel vasculitis and cranial-limited vasculitis), time period (March 2020 to February 2021, March 2021 to December 2021 and January 2022 to February 2022), comorbidities (arterial hypertension, cardiovascular disease [including coronary artery disease, cerebrovascular disease, peripheral artery disease and aortic atherosclerosis], lung disease [including interstitial lung disease, chronic obstructive pulmonary disease, asthma or other lung diseases], diabetes, chronic kidney disease, or cancer), number of comorbidities and body mass index (BMI; obese [BMI ≥ 30 kg/m2]).
Ethical standards
The study was approved by the National medical ethics committee, approval number 0120–554/2020/3.
All patients provided their consent for the use of their demographic and clinical data.
Statistical analysis
Descriptive statistics were used to analyse the studied population. The results were expressed as medians and interquartile ranges (IQR) for metric continuous variables with skewed distribution and as numbers and proportions for categorical variables. To test the differences between groups of patients, we used the Mann–Whitney U-test for metric, Fisher’s exact test for categorical variables and Kruskal–Wallis test for the comparison of continuous variables between the groups. The significance threshold selected in all analyses was set at 0.05. Jamovi (Sydney, Australia) software version 2.3.0 was used for statistical calculations.
Results
GCA patients
During the 102-month period, we identified 314 new GCA patients, 49 of whom died before March 2020. Of the remaining 265 patients (69.4% women), temporal artery biopsy was performed in 135 patients and was consistent with GCA in 112 patients (83.0%). Colour Doppler sonography of examined arteries was consistent with vasculitis for GCA in 255 out of 265 patients (96.2%). According to ultrasound, 165 (64.7%) patients had cranial-limited GCA, and 90 (35.3%) patients had extracranial large vessel GCA. Using 18F-FDG PET/CT, we found large vessel vasculitis in 32 out of 38 imaged patients (84.2%).
COVID-19
SARS-CoV-2 infection was documented by PCR and/or RAgT in 55 (20.8%) GCA patients (69.1% females) with overall 57 confirmed SARS-CoV-2 cases, as two patients developed two episodes of SARS-CoV-2 infection (one patient had two mild COVID-19 episodes, and in another patient, both COVID-19 episodes were severe). The median (IQR) patient age at the time of infection was 78.2 (69.1; 83.7) years, and median (IQR) GCA duration time was 3.8 (2.5; 5.9) years. Overall, 20 patients (36.3%) had large vessel GCA, and the rest had cranial-limited GCA. At the time of SARS-CoV-2 infection, GCA was in stable remission in 53 patients, 24 without immunosuppressive therapy, 11 on glucocorticoid monotherapy (10 on prednisolone-equivalent dose 1–5 mg/day and one on 7.5 mg/day), 12 on leflunomide monotherapy (10 mg/day), 5 on glucocorticoids (5 mg/day prednisolone equivalent) and leflunomide (10 or 20 mg/day), one on ustekinumab monotherapy and none on methotrexate or tocilizumab. In two patients, GCA relapsed shortly before the documented SARS-CoV-2 infection: one patient relapsed 6 weeks before SARS-CoV-2 infection and was treated at the time of infection with a prednisolone-equivalent dose of 30 mg/day and leflunomide 20 mg/day, and another patient had a relapse of polymyalgia rheumatica symptoms 3 weeks before SARS-CoV-2 infection and was treated with a prednisolone-equivalent dose of 10 mg/day at the time of COVID-19. We documented 44 out of 57 (77.2%) episodes of mild COVID-19 and 13 out of 57 (22.8%) episodes of severe COVID-19, and one of these patients died. In 9 out of 13 severe COVID-19 episodes, patients required supplemental oxygen, but none of the patients was treated in the intensive care unit. Two patients suffered a cerebrovascular accident, and one developed pulmonary embolism and myocardial infarction timely associated with COVID-19. Detailed data on the clinical manifestations of COVID-19 were available for 53 (93.0%) patients and are presented based on the time frame of proven infection (i.e. before COVID-19 vaccination period, during vaccination period, during Omicron period) in Table 1. Ten (76.9%) patients who had severe COVID-19 were diagnosed before March 2021. Interestingly, 18 out of 57 episodes were documented during the 2-month Omicron period (i.e. on average 9 episodes per month), whereas there were 28 (i.e. 2.3 per month) and 11 episodes (i.e. 1.1 per month) documented during the pre-vaccination and vaccination periods, respectively. The three (time-defined) groups of patients differed significantly in the recorded frequency of myalgias (p = 0.002), arthralgias (p = 0.002), anosmia (p = 0.004) and dysgeusia (p = 0.003). The difference in the frequency of respiratory insufficiency was borderline (p = 0.049).
Table 1.
Symptoms of SARS-CoV-2 infection in 53 GCA patients
| Characteristic | Period (month/year) | p-value | |||
|---|---|---|---|---|---|
| 3/2020 to 2/2022 | 3/2020 to 2/2021 | 3/2021 to 12/2021 | 1/2022 to 2/2022 | ||
| Documented COVID-19 episodes | 57 (100.0%) | 28 (49.1%) | 11 (19.3%) | 18 (31.5%) | |
| Available COVID-19 episodes for analysis | 53 (93.0%) | 24 | 11 | 18 | |
| Female gender | 75.5% | 79.2% | 72.7% | 72.2% | 0.850 |
| Age | 78.2 (69.1; 83.7) | 73.6 (69.1; 82.1) | 79.6 (78.1; 84.6) | 76.6 (71.4; 82.6) | 0.834 |
| Severe COVID-19 | 13 (22.8%) | 9 (32.1%) | 1 (9.1%) | 3 (16.7%) | 0.120 |
| Duration* | 10 (4; 14) | 10 (8; 15) | 10 (7; 19) | 10 (3; 13) | 0.142 |
| Fever | 45.3% | 54.2% | 27.3% | 44.4% | 0.331 |
| Headache | 22.6% | 33.3% | 9.1% | 16.7% | 0.185 |
| Myalgias | 30.2% | 54.2% | 18.2% | 5.6% | 0.002 |
| Arthralgias | 26.4% | 50.0% | 9.1% | 5.6% | 0.002 |
| Fatigue | 54.7% | 70.8% | 54.5% | 33.3% | 0.054 |
| Rhinitis | 32.1% | 25.0% | 27.3% | 44.4% | 0.381 |
| Throat pain | 17.0% | 25.0% | 0.0% | 16.7% | 0.188 |
| Anosmia | 26.4% | 45.8% | 27.3% | 0.0% | 0.004 |
| Dysgeusia | 20.8% | 41.7% | 9.1% | 0.0% | 0.003 |
| Chest pain | 15.1% | 25.0% | 9.1% | 5.6% | 0.180 |
| Dyspnoea | 20.8% | 33.3% | 18.2% | 5.6% | 0.087 |
| Cough | 47.2% | 50.0% | 36.4% | 50.0% | 0.722 |
| Respiratory insufficiency | 18.9% | 33.3% | 9.1% | 5.6% | 0.049 |
| Abdominal pain | 13.2% | 20.8% | 9.1% | 5.6% | 0.317 |
| Nausea, vomiting | 0.0% | 0.0% | 0.0% | 0.0% | N/A |
| Diarrhoea | 11.3% | 20.8% | 9.1% | 0.0% | 0.105 |
| Thrombotic complications | 5.7% | 4.2% | 0.0% | 11.1% | 0.414 |
Data are median (IQR) or n (%). *Recorded in days. N/A not applicable
Baseline demographic characteristics, GCA disease duration time, medications and comorbidities at the time of mild or severe SARS-CoV-2 infection are presented in Table 2. There were no significant differences in sex, age, GCA duration, GCA type and GCA treatment between patients with mild and severe COVID-19. We found a significant association between severe COVID-19 and the presence of arterial hypertension (p = 0.022), cardiovascular disease (p = 0.043) and obesity (p = 0.038). There were no significant differences in other comorbidities between patients with mild and severe COVID-19.
Table 2.
Baseline demographic characteristics, medication and comorbidities of GCA patients by COVID-19 severity
| Characteristics, medication and comorbidities | Mild COVID-19 44 episodes (77.2%) |
Severe COVID-19 13 episodes (22.8%) |
p-value |
|---|---|---|---|
| Female | 31 (73.8%) | 9 (69.2%) | 0.734 |
| Age, years | 75.6 (68.8; 82.3) | 82.9 (78.4; 84.8) | 0.084 |
| GCA duration, years | 4.0 (2.5; 5.8) | 3.3 (2.6; 5.5) | 0.726 |
| Large vessel vasculitis | 17 (40.5%) | 3 (23.1%) | 0.333 |
| Medication | |||
| No treatment | 19 (43.2%) | 5 (38.5%) | 1.000 |
| Glucocorticoids | 12 (27.3%) | 7 (53.9%) | 0.099 |
| Prednisolone 1–5 mg/day | 11 (25.0%) | 5 (38.5%) | 0.483 |
| Prednisolone 6–9 mg/day | 1 (2.3%) | 0 | 1.000 |
| Prednisolone ≥ 10 mg/day | 1 (2.3%) | 1 (7.7%) | 0.407 |
| Leflunomide | 15 (34.1%) | 4 (30.8%) | 1.000 |
| Leflunomide 10 mg/day | 13 (29.6%) | 2 (15.4%) | 0.478 |
| Leflunomide 20 mg/day | 2 (4.6%) | 2 (15.4%) | 0.221 |
| Methotrexate | 0 | 0 | |
| Ustekinumab | 1 (2.3%) | 0 | 1.000 |
| Tocilizumab | 0 | 0 | |
| Therapeutic schemes | |||
| Glucocorticoid monotherapy | 9 (20.5%) | 4 (30.8%) | 0.466 |
| Leflunomide monotherapy | 12 (27.3%) | 1 (7.7%) | 0.259 |
| Glucocorticoid + leflunomide | 3 (6.8%) | 3 (23.1%) | 0.125 |
| Ustekinumab monotherapy | 1 (2.3%) | 0 | 1.000 |
| Number of comorbidities | |||
| 0 | 9 (20.5%) | 0 | 0.101 |
| 1 | 16 (36.4%) | 4 (30.8%) | 1.000 |
| ≥ 2 | 19 (43.2%) | 9 (69.2%) | 0.123 |
| Comorbidities | |||
| Hypertension | 25 (56.8%) | 12 (92,3%) | 0.022 |
| Cardiovascular disease* | 10 (22.7%) | 7 (53.8%) | 0.043 |
| Diabetes | 11 (25.0%) | 4 (30.8%) | 0.727 |
| Chronic kidney disease | 5 (11.4%) | 2 (15.4%) | 0.653 |
| Lung disease† | 11 (25.0%) | 5 (38.5%) | 0.483 |
| Body mass index ≥ 30 kg/m2 | 5 (11.4%) | 5 (38.5%) | 0.038 |
Data are median (IQR) or n (%). *Includes coronary artery disease, cerebrovascular disease, peripheral artery disease and aortic atherosclerosis. †Includes interstitial lung disease, chronic obstructive pulmonary disease, asthma or other lung diseases
Of 257 GCA patients eligible for vaccination against SARS-CoV-2, 217 (84.4%) were vaccinated by the end of February 2022. Fifteen patients developed COVID-19 after receiving anti-SARS-CoV-2 vaccine (6.9% breakthrough rate), of which two (13.3%) suffered from COVID-19 before and 13 (86.7%) after appearance of the Omicron variant in Slovenia.
Discussion
To our knowledge, we report the largest single-centre cohort of patients with GCA and their COVID-19 outcomes to date.
More than a fifth of our GCA patients had severe COVID-19 course. We observed a higher rate of severe COVID-19 before March 2021, a finding that coincides with the anti-SARS-CoV-2 vaccination, which began in Slovenia at the end of December 2020 for the elderly and was expanded soon after to all patients with chronic diseases and the rest of the population. These data extend previous observations from cohorts of patients with rheumatic diseases [13, 19]. In recently published data from the COVID-19 Global Rheumatology Alliance registry, 36.1% of patients with GCA were hospitalized and 20.3% died. Patients in this cohort were diagnosed with COVID-19 between October 2020 and April 2021 [13]. Compared with the data from our study, which extends over the period of the fourth wave of the coronavirus pandemic and covers the time period of prevalent SARS-CoV-2 Omicron variant, our results provide additional support for previously published data of an improvement in outcomes over time, which is likely multifactorial, related to improved treatments and management of COVID-19, increased vaccination rate, increased testing capacity and reduced pathogenicity of Omicron variant [13, 19–21]. By comparing the three designated COVID-19 periods, we observed a reduction in frequency of myalgias, arthralgias, anosmia and dysgeusia, as well as a tendency of reduced frequency of respiratory insufficiency towards the vaccination period and especially Omicron period. These findings correlate with well-described clinical features of Omicron variant infection in general population [22–24]. Even though Omicron variant has been currently associated with milder clinical presentation, we confirmed 30% of all severe COVID-19 cases during this time period alone, which might be due to a generally large proportion of infected patients in this time period, but also suggest that infection with Omicron variant can present with unfavourable course. In the 24-month period of the pandemic, almost one-third of SARS-CoV-2 infections were confirmed in the 2-month period of predominant Omicron variant, a finding that reflects high infectivity of the Omicron variant [25]. The majority of patients who were infected with SARS-CoV-2 after vaccination were identified during the Omicron period, a finding consistent with reported limited protection against symptomatic disease caused by Omicron variant after vaccination [26, 27].
To date, there are only few other available data on cohorts of patients with GCA. A French study conducted early in the pandemic reported 148 patients with large vessel vasculitis (GCA or Takayasu arteritis), of whom 8 had confirmed SARS-CoV-2 infection, 37.5% required hospitalization and 12.5% died [28]. A study performed in Italy early in the pandemic reported 4 cases of COVID-19 among 162 patients with large vessel vasculitis, of which 2 required hospitalization and none died [29].
In our cohort, patients are exposed to leflunomide, and low doses of glucocorticoids did not appear to be at higher risk for severe COVID-19. In an observational study based on the data from the COVID-19 Global Rheumatology Alliance registry, the authors reported that a prednisolone-equivalent dose of > 10 mg/day was associated with death, particularly in the connective tissue diseases and vasculitis subgroups [30]. In our cohort, only two patients received a prednisolone-equivalent dose of ≥ 10 mg/day, so it is not possible to draw any conclusions for higher prednisolone doses and COVID-19 outcome. In accordance with our local guidelines, we favour leflunomide over methotrexate as glucocorticoid-sparing agent [31]. In addition, as previously shown [30], treatment with leflunomide was not associated with worse COVID-19 outcomes in our study. There are some reports that leflunomide reduces the duration of viral shedding, duration of hospitalization and severity of infection indicating the possibility of a potentially protective effect against severe COVID-19, probably as a result of preventing cytokine storm, and has also been considered a candidate for the treatment of COVID-19 [32–34]. Further studies are needed to evaluate this putative protective effect.
Age, comorbidities and disease activity are well-defined factors associated with worse COVID-19 outcome in general and in patients with rheumatic diseases [13, 30, 35–37]. Because of the similar age distribution of the patients in our cohort and low percentage of patients with a disease flare at the time of infection, it was not possible to evaluate age or high disease activity as a risk factor for severe COVID-19. We found that patients with GCA and arterial hypertension, cardiovascular disease or obesity were at higher risk for severe COVID-19, as shown by several previous studies in patients with rheumatic diseases [35, 37, 38]. We did not find a significant association between other comorbidities and severe COVID-19, although prior data in patients with rheumatic diseases show that diabetes, lung disease, chronic kidney disease among others are known risk factors for severe COVID-19; however, to our knowledge, there are as yet no data for the subgroup of patients with GCA [13, 35–38].
Our study was limited by the small sample size of COVID-19-positive GCA patients (n = 55). Due to low number of patients in the subgroups, also a multivariate analysis was not feasible. It lacks a population-based comparison group. We did not routinely test patients for SARS-CoV-2 infection, so asymptomatic patients, patients with milder infection or patients who could not reach a testing centre may be underrepresented. This could lead to selection bias based on factors such as geographic location and disease severity. We evaluated the COVID-19 disease course by telephone survey and could not reach all patients. Other data were mostly collected with a manual medical record review where, especially in the search for comorbidities, there is a possibility of missing detailed data. In this regard, the results and conclusions should be interpreted with caution. Despite the limitations, this study significantly contributes to the growing knowledge of the clinical course of COVID-19 and risk factors in patients with GCA.
In conclusion, in this observational single-centre study, we found a lower incidence of severe COVID-19 in patients with GCA than in previous studies. Treatment with leflunomide and low doses of glucocorticoids were not associated with severe COVID-19 in our cohort. Further studies are required to evaluate the outcomes of COVID-19 in the subgroup of patients with GCA.
Author contribution
All authors contributed to the study conception and design. Material preparation and data collection were performed by AH, MT and JK. Analyses were performed by AH and JK. The first draft of the manuscript was written by AH and JK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Slovenian Research Agency (ARRS) for the National Research Program [P3-0314].
Data availability
Data is available upon request.
Declarations
Disclosures
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jennette JC, Falk RJ, Bacon PA, et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013;65:1–11. doi: 10.1002/art.37715. [DOI] [PubMed] [Google Scholar]
- 2.Hayreh SS, Zimmerman B, Kardon RH. Visual improvement with corticosteroid therapy in giant cell arteritis. Report of a large study and review of literature. Acta Ophthalmol Scand. 2002;80:355–367. doi: 10.1034/j.1600-0420.2002.800403.x. [DOI] [PubMed] [Google Scholar]
- 3.Diamantopoulos AP, Haugeberg G, Lindland A, Myklebust G. The fast-track ultrasound clinic for early diagnosis of giant cell arteritis significantly reduces permanent visual impairment: towards a more effective strategy to improve clinical outcome in giant cell arteritis? Rheumatology. 2016;55:66–70. doi: 10.1093/rheumatology/kev289. [DOI] [PubMed] [Google Scholar]
- 4.Luther R, Skeoch S, Pauling JD, et al (2020) Increased number of cases of giant cell arteritis and higher rates of ophthalmic involvement during the era of COVID-19. Rheumatol Adv Pract 4. 10.1093/rap/rkaa067 [DOI] [PMC free article] [PubMed]
- 5.Mackie SL, Brouwer E, Conway R, et al. Clinical pathways for patients with giant cell arteritis during the COVID-19 pandemic: an international perspective. Lancet Rheumatol. 2021;3:e71–e82. doi: 10.1016/S2665-9913(20)30386-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baymakova M, Demirev A, Kostadinova I, et al. Giant-cell arteritis without cranial manifestations presenting as fever of unknown origin: a diagnostic value of 18F-FDG PET/CT. Clin Ter. 2018;169:e274–e276. doi: 10.7417/CT.2018.2092. [DOI] [PubMed] [Google Scholar]
- 7.Balink H, Bennink RJ, van Eck-Smit BLF, Verberne HJ. The role of 18F-FDG PET/CT in large-vessel vasculitis: appropriateness of current classification criteria? Biomed Res Int. 2014;2014:687608. doi: 10.1155/2014/687608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chrapko BE, Chrapko M, Nocuń A, et al. Role of 18F-FDG PET/CT in the diagnosis of inflammatory and infectious vascular disease. Nucl Med Rev Cent East Eur. 2016;19:28–36. doi: 10.5603/NMR.2016.0006. [DOI] [PubMed] [Google Scholar]
- 9.WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. (6 February 2022, date last accessed)
- 10.Ahmed S, Gasparyan AY, Zimba O. Comorbidities in rheumatic diseases need special consideration during the COVID-19 pandemic. Rheumatol Int. 2021;41:243–256. doi: 10.1007/s00296-020-04764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grainger R, Machado PM, Robinson PC. Novel coronavirus disease-2019 (COVID-19) in people with rheumatic disease: epidemiology and outcomes. Best Pract Res Clin Rheumatol. 2021;35:101657. doi: 10.1016/j.berh.2020.101657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng J, Yu X, Bao H, et al (2021) Chronic diseases as a predictor for severity and mortality of COVID-19: a systematic review with cumulative meta-analysis. Front Med 8. 10.3389/fmed.2021.588013 [DOI] [PMC free article] [PubMed]
- 13.Sattui SE, Conway R, Putman MS, et al. Outcomes of COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica from the COVID-19 Global Rheumatology Alliance physician registry: a retrospective cohort study. Lancet Rheumatol. 2021;3:e855–e864. doi: 10.1016/S2665-9913(21)00316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Driscoll M, Ribeiro Dos Santos G, Wang L, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 15.Tedeschi SK, Jin Y, Vine S, et al (2021) Giant cell arteritis treatment patterns and rates of serious infections. Clin Exp Rheumatol 2021 Dec 13. Online ahead of print. PMID: 34905480 [DOI] [PMC free article] [PubMed]
- 16.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 17.EULAR COVID-19 Registry. https://www.eular.org/eular_covid_19_registry.cfm (9 March 2022, date last accessed)
- 18.National Laboratory of Health, Enviroment and Food, Slovenia. https://www.nlzoh.si/objave/sledenje-razlicicam-sars-cov-2-43/(13 March 2022, date last accessed)
- 19.Jorge A, D’Silva KM, Cohen A, et al. Temporal trends in severe COVID-19 outcomes in patients with rheumatic disease: a cohort study. Lancet Rheumatol. 2021;3:e131–e137. doi: 10.1016/S2665-9913(20)30422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinato DJ, Patel M, Scotti L, et al. Time-dependent COVID-19 mortality in patients with cancer. JAMA Oncol. 2021 doi: 10.1001/jamaoncol.2021.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung C, Kmiec D, Koepke L, et al. Omicron: what makes the latest SARS-CoV-2 variant of concern so concerning? J Virol. 2022 doi: 10.1128/jvi.02077-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al Maqbali M, Al badi K, Al Sinani M, et al (2021) Clinical features of COVID-19 patients in the first year of pandemic: a systematic review and meta-analysis. Biol Res Nurs 109980042110558. 10.1177/10998004211055866 [DOI] [PMC free article] [PubMed]
- 23.Maisa A, Spaccaferri G, Fournier L, et al. First cases of Omicron in France are exhibiting mild symptoms, November 2021–January 2022. Infect Dis Now. 2022 doi: 10.1016/j.idnow.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long B, Carius BM, Chavez S, et al. Clinical update on COVID-19 for the emergency clinician: presentation and evaluation. Am J Emerg Med. 2022;54:46–57. doi: 10.1016/j.ajem.2022.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Berger NA, Kaelber DC, et al. COVID infection rates, clinical outcomes, and racial/ethnic and gender disparities before and after Omicron emerged in the US. medRxiv. 2022 doi: 10.1101/2022.02.21.22271300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araf Y, Akter F, Tang Y, et al. Omicron variant of SARS-CoV-2: genomics, transmissibility, and responses to current COVID-19 vaccines. J Med Virol. 2022 doi: 10.1002/jmv.27588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022 doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Comarmond C, Leclercq M, Leroux G, et al (2020) Correspondence on ‘impact of COVID-19 pandemic on patients with large-vessels vasculitis in Italy: a monocentric survey.’ Ann Rheum Dis annrheumdis-2020–219407. 10.1136/annrheumdis-2020-219407 [DOI] [PubMed]
- 29.Tomelleri A, Sartorelli S, Campochiaro C, et al. Impact of COVID-19 pandemic on patients with large-vessel vasculitis in Italy: a monocentric survey. Ann Rheum Dis. 2020;79:1252–1253. doi: 10.1136/annrheumdis-2020-217600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hočevar A, Ješe R, Rotar Ž, Tomšič M. Does leflunomide have a role in giant cell arteritis? An open-label study. Clin Rheumatol. 2019;38:291–296. doi: 10.1007/s10067-018-4232-x. [DOI] [PubMed] [Google Scholar]
- 32.Moradi S, Masoumi M, Mohammadi S, et al. Prevalence of coronavirus disease 2019 in rheumatic patients and evaluation of the effect of disease-modifying anti-rheumatic drugs. Intern Emerg Med. 2021;16:919–923. doi: 10.1007/s11739-020-02535-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Guo H, Li Y, et al. Efficacy and safety of leflunomide for refractory COVID-19: a pilot study. Front Pharmacol. 2021;12:581833. doi: 10.3389/fphar.2021.581833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaur H, Sarma P, Bhattacharyya A, et al. Efficacy and safety of dihydroorotate dehydrogenase (DHODH) inhibitors “leflunomide” and “teriflunomide” in Covid-19: a narrative review. Eur J Pharmacol. 2021;906:174233. doi: 10.1016/j.ejphar.2021.174233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos CS, Morales CM, Álvarez ED, et al. Determinants of COVID-19 disease severity in patients with underlying rheumatic disease. Clin Rheumatol. 2020;39:2789–2796. doi: 10.1007/s10067-020-05301-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyrich KL, Machado PM. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol. 2021;17:71–72. doi: 10.1038/s41584-020-00562-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan EH, Sena AG, Prats-Uribe A, et al. COVID-19 in patients with autoimmune diseases: characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatology. 2021;60:SI37–SI50. doi: 10.1093/rheumatology/keab250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available upon request.


