Abstract
B-cells play a key role in outcomes of cancer patients and responses to checkpoint blockade immunotherapies. However, the effect of radiation therapy on B-cell populations is poorly understood. Here we characterize the effects of radiation on the development, survival, and phenotype of physiologic B-cell subsets. Naïve and immunized tumor bearing and non-tumor bearing mice were treated with large field or focal stereotactic radiation and distinct B-cells subsets of varying developmental stages were analyzed by flow cytometry and RT-PCR. We first report that focal stereotactic radiation is highly superior to large field radiation at inducing tumor infiltration of B-cells, CD8+ T cells, and macrophages. We observed that radiation impacts B-cell development in the bone marrow, increasing frequencies of early pro-B-cells and late pro-B-cells while inducing upregulation of PD-1. We then demonstrate that class-switched B-cells and plasma cells are highly resistant to radiation therapy compared to naïve B-cells and upregulate activation markers PD-L2 and MHC II after radiation. Mechanistically, radiation upregulates Xbp1 and Bcl6 in plasma cells, conferring radioresistance. Furthermore, using an immunization approach, we demonstrate that radiation enhances activation-induced cytidine deaminase (AID) mediated class switching and somatic hypermutation in primed B-cells. In conclusion, these data demonstrate that stereotactic radiation is superior to large field radiation at inducing infiltration of immune cells into tumors and that plasma cells and class-switched B-cells are highly resistant to radiation therapy. These results represent the most comprehensive analysis of the effects of radiation on B-cells to date and identify novel mechanisms by which radiation modulates immune cells within the tumor microenvironment.
Summary:
B-cells play key roles in cancer patient survival and responses to checkpoint blockade immunotherapy; however, the effects of radiation on B-cell survival and phenotypes are unclear. Here we perform a comprehensive analysis of the effects of radiation on B-cells and discover that class-switched B-cells and plasma cells are highly resistant to radiation therapy and that radiation triggers class switching in B cells and modulates B-cell development.
Introduction
Radiation therapy modulates the tumor microenvironment by inducing tumor cell death, release of inflammatory cytokines, and activation of vascular remodeling and repair pathways1–3. The DNA damage response and immunogenic cell death induced by radiation have also been demonstrated to cause upregulation of MHC and enhance antigen presentation4, 5, leading to the many ongoing trials combining radiation with immunotherapy6, 7. However, radiation has also been demonstrated to increase expression of cell adhesion molecules on tumor vasculature and release chemokines which can drive immune cell infiltration into tumors8, 9. It is known that tumors with little to no immune cell infiltrate (‘Immune Desert’ or ‘Immune Excluded’) are resistant or refractory to checkpoint blockade immunotherapies10, 11. Thus, the distinct ability for radiation to induce a robust immune cell infiltrate may have unique importance when used in combination with immunotherapies.
While most studies investigating the effects of radiation on immune cell infiltrates have focused on T-cells or myeloid cells, the effects of radiation on other immune cells, including B-cells is poorly understood. Recent seminal studies have identified a key role for B-cells and antibody responses in outcomes of cancer patients12–16. B cells are professional antigen presenting cells that also produce antibodies that can independently alter the function of cancer cells, activate the complement cascade, as well as contribute to NK cell mediated tumor killing via antibody-dependent cell-mediated cytotoxicity. In many cancer mouse models, about a third of tumor-draining lymph nodes cells are B cells17, indicating that B-cells constitute a major component of the adaptive immune system. In addition therapeutic immune checkpoint blockade may also target B cells, since PD-1, PD-L1, CTLA-4, and the B7 molecules are expressed on B cells 18 19 20. Given these findings, recent reviews have highlighted the importance of B-cells in oncology and patient outcomes21. Nevertheless, the intrinsic effects of radiation on the survival or frequency of B-cells and B-cell subsets in the tumor microenvironment remains unclear. It is commonly believed that any immune cell within a radiation treatment field is destroyed by high doses of radiation. Indeed, lymphocytes are one of the most radiosensitive cells in the mammalian body22. However, it has been clearly demonstrated that activated T-cells in the tumor microenvironment are resistant to radiation and maintain their effector function even after high doses of radiation23. Whether the same holds true for B-cells in the tumor microenvironment is unknown.
The effect of radiation on B-cell development and differentiation in the bone marrow (BM) and spleen is also poorly understood. B-cell development is guided by sequential events leading to assembly, expression, and signaling of the B-cell antigen receptor (BCR). Importantly, heavy and light chain immunoglobulin genes are rearranged at the pro-B and pre-B stages, respectively, through V(D)J recombination which depends on RAG-1 and RAG-2 DNA endonucleases of the non-homologous end-joining DNA repair pathway24, 25. Complete surface IgM is expressed at the immature stage in the BM and further development is guided by positive and negative selection though the BCR with the goal of generating immune-competent naïve B cells that do not react to self-antigens 25, 26. It has been estimated that 1–2 × 107 immature B cells are generated daily in the adult mouse and leave the BM as transitional B cells 27, but only about 3% enter the pool of mature B-cells. At multiple steps, transcription factors (TFs) orchestrate the B-cell fate specification 28, 29. Naïve B-cells, after leaving BM, are activated by antigen-presenting dendritic cells (DC), proliferate rapidly and form germinal centers (GCs) under the help of follicular helper T (Tfh) cells, where the activated B-cells undergo massive proliferation, somatic hypermutation (SHM), and class-switch recombination (CSR) and eventually differentiate into memory B-cells (Bmem) or Plasma Cells (PC)30. The expression of BCL6/BACH2/PAX5 maintain the B-cell phenotype and promote their proliferation and SHM, whereas IRF4/PRDM1/XBP1 reduce proliferation and facilitates CSR and differentiation toward Bmem and PCs 31. Interestingly, radiation activates DNA damage repair and response pathways including non-homologous end-joining which is required for B-cell development; however, the effect of radiation on these transcriptional pathways as they guide B-cell development remains poorly understood.
Here we set out to characterize the effects of radiation on the survival and phenotype of B-cells and physiologic B-cell subsets. Using a squamous cell carcinoma model system, wildtype naïve and tumor bearing mice were treated with varying radiation field sizes and doses of radiation to the bone marrow, spleen, and flank tumors. B-cell populations were quantified, phenotyped, and sorted using multiparametic flow cytometry and subjected to gene expression analysis. We observed differential effects of radiation on B-cell subpopulations in tumor, spleen (SP), lymph nodes (LN), and BM. By sorting B-cell subsets including memory populations and plasma cells we dissect the time and dose-dependent effects of radiation on B-cell apoptosis and differentiation/maturation pathways. Remarkably we found that stereotactic radiation is highly superior to large field radiation at inducing immune cell infiltrates. Furthermore, we discovered that plasma cells and class-switched B-cells are highly resistant to radiation therapy and that radiation induces class-switch recombination and somatic hypermutation in B-cells.
Materials and Methods
Cell lines and reagents
AT-84-E7-OVA cell line has been created from AT-84-E7 cells, kindly obtained from Dr. Aldo Venuti (Regina Elena National Cancer Institute, Italy) as described previously. MC38 mouse colon carcinoma cell line was purchased from the American Type Culture Collection. Cells were cultured at 37°C, 5% CO2, in full humidity (98%). MC38 cells were cultured in RPMI 1640 media supplemented with 10% FBS, 1% L-Glutamine, 1% Penicillin/Streptomycin, for AT-84-E7-OVA 1% Sodium Pyruvate, Geneticin (200 μg/ml), and Blasticidin (3 μg/ml) were also added.
Mice and in vivo treatments
For the MC38 colon carcinoma studies, 5 x 105 tumor cells were subcutaneously implanted on the right flanks of 8- to 10-week-old female and male C57BL/6 mice (The Jackson Laboratory, CA) on day 0. Six days after implantation the mice were randomly assigned to two groups for 10Gy stereotactic or large field radiotherapy treatment (n = 4 female and 2 male mice per group). For the experiment with bone marrow, six days after implantation the mice were randomly assigned to three groups (n = 5 mice per group) for anti-PD-L1 (10F.9G2, Bio X Cell) at 5mg/kg every 3 days for a total of 3 intraperitoneal injections per mouse, starting on day 6; or 12Gy large field radiotherapy treatment on right flank tumor on day 6, or combined treatment RT and aPD-L1.
For AT-84-E7-OVA head and neck carcinoma studies, 5 x 105 tumor cells were subcutaneously implanted on the right flanks of 8- to 10-week-old female C3H/HeN mice (Charles River, Wilmington, MA) on day 0. Six days after implantation the mice were randomly assigned to two groups (n = 5 mice per group) for 10Gy stereotactic or large field radiotherapy treatment.
Tumor was measured by means of electronic caliper every 3 days and a volume was calculated using (l x w^2)/2 formula.
For the experiment with immunization, mice (n = 5 mice per group) were injected intraperitoneally with 50 mg OVA-alum and 14 days later were radiated with 8Gy x1 on spleen. The experiment was terminated after 24- or 48 hours. Spleens were harvested, cells extracted.
For in vitro experiments cells were extracted from C3H naïve mice (n = 2 mice per experiment; three replicates of each experiment), B-cells were purified with MACS separation kit and irradiated with 2, 8, 20Gy. After RT, B cells were cultured at 37°C, 5% CO2 in RPMI 1640 media supplemented with 10% FBS, 1% L-Glutamine, 1% Penicillin/Streptomycin for 2-, 4-, and 6 hours. After that time cells were harvested and suspended in Trizol and frozen at −80°C. RNA was extracted and stored at −80°C.
The study was completed under the guidance for the care and use of laboratory animals specified by the National Institutes of Health. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of California at San Diego.
Human studies
Two fresh human PBMCs samples were obtained under IRB approved protocol from clinical trial HRPP 151570 at University of California at San Diego, Moores Cancer Cen-ter. Both PBMC samples were negatively selected on MACS to isolate B cells and split into two equal parts, one was an untreated control, and the other was radiated with 8Gy. After 6 hours of incubation at 37°C, 5% CO2 in RPMI 1640 media supplemented with 10% FBS, 1% L-Glutamine, 1% Penicillin/Streptomycin cells were harvested and suspended in Trizol and frozen at −80C. RNA was extracted and processed as the other samples to determine genes expression.
Irradiation
Large field conventional irradiation of mice was performed with a Cs-137 Irradiator (2.53 cGy/min) (JL Shepherd and Associates, San Francisco, CA). Stereotactic body radiation therapy (SBRT), delivering the radiation therapy (RT) (225 kV/13 mA) with millimeter precision and minimizing dose to surrounding tissue structures, was performed with SmART Irradiator (Precision X-Ray Irradiation, Canada). All RT was per-formed at University of California at San Diego, Moores Cancer Center.
Tissue processing, cell surface staining, flow cytometry, and cell sorting
Tumors were dissected, minced, and resuspended in RPMI 1640 media supplemented with Collagenase-D (1 mg/mL; Roche) and incubated at 37 °C for 60 min with shaking and filtered through a 70-μm nylon mesh filter to generate single-cell suspensions. Inguinal LN, SP, and BM from tumor site femur were also resected and mechanically disrupted through a 70-μm nylon mesh filter to generate single-cell suspensions. Erythrocytes from SP and BM were lysed with ACK buffer (Gibco, USA). For in vitro experiments, this was followed by magnetic enrichment of B-cells using Pan B Cell Isolation Kit II (Miltenyi, Negative Selection Kit). Single-cell suspensions from all harvested organs were stained with relevant antibodies for 30 min on ice and washed twice with PBS. Antibodies for mouse B220/CD45R (RA3-6B2), CD45.2 (104), CD19 (6D5), IgM (RMM-1), IgD (11-26c.2a), IgG (Poly4053), CD3e (145-2C11), CD8a (53-6.7), CD11b (M1/70), CD11c (N418), Gr-1 (RB6-8C5), CD24 (M1/69), CD27 (LG.3A10), CD138 (281-2), CD1d (1B1), CD5 (53-7.3), MHC II (2G9), PD-L2 (TY25), NK1.1 (PK136), F4/80 (BM8), PD-1 (29F.1A12), Ter119 (Ter119), CD43 (S7), Ly6C ( HK1.4), Ly6D (49-H4), Ly51 (BP-1) (6C3) were from BioLegend and CD93 (AA4.1) from BD. Streptavidin with biotinylated-ovalbumin at approximately 1:10 molar ratio was used. Apoptosis was assessed by Annexin V/PI test according to the manufacture’s protocol (BioLegend). Data were acquired on LSRII (BD) and analyzed with FlowJo v10 software. Sorting was performed on a FACSAria II (BD) at the Flow Cytometry Facility at University of California at San Diego, Moores Cancer Center.
Gene expression analysis
RNA was extracted from B-cells, isolated from mouse spleen, using Trizol (Invitrogen, CA, USA), according to the manufacturer’s instructions. The RNA concentration was measured on Qubit (Thermo Fisher Scientific, Waltham, MA). RNA (100 ng) was used for cDNA synthesis (Superscript IV VILO, Thermo Fisher Scientific, CA). Real-time PCR was performed in an optical 96-well plate with an CFX96 (Bio-Rad). Each 20 μl reaction contained 10 μl iTaq SYBR Green Master Mix (Bio-Rad), 2 μl gene-specific forward and reverse primers (10 μM), 4 μl cDNA and 4 μl nuclease-free water. Validated PrimeTime qPCR primer pairs (IDT, CA, USA) were used for each gene. Data was obtained as threshold cycle (Ct) values. The average Ct value was used for ΔCt (delta Ct value, dCt) measurement. Afterwards, the difference between the Ct value of each gene of interest and the average Ct value for a housekeeping gene in each sample was measured. The relative expression of each gene was quantified by the comparative cycle threshold method (ΔΔCt, ddCt), using Actb as an endogenous control (a housekeeping gene) 32. Data for 2, 8, and 20Gy is normalized to 0Gy. Regarding the heatmap with 0Gy non-class switched and class-switched cells are normalized to naïve B cells. To show the differences between naïve B cells and other B cell subsets, the value for naïve cells was set to zero on the heatmap as a reference value.
OVA-specific serum immunoglobulin IgG1 level
Serum was collected from treatment-matched mice. High-binding 96-well plate was coated with ovalbumin, ready to use (# 500830, Cayman Chemical). Serum samples were used at the indicated dilutions according to the manufacturer protocol. Plates were washed three times, and HRP-coupled secondary antibody recognizing IgG1 was added. Plate was washed three times and bound antibody was detected using 3,3’5,5’-tetramethylbenzidine substrate.
Statistical analysis
Statistical analysis was performed in GraphPad Prism 8.0 software (GraphPad, San Diego, CA). One-way ANOVA with a Turkey’s post-hoc test for multiple comparisons were conducted for flow cytometry analysis. The asterisks denote statistical significance (nonsignificant or ns, P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001; and ****P < 0.0001). All data are reported as mean ± standard error of the mean (S.E.M.). For all experiments, each experiment was repeated independently with similar results for at least three times.
Results
Stereotactic focal RT significantly enhances B-cell and T-cell immune cell infiltration when compared to Large Field RT.
In order to study the effect of radiation on the tumor microenvironment we first evaluated whether differences in radiation field size impacted immune cell infiltration. A recent pre-clinical study demonstrated that large field tumor irradiation encompassing draining lymph nodes severely diminished T-cell infiltration when compared to focal tumor only radiation33. However, whether the same holds true for B-cell infiltration is unknown. C3H/HeN mice harboring AT-84-E7/OVA tumors were established as previously described. Flank tumors were then treated with large field irradiation (10 Gy) including inguinal draining LN or stereotactic tumor only focal radiation (10 Gy) using the SmART stereotactic small animal irradiator. Tumors were then excised at day 7 and 14 after irradiation and analyzed for immune cell infiltration by flow cytometry (Suppl. Fig 4C). At day 7 we observed that both stereotactic as well as large field irradiation increased intratumoral infiltration of T-cells and B-cells; however stereotactic radiation resulted in a dramatically increased absolute tumor infiltrate of T-cells, B-cells and Macrophages compared to large field radiation (Fig 1A–C,E). Furthermore, by day 14 we observed that stereotactic focal irradiation maintained a dramatic increase in infiltrating T-cells and B-cells, while the impact of large field radiation was attenuated or even slightly reduced compared to untreated (Fig 1A–C). Moreover, we observed dramatic overall increases in absolute intra-tumoral immune cell number at day 14 compared to day 7 (Fig 1A–C), consistent with known pattern and time course of radiation induced tumor infiltrates. We observed a similar trend using a separate MC38 colon cancer tumor model on the C57BL/6 background and analyzed infiltration of immune cells by flow cytometry (Fig 1D) as well as immunostaining (Fig 1E, Suppl Fig 5A–B). Of note, we did not identify significant changes in intratumoral macrophage or NK cells. Taken together these findings demonstrate that focal stereotactic radiation may be superior to larger field conventional radiation at inducing both B-cell and T-cell infiltration into tumors.
Figure 1: Stereotactic focal radiation enhances tumor immune cell infiltration.

(A) The number of all T cells and CD8+ T population in tumor; n = 5 mice in each group. (B) The number of macrophages in tumor; n = 5 mice in each group. (C) The number of B-cells in tumor (n = 5 mice in each group). The data indicates the number of cells before and after radiation. We show two time points Day 7 and Day 14 after stereotactic focal or large field radiation on C3H mice (female) with AT-84-E7/OVA flank tumor. (D) The number of CD8+ T cells, B cells, dendritic cells, macrophages, and NK cells in MC38 tumor injected into flank of C57BL/6 mice; n = 6 mice per group, 2 male and 4 female. (E) B and T cells infiltrating the MC38 tumor confirmed by immunohistochemistry staining. CD3+ T cell (green) and B220+ B cell (red)) in MC38 tumor were stained in multiplex immunofluorescent staining panel. DAPI stain indicate the cell nuclei. Representative images were shown in 20 x magnification. Scare bar indicates 50 mm.
Radiation modulates B-cell development in BM of tumor-bearing mice.
While the effects of RT on T cell activity in HNSCC have been studied9, little is known how RT affects B-cell development and B-cell precursors in the bone marrow. In order to evaluate the effects of RT on B-cell development we irradiated BM of naïve and tumor-bearing mice and analyzed B-cell populations by flow cytometry (Suppl Fig 1A). We observed that RT increases the frequency of CD43+B220+BP-1−CD24+ Pro-B cells (early and late Pro-B cells), while decreasing populations of CD43+B220+BP-1−CD24− Pre-pro B cells (the earliest population) in C3H mice with AT-84-E7-OVA tumor (Fig 2A–B). In C57BL/6 mice with MC38 tumor, after RT the number of Pre-pro-B cells also decreased (Fig. 2C–D). Pre-B cells represent the next stage of development after Pro-B cells and they are the earliest cell type that create pre-B cell receptors (pre-BCR), which stimulate the proliferation and continued maturation of the developing B-cells26. We found that the frequency of CD43−B220+IgD−IgM− Pre-B cell populations are largely unaffected by RT (Fig 2B, D). Interestingly in C3H mice with AT-84-E7-OVA at day 7 after RT, we saw a significant decrease in immature B-cells which reversed at day 14 to a significant increase. Ultimately, mature naïve B-cells at day 7 and day 14 were largely unaffected by RT (Fig 2B), perhaps due to the cumulative effects on proliferation versus cell death of precursor and progenitor populations. We also observed that radiation can modulate B-cell development in mice on C57BL/6 background (Fig 2D).
Figure 2: Stereotactic focal radiation modulates B-cell development.

(A) Gating strategy and frequency of pro-B cells in BM of CH3 mice with AT-84-E7/OVA tumor on Day 7 (n = 5) and 14 (n = 5) after radiation 12Gy x 1, untreated mice are a control (n = 5). (B) Gating strategy and frequency of pre-B cells, immature, and mature naïve B-cells in BM of CH3 mice with AT-84-E7/OVA tumor on Day 7 and 14 after radiation, untreated mice are a control. (C) The frequency of pre-pro-B-cells and pro-B-cells subtypes in BM of C57BL/6 mice with MC38 tumor after radiation 12Gy x 1. (D) The frequency of pre-B-cells, immature, and naïve mature B-cells subtypes in BM of C57BL/6 mice with MC38 tumor after radiation 12Gy x 1.
Plasma cells are highly resistant to radiation therapy.
We next turned to study the effects of radiation on survival and frequency of mature B-cell subsets in the periphery. Specifically, we analyzed the frequencies of B-regulatory cells (B regs) (CD5+CD1dhigh) (Fig. 3C, F), memory-like B-cells (Bmem) (Fig. 5), transitional (T1, T2) (Suppl Fig 3A–B), mature (IgM−IgD+) (Suppl Fig 3A–B), and PCs (Fig. 3B, D) (gating strategy in Supp Fig 1B, 2). We first observed a dose-dependent decrease in total B-cells in the spleen of irradiated mice at 24 and 48 hours consistent with the known radiosensitivity of unselected B-cells (Fig. 3A). However, in contrast, we observed a striking dose dependent increase in the frequency of CD138+ plasma cells in the spleen after RT at 24 and 48 hours (Fig 3B). To determine whether these plasma cells were resistant to radiation therapy, we performed Annexin-V/PI staining and observed that both IgG−CD138+ and IgG+CD138+ plasma cells are almost entirely resistant to radiation induced apoptosis or cell death, even at high doses of 20 Gy (Fig 3C). Interestingly, we observed that irradiated plasma cells have increased surface expression CD138 supporting the radioresistance of plasma cells (Fig. 3E). Importantly, the radioresistance of plasma cells did not extend to other B-cell subtypes, for example CD5+CD1dhigh B-regulatory cells were highly sensitive to radiation induced apoptosis and cell death (Fig 3D, F). Taken together these results demonstrate that naïve and resting B-cells are highly sensitive to radiation induced apoptosis, however plasma cells are remarkably resistant to even high doses of radiation. To characterize the activity and functional output of these plasma cells we quantified anti-Ovalbumin IgG1 levels in serum of OVA-immunized C57BL/6 mice after RT. We observed significant increases in OVA specific IgG1 concentrations approx. 27 days after irradiation, indicating that the functionality of radioresistant plasma cells was maintained and enhanced after RT(Fig. 3G).
Figure 3: Plasma cells are highly resistant to radiation.
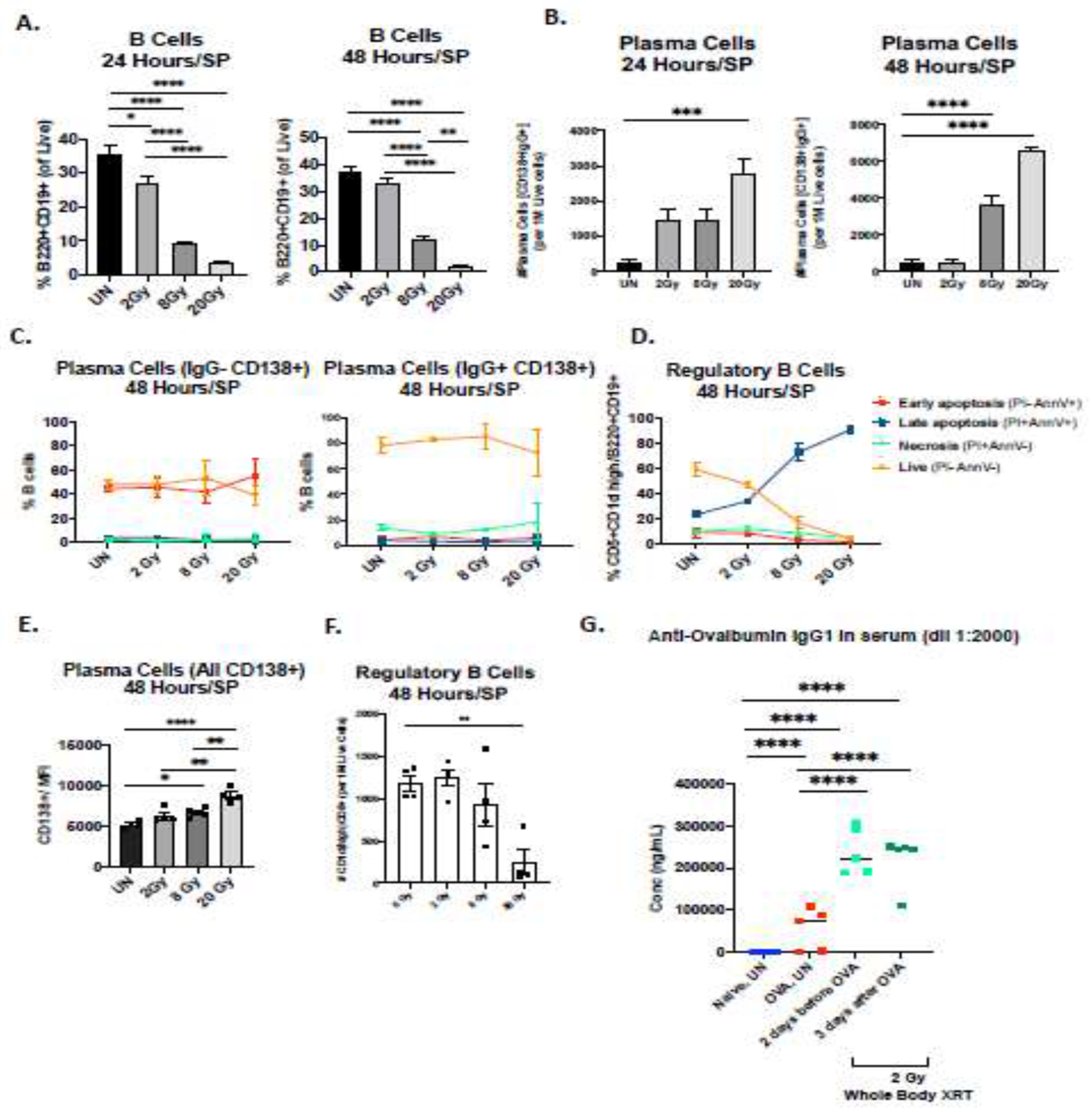
(A) The frequency of all B-cells in SP of naïve C3H mice at 24- and 48 hours after large field radiation showing a dose dependent sensitivity to radiation. We treated mice (n = 5 per group) on SP in three doses: 2, 8, 20Gy. (B) Plasma cells (CD138+IgG+) in radiated SP show dose-dependent resistance to radiation. The number of cells was normalized to 1M of live cells. (C) Percent of PCs in each group that were live (Ann−/PI−), in early apoptosis (Ann+/PI−), in late apoptosis (Ann+/PI+) and necrosis (Ann−/PI+) (D). Regulatory B-cells (CD1dHigh CD5+) in SP radiated with large field radiation, viability and apoptosis. (E) MFI (Median) of all CD138+ plasma cells after 48 hours of large field SP radiation in naïve mice. (F) The number of regulatory B-cells have been measured after 48 hours. (G) Functional humoral effect of radiation on immunized mice; n = 5 mice per group, 2Gy x 1 the whole-body radiation.
Figure 5: Activated memory-like B-cells are resistant to and activated by radiation.

B-cells isolated from SP of OVA-immunized mice radiated in vivo with three doses 2, 8, 20Gy and harvested 6 hours after treatment. (A) The gating of Bmem-like cells in SP. (B) Percent of class switched and non-class switched B-cells in radiated SP (large field); upper panel, PD-L2 activation in SP; middle panel, and MHC II surface marker in SP; lower panel. (C) The gating of Bmem-like cells in LN. (D) PD-L2 activation marker in class-switched and non-class switched B-cells in LN. Each test was performed on C3H mice (female); n = 5/group.
Radiation modulates B-cell apoptotic gene profile.
In order to investigate the molecular mechanisms underlying the radiosensitivity of B-cells we performed RT-PCR on ex-vivo B-cells isolated from mouse spleens and irradiated with 0Gy, 2Gy, 8Gy, or 20Gy (Fig 4A). Compared to 0 Gy, we observed that radiation induced significant changes in expression of multiple B-cell genes and pathways involved in B-cell signaling, survival, and apoptosis (Fig 4A). Specifically, we observed in bulk populations that Fas mRNA was rapidly upregulated in B-cells at 2 hours after irradiation and remained elevated at 4 hours with higher doses of 8 Gy and 20 Gy of radiation (Fig 4C). We also observed that expression of the pro-apoptotic gene Bax as well as p53, Caspase 3, and Caspase 9 increased over time after irradiation in a dose-dependent fashion (Fig 4A, C). Our data on B-cells obtained from C3H naïve mouse spleens that were irradiated in vitro shows significant activation of initiator Casp 9 and effectors Casp 3 and Casp 7 post-RT and we also confirmed similar findings in mice on C57BL/6 background (Suppl Fig. 6A). To confirm whether these findings translated to human B-cells we analyzed the effects of radiation on freshly isolated B-cells from human PBMC. We observed significant and conserved changes in apoptosis gene expression in human B-cells after irradiation, including upregulation of Fas, Bax, and Traf1, and down regulation of Pik3r1 and Survivin. These data clearly demonstrate that radiation induces dose and time dependent modification of genes involved in B-cell apoptosis.
Figure 4: Radiation modulates apoptotic gene expression in B-cells and sorted B-cell subsets.

(A) Heat map Log2 fold change. B-cells isolated from SP were radiated in vitro with three doses 2, 8, 20Gy and harvested at three time points 2, 4 and 6 hours after treatment. The expression of genes after treatment was normalized to 0Gy. (B) Apoptosis gene expression in human B cells. B-cells isolated from PBMCs were irradiated with 8Gy and gene expression was analyzed 6 hours after irradiation and compared to non-irradiated matched control. (C) Fas and Bax mRNA (in vitro) have been extracted from the heat map (A) to show the dynamics of irradiation effect on B cells. (D) Heat map Log2 fold change of apoptotic genes. B-cells isolated from SP of C3H OVA-immunized mice radiated in vivo with three doses 2, 8, 20Gy and harvested 6 hours after treatment. The expression of genes after treatment was normalized to OGy. (E) Expression of transcription factors (TFs) Xbp1 and Bcl6 in naïve B-cells versus PCs before and 6 hours after radiation. These two TFs responsible for PC’s radioresistance depict high expression of mRNA. The expression has been measured by means of qRT-PCR in triplicates. All TFs (E, F, G) were measured in B-cells from C3H OVA-immunized mice 6 hours after treatment. (F) Heat map Log2 fold change of TFs. Left panel shows the basic genes expression without radiation, right panel shows the same panel of genes and their expression after 6 hours post treatment first normalized to the endogenous control Actb and then normalized to 0Gy.
To dissect the effects of radiation on B-cell subsets in more detail, we sorted and isolated the following individual B-cell subpopulations from irradiated mouse spleens: Naïve B-cells, Non-class switched memory, Class-Switched memory, and plasma cells (Supp Fig 1B). RT-PCR performed on sorted B-cell subsets revealed marked differences in baseline gene expression and changes after radiation (Fig 4D–F). One of the key transcription factors required for B-cell differentiation and antibody production is XBP-1 and we observed significant differences in expression of XBP-1 after RT between subsets including naïve B cells and plasma cells (Fig 4F & 4E Left Panel). We also observed that Birc5 (survivin) which suppresses Casp9 and effector Casp3 and 7 was upregulated in putative memory B-cells and PCs and likely contributes to their radioresistance (Fig 4F). Additionally, we found significant upregulation of XBP-1 and Bcl6 in plasma cells compared to naïve cells after irradiation (Fig 4F Right & 4E). These gene changes are consistent with and support a molecular mechanism underlying the radioresistance of plasma cells and the ability of radiation therapy to modulate expression of genes critical for B-cell differentiation.
Activated Class switched and putative memory B-cells are also resistant to Radiation.
Mature B-cells and memory B-cells can express IgM or undergo recombination and class switching to produce IgG, IgA, or IgE antibodies. The effect of radiation on these subtypes of mature B-cells or memory B-cells is unknown. When we analyzed the effect of radiation on CD27+IgD+ Non-classed switched and CD27+IgD− Class switched putative memory B-cells (Fig 5A) we observed striking differences in the radiosensitivity and frequency of these populations. Non-class switched B-cells were diminished after 8Gy or 20Gy, while the relative frequency of class-switched B-cells was markedly increased after 8Gy or 20Gy (Fig 5B upper panels). PD-L2 is a known activation marker for B-cells34. Interestingly we observed that radiation induced a dose dependent upregulation of PD-L2 expression in both class switched and non-class switched B-cells (Fig 5B middle panels). We observed a very high frequency of MHC Class II expression on resting B-cells and radiation resulted in a slight further increase in overall expression of MHC Class II on these populations (Fig 5B lower panels). In the lymph node we observed that radiation can also upregulate PD-L2 expression on B-cells, however 8Gy appeared optimal for inducing PD-L2 upregulation on non-class switched B-cells in the LN (Fig 5C), perhaps suggesting differential effects of radiation depending on surrounding stroma and microenvironment between spleen and LN (Fig 5C, D).
Primed B-cells but not naïve B-cells are induced to undergo class switching by RT.
Given the observed effects of RT on apoptotic gene pathways in B-cell subsets (Fig 4) we sought to determine whether RT impacts transcription factors regulating B-cell differentiation and function. To further dissect the effects of RT on resting versus activated B-cell subsets, we employed an immunization approach using OVA antigen with alum adjuvant and compared transcriptional profiles of sorted B-cell subsets from naïve mice or immunized mice (Fig 6A, B). Interestingly, when comparing non-immunized to immunized mice we observed a significant upregulation of activation-induced cytidine deaminase (AID) in resting non-class switched and class switched mature B-cells after immunization (Fig 6A, B left panels). AID is a master regulator of class switch recombination (CSR) and somatic hypermutation (SHM) in B-cells35. When analyzing the expression of key signaling genes, we observed that 8Gy and 20 Gy of radiation enhanced relative expression of Aid, Btk, Mek1 and Lyn, in class switched B-cells but not in non-class switched B-cells from immunized mice compared to non-immunized mice (Fig 6A & B right panels, D). Furthermore, we also observed higher expression of Bach2 and Pax5 which also play key roles in SHM (Fig 6B, C). Zbtb24 is a transcription factor which controls the in vivo Bmem development of human GC-B cells36, and we also observed increased expression in non-class switched and class switched B-cells after 8Gy and 20Gy (Fig 6B, C). We also observed comparable findings using mice on C57BL/6 background (Suppl Fig. 6). The ability for radiation to modulate genes involved in CSR and SHM in B-cells represents a novel mechanism of activation by which radiation could augment B-cell mediated anti-tumor immune responses and development of memory B-cells.
Figure 6: Primed B-cells but not antigen naïve B-cells are induced to undergo class switching by radiation.

(A) Heat maps of genes expression in B-cell subtypes at 6 hours after SP large field radiation – non-immunized (naïve) mice (A) and alum/OVA-immunized C3H female mice (B); n = 5/group. (C) Selected TFs mRNA showing higher expression in class switching cells thus showing that radiation can trigger class switching recombination. TFs were measured in sorted B cells from radiated SP of immunized mice; n = 5/group (D) Gene expression fold difference between primed and non-primed B-cells; n = 5/group.
Discussion
The effects of radiation on tumor associated immune cells, tumor vasculature, and extravasation of infiltrating immune cells are becoming better understood37. Multiple groups have reported an increased infiltration of immune cells into tumors approximately 7–10 days after irradiation5, 8, 38, 39. The mechanisms underlying this involve radiation induced upregulation of cell adhesion molecules including VCAM-1 and ICAM-1 on tumor vasculature as well as release of chemokines and chemoattractant including CCL2, CCL4, CXCL9, CXCL10, CXCL16, and MCP-1 which can drive immune cell extravasation and infiltration into irradiated tumors9, 39–42 However, the vast majority of these studies focus on T-cell or Myeloid cell infiltration and have not analyzed the effects of radiation on B-cell infiltration. Furthermore, very few studies have evaluated the effects of large field versus stereotactic focused radiation on tumor infiltrates33. Our data is among the first to demonstrate that radiation can induce robust B-cell infiltrates and that focal stereotactic radiation is superior to large field conventional radiation at inducing tumor immune cell infiltrates (Fig 1). While B-cells traditionally reside in the spleen, bone marrow, and lymph nodes, it has been demonstrated that intratumoral B-cells can modulate anti-tumor immune responses43. Thus the ability for radiation to induce B-cell infiltrates is a key finding that deserves further investigation as a strategy to enhance antitumor immunity.
While the prevailing thought is that all immune cells within an irradiated field are killed, recent data has disproven this belief and clearly demonstrated that subsets of T-cells within an irradiated tumor are resistant to radiation and can continue their effector functions23. Using detailed longitudinal imaging of fluorescently marked T-cells, Arina A. et al., demonstrated that preexisting tumor T-cells are not eliminated by radiation and instead appear essential to the antitumor effects of radiation therapy23. This central finding in T-cells has profound implications for the use of radiation in combination with immunotherapies. Nevertheless, the effect of radiation on B-cell survival and function remains poorly understood.
To better understand the radiosensitivity of B-cells it is of critical importance to quantify the frequency and absolute number of B-cell subsets under physiological conditions after RT. Our experiments based on irradiation of BM derived B-cells demonstrated that frequencies of pro-/pre-B-cells and immature B-cells increase after RT, and that radiation also modulates phenotypic and activation markers. Interestingly, we observed possible differences in RT treatment between C57BL/6 male and female mice with MC38 tumor although the number of mice in both groups was too small to draw conclusions. (Suppl Fig 4A–B). We also observed that Pre-pro-B cells and early/late-pro-B cells corresponding to the earliest stages of mouse B-lymphocyte development were the most radiation sensitive B-cell subpopulations. Our work is consistent with early studies demonstrating that later stages of human as well as mouse B-lymphocytes development were more resistant to RT44. It is known that lymphoid progenitors and stem cells are very sensitive to RT45, but interestingly, pre-B cells are the most resistant to radiation among early stages of B-cell subsets. Following Uckun et al. (1991) 44 differentiated cells are in general much more RT resistant than undifferentiated ones, and this influence of the degree of cellular differentiation on radiation sensitivity cannot be explained solely by differences in the proliferative or apoptotic activity of cells. Of note, our data is supported by historical data which indicated changes in B-cell development after whole body irradiation. Specifically, Park and Osmond found that early B-cell precursors increased in murine bone marrow during a “wave of post-irradiation regeneration.” 45 Bouteiller PP et al., reported an increase in surface immunoglobulin on B-cells from mice that had received whole body irradiation, and concluded that this effect was due to modified maturation and not enhanced proliferation46. However, these early studies did not isolate or analyze distinct subsets of mature B-cells and also did not evaluate the effects of modern focal or stereotactic radiation. Thus, our results clarify the effects of radiation on B-cell subpopulations including developing B-cells in the bone marrow. Future work is deserved to analyze the effects of radiation on B-cell development given that intensity modulated radiation (IMRT) which is used clinically to treat patients can expose large volumes of bone marrow to low doses of radiation.
It is known that upon immunological challenge, B lymphopoiesis increases, as measured by cell number, cell division, and turnover 47 Our present study supports the concept that B-cell differentiation and function in the periphery is regulated by a feedback mechanism which can be impacted by RT. For example, our data demonstrate that tumor irradiation can result in the mobilization of B cells in TME as well as increase BCR expression, especially after immunization. Additionally, we demonstrate that RT activates, in direct dose-dependent way, T1 and mature B-cell in SP. Indeed, the T1 population seems to be more resistant to RT and we observed a dose-dependent increase in population’s frequency, which might be due to BCR activation or PD-L2 activation (Suppl Fig 3). However, we did observe a dose dependent decrease in unselected bulk B-cell populations in the spleen after radiation therapy (Fig 3A), indicating that these findings are limited to select B-cell subsets.
As compared to T-cells, significantly less attention has been given to the role of B-cells in host defense against tumors, but critically, B-cells can regulate other immune cells and also generate potent anti-tumor humoral immune responses. For example, of particular importance is the role they play in concert with CD4+ memory T-cells 48, 49 to promote survival and proliferation of activated CD8+ T-cells through CD27/CD70 interactions 49 In clinical studies, tumor-infiltrating B-cells correlate with survival in ovarian cancer, and survival was higher when tumors contained both CD20+ and CD8+ cells, which suggests coordinated immune cross-talk between these two populations50. Recent work identified a significant overall survival advantage in head and neck cancer patients who have high levels of intratumoral B-cells. Additionally, recent seminal studies have identified key roles for B-cells in responses to checkpoint blockade immunotherapy across multiple disease types12–15. In particular in head and neck cancer recent studies have demonstrated the importance of B-cells and tertiary lymphoid structures in patient outcomes16.
Since tertiary lymphoid structures have been identified and correlated with responses and patient outcomes, this brings forth the question regarding the effects of RT on TLS. Boivin et al. reported in medullary breast cancer models that RT induced an acute and transient depletion of TLS which was followed by a restoration phase51. This report highlights the plasticity of plasticity of TLS and their reappearance 14 days post-RT which is in line with our data and findings demonstrating increased immune cell infiltrates at 14 days post radiation. Although our study did not focus on TLS, future work is certainly deserved to study the impact and outcomes of radiation and checkpoint blockade on TLS and further elucidate the role that B-cells and TLS play in anti-tumor immunity.
Continuous BCR signaling is required for the survival of peripheral B-cells including all follicular and marginal zone B-cells. Although we did not investigate BCR activation in transitional and all subsets of mature B-cells, we were very interested in the mechanism of RT resistance in mature B-cells, long-lived antigen-experienced populations including a group of long-lived PCs as well as a separate group Bmem-like cells. Short-lived plasma cells are located mainly in GC centers and since they are not thought to migrate heavily, we can assume that due to RT activation, the number of PCs increased 52 53. Moreover, we observed a larger humoral effect of RT in the increased level of IgG1 after the whole-body radiation, comparing to a spleen focal RT. These data support a direct activation radioresistance/survival mechanism as opposed to effects dominated by differential cellular migration.
CD138, also known as syndecan-1, is known to promote survival of plasma cells by enhancing pro-survival cytokine signaling54. Interestingly, we showed that irradiated plasma cells have increased surface expression CD138 supporting the radioresistance of plasma cells. Our results show that PCs are the most RT-resistant B-cell subtype followed by Bmem-like cells, and we identified that transcription factors Xbp1 and Bcl6 are associated with this phenomenon. One of the key transcription factors required for B-cell differentiation and antibody production is XBP-155 and XBP-1 also regulates the unfolded protein response (UPR) which is critical to manage the massive immunoglobulin protein production in antibody producing B-cells and Plasma cells. Indeed, XBP-1 KO mice have an almost complete absence of plasma cells and circulating antibodies, and XBP-1 deficient B-cells fail to signal effectively though the B-cell receptor55. Consistent with this we observed that resting naïve cells had little to no Xbp1 expression compared to plasma cells. However, we observed that radiation induced a significant fold-increase in Xbp1 expression in plasma cells. It is likely that the residual B-cells after RT are plasma cells and memory cells carrying effective BCRs and are thus resistant to apoptosis. Our results are corroborated by historical reports of radioresistant PCs described by Miller and Cole (1967) using only microscopy56. Our current work significantly expands upon this report and details molecular mechanisms of action. Specifically, the expression of Aid and Bcl6 indicate that SHM and CSR machinery are activated 57, and these processes are also critical for B-cell affinity maturation. Increased expression of Aid, Bach2, Pax5, Sfpi and Zbtb24 indicates increased CSR, but interestingly there is also an evidence that even when B-cells are forced to class switch to IgG1, this uncouples the effects of SHM and CSR, and skews GC B-cells to predominantly differentiate into PCs 58. Radiation additionally induces several pro-survival signaling pathways involved in class-switching, e.g. BCR/Btk pathway overlapping with Ras/Raf/Mek/Erk. Due to cell membrane stress during RT with Fas and ceramide activation, the Pi3k/Akt pathway is also modulated. Most of the genes mentioned above are upregulated in class-switched subpopulations which shows selective activation of pro-survival pathways (radioresistance). Coordinated immune responses involving multiple arms of the innate and adaptive immune system may be critical for tumor control, thus the effects of radiation on B-cells and radioresistance of antibody producing plasma cells has important implications when incorporating immunotherapy into cancer treatment paradigms in the curative setting.
In summary, this study characterizes the effects of radiation on B-cell subpopulations during development in the BM as well as maturation and differentiation in the periphery and tumor microenvironment. Importantly this is the first report to our knowledge to demonstrate that modern focal or stereotactic radiation is superior to conventional large field radiation at inducing B-cell infiltration into tumors. We then establish that host plasma cells and class switched memory B-cells are highly resistant to radiation therapy. Our gene expression profiling in sorted B-cell subsets identified that radiation modulates key pathways and transcription factors involved in apoptosis, differentiation, and maturation, including class switch recombination and somatic hypermutation which are critical for B-cell function. Overall, these findings have novel implications for the effects of radiation on the immune system and how radiation can impact B-cells involved in anti-tumor immune responses.
Supplementary Material
Acknowledgements
We thank the Moores Cancer Center and La Jolla Institute for Immunology for the access to Flow Cytometry Cores. This work was supported in part by1KL2TR001444, R01 DE028563, and 1U01 DE028227-01.
Conflict of Interest Statement:
A.B.S. reports research funding and honoraria from Pfizer and Varian Medical Systems, and consultant fees from AstraZeneca and Merck. A.B.S. is the scientific founder and has an equity interest in Toragen Inc. outside the submitted work. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement for this Work
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. Aug 2018;39(8):644–655. doi: 10.1016/j.it.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darragh LB, Oweida AJ, Karam SD. Overcoming Resistance to Combination Radiation-Immunotherapy: A Focus on Contributing Pathways Within the Tumor Microenvironment. Front Immunol. 2018;9:3154. doi: 10.3389/fimmu.2018.03154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. Oct 2015;16(13):e498–509. doi: 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 4.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. May 15 2006;203(5):1259–71. doi: 10.1084/jem.20052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res. Apr 2015;3(4):345–55. doi: 10.1158/2326-6066.CIR-14-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bristow RG, Alexander B, Baumann M, et al. Combining precision radiotherapy with molecular targeting and immunomodulatory agents: a guideline by the American Society for Radiation Oncology. Lancet Oncol. May 2018;19(5):e240–e251. doi: 10.1016/S1470-2045(18)30096-2 [DOI] [PubMed] [Google Scholar]
- 7.Cushman TR, Caetano MS, Welsh JW, Verma V. Overview of ongoing clinical trials investigating combined radiotherapy and immunotherapy. Immunotherapy. Aug 2018;10(10):851–0. doi: 10.2217/imt-2018-0019 [DOI] [PubMed] [Google Scholar]
- 8.Filatenkov A, Baker J, Mueller AM, et al. Ablative Tumor Radiation Can Change the Tumor Immune Cell Microenvironment to Induce Durable Complete Remissions. Clin Cancer Res. Aug 15 2015;21(16):3727–39. doi: 10.1158/1078-0432.CCR-14-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyauchi S, Sanders PD, Guram K, et al. HPV16 E5 Mediates Resistance to PD-L1 Blockade and Can Be Targeted with Rimantadine in Head and Neck Cancer. Cancer Res. Feb 15 2020;80(4):732–746. doi: 10.1158/0008-5472.CAN-19-1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce JA, Fearon DT. T cell exclusion, immune privilege, and the tumor microenvironment. Science. Apr 3 2015;348(6230):74–80. doi: 10.1126/science.aaa6204 [DOI] [PubMed] [Google Scholar]
- 11.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. Jan 18 2017;541(7637):321–330. doi: 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 12.Helmink BA, Reddy SM, Gao J, et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature. Jan 2020;577(7791):549–555. doi: 10.1038/s41586-019-1922-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. Jan 2020;577(7791):561–565. doi: 10.1038/s41586-019-1914-8 [DOI] [PubMed] [Google Scholar]
- 14.Wieland A, Patel MR, Cardenas MA, et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature. Nov 18 2020;doi: 10.1038/s41586-020-2931-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petitprez F, de Reynies A, Keung EZ, et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature. Jan 2020;577(7791):556–560. doi: 10.1038/s41586-019-1906-8 [DOI] [PubMed] [Google Scholar]
- 16.Ruffin AT, Cillo AR, Tabib T, et al. B cell signatures and tertiary lymphoid structures contribute to outcome in head and neck squamous cell carcinoma. Nat Commun. Jun 7 2021;12(1):3349. doi: 10.1038/s41467-021-23355-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q, Grover AC, Donald EJ, et al. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. J Immunol. Aug 1 2005;175(3):1424–32. doi: 10.4049/jimmunol.175.3.1424 [DOI] [PubMed] [Google Scholar]
- 18.Postow MA, Callahan MK, Wolchok JD. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. Jun 10 2015;33(17):1974–82. doi: 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanoni D, Tavecchio S, Recalcati S, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. Jan 30 2011;134(2):157–60. doi: 10.1016/j.imlet.2010.09.022 [DOI] [PubMed] [Google Scholar]
- 20.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalapour S, Karin M. The neglected brothers come of age: B cells and cancer. Semin Immunol. Jun 29 2021:101479. doi: 10.1016/j.smim.2021.101479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trowell OA. The sensitivity of lymphocytes to ionising radiation. J Pathol Bacteriol. Oct 1952;64(4):687–704. doi: 10.1002/path.1700640403 [DOI] [PubMed] [Google Scholar]
- 23.Arina A, Beckett M, Fernandez C, et al. Tumor-reprogrammed resident T cells resist radiation to control tumors. Nat Commun. Sep 2 2019;10(1):3959. doi: 10.1038/s41467-019-11906-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. May 1 1991;173(5):1213–25. doi: 10.1084/jem.173.5.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Edry E, Melamed D. Receptor editing in positive and negative selection of B lymphopoiesis. J Immunol. Oct 1 2004;173(7):4265–71. doi: 10.4049/jimmunol.173.7.4265 [DOI] [PubMed] [Google Scholar]
- 26.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. 2006. [DOI] [PubMed]
- 27.Carsetti R, Kohler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment. J Exp Med. Jun 1 1995;181(6):2129–40. doi: 10.1084/jem.181.6.2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melchers F Checkpoints that control B cell development. J Clin Invest. Jun 2015;125(6):2203–2210. doi: 10.1172/Jci78083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choukrallah MA, Matthias P. The interplay between chromatin and transcription factor networks during B cell development: who pulls the trigger first? Front Immunol. 2014;5:156–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basso K, Dalla-Favera L. Roles of BCL6 in normal and transformed germinal center B cells. Immunol Rev. 2010;247(1):172–83. [DOI] [PubMed] [Google Scholar]
- 31.Suan D, Sundling C, Brink R. Plasma cell and memory B cell differentiation from the germinal center. Curr Opin Immunol. 2017;45:97–102. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. Dec 2001;25(4):402–8. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 33.Marciscano AE, Ghasemzadeh A, Nirschl TR, et al. Elective Nodal Irradiation Attenuates the Combinatorial Efficacy of Stereotactic Radiation Therapy and Immunotherapy. Clin Cancer Res. Oct 15 2018;24(20):5058–5071. doi: 10.1158/1078-0432.CCR-17-3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thibult ML, Mamessier E, Gertner-Dardenne J, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol. Feb 2013;25(2):129–37. doi: 10.1093/intimm/dxs098 [DOI] [PubMed] [Google Scholar]
- 35.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. Sep 1 2000;102(5):553–63. doi: 10.1016/s0092-8674(00)00078-7 [DOI] [PubMed] [Google Scholar]
- 36.Yoon HS, Scharer CD, Majumder P, et al. ZBTB32 Is an Early Repressor of the CIITA and MHC Class II Gene Expression during B Cell Differentiation to Plasma Cells. Journal of Immunology. Sep 1 2012;189(5):2393–2403. doi: 10.4049/jimmunol.1103371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menon H, Ramapriyan R, Cushman TR, et al. Role of Radiation Therapy in Modulation of the Tumor Stroma and Microenvironment. Front Immunol. 2019;10:193. doi: 10.3389/fimmu.2019.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dovedi SJ, Cheadle EJ, Popple AL, et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin Cancer Res. Sep 15 2017;23(18):5514–5526. doi: 10.1158/1078-0432.CCR-16-1673 [DOI] [PubMed] [Google Scholar]
- 39.Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol. Mar 1 2008;180(5):3132–9. doi: 10.4049/jimmunol.180.5.3132 [DOI] [PubMed] [Google Scholar]
- 40.Quarmby S, Hunter RD, Kumar S. Irradiation induced expression of CD31, ICAM-1 and VCAM-1 in human microvascular endothelial cells. Anticancer Res. Sep- Oct 2000;20(5B):3375–81. [PubMed] [Google Scholar]
- 41.Matsumura S, Demaria S. Up-regulation of the pro-inflammatory chemokine CXCL16 is a common response of tumor cells to ionizing radiation. Radiat Res. Apr 2010;173(4):418–25. doi: 10.1667/RR1860.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriconi F, Christiansen H, Raddatz D, et al. Effect of radiation on gene expression of rat liver chemokines: in vivo and in vitro studies. Radiat Res. Feb 2008;169(2):162–9. doi: 10.1667/RR1006.1 [DOI] [PubMed] [Google Scholar]
- 43.Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol. May 2020;20(5):294–307. doi: 10.1038/s41577-019-0257-x [DOI] [PubMed] [Google Scholar]
- 44.Uckun FM, Mitchell JB, Obuz V, et al. Radiation sensitivity of human B-lineage lymphoid precursor cells. Int J Radiat Oncol Biol Phys. Nov 1991;21(6):1553–60. doi: 10.1016/0360-3016(91)90332-x [DOI] [PubMed] [Google Scholar]
- 45.Park YH, Osmond DG. Post-irradiation regeneration of early B-lymphocyte precursor cells in mouse bone marrow. Immunology. Mar 1989;66(3):343–7. [PMC free article] [PubMed] [Google Scholar]
- 46.Le Bouteiller PP, Asherson GL, Edwards AJ. Control of B-cell maturation in mice. I. Increased B-cell maturation in vitro by bone marrow protected during whole body irradiation. Immunology. 1981;42(2):267–76. [PMC free article] [PubMed] [Google Scholar]
- 47.Opstelten D, Osmond DG. Regulation of pre-B cell proliferation in bone marrow: immunofluorescence stathmokinetic studies of cytoplasmic mu chain-bearing cells in anti-IgM-treated mice, hematologically deficient mutant mice and mice given sheep red blood cells. Eur J Immunol. Jun 1985;15(6):599–605. doi: 10.1002/eji.1830150613 [DOI] [PubMed] [Google Scholar]
- 48.Whitmire JK, Asano MS, Kaech SM, et al. Requirement of B cells for generating CD4+ T cell memory. J Immunol. Feb 15 2009;182(4):1868–76. doi: 10.4049/jimmunol.0802501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deola S, Panelli MC, Maric D, et al. Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. Journal of Immunology. Feb 1 2008;180(3):1362–1372. doi:DOI 10.4049/jimmunol.180.3.1362 [DOI] [PubMed] [Google Scholar]
- 50.Miklne K, Kobel M, Kalloger SE, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLOS ONE. 2009;4:e6412–e6412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boivin G, Kalambaden P, Faget J, et al. Cellular Composition and Contribution of Tertiary Lymphoid Structures to Tumor Immune Infiltration and Modulation by Radiation Therapy. Front Oncol. 2018;8:256. doi: 10.3389/fonc.2018.00256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manz RA, Thiel A, Radbruch A. Lifetime of plasma cells in the bone marrow. Nature. 1997;388(6638):133–134. doi: 10.1038/40540 [DOI] [PubMed] [Google Scholar]
- 53.Kuby Jea. Kuby Immunology. W.H. Freeman; 2007. [Google Scholar]
- 54.McCarron MJ, Park PW, Fooksman DR. CD138 mediates selection of mature plasma cells by regulating their survival. Blood. May 18 2017;129(20):2749–2759. doi: 10.1182/blood-2017-01-761643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu CC, Dougan SK, McGehee AM, Love JC, Ploegh HL. XBP-1 regulates signal transduction, transcription factors and bone marrow colonization in B cells. EMBO J. Jun 3 2009;28(11):1624–36. doi: 10.1038/emboj.2009.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller Iii JJ, Cole LJ. The radiation resistance of long-lived lymphocytes and plasma cells in mouse and rat lymph nodes. The Journal of Immunology. 1967;98(5):982–990. [PubMed] [Google Scholar]
- 57.Xu Z, Pone EJ, Al-Qahtani A, Park SR, Zan H, Casali P. Regulation of aicda expression and AID activity: relevance to somatic hypermutation and class switch DNA recombination. Crit Rev Immunol. 2007;27(4):367–97. doi: 10.1615/critrevimmunol.v27.i4.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gitlin AD, Von Boehmer L, Gazumyan A, Shulman Z, Oliveira TY, Nussenzweig MC. Independent role of switching and hypermutation in the development and persistance of B lymphocyte memory. Immunity. 2016;44:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.


