Abstract
Background
Most conventional, oral, preventive treatments for migraine are non-specific and ~50% of patients discontinue them within six months. In 2018, the Food and Drug Administration approved three preventive migraine treatments: monoclonal antibodies (mAb) targeting the calcitonin gene-related peptide (CGRP) pathway implicated in migraine; galcanezumab and fremanezumab which target CGRP ligand; and erenumab which targets CGRP receptor. Real-world treatment patterns for CGRP mAb are limited.
Purpose
To compare real-world treatment patterns for CGRP mAb, specifically galcanezumab versus standard-of-care (SOC) migraine preventive treatments.
Patients and methods
This retrospective, observational study included 12-month baseline and 6- and 12-month follow-up analyses using IBM® MarketScan® databases. Patients identified were aged ≥18 years with ≥1 claim (first claim=index) for CGRP mAb (erenumab, fremanezumab, or galcanezumab) or SOC preventives (eg, antiepileptics, beta-blockers, antidepressants, or onabotulinumtoxinA) as index drugs between May/01/2018 and June/30/2019. Propensity score matching was used to address confounding by observed covariates. Outcomes analyzed included proportion of days covered (PDC), persistence (≤60-day gap), and first non-index drug switch. Descriptive, chi-square (categorical), and t-test (continuous) analyses were conducted.
Results
The study included 3082 (CGRP mAb versus SOC) and 421 (galcanezumab versus SOC) matched patient pairs with 12-month follow-up. Mean age across cohorts ranged 43.2–44.4 years (females: 85.7–88.6%). Compared with SOC, the CGRP mAb cohort had higher mean persistence (212.5 vs 131.9 days), adherence (PDC: 55.1% vs 35.2%), and more patients were adherent with PDC ≥80% (32.7% vs 18.7%) (all p <0.001). During 12-month follow-up, fewer patients discontinued CGRP mAb versus SOC (58.8% vs 77.6%, p <0.001). Galcanezumab versus SOC comparisons yielded similar results. In the CGRP mAb cohort, most switchers (28.3%) used galcanezumab as subsequent treatment. Largely similar results were observed for 6-month follow-up cohorts.
Conclusion
Patients on CGRP mAb and specifically galcanezumab showed higher adherence and persistence than patients on SOC migraine preventive treatments.
Keywords: CGRP, persistence, adherence, discontinuation, switch
Plain Language Summary
What was known before?
More than 1 in 4 patients with migraine are eligible for migraine preventive treatments, yet around 1 in 10 patients report using them.
Conventional preventive treatments are not migraine-specific; taken orally daily or injected quarterly by trained staff; more than half of patients stop using oral medications within six months.
Calcitonin gene-related peptide (CGRP) levels increase around the brain during migraine attack causing pain.
CGRP pathway restriction using CGRP monoclonal antibodies (mAbs), laboratory-made proteins binding to CGRP receptor (erenumab) or ligand (fremanezumab and galcanezumab), provide migraine-specific preventive treatment.
In 2018, the United States Food and Drug Administration approved self-injected monthly/quarterly CGRP mAbs.
What does this study add?
This study compared 12-month prescription claims pattern of patients with migraine newly starting CGRP mAb including galcanezumab versus those newly starting conventional (standard-of-care [SOC]) migraine preventive treatments.
Patients on CGRP mAb and specifically galcanezumab were more likely to continue their treatment than patients on SOC over 12 months.
At 12 months, more than 75% of patients stopped SOC, while around half of patients stopped newly initiated CGRP mAb preventive treatment.
Most patients who moved to another preventive treatment after starting a non-galcanezumab CGRP mAb started galcanezumab as a following treatment.
Interpretation
Compared with SOC, patients are more likely to continue using CGRP mAb preventive treatments.
Study did not measure CGRP mAbs’ effectiveness. Future research should explore reasons for continuing CGRP mAbs. Patients may possibly find CGRP mAb to be more effective than SOC in managing their headache.
Introduction
Migraine is a recurrent headache disorder manifesting in attacks, each lasting 4 to 72 hours.1 It is a common disabling condition typically characterized by unilateral, pulsating headache, and is accompanied by i) nausea and/or vomiting, or ii) photophobia and phonophobia.1 It is the second leading cause of years lived with disability worldwide, and the first among women under 50 years of age.2 In the United States (US), overall prevalence of migraine (2019 estimate) was 16.7%.3 Each year, migraine occurs in 12% of the US population, disproportionately affecting approximately 18% of women versus 6% of men.4
Migraine gravely impacts functional ability at work or school, home, and social events, especially in patients experiencing four or more migraine headache days per month.5,6 Current medications for migraine fall under two broad categories: acute medications for symptomatic relief taken during migraine attacks; and preventive medications, which are taken regularly to reduce the severity or frequency of migraine attacks.7–9 As per the American Headache Society (AHS) 2019 and 2021 position statements, and per expert consensus in the Lipton et al, 2007 study, migraine preventive treatments may be beneficial and should be considered for any of the following scenarios: four or more headache days per month (or two or more headache days per month with severe disability); attacks significantly interfering with daily activity; contraindication, failure, or overuse of acute medications; adverse events with acute medications; and patient preference.7,10,11 However, most of the preventive medications recommended by the American Academy of Neurology (AAN) are not specifically developed to treat migraine.9 This includes antiepileptics, beta-blockers, antidepressants, and alpha-agonists administered orally almost daily.9 Patients show low six-month treatment adherence (26–29%) and persistence (25%) to conventional, oral, preventive treatments for migraine, with more than half of patients discontinuing treatment within six months.12–15 This may be due to common issues including inconsistent or suboptimal efficacy, adverse events, contraindications, and pharmacological interactions.11,16,17
In 2018, the US Food and Drug Administration (FDA) approved three monoclonal antibodies (mAb) targeting the calcitonin gene-related peptide (CGRP) pathway implicated in migraine pathophysiology.18–21 These subcutaneously self-injected mAbs either target CGRP receptor (erenumab, monthly dosing) or CGRP ligand (fremanezumab, monthly/quarterly dosing; and galcanezumab, monthly dosing).19–21 Considering limited real-world evidence and cost-effectiveness of CGRP mAbs relative to oral preventives, the AHS 2019 and 2021 position statements require patients to have failed two or more standard-of-care (SOC) preventive medications due to intolerance or inadequate response before initiating CGRP mAb.7,11 In 2020, intravenously administered eptinezumab (quarterly dosing) was the fourth FDA-approved CGRP mAb, which targets the CGRP ligand.22 In addition to mAbs, small molecule CGRP receptor antagonists, known as gepants, are currently in development for preventive treatment of migraine, with only rimegepant (once every other day) and atogepant (once daily dosing) approved by the FDA for preventive use in 2021.23–25
Given the recent approval and post-marketing use of CGRP inhibitors, there is a gap in knowledge about the real-world treatment patterns for patients initiating a CGRP mAb. Further, there are gaps in the literature on how treatment patterns differ across specific CGRP inhibitor agents as compared with SOC preventive treatments for migraine. This study compared treatment patterns, including adherence, persistence, and switching among adult patients with migraine initiating self-injected CGRP mAb, and specifically galcanezumab versus SOC preventive treatments for migraine.
Materials and Methods
Study Design and Data Sources
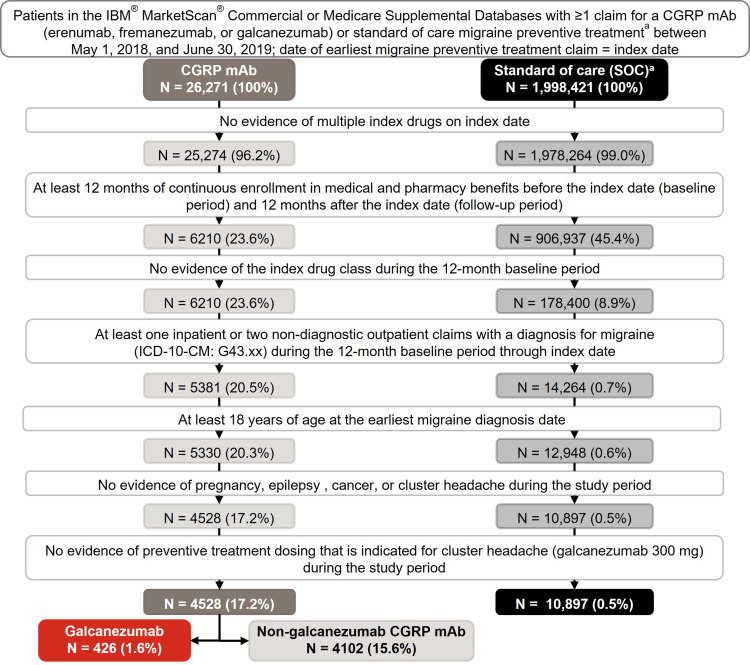
This US real-world analysis was a retrospective, observational cohort study using 2017 to 2020 administrative claims data from the IBM® MarketScan® Commercial and Medicare Supplemental Databases (Figure 1 and Supplementary Figure 1). The study assessed 6-month and 12-month treatment patterns in adults with migraine newly initiating CGRP mAb (erenumab, fremanezumab, or galcanezumab), or SOC migraine preventive treatments.9 SOC included drugs with established efficacy (level A drugs), drugs that are probably effective (level B drugs), and other non-specific drug (onabotulinumtoxinA) (Table 1).9 The National Drug Codes list was obtained from First Databank®. Considering the FDA approvals for eptinezumab in 2020, rimegepant and atogepant in 2021, and lack of real-world clinical practice or fully adjudicated data available during the study period, these drugs were not included in the current study.
Figure 1.
Patient selection and data attrition for 12-month follow-up.
Notes: aConventional preventive treatments for migraine in SOC cohort included level A drugs: antiepileptic drugs (divalproex sodium, sodium valproate, topiramate), beta-blockers (metoprolol, propranolol, timolol); level B drugs: antidepressants (amitriptyline, venlafaxine), beta-blockers (atenolol, nadolol); and other non-specific drug: onabotulinumtoxinA.9.
Abbreviations: CGRP mAb, calcitonin gene-related peptide ligand or receptor-targeted monoclonal antibody; ICD-10-CM, International Classification of Diseases, 10th Revision, Clinical Modification; N, number of patients identified at each selection step; SOC, standard-of-care.
Table 1.
Conventional Preventive Medications for Migraine by Drug Class and Drug Category
| Drug Category Drug Class |
Conventional Preventive Treatments for Migraine (Standard-of-Care [SOC]) Cohort | Level C Possibly Effective | Level U Inadequate or Conflicting Data | Other Established as Possibly or Probably Ineffective | ||
|---|---|---|---|---|---|---|
| Level A Established Efficacy | Level B Probably Effective | Other | ||||
| Antiepileptics | Divalproex Na or Na valproate Topiramate |
Carbamaze-pine | Gabapentin | Lamotrigine Clonazepam Oxcarbazepine |
||
| Beta-blockers | Metoprolol Propranolol Timolol |
Atenolol Nadolol |
Nebivolol Pindolol |
Bisoprolol | Acebutolol | |
| Antidepressants | Amitriptyline Venlafaxine |
Fluoxetine Fluvoxamine Protriptyline |
Clomipramine | |||
| Non-specific | Onabotulinum-toxinA | |||||
| ACE inhibitor | Lisinopril | |||||
| Angiotensin receptor blockers | Candesartan | Telmisartan | ||||
| Alpha-Agonists | Clonidine Guanfacine |
|||||
| Antithrombotic | Coumadin | |||||
| Calcium Channel Blockers | Nicardipine Nifedipine Nimodipine Verapamil |
|||||
| Diuretic | Acetazolamide | |||||
| NSAID | Nabumetone | |||||
Note: Reference table modified from Silberstein et al, 2012.9.
Abbreviations: ACE, angiotensin-converting enzyme; Na, sodium; NSAID, nonsteroidal anti-inflammatory drug; SOC, standard-of-care.
IBM® MarketScan® databases include healthcare experience of approximately 27 million unique individuals obtained annually and contain inpatient, outpatient, and pharmacy claims, providing a complete assessment of patients’ treatment patterns and clinical outcomes. All database records were de-identified and fully compliant with US patient-confidentiality requirements, including the Health Insurance Portability and Accountability Act of 1996. The study used only de-identified patient records, and therefore did not require Institutional Review Board approval and patient informed consent to conduct this study.
Patient Selection
Patients selected were adults aged ≥18 years at earliest migraine diagnosis date with ≥1 claim for CGRP mAb (erenumab, fremanezumab, or galcanezumab) or SOC preventive treatments for migraine (Table 1) between May 01, 2018, and June 30, 2019. Index date was the date of earliest migraine treatment claim within this time, and the drug claimed for on the index date was identified as the index drug. Galcanezumab cohort was a subset of the CGRP mAb cohort, where galcanezumab was the index drug. Patients were required to have continuous enrollment in medical and pharmacy benefits for 12 months pre-index (baseline) and 12 months post-index (12-month follow-up); a subset of patients with continuous enrollment for 6 months after index date (6-month follow-up) were also identified (Figure 1 and Supplementary Figure 1). Only those patients with complete claims, enrollment, and demographics data were included. Migraine diagnosis required ≥1 inpatient or ≥2 non-diagnostic outpatient claims with a diagnosis for migraine based on the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code in G43.xx range during baseline through index date. Patients with evidence of the index drug class during baseline or evidence of multiple index drugs on index date were excluded. Patients were, thus, newly initiated on the index drug but may have had non-index drug classes for other migraine preventive treatments during baseline. Patients with evidence of pregnancy, epilepsy, cancer, cluster headache, or preventive treatment with dosing indicated for cluster headaches (galcanezumab 300mg) anytime during the study period were excluded.
Study Measures
Baseline Demographics and Clinical Features
Baseline demographics measured on the index date included age, sex, geographical region, insurance plan type, and provider type. Provider type was based on the closest non-pharmacy claim to the index date, within 45 days of index date in either direction, and categorized as neurology, primary care, acute hospital care or emergency room, radiology/laboratory/pathology, and other/unknown. Primary care provider included family practice, internal medicine, obstetrics/gynecology, and nurse practitioner. Baseline clinical features assessed during the 12-month pre-index period included Deyo-Charlson Comorbidity Index, comorbidity, and preventive and acute medications for migraine.7,9,26 Comorbid medical conditions were identified by presence of ≥1 inpatient or non-diagnostic outpatient medical claim with an ICD-10-CM diagnosis code in any position. Comorbid conditions present in ≥10% of patients in either of the cohorts were reported.
Preventive and Acute Medications for Migraine
Baseline and follow-up preventive and acute medications for migraine analyzed were based on the 2012 guidelines published by the AAN, and the 2019 AHS position statement.7,9 Preventive medications for migraine included CGRP mAbs: erenumab, fremanezumab, and galcanezumab; and other preventive drug categories: level A, level B, other non-specific (onabotulinumtoxinA), level C, level U, and other medications that are established as possibly or probably ineffective (Table 1). Acute medications for migraine analyzed included ergotamine derivatives, nonsteroidal anti-inflammatory drugs, opioids, and triptans, among others (Table 2). Medications were identified based on ≥1 outpatient prescription claim or ≥1 medical claim with a procedure code for the administered medication during baseline and follow-up periods. Patients with claims for specific drug categories and number of unique preventive treatments for migraine per patient in each cohort during baseline and follow-up periods were analyzed.
Table 2.
Acute Medications for Migraine by Drug Class
| Drug Class | Specific Drugs Included | |
|---|---|---|
| Established Efficacy | Ergotamine derivatives | Dihydroergotamine |
| Nonsteroidal anti-inflammatory drugs | Aspirin, diclofenac, ibuprofen, naproxen | |
| Opioids | Buprenorphine, butorphanol, codeine, dezocine, fentanyl, hydrocodone, hydromorphone, levomethadyl, levorphanol, meperidine, methadone, morphine, nalbuphine, oxycodone, oxymorphone, pentazocine, propoxyphene, tapentadol, tramadol | |
| Triptans | Almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan, zolmitriptan | |
| Probably Effective | Antiemetics | Droperidol, chlorpromazine, metoclopramide, prochlorperazine, promethazine |
| Acetaminophen combinations | Codeine with acetaminophen, tramadol with acetaminophen | |
| Ergotamine/other forms of dihydroergotamine | Ergotamine | |
| Isometheptene-containing compounds | Isometheptene | |
| Magnesium, intra-venous | Magnesium | |
| Nonsteroidal anti-inflammatory drugs | Flurbiprofen, ketoprofen, and ketorolac intra-venous and intra-muscular |
Note: Reference data source was 2019 American Headache Society position statement.11.
Treatment Patterns
Index Drug Adherence, Persistence, and Discontinuation
Treatment patterns for CGRP mAb, galcanezumab, and SOC initiators were analyzed during follow-up. Number of index drug fills during follow-up was calculated for each cohort. Treatment adherence was evaluated by measuring proportion of days covered (PDC) and medication possession ratio (MPR). PDC was calculated as number of days with index drug on-hand or number of days exposed to drug, divided by number of days in the follow-up period, regardless of discontinuation. MPR was calculated as the ratio of sum of days’ supply from all prescriptions divided by total number of days in the follow-up period. Overlapping days’ supply and days’ supply after the end of follow-up were not included. MPR was capped at 100%. Patients with PDC or MPR of ≥80% were considered treatment adherent.
Persistence to index drug was defined as number of days of continuous therapy from the index date until the end of the follow-up period, allowing for a maximum gap between fills of 60 days (60-day gap). Patients persistent to index drug until the end of follow-up, and number of days of persistent index drug use among all patients in the cohort were calculated. Discontinuation of index drug was defined as failure to refill the index drug within 60-day gap after the days’ supply from previous fills were depleted. A sensitivity analysis using 45-gap day rule for discontinuation of index drug was conducted. Treatment duration was defined as time in days from index date to last day’s supply of index drug allowing for specific gap days and included observations censored at the end of follow-up.
Restart of Index Drug and Switch to Non-Index Drug After Discontinuation
Among patients who discontinued index drug (60-day gap), patients who restarted the index drug or switched to non-index drug for migraine preventive treatment were analyzed. Restart of index drug occurred when patients had a fill for their index drug after their discontinuation date and no later than the end of the study period. Time to first restart was calculated as days between discontinuation date and restart date of index drug. Treatment switch was defined as first switch to a non-index migraine preventive treatment that was not a part of the index treatment regimen any time during the follow-up period. Index treatment regimen comprised all the drugs a patient was on ±30 days of index date. Treatment switch could occur within the CGRP class, as well as to a different class of migraine preventive treatment (Table 1). Time to first switch was calculated as days between index date and first switch date to a non-index drug that was not part of the index treatment regimen.
Statistical Analyses
Propensity Score Matching
To control for selection bias and confounders due to the observational nature of the study, propensity score matching was used.27 The propensity score was defined as the probability of receiving a treatment conditional on the patient’s observed baseline characteristics. Propensity scores were estimated using multivariable logistic regression with receiving CGRP mAb or specifically galcanezumab as dependent variable and following baseline characteristics as independent variables: demographics (age, sex, region, plan type, provider type); comorbidity (chronic migraine, anxiety, depression, hypertension, sleep disorders); number of unique migraine preventive drug classes; and Deyo-Charlson Comorbidity index score. Greedy nearest neighbor 1:1 matching with a caliper of 0.25 standard deviation of the logit of propensity score was used to create the following cohorts: i) CGRP mAb and SOC; ii) galcanezumab and SOC. To balance cohorts for comparison, propensity scores were matched for 6-month and 12-month follow-up cohorts separately. Standardized difference (Std. Diff) of greater than 10% in absolute value and variance ratio outside the range of 0.55 and 2.00 was considered for potential imbalances that warranted further investigation or adjustment.
Significance Level, Hypotheses Testing, and Multiplicity Adjustment
Statistical analyses were performed using WPS Analytics version 4.1 (World Programming, United Kingdom) and R (Vienna, Austria). All analyses were planned, and significance level was set at α = 0.05 a priori. Descriptive analyses were carried out pre- and post-matching with mean, standard deviation, number of observations, and percentage reported. Continuous and categorical variables were statistically compared using Student’s t-test and chi-square test, respectively. Multiplicity adjustments by Holm method were performed only for comparing adherence measured using PDC. Index drug persistence (time to discontinuation) over 6- and 12-month follow-up periods was described using Kaplan-Meier (KM) curves. Differences between groups were assessed using a Log rank test. Patients who did not discontinue the index drug were censored at the end of follow-up.
Results
Patient Sample and Baseline Characteristics
In the 12-month follow-up cohort, 4568 patients using CGRP mAb (galcanezumab, n = 426) as the index drug, and 10,897 patients using SOC were identified (Figure 1). Before matching, patients on CGRP mAb (45.1 years, Std. Diff = 0.323) and specifically galcanezumab (43.8 years, Std. Diff = 0.210) were older than patients on SOC (41.3 years); 85.1% to 86.4% of the patients were female (Tables 3 and 4). More patients in the CGRP mAb cohort visited a neurologist closest to index date than in the SOC cohort (31.2% vs 25.9%, Std. Diff = 0.117). Fewer patients in the CGRP mAb (22.6%, Std. Diff = 0.425) and galcanezumab (26.1%, Std Diff = 0.342) cohorts visited a primary care physician versus the SOC cohort (42.0%). Chronic migraine rates were higher in CGRP mAb (69.0%, Std. Diff = 1.083) and galcanezumab (58.5%, Std. Diff = 0.811) cohorts than SOC cohort (21.6%).
Table 4.
Patient Demographics and Clinical Characteristics Pre- and Post-Matching of Covariates for Galcanezumab and SOC 12-Month Follow-Up Cohort
| Demographics and Clinical Characteristics | Pre-Matching | Post-Matching | ||||
|---|---|---|---|---|---|---|
| GMB (N=426) | SOC (N=10,897) | Std Diff | GMB (N=421) | SOC (N=421) | Std Diff | |
| Age (years), mean (SD) | 43.8 (11.4) | 41.3 (12.3) | 0.210 | 43.7 (11.4) | 43.2 (11.8) | 0.049 |
| Female, n (%) | 368 (86.4) | 9275 (85.1) | 0.036 | 363 (86.2) | 373 (88.6) | 0.072 |
| Region, n (%) | ||||||
| Northeast | 65 (15.3) | 1726 (15.8) | 0.016 | 65 (15.4) | 73 (17.3) | 0.051 |
| North central | 93 (21.8) | 2476 (22.7) | 0.021 | 92 (21.9) | 85 (20.2) | 0.041 |
| South | 216 (50.7) | 5246 (48.1) | 0.051 | 212 (50.4) | 207 (49.2) | 0.024 |
| West | 51 (12.0) | 1427 (13.1) | 0.034 | 51 (12.1) | 56 (13.3) | 0.036 |
| Unknown | 1 (0.2) | 22 (0.2) | 0.007 | 1 (0.2) | 0 (0) | 0.069 |
| Insurance plan type, n (%) | ||||||
| Comprehensive/ indemnity | 22 (5.2) | 415 (3.8) | 0.066 | 22 (5.2) | 11 (2.6) | 0.135 |
| EPO/PPO | 219 (51.4) | 5653 (51.9) | 0.009 | 216 (51.3) | 214 (50.8) | 0.010 |
| POS/POS with capitation | 18 (4.2) | 712 (6.5) | 0.102 | 18 (4.3) | 24 (5.7) | 0.066 |
| HMO | 51 (12.0) | 1480 (13.6) | 0.048 | 51 (12.1) | 39 (9.3) | 0.092 |
| CDHP/HDHP | 113 (26.5) | 2495 (22.9) | 0.084 | 111 (26.4) | 129 (30.6) | 0.095 |
| Other/unknown | 3 (0.7) | 142 (1.3) | 0.060 | 3 (0.7) | 4 (1.0) | 0.026 |
| Provider, n (%) | ||||||
| Neurology | 127 (29.8) | 2827 (25.9) | 0.086 | 126 (29.9) | 140 (33.3) | 0.072 |
| Primary carea | 111 (26.1) | 4578 (42.0) | 0.342 | 111 (26.4) | 111 (26.4) | 0.000 |
| Acute hospital care, ER | 40 (9.4) | 549 (5.0) | 0.169 | 38 (9.0) | 33 (7.8) | 0.043 |
| Radiology, laboratory, pathology | 19 (4.5) | 283 (2.6) | 0.101 | 18 (4.3) | 9 (2.1) | 0.122 |
| Other/unknown | 129 (30.3) | 2660 (24.4) | 0.132 | 128 (30.4) | 128 (30.4) | 0.000 |
| DCI, mean (SD) | 0.5 (1.1) | 0.4 (0.9) | 0.112 | 0.5 (1.1) | 0.6 (1.1) | 0.042 |
| Chronic clinical conditions, n (%) | ||||||
| Anxiety | 134 (31.5) | 3496 (32.1) | 0.013 | 132 (31.4) | 130 (30.9) | 0.010 |
| Asthma | 56 (13.1) | 1227 (11.3) | 0.058 | 54 (12.8) | 45 (10.7) | 0.066 |
| Chronic migraine | 249 (58.5) | 2355 (21.6) | 0.811 | 245 (58.2) | 251 (59.6) | 0.029 |
| Depression | 116 (27.2) | 2573 (23.6) | 0.083 | 116 (27.6) | 112 (26.6) | 0.021 |
| Hypertension | 109 (25.6) | 2729 (25.0) | 0.012 | 107 (25.4) | 98 (23.3) | 0.050 |
| Nausea | 65 (15.3) | 1993 (18.3) | 0.081 | 65 (15.4) | 76 (18.1) | 0.070 |
| Obesity | 62 (14.6) | 1772 (16.3) | 0.047 | 62 (14.7) | 78 (18.5) | 0.102 |
| Osteoarthritis | 44 (10.3) | 909 (8.3) | 0.068 | 43 (10.2) | 50 (11.9) | 0.053 |
| Sleep disorder | 124 (29.1) | 2214 (20.3) | 0.205 | 122 (29.0) | 118 (28.0) | 0.021 |
Notes: Covariates with Std Diff ≥0.1 are bolded, suggesting potential imbalance between cohorts. The 1:1 propensity score matched GMB and SOC cohorts were well-balanced. aIncludes family practice, internal medicine, obstetrics/gynecology, and nurse practitioner.
Abbreviations: CDHP, consumer-driven health plan; CGRP mAb, calcitonin gene-related peptide ligand or receptor-targeted monoclonal antibody; DCI, Deyo-Charlson comorbidity index; EPO, exclusive provider organization; ER, emergency room; GMB, galcanezumab; HDHP, high deductible health plan; HMO, health maintenance organization; N, number of patients in the cohort; n, number of patients in each category; POS, point of service; PPO, preferred provider organization; SD, standard deviation; SOC, standard-of-care; Std Diff, standardized difference.
After 1:1 propensity score matching, the CGRP mAb and SOC matched population comprised 3082 patients each (Table 3); the galcanezumab and SOC cohorts consisted of 421 patients each (Table 4). In these matched cohorts, mean (standard deviation) age ranged from 43.2 to 44.4 (11.3 to 12.0) years; the majority of patients were female (range: 85.7–88.6%). Most patients resided in the South (43.9–50.4%) or North-Central (20.0–23.1%) region of the US. More CGRP mAb than SOC initiators resided in the North-East region of the US (22.0% vs 16.2%, Std Diff = 0.148). Across cohorts (range), most common provider type closest to index date was neurologist (29.9–33.3%) and primary care provider (25.0–26.4%). Proportion of patients with chronic migraine across matched cohorts ranged from 54.8% to 59.6%. The top three chronic comorbid conditions were anxiety (30.9–33.2%), sleep disorder (25.2–29.0%), and depression (26.6–27.6%).
Table 3.
Patient Demographics and Clinical Characteristics Pre- and Post-Matching of Covariates for CGRP mAb and SOC 12-Month Follow-Up Cohort
| Demographics and Clinical Characteristics | Pre-Matching | Post-Matching | ||||
|---|---|---|---|---|---|---|
| CGRP mAb (N=4528) | SOC (N=10,897) | Std Diff | CGRP mAb (N=3082) | SOC (N=3082) | Std Diff | |
| Age (years), mean (SD) | 45.1 (11.3) | 41.3 (12.3) | 0.323 | 44.4 (11.3) | 44.2 (12.0) | 0.018 |
| Female, n (%) | 3904 (86.2) | 9275 (85.1) | 0.032 | 2641 (85.7) | 2676 (86.8) | 0.033 |
| Region, n (%) | ||||||
| Northeast | 1071 (23.7) | 1726 (15.8) | 0.197 | 679 (22.0) | 500 (16.2) | 0.148 |
| North central | 930 (20.5) | 2476 (22.7) | 0.053 | 617 (20.0) | 713 (23.1) | 0.076 |
| South | 1899 (41.9) | 5246 (48.1) | 0.125 | 1354 (43.9) | 1424 (46.2) | 0.046 |
| West | 617 (13.6) | 1427 (13.1) | 0.016 | 427 (13.9) | 435 (14.1) | 0.007 |
| Unknown | 11 (0.2) | 22 (0.2) | 0.009 | 5 (0.2) | 10 (0.3) | 0.033 |
| Insurance plan type, n (%) | ||||||
| Comprehensive/ indemnity | 153 (3.4) | 415 (3.8) | 0.023 | 105 (3.4) | 101 (3.3) | 0.007 |
| EPO/PPO | 2604 (57.5) | 5653 (51.9) | 0.113 | 1734 (56.3) | 1730 (56.1) | 0.003 |
| POS/POS with capitation | 273 (6.0) | 712 (6.5) | 0.021 | 198 (6.4) | 169 (5.5) | 0.040 |
| HMO | 500 (11.0) | 1480 (13.6) | 0.077 | 339 (11.0) | 345 (11.2) | 0.006 |
| CDHP/HDHP | 960 (21.2) | 2495 (22.9) | 0.041 | 674 (21.9) | 694 (22.5) | 0.016 |
| Other/unknown | 38 (0.8) | 142 (1.3) | 0.045 | 32 (1.0) | 43 (1.4) | 0.033 |
| Provider, n (%) | ||||||
| Neurology | 1414 (31.2) | 2827 (25.9) | 0.117 | 944 (30.6) | 993 (32.2) | 0.034 |
| Primary carea | 1022 (22.6) | 4578 (42.0) | 0.425 | 800 (26.0) | 771 (25.0) | 0.022 |
| Acute hospital care, ER | 310 (6.8) | 549 (5.0) | 0.077 | 186 (6.0) | 188 (6.1) | 0.003 |
| Radiology, laboratory, pathology | 201 (4.4) | 283 (2.6) | 0.100 | 132 (4.3) | 90 (2.9) | 0.073 |
| Other/unknown | 1581 (34.9) | 2660 (24.4) | 0.232 | 1020 (33.1) | 1040 (33.7) | 0.014 |
| DCI, mean (SD) | 0.4 (0.9) | 0.4 (0.9) | 0.029 | 0.5 (0.9) | 0.4 (0.9) | 0.012 |
| Chronic clinical conditions, n (%) | ||||||
| Anxiety | 1483 (32.8) | 3496 (32.1) | 0.014 | 1011 (32.8) | 1023 (33.2) | 0.008 |
| Asthma | 594 (13.1) | 1227 (11.3) | 0.057 | 424 (13.8) | 330 (10.7) | 0.093 |
| Chronic migraine | 3125 (69.0) | 2355 (21.6) | 1.083 | 1749 (56.7) | 1690 (54.8) | 0.039 |
| Depression | 1249 (27.6) | 2573 (23.6) | 0.091 | 820 (26.6) | 828 (26.9) | 0.006 |
| Hypertension | 1140 (25.2) | 2729 (25.0) | 0.003 | 798 (25.9) | 810 (26.3) | 0.009 |
| Nausea | 816 (18.0) | 1993 (18.3) | 0.007 | 564 (18.3) | 563 (18.3) | 0.001 |
| Obesity | 650 (14.4) | 1772 (16.3) | 0.053 | 454 (14.7) | 519 (16.8) | 0.058 |
| Osteoarthritis | 517 (11.4) | 909 (8.3) | 0.103 | 317 (10.3) | 331 (10.7) | 0.015 |
| Sleep disorder | 1272 (28.1) | 2214 (20.3) | 0.182 | 841 (27.3) | 778 (25.2) | 0.046 |
Notes: Covariates with Std Diff ≥0.1 are bolded, suggesting potential imbalance between cohorts. The 1:1 propensity score matched CGRP mAb and SOC cohorts were well-balanced. aIncludes family practice, internal medicine, obstetrics/gynecology, and nurse practitioner.
Abbreviations: CDHP, consumer-driven health plan; CGRP mAb, calcitonin gene-related peptide ligand or receptor-targeted monoclonal antibody; DCI, Deyo-Charlson comorbidity index; EPO, exclusive provider organization; ER, emergency room; GMB, galcanezumab; HDHP, high deductible health plan; HMO, health maintenance organization; N, number of patients in the cohort; n, number of patients in each category; POS, point of service; PPO, preferred provider organization; SD, standard deviation; SOC, standard-of-care; Std Diff, standardized difference.
Baseline and Follow-Up Preventive and Acute Medications Used for Migraine in Matched Population
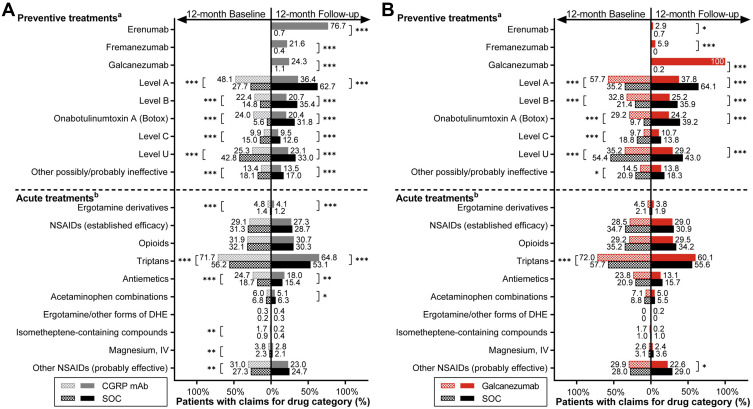
During the baseline period, patients initiating CGRP mAb versus SOC received a significantly greater number (mean per patient) of unique preventive drugs (1.6 vs 1.4) and drug classes (1.4 vs 1.2) for migraine (both p < 0.001). Level A and level U drugs were the most prescribed preventive drug categories in CGRP mAb (48.1%) and SOC (42.8%) cohorts, respectively (Figure 2A). Additionally, a significantly greater proportion of CGRP mAb versus SOC initiators received several acute medications. These included triptans (71.7% vs 56.2%), ergotamine derivatives (4.8% vs 1.4%), antiemetics (24.7% vs 18.7%) (all p < 0.001), and other probably effective NSAIDs (31.0% vs 27.3%, p = 0.001).
Figure 2.
Medications used during 12-month baseline and 12-month follow-up period in matched (A) CGRP mAb and SOC and (B) galcanezumab and SOC cohorts.
Notes: a,bRefer to Tables 1 and 2 for medication details under each treatment category. Chi-square test was used to compare cohorts during baseline and during follow-up. Temporal comparisons were not conducted. *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: CGRP mAb, calcitonin gene-related peptide ligand or receptor-targeted monoclonal antibody; DHE, dihydroergotamine; IV, intravenous; NSAIDs, nonsteroidal anti-inflammatory drugs; SOC, standard-of-care.
In the 12-month follow-up, patients who initiated CGRP mAb versus SOC received fewer mean per patient prescriptions of unique preventive drugs (1.4 vs 2.1) and drug classes (1.2 vs 1.8) for migraine (both p < 0.001) (Figure 2A). Significantly fewer CGRP mAb versus SOC initiators received conventional migraine preventive medications in all drug categories. However, significantly more CGRP mAb versus SOC initiators received the following acute medications for migraine, triptans (64.8% vs 53.1%, p < 0.001), antiemetics (18.0% vs 15.4%, p = 0.007), and ergotamine derivatives (4.1% vs 1.2%, p < 0.001). Among CGRP mAb initiators, 76.7% received erenumab, 21.6% received fremanezumab, and 24.3% received galcanezumab during follow-up.
Galcanezumab versus SOC comparisons revealed similar findings to CGRP mAb versus SOC. However, during baseline, galcanezumab versus SOC initiators received a similar mean number of unique preventive drugs and drug classes for migraine. Further, significantly (all p < 0.001) more galcanezumab versus SOC initiators received preventive drugs in level A category (57.7% vs 35.2%), level B category (32.8% vs 21.4%), onabotulinumtoxinA treatment (29.2% vs 9.7%), and acute treatment of triptans (72.0% vs 57.7%) (Figure 2B). Conversely, significantly fewer galcanezumab versus SOC initiators received preventive drug categories belonging to level C (9.7% vs 18.8%, p < 0.001), level U (35.2% vs 54.4%, p < 0.001), and other possibly or probably ineffective preventive treatments for migraine (14.5% vs 20.9%, p = 0.015).
With regards to 12-month follow-up medications used, similar to CGRP mAb, patients starting galcanezumab versus SOC received significantly fewer number of mean unique preventive drugs (1.6 vs 2.3, p < 0.001) and drug classes (1.4 vs 2.0, p < 0.001) for migraine. Numerically fewer patients received conventional preventive treatments after starting galcanezumab than during the baseline period (Figure 2B). Overall, 12-month follow-up data showed that significantly fewer galcanezumab versus SOC initiators received preventive drugs (all p < 0.001) in level A category (37.8% vs 64.1%), level B category (25.2% vs 35.9%), level U category (29.2% vs 43.0%), and onabotulinumtoxinA treatment (24.2% vs 39.2%), and acute treatment of other probably effective NSAIDs (22.6% vs 29.0%, p = 0.033). In addition, 2.9% and 5.9% of galcanezumab initiators received erenumab and fremanezumab, respectively.
Collectively, the most prescribed migraine preventive medication during follow-up in SOC initiators was level A drugs (62.7% to 64.1%); level B drugs (35.4% to 35.9%), onabotulinumtoxinA (31.8% to 39.2%), and level U drugs (33.0% to 43.0%) were almost equally prescribed (Figure 2A and B).
Treatment Patterns in Matched Population
Index Drug Refill and Treatment Adherence
During 12-month follow-up, CGRP mAb versus SOC initiators refilled their index drug almost twice as often (7.0 vs 4.1, p < 0.001) (Table 5). At 12 months, CGRP mAb initiators had significantly higher adherence than SOC initiators with mean PDC 55.1% vs 35.2%, and mean MPR 57.8% vs 36.9%, respectively (both p < 0.001). In the CGRP mAb versus SOC cohort, 32.7% vs 18.7% of the patients had a PDC ≥80% and 36.7% vs 21.0% of the patients had an MPR ≥80% (both p < 0.001); thus, more patients on CGRP mAb were treatment adherent. While similar results were observed for galcanezumab initiators versus SOC initiators, most values were numerically higher for galcanezumab compared with CGRP mAb cohort (Table 5). Mean index drug refills during 12-month follow-up were more than twice as often (mean: 8.4 versus 4.1, p < 0.001) in galcanezumab versus SOC initiators. At 12 months, galcanezumab initiators had significantly higher mean PDC (63.7% vs 33.7%) and MPR (66.4% vs 35.6%) than SOC initiators (both p < 0.001). In addition, significantly more patients were treatment adherent in the galcanezumab versus SOC cohort, based on number of patients who achieved PDC ≥80% (44.2% vs 17.3%) or MPR ≥80% (48.7% vs 19.7%) (both p < 0.001).
Table 5.
Treatment Patterns in Patients with Migraine Prescribed Index Treatment of Galcanezumab, CGRP mAb, or SOC During 12-Month Follow-Up
| Treatment Patterns During 12-Month Follow-Up Period | CGRP mAb (N=3082) | SOC (N=3082) | p-value | GMB (N=421) | SOC (N=421) | p-value |
|---|---|---|---|---|---|---|
| Number of index drug fills, mean (SD) | 7.0 (4.2) | 4.1 (3.5) | <0.001 | 8.4 (4.1) | 4.1 (3.4) | <0.001 |
| Adherence | ||||||
| PDC, % mean (SD) | 55.1 (31.2) | 35.2 (34.2) | <0.001 | 63.7 (30.6) | 33.7 (33.9) | <0.001 |
| PDC ≥ 80% (adherent), n (%) | 1008 (32.7) | 577 (18.7) | <0.001 | 186 (44.2) | 73 (17.3) | <0.001 |
| MPR, % mean (SD) | 57.8 (32.9) | 36.9 (36.0) | <0.001 | 66.4 (32.1) | 35.6 (36.0) | <0.001 |
| MPR ≥ 80% (adherent), n (%) | 1130 (36.7) | 647 (21.0) | <0.001 | 205 (48.7) | 83 (19.7) | <0.001 |
| Persistence, 60-day gap | ||||||
| Days of persistent use among all patients, mean (SD) | 212.5 (139.7) | 131.9 (140.2) | <0.001 | 252.3 (140.6) | 127.3 (139.4) | <0.001 |
| Patients persistent on index drug at end of follow-up, n (%) | 1269 (41.2) | 691 (22.4) | <0.001 | 239 (56.8) | 87 (20.7) | <0.001 |
| Discontinuation, 45-day gap | ||||||
| Patients that discontinued index drug during follow-up, n (%) | 1963 (63.7) | 2464 (79.9) | <0.001 | 206 (48.9) | 344 (81.7) | <0.001 |
| Treatment duration days, mean (SD) | 198.5 (138.0) | 124.4 (135.4) | <0.001 | 236.7 (143.1) | 119.5 (134.2) | <0.001 |
| Discontinuation, 60-day gap | ||||||
| Patients that discontinued index drug during follow-up, n (%) | 1813 (58.8) | 2391 (77.6) | <0.001 | 182 (43.2) | 334 (79.3) | <0.001 |
| Treatment duration days, mean (SD) | 210.4 (137.8) | 131.0 (138.8) | <0.001 | 249.9 (139.0) | 126.4 (137.9) | <0.001 |
| Among patients who discontinued index drug, 60-day gap | ||||||
| Patients who restarted index drug during follow-up, n (%) | 495 (27.3) | 837 (35.0) | <0.001 | 58 (31.9) | 124 (37.1) | 0.232 |
| Days from discontinuation date to first restart, mean (SD) | 120.2 (59.2) | 113.3 (53.7) | <0.001 | 114.6 (59.9) | 114.3 (51.1) | 0.943 |
| Patients with a switch to non-index drug during follow-up, n (%)a | 1049 (57.9) | 861 (36.0) | <0.001 | 90 (49.5) | 139 (41.6) | 0.087 |
| Days from index date to first switch, mean (SD) | 184.4 (85.9) | 164.2 (97.3) | <0.001 | 187.5 (96.7) | 149.2 (95.6) | <0.001 |
Notes: The 1:1 propensity score matched CGRP mAb vs SOC and GMB vs SOC cohorts were compared separately using Chi-square test for categorical variables and Student’s t-test for continuous variables. aSwitching could occur within the CGRP class, as well as to a different class of preventive treatment for migraine.
Abbreviations: GMB, galcanezumab; CGRP mAb, calcitonin gene-related peptide ligand or receptor-targeted monoclonal antibody; MPR, medical possession ratio; N, number of patients in the cohort; n, number of patients in each category; PDC, proportion of days covered; SD, standard deviation; SOC, standard-of-care; vs, versus.
Persistence and Discontinuation of Index Treatment
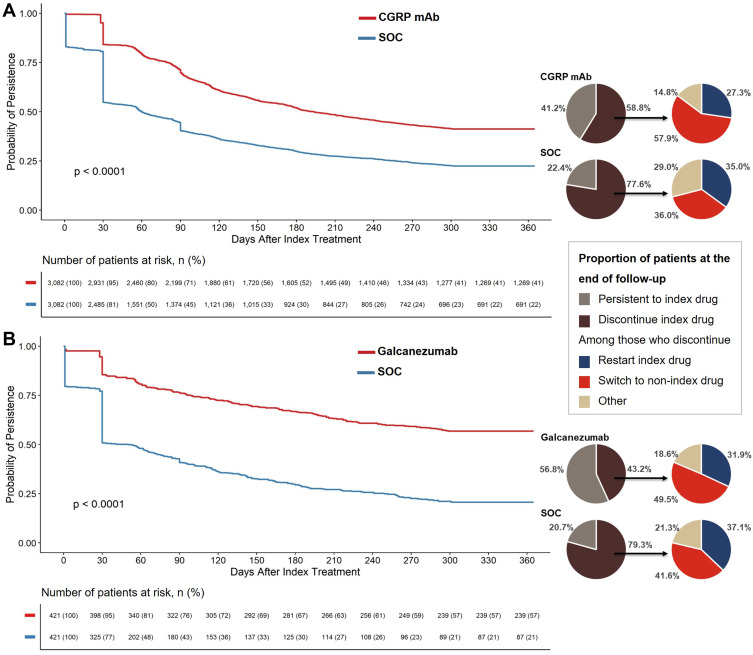
During 12-month follow-up, the CGRP mAb cohort had significantly higher persistence (60-day gap) than the SOC cohort (212.5 vs 131.9 days; p < 0.001) (Table 5). CGRP mAb initiators were also significantly less likely to discontinue their index treatment than SOC initiators (log-rank p < 0.0001) throughout the follow-up period (Figure 3A). Within a month of starting index treatment, almost half of SOC initiators discontinued their index treatment; 16% of patients discontinued CGRP mAb index treatment. At the end of 12-month follow-up, significantly fewer patients discontinued CGRP mAb compared with SOC (58.8% vs 77.6%, p < 0.001). Likewise, the patients on CGRP mAb treatment had longer mean treatment duration versus patients on SOC (210.4 days vs 131.0 days, p < 0.001).
Figure 3.
Proportion of patients that remain persistent to and discontinue index drug during 12-month follow-up.
Notes: Kaplan-Meier curves with Log rank test p-values are provided for comparing persistence to index drug (A) CGRP mAb vs SOC and (B) galcanezumab vs SOC, allowing for maximum 60-day gap between fills, during the 12-month follow-up period. Accompanying pie charts provide proportion of the patients that remain persistent to and who discontinue index drug (60-day gap rule) at the end of follow-up; and among those who discontinued index drug, proportion of the patients who restart index treatment or switch to non-index treatment during follow-up. Chi-square test was used to compare proportion of patients at the end of follow-up represented in the pie charts. All comparisons for CGRP mAb vs SOC cohort were significant with p < 0.001. Proportion of patients in galcanezumab vs SOC cohort persistent to and who discontinued index drug were significantly different (p < 0.001), while restart and switch rates were not significantly different (p > 0.05).
Abbreviations: CGRP mAb, calcitonin gene-related peptide ligand or receptor-targeted monoclonal antibody; n, number of patients at risk for each time point; SOC, standard-of-care; vs, versus.
Similar results were observed for matched galcanezumab versus SOC initiators. During 12-month follow-up, galcanezumab initiators were persistent on their index drug for significantly longer than SOC initiators (mean 252.3 vs 127.3 days, p < 0.001) (Table 5). As presented by the Kaplan-Meier curve, persistence rates fell over the 12-month follow-up period for all cohorts (Figure 3). Yet, galcanezumab initiators were significantly less likely to discontinue treatment than SOC initiators (log-rank p < 0.0001) (Figure 3B). Almost half of SOC initiators discontinued their index treatment within a month of starting it, while only around 14% of galcanezumab initiators discontinued galcanezumab. At the end of 12-month follow-up, galcanezumab discontinuation rates were lower than SOC discontinuation rates (43.2% vs 79.3%, p < 0.001). Correspondingly, mean treatment duration was significantly longer for galcanezumab versus SOC (249.9 days vs 126.4 days, p < 0.001).
Sensitivity analysis allowing for a shorter gap of 45 days between fills showed similar results with a slight increase in discontinuation rates in all cohorts (Table 5).
Restart of Index Drug and Switch to Non-Index Drug After Discontinuation
Among patients who discontinued CGRP mAb (versus SOC) index treatment during the 12-month follow-up period, significantly fewer patients restarted index drug (27.3% vs 35.0%) while significantly more patients switched to non-index drug (57.9% vs 36.0%) (both p < 0.001) (Table 5 and Figure 3A). Among patients who restarted or switched treatments, those in the CGRP mAb cohort had a significantly longer mean time to first restart of their index drug (120.2 days vs 113.3 days) or first switch to non-index drug (184.4 days vs 164.2 days) versus the SOC cohort, respectively (both p < 0.001).
Of the patients who discontinued index drug in the galcanezumab versus SOC cohort, a similar proportion restarted index drug and numerically more switched to non-index drug (Table 5 and Figure 3B). Among patients who restarted index drug in the galcanezumab versus SOC cohort, the mean number of days until first restart of the index drug was also similar. However, among patients who switched to non-index drug, galcanezumab initiators took a significantly longer time to switch to a non-index drug than SOC initiators (mean, 187.5 days vs 149.2 days, p < 0.001).
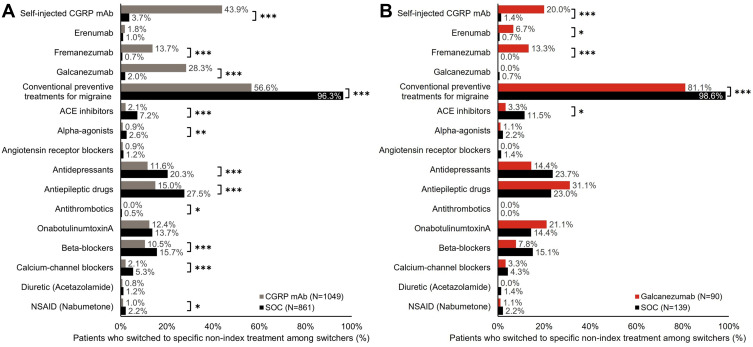
Switching to Specific Non-Index Drug Class Among Switchers
Comparing switching patterns of matched CGRP mAb and SOC cohorts during 12-month follow-up revealed substantial differences (Figure 4A). More patients switching from CGRP mAb index treatment switched to a different CGRP mAb (43.9% vs.3.7%, p < 0.001) compared with patients switching from SOC index treatment; fewer patients switched to conventional preventive drug classes (56.6% vs 96.3%, p < 0.001). Among switchers in the CGRP mAb versus SOC cohort, significantly (both p < 0.001) more patients switched to galcanezumab (28.3% vs 2.0%), or fremanezumab (13.7% vs 0.7%), and fewer patients switched to one of the conventional preventive drug classes. No substantial difference was seen in switching rates in the CGRP mAb versus SOC cohort to erenumab. The top three conventional preventive drug classes switched to after initiating CGRP mAb were antiepileptic drugs (15.0%), onabotulinumtoxinA (12.4%), and antidepressants (11.6%).
Figure 4.
Switch to non-index preventive migraine drug class among patients who switched treatment in (A) CGRP mAb vs SOC and (B) galcanezumab vs SOC cohorts during 12‑month follow-up.
Notes: Treatment switch could occur within the CGRP class, as well as to a different class of preventive treatments for migraine. Refer to Table 1 for details on preventive drug class. Proportion of patients were compared using Chi-square test. *p < 0.05, **p < 0.01, ***p < 0.001.
Abbreviations: ACE, angiotensin-converting enzyme; CGRP mAb, calcitonin gene-related peptide ligand or receptor-targeted monoclonal antibody; N, number of patients who switched to non-index drug; NSAID, nonsteroidal anti-inflammatory drug; SOC, standard-of-care; vs, versus.
Significantly more switchers in the galcanezumab versus SOC cohort switched to other CGRP mAb drugs (20.0% vs.1.4%), while significantly fewer switched to conventional preventive drug classes (81.1% vs 98.6%) (both p < 0.001) (Figure 4B). Of the switchers in the galcanezumab versus SOC cohort, significantly more patients switched to erenumab (6.7% vs 0.7%, p = 0.016) or fremanezumab (13.3% vs 0.0%, p < 0.001), and fewer switched to angiotensin converting enzyme inhibitor drug class. In the galcanezumab cohort, the top three conventional preventive drug classes switched to were antiepileptic drugs (31.1%), onabotulinumtoxinA (21.1%), and antidepressants (14.4%). Collectively in the SOC cohort, the top three non-index drug classes (range) switched to were antiepileptic drugs (23.0–27.5%), antidepressants (20.3–23.7%), and beta-blockers (15.1–15.7%).
Patient Sample, Baseline Characteristics, and Treatment Patterns for 6-Month Follow-Up Cohorts
For the 6-month follow-up cohort, 12,681 patients on CGRP mAb (galcanezumab n = 3253) index drug and 21,474 patients on SOC were identified (Supplementary Figure 1). Post matching, CGRP mAb versus SOC matched population comprised 7867 patients each; and galcanezumab versus SOC matched population comprised 2986 patients each. The baseline patient demographics and outcomes for the 6-month follow-up cohorts showed largely similar trends to the 12-month follow-up cohorts (Supplementary Tables 1–3 and Supplementary Figures 1–3).
Discussion
This study compared real-world treatment patterns for patients with migraine initiating CGRP mAb and specifically galcanezumab versus SOC migraine preventive treatments. The key findings are summarized as follows: i) approximately 50% of patients discontinued their index SOC treatment within a month of starting it, while less than 20% of patients discontinued CGRP mAb or specifically galcanezumab index treatment. ii) Compared with patients on SOC, patients on CGRP mAb or specifically galcanezumab had higher treatment adherence, persistence, and were less likely to discontinue their treatment over 6 and 12 months of follow-up. iii) Among switchers in the CGRP mAb 12-month follow-up cohort, the most common non-index drug or drug class switched to was galcanezumab. iv) Among switchers in the galcanezumab 12-month follow-up cohort, the top three non-index treatments switched to were antiepileptics, onabotulinumtoxinA, and antidepressants, followed by a different CGRP mAb, fremanezumab.
About half of the patients in the current study had chronic migraine and frequently reported comorbidities were anxiety, depression, and sleep disorders. Several reports have highlighted the strong association between migraine and these comorbidities; future studies are warranted to understand if there is any overlapping pathophysiology.4,28–30 While majority of the SOC initiators received level A drugs (antiepileptics and beta-blockers) during 12-month follow-up as per the AAN guidelines, it was interesting to observe similar prescription rates for level B, onabotulinumtoxinA and level U drugs.9 This may be partly explained by comorbid anxiety and depression in the patient cohorts, which were possibly prescribed antidepressants and other drugs from the level U drug category for treating comorbidities.
The treatment pattern findings from this study among patients initiating SOC preventive treatments are in line with previous reports, though slightly greater adherence and persistence was observed in the current study.12,14,15 A previous US claims report analyzing patients initiating antidepressants, beta-blockers, and antiepileptics between 2008 and 2012, showed a sharp decline in persistence to oral preventive treatments for migraine within 30 days of initiation; persistence fell to 25% at six months and 14% at 12 months.14 Adherence rates in the same cohort ranged between 26–29% at six months and fell to 17–20% at 12 months after initiating conventional oral preventive treatments for migraine.15 This is also consistent with results from the second international burden of migraine study conducted in 2010 using a web-based survey.12 The survey results showed that less than half of patients with migraine take preventive medications, with the most common drug classes being antidepressants, beta-blockers, and antiepileptics.12 While the current study was not designed to compare the galcanezumab versus CGRP mAb cohort, results do suggest the galcanezumab cohort may have higher treatment adherence and persistence.
In the current study, the majority of patients initiating CGRP mAb treatment had erenumab as their index drug. Thus, numerical differences observed between the galcanezumab and the CGRP mAb cohorts may primarily be driven by erenumab. Thus far, few studies report real-world treatment patterns for CGRP mAbs.31,32 Reports show (PDC/MPR ≥80%) 31%/42% of patients on erenumab, and 82.8%/84.4% on quarterly and 72.9%/77.8% on monthly fremanezumab were treatment adherent at the end of at least 180-day follow-up.31,32 For monthly/quarterly fremanezumab and galcanezumab, discontinuation rates over ≥6 months (23.4%/15.6% and 25.6%), and in patients who discontinued, restart (10.2%/6.9% and 17.0%) and switch rates (44.4%/44.8% and 35.3%) were comparable.32 After initiating erenumab, 48.7% and 36.1% of patients discontinued one or more acute and preventive treatments for migraine, respectively.31 The temporal comparisons to assess treatment use during baseline and follow-up were out of scope for the current study and were not evaluated.
During follow-up, compared with SOC cohort, fewer patients on CGRP mAb, specifically galcanezumab, used other conventional preventive treatments. The proportion of patients prescribed acute medications in the galcanezumab or CGRP mAb cohort versus SOC cohort during follow-up were not substantially different. Although, more patients on CGRP mAb versus SOC used triptans, antiemetics, and ergotamine derivatives. The study objective did not include time to discontinue acute medications from index date, which will be considered in future studies to understand the real-world effectiveness of CGRP mAbs. While the erenumab study had less stringent selection criteria compared with the current study and did not compare patients on SOC treatments, the findings are consistent between studies. Future studies comprising longer follow-up time and larger patient cohorts may help characterize factors associated with improved adherence and persistence to CGRP mAb preventive treatments for migraine. Understanding reasons for discontinuing treatment is important to patients, clinicians, health policy makers, payers, and other stakeholders to appropriately address preventive treatment non-compliance observed in patients with migraine.33
Findings from the large survey-based American Migraine Prevalence and Prevention study published in 2007 revealed that while preventive treatment may be considered for more than one in four patients with migraine, most patients who might benefit did not have access to preventive treatments.10 Further, the focus group study conducted in 2019 in the US, UK, and Germany, in patients using or who had used preventive treatments for migraine showed that the top priority for patients was to reduce migraine attack severity and frequency.34 Patients also reported dissatisfaction with oral preventive treatments due to intolerance or adverse events and were willing to self-inject efficacious and tolerable preventive treatments via syringes or autoinjectors.34 However, most of the patients in the study group lacked access to efficacious preventive treatments for migraine. The AHS 2021 consensus statement suggests preventive treatments in patients severely affected due to migraine for two or more days in a month, with any level A/B drugs categories as first- and second-line treatment, followed by CGRP mAb as third line treatment.35 However, considering emerging literature on real-world treatment adherence and persistence with CGRP mAb over conventional preventives along with treatment effectiveness, the preventive treatment landscape for migraine may need to be re-evaluated.16 One of the major challenges is the relatively high cost associated with CGRP mAb, and more real-world data studies supporting CGRP mAb over conventional preventive treatments may add credence to reassess requirements to prescribe CGRP mAb treatments.
Strengths and Limitations of the Study
This study addresses critical gaps in literature regarding real-world treatment patterns for CGRP mAb and specifically galcanezumab treatment compared with SOC preventive treatments for migraine. One of the key strengths of the study is use of propensity score matched cohorts to address selection or confounder bias, inherent with observation studies. For the SOC cohort, the study included index drugs that have favorable evidence-based efficacy; this ensured appropriate comparisons as drugs with inconsistent or poor efficacy may skew the results. Similar trends in 12-month and 6-month follow-up cohorts, as well as galcanezumab and CGRP mAb cohorts add credibility to the study findings.
The study has the following limitations. The study sample is representative of only individuals with commercial health or private Medicare supplemental insurance, and the findings may not be inferred for the general US population. Migraines, comorbidities, or medication use could have potentially been misclassified due to data coding or data entry error, or both. As the study used claims databases, filled prescriptions may not have translated to patients taking medications; prescriptions that are written but not filled were not captured. Considering a substantial study population had anxiety, depression, or other comorbidities, this may have confounded the results, treatment choice, and index drug adherence and persistence across all cohorts. However, considering the rate of anxiety, depression, and other comorbidities were largely comparable between cohorts, it might not have affected the differences observed between matched cohorts. Some medications have on- and off-label indications, and the study could not determine which indication medications were prescribed for; this may have affected follow-up medications used and switching outcomes as some prescriptions may have been for comorbid conditions. Migraine treatment status was based on observable treatment and diagnosis data, which may not reflect true clinical course of disease and treatment.
Conclusion
This study demonstrated higher treatment adherence and persistence to CGRP mAbs and specifically galcanezumab over SOC migraine preventive treatments. At the end of 12-month follow-up, discontinuation rates were lower for CGRP mAb (59%) and specifically galcanezumab (43%) than for the SOC (80%) cohort. This highlights that overall persistence to migraine preventives is still low, and to improve treatment compliance, encourages future studies differentiating persistent and non-persistent patient characteristics, and patient surveys to understand reasons for discontinuing migraine preventive treatments. With emerging real-world evidence supporting CGRP mAb as a preventive treatment for migraine, the current migraine therapeutic landscape may need revisiting to ensure faster access to adequate treatments.
Acknowledgments
The authors would like to acknowledge Shonda A. Foster and Janet H. Ford, employees of Eli Lilly and Company for their contributions toward the study conception, design, and implementation, and Minal Jaggar, PhD, an employee of Eli Lilly Services India Pvt. Ltd. for medical writing support.
Funding Statement
This study was funded by Eli Lilly and Company.
Data Sharing Statement
The data that support the study findings were provided by IBM®. Restrictions apply to the availability of these data, which were used under license for this study and therefore are not publicly available. Requests may be sent to IBM® for more information on data availability and licensing.
Author Contributions
Oralee J. Varnado, Wenyu Ye, Kory Schuh, and Richard Wenzel: data interpretation and critical revision for important intellectual content. Janna Manjelievskaia and Allison Perry: study design, data acquisition, analyses, interpretation, and critical revision for important intellectual content. All authors made substantial contributions to conception and design, acquisition of data, or analyses and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
Oralee J Varnado, Wenyu Ye, and Kory Schuh are employees and minor shareholders of Eli Lilly and Company. Richard Wenzel is a former employee of Eli Lilly and Company. Janna Manjelievskaia and Allison Perry are employees of IBM Watson Health, which was contracted by Eli Lilly and Company to conduct the analyses.
References
- 1.IHS. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 2.Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Lifting the burden: the global campaign against H. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137–140. doi: 10.1186/s10194-020-01208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safiri S, Pourfathi H, Eagan A, et al. Global, regional, and national burden of migraine in 204 countries and territories, 1990 to 2019. Pain. 2021;163(2):e293–e309. [DOI] [PubMed] [Google Scholar]
- 4.Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631–649. doi: 10.1016/j.ncl.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 5.Ford JH, Foster SA, Nichols RM, et al. A real-world analysis of patient-reported outcomes in patients with migraine by preventive treatment eligibility status in the US and Europe. JPRO. 2020;4(1):53. doi: 10.1186/s41687-020-00221-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbs SN, Shah S, Deshpande CG, et al. United States Patients’ perspective of living with migraine: country-specific results from the global ”My Migraine Voice” survey. Headache. 2020;60(7):1351–1364. doi: 10.1111/head.13829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59(1):1–18. [DOI] [PubMed] [Google Scholar]
- 8.Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: the American headache society evidence assessment of migraine pharmacotherapies. Headache. 2015;55(1):3–20. [DOI] [PubMed] [Google Scholar]
- 9.Silberstein SD, Holland S, Freitag F, et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337–1345. doi: 10.1212/WNL.0b013e3182535d20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipton RB, Bigal ME, Diamond M, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343–349. doi: 10.1212/01.wnl.0000252808.97649.21 [DOI] [PubMed] [Google Scholar]
- 11.Ailani J, Burch RC, Robbins MS; Board of Directors of the American Headache S. The American Headache Society Consensus Statement: update on integrating new migraine treatments into clinical practice. Headache. 2021;61(7):1021–1039. doi: 10.1111/head.14153 [DOI] [PubMed] [Google Scholar]
- 12.Blumenfeld AM, Bloudek LM, Becker WJ, et al. Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the second international burden of migraine study (IBMS-II). Headache. 2013;53(4):644–655. doi: 10.1111/head.12055 [DOI] [PubMed] [Google Scholar]
- 13.Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharm. 2014;20(1):22–33. doi: 10.18553/jmcp.2014.20.1.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478–488. doi: 10.1177/0333102414547138 [DOI] [PubMed] [Google Scholar]
- 15.Hepp Z, Dodick DW, Varon SF, et al. Persistence and switching patterns of oral migraine prophylactic medications among patients with chronic migraine: a retrospective claims analysis. Cephalalgia. 2017;37(5):470–485. doi: 10.1177/0333102416678382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Negro A, Martelletti P. Patient selection for migraine preventive treatment with anti-CGRP(r) monoclonal antibodies. Expert Rev Neurother. 2019;19(8):769–776. doi: 10.1080/14737175.2019.1621749 [DOI] [PubMed] [Google Scholar]
- 17.Ha H, Gonzalez A. Migraine headache prophylaxis. Am Fam Physician. 2019;99(1):17–24. [PubMed] [Google Scholar]
- 18.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28(2):183–187. doi: 10.1002/ana.410280213 [DOI] [PubMed] [Google Scholar]
- 19.Aimovig™ (erenumab-aooe subcutaneous injection) [package insert]. Thousand Oaks, CA: Amgen Pharmaceuticals; February, 2021. [Google Scholar]
- 20.Ajovy™ (fremanezumab-vfrm subcutaneous injection) [package insert]. North Wales PA: TEVA Pharmaceuticals USA; January, 2020. [Google Scholar]
- 21.Emgality™ (galcanezumab-gnlm subcutaneous injection) [package insert]. Indianapolis IN: Eli Lilly and Company; June, 2019. [Google Scholar]
- 22.VYEPTI™ (eptinezumab-jjmr intravenous injection) [package insert]. Bothell WA: Lundbeck Seattle BioPharmaceuticals, Inc.; February, 2020. [Google Scholar]
- 23.Moreno-Ajona D, Perez-Rodriguez A, Goadsby PJ. Gepants, calcitonin-gene-related peptide receptor antagonists: what could be their role in migraine treatment? Curr Opin Neurol. 2020;33(3):309–315. doi: 10.1097/WCO.0000000000000806 [DOI] [PubMed] [Google Scholar]
- 24.Nurtec ODT™ (rimegepant orally disintegrating tablet) [package insert]. New Haven, CT: Biohaven Pharmaceuticals Inc.; May, 2021. [Google Scholar]
- 25.QULIPTA™ (atogepant, oral tablets) [package insert]. Dublin, Ireland: AbbVie; September, 2021. [Google Scholar]
- 26.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 27.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265–2281. doi: [DOI] [PubMed] [Google Scholar]
- 28.Caponnetto V, Deodato M, Robotti M, et al. Comorbidities of primary headache disorders: a literature review with meta-analysis. J Headache Pain. 2021;22(1):71. doi: 10.1186/s10194-021-01281-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radat F. What is the link between migraine and psychiatric disorders? From epidemiology to therapeutics. Rev Neurol (Paris). 2021;177(7):821–826. doi: 10.1016/j.neurol.2021.07.007 [DOI] [PubMed] [Google Scholar]
- 30.Altamura C, Corbelli I, de Tommaso M, et al. Pathophysiological bases of comorbidity in migraine. Front Hum Neurosci. 2021;15:640574. doi: 10.3389/fnhum.2021.640574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hines DM, Shah S, Multani JK, Wade RL, Buse DC, Bensink M. Erenumab patient characteristics, medication adherence, and treatment patterns in the United States. Headache. 2021;61(4):590–602. doi: 10.1111/head.14068 [DOI] [PubMed] [Google Scholar]
- 32.Tangirala K, Cohen JM, Pandya S, Krasenbaum LJ, Thompson SF, Chen -C-C. Real-world adherence, persistence, switching, and reinitiation by quarterly and monthly dosing regimen in patients prescribed AJOVY in US physician practices (2145). Neurology. 2021;96(15 Supplement):2145. [Google Scholar]
- 33.Ashina M, Katsarava Z, Do TP, et al. Migraine: epidemiology and systems of care. Lancet. 2021;397(10283):1485–1495. doi: 10.1016/S0140-6736(20)32160-7 [DOI] [PubMed] [Google Scholar]
- 34.Seo J, Smith CA, Thomas C, et al. Patient perspectives and experiences of preventive treatments and self-injectable devices for migraine: a focus group study. Patient. 2021;15(1):93–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eigenbrodt AK, Ashina H, Khan S, et al. Diagnosis and management of migraine in ten steps. Nat Rev Neurol. 2021;17(8):501–514. doi: 10.1038/s41582-021-00509-5 [DOI] [PMC free article] [PubMed] [Google Scholar]