Abstract
Purpose
Programmed cell death protein 1 (PD-1) inhibitors have shown a therapeutic effect in the treatment of advanced cervical cancer in clinical trials. However, the clinical characteristics associated with response remain undetermined. This study aimed to evaluate the efficacy and prognostic factors of PD-1 inhibitors in patients with advanced cervical cancer in clinical practice.
Patients and Methods
The study enrolled patients with recurrent or metastatic cervical cancer treated with PD-1 inhibitors at our center between March 2018 and November 2020. The primary outcomes were the objective response rate (ORR) and progression-free survival (PFS). Secondary endpoints were overall survival (OS) and safety. In addition, independent prognostic factors were identified by multivariate regression analysis.
Results
A total of 102 patients were included, and the ORR and disease control rate (DCR) were 51.0% and 66.7%, respectively. Median PFS was 11.0 months (95% CI, 1.7–20.4), while median OS was not achieved. Multivariate analysis indicated that factors associated with a better prognosis (ORR and PFS) included squamous cell carcinoma, a time to recurrence >6 months, and PD-1 plus chemotherapy and anti-angiogenic drugs (p < 0.05). Lines of therapy were independent factors for ORR but not for PFS. We also observed a tendency for longer PFS in patients with lung metastases and lymph node metastases only. Treatment-related adverse events (AEs) were well tolerated and primarily included thrombocytopenia, hepatic dysfunction, anemia, and leukopenia.
Conclusion
PD-1 inhibitors demonstrated beneficial efficacy and safety in advanced cervical cancer, particularly for patients with squamous cell carcinoma, a time to recurrence >6 months, or PD-1 plus chemotherapy and anti-angiogenic drugs. Further studies are needed to confirm the long-term outcomes.
Keywords: cervical cancer, PD-1, chemotherapy, anti-angiogenic therapy, immune checkpoint inhibitors
Introduction
Cervical cancer is the fourth most common and deadliest malignancy among women, with more than 604,000 new cases and 342,000 deaths reported globally in 2020.1 In China, 111,000 women were diagnosed with cervical cancer in 2015, with 34,000 deaths.2 Although prophylactic vaccination and screening programs for human papillomavirus are increasingly available, more than 70% of diagnosed cervical cancer cases in developing countries are diagnosed at an advanced stage, and 15–61% of women develop metastatic disease, usually within the first 2 years of completing treatment, with a 5-year survival of 17%.3,4 Platinum-based chemotherapy is the first-line treatment, and the addition of bevacizumab to chemotherapy has been shown to improve survival, as reported in the Gynecologic Oncology Group 240 trial. However, despite the current advances in various treatments such as chemotherapy and targeted therapies, the prognosis for patients who progress after first-line therapy remains dismal with limited treatment options. Hence, effective therapies must be developed for patients with recurrent or metastatic cervical cancer.
Recently, immune checkpoint inhibitors have shown antitumor activity in multiple tumor types, representing a shift in the treatment paradigm. Programmed cell death protein/ligand 1 (PD-1/L1) plays an important role in cancer by allowing cancer cells to avoid immunosurveillance by tumor-specific T cells.5,6 Clinical studies have shown that immune checkpoint inhibitors have promising antitumor activity in cervical cancer. According to the KEYNOTE-028 and KEYNOTE-158 trials, the United States Food and Drug Administration approved pembrolizumab as second-line therapy for PD-L1 positive recurrent or metastatic cervical cancer.7,8 The randomized Phase III KEYNOTE-826 trial reported that pembrolizumab plus chemotherapy with or without bevacizumab exhibited significantly longer progression-free and overall survival than placebo plus chemotherapy with or without bevacizumab as a first-line treatment for recurrent and metastatic cervical cancer.9 In addition, several other immune checkpoint inhibitors, including nivolumab,10–12 atezolizumab,13 camrelizumab,14 cemiplimab,15,16 and balstilimab,17,18 have been studied as monotherapy or as part of combination therapy for cervical cancer. However, the objective response rate (ORR) of immune checkpoint inhibitor monotherapy is 4–26%, and progression-free survival (PFS) is 2–5.1 months; although both ORR and PFS are significantly improved with immune checkpoint inhibitor combination therapy (0–65.9% and 2.9–13.8 months, respectively)—only a subset of patients showed a strong and prolonged response. Identifying the profile of patients who may benefit from this treatment has been a concern for clinicians, and only a few studies have been able to achieve this. Therefore, we conducted the present study not only to assess the efficacy and safety of PD-1 in patients with recurrent or metastatic cervical cancer but also to analyze the various prognostic factors in different subgroups to identify patients receiving the most benefit.
Patients and Methods
Study Cohort
Eligible patients were histologically diagnosed with recurrent or metastatic cervical cancer treated using anti-PD-1 therapy at the Cancer Center, Wuhan Union Hospital, China, between March 2018 and November 2020. Patients included had at least one measurable lesion after prior lines of treatment and had completed one or more post-baseline tumor assessments. We excluded patients with missing follow-up or post-baseline assessment data. Clinical data were retrospectively collected from patient records and follow-up visits. This retrospective study was approved by the Ethics Committee of Wuhan Union Hospital, China (approval number: 2021–0047) and was conducted in accordance with the Declaration of Helsinki. All patients signed informed consent in this study.
Treatment Regimens
Patients received PD-1 as a monotherapy or in combination with chemotherapy or chemotherapy and anti-angiogenic therapy. PD-1 inhibitor therapy consisted of 200 mg of camrelizumab or sintilimab administered intravenously every 3 weeks. Specifically, 85 patients received camrelizumab, and 27 patients received sintilimab. In addition, patients were treated with 4–6 cycles of paclitaxel (175 mg/m2) and cisplatin (50 mg/m2) or carboplatin AUC 5 administered intravenously every 3 weeks, followed by maintenance treatment using immune checkpoint inhibitors every 3 weeks with or without daily administration of anti-angiogenic drugs. The anti-angiogenic drug administered was oral apatinib at a dose of 250 mg once daily. The total exposure to PD-1 and anti-angiogenic drugs did not exceed 2 years or was conducted until further disease progression, death, intolerable adverse events, or physician/patient decision.
Assessments
Tumor response was assessed using the immune-related response evaluation criteria in solid tumors (iRECIST) after every two cycles of therapy and included complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD).19 If radiologic imaging indicated PD, a repeat assessment was performed after 4 weeks. The ORR was defined as the proportion of patients with CR+PR, and DCR was defined as the proportion of patients with CR+PR+SD. Progression-free survival was defined as the period from the first date of treatment to disease progression or death from any cause. Overall survival was defined as the time from initiation of immunotherapy to the date of death from any cause. Safety was assessed using the Common Terminology Criteria for Adverse Events (version 5.0). The primary outcomes were the ORR and PFS, with overall survival and safety as secondary endpoints.
Statistical Analysis
Continuous variables were presented as the median and range, and categorical variables were expressed as the frequency and percentage. Clinical variables associated with the ORR were evaluated using the Chi-squared test or Fisher’s exact test. Progression-free and overall survival were compared using Kaplan–Meier analysis and the Log rank test. Further multivariate logistic regression analysis and Cox proportional hazards regression were used to identify factors with p<0.05 in ORR and PFS, respectively. All analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA).
Results
Clinical Characteristics and Treatment Strategies
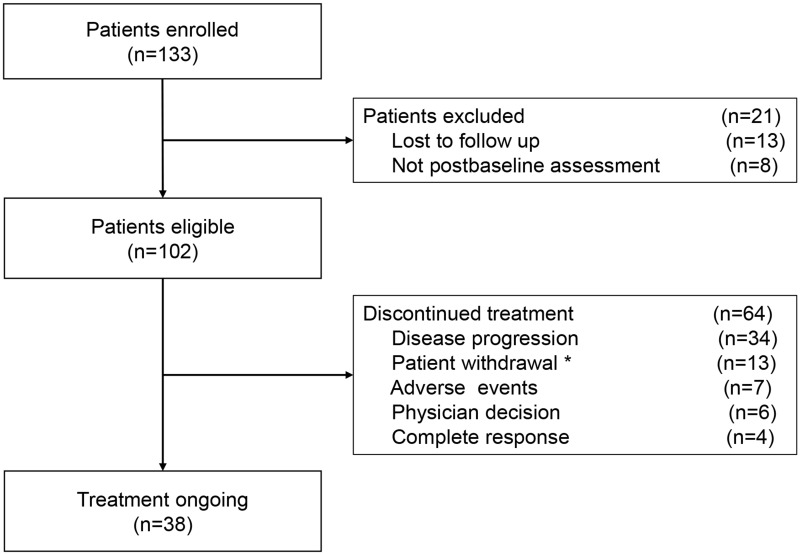
Between the study period from March 2018 to November 2020, 133 patients with recurrent or metastatic cervical cancer received anti-PD-1 treatment at our center. Of these, 13 patients had no follow-up data, and 8 had no post-baseline assessment, leaving 102 eligible patients (Figure 1). The median age was 52 years (range, 22–78). Detailed patient characteristics are presented in Table 1. The median follow-up was 18 months (range, 2–28). Overall, 64 patients (62.7%) discontinued treatment during the study, mostly because of progressive disease (n = 34, 53.1%) (Figure 1). The median number of PD-1 inhibitor cycles was 5 (range, 1–36).
Figure 1.
Flow diagram of patient selection. *Thirteen patients withdrew treatment, eight of whom did not continue treatment due to financial problems, and five failed to complete treatment due to COVID-19.
Table 1.
Patient Baseline Characteristics
| Characteristic | Patients (n = 102) (%) |
|---|---|
| Median age (range), years | 52 (22–78) |
| Histology | |
| Squamous | 75 (73.5) |
| Adenocarcinoma | 27 (26.5) |
| Disease status | |
| Recurrent | 48 (47.1) |
| Metastatic | 54 (52.9) |
| Location of metastasis | |
| Lymph node only | 15 (14.7) |
| Lung only | 12 (11.8) |
| Lung and lymph node | 9 (8.8) |
| Bone | 7 (6.9) |
| Liver | 4 (3.9) |
| Multi-site | 17 (16.7) |
| Time to recurrence | |
| ≤ 6 months | 44 (43.1) |
| > 6 months | 58 (56.9) |
| Previous therapy | |
| Surgery | 47 (46.1) |
| Radiotherapy | 80 (78.4) |
| Chemotherapy | 92 (90.2) |
| Anti-angiogenic therapy | 19 (18.6) |
| Lines of therapy | |
| 1 | 35 (34.3) |
| 2 | 39 (38.2) |
| ≥ 3 | 28 (27.5) |
| Treatment pattern | |
| PD-1 monotherapy | 24 (23.5) |
| PD-1+chemotherapy | 26 (25.5) |
| PD-1+chemotherapy+anti-angiogenic therapy | 52 (51.0) |
Notes: Data are presented as No(%) unless otherwise indicated. Time to recurrence, recurrence occurred after first-line therapy.
Abbreviation: PD-1, programmed death-1.
Antitumor Activity
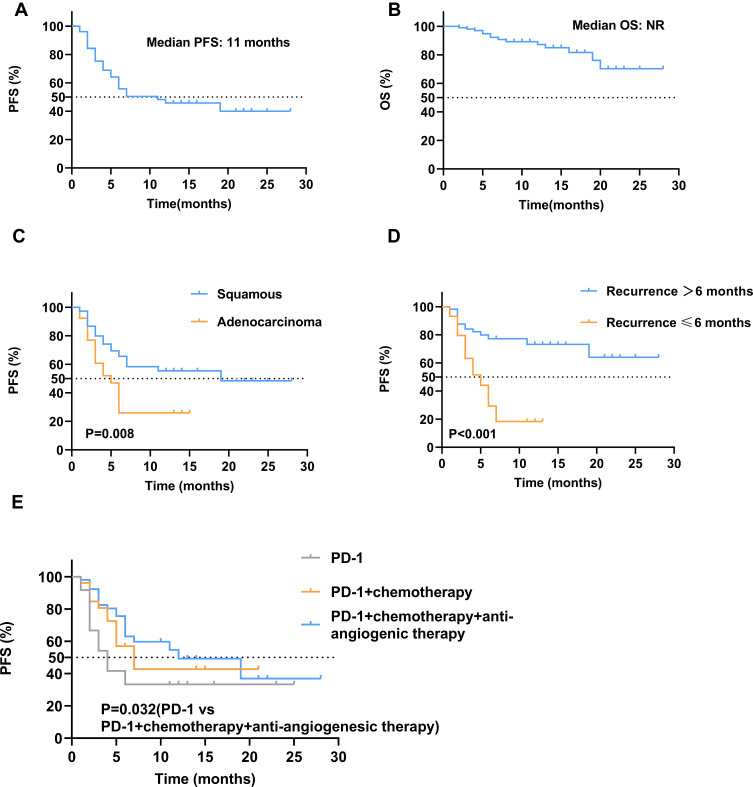
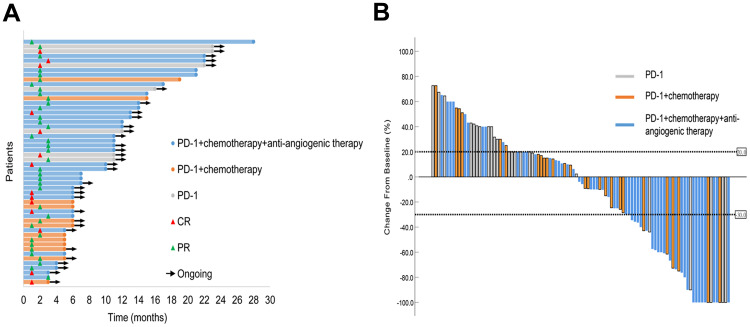
At the final follow-up date of June 30, 2021, 14 patients achieved a CR, 38 patients showed a PR, and 17 patients had an SD, with an ORR of 51.0% (95% CI, 41.1–60.8) and a DCR of 66.7% (95% CI, 57.7–76.6) (Table 2). The median time to response for CR and PR was 1.9 months (range, 1–6), and the duration of response was 9.4 months (range, 2–27). The median PFS was 7 months (95% CI, 1.7–9.5), and the PFS rate at 12 months was 64.3%. The median OS was not reached with 12-month OS estimates of 87.6% (Figure 2). The duration of objective response to PD-1 inhibitors and the best change from baseline in tumor size for all patients are presented in Figure 3.
Table 2.
Relationship Between Clinical Factors and Objective Response Rate and Progression-Free Survival in Patients
| Variables | No | CR | PR | SD | PD | ORR (%) | P | mPFS (m) | P |
|---|---|---|---|---|---|---|---|---|---|
| Total | 102 | 14 | 38 | 17 | 33 | 51 | / | 11 | / |
| Age | |||||||||
| <50 | 41 | 7 | 15 | 3 | 16 | 53.7 | 0.658 | 7 | 0.558 |
| ≧50 | 61 | 7 | 23 | 14 | 17 | 49.2 | 11 | ||
| Histology | |||||||||
| Squamous cell | 75 | 13 | 30 | 12 | 20 | 57.3 | 0.032 | 19 | 0.008 |
| Adenocarcinoma | 27 | 1 | 8 | 5 | 13 | 33.3 | 5 | ||
| Disease status | |||||||||
| Recurrent | 48 | 6 | 16 | 8 | 18 | 45.8 | 0.328 | 6 | 0.302 |
| Metastatic | 54 | 8 | 22 | 9 | 15 | 55.6 | NR | ||
| Location of metastasis | |||||||||
| Lung/lymph node only | 36 | 7 | 16 | 5 | 8 | 63.9 | 0.044 | NR | 0.003 |
| Non-lung/lymph node | 66 | 7 | 22 | 11 | 26 | 43.9 | 6 | ||
| Previous therapy | |||||||||
| Surgery, radiotherapy, and chemotherapy | 47 | 6 | 16 | 8 | 17 | 46.8 | 0.436 | 6 | 0.660 |
| Radiotherapy and chemotherapy | 55 | 8 | 22 | 9 | 16 | 54.5 | 11 | ||
| Time to recurrence | |||||||||
| ≤ 6 months | 44 | 4 | 10 | 9 | 21 | 31.8 | 0.001 | 5 | <0.001 |
| > 6 months | 58 | 10 | 28 | 8 | 12 | 65.5 | NR | ||
| Lines of therapy | |||||||||
| 1 | 36 | 9 | 20 | 3 | 4 | 80.6 | <0.001 | NR | 0.010 |
| 2 | 38 | 2 | 13 | 6 | 17 | 39.5 | 7 | ||
| ≥ 3 | 28 | 3 | 5 | 8 | 12 | 28.6 | 4 | ||
| Treatment pattern | |||||||||
| PD-1 | 24 | 3 | 4 | 4 | 13 | 29.2 | 0.003 | 4 | 0.032 |
| PD-1+chemotherapy | 26 | 2 | 10 | 6 | 8 | 46.2 | 7 | ||
| PD-1+chemotherapy+anti-angiogenic therapy | 52 | 9 | 24 | 7 | 12 | 63.5 | 12 |
Abbreviations: CR, complete response; mPFS(m), median progression-free survival (months); NR, not reached; ORR, overall response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 2.
Kaplan–Meier plot of progression-free survival (A) and overall survival (B) in all patients; Kaplan–Meier plot of progression-free survival by clinical factors, (C) histology, (D) time to recurrence, and (E) treatment pattern. NR, not reached; OS, overall survival; PD-1, programmed death-1; PFS, progression-free survival.
Figure 3.
(A) Duration of responses in patients who achieved an objective response. The swimmer plots show the time to first response and the duration of the first response. (B) Waterfall plot of the best percentage change in tumor size from baseline. CR, complete response; PR, partial response.
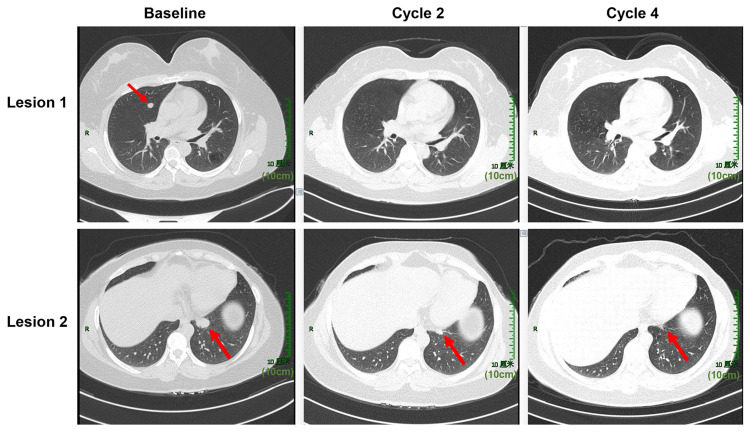
To explore the clinical factors that may improve the efficacy of PD-1, the relationship between clinical characteristics with ORR and PFS in patients is shown in Table 2. Significant differences in ORR and PFS were identified for patients with squamous cell cancer vs adenocarcinoma (ORR: 57.3% vs 33.3%, p = 0.032; PFS: 19 vs 5 months, p = 0.008), time to recurrence ≤ 6 months vs time to recurrence > 6 months (ORR: 31.8 vs 65.5%, p = 0.001; PFS: 5 months vs not reached, p < 0.001), first-line vs second-line therapy vs subsequent lines of therapy (ORR: 80.6% vs 39.5% vs 28.6%, p < 0.001; PFS: NR vs 7 months vs 4 months, p = 0.032), and anti-PD-1 monotherapy vs anti-PD-1 + chemotherapy vs anti-PD-1 + chemotherapy + anti-angiogenic therapy (ORR: 29.2 vs 46.2 vs 63.5%, p = 0.003; PFS: 4 vs 7 vs 12 months, p = 0.032). Multivariate analysis indicated that squamous cell cancer, time to recurrence > 6 months, and anti-PD-1 + chemotherapy + anti-angiogenic therapy were significantly associated with favorable ORR and PFS (Table 3). Lines of therapy were an independent positive factor for ORR but not for PFS. We also observed a tendency for longer PFS in patients with lung metastases and lymph node metastases only. Anti-PD-1 + chemotherapy showed superior ORR and PFS benefits over PD-1 monotherapy but without statistical significance. There was no significant effect of age, disease status, and previous therapy on ORR and PFS. Differences between these subgroups in OS need to be demonstrated by long-term follow-up. Figure 4 illustrates a case of effective treatment in a patient with squamous cell cancer who developed lung metastasis after 9 months of first-line systemic therapy and achieved PR after two cycles of anti-PD-1 + chemotherapy + anti-angiogenic therapy.
Table 3.
Multivariate Logistic Regression Analysis of Clinical Factors on ORR and Multivariate Cox Proportional Hazards Regression Analysis of Clinical Factors on PFS
| Variables | ORR | PFS | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | HR | 95% CI | P | |
| Histology | ||||||
| Squamous | Ref | Ref | ||||
| Adenocarcinoma | 0.306 | 0.099–0.943 | 0.039 | 2.022 | 1.076–3.802 | 0.029 |
| Location of metastasis | ||||||
| Lung/lymph node only | Ref | Ref | ||||
| Non-lung/lymph node | 0.580 | 0.192–1.754 | 0.335 | 1.786 | 0.819–3.896 | 0.145 |
| Time to recurrence | ||||||
| ≤ 6 months | Ref | Ref | ||||
| > 6 months | 3.277 | 1.200–8.947 | 0.021 | 0.287 | 0.144–0.574 | <0.001 |
| Lines of therapy | ||||||
| 1 | Ref | Ref | ||||
| 2 | 0.153 | 0.042–0.549 | 0.004 | 1.596 | 0.684–3.671 | 0.282 |
| ≥ 3 | 0.066 | 0.015–0.288 | <0.001 | 1.916 | 0.780–4.703 | 0.156 |
| Treatment pattern | ||||||
| PD-1 | Ref | Ref | ||||
| PD-1+chemotherpy | 0.922 | 0.214–3.966 | 0.913 | 0.722 | 0.315–1.653 | 0.441 |
| PD-1+chemotherapy+anti-angiogenic therapy | 3.759 | 1.095–12.130 | 0.035 | 0.451 | 0.214–0.947 | 0.040 |
Abbreviations: HR, hazard ratio; PFS, median progression-free survival; OR, odds ratio; ORR: objective response rate.
Figure 4.
Chest computed tomography images at baseline, cycles 2 and 4 are shown for a 49-year-old woman with squamous cervical cancer who developed lung metastasis after 9 months of first-line systemic therapy. She was treated with camrelizumab combined with paclitaxel, cisplatin, and apatinib for four cycles, followed by camrelizumab and apatinib as maintenance therapy. Tumor lesions in her lung clearly shrank after two cycles and were significantly reduced after four cycles.
Adverse Events
Adverse events occurred in 56 patients (54.9%), 16.7% of whom were grade 3–5 (Table 4). The most common adverse events were thrombocytopenia (14.7%), hepatic dysfunction (13.7%), anemia (11.8%), and leukopenia (11.8%). Only one patient died during treatment. This patient had postoperative vaginal residual recurrence with a large tumor, which regressed rapidly after PD-1 treatment. Death was caused by suddenly massive vaginal bleeding, which we suspected to have been due to tumor necrosis or the side effects of anti-angiogenic drugs. The immune-related adverse events observed in 27 patients (26.5%) were mostly hepatic dysfunction (12.7%), rash (8.8%), and hypothyroidism (7.8%). Three patients (2.9%) experienced grade 3–5 adverse events, including two patients with hepatitis dysfunction and one with myocarditis. These results indicated that PD-1 inhibitors were tolerable in the real world.
Table 4.
Treatment-Related Adverse Events in Total Patients
| Adverse Event | Any Grade n (%) | Grade 3–5 n (%) |
|---|---|---|
| Total | 56 (54.9) | 17 (16.7) |
| Thrombocytopenia | 15 (14.7) | 8 (8.8) |
| Hepatic dysfunction | 14 (13.7) | 4 (3.9) |
| Anemia | 12 (11.8) | 5 (4.9) |
| Leukopenia | 11 (10.8) | 3 (2.9) |
| Rash | 9 (8.8) | 0 (0) |
| Renal dysfunction | 8 (8.8) | 3 (2.9) |
| Hypothyroidism | 8 (7.8) | 0 (0) |
| Diarrhea | 7 (6.9) | 1(1.0) |
| Hemorrhage | 7 (6.9) | 1(1.0) |
| Hypertension | 6 (5.9) | 0 (0) |
| Fever | 6 (5.9) | 0 (0) |
| Hand-foot syndrome | 5 (4.9) | 0 (0) |
| Myalgia | 4 (3.9) | 0 (0) |
| Fistula | 4 (3.9) | 1 (1.0) |
| Fatigue | 4 (3.9) | 0 (0) |
| Vomiting | 3 (2.9) | 0 (0) |
| Hyperthyroidism | 2 (2.0) | 0 (0) |
| Reactive cutaneous capillary endothelial proliferation | 2 (2.0) | 0 (0) |
| Constipation | 2 (2.0) | 0 (0) |
| Myocarditis | 1 (1.0) | 1 (1.0) |
| Proteinuria | 1 (1.0) | 0 (0) |
| Thrombosis | 1 (1.0) | 1 (1.0) |
| Immune-related adverse event | ||
| Hepatic dysfunction | 13 (12.7) | 2 (2.0) |
| Rash | 9 (8.8) | 0 (0) |
| Hypothyroidism | 8 (7.8) | 0 (0) |
| Reactive cutaneous capillary endothelial proliferation | 2 (2.0) | 0 (0) |
| Myocarditis | 1 (1.0) | 1 (1.0) |
Adverse events of any grade were observed in 9 (37.5%) of 24 patients receiving PD-1 alone, 14 (53.8%) of 26 patients receiving PD-1 plus chemotherapy, and 32 (61.5%) of 52 patients treated with PD-1 plus chemotherapy and anti-angiogenic therapy (Table S1). Grade 3–5 adverse events occurred in 3 (12.5%), 4 (15.4%) and 10 (19.2%) patients, respectively. The most common adverse events in patients receiving PD-1 alone were hepatic dysfunction (12.5%) and anemia (12.5%). The most common adverse events attributed to PD-1 plus chemotherapy included thrombocytopenia (11.5%) and anemia (11.5%). Among patients receiving PD-1 combined with chemotherapy and anti-angiogenic therapy, the most common adverse events were thrombocytopenia (21.2%), hepatic dysfunction (17.3%), and leukopenia (15.4%). Adverse events in the three treatment groups were similar to those in overall patients.
Discussion
In the present study, PD-1 inhibitors showed effective antitumor activity in real-world populations with recurrent or metastatic cervical cancer. Squamous cell carcinoma, a time to recurrence > 6 months, or PD-1 plus chemotherapy and anti-angiogenic drugs were further independent prognostic predictors of outcomes. In addition, adverse events associated with the treatment were controllable.
Previous clinical studies reported that the ORR of recurrent or metastatic cervical cancer treated with immune checkpoint inhibitor monotherapy was 4–26%, and median PFS was 2–5.1 months.7,8,10–12,17,20 Compared with previous results, the ORR of PD-1 inhibitor monotherapy in our study was slightly higher. There are some possible reasons for this. First, in previous studies, PD-1 inhibitors were used as second-line or further-line therapy, whereas in our study, PD-1 was used as first-line, second-line, or further treatment. This supported our findings that PD-1 was more effective in patients treated in first-line therapy. Second, patients in previous studies were Caucasians or African Americans who used pembrolizumab, nivolumab, cemiplimab, or balstilimab, whereas the patients in our study were Chinese using camrelizumab or sintilimab. These differences in patient characteristics and PD-1 inhibitor drugs used could contribute to the different outcomes.
Several clinical trials are testing the efficacy of PD-1 inhibitor combination therapy for treating advanced cervical cancer. Single-agent anti-PD-1 balstilimab as monotherapy or in combination with anti-CTLA-4 zalifrelimab showed that both regimens were well-tolerated, and the responses were more common in patients with PD-L1 positive and squamous cell carcinoma cervical cancer.18 The present study also showed that patients with squamous cell carcinoma responded better than those with adenocarcinoma, consistent with the findings of previous studies. The expression of PD-L1 in tumor cells is relevant to the objective response to PD-1/PD-L1 inhibitors, with PD-L1 expression identified in 54%–80% of patients with squamous cell cervical cancer.21,22 We also found that patients with metastases restricted to lung and lymph nodes only had significantly better outcomes, demonstrating the association between the location of metastatic disease with immunotherapy response, consistent with a retrospective study by Miller et al.23 In addition, we observed that patients who relapsed within 6 months had a poor prognosis for PD-1 blockades. This suggested that patients with rapid progression were less likely to respond to immunotherapy, an issue that needs further investigation. The Camrelizumab Plus Apatinib (CLAP) trial reported an ORR of 55.6% for recurrent or metastatic cervical cancer, with a median PFS of 8.8 months; duration of response and overall survival were not reached.14 The KEYNOTE-826 study determined median PFS to be 10.4 months in a pembrolizumab group and 8.2 months in a placebo group in an intention-to-treat population that was also receiving chemotherapy with or without bevacizumab; overall survival at 24 months was 50.4 and 40.4%, respectively.9 These results are comparable to our findings. In the present study, we combined anti-PD-1 with chemotherapy or chemotherapy and anti-angiogenic drugs, showing an advantage of combination immunotherapy over immune checkpoint inhibitors alone, with chemotherapy and anti-angiogenic drugs providing significant additional therapeutic effects. For example, a 49-year-old patient diagnosed with squamous cell cervical cancer (FIGO stage IIB) was found to have lung metastases after 9 months of concurrent chemoradiotherapy, with genetic test results showing 0% PD-L1 expression (IHC), microsatellite stability (MSS), and a tumor mutational load (TMB) of 1.2 Muts/Mb. Although this patient had negative PD-L1 expression, stable microsatellites, and a low TMB, she was not a suitable candidate for immune monotherapy. We treated her with camrelizumab combined with paclitaxel, cisplatin, and apatinib for four cycles, followed by camrelizumab and apatinib as maintenance therapy. Tumors in her lung clearly shrank after two cycles and were significantly reduced after four cycles, and she tolerated treatment well. The favorable outcome of this case suggested that PD-1 combined with chemotherapy and anti-angiogenic therapy had synergistic and promising effects for the treatment of advanced distant metastatic cervical cancer. The clinical experience and data accumulated in this study further validated our hypothesis and helped us to screen a potential beneficiary population for immunotherapy. Other phase III trials for this combination therapy are currently underway, including the FERMATA (anti-PD-1 in combination with chemotherapy with bevacizumab)24 and BEATcc (platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab) studies.25 We expect that the findings from these clinical trials will confirm the findings of our study in the near future.
Anti-PD-1 treatment showed acceptable safety profiles, either alone or in combination with other therapies, consistent with previous studies. No new safety signals were observed.7,9,14 The frequency of any adverse event was 54.9% (n = 56), with the most common adverse effects being thrombocytopenia, hepatic dysfunction, anemia, and leukopenia. The incidence of thrombocytopenia, leukopenia, hemorrhage, hypertension, hand-foot syndrome, and fistulas was relatively higher in combination therapies than that in checkpoint inhibitor monotherapy, which might be attributable to additive toxicity when combining chemotherapy or anti-angiogenic therapy. However, most adverse events were grade 1–2 and could be ameliorated with supportive care, dose reduction, or time delay. Thus, anti-PD-1 antibodies appeared to be safe for use in cervical cancer.
We acknowledge that the present study has several limitations. First, the single-center and retrospective design of the study decreased the power to draw reliable conclusions and may have introduced bias. Second, only Chinese patients were included, limiting the generalizability of our findings. Third, biomarkers such as PD-L1 expression were not available. However, since the clinical benefit of PD-L1 expression has been shown to guide immunotherapy,26 we are currently investigating these biomarkers, which will be addressed in future work.
Conclusions
We evaluated the real-world effectiveness and safety of PD-1 inhibitor treatment for recurrent or metastatic cervical cancer. We demonstrated a positive therapeutic effect in improving the objective response rate and progression-free survival, particularly for patients with squamous cell cancer and a time to recurrence > 6 months treated with PD-1 inhibitors in combination with chemotherapy and anti-angiogenic therapy. However, further research is needed to evaluate the therapeutic effect of this treatment on overall survival, as well as to develop new treatment strategies for immune checkpoint inhibitors in advanced cervical cancer.
Funding Statement
National Natural Science Foundation of China (Grant No. 81974463).
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Statement
This retrospective study was approved by the Ethics committee of Wuhan Union Hospital (approval no.0047) and was conducted in accordance with the Declaration of Helsinki. All patients signed informed consent to participate in this study.
Author Contributions
All authors made substantial contributions to conception and design, collection and assembly of data, or analysis and interpretation of data; took part in manuscript writing; agreed to submit to the current journal; and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Gu W, Yang L, et al. NanoTechnology in the management of cervical cancer. Rev Med Virol. 2015;25(Suppl 1):72–83. [DOI] [PubMed] [Google Scholar]
- 4.Pfaendler KS, Tewari KS. Changing paradigms in the systemic treatment of advanced cervical cancer. Am J Obstet Gynecol. 2016;214(1):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. [DOI] [PubMed] [Google Scholar]
- 6.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frenel JS, Le Tourneau C, O’Neil B, et al. Safety and Efficacy of Pembrolizumab in Advanced, Programmed Death Ligand 1-Positive Cervical Cancer: results From the Phase Ib KEYNOTE-028 Trial. J Clin Oncol. 2017;35(36):4035–4041. [DOI] [PubMed] [Google Scholar]
- 8.Chung HC, Ros W, Delord JP, et al. Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Cervical Cancer: results From the Phase II KEYNOTE-158 Study. J Clin Oncol. 2019;37(17):1470–1478. [DOI] [PubMed] [Google Scholar]
- 9.Colombo N, Dubot C, Lorusso D, et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N Engl J Med. 2021;385(20):1856–1867. [DOI] [PubMed] [Google Scholar]
- 10.Naumann RW, Hollebecque A, Meyer T, et al. Safety and Efficacy of Nivolumab Monotherapy in Recurrent or Metastatic Cervical, Vaginal, or Vulvar Carcinoma: results From the Phase I/II CheckMate 358 Trial. J Clin Oncol. 2019;37(31):2825–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santin AD, Deng W, Frumovitz M, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol. 2020;157(1):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura K, Hasegawa K, Katsumata N, et al. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: multicenter, open-label Phase 2 trial. Cancer Sci. 2019;110(9):2894–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman CF, Snyder Charen A, Zhou Q, et al. Phase II study of atezolizumab in combination with bevacizumab in patients with advanced cervical cancer. J Immunother Cancer. 2020;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan C, Shen J, Wang Y, et al. Camrelizumab Plus Apatinib in Patients With Advanced Cervical Cancer (CLAP): a Multicenter, Open-Label, Single-Arm, Phase II Trial. J Clin Oncol. 2020;38(34):4095–4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rischin D, Gil-Martin M, González-Martin A, et al. PD-1 blockade in recurrent or metastatic cervical cancer: data from cemiplimab Phase I expansion cohorts and characterization of PD-L1 expression in cervical cancer. Gynecol Oncol. 2020;159(2):322–328. [DOI] [PubMed] [Google Scholar]
- 16.Tewari KS, Monk BJ, Vergote I, et al. VP4-2021: EMPOWER-Cervical 1/GOG-3016/ENGOT-cx9: interim analysis of phase III trial of cemiplimab vs. investigator’s choice (IC) chemotherapy (chemo) in recurrent/metastatic (R/M) cervical carcinoma. Ann Oncol. 2021;32(7):940–941. [Google Scholar]
- 17.O’Malley DM, Oaknin A, Monk BJ, et al. Phase II study of the safety and efficacy of the anti-PD-1 antibody balstilimab in patients with recurrent and/or metastatic cervical cancer. Gynecol Oncol. 2021;163(2):274–280. [DOI] [PubMed] [Google Scholar]
- 18.O’Malley DM, Oaknin A, Monk BJ, et al. Single-agent anti-PD-1 balstilimab or in combination with anti-CTLA-4 zalifrelimab for recurrent/metastatic (R/M) cervical cancer (CC): preliminary results of two independent phase II trials. Ann Oncol. 2020;31:S1164–S1165. [Google Scholar]
- 19.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. [DOI] [PubMed] [Google Scholar]
- 20.Choi MC, Kim YM, Lee JW, et al. Real-World Experience of Pembrolizumab Monotherapy in Patients with Recurrent or Persistent Cervical Cancer: a Korean Multi-Center Retrospective Study (KGOG1041). Cancers. 2020;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mezache L, Paniccia B, Nyinawabera A, Nuovo GJ. Enhanced expression of PD L1 in cervical intraepithelial neoplasia and cervical cancers. Mod Pathol. 2015;28(12):1594–1602. [DOI] [PubMed] [Google Scholar]
- 22.Heeren AM, Punt S, Bleeker MC, et al. Prognostic effect of different PD-L1 expression patterns in squamous cell carcinoma and adenocarcinoma of the cervix. Mod Pathol. 2016;29(7):753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller KM, Filippova OT, Hayes SA, et al. Pattern of disease and response to pembrolizumab in recurrent cervical cancer. Gynecol Oncol Rep. 2021;37:100831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Efficacy and Safety of BCD-100 (Anti-PD-1) in Combination With Platinum-Based Chemotherapy With and Without Bevacizumab as First-Line Treatment of Subjects With Advanced Cervical Cancer (FERMATA). Available from: https://clinicaltrials.gov/ct2/show/NCT03912415. Accessed 20 July 2019.
- 25.Grau JF, Farinas-Madrid L, Oaknin A. A randomized phase III trial of platinum chemotherapy plus paclitaxel with bevacizumab and atezolizumab versus platinum chemotherapy plus paclitaxel and bevacizumab in metastatic (stage IVB), persistent, or recurrent carcinoma of the cervix: the BEATcc study (ENGOT-Cx10/GEICO 68-C/JGOG1084/GOG-3030). Int J Gynecol Cancer. 2020;30(1):139–143. [DOI] [PubMed] [Google Scholar]
- 26.Nadal E, Massuti B, Domine M, Garcia-Campelo R, Cobo M, Felip E. Immunotherapy with checkpoint inhibitors in non-small cell lung cancer: insights from long-term survivors. Cancer Immunol Immunother. 2019;68(3):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]