Abstract
Introduction
Point of care ultrasound (POCUS) has become more common for rapid evaluation. Applications are limited by lack of training of users, difficulty maintaining ultrasound competencies, access to equipment for optimal imaging, and limitations in quality control. Such barriers exist in low-resource, underserved health care settings.
Objective
The aim was to explore the use of POCUS in under-resourced health care settings, such as rural and remote locations in Australia and other countries. Key variables include health outcomes, quality of care, service availability, examinations types performed, equipment used, who performs the examinations, and the ultrasound training received.
Methods
Literature was identified via CINAHL, Cochrane, Embase, Medline, PubMed, SCOPUS, and Web of Science, plus grey literature. Recommended guidelines were followed, and only research-based articles were included, with searches limited to English language and 2010–present.
Results
After screening, 23 articles were reviewed. No studies had low risk of bias and, overall, the quality was poor and only two studies used random sampling. The majority were from developing countries, with only one performed in Australia. Echocardiographic screening in schools was common. Others included emergency department (ED) patients, abdominal aorta screening, obstetric scans, and intensive care unit (ICU) management. Operators included ED doctors, medical students, nurses, community healthcare workers and general practitioners, who received limited training in protocol-driven scanning, often monitored by experts. In comparison to clinical assessment, standard ultrasound or other imaging, accuracy was of the order of 70–95%, depending on the condition, with high efficacy in improving patient care.
Conclusion
Lack of studies of POCUS in Australia and other developed countries suggests a need for further research. Current evidence supports use of limited ultrasound using portable machines in locations with limited access to diagnostic ultrasound performed by sonographers, which has the potential to improve health outcomes in under-resourced communities in Australia and elsewhere.
Keywords: point-of-care, POCUS, sonography, mobile, handheld, rural health
Introduction and Background
Ultrasound is a valuable imaging modality that has been used increasingly for a variety of diagnostic procedures in medicine since becoming more common place in the 1960s.1 While most commonly used for obstetric scans in the early days, its uses now extend beyond obstetrics into many facets of medicine. Through recent technological improvements, ultrasound machines have also become more portable, user friendly and affordable, including hand-held devices.2 Ultrasound examinations are usually performed by sonographers, health professionals with extensive training, or by radiologists and other specialist medical practitioners, somewhat limiting access and availability of the modality. As the technology has changed, however, access to ultrasound has broadened to include other practitioners with appropriate training and equipment.3,4 The advances have allowed ultrasound machines to be used by a range of health professionals at a patient’s bedside for a variety of examination types, increasing the diagnostic utility of ultrasound at the point-of-care.
As a consequence of the technological evolution, point of care ultrasound (POCUS) has become increasingly common, particularly in emergency medicine and in locations remote from mainstream, usual care.4 Scanning protocols suited to such applications and relevant training programs have been developed. Many undergraduate and postgraduate medical education programs now integrate some basic ultrasound training into the curriculum.5 Nevertheless, the application of POCUS is limited by several factors, including: lack of training of users in the scans they need to perform; shortage of time for practitioners to maintain their US competencies; access to the equipment, particularly the standard of ultrasound machines necessary for acquiring best possible images;6 and limited availability of maintenance, repair and quality control of the ultrasound devices and images. Barriers such as these are prevalent in low-resource or underserved health care settings, such as in rural and remote locations.7
The World Health Organisation (WHO) defines underserved areas as ‘geographical areas where populations have limited access to qualified health-care providers and quality health-care services’.8 Under that definition, the WHO includes
remote and rural areas, small or remote islands, urban slums, conflict and post-conflict zones, refugee camps, minority and indigenous communities, and any place that has been severely affected by a major natural or man-made disaster8.
As of 2020, approximately 44% of the world fits this description, making this a relevant issue across the world,9 including within developed countries. Much of rural and remote Australia, for example, fits the definition of being underserved, particularly by virtue of limited staffing, especially specialist medical and allied health service providers, lack of available facilities, equipment and health care resources, and relatively poor patient outcomes compared with urban populations.10,11
As in other countries, in Australia, rural or remote areas are classified as according to the government’s classification systems,12 which are used this to prioritise and incentivise staffing, including allocation of funding for education and training.13 Such incentives, however, do not generally extend to support for medical imaging practitioners.14 This can lead to limitations on the range of services and resources available for the variety of patient presentations that a rural health service faces. One measure to address these limitations involves the local health professionals, other than medical imaging practitioners, acquiring a broader skillset and extending their role beyond traditional professional boundaries. This usually requires a time and monetary commitment to undertake further training, on top of the cost of acquiring the necessary medical imaging equipment. There is a risk that, in order to bridge the affordability-gap, concessions may be made in training, as well as in equipment type, capability and quality, potentially leading to sub-optimal patient outcomes.
Previous Reviews
There have been other literature reviews performed evaluating various specific aspects of ultrasound use in resource-limited settings. Obstetric examinations were the focus of some recent reviews, which concluded that ultrasound has the potential to improve aspects of foetal, perinatal and maternal health.15,16 While the potential was recognised, appropriate support and monitoring were recommended to ensure positive outcomes. In both reviews, limited training of providers and inappropriate use of ultrasound were raised as significant concerns related to the use of ultrasound to determine foetal sex, inadequate image quality and consequent misdiagnosis. Those concerns are increased with the growing availability of low-cost portable ultrasound devices, particular when used by practitioners with limited knowledge and experience.
Two other reviews focused on the potential impact on ultrasound use of tele-communication technology.17,18 While the studies included were potentially biased due to self-reporting, both studies concluded that tele-ultrasound has a potential future role in imaging. Neither of those reviews had a specific focus on resource-limited settings, though both acknowledged the potential benefits in remote locations as transmission and reception limitations are overcome and the image quality of portable ultrasound devices improves. A further review explored the impact of tele-ultrasound in rural settings,19 though not specifically POCUS. Again, the quality of the evidence was lacking but the authors concluded that the image quality was satisfactory for diagnosis and patient management.
In further reviews, the search and exclusion criteria used led to a very specific field of interest and limited retrieval of relevant literature. Telford et al20 explored articles comparing standard echocardiography machines with handheld equipment, finding high levels of accuracy for handheld ultrasound in detecting definite rheumatic heart disease, though poor accuracy for detection of borderline disease. That review included metropolitan and rural areas, with more focus on proof of concept for handheld devices, rather than the potential benefit of portable devices in low-resource settings. Similarly, Marangou et al21 explored the use of echocardiography in Indigenous and underprivileged populations, highlighting the need for more accessible ultrasound technology for screening and diagnosis of disease in those populations. Both reviews focused on particular conditions and examination types, rather than the variety of uses of ultrasound.
Becker et al22 performed a systematic review in 2016 with a focus on portable ultrasound in low-and middle-income countries, as defined by the WHO, which included Argentina, Bangladesh, Nepal, South Africa, and Thailand. They found that ultrasound is used for a variety of patient presentations and conditions in low-resource areas, including screening for heart disease, various emergency and trauma presentations, and both within and outside the hospital setting. That review also reflected on the types of ultrasound machines being used. It was concluded that further higher quality research is needed to better inform the general use recommendations, as well as the scope and impact of handheld, portable ultrasound devices. That study, as well as others that delved into ultrasound use in underserved areas,23,24 provides relevant background relating to the use of ultrasound in resource-limited settings. However, those studies do not necessarily translate into the Australian rural and remote health care environment, which is the context of interest to the authors of this systematic review.
Aim of This Review
Building on the previous research above, this systematic review aims to explore the use of POCUS in relatively under-resourced health care settings, with particular reference to rural and remote contexts in developed countries, such as Australia. A range of study types will be included in the review, so comparison may be made with mainstream, conventional ultrasound service provision, including in metropolitan locations, or pre-existing services, which may include having no prior ultrasound services available. Principal outcome variables may include health outcomes, quality of care, and service access and availability, provided relevant data is reported. Other descriptive variables that will be collated include: the types of ultrasound examinations performed; the types of equipment used; who was performing the examinations; and, if not performed by a sonographer with formal tertiary education, the type of training received for their ultrasound imaging role.
Methods
This systematic review was registered with PROSPERO Centre for Systematic Reviews and Dissemination (ID: 273142; 09/09/21). The method used was based on the Cochrane Guidelines for Systematic Reviews25 and reporting is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA; 2020 Statement).26 Following development of the above study aim in PICO (Population, Intervention, Comparison and Outcomes)27 format and identification of relevant search terms, a consultation was held with a University Research Liaison Librarian to design a search strategy. Key search terms, with relevant truncations were linked using Boolean operators, as below:
(Ultraso*.mp. OR (exp ultrasound/) OR sonogr*.mp.) AND (rural.mp. OR resource.mp.) AND (access*.mp. OR avail*.mp.) AND (portable mp OR handheld.mp. OR pocket.mp. OR mobile.mp. OR (point-of-care.mp. OR (“point of care ultrasound”/)))
In order to minimise the risk of publication bias and the capture as many relevant studies as possible, searches were performed on seven electronic databases (CINAHL, Cochrane, Embase, Medline, PubMed, SCOPUS, and Web of Science). These were accessed via the University of Newcastle’s library between December 2020 and October 2021 by first author (LS), the last search having been performed on 16th of October 2021.
Variations of words or database syntax were occasionally required for an individual database, but the results reflected the search as intended. To address the use of “‘rural’ OR ‘resource’”, the authors discussed various terms and decided on these key words to best represent resource-limited environments. While this expanded search results to a variety of articles mentioning resources, it was deemed appropriate to ensure no articles were missed that addressed resource limitations in different words. As mentioned in the introduction, advances of ultrasound technology have provided more mobile, accessible, and affordable ultrasound units, particularly in the last decade or so. Becker et al found that most relevant articles for their review were published after 2010,22 so that time period was the focus of this review. An example of the search strategy is shown in Table 1, including the number of records identified with each search string. Data base search results were supplemented by a hand-searching references lists of peer-reviewed journal articles, especially other relevant reviews of literature. Grey literature was sourced and subject to the same limitations as database searching.
Table 1.
Embase via Ovid Search (Last Performed October 2021)
| Search | Keywords | Records |
|---|---|---|
| S1 | Exp ultrasound/ | 200,456 |
| S2 | Ultraso*.mp. | 678,821 |
| S3 | S1 or S2 | 678,821 |
| S4 | Sonogr*.mp. | 84,038 |
| S5 | S3 or S4 | 716,489 |
| S6 | Rural.mp. | 212,517 |
| S7 | Resource.mp. | 247,299 |
| S8 | S6 or S7 | 451,759 |
| S9 | S5 and S8 | 4505 |
| S10 | Access*.mp. | 798,212 |
| S11 | Avail*.mp. | 1,792,438 |
| S12 | S10 or S11 | 2,476,007 |
| S13 | S9 and S12 | 1275 |
| S14 | Portablemp. | 43,939 |
| S15 | Handheld.mp. | 9518 |
| S16 | Pocket.mp. | 60,874 |
| S17 | Mobile.mp. | 159,607 |
| S18 | Point-of-care.mp. or “point of care ultrasound”/ | 39,146 |
| S19 | S14 or S15 or S16 or S17 or S18 | 304,283 |
| S20 | S13 and S19 | 270 |
| S21 | Limit S20 to (human and English language and yr=“2010 -Current”) | 247 |
Note: *Truncation symbol for this search and will search all words with the preceding letters .mp. indicates a multi-purpose search across all fields.
Records from all databases, including electronic copies of abstract and full-text articles, were compiled using Endnote 20 software (Clarivate, Philadelphia: https://endnote.com/contact/) and then exported to Covidence (Veritas Health Innovation, Melbourne: https://www.covidence.org/). After removal of duplicates, title and abstract screening was carried out by both authors (LS and TS), with conflicts resolved by consensus. Inclusion and exclusion criteria were as shown in Table 2. Types of articles excluded were non-research-based reviews, case reports, editorials and discussion papers. Remaining eligible records then underwent full-text screening in Covidence, again by both authors and with conflict resolution, as necessary. Subsequently, all retained articles were subject to quality assessment using standardised risk of bias criteria based on the Cochrane RoB2 tool.28 Data extraction and synthesis used a template constructed in Covidence to record first author, year of publication, country of study, study type, study aims, population description, sample size study methods, type of ultrasound equipment used, provider training, and the results of the study.
Table 2.
Inclusion and Exclusion Criteria Used in This Systematic Review
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
• Point-of-care ultrasound used • Mobile ultrasound device used • Rural location • Low-resource setting • Study type - randomised control trial - cohort study - case controlled - cross-sectional |
• Pre-2010 • non-English language • non-human • No outcome measures • Not rural • Not portable US • Niche or irrelevant to Australian experience • Study type - case studies - not peer reviewed - report, opinion piece or editorial - surveys - reviews |
Results
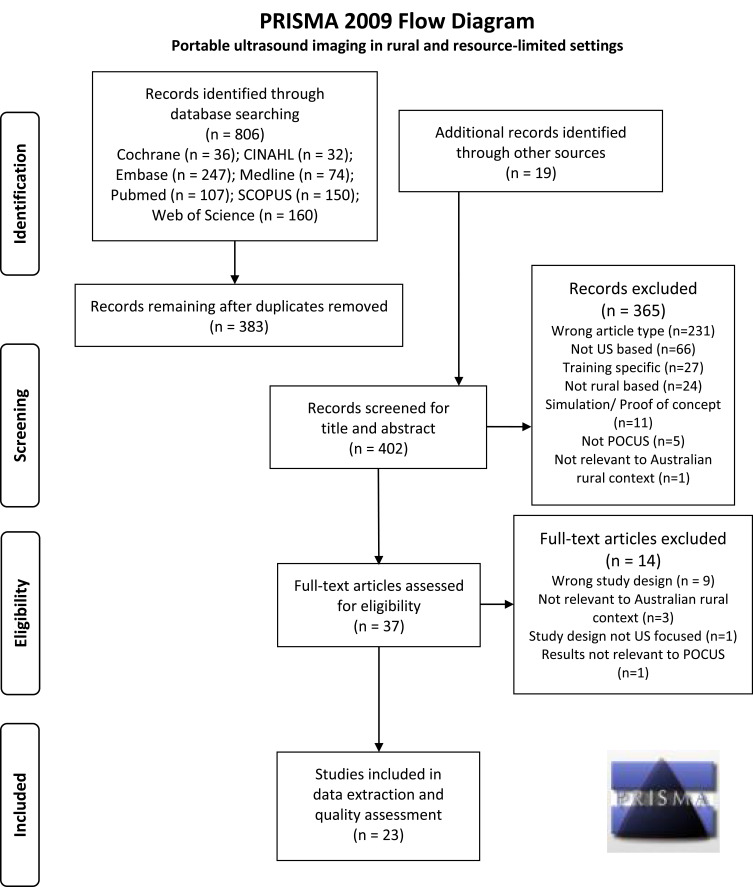
Figure 1 shows the PRISMA flowchart of the articles screened, with the initial searches of the various databases yielding 806 articles. After screening was completed, 23 articles remained eligible and were included for quality assessment and data extraction. The results of quality assessment are shown in Table 3. None of the studies had a low risk of bias in all seven assessment criteria of the RoB2 tool and, overall, the quality of studies was poor, with a high risk of bias being common. One study, by Ketelaars et al,29 was considered to have a high risk of bias in all categories. For two other studies, no criteria were considered to meet the expectations for low risk, although there was uncertainty for some criteria. Seven of the studies appeared to have low risk of bias in a majority of the criteria but none had low risk in all categories.
Figure 1.
PRISMA flow diagram showing the process used and records included and excluded at the various stages of this systematic review.
Notes: Adapted from Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode26.
Table 3.
Risk of Bias Quality Assessment of Studies Included in This View, According to the Cochrane RoB2 Criteria.28
| Study Author and Year | Assessment Criteria | ||||||
|---|---|---|---|---|---|---|---|
| Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Sources of Bias | |
| Amatya et al. 201843 | High | High | Unsure | Low | Unsure | Unsure | High |
| Baker et al. 202146 | High | High | High | High | Low | High | High |
| Beaton et al. 201434 | High | Low | Low | Low | Low | Low | High |
| Beaton et al. 201535 | Low | Low | High | Low | Low | Low | High |
| Bhavnani et al. 201840 | Low | Low | High | Low | Low | Low | High |
| Blois 201233 | High | High | High | High | Low | Low | High |
| Chavez et al. 201541 | High | High | Low | Low | Low | Low | High |
| Dalmacion et al. 201842 | Low | High | High | High | Low | High | Low |
| Godown et al. 201536 | Low | Low | High | Low | Low | High | High |
| Ketelaars et al. 201329 | High | High | High | High | High | High | High |
| Kissoon et al. 202044 | High | High | High | High | Low | Low | High |
| Lu et al. 201537 | Low | High | High | Low | Low | Low | High |
| Nixon et al. 20187 | High | High | High | High | Unsure | Unsure | High |
| Nixon et al. 201832 | High | High | High | Low | High | Unsure | High |
| Ploutz et al. 201630 | High | Low | High | Low | Low | Low | High |
| Pontet et al. 201931 | Low | Low | Unsure | High | Low | Low | High |
| Prager et al. 201847 | High | High | High | High | Unsure | Unsure | High |
| Reynolds et al. 201845 | High | High | High | High | Low | Low | High |
| Roberts et al. 201438 | High | High | High | Low | Low | Low | High |
| Singh et al. 201339 | High | High | High | Low | Unsure | Unsure | High |
| Smith et al. 20106 | High | High | High | High | Low | Low | High |
| Umuhire et al. 201948 | High | High | High | High | Low | Low | High |
| Zanatta et al. 201549 | High | High | High | High | Unsure | Unsure | Unsure |
The results of the data extraction are shown in Table 4, with details expanded upon below. Given the focus on resource-limited regions, the majority of the studies were performed in developing countries. Only one study had been performed in Australia. Most were cross-sectional and descriptive cohort studies, of which four were retrospective and the rest prospective. There was one prospective comparative study, by Ploutz et al,30 but only two studies employed some form of random sampling, while only one, by Pontet et al,31 had a control group comparator. In total, there were 18,893 patients or study subjects, taking into account that Nixon et al reported two studies with different outcomes measures on the same study cohort.7,32 In total, about 4000 of the study subjects (21%) were school children, most being in screening studies for cardiac abnormalities.
Table 4.
Summary of the Characteristics of the Studies Included in This Systematic Review Following Data Extraction
| Author (s); Journal; Year; Country | Study Types and Relevant Aims | Population; Sample | Method; Equipment; Provider Training | Results |
|---|---|---|---|---|
| Amatya et al; Int J Emerg Med; 2018; Nepal43 |
Prospective cross-sectional study: ● Evaluate POCUS sensitivity for dx of pneumonia compared with CXR; ● Assess reliability of clinicians’ image interpretation. |
● Patients suspected of pneumonia attending ED at Patan Hospital; ● Convenience sample of 62 pts, 44 diagnosed with pneumonia on CT. |
● Patients evaluated with bedside lung US, chest X-ray, with CT as the gold standard; ● Sonosite M Turbo US machine; ● Emergency physician trained in US |
● Lung US = 91% sensitivity and 61% specificity; ● CXR = 73% sensitivity and 51% specificity (p=0.01); ● Inter-rater reliability between expert reviewer and sonographer was 0.79. |
| Baker et al; Tropical Doctor; 2021; Uganda46 | Cross-sectional study: ● Assess POCUS effectiveness at mobile outreach clinics by: ◦ evaluating type of examinations performed; ◦ how often findings altered pre-test Dx & Tx plan. |
● Patients presenting to mobile health clinics in Masindi region of Western Uganda; ● Convenience sample of 144 pts, with 177 scans performed. |
● Scans deemed necessary and questionnaire completed by attending physician; ● Philips Lumify US probe attached to Samsung Galaxy tablets; ● Clinics staffed by American board-certified physicians + 2nd and 3rd year residents. Two had completed emergency US fellowships. |
● Most frequent scans were: cardiac, obstetric, abdominal, MSK/ST, biliary, urinary tract, pleural, testicular, aorta, thyroid, eFast, FASH; ● In 73%, diagnosis either confirmed (50%) or changed (23%); ● In 53%, US findings changed Tx plan. |
| Beaton et al; J of the American Society of Echocardiography; 2014; Uganda34 | Prospective observational study: ● Determine sensitivity and specificity of focused echocardiography with HAND US versus STAND US units |
● Run in Kampala, Uganda; ● 125 participants in the comparison study: ◦ 60 part of a rheumatic heart disease (RHD) follow-up; and ◦ 65 asymptomatic children for a screening study. |
● Scanned by HAND & STAND US machines with WHF 2012 echocardiography protocol. Images interpreted separately and findings compared; ● HAND performed with GE Vscan versus STAND GE Vivid-I; ● Paediatric cardiologist performed all examinations. |
● HAND = 90.2% sensitivity and 92.9% specificity for detecting RHD; ● HAND overestimated mitral valve morphologic abnormalities; ● False-negative results (n = 4) due primarily to underestimation of mitral regurgitation length. |
| Beaton et al; Eur Heart J Cardiovasc Imaging; 2015; Uganda35 | Prospective Comparative study: ● Evaluate performance of HAND compared to STAND for early diagnosis of rheumatic heart disease (RHD) in a field screening study. |
● Students from 5 schools in Gulu, Uganda; ● 1420 children scanned, either randomly selected (10%) or previously diagnosed with RHD. |
● STAND, then HAND GE Vivid Q/I & Philips CX-50; ● HAND performed with GE Vscan; ● Performed by 5 paediatric cardiologists, 4 paediatric cardiology fellows and 3 senior echo technologists. |
● HAND = 78.9% sensitivity and 87.2% specificity for RHD compared to STAND. ● 97.9% sensitive for definite RHD; ● Inter- and intra-reviewer agreement ranged between 66–83 and 71.4–94.1%, respectively. |
| Bhavani et al; JACC Cardiovasc Imaging; 2018; India40 | Randomised trial: ● Compare outcomes of mHealth (smartphone-connected devices and pocket echocardiography) versus standard echocardiography for heart disease. |
● Heart disease clinics in Bangalore; India ● All participants had known structural heart disease ● 253 participants randomised: ◦139 to mHealth; ◦114 to standard care. |
● All patients had comprehensive echocardiographs; ● GE Vscan, GE Vivid-E9 & Philips ie33 used for m-Health; ● mHealth by local physicians; ● Sonographers performed standard echograms. |
● mHealth group had: ● shorter referral time for interventions; ● increased probability of interventions; ● lower risk of hospitalisation/death. ● Could be due to over-diagnosis in initial scans. |
| Blois; Can Fam Phys; 2012; Canada33 | Prospective cross-sectional study: ● Evaluate safety and efficacy of US screening for abdominal aortic aneurysm (AAA) by rural family physician. |
● Study performed in Grand Forks and Revelstoke, British Columbia, Canada. ● 45 participants screened ● Participants recruited if at least 1 risk factor for AAA. |
● Portable US aortic screening with Sonosite TITAN US machine; ● Follow-up by US expert using hospital US equipment; ● Screening performed by GP and follow-up by Sonographers. |
● Sensitivity & specificity of GP scans = 100%; ● The mean absolute difference between scan diameter = 0.2cm; ● Correlation = 0.81 (high) |
| Chavez et al; Lung; 2015; Nepal & Peru41 | Prospective cross-sectional study: ● Assess agreement between WHO pneumonia algorithm and POCUS for pneumonia; ● Determine feasibility of POCUS by GPs in for diagnosis of pneumonia. |
● Performed in Sarlahi District, Nepal & Lima, Peru; ● 378 patients; ◦127 controls; ◦82 without clinical pneumonia; ◦169 with pneumonia. |
● Chest US using international protocols for lung consolidation; ● SonoSite MicroMAxX & M-Turbo US machines used; ● GPs performed scans after US course developed and delivered. |
● WHO algorithm had 69.6% sensitivity and 59.6% specificity; ● Positive & negative likelihood ratios of 1.73 and 0.51 for pneumonia on POCUS; ● Inter-observer agreement for POCUS interpretation between GPs was high. |
| Dalmacion et al; BMC Pregnancy & Childbirth; 2018; Philippines42 | Prospective Cross-Sectional Study: ● Assess benefits of pregnancy US for early detection of maternal/neonatal conditions; ● Estimate agreement between trainees and trainers, and hand versus reference US scans; ● Estimate maternal and neonatal deaths averted by prenatal handheld US. |
● Performed in Parañaque City & Tagum city; ● 460 participants randomly selected from a list of pregnant women at 20–24 weeks. |
● Obstetric scan assessed: number of foetuses; foetal viability; presentation; placenta location; amniotic fluid volume. ● Confirmation by trainers; ● Handheld US by GE Vscan with GE Logic Prem as reference; ● Community healthcare workers undertook 3 training modules; ● Expert trainers and sonologist. |
● 146 (31.7%) showed abnormalities; ● Approx. 95% agreement between trainees and trainers; ● 99% agreement between Handheld and reference scan; ● Handheld US could have possibly averted 6.3% maternal deaths and 14.6% neonatal deaths. |
| Godown et al; Pediatrics; 2015; Uganda36 | Observational Cross-sectional Study: ● Determine incremental value of HAND over auscultation to detect rheumatic heart disease (RHD). |
● Performed in 5 different schools in Gulu, Uganda; ● 1317 participants out of 4773 screened; ◦Children aged 5–17 years; ◦Any child with RHD indications plus randomly selected group of normal. |
● Initial screened with STAND; ● Subset scanned with HAND & auscultation; ● Compared using STAND; ● HAND used GE Vscan; ● STAND used GE Vivid Q/I & Philips CX-50; ● Performed by paediatric cardiologists, senior cardiology fellows or sonographer. |
● Sensitivity of HAND = 97.8% for definite RHD, borderline or definite = 78.4% and pathologic aortic insufficiency = 81.8%; ● Sensitivity of auscultation was 22.2%, 16.4% and 13.6% respectively. |
| Ketelaars et al; J Emerg Med; 2013; Netherlands29 | Retrospective cross-sectional study: ● Evaluate impact of US on treatment plan in a Helicopter Emergency Medical Service (HEMS). |
● Performed by Dutch HEMS across 3895 miles 2 service area in eastern Netherlands; ● 281 patients with US as deemed necessary by HEMS team. |
● Scans of the chest and abdomen following with standardised method including thorax, heart, pericardium and aorta; ● Sonosite Micromaxx; ● Doctors trained in emergency US |
● 326 examinations performed on 281 (11%) patients; ● Treatment plan changed in 60 (21%) patients. |
| Kissoon et al; PLoS ONE; 2013; Guyana44 | Cross-sectional observational descriptive analysis; ● Quantify use of US & impact on care in local emergency department (ED). |
● Performed in ED at Georgetown Public Hospital; ● 173 patients with 426 individual scans performed; ◦ Patients deemed needing US by attending physician. |
● Data recorded on each US exam, including further treatment and disposition; ● POCUS equipment unspecified; ● Scans performed by Registrars, Residents & General Medical Officers. |
● 196 (46%) of studies positive; ● US changed final patient disposition for 276 (64.8%); ● US aided the decision to admit (22.8%), discharge (22.8%), specialist review (14.3%) and surgery (23.4%). |
| Lu et al; J Amer Soc of Echo; 2015; Uganda37 | Prospective cross-sectional study: ● Determine best simplified screening criteria for rheumatic heart disease (RHD) using HAND. |
● Performed in 5 different schools in Gulu, Uganda; ● 1439 participants for HAND: ◦ 447 randomly assigned, 992 for indications; ◦ Children aged 5–17 years; ◦ Children with RHD indications, plus randomly selected group normal. |
● Initial screening with STAND using GE Vivid Q/I or Philips CX-50; ● Subset underwent HAND with GE Vscan; ● STAND & HAND performed by paediatric cardiologists, fellows, and experienced sonographers. |
● Combined criteria of mitral regurgitation jet length ≥ 15cm or any aortic insufficiency sensitivity = 73.3% and specificity = 82.4%; ● Sensitivity for definite RHD = 97.9%; ● With prevalence of 4%, subsequent STAND of positive HAND studies would reduce STAND by 80%. |
| Nixon et al; J Prim Health Care; 2018; New Zealand7 |
Retrospective cross-sectional study: ● Characterise POCUS use in Rural Hospitals, with focus on volume and range of studies; ● Secondary aim of providing perspective of participating doctors. |
● Performed at 6 rural hospitals; ● 1014 patient results included; ● Patients deemed needing US by the attending physician. |
● Form retrospectively completed with patient details and findings; ● POCUS equipment unspecified; ● All participating doctors were senior rural generalists with formal POCUS training. |
● Most common scans: cardiac (18%); IVC/JVP (14%); gallbladder (13%); renal (11%); FAST (7%); bladder (6%); leg veins (6%); and lungs (5%); ● Doctors considered POCUS had a positive, significant effect due to diagnostic certainty; ● Challenges included: skills maintenance; lack of system for set-up; lack of quality control. |
| Nixon et al; Aust J Rural Health; 2018; New Zealand32 | Retrospective cross-sectional study: ● Assess POCUS skills of rural doctors in obtaining and interpreting images; ● Secondary aim to assess impact of POCUS on diagnostic decision making and patient management. |
● Study performed in 6 rural hospitals of NZ; ● 1014 patients, with 1248 examinations; ● Patients deemed needing US by the attending physician. |
● Form retrospectively completed with patient details and findings; ● POCUS equipment unspecified; ● All participating doctors were senior rural generalists with formal POCUS training. |
● 90% of images correctly interpreted compared to formal imaging or final diagnosis; ● 87% of scans contributed to or changed the diagnosis; ● 4% reduction in patients needing admission or transfer larger hospital; ● 71% POCUS scans had positive impact on patient care; ● 3% scans had potential for harm. |
| Ploutz et al; Heart; 2016; Uganda30 | Prospective cross-sectional comparative study: ● Evaluate performance of simplified echo screening by non-experts. |
● Two public primary schools in Gulu participated; ● 1002 primary students screened, with 956 paired exams in the final study. |
● HAND using GE Vscan by non-experts and STAND with GE Vivid Q by experts; ● HAND studies performed by 2 nurses with intensive training; ● Cardiologists performed STAND. |
● 95.5% of children were normal; ● 33% with borderline RHD & 12% with definite RHD; ● Simplified approach had sensitivity = 74.4% and specificity = 78.8% for any RHD; ● Sensitivity for definite RHD = 90.9%. |
| Pontet et al; US J; 2019; Uruguay31 | Prospective randomised control trial: ● Analyse diagnostic and treatment implications of POCUS-driven protocol in first 5 days of Intensive Care Unit (ICU) admission. |
● Two major referral hospitals in Montevideo; ● 80 participants, randomised in two groups, POCUS versus control; ● All patients were 18 years or older and required mechanical ventilation on ICU admission. |
● POCUS group underwent standardised US-driven protocol; ● Control group received conventional management. ● GE Logiq-e US used; ● US-trained Intensivist performed POCUS. |
● POCUS group used fewer resources in first 5 days in ICU; ● POCUS gave better characterisation of admission diagnosis in 35% and changed in management in 60%; ● POCUS group had lower fluid balance at 48 and 96 hours post-admission and less ventilation (51±57 days vs 88±94). |
| Prager et al; Prehosp & Disaster Med; 2018; Canada47 | Retrospective cross-sectional study: ● Characterise the use of POCUS at a remote, multi-day music festival; ● Secondary aim to consider impact on patient care and resource utilisation. |
● Study performed in Pemberton, British Columbia; ● When available, POCUS used on 28 patients from the 686 who presented. |
● Patients evaluated by physicians and POCUS performed as necessary; ● Structured survey of patient and scan details completed; ● GE Vscan was used; ● Physicians self-reported US training/experience - 9 novices, 6 intermediates, and 2 advanced. |
● POCUS narrowed diagnosis in 64% of cases, altered diagnosis in 21%, and changed management in 39%; ● Burden on resources utilization reduced in 46% of cases; ● Absolute risk reduction of 1.3% for hospital transferred patients. |
| Reynolds et al; PLoS ONE; 2018; Tanzania45 | Prospective cross-sectional study: ● Characterise utilisation and impact of POCUS on ED clinical decision-making. |
● Performed at Muhimbili National Hospital; ● POCUS performed on 784 pts, with 986 studies completed; ● Any ED patient requiring US was included, when available, if research assistant also present. |
● POCUS performed with protocols used by all providers; ● Patient & scan details recorded; ● Sonosite mTurbo used; ● Doctors performing scans included 12 specialists, 10 residents and 17 registrars. |
● Cases included trauma, abdo/ pelvic pain, respiratory signs; ● Most common scans were eFAST, cardiac, female pelvic studies; ● POCUS changed diagnosis or management in 29% of cases; ● Rates of change increased to 45% if patients had multiple POCUS studies performed. |
| Roberts et al; Circulation; 2014; Australia38 | Prospective cross-sectional study: ● Define prevalence of RHD in remote Indigenous children; ● Compare echo findings with non-indigenous children in the same region. |
● Performed in Northern & Central Australia; ● 5237 scans performed, with 4999 included in the study; ● High-risk cohort included Indigenous children in remote areas; ● Low-risk cohort from local cities with above average socio-economic status. |
● Basic demographic details obtained and abbreviated echo protocol used on GE Vivid e/I US machines; ● If any abnormality, a more intensive echo performed; ● Scans reported blindly by cardiologists; ● Experienced cardiac sonographers performed echoes. |
● Of 3946 high-risk children, 34 met WHF criteria for definite RHD with prevalence of 86/1000; ● 66 were borderline RHD, with prevalence of 167/1000; ● Of 1053 low-risk children, none met the criteria for definite RHD, and 5 for borderline RHD. |
| Singh et al; J Amer Soc of Echo; 2013; India39 | Prospective cross-sectional study: ● Assess feasibility of focused echo studies with long-distance assessment of images for patients with cardiovascular disease. |
● Performed in a remote rural community in northern India; ● Patients recruited for study if symptomatic; ● 1021 US exams interpreted. |
● Protocol driven echocardiographic studies performed and uploaded to web-based platform for remote interpretation; ● GE Vscan & Vivid I/Q US used; ● Volunteer expert sonographers. |
● Median time to interpretation was 11:44 hours; ● 207 scans (20.3%) had minor and 170 (16.7%) major abnormalities. |
| Smith et al; South African Med J; 2010; South Africa6 | Prospective observational Study: ● Assess use and accuracy of an existing US machine for FAST scanning in an emergency department (ED). |
● Performed in rural KwaZulu-Natal, Nqwelezane Hospital; ● 72 scans included; ● Included blunt or penetrating thoracic or abdominal trauma, if a trained doctor was available. |
● FAST scan performed, with findings confirmed by either CT or laparotomy; ● Aloka SSD 500 B-scan machine; ● Three ED doctors accredited for FAST performed the scans. |
● 72 FAST scans, 52 for blunt and 20 for penetrating trauma; ● 20.8% were positive; ● FAST had 100% specificity and overall sensitivity = 71.4%; ● 81.3% sensitivity for blunt, and 62.5% for penetrating injuries. |
| Umuhire et al; US J; 2019; Rwanda48 | Prospective cross-sectional observational study: ● Determine proportion of acute dyspnoea cases where US changed the diagnosis; ● Determines if multi-organ US improve accuracy and confidence in dyspnoea. |
● Performed at University Teaching Hospital of Kigali; ● Patients presenting to the ED with breathlessness; ● 100 patients scanned, 99 used in final results. |
● Physical examination performed; ● Another doctor performed Heart, IVC, Lungs, FASH & DVT US, with SonoSite M-Turbo US machine; ● Lead ‘sonographer’ had received US training, was enrolled in US fellowship and had passed ultrasound clinical skills assessment. |
● Most common diagnoses were acute decompensated heart failure (26.3%) and pneumonia (21.2%); ● US altered diagnosis in 66% of cases; ● Physician diagnostic accuracy increased from 34.7% to 88.8%; ● Mean diagnostic confidence increased from 3.5 to 4.7 (Likert). |
| Zanatta et al; Emerg Care J; 2015; Italy49 | Cross sectional observational study: ● Evaluate role of Critical Care Ultrasound (CCUS) on diagnostic and therapeutic procedures in the emergency department. |
● Cazzavillan Hospital, Arzignano; ● 241 patients scanned; ● Patients presenting with: shock, cardiac arrest, dyspnoea, non-traumatic chest pain, abdominal pain, abdominal or chest trauma, and suspected DVT. |
● Initial clinical assessment then POCUS performed by the same physician; ● Esaote MyLab 30 US machine; ● Operators were certified emergency physicians who had completed competency training for Ultrasound in Life Support. |
● Final diagnosis compared with CCUS vs clinical assessment was respectively (P=0.014): ◦ dyspnea - 82.5% vs 49.1%; ◦ thoracic pain - 71.9% vs 40.6%; ◦ abdominal pain - 76.2% vs 45%; ◦ suspected DVT - 80.0% vs 43.6%; ◦ shock - 80.0% vs 20%. ● eFAST correctly ruled out trauma complications in 81.1%. |
Abbreviations: CXR, Chest X-ray; CT, Computed Tomography; DVT, Deep Vein Thrombosis; Dx, Diagnosis; ED, Emergency Department; (e) FAST, (extended) Focussed Assessment with Sonography in Trauma; FASH, Focussed Assessment with Sonography for HIV-associated tuberculosis; GP, General Practitioner; HAND, Hand-held US unit; IVC/JVP, Inferior Vena Cava/Jugular Venous Pulse; MSK/ST, Musculoskeletal/Soft Tissue; STAND, Portable US unit; Tx, Treatment; US, Ultrasound; WHF, World Heart Foundation; WHO, World Health Organisation.
Setting and Context
As mentioned above, most studies were performed in developing countries that face challenges related to limited healthcare resource access and availability, including limited access to medical imaging services. As shown in Table 4, eight of those studies were performed in Africa, six in Uganda alone, while others were based in Nepal, India, The Philippines and South America. Another eight studies were performed in rural and remote regions of countries with a westernised health care system, in Canada, Europe, Australia and New Zealand.
Although cardiac screening in schools was reported in several studies, most studies took place in hospitals, usually in the emergency department (ED), or in other health care settings, such as General Practice (GP) clinics. One study looked at the use of POCUS in a helicopter retrieval service, finding that its use changed treatment plans in 21% of patients.29 The ED studies commonly investigated the diagnostic accuracy of the ultrasound examinations compared with other diagnostic tests, as well as effects on patient management and disposition for definitive care. The one prospective randomised control trial investigated the use of ultrasound in the Intensive Care Unit (ICU) of two major referral hospitals and found lower overall utilisation of resources for the POCUS cohort compared to the control group.31 In that study, while mortality rates were the same in both cohorts, there was a decreased ICU length of stay in the POCUS group.
In 52% (12/23) of the studies, ultrasound equipment was provided specifically for the purpose of the study, reflecting a lack of access under normal circumstances. This is assumed to have been the case for the screening studies, particularly for those performed in schools. In six studies, previous ownership of equipment was either stated or implied, although in other studies no mention was made of how the ultrasound equipment was acquired.
Examination Types
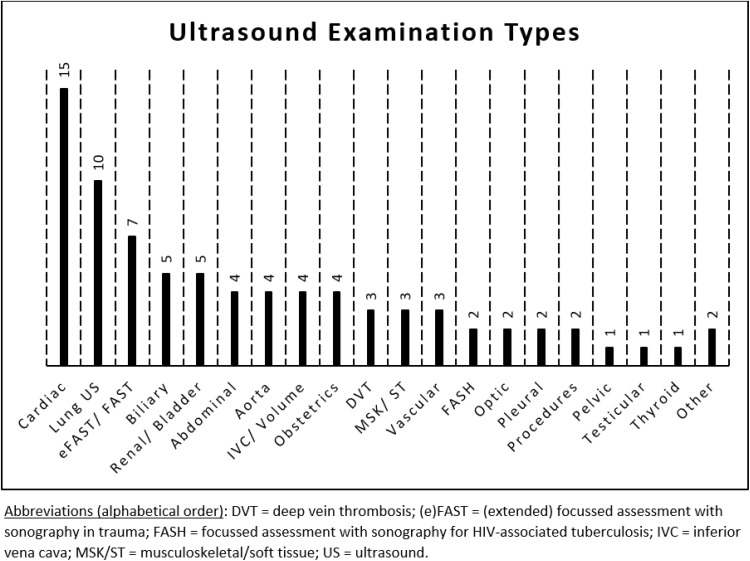
There was a broad range of ultrasound examination types performed across the different studies. Most studies used POCUS for ED patients (10/23) or for echocardiography (8/23), while other examinations types included lung ultrasound (2/23), abdominal aorta screening (1/23), obstetric scans (1/23), and ICU diagnosis and management (1/23). Many of the studies involved multiple examination types, with a summary shown in the bar chart in Figure 2, cardiac scanning being the most common, documented in 15 different studies.
Figure 2.
Barchart showing the range of ultrasound examination types and the number of articles in this systematic review that included each type of examination.
Diagnostic accuracy and utility were generally high. All screening studies were able to successfully confirm or exclude the conditions of interest compared to a gold standard. For example, Blois performed aortic screening at a GP clinic, comparing the findings with those for an examination performed by a trained sonographer and found 100% sensitivity and specificity.33 Other screening studies were echocardiographs that compared findings for different patient presentations, scanning protocols, operators, or equipment and comparisons.30,34–40 Patients who screen positively for the abnormality in question underwent appropriate diagnostic follow-up and treatment.
Practitioners and Training
There was a broad range of POCUS imaging providers, with various prior qualifications and experience, including 10 studies involving ED doctors with varying levels of experience. Other studies included medical students, nursing staff, community healthcare workers and general practitioners (GPs). The screening studies were performed or closely monitored by experts, who were sonographers, specialist echocardiographers, paediatric cardiologists, cardiology specialist trainees or experienced ICU specialists.
Training of ultrasound providers for specifically for POCUS was described in four of the articles, which included: nurses with limited ultrasound experience trained in a echocardiography protocol;30 GPs undertaking standardised training course for detection of pneumonia;41 community health care workers receiving device-specific training in obstetric scanning;42 and, local physicians training in the use of a pocket ultrasound device for echocardiography using a previously published protocol.40 Training courses used either classroom or computer-based learning modules, taught by specialists, with a practical component completed under supervision. Studies reported high rates of success. For example, the study by Dalmacion et al42 compared the finding of obstetric scans performed by community health workers using handheld portable ultrasound equipment with examinations performed by experts, finding 99% agreement. Significantly, the authors of that study concluded that POCUS had the potential to avoid an estimated 6.3% of maternal and 14.6% of neonatal deaths.42
Outcomes Measures
The results of each study in terms of various outcome measures are shown in the far-right column of Table 4. In several descriptive studies, the outcome was reported simply in terms of the types of examinations performed. Other studies reported the sensitivity and specificity of POCUS compared to other diagnostic examinations, such as auscultation,36 CT,43,44 specialist consultation and surgery.45 Several studies reported comparisons between different types of ultrasound equipment, such as hand-held (HAND) compared to standard mobile units (STAND) or between portable units and conventional ultrasound performed by sonographers or other experts.
Overall, there were conclusive benefits from the use of POCUS in a variety of clinical contexts. In the ED, it has been found that focussed assessment with sonography in trauma (FAST) had 81% sensitivity for detection of free intra-abdominal fluid in blunt trauma and 63% sensitivity in penetrating injuries, while the specificity was 100%.6 Also in the ED setting, Amatya et al43 concluded that lung ultrasound had a better sensitivity and specificity than plain chest radiographs, while in the studies by Chavez et al41 lung ultrasound performed by GPs using a standardised protocol had 70% sensitivity and 60% specificity.
Changes to patient management pathways was also used as an outcome measure in some studies,29,44–49 as confirmation or a change in diagnosis has potential to alter treatment and disposition of patients. Again, the findings of the individual studies are given in Table 4; however, it was found that POCUS contributed to diagnosis and management in as much as 87%32 or changed the final disposition of patients in up to 65% of cases.44
Various methods were used to assess the quality and accuracy of examinations and in most studies (17/23) images were assessed by experts. In other studies, such as that by Pontet et al.31 POCUS was performed to monitor ventilated patients in ICU, with scans performed by an intensive care physician, so diagnosis and image quality were less relevant, with the emphasis instead on changes in patient condition in comparison to initial imaging on admission to ICU. The expertise in several studies involved follow-up scans performed by sonographers using more sophisticated ultrasound equipment, which common in the screening studies, and interpretation of images by expert trainers, clinical specialists or sonologists. Nixon et al32 used experts to evaluate diagnostic image quality and according to those experts the rural generalist doctors who performed the POCUS examinations correctly interpreted 90% of the images compared with formal imaging or the final diagnosis.
Discussion
The aim of this review was to investigate the use of portable ultrasound imaging devices in under-resourced healthcare contexts, particular where comparisons may be drawn to rural and remote healthcare settings in Australia, the country of origin of the review. The study was motivated by, firstly, a perception that the use of ultrasound performed by non-sonographers has proliferated in recent times and, secondly, that ultrasound has potential to appreciably increase diagnostic capabilities in locations where service access and availability is limited and, thus, improve patient outcomes. In fact, only one of the studies included in this review was performed in Australia,38 although others were performed in other developed countries. Most of the studies were performed in under-resourced settings in developing countries. The challenges facing low-resource settings in healthcare transcend country borders, however, with accessibility and resource constraints being a general limitation to health outcomes for populations in poorly resourced regions of the world.50 The lack of evidence for the use of POCUS in rural and remote Australia may be reflective of a lack of research in this field, rather than a lack of the use of ultrasound in diagnosis. It is, therefore, suggested that future research should explore questions related to the use the POCUS in rural and remote areas of Australia and other developed countries, in order to fill the apparent gap in the literature.
As in Figure 2, a wide variety of ultrasound examination types are performed using POCUS in the settings included in this review, often using prescribed protocols and targeting particular conditions. Cardiac examinations were the most common, particular echocardiographic screening studies, although cardiac scans were also performed emergency departments for various patient presentations. Screening studies were mainly performed in developing countries, with examinations performed by non-expert operators, and were aimed at early detection of cardiac abnormalities in school children, generally using a focused scanning protocol to detect morphological and physiological changes related to rheumatic heart disease.51 One of the echocardiographic screening studies was performed in Australia, confirming health disparities between the at risk young Indigenous population and a non-Indigenous urban population.38 Another screening study performed in a developed country involved scans performed in a GP clinic in rural Canada by appropriately trained health professionals and intended to confirm or exclude abdominal aortic aneurysms in an older adult population.33 These screening studies and the use of limited, focused scanning protocols demonstrated the potential use of POCUS as a tool for early detection of disease, thus avoiding late-stage diagnosis, a recognised problem in rural areas where access is limited, though principally in relation to cancer diagnosis rather than other conditions.52
Other than cardiac examinations, lung ultrasound and FAST or eFAST scans were also common in emergency departments, in particular for confirming or excluding the presence of lung consolidation and intrathoracic or intra-abdominal fluid collections, particularly in trauma management.53 The evidence supports the use of POCUS in emergency departments to increase confidence in the diagnosis, expedite treatment, to better target patient disposition for definitive care, and for better allocation of resources. Similarly, in other critical care settings, such as in the ICU, the use of ultrasound as a monitoring tool can improve patient outcomes and overall resource allocation.31 Though performed in metropolitan ICUs, other studies support the finding that POCUS has important applications in monitoring, diagnosis and procedural guidance in critical care.54
Ultrasound technology is evolving rapidly, including increasingly sophisticated telemedicine applications17,18,55 and the use of artificial intelligence (AI) and automated machine learning.56 These new applications include real-time, remote image guidance and volume sweep imaging (VSI),19,57,58 with standardisation of scanning protocols and measurements.59,60 Such technological advances, together with wider availability of low cost, hand-held ultrasound equipment, promise to increase the access to ultrasound for a variety of non-expert users.61,62 The advances may be of benefit in low-resource, rural and remote health care settings,19 provided high quality of examinations and standards of care are assured.
While in some of the studies included in this review expert sonographers performed the examinations, in many of the studies the operators performing the scans were non-sonographers who had undergone training in a particular examination type and were following a given protocol. In several cases, those operators were working beyond their traditional health professional scope of practice, which may be a way to bridge the gap in low resource settings, where ultrasound services provided by a sonographer may be unavailable. This raises issues about the perceived need to clearly defined practice roles and boundaries, as acknowledged in the cases of other extended scope of practice roles.63 Patient safety is of paramount importance and, while ultrasound is a safe imaging modality with no known adverse effects, unless high exposure levels occur,64 diagnostic accuracy is still an important consideration.
Studies included in this review used various means to ensure the quality of the examinations was adequate for diagnosis, such as training regimens, adherence to protocols, or having guidance by expert trainers or other senior health professionals. Nevertheless, a variety of problems can occur if regular equipment maintenance and quality control testing is not carried out, which can compromise diagnostic accuracy.7 In some studies, particularly screening studies, a second examination was performed by an experienced sonographer on a standard ultrasound device to confirm the diagnosis and ensure optimum quality images were produced. Previously published review articles have found that legal actions related to the use of POCUS had only been brought against emergency physicians where they had failed to perform an ultrasound examination that was within scope in a timely manner.65,66 No legal actions related to malpractice or misdiagnosis when using POCUS were found in this review.
This review has found that portable ultrasound has a wide range of uses in rural and remote, low-resource settings. In the studies reviewed, POCUS was performed a range of health care settings, including emergency departments, GP clinics, schools, a helicopters retrieval service, and in the medical station at a music festival, demonstrating the versatility and flexibility of the equipment used. Patient outcomes across the studies were generally improved, with clinical information gained through early diagnosis of conditions in emergency care or screening studies. It is concluded that, in locations where there is limited access to diagnostic ultrasound examinations performed by sonographers or medical specialists, the use of limited ultrasound examinations performed by non-sonographers on small portable machines, including hand-held devices, has potential to improve diagnostic accuracy and patient care, as well as the overall health outcomes of under-resourced communities, including in rural and remote areas of Australia and other developed countries.
While non-sonographer ultrasound performed on small portable units is prevalent in many high-resource, urban healthcare settings, anecdotally, its use in low-resource settings in Australia is less common. Its increased use may be a step toward better healthcare equity of access between urban and rural populations. This may be increasingly the case as the technology continues to advance; however, this progress must be accompanied by some caveats in order to ensure that rural communities are not exposed to poor practice. Primarily, it is recommended that limited ultrasound practice is specific to certain examination types that are relevant to community needs, and that the education and training programs are of high quality. Practice should be regularly monitored, with practitioners required to undertake recurrent assessment of their competency to perform ultrasound, as well as regular quality control monitoring of equipment and image quality. If such measures are in place, POCUS may become a key component of diagnosis in rural healthcare.
Strengths and Limitations of This Review
Though this review has shed light on the widespread use of POCUS in under-resourced health care settings and the wide range of examination types performed, a large number of the studies lacked consistency and reliability in terms of the outcome variables evaluated. While comprehensive, the lack of consistency in study type did not lend itself to a more detailed meta-analysis. Overall, the quality of the studies extracted was low, as indicated in the risk of bias table. There were only two studies fitting the inclusion criteria that involved some form of randomisation. Self-reporting of outcome measures was common and there was minimal blinding of participants, as blinding is not possible when the physicians assessing patients clinically were themselves performing the ultrasound examinations. On the issue of self-reporting the authors of one of the studies commented that their research would ‘be expected to underestimate the actual impact of POCUS’, as the providers were likely to be influenced by “pre-ultrasound diagnostic impression consistent with ultrasound findings”.45 In another study, the authors acknowledged that because the physicians knew that research was underway, they may have been inclined to respond favourable about the diagnostic value of the ultrasound examinations.47
A key strength of this review, however, is the depth of the searches, with articles extracted across seven databases, as well as being sourced through bibliographical and grey literature searches. This provides certainty about the scope of the search strategy and is beyond the search parameters used in other literature reviews found on related topics. It should also be noted, however, that only studies reported in English language were included in the review so some relevant studies published in other languages may have overlooked.
Conclusions
Very little research was found about the use of POCUS that was directly relevant to the Australian rural and remote healthcare experience, necessitating extrapolation of the findings of studies performed in other countries and locations where the health system is under-resourced. The variety of papers found from across the world indicates the vast array of settings in which portable ultrasound is used, and the reported improvements to patient management and health outcomes as a result of the implementation of POCUS. Although there are various studies demonstrating the accessibility, utility and importance of POCUS in low-resource settings, there is a need for more high-quality research to investigate both the benefits and risks of this valuable diagnostic tool. In rural and remote locations in Australia, as well as in other countries where distance from mainstream health services is a serious problem, technological developments have potential to lessen the negative impact of some healthcare access and availability issues. It is highly probable that technology will evolve even further and that small portable and hand-held ultrasound units will be available for use by an increasingly wide variety of users. Practitioners may be encouraged to adopt innovative technological solutions; however, implementation should be informed by sound research evidence.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kane D, Grassi W, Sturrock R, Balint PV. A brief history of musculoskeletal ultrasound: ‘From bats and ships to babies and hips’. Rheumatology. 2004;43(7):931–933. doi: 10.1093/rheumatology/keh004 [DOI] [PubMed] [Google Scholar]
- 2.Wright J, Noriega O, Ho H. The application of hand-held ultrasound scanner in teaching of telemedicine and rural medicine. Donald School J Ultrasound Obstet Gynecol. 2014;8(1):87–91. doi: 10.5005/jp-journals-10009-1340 [DOI] [Google Scholar]
- 3.Wise J. Everyone’s a radiologist now. BMJ. 2008;336(7652):1041–1043. doi: 10.1136/bmj.39560.444468.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore CL, Copel JA. Point-of-care ultrasonography. N Engl J Med. 2011;364(8):749–757. doi: 10.1056/NEJMra0909487 [DOI] [PubMed] [Google Scholar]
- 5.Smallwood N, Dachsel M. Point-of-care ultrasound (POCUS): unnecessary gadgetry or evidence-based medicine? Clin Med. 2018;18(3):219–224. doi: 10.7861/clinmedicine.18-3-219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith ZA, Postma N, Wood D. FAST scanning in the developing world emergency department. S Afr Med J. 2010;100(2):105‐108. doi: 10.7196/SAMJ.3821 [DOI] [PubMed] [Google Scholar]
- 7.Nixon G, Blattner K, Muirhead J, Finnie W, Lawrenson R, Kerse N. Scope of point-of-care ultrasound practice in rural New Zealand. J Prim Health Care. 2018;10(3):224–236. doi: 10.1071/HC18031 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organisation (WHO). Increasing Access to Health Workers in Remote and Rural Areas Through Improved Retention: Global Policy Recommendations. Geneva: WHO; 2010. [PubMed] [Google Scholar]
- 9.World Bank Group. Rural population (% of total population). World Bank; 2020. Available from: https://data.worldbank.org/indicator/SP.RUR.TOTL.ZS?end=2020&start=2020&view=bar. Accessed February 27, 2022.
- 10.Australian Institute of Health and Welfare (AIHW). Rural & remote health. AIHW, Australian Government; 2019. Available from: https://www.aihw.gov.au/reports/rural-remote-australians/rural-remote-health. Accessed March 13, 2022.
- 11.Australian Institute of Health and Welfare (AIHW). Allied health workforce 2012. AIHW, Australian Government; 2013. Available from: https://www.aihw.gov.au/reports/workforce/allied-health-workforce-2012/summary. Accessed March 13, 2022.
- 12.Beks H, Walsh S, Alston L, et al. Approaches used to describe, measure and analyze place of practice in dentistry, medical, nursing and allied health rural graduate workforce research in Australia: a systematic scoping review. Int J Environ. 2022;19(3):1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Australian Government Department of Health. Stronger rural health strategy. department of health; 2019. Available from: https://www.health.gov.au/health-topics/rural-health-workforce/stronger-rural-health-strategy?utm_source=health.gov.au&utm_medium=callout-auto-custom&utm_campaign=digital_transformation. Accessed March 13 2022.
- 14.National Rural Health Alliance (NRHA). Medical practitioners in rural, regional & remote Australia. NRHA; 2019. Available from: https://www.ruralhealth.org.au/. Accessed October, 2021. [Google Scholar]
- 15.Luntsi G, Ugwu AC, Nkubli FB, Emmanuel R, Ochie K, Nwobi CI. Achieving universal access to obstetric ultrasound in resource constrained settings: a narrative review. Radiography. 2020;27(2):709–715. doi: 10.1016/j.radi.2020.10.010 [DOI] [PubMed] [Google Scholar]
- 16.Kim ET, Singh K, Moran A, Armbruster D, Kozuki N. Obstetric ultrasound use in low and middle income countries: a narrative review. Reprod Health. 2018;15(1):15. doi: 10.1186/s12978-018-0460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh-Feiley G, Eadie L, Wilson P. Telesonography in emergency medicine: a systematic review. PLoS One. 2018;13(5):5. doi: 10.1371/journal.pone.0194840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salerno A, Tupchong K, Verceles AC, McCurdy MT. Point-of-care teleultrasound: a systematic review. Telemed J E Health. 2020;26(11):1314–1321. doi: 10.1089/tmj.2019.0177 [DOI] [PubMed] [Google Scholar]
- 19.Britton N, Miller MA, Safadi S, Siegel A, Levine AR, McCurdy MT. Tele-ultrasound in resource-limited settings: a systematic review. Front Public Health. 2019;7:244. doi: 10.3389/fpubh.2019.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Telford LH, Abdullahi LH, Ochodo EA, Zuhlke LJ, Engel ME. Standard echocardiography versus handheld echocardiography for the detection of subclinical rheumatic heart disease: a systematic review and meta-analysis of diagnostic accuracy. BMJ Open. 2020;10(10):10. doi: 10.1136/bmjopen-2020-038449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marangou J, Beaton A, Aliku TO, Nunes MCP, Kangaharan N, Remenyi B. Echocardiography in indigenous populations and resource poor settings. Heart Lung Circ. 2019;28(9):1427–1435. doi: 10.1016/j.hlc.2019.05.176 [DOI] [PubMed] [Google Scholar]
- 22.Becker DM, Tafoya CA, Becker SL, Kruger GH, Tafoya MJ, Becker TK. The use of portable ultrasound devices in low- and middle-income countries: a systematic review of the literature. Trop Med Int Health. 2016;21(3):294–311. doi: 10.1111/tmi.12657 [DOI] [PubMed] [Google Scholar]
- 23.Sippel S, Muruganandan K, Levine A, Shah S. Review article: use of ultrasound in the developing world. Int J Emerg Med. 2011;4(1):72. doi: 10.1186/1865-1380-4-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groen RS, Leow JJ, Sadasivam V, Kushner AL. Review: indications for ultrasound use in low- and middle-income countries. Trop Med Int Health. 2011;16(12):1525–1535. doi: 10.1111/j.1365-3156.2011.02868.x [DOI] [PubMed] [Google Scholar]
- 25.Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2. Cochrane; 2021. Available from: www.training.cochrane.org/handbook. Accessed September 2021. [Google Scholar]
- 26.Page MJ, McKenzie JE, Bossuyt PM, et al. 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:71. PMID: 33782057; PMCID: PMC8005924. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollock A, Berge E. How to do a systematic review. Int J Stroke. 2018;13(2):138–156. doi: 10.1177/1747493017743796 [DOI] [PubMed] [Google Scholar]
- 28.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. PMID: 31462531. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 29.Ketelaars R, Hoogerwerf N, Scheffer GJ. Prehospital chest ultrasound by a Dutch helicopter emergency medical service. J Emerg Med. 2013;44(4):811–817. doi: 10.1016/j.jemermed.2012.07.085 [DOI] [PubMed] [Google Scholar]
- 30.Ploutz M, Lu JC, Scheel J, et al. Handheld echocardiographic screening for rheumatic heart disease by non-experts. Heart. 2016;102(1):35–39. doi: 10.1136/heartjnl-2015-308236 [DOI] [PubMed] [Google Scholar]
- 31.Pontet J, Yic C, Díaz-Gómez JL, et al. Impact of an ultrasound-driven diagnostic protocol at early intensive-care stay: a randomized-controlled trial. Ultrasound J. 2019;11(1):24. PMID: 31595353; PMCID: PMC6783485. doi: 10.1186/s13089-019-0139-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nixon G, Blattner K, Koroheke-Rogers M, et al. Point-of-care ultrasound in rural New Zealand: safety, quality and impact on patient management. Aust J Rural Health. 2018;26(5):342–349. PMID: 30303278. doi: 10.1111/ajr.12472 [DOI] [PubMed] [Google Scholar]
- 33.Blois B. Office-based ultrasound screening for abdominal aortic aneurysm. Can Fam Physician. 2012;58(3):e172–e178. [PMC free article] [PubMed] [Google Scholar]
- 34.Beaton A, Aliku T, Okello E, et al. The utility of handheld echocardiography for early diagnosis of rheumatic heart disease. J Am Soc Echocardiogr. 2014;27(1):42–49. PMID: 24183541. doi: 10.1016/j.echo.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 35.Beaton A, Lu JC, Aliku T, et al. The utility of handheld echocardiography for early rheumatic heart disease diagnosis: a field study. Eur Heart J Cardiovasc Imaging. 2015;16(5):475–482. PMID: 25564396; PMCID: PMC4542771. doi: 10.1093/ehjci/jeu296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godown J, Lu JC, Beaton A, et al. Handheld echocardiography versus auscultation for detection of rheumatic heart disease. Pediatrics. 2015;135(4):e939–44. PMID: 25780068. doi: 10.1542/peds.2014-2774 [DOI] [PubMed] [Google Scholar]
- 37.Lu JC, Sable C, Ensing GJ, et al. Simplified rheumatic heart disease screening criteria for handheld echocardiography. J Am Soc Echocardiogr. 2015;28(4):463–469. doi: 10.1016/j.echo.2015.01.001 [DOI] [PubMed] [Google Scholar]
- 38.Roberts K, Maguire G, Brown A, et al. Echocardiographic screening for rheumatic heart disease in high and low risk Australian children. Circulation. 2014;129(19):1953–1961. doi: 10.1161/CIRCULATIONAHA.113.003495 [DOI] [PubMed] [Google Scholar]
- 39.Singh S, Bansal M, Maheshwari P, et al. American society of echocardiography: remote echocardiography with web-based assessments for referrals at a distance (ASE-REWARD) Study. J Am Soc Echocardiogr. 2013;26(3):221–233. doi: 10.1016/j.echo.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 40.Bhavnani SP, Sola S, Adams D, Venkateshvaran A, Dash PK, Sengupta PP. A randomized trial of pocket-echocardiography integrated mobile health device assessments in modern structural heart disease clinics. JACC Cardiovasc Imaging. 2018;11(4):546–557. doi: 10.1016/j.jcmg.2017.06.019 [DOI] [PubMed] [Google Scholar]
- 41.Chavez MA, Naithani N, Gilman RH, et al. Agreement between the world health organization algorithm and lung consolidation identified using point-of-care ultrasound for the diagnosis of childhood pneumonia by general practitioners. Lung. 2015;193(4):531–538. PMID: 25921013. doi: 10.1007/s00408-015-9730-x [DOI] [PubMed] [Google Scholar]
- 42.Dalmacion GV, Reyles RT, Habana AE, et al. Handheld ultrasound to avert maternal and neonatal deaths in 2 regions of the Philippines: an iBuntis® intervention study. BMC Pregnancy Childbirth. 2018;18(1):32. PMID: 29347926; PMCID: PMC5774122. doi: 10.1186/s12884-018-1658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amatya Y, Rupp J, Russell FM, Saunders J, Bales B, House DR. Diagnostic use of lung ultrasound compared to chest radiograph for suspected pneumonia in a resource-limited setting. Int J Emerg Med. 2018;11(1):1. doi: 10.1186/s12245-018-0170-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kissoon DV, Jagjit SD, Bales BD, Luke-Blyden Z, Boyd JS, Rupp JD. Observational descriptive study of ultrasound use and its impact on clinical decisions in the accident and emergency department at Georgetown public hospital corporation. PLoS One. 2020;15(5):e0233379. doi: 10.1371/journal.pone.0233379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reynolds TA, Amato S, Kulola I, Chen C-J-J, Mfinanga J, Sawe HR. Impact of point-of-care ultrasound on clinical decision-making at an urban emergency department in Tanzania. PLoS One. 2018;13(4):e0194774. doi: 10.1371/journal.pone.0194774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker DE, Nolting L, Brown HA. Impact of point-of-care ultrasound on the diagnosis and treatment of patients in rural Uganda. Trop Dr. 2021;2021:49475520986425. [DOI] [PubMed] [Google Scholar]
- 47.Prager R, Sedgwick C, Lund A, et al. Prospective evaluation of point-of-care ultrasound at a remote, multi-day music festival. Prehosp Disaster Med. 2018;33(5):484–489. doi: 10.1017/S1049023X18000821 [DOI] [PubMed] [Google Scholar]
- 48.Umuhire OF, Henry MB, Levine AC, Cattermole GN, Henwood P. Impact of ultrasound on management for dyspnea presentations in a Rwandan emergency department. Ultrasound J. 2019;11(1):18. doi: 10.1186/s13089-019-0133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanatta M, Benato P, De Battisti S, Pirozzi C, Cianci V. Point-of-care critical ultrasound in a rural emergency department. Emerg Care J. 2015;11(2):26–31. doi: 10.4081/ecj.2015.5017 [DOI] [Google Scholar]
- 50.Strasser R. Rural health around the world: challenges and solutions. Fam Pract. 2003;20(4):457–463. PMID: 12876121. doi: 10.1093/fampra/cmg422 [DOI] [PubMed] [Google Scholar]
- 51.Mirabel M, Bacquelin R, Tafflet M, et al. Screening for rheumatic heart disease: evaluation of a focused cardiac ultrasound approach. Circ Cardiovasc Imaging. 2015;8(1):e002324. doi: 10.1161/CIRCIMAGING.114.002324 [DOI] [PubMed] [Google Scholar]
- 52.Zhou K, Pickering TA, Gainey CS, et al. Presentation, management, and outcomes across the rural-urban continuum for hepatocellular carcinoma. JNCI Cancer Spectr. 2021;5(1):pkaa100. doi: 10.1093/jncics/pkaa100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Netherton S, Milenkovic V, Taylor M, Davis PJ. Diagnostic accuracy of eFAST in the trauma patient: a systematic review and meta-analysis. CJEM. 2019;21(6):727–738. PMID: 31317856. doi: 10.1017/cem.2019.381 [DOI] [PubMed] [Google Scholar]
- 54.Zieleskiewicz L, Muller L, Lakhal K, et al. CAR’Echo and AzuRea Collaborative Networks. Point-of-care ultrasound in intensive care units: assessment of 1073 procedures in a multicentric, prospective, observational study. Intensive Care Med. 2015;41(9):1638–1647. PMID: 26160727. doi: 10.1007/s00134-015-3952-5 [DOI] [PubMed] [Google Scholar]
- 55.Dougherty A, Kasten M, DeSarno M, et al. Validation of a telemedicine quality assurance method for point-of-care obstetric ultrasound used in low-resource settings. J Ultrasound Med. 2021;40(3):529–540. PMID: 32770709. doi: 10.1002/jum.15429 [DOI] [PubMed] [Google Scholar]
- 56.Seetharam K, Kagiyama N, Sengupta PP. Application of mobile health, telemedicine and artificial intelligence to echocardiography. Echo Res Pract. 2019;6(2):R41–R52. doi: 10.1530/ERP-18-0081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferrer J, Chaumont T, Trujillo L, et al. New tele-diagnostic model using volume sweep imaging for rural areas. Annu Int Conf IEEE Eng Med Biol Soc. 2017;2017:2622–2625. PMID: 29060437. doi: 10.1109/EMBC.2017.8037395 [DOI] [PubMed] [Google Scholar]
- 58.Marini TJ, Oppenheimer DC, Baran TM, et al. New ultrasound telediagnostic system for low-resource areas: pilot results from Peru. J Ultrasound Med. 2021;40(3):583–595. PMID: 32798267. doi: 10.1002/jum.15420 [DOI] [PubMed] [Google Scholar]
- 59.Saavedra AC, Arroyo J, Tamayo L, Egoavil M, Ramos B, Castaneda B. Automatic ultrasound assessment of placenta previa during the third trimester for rural areas. IEEE Int Ultrason Symp. 2020;1–4. doi: 10.1109/IUS46767.2020.9251764 [DOI] [Google Scholar]
- 60.van den Heuvel TLA, Petros H, Santini S, de Korte CL, van Ginneken B. Automated fetal head detection and circumference estimation from free-hand ultrasound sweeps using deep learning in resource-limited countries. Ultrasound Med Biol. 2019;45(3):773–785. doi: 10.1016/j.ultrasmedbio.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 61.Burleson SL, Swanson JF, Shufflebarger EF, et al. Evaluation of a novel handheld point-of-care ultrasound device in an African emergency department. Ultrasound J. 2020;12(1):53. PMID: 33284368; PMCID: PMC7721766. doi: 10.1186/s13089-020-00200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baribeau Y, Sharkey A, Chaudhary O, et al. Handheld point-of-care ultrasound probes: the new generation of POCUS. J Cardiothorac Vasc Anesth. 2020;34(11):3139–3145. PMID: 32736998; PMCID: PMC7340048. doi: 10.1053/j.jvca.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Smith T, McNeil K, Mitchell R, Boyle B, Ries N. A study of macro-, meso- and micro-barriers and enablers affecting extended scopes of practice: the case of rural nurse practitioners in Australia. BMC Nurs. 2019;18(1):14. doi: 10.1186/s12912-019-0337-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izadifar Z, Babyn P, Chapman D. Mechanical and biological effects of ultrasound: a review of present knowledge. Ultrasound Med Biol. 2017;43(6):1085–1104. doi: 10.1016/j.ultrasmedbio.2017.01.023 [DOI] [PubMed] [Google Scholar]
- 65.Blaivas M, Pawl R. Analysis of lawsuits filed against emergency physicians for point-of-care emergency ultrasound examination performance and interpretation over a 20-year period. Am J Emerg Med. 2012;30(2):338–341. doi: 10.1016/j.ajem.2010.12.016 [DOI] [PubMed] [Google Scholar]
- 66.Stolz L, O’Brien KM, Miller ML, Winters-Brown ND, Blaivas M, Adhikari S. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med. 2015;16(1):1–4. doi: 10.5811/westjem.2014.11.23592 [DOI] [PMC free article] [PubMed] [Google Scholar]