Abstract
Background
Various neurological sequalae have been described following COVID-19 vaccination. Here we describe the first case of untreated post COVID-19 vaccine encephalitis with spontaneous resolution of contrast enhancing hyperintensities on MRI concomitant with clinical improvement.
Case Presentation
A 59-year-old woman presented with a two-day history of unsteady gait, incoordination, visual symptoms, and lethargy. She had received AZD1222 (AstraZeneca) and mRNA-1273 (Moderna) COVID-19 vaccines at 3 months and 12 days, respectively, before presentation. Brain MRI showed no abnormality on the non-enhanced sequences, but numerous enhancing lesions in the cerebral cortex, deep grey matter, brainstem, and cerebellum. Treatment was expectant, the patient improved clinically over 10 days, and repeat MRI showed near complete resolution of the imaging abnormality.
Conclusions
We describe neurological deterioration 12 days after a second dose of COVID-19 vaccine. There was no evidence of edema or demyelinating lesions in the brain on MRI, but there was extensive contrast-enhancement indicating loss of blood-brain barrier (BBB) integrity. This provides a potential in vivo, clinical-imaging correlate of the post-mortem evidence that SARS-CoV-2 spike protein may induce loss of BBB permeability. While this adds to the list of rare adverse neurological reactions to COVID-19 vaccination, the benefits of receiving the vaccine far outweigh these risks.
A 59-year-old woman presented to the emergency department with a two-day history of unsteady gait, incoordination, dizziness, binocular diplopia, perioral paresthesias, right hand numbness, and lethargy. Her vital signs were normal and without signs of a systemic inflammatory response syndrome (temperature 36.4°C, blood pressure 127/73, heart rate 60 bpm, respiratory rate 18, O2 saturation 94% on room air). The patient's mental status exam was normal and, although no formal cognitive testing was performed, she was attentive, verbally communicative and followed commands. She had no apparent deficits in memory, language, or changes in personality and behaviour. Neurological exam revealed normal visual fields to confrontation, normal reactive and equal pupils to light and accommodation, normal extra-ocular movements with no ophthalmoplegia, sustained left-beating horizontal nystagmus, decreased pin sensation of the bottom lip along CN V3 distribution, no facial droop, tongue and uvula were midline. Motor examination, including tone, bulk and power, was normal. Reflexes were normal and symmetrical throughout. Sensory examination revealed a reduced pin sensation in the right hand along the recurrent median nerve distribution with a positive Tinel's sign but otherwise normal vibration and proprioception in extremities. Cerebellar testing revealed bilateral dysdiadokokinesia on rapid alternating movements, bilateral dysmetria with heel-to-shin bilaterally, absent rebounding, wide based ataxic gait with lateral veering and reduced stride length. Past medical history included fibromyalgia, migraines, and carpel tunnel syndrome. There was no family history of neurological or autoimmune disorders. The patient received AZD1222 (AstraZeneca) and mRNA-1273 (Moderna) COVID-19 vaccines at 3 months and 12 days, respectively, before presentation.
Complete blood count showed a normal hemoglobin (124g/L; normal 120-160), a mildly elevated white blood cell count (11.8 × 109/L; normal 4.0 – 11.0) with mild neutrophilia (8.3 × 109/L; normal 2.0 – 7.5), and a normal platelet count (276 × 109/L; normal 150-400). Nasopharyngeal swab polymerase chain reaction (PCR) testing for COVID-19 was negative.
Cerebrospinal fluid (CSF) had mildly elevated protein (0.56 g/L; normal 0.15 – 0.45), mildly elevated glucose (4.8 mmol/L; normal 2.2 – 4.4), and lymphocytic pleocytosis (WBC: 14.0 × 106/L; normal 0 – 5) (81% lymphocytes). CSF bacterial and fungal cultures and PCR assay (Bio-Fire Film-Array, Idaho Technology, Salt Late City, Utah) for viral infections (including HSV1/2 and VZV) were negative. CSF IgG was normal and oligoclonal bands were absent. CSF neuronal autoantibody testing (including anti-GAD65, anti-Yo, and Anti-Hu) was negative. CSF cytology showed no malignant cells, but a predominant population of T-cells and small number of polytype B-cells consistent with a reactive lymphoid population.
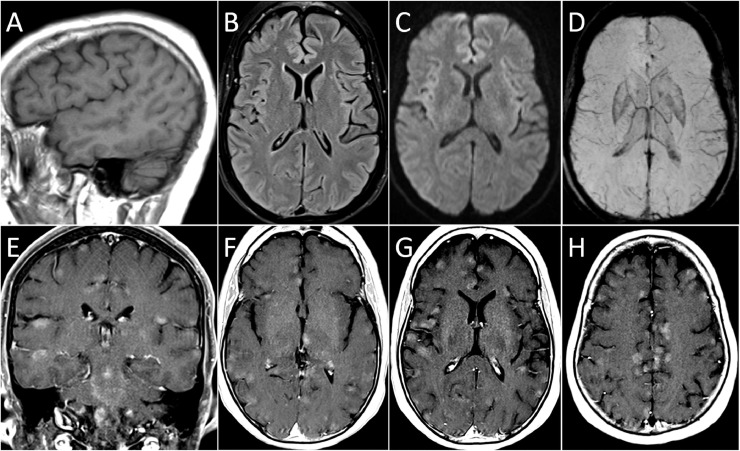
Brain magnetic resonance imaging (MRI) showed no abnormality on the non-enhanced sequences (including diffusion, susceptibility, T1, and T2-weighted FLAIR), but there were numerous focal regions of contrast enhancement in the cerebral cortex, deep grey matter, brainstem, and cerebellum (Fig. 1 ). Chest, abdomen and pelvis computed tomography, pelvic ultrasound, and mammography showed no malignancy.
Fig. 1.
Brain MRI at Presentation
Brain MRI at presentation. There is no abnormality on non-enhanced sagittal T1 (A), axial T2-weighted FLAIR (B), diffusion-weighted (C) and susceptibility-weighted (minimum intensity projection, D) images. Contrast-enhanced T1-weighted images in the coronal (E) and axial (F to E) planes show multiple focal, poorly defined regions of contrast enhancement in the cerebral cortex, deep grey matter, brainstem, and cerebellum.
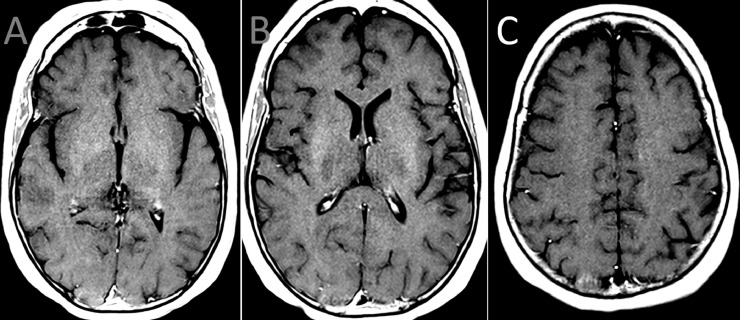
Treatment was expectant, with no empiric corticosteroids or antimicrobials. After 10 days in hospital, the patient's gait had considerably improved and her other symptoms had resolved. Repeat MRI showed near-complete resolution of the enhancing lesions (Fig. 2 ). She was discharged from hospital. At two-month follow-up, she continued to use a walker, but with ongoing gradual improvement in gait. She had no recurrence of her other symptoms.
Fig. 2.
Repeat Brain MRI 10 days after presentation
Brain MRI follow-up 10 days after presentation. Contrast-enhanced T1-weighted images in the axial plane (A to C) shows complete resolution of the enhancing lesions in the brain.
We have described a patient with neurological deterioration 12 days after receiving a second dose of COVID-19 vaccine. There was no evidence of edema or demyelinating lesions in her brain on MRI, but there was extensive contrast-enhancement indicating loss of blood-brain barrier (BBB) integrity. Total CSF protein was only mildly elevated, but the gadolinium-based MRI contrast agent has molecular weight approximately 100 times smaller than albumin, so BBB permeability to MRI contrast and permeability to serum proteins such as albumin may not correspond.
While COVID-19 infection can present with various neuroimaging findings,1 cases of post COVID-19 vaccine encephalitis are exceedingly scarce.2 , 3 Prior case studies suggest that the time between vaccination and symptom onset may be approximately 7-14 days.2 , 3 The mechanism of post-COVID-19 vaccine encephalitis is unknown. A post-mortem study found that SARS-CoV-2 spike proteins may trigger a pro-inflammatory reaction in brain endothelial cells causing a loss of permeability in the BBB.4 Mouse models have also shown that SARS-CoV-2 spike proteins cross the BBB with preferential distribution in the brainstem, cerebellum, and frontal cortex.5 We speculate that our patient developed transient, autoimmune-mediated BBB dysfunction triggered by vaccination. To our knowledge, there are also no published case reports with repeat imaging demonstrating near total rapid resolution of the contrast enhancing lesions without therapy.
From a clinical perspective, the patient's presentation was consistent with a rhombencephalitis. She fulfilled the diagnostic criteria for possible autoimmune encephalitis6 with subacute onset of lethargy, focal CNS symptoms, CSF pleocytosis, MRI features suggestive of encephalitis, and reasonable exclusion of alternative causes. While rare neurological complications following COVID-19 vaccines have been reported and it is prudent for clinicians to be able to recognize these events,7 , 8 it is also important to emphasize that COVID-19 infection itself poses a significantly greater risk for these same reactions both in terms of frequency and severity.9 Hence, the benefits of COVID-19 vaccination far outweigh the potential for developing rare adverse neurological reactions.10
Competing interests
Not applicable
Funding
Not applicable
Acknowledgements
Not applicable
Footnotes
SUBMISSION TO: Journal: Journal of Neuroradiology
References
- 1.Katal S, Balakrishnan S, Gholamrezanezhad A. Neuroimaging and neurologic findings in COVID-19 and other coronavirus infections: a systematic review in 116 patients. J Neuroradiol. 2021;48(1):43–50. doi: 10.1016/j.neurad.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zuhorn F, Graf T, Klingebiel R, Schäbitz WR, Rogalewski A. Postvaccinal Encephalitis after ChAdOx1 nCov-19. Ann Neurol. 2021;90(3):506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takata J, Durkin SM, Wong S, Zandi MS, Swanton JK, Corrah TW. A case report of ChAdOx1 nCoV-19 vaccine-associated encephalitis. BMC Neurol. 2021;21(1):485. doi: 10.1186/s12883-021-02517-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, Razmpour R, Hale JF, Galie PA, Potula R, Andrews AM, Ramirez SH. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol Dis. 2020;146:1–12. doi: 10.1016/j.nbd.2020.105131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24(3):368–378. doi: 10.1038/s41593-020-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler M, Tamborska A, Wood GK, Ellul M, Thomas RH, Galea I, Pett S, Singh B, Solomon T, Pollak TA, Michael BD, Nicholson TR. Considerations for causality assessment of neurological and neuropsychiatric complications of SARS-CoV-2 vaccines: from cerebral venous sinus thrombosis to functional neurological disorder. J Neurol Neurosurg Psychiatry. 2021;92(11):1144–1151. doi: 10.1136/jnnp-2021326924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patone M, Handunnetthi L, Saatci D, Pan J, Katikireddi SV, Razvi S, Hunt D, Mei XW, Dixon S, Zaccardi F, Khunti K, Watkinson P, Coupland CAC, Doidge J, Harrison DA, Ravanan R, Sheikh A, Robertson C, Hippisley-Cox J. Neurological complications after first dose of COVID-19 vaccines and SARS-CoV-2 infection. Nat Med. 2021;Oct 25 doi: 10.1038/s41591-021-01556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, Reis BY, Balicer RD. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marsh EB, Kornberg M, Kessler K, Haq I, Patel AD, Nath A, Schierman B, Jones LK., Jr Quality Committee of the American Academy of Neurology. COVID-19 and Vaccination in the Setting of Neurologic Disease: An Emerging Issue in Neurology. Neurology. 2021;97(15):720–728. doi: 10.1212/WNL.0000000000012578. [DOI] [PMC free article] [PubMed] [Google Scholar]