Abstract
Free radical release due to oxidative stress is gaining importance in the field of viral pathogenesis. Recent studies suggest the involvement of oxidative stress and ROS levels in regulating disease virulence during RNA virus infection. Most of the RNA virus infections lead to vascular dysfunction and disease severity. However, the biology of free radicals in maintaining vascular endothelium integrity is not completely understood. In the present review, we discuss some of the common features in positive-strand RNA virus infections such as dengue and SARS-CoV-2 and suggest that anti-oxidant therapy could pave the way to develop therapeutic strategies in combating emerging and re-emerging RNA viruses.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-022-01269-x.
Keywords: RNA virus, ROS, Anti-oxidant therapy, COVID-19, Endothelial activation
One-third of the virus genera are made up of positive single-strand RNA (+ ssRNA) viruses. This includes clinically important and deadly pathogens like severe acute respiratory syndrome coronavirus–2 (SARS-CoV-2), dengue virus (DENV), and hepatovirus A. The viruses utilize host factors for initial access, cell invasion, and replication processes. Perhaps most importantly, studies have suggested that these RNA viruses, upon entry into the host, make use of oxidative stress–induced ambience for their genome capping and replication, thereby contributing towards disease severity (Reshi et al. 2014). In addition, + ssRNA can also alter the gene expression or reprogram the function of host-cell defense mechanisms by co-opting host factors (Nagy and Pogany 2011). Thus, anti-oxidant therapy might be a potential therapeutic approach in combatting RNA viruses.
The endothelium is a continuous monolayer of endothelial cells (ECs) aligned along the direction of blood flow and plays an important role in regulating vascular integrity within the blood vessel wall (Shin et al. 2017). Upon stimulation, the endothelium undergoes a specific alteration in its phenotype referred to as “endothelial activation” which is characterized by enhanced expression of endothelial selectins, increased endothelial-leukocyte interaction, and permeability. Evidence suggests that ROS-mediated modulation of signal transduction pathways (activation of transcription factor AP-1 and activation of NFκB and p38 MAPK pathways) on ECs are key signaling mechanisms for endothelial activation (Alom-Ruiz et al. 2008). Oxidative stress–induced activation of ECs and platelets is presumed to contribute towards disease virulence during virus infection. The endothelium could dynamically elicit responses that may contribute to the hyper-inflammation and altered vascular permeability during ssRNA viral infection, especially in the case of DENV infection (Dalrymple and Mackow 2012b, a). Thus, a proper understanding of the participation of ECs in stabilizing fluid barrier functions of the endothelium may lead to developing a therapeutic approach for reducing vascular leakage in the severe form of the disease (Dalrymple and Mackow 2012a). Though there are several factors like cellular interactions, cell–cell aggregation, and direct binding of the viral proteins that are presumed to be the causes for endothelial dysfunction, the actual mechanism remains obscure. Most importantly, the activation of ECs during the critical phase of infection when there is no virus in circulation makes us assume that there exist alternative means of host responsive factors that regulate endothelial permeability.
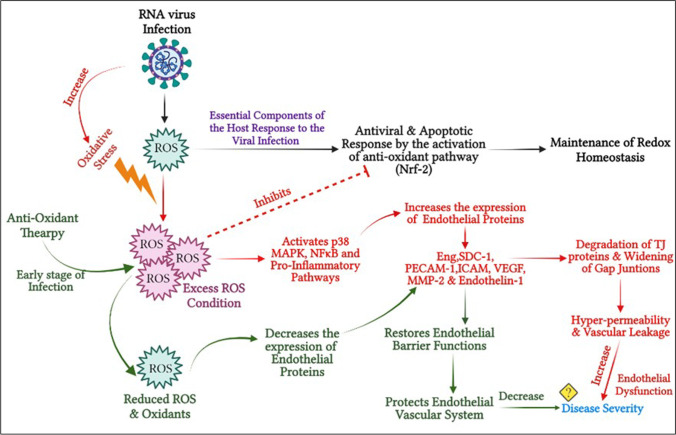
An imbalance in the production of reactive oxygen species (ROS) and the inability of the host to detoxify ROS results in oxidative stress. The resulting oxidative stress is associated with pro-inflammatory cytokine release, as was reported for severe cases of dengue fever (Soundravally et al. 2014). Recently, we have reviewed the generation of ROS by endoplasmic reticulum (ER) and mitochondria that leads to the hyper-inflammatory response and disease severity in ssRNA viral infections like dengue, HIV, HBV, and HCV (Pillai et al. 2019). On one hand, some of the ssRNA infections alter the status of the mitochondrial chaperone prohibitin, resulting in dysregulation of the mitochondrial respiratory chain leading to the cause of ROS overproduction (Dang et al. 2011). On the other hand, virus replication is directly associated with protein oxidation in the ER and enhances ROS production and oxidative stress (Paracha et al. 2013). This leads to oxidative stress–induced cellular damage and disease severity (Reshi et al. 2014). A recent study has shown that the envelope (E) protein of SARS-CoV-2 binds to the extracellular iron or haem. This E-protein-haem bounded complex in turn produces oxygen and water and then converts them to O2−, H2O2, and hydroxyl radicals leading to ROS attack (Wenzhong and Hualan 2021). In addition, excessive production of ROS disrupts the lysosomal membrane and releases hydrolases. This results in autophagy in phagocytic cells and causes subsequent cell death followed by a strong cytokine storm and organ failure (Wenzhong and Hualan 2021).
Interestingly, ROS is identified as an essential component of the host response to viral infections. For instance, recent experimental findings have suggested that DENV infection induces intracellular ROS levels that regulate the activation of innate antiviral immune responses and stimulate apoptosis. Thus, a further understanding of the molecular details underlying the biological targets of ROS during DENV infection may facilitate the identification of novel treatment strategies for dengue-associated diseases. Parallel activation of antioxidant pathways regulated by Nrf2 also contributes to the regulatory control of antiviral and apoptotic responses by maintaining redox homeostasis. However, excess ROS can hamper this equilibrium and thus ROS was identified as an essential component of the host response to DENV infection (Olagnier et al. 2014; Pillai et al. 2019). Another important downstream pathway of ROS is the MAPK signaling pathway (Son et al. 2011). Recently, it has been demonstrated that the NS1 protein of DENV activates the p38 MAPK pathway, thereby contributing to the hyper-permeability of ECs in vitro (Barbachano-Guerrero et al. 2020). Similarly, the p38 MAPK pathway was reported to be upregulated in SARS-CoV-2 (Ma et al. 2020), SARS-CoV (Kopecky-Bromberg et al. 2006), and other respiratory viral infections (Börgeling et al. 2014). In this view, Grimes and Grimes et al. suggested MAPK inhibitors could be an effective therapeutic and promising approach for the management of coronavirus disease 2019 (COVID-19) (Grimes and Grimes 2020). An overview of the role of ROS, various associated signaling pathways, and disease severity during RNA virus infection is depicted in Fig. 1. Potentially useful inhibitors targeting some of the signaling pathways during ssRNA virus infection are listed in Table S1.
Fig. 1.
Schematic representation of ROS production and its role in virus disease pathogenesis
Endothelial dysfunction and SARS-CoV-2
In the case of SARS-CoV-2 infection, the participation of EC and EC injury in the disease pathogenesis has been described by Varga et al. (2020). In this line, biopsy of SARS-CoV-2-infected lungs exhibited mononuclear and polymorphonuclear aggregation accompanied by apoptotic ECs (Varga et al. 2020). Pulmonary endothelium serves as a selective barrier between the plasma and interstitium. Any drastic change in the endothelium will have an effect on the barrier function that leads to lung injury and pulmonary edema. In the case of COVID-19 pathogenesis, the molecular mechanism of endothelial dysfunction/dysregulation is not completely understood.
From the available literature on the disease mechanism exhibited by SARS-CoV-2, it shares some of the below-mentioned properties with dengue viral infection in terms of disease pathogenesis.
-
(i)
Activation of ECs and vascular dysfunction in severe COVID-19 cases — the vascular tone which is regulated by the endothelium is affected by the infection of the ECs by the virus and the presence of viral inclusion structures in ECs (Varga et al. 2020); expression of ACE2 receptors by ECs favors the virus to infect ECs and leads to cell death (Beyerstedt et al. 2021).
-
(ii)
Activation of the coagulation pathway with disseminated intravascular coagulation in severe cases (Zhou et al. 2021).
-
(iii)
Patients with severe disease had thrombocytopenia (low platelet count) as compared to those of non-severe cases (Lippi et al. 2020).
-
(iv)
Cellular interactions and adhesions of platelet-leukocyte and ECs during the infection (Canzano et al. 2021; Mariappan et al. 2021; Balakrishna Pillai et al. 2022).
-
(v)
The coagulopathy observed in severe cases may be due to the results of inflammatory responses and endothelial activation or damage (Zhou et al. 2021).
-
(vi)
Elevated fibrin degradation products (D-dimer) and fibrinogen in severe cases (Poudel et al. 2021).
-
(vii)
Excessive production of ferritin, macrophage activation, and ROS production leads to the release of free radicals, which convert Fe (II) to Fe (III) resulting in cellular apoptosis and coagulation. Elevated oxidative stress is associated with pro-inflammatory cytokines and cytokine storms (Perricone et al. 2020).
-
(viii)
ROS is also capable of activating calcium and NF-κB signaling to induce adhesion molecules and pro-inflammatory cytokines, which can increase vascular permeability and promote leukocyte adhesion. A recent study suggests that oxidative stress caused by Nox2 activation contributes to COVID-19 pathogenesis and is associated with thrombotic events in COVID-19 patients (Violi et al. 2020). Therefore, the beneficial effect of antioxidant drugs on endothelial function should be considered for the treatment of COVID-19 in the future.
Could neutralizing free radicals stop cytokine storm and disease severity in COVID?
Though there are many studies on the association of oxidative stress response and viral infection, it is currently not known how the free radicals released by mitochondria and ER could trigger the hyper-inflammatory response. To the same degree, how the excess production or decreased scavenging of ROS and the inflammatory signal could activate macrophages, mast cells, leukocytes, platelets, and ECs and dysfunction is not clear. For instance, during inflammation, chemical substances released by macrophages and subsequently by mast cells activate EC signaling pathways, which target structural elements such as actin and myosin that regulate vascular permeability. Evidence indicates that SARS-CoV-2 infection could affect capillary endothelium by inducing endothelial inflammation and contribute to COVID-19 severity (Jin et al. 2020). When vascular endothelium is exposed to various blood-borne pathogens or agents, the activated neutrophils could release a large amount of ROS into the circulation via membrane-bound NADPH oxidase during the neutrophil respiratory burst, causing host tissue injury and endothelial barrier dysfunction. In addition to this, vascular endothelium is the primary target for oxidants released by the activated blood cells at the site of injury or inflammation. These cellular events lead to reduced nitric oxide bioavailability, impairment of vascular tone, and alteration in endothelial phenotype (upregulation of adhesion molecules, MMP activity, formation of intercellular gaps, hyper-permeability, and leukocyte transmigration) (Alom-Ruiz et al. 2008; Incalza et al. 2018). Thus, in excess conditions, ROS might play a crucial role in the activation of ECs, resulting in vascular leakage as observed in dengue and COVID-19 patients. In this context, serotonin inhibitors, a class of anti-depressants with potential anti-viral, immune-modulatory, and anti-oxidant properties, are proposed to alleviate SARS-CoV-2 disease virulence (Hamed and Hagag 2020). In addition, treatment and/or supplementation with antioxidant drugs or compounds has shown to reduce the expression of various endothelial cell proteins (MMP-9, VEGF, PECAM-1, ET-1, and Syndecan-1) thereby protecting the vascular endothelial membrane (Reiter et al. 2010; Fang et al. 2013; Lee et al. 2014; Almatroodi et al. 2020). More research should be done on the antioxidant-oxidant status that is specifically altered in their expression during the course of viral infection, for developing modalities based on anti-oxidant-based COVID-19 and dengue management. Some of the potential antioxidants like curcumin, N-acetyl-L-cysteine, zinc, resveratrol, catechin, vitamin C, and vitamin D are currently under various phases of clinical trials in controlling respiratory virus severity (Delić 2021; Sherkawy 2021; Lai-Becker 2022). Most importantly, a patient suffering from a severe form of COVID-19 along with pneumonia cannot be amenable to any anti-oxidant therapy. Anti-oxidants should be administered in the early course of infection, before the development of pneumonia, which helps to prevent excessive ROS release (Lapenna 2021) and hyper-inflammatory responses, thus alleviating the disease severity caused by RNA viruses (Soto et al. 2020). In the above context, a list of antioxidant drugs and herbal extracts undergoing pre-clinical evaluation against RNA viral disease is mentioned in Table S2.
Thus, anti-oxidant therapy could help the supportive strategies, thereby improving the outcomes in patients with COVID-19 and other diseases caused by RNA viruses. Developing strategies for supplementing antioxidant therapy may augment the current disease management of debilitating positive-strand RNA virus diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge the infrastructure support provided by Sri Balaji Vidyapeeth for writing this manuscript.
Abbreviations
- ssRNA
Single strand RNA
- ECs
Endothelial cells
- ROS
Reactive oxygen species
- ACE2
Angiotensin converting enzyme 2
- DENV
Dengue virus
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus virus–2
- Nrf 2
Nuclear factor erythroid 2-related factor 2
- COVID-19
Coronavirus disease 2019
- Nox2
NADPH oxidase 2
Author contribution
All authors contributed equally in writing and reviewing the manuscript.
Funding
Not applicable.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Almatroodi SA, Almatroudi A, Khan AA, et al. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules. 2020;25:3146. doi: 10.3390/molecules25143146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alom-Ruiz SP, Anilkumar N, Shah AM. Reactive oxygen species and endothelial activation. Antioxid Redox Signal. 2008;10:1089–1100. doi: 10.1089/ars.2007.2007. [DOI] [PubMed] [Google Scholar]
- Anish N, Rajshree Roy C (2020) Piperine, an alkaloid of black pepper seeds can effectively inhibit the antiviral enzymes of dengue and Ebola viruses, an in silico molecular docking study. Virusdisease 31:308–315. 10.1007/s13337-020-00619-6 [DOI] [PMC free article] [PubMed]
- Balakrishna Pillai AK, Chu JJH, Mariappan V, JeanPierre AR. Platelets in the pathogenesis of flavivirus disease. Curr Opin Virol. 2022;52:220–228. doi: 10.1016/j.coviro.2021.12.007. [DOI] [PubMed] [Google Scholar]
- Barbachano-Guerrero A, Endy TP, King CA. Dengue virus non-structural protein 1 activates the p38 MAPK pathway to decrease barrier integrity in primary human endothelial cells. J Gen Virol. 2020;101:484–496. doi: 10.1099/jgv.0.001401. [DOI] [PubMed] [Google Scholar]
- Berretta AA, Silveira MAD, Cóndor Capcha JM, De Jong D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed Pharmacother. 2020;131:110622. doi: 10.1016/j.biopha.2020.110622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyerstedt S, Casaro EB, Rangel ÉB (2021) COVID-19: angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur J Clin Microbiol Infect Dis 40:905–919. 10.1007/s10096-020-04138-6 [DOI] [PMC free article] [PubMed]
- Börgeling Y, Schmolke M, Viemann D, et al. Inhibition of p38 mitogen-activated protein kinase impairs influenza virus-induced primary and secondary host gene responses and protects mice from lethal H5N1 infection. J Biol Chem. 2014;289:13–27. doi: 10.1074/jbc.M113.469239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canzano P, Brambilla M, Porro B, et al. Platelet and endothelial activation as potential mechanisms behind the thrombotic complications of COVID-19 patients. JACC Basic Transl Sci. 2021;6:202–218. doi: 10.1016/j.jacbts.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra PK, Gerlach SL, Wu C, et al. Mesenchymal stem cells are attracted to latent HIV-1-infected cells and enable virus reactivation via a non-canonical PI3K-NFκB signaling pathway. Sci Rep. 2018;8:14702. doi: 10.1038/s41598-018-32657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih-Ching Y, Chang-Jer W, Chen-Yen C, Chiang-Ting C (2021) Green tea polyphenol catechins inhibit coronavirus replication and potentiate the adaptive immunity and autophagy-dependent protective mechanism to improve acute lung injury in mice. Antioxidants (Basel, Switzerland) 10:928. 10.3390/antiox10060928 [DOI] [PMC free article] [PubMed]
- Chiou W-F, Chen C-C, Wei B-L. 8-Prenylkaempferol suppresses influenza A virus-induced RANTES production in A549 cells via blocking PI3K-mediated transcriptional activation of NF-κB and IRF3. Evid Based Complement Alternat Med. 2011;2011:920828. doi: 10.1093/ecam/nep066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple NA, Mackow ER. Roles for endothelial cells in dengue virus infection. Advances in Virology. 2012;2012:e840654. doi: 10.1155/2012/840654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple NA, Mackow ER. Endothelial cells elicit immune-enhancing responses to dengue virus infection. J Virol. 2012;86:6408–6415. doi: 10.1128/JVI.00213-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang S-S, Sun M-Z, Yang E, et al. Prohibitin is overexpressed in Huh-7-HCV and Huh-7.5-HCV cells harboring in vitro transcribed full-length hepatitis C virus RNA. Virol J. 2011;8:424. doi: 10.1186/1743-422X-8-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delić N (2021) Mucolytic agents and ventilator-associated pneumonia in patients on invasive mechanical ventilation due to severe acute respiratory syndrome coronavirus 2. Identifier: NCT04755972. https://clinicaltrials.gov/ct2/show/NCT04755972
- Dong W, Xie W, Liu Y, et al. Receptor tyrosine kinase inhibitors block proliferation of TGEV mainly through p38 mitogen-activated protein kinase pathways. Antiviral Res. 2020;173:104651. doi: 10.1016/j.antiviral.2019.104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droebner K, Pleschka S, Ludwig S, Planz O. Antiviral activity of the MEK-inhibitor U0126 against pandemic H1N1v and highly pathogenic avian influenza virus in vitro and in vivo. Antiviral Res. 2011;92:195–203. doi: 10.1016/j.antiviral.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Dunn EF, Fearns R, Connor JH. Akt inhibitor Akt-IV blocks virus replication through an Akt-independent mechanism. J Virol. 2009;83:11665–11672. doi: 10.1128/JVI.01092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Sang H, Yuan N, et al. Ethanolic extract of propolis inhibits atherosclerosis in ApoE-knockout mice. Lipids Health Dis. 2013;12:123. doi: 10.1186/1476-511X-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkhondeh T, Folgado SL, Pourbagher-Shahri AM, et al. The therapeutic effect of resveratrol: focusing on the Nrf2 signaling pathway. Biomed Pharmacother. 2020;127:110234. doi: 10.1016/j.biopha.2020.110234. [DOI] [PubMed] [Google Scholar]
- Ferder L, Martín Giménez VM, Inserra F, et al. Vitamin D supplementation as a rational pharmacological approach in the COVID-19 pandemic. Am J Physiol Lung Cell Mol Physiol. 2020;319:L941–L948. doi: 10.1152/ajplung.00186.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiler J, Michaelis M, Naczk P, et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Gong J, Shen X, Chen C, et al. Down-regulation of HIV-1 infection by inhibition of the MAPK signaling pathway. Virol Sin. 2011;26:114–122. doi: 10.1007/s12250-011-3184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes JM, Grimes KV. p38 MAPK inhibition: a promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg RC, Jordan Steel J, Moon SL, et al. Oxidative stress influences positive strand RNA virus genome synthesis and capping. Virology. 2015;475:219–229. doi: 10.1016/j.virol.2014.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haasbach E, Müller C, Ehrhardt C, et al. The MEK-inhibitor CI-1040 displays a broad anti-influenza virus activity in vitro and provides a prolonged treatment window compared to standard of care in vivo. Antiviral Res. 2017;142:178–184. doi: 10.1016/j.antiviral.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Hall A, Troupin A, Londono-Renteria B, Colpitts TM. Garlic organosulfur compounds reduce inflammation and oxidative stress during dengue virus infection. Viruses. 2017;9:E159. doi: 10.3390/v9070159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamed MGM, Hagag RS. The possible immunoregulatory and anti-inflammatory effects of selective serotonin reuptake inhibitors in coronavirus disease patients. Med Hypotheses. 2020;144:110140. doi: 10.1016/j.mehy.2020.110140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang BX, Shaw G, Fang W, Han B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J Glob Antimicrob Resist. 2020;23:256–262. doi: 10.1016/j.jgar.2020.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu AC-Y, Starkey MR, Hanish I, et al. Targeting PI3K-p110α suppresses influenza virus infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:1012–1023. doi: 10.1164/rccm.201501-0188OC. [DOI] [PubMed] [Google Scholar]
- Incalza MA, D’Oria R, Natalicchio A, et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vascul Pharmacol. 2018;100:1–19. doi: 10.1016/j.vph.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Jarosz M, Olbert M, Wyszogrodzka G, et al. Antioxidant and anti-inflammatory effects of zinc. Zinc-Dependent NF-Κb Signaling Inflammopharmacol. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Ji W, Yang H, et al. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transduct Target Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky-Bromberg SA, Martinez-Sobrido L, Palese P. 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen-activated protein kinase. J Virol. 2006;80:785–793. doi: 10.1128/JVI.80.2.785-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai-Becker M (2022) N-acetylcysteine for Attenuation of COVID Symptomatology. Identifier: NCT05074121. https://clinicaltrials.gov/ct2/show/NCT05074121
- Lapenna D (2021) Antioxidant therapy in COVID-19: the crucial role of early treatment and antioxidant typology. Clin Infect Dis 73:2370–2371. 10.1093/cid/ciab055 [DOI] [PMC free article] [PubMed]
- Lee HS, Jun J-H, Jung E-H, et al. Epigalloccatechin-3-gallate inhibits ocular neovascularization and vascular permeability in human retinal pigment epithelial and human retinal microvascular endothelial cells via suppression of MMP-9 and VEGF activation. Molecules. 2014;19:12150–12172. doi: 10.3390/molecules190812150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zang R, Ma Y, et al. Xanthohumol is a potent pan-inhibitor of coronaviruses targeting main protease. Int J Mol Sci. 2021;22:12134. doi: 10.3390/ijms222212134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ying Y. The inhibitory effect of curcumin on virus-induced cytokine storm and its potential use in the associated severe pneumonia. Front Cell Dev Biol. 2020;8:479. doi: 10.3389/fcell.2020.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas K, Fröhlich-Nowoisky J, Oppitz N, Ackermann M. Cinnamon and hop extracts as potential immunomodulators for severe COVID-19 cases. Front Plant Sci. 2021;12:589783. doi: 10.3389/fpls.2021.589783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Pan W, Li R, et al. Liu Shen capsule shows antiviral and anti-inflammatory abilities against novel coronavirus SARS-CoV-2 via suppression of NF-κB signaling pathway. Pharmacol Res. 2020;158:104850. doi: 10.1016/j.phrs.2020.104850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magesh S, Chen Y, Hu L. Small molecule modulators of Keap1-Nrf2-ARE pathway as potential preventive and therapeutic agents. Med Res Rev. 2012;32:687–726. doi: 10.1002/med.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda G, Rojo AI, Martínez-Klimova E, et al. Nordihydroguaiaretic acid: from herbal medicine to clinical development for cancer and chronic diseases. Front Pharmacol. 2020;11:151. doi: 10.3389/fphar.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariappan V, Adikari S, Shanmugam L, et al. Expression dynamics of vascular endothelial markers: endoglin and syndecan-1 in predicting dengue disease outcome. Transl Res. 2021;232:121–141. doi: 10.1016/j.trsl.2021.02.001. [DOI] [PubMed] [Google Scholar]
- Meltzer DO, Best TJ, Zhang H, et al. Association of vitamin D levels, race/ethnicity, and clinical characteristics with COVID-19 test results. JAMA Netw Open. 2021;4:e214117. doi: 10.1001/jamanetworkopen.2021.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrityunjaya M, Pavithra V, Neelam R, et al. Immune-boosting, antioxidant and anti-inflammatory food supplements targeting pathogenesis of COVID-19. Front Immunol. 2020;11:570122. doi: 10.3389/fimmu.2020.570122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy PD, Pogany J. The dependence of viral RNA replication on co-opted host factors. Nat Rev Microbiol. 2011;10:137–149. doi: 10.1038/nrmicro2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki Y, Kono I, Kuriki T, et al. Bovine lactoferrin ameliorates ferric nitrilotriacetate-induced renal oxidative damage in rats. J Clin Biochem Nutr. 2012;51:84–90. doi: 10.3164/jcbn.11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olagnier D, Peri S, Steel C, et al. Cellular oxidative stress response controls the antiviral and apoptotic programs in dengue virus-infected dendritic cells. PLoS Pathog. 2014;10:e1004566. doi: 10.1371/journal.ppat.1004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paracha UZ, Fatima K, Alqahtani M, et al. Oxidative stress and hepatitis C virus. Virol J. 2013;10:251. doi: 10.1186/1743-422X-10-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Mazliah D, Albareda MC, Alvarez MG, et al. Allopurinol reduces antigen-specific and polyclonal activation of human T cells. Front Immunol. 2012;3:295. doi: 10.3389/fimmu.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone C, Bartoloni E, Bursi R, et al. COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res. 2020;68:213–224. doi: 10.1007/s12026-020-09145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai AB, Muthuraman KR, Mariappan V, et al. Oxidative stress response in the pathogenesis of dengue virus virulence, disease prognosis and therapeutics: an update. Arch Virol. 2019;164:2895–2908. doi: 10.1007/s00705-019-04406-7. [DOI] [PubMed] [Google Scholar]
- Polonikov A. Endogenous deficiency of glutathione as the most likely cause of serious manifestations and death in COVID-19 patients. ACS Infect Dis. 2020;6:1558–1562. doi: 10.1021/acsinfecdis.0c00288. [DOI] [PubMed] [Google Scholar]
- Poudel A, Poudel Y, Adhikari A, et al. D-dimer as a biomarker for assessment of COVID-19 prognosis: D-dimer levels on admission and its role in predicting disease outcome in hospitalized patients with COVID-19. PLoS ONE. 2021;16:e0256744. doi: 10.1371/journal.pone.0256744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratomo IP, Ariane A, Tedjo A, et al. Xanthine oxidase inhibition in SARS-CoV-2 infection: the mechanism and potency of allopurinol and febuxostat in COVID-19 management. Med J Indones. 2021;30:75–80. doi: 10.13181/mji.rev.204641. [DOI] [Google Scholar]
- Rahban M, Habibi-Rezaei M, Mazaheri M, et al. Anti-viral potential and modulation of Nrf2 by curcumin: pharmacological implications. Antioxidants (basel) 2020;9:E1228. doi: 10.3390/antiox9121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman MU, Rashid S, Arafah A, et al. Piperine regulates Nrf-2/Keap-1 signalling and exhibits anticancer effect in experimental colon carcinogenesis in Wistar rats. Biology (basel) 2020;9:E302. doi: 10.3390/biology9090302. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reiter CEN, Kim J, Quon MJ. Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1. Endocrinology. 2010;151:103–114. doi: 10.1210/en.2009-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshi ML, Su Y-C, Hong J-R. RNA viruses: ROS-mediated cell death. Int J Cell Biol. 2014;2014:467452. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schink A, Neumann J, Leifke AL, et al. Screening of herbal extracts for TLR2- and TLR4-dependent anti-inflammatory effects. PLoS ONE. 2018;13:e0203907. doi: 10.1371/journal.pone.0203907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherkawy SM (2021) Effect of N-acetylcysteine on oxidative stress and occurrence of complications in patients with COVID 19 infections. Identifier: NCT04792021. https://clinicaltrials.gov/ct2/show/NCT04792021
- Shin YM, Shin HJ, Heo Y, et al. Engineering an aligned endothelial monolayer on a topologically modified nanofibrous platform with a micropatterned structure produced by femtosecond laser ablation. J Mater Chem B. 2017;5:318–328. doi: 10.1039/c6tb02258h. [DOI] [PubMed] [Google Scholar]
- Son Y, Cheong Y-K, Kim N-H, et al. Mitogen-activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto ME, Guarner-Lans V, Soria-Castro E, et al. Is antioxidant therapy a useful complementary measure for Covid-19 treatment? An Algorithm for Its App Medicina (kaunas) 2020;56:E386. doi: 10.3390/medicina56080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Acosta R, Bautista-Carbajal P, Syed GH, et al. Nordihydroguaiaretic acid (NDGA) inhibits replication and viral morphogenesis of dengue virus. Antiviral Res. 2014;109:132–140. doi: 10.1016/j.antiviral.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Soundravally R, Hoti SL, Patil SA, et al. Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence. Int J Infect Dis. 2014;18:68–72. doi: 10.1016/j.ijid.2013.09.022. [DOI] [PubMed] [Google Scholar]
- Sreekanth GP, Chuncharunee A, Sirimontaporn A, et al. SB203580 modulates p38 MAPK signaling and dengue virus-induced liver injury by reducing MAPKAPK2, HSP27, and ATF2 phosphorylation. PLoS ONE. 2016;11:e0149486. doi: 10.1371/journal.pone.0149486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrenner H, Al-Quraishy S, Dkhil MA, et al. Dietary selenium in adjuvant therapy of viral and bacterial infections. Adv Nutr. 2015;6:73–82. doi: 10.3945/an.114.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai WM, Yong WP, Lim C, et al. A phase Ib study of selumetinib (AZD6244, ARRY-142886) in combination with sorafenib in advanced hepatocellular carcinoma (HCC) Ann Oncol. 2016;27:2210–2215. doi: 10.1093/annonc/mdw415. [DOI] [PubMed] [Google Scholar]
- Tomo S, Saikiran G, Banerjee M, Paul S. Selenium to selenoproteins - role in COVID-19. EXCLI J. 2021;20:781–791. doi: 10.17179/excli2021-3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violi F, Oliva A, Cangemi R, et al. Nox2 activation in Covid-19. Redox Biol. 2020;36:101655. doi: 10.1016/j.redox.2020.101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen Y, Gao N, et al. Inhibitory effect of glutathione on oxidative liver injury induced by dengue virus serotype 2 infections in mice. PLoS ONE. 2013;8:e55407. doi: 10.1371/journal.pone.0055407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzhong L Hualan L (2021) COVID-19: captures iron and generates reactive oxygen species to damage the human immune system. Autoimmunity 54:213–224. 10.1080/08916934.2021.1913581 [DOI] [PMC free article] [PubMed]
- Wessels I, Rolles B, Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakhchali M, Taghipour Z, Mirabzadeh Ardakani M, et al. Cinnamon and its possible impact on COVID-19: the viewpoint of traditional and conventional medicine. Biomed Pharmacother. 2021;143:112221. doi: 10.1016/j.biopha.2021.112221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Wei J, Huang T, et al. Resveratrol inhibits the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother Res. 2021;35:1127–1129. doi: 10.1002/ptr.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H-S, Wu T-C, Sang W-W, Ruan Z. EGCG inhibits Tat-induced LTR transactivation: role of Nrf2, AKT, AMPK signaling pathway. Life Sci. 2012;90:747–754. doi: 10.1016/j.lfs.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Zhang J, Shen X, Wang K, et al. Antioxidant activities and molecular mechanisms of the ethanol extracts of Baccharis propolis and Eucalyptus propolis in RAW64.7 cells. Pharm Biol. 2016;54:2220–2235. doi: 10.3109/13880209.2016.1151444. [DOI] [PubMed] [Google Scholar]
- Zhou X, Cheng Z, Luo L, et al. Incidence and impact of disseminated intravascular coagulation in COVID-19 a systematic review and meta-analysis. Thromb Res. 2021;201:23–29. doi: 10.1016/j.thromres.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimecki M, Actor JK, Kruzel ML. The potential for Lactoferrin to reduce SARS-CoV-2 induced cytokine storm. Int Immunopharmacol. 2021;95:107571. doi: 10.1016/j.intimp.2021.107571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.
Not applicable.