Abstract
SCH56592 (SCH) was evaluated in an immunosuppressed rabbit model of invasive aspergillosis. SCH was more effective than similar doses of itraconazole and as effective as amphotericin B in the clearance of Aspergillus spp. from tissues. Compared with controls, SCH regimens reduced mortality, improved survival, and significantly reduced tissue colony counts.
Invasive aspergillosis is among the leading fungal diseases leading to death in immunocompromised patients, including hematology, transplant, cancer, and AIDS patients, but is rarely found in hosts with intact immune systems (2, 8). Amphotericin B and itraconazole are the only commercially available antifungal agents that have been shown to be effective against Aspergillus spp., yet each has drawbacks (8). Amphotericin B remains the drug of choice for the treatment of invasive aspergillosis, but therapy with amphotericin B is often toxic and may be ineffective, particularly in immunosuppressed hosts (8). Itraconazole, while less toxic than amphotericin B, has variable bioavailability in certain patient groups, and itraconazole resistance in A. fumigatus has been recently described (1, 3, 10). One approach to improving antifungal therapies has been in the development of the newer azoles. These agents offer several potential advantages over amphotericin B, including offering oral therapy, reduced toxicity, and a broad therapeutic index (5, 6). One of the newer azoles is SCH56592 (SCH), a triazole compound with a broad spectrum of antifungal activity, a long terminal half-life, and excellent absorption following oral administration (1, 17).
We used a rabbit model of invasive aspergillosis to evaluate the efficacy of antifungal therapy in this disease (14–16). In this lethal model, rabbits are made leukopenic and are further immunocompromised with steroid therapy. Extensive infection develops in liver, kidney, lung, and brain, similar to the progression of clinically disseminated invasive aspergillosis (12, 13). In this study, we assessed the activity of SCH in experimental invasive aspergillosis and compared its efficacy to the efficacies of amphotericin B and itraconazole. In addition, we compared the in vitro activity of SCH with the activities of amphotericin B and itraconazole against several Aspergillus spp. isolates.
New Zealand White female rabbits (2.5 kg) were immunosuppressed as previously described (14, 15) with a single intravenous (i.v.) 200-mg dose of cyclophosphamide (Pharmacia, Inc., Kalamazoo, Mich.) injected through a lateral ear vein and daily subcutaneous 10-mg doses of triamcinolone acetonide (Steris Laboratories, Inc., Phoenix, Ariz.). With this immunosuppressive regimen, the rabbits have reduced total leukocyte counts through day 7 (15). Twenty-four hours after immunosuppression, groups of 8 to 10 rabbits were challenged i.v. through a lateral ear vein with a lethal inoculum of 106 Aspergillus fumigatus conidia. Each group contained at least one untreated control rabbit, for which the lethal challenge was fatal within 5 days of challenge, with a mean survival of 3.9 ± 0.2 days (range 3 to 5 days) after challenge. Ceftazidime (200 mg) (SmithKline Beecham Pharmaceuticals, Philadelphia, Pa.) was administered intramuscularly daily beginning on the day of challenge in order to prevent intercurrent bacterial infection.
Antifungal therapy with amphotericin B (Fungizone; Bristol-Myers Squibb Co., Princeton, N.J.), SCH (Schering-Plough Research Institute, Kenilworth, N.J.), or itraconazole cyclodextrin solution (Janssen Research Foundation, Beerse, Belgium) was begun 24 h after challenge with A. fumigatus conidia and was continued for 5 days. Amphotericin B was diluted with 5% dextrose in sterile water at a ratio of 1 mg of drug to 10 ml of diluent and was given i.v. over 15 to 30 min through a lateral ear vein at a dose of 1.0 mg/kg/day. SCH was suspended in 0.4% methyl cellulose solution (4.0 g of methyl cellulose [Sigma Chemical, St. Louis, Mo.], 5.6 ml of Tween 80 [Sigma], and 9.0 g of NaCl [Sigma] per liter of H2O) and was administered via gastric gavage tube (Monoject, St. Louis, Mo.) as a suspension at doses of 2.5 (SCH 2.5) or 10 (SCH 10) mg/kg/day. Itraconazole was also administered via gastric gavage at 10 mg/kg/day.
Organ cultures and histopathology were performed at the time of sacrifice (72 h after completion of therapy in the treated rabbits). Rabbits were sacrificed by terminal exsanguination after being anesthetized with 35 mg of ketamine HCl per kg (Fort Dodge Laboratories, Inc., Fort Dodge, Iowa) and 10 mg of xylazine per kg (Bayer Corporation, Agriculture Division, Shawnee Mission, Kans.). Organs (brain, lung, liver, and kidneys) were removed aseptically following sacrifice and were cultured to determine the degree of infection with A. fumigatus. Organs were considered positive when three or more colonies of A. fumigatus were present on minced tissues planted directly on Sabouraud dextrose agar (Sab-Dex) plates (Becton Dickinson and Co., Cockeysville, Md.) or when semiquantitative cultures of tissue homogenates contained over 30 CFU per g of tissue (14). In combination, these two methods detected A. fumigatus at 3 to 20,000 CFU/g of tissue (7). Samples of each organ were finely chopped (manually), weighed, diluted 1:10 (wt/vol) with sterile saline, and homogenized for 25 s with an electric tissue homogenizer (IKA-Works, Inc., Cincinnati, Ohio). Duplicate 0.1- and 1.0-ml samples of the organ homogenate were plated on Sab-Dex and incubated at 37°C for 48 h, and colonies were counted.
A. fumigatus isolate P160, a clinical isolate which had been used in previous animal studies, was grown on Sab-Dex slants at 37°C for 24 h. For injection into the rabbits, conidia were harvested by a sterile saline wash of the slant surface, with conidia being dislodged by gentle rubbing with a sterile glass rod. The resultant conidial suspension was adjusted to the desired concentration of 106 conidia/ml by hemacytometer count, which was verified by duplicate serial plating on Sab-Dex plates for colony counts.
Additionally, broth macrodilution MICs were obtained for 23 clinical isolates of Aspergillus spp. and were tested against amphotericin B, itraconazole, and SCH following the protocol recommended by the National Committee for Clinical Laboratory Standards (9). Included in this study were A. fumigatus (11 isolates), Aspergillus niger (five isolates), Aspergillus flavus (five isolates), and Aspergillus terreus (2 isolates). Testing was performed by the Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio.
The Fisher exact test and the Wilcoxon rank sum test were used where appropriate. Statistical significance was defined as P < 0.05, adjusting for multiple dose comparisons. Specifically, 10 drug group comparisons were made for each organ evaluated so that the level of significance was P < 0.005.
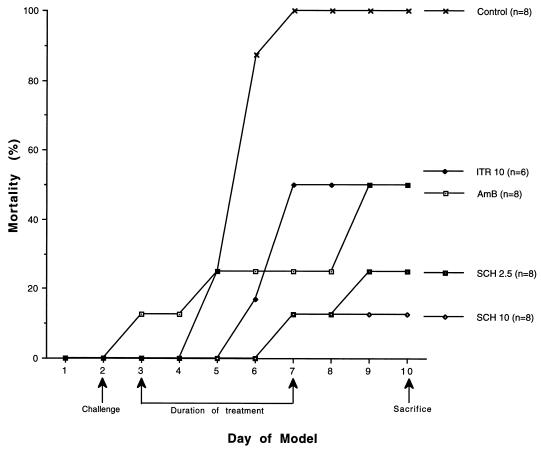
In this series of experiments, antifungal therapies with amphotericin B, itraconazole, and SCH begun 24 h after lethal A. fumigatus challenge improved survival as compared to untreated infected controls (Fig. 1). By day 6, rabbits receiving SCH 2.5 or SCH 10 showed an increase in survival over those treated with either amphotericin B or itraconazole versus controls. By the 5th day following challenge, death occurred in all untreated control rabbits while half of those treated with either amphotericin B or itraconazole survived to day 9. Therapy with SCH 2.5 or SCH 10 reduced mortality to two of eight (25%) and one of eight (13%) rabbits, respectively. All antifungal treatment regimens used in these experiments increased the mean days of survival versus controls for these rabbits. Survival after SCH 10 was significantly improved as compared with itraconazole, SCH 2.5, and untreated controls (P < .001). In this model of lethal infection, SCH therapy significantly prolonged survival as compared to untreated controls or to those rabbits treated with either itraconazole at 10 mg/kg/day or amphotericin B at 1 mg/kg/day.
FIG. 1.
Cumulative mortality of rabbits treated with SCH, itraconazole (ITR), and amphotericin B (AmB). Rabbits were challenged on day 2. Controls received no antifungal therapy. Treated rabbits received 1 mg of Amb per kg/day (AmB 1), 10 mg of ITR per kg/day (ITR 10), or SCH 2.5 or SCH 10 initiated 24 h after challenge, daily for 5 days. Rabbits were sacrificed 72 h after the end of treatment.
Semiquantitative cultures of liver, lung, kidney, and brain are shown in Table 1. The kidneys, liver, and lungs of all untreated control animals were extensively infected. Both SCH 10 and amphotericin B significantly reduced tissue burdens of Aspergillus (P < 0.001). SCH 10 reduced the tissue burden of Aspergillus 100- to 1,000-fold versus controls but was not significantly different from amphotericin B. In brain tissue, a 10-fold reduction in colony counts was obtained with both SCH 10 and amphotericin B as compared to controls, yet neither SCH 2.5 nor itraconazole differed from controls in this tissue.
TABLE 1.
Semiquantitative organ cultures of rabbits treated with antifungal therapy begun 24 h after challenge and sacrificed 72 h after the completion of therapy
| Group (no. of rabbits) | Colony countsa (no. of positive cultures/no. of rabbits cultured) in:
|
|||
|---|---|---|---|---|
| Liver | Lung | Kidney | Brain | |
| Control (8) | 3.5 ± 0.2 (8/8) | 2.8 ± 0.2 (8/8) | 2.9 ± 0.1 (8/8) | 1.7 ± 0.4 (7/8) |
| ITR 10 (6) | 3.4 ± 0.3 (6/6) | 3.0 ± 0.3 (6/6) | 3.1 ± 0.2 (6/6) | 1.5 ± 0.5 (4/6) |
| SCH 2.5 (8) | 2.6 ± 0.4 (8/8) | 2.9 ± 0.3 (8/8) | 2.8 ± 0.4 (7/8) | 1.7 ± 0.4 (7/8) |
| SCH 10 (8) | 0.5 ± 0.3b (2/8) | 1.3 ± 0.4 (5/8) | 0.8 ± 0.4b (4/8) | 0.6 ± 0.4 (3/8) |
| AmB 1.0 (8) | 0.8 ± 0.4b (3/8) | 1.2 ± 0.4 (6/8) | 0.3 ± 0.2b (2/8) | 0.4 ± 0.2 (3/8) |
Given as mean log10 CFU/gm of tissue ± standard error.
P < 0.001 versus controls, ITR 10, and SCH 2.5, as calculated by Wilcoxon Rank Sum test.
Culture results from rabbits treated with SCH, itraconazole, and amphotericin B are also shown in Table 1. SCH 10 and amphotericin B were more effective than SCH 2.5 and itraconazole at sterilizing liver and kidney tissues as compared to controls, and these therapies were not significantly different. Additionally, SCH 10 and amphotericin B were more effective in reducing positive cultures (A. fumigatus being undetectable in three of eight and two of eight cultures following these respective treatments) in lung tissue than were either SCH 2.5 or itraconazole as compared to controls. Itraconazole and SCH 2.5, on the other hand, were virtually indistinguishable from controls in reducing tissue burden; however, liver counts were reduced almost 10-fold by SCH 2.5 as compared to controls. The ability of SCH to approximate the ability of amphotericin B to reduce fungal burden in this model, coupled with the reduced mortality attributed to either dose of SCH, suggests that this compound may have clinical usefulness in the treatment of invasive aspergillosis.
Antifungal susceptibility testing with amphotericin B, itraconazole, and SCH was performed according to the National Committee for Clinical Laboratory Standards' M-38P broth macrodilution protocol on 23 Aspergillus spp. clinical isolates, including one A. fumigatus isolate used to infect the rabbits in this model. All isolates examined showed susceptibility to each of the three antifungal drugs tested. The geometric mean MICs at 48 h for 11 isolates of A. fumigatus with amphotericin B, itraconazole, and SCH were 0.4695 (range, 0.25 to 0.5), 0.2804 (range, 0.125 to 1), and 0.0831 (range, 0.06 to 0.5), respectively. For five isolates of A. niger, the geometric mean MICs were 0.500 for amphotericin B (range, 0.25 to 1), 0.4352 for itraconazole (range, 0.25 to 0.5), and 0.1649 for SCH (range, 0.125 to 0.25); for five isolates of A. flavus, the MICs were 1.1487 for amphotericin B (range, 1 to 2), 0.0798 for itraconazole (range, 0.06 to 0.25), and 0.0805 for SCH (range, 0.06 to 0.125); and for two isolates of A. terreus, the MICs were 1.414 for amphotericin B (range, 1 to 2), 0.4243 for itraconazole (range, 0.03 to 0.06), and 0.0612 for SCH (range, 0.03 to 0.125). The geometric mean MICs for SCH were lower than those for amphotericin B and itraconazole in nearly all cases, indicating the excellent potency of this new drug. These results support other reports of in vitro Aspergillus sp. susceptibility to SCH (4, 10), with similar MIC ranges and geometric mean MICs. With Aspergillus spp. and other filamentous fungi, reliable correlations of in vitro MIC data with in vivo clinical responses remain controversial and should be validated (4, 11).
Effective therapy for invasive aspergillosis remains elusive (6). The “gold standard” of aspergillosis therapy, amphotericin B, is often ineffective in certain patients and may prove toxic (3, 8) while itraconazole, often used for the treatment of this disease, may be erratically absorbed and ineffective (1, 10). One response to this situation has been the development of new antifungal drugs. One of these, SCH, was evaluated in a rabbit model of infectious aspergillosis. SCH demonstrated significant efficacy in treating experimental invasive aspergillosis in a dose response fashion, and this drug was superior to a similar dose of itraconazole. SCH approximated the efficacy of amphotericin B in this lethal model of invasive aspergillosis and reduced mortality more than amphotericin B. SCH should be evaluated for use in the treatment of invasive aspergillosis.
Acknowledgments
This work was supported by a grant from the Schering-Plough Research Institute.
We thank the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio for performing antifungal susceptibility testing.
REFERENCES
- 1.Connolly P, Wheat J, Schnizlein-Bick C, Durkin M, Kohler S, Smedema M, Goldberg J, Brizendine E, Loebenburg D. Comparison of a new triazole antifungal agent, Schering 56592, with itraconazole and amphotericin B for treatment of histoplasmosis in immunocompetent mice. Antimicrob Agents Chemother. 1999;43:322–328. doi: 10.1128/aac.43.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 3.Denning D W, Radford S A, Oakley K L, Hall L, Johnson E M, Warnock D W. Correlation between in-vitro susceptibility testing to itraconazole and in-vivo outcome of Aspergillus fumigatus infection. J Antimicrob Chemother. 1997;40:401–414. doi: 10.1093/jac/40.3.401. [DOI] [PubMed] [Google Scholar]
- 4.Espinel-Ingroff A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998;36:2950–2956. doi: 10.1128/jcm.36.10.2950-2956.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graybill J R. New antifungal agents. Eur J Clin Microbiol Infect Dis. 1989;5:402–412. doi: 10.1007/BF01964056. [DOI] [PubMed] [Google Scholar]
- 6.Graybill J R, Bocanegra R, Najvar L K, Luther M F, Loebenberg D. SCH56592 treatment of murine invasive aspergillosis. J Antimicrob Chemother. 1998;42:539–542. doi: 10.1093/jac/42.4.539. [DOI] [PubMed] [Google Scholar]
- 7.Graybill J R, Kaster S R. Experimental murine aspergillosis: comparison of amphotericin B and a new polyene antifungal drug, SCH 28191. Am Rev Respir Dis. 1984;129:292–295. [PubMed] [Google Scholar]
- 8.Latgé J-P. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev. 1999;12:310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 10.Oakley K L, Morrissey G, Denning D W. Efficacy of SCH-56592 in a temporarily neutropenic murine model of invasive aspergillosis with an itraconazole-susceptible and itraconazole-resistant isolate of Aspergillus fumigatus. Antimicrob Agents Chemother. 1997;41:1504–1507. doi: 10.1128/aac.41.7.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odds T C, Van Gerven F, Espinel-Ingroff A, Bartlett M S, Ghannoum M A, Lancaster M V, Pfaller M A, Rex J H, Rinaldi M G, Walsh T J. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob Agents Chemother. 1998;42:282–288. doi: 10.1128/aac.42.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patterson T F, Fothergill A W, Rinaldi M G. The efficacy of itraconazole solution in experimental invasive aspergillosis. Antimicrob Agents Chemother. 1993;37:2307–2310. doi: 10.1128/aac.37.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson T F, George D, Ingersoll R, Miniter P, Andriole V T. The efficacy of SCH-39304 in experimental invasive aspergillosis. Antimicrob Agents Chemother. 1991;35:1985–1988. doi: 10.1128/aac.35.10.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson T F, Miniter P, Dijkstra J, Szoka F C, Ryan J L, Andriole V T. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol-sulfate complexes. J Infect Dis. 1989;159:717–724. doi: 10.1093/infdis/159.4.717. [DOI] [PubMed] [Google Scholar]
- 15.Patterson T F, Miniter P, Ryan J L, Andriole V T. Effect of immunosuppression and amphotericin B on aspergillus antigenemia in an experimental model. J Infect Dis. 1988;158:415–422. doi: 10.1093/infdis/158.2.415. [DOI] [PubMed] [Google Scholar]
- 16.Sabetta J R, Miniter P, Andriole V T. The diagnosis of invasive aspergillosis by an enzyme-linked immunosorbent assay for circulating antigen. J Infect Dis. 1985;152:946–953. doi: 10.1093/infdis/152.5.946. [DOI] [PubMed] [Google Scholar]
- 17.Sugar A M, Xiu-Ping L. In vitro and in vivo activities of SCH 56592 against Blastomyces dermatitidis. Antimicrob Agents Chemother. 1996;40:1314–1316. doi: 10.1128/aac.40.5.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]