Abstract
Contributing to organ formation and tissue regeneration, extracellular matrix (ECM) constituents provide tissue with three-dimensional (3D) structural integrity and cellular-function regulation. Containing the crucial traits of the cellular microenvironment, ECM substitutes mediate cell–matrix interactions to prompt stem-cell proliferation and differentiation for 3D organoid construction in vitro or tissue regeneration in vivo. However, these ECMs are often applied generically and have yet to be extensively developed for specific cell types in 3D cultures. Cultured cells also produce rich ECM, particularly stromal cells. Cellular ECM improves 3D culture development in vitro and tissue remodeling during wound healing after implantation into the host as well. Gaining better insight into ECM derived from either tissue or cells that regulate 3D tissue reconstruction or organ regeneration helps us to select, produce, and implant the most suitable ECM and thus promote 3D organoid culture and tissue remodeling for in vivo regeneration. Overall, the decellularization methodologies and tissue/cell-derived ECM as scaffolds or cellular-growth supplements used in cell propagation and differentiation for 3D tissue culture in vitro are discussed. Moreover, current preclinical applications by which ECM components modulate the wound-healing process are reviewed.
Keywords: decellularized extracellular matrix, 3D culture, organoids, tissue repair
Introduction
As a three-dimensional (3D) network in biology, extracellular matrix (ECM) provides a microenvironment to cells for homeostasis, ingrowth, tissue formation, and repair [1]. Each tissue or organ has its own ECM with a distinct composition, which is generated in the early stages of embryonic development and constantly remodeled to control 3D tissue homeostasis [2]. Tissue-specific ECM offers optimal cell–cell and cell–ECM interactions by mimicking native signaling events [3]. Cell–ECM interactions are crucial for modulating cell behaviors, functions, and fates [4]. During tissue repair, quantitative and qualitative changes occur in ECM compounds during 3D tissue remodeling, which is regulated by specific enzymes, including matrix metalloproteinases (MMPs) [5].
The principle of cell-based bioengineering aims to (1) develop in vitro 3D culture models, such as organoid formation; and (2) regenerate damaged tissues and organs with a combination of cells and ECM scaffolds. Previous studies have reported the use of various synthetic scaffolds mimicking the 3D ECM for tissue regeneration. For example, the pLOXL1-Lipo@PLCL-HA co-delivery system reportedly promotes pelvic-floor repair in rabbits [6], and 3D electrospun short fibrous sponges are demonstrated to possess good 3D adhesion onto chronic diabetic wounds in rats [7]. However, clinical applications for biomaterials remain hampered probably because of the “inertness” of synthetic ECM scaffolds [8,9]. Conversely, natural ECM contains useful structural and biochemical information, providing sufficient bioactive cues to trigger cell functions needed for tissue regeneration [10,11]. Natural ECM scaffolds are generated from decellularized ECM (dECM), either from decellularized cells (C-ECM) or decellularized tissue-specific ECM (TS-ECM) [12].
Considering the numerous advantages of dECM for cell growth and differentiation because of the retention of biochemical cues, dECM products have become an attractive platform for several bioengineering applications [13]. Nowadays, dECM applications in pioneering scaffold-manufacturing techniques such as 3D cell printing and electrospinning also bring the field closer to clinical translation. 3D cell printing, also known as bioprinting, enables the recapitulation of the unique features of human tissues and organs through the design of bioink and polymerization techniques [14,15]. Bioink is a formulation of cellular components and biomaterials [14,16]. These biomaterials could satisfy the requirements to print cell-laden constructs; however, tissue- and organ-specific dECM-based bioinks can recapitulate a cell-supportive microenvironment niche in 3D cell-printed constructs [16]. The use of the bioprinting method for printing of cell-laden structures can reportedly provide an optimized microenvironment for 3D-structured tissue growth [17]. Thus, the new paradigm of dECM-based bioinks has been deemed as a powerful modern technology. Recently, electrospinning has attracted notable attention as another scaffold manufacturing technique. Electrospinning is a high-throughput technique that fabricates high-porosity fibrous scaffolds with nano-/microsized ultrafine fibers, whose morphology and structure mimic those of natural ECM [13,18,19]. The retention of architecture in electrospinning is beneficial for cell growth and alignment, but the biomechanical components in dECM may play a great role in cell differentiation [13]. Moreover, dECM is often difficult to scale up to clinically desired shapes due to its physiochemical properties. Thus, the combination of dECM and electrospinning can reduce the limitations of dECM scaffolds and provide them with tunability.
Despite the broad use of ECM, its exact mechanisms for tissue repair remain elusive. This review discusses the characteristics and mechanisms of tissue- or cell-specific ECM, along with the preparation for 3D organoid models and preclinical applications of tissue repair. Furthermore, we address challenges in clinical application and future directions.
Physiologic roles of TS-ECM in organ formation
ECM remodeling is crucial to organ formation and development. Among various organs, the intestine is an example of how ECM regulates normal organ morphogenesis [5]. In anurans tadpoles, the basement membrane of the tubular intestine thickens during intestinal metamorphosis. When induced by thyroid hormone, ECM proteins (including collagen, laminin, and fibronectin) increase, thereby inhibiting epithelial cell apoptosis in tadpoles [20]. Similarly, ECM remodeling is observed to play a central role in intestinal morphogenesis in rat [21] and mouse [22] models. Alternatively, other organs such as the lungs and the mammary and submandibular glands develop by epithelial branching. The branching process establishes the structure of these organs, and this process involves the repetitive formation of epithelial clefts and buds. The formation invades adjacent embryonic ECM, and the ECM composition and distribution shift over time. Thus, ECM remodeling provides structural integrity and regulates multiple cellular processes, such as cell growth, cell motility, and cell shape [23]. Meanwhile, the dysregulation of ECM components, structure, stiffness, and abundance may contribute to pathological conditions and exacerbate disease progression. For example, heavy scar formation is associated with abnormal ECM deposition [24], whereas osteoarthritis is linked to excessive ECM degradation [25].
ECM composition
ECM displays a 3D macromolecular network providing both structural support and biomechanical signaling to mediate cell behaviors, such as adhesion, proliferation, migration, and differentiation [26–28]. ECM consists of collagens, fibronectin (FN), laminins, elastin, proteoglycans (PGs), glycosaminoglycans (GAGs), and several other glycoproteins [29].
In mammalian tissues, ECM is generally divided into two types based on location and composition: (1) the interstitial connective tissue matrix, which surrounds and supports most stromal cells, thereby providing structural scaffolding for tissues, such as skeletal, and smooth muscle tissues [5]; and (2) the basement membrane, which primarily supports the epithelium and separates it from the environmental stroma, such as tubular and hollow structure tissues [5,30]. Although the ratios of ECM composition and structure vary among different organs or tissues, common biomacromolecules have been extensively studied (Table 1). The most dominant and abundant protein within tissue ECM is collagen [31]. Specifically, collagen type I functions in forming fibrils, collagen type II is rich in cartilage, and collagen type IV serves as a constituent part of the basement membrane [32]. Collagen types I and II are the main components of ECM. FN is a ubiquitous ECM glycoprotein that plays a critical role in attaching onto cells through binding between ligands and receptors. Thus, FN can provide molecules within the ECM with adhesion sites, such as collagens, integrins, proteoglycan, and heparan sulfate [29]. Laminins also serve as adhesive sites for ECM biomacromolecules and receptors located on the cell surface [29]. Elastin fibers are large ECM structures that undergo repeated stretching forces and thus provide recoil to tissues [33]. GAGs are usually covalently bonded to proteins to form PGs, which are vital molecules in tissue development and homeostasis [34]. Hyaluronan (HA) is a linear form of GAG containing repetitive disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid. As a major constituent of the pericellular matrix of many cell types, HA attaches onto its cellular receptors or binds to its own synthases, thereby influencing various cell functions [35].
Table 1.
Composition of ECM
| ECM protein | Tissue sources | Functions |
|---|---|---|
| Collagen | Resists tensile and shearing forces, affects various cellular functions [29,36] | |
| Collagen I (80%) | Skin, tendon, internal organs, organic parts of bone | |
| Collagen II | Cartilage | |
| Collagen III | Bone marrow, lymphoid tissues | |
| Collagen IV | Basement membrane | |
| Collagen V | Hair, surfaces of cells | |
| Fibronectin | Plasma, surfaces of cells | Cell adhesion sites, influences cellular behaviors [29,37] |
| Laminin | Basal lamina, placenta | Cell adhesion sites [29] |
| Elastin | Blood vessels, ligaments, skin, lung, bladder, elastic cartilage | Recoil [33] |
| Proteoglycans | Connective tissues, intracellular compartments, surfaces of cells | Resists compressive forces, provides recoil and participates in cell signaling and cellular behaviors [29,36] |
| Hyaluronan | Placenta, amniotic fluid, vitreous body, articular cartilage, dermis of skin | Lubricates, absorbs shock, affects cellular behaviors and signaling molecules [38,39] |
The composition of ECM is constantly updated. Matrix-bound nanovesicles, a subgroup of extracellular vesicles, have been recently found within ECM. They are embedded within it and have a tissue-specific microRNA cargo and membrane lipid structure that can play a significant role in the regulation of inflammation and healing processes [40].
Role of ECM in inducing stem-cell fate
Accurately guiding stem cells to give rise to target cells is challenging due to the lack of defined inductors. As a natural niche, ECM provides a dynamic microenvironment for cell replication and differentiation when stem cells are activated [36]. The dynamic interaction in the microenvironment is also deemed as “dynamic reciprocity” [41]. With cell–ECM communication, ECM regulates stem-cell fate through structural support, biochemical composition, growth factors, and biomechanical factors [4] (Fig. 1) (Table 2).
Fig. 1.
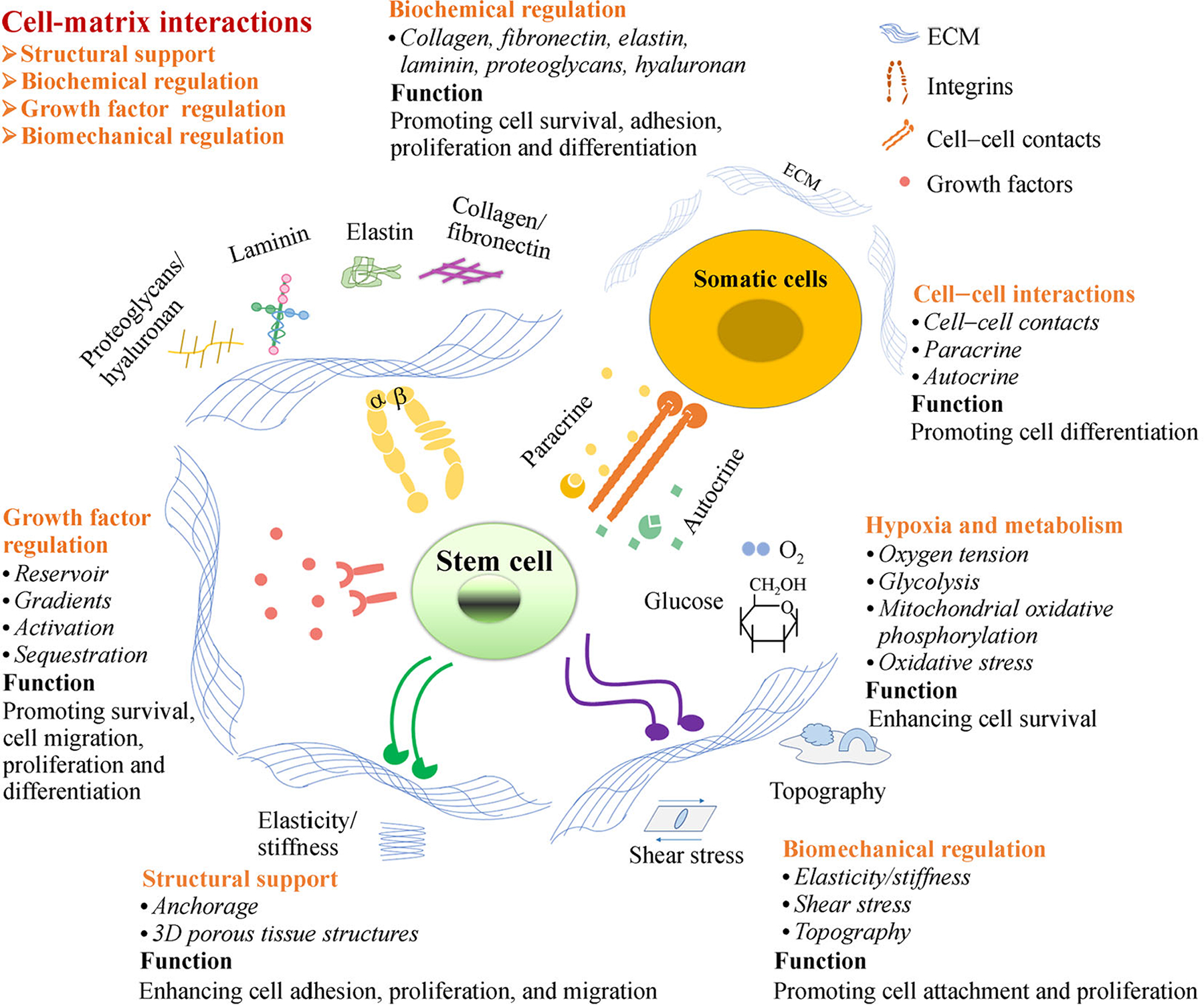
Role and composition of stem-cell niche. The stem-cell niches retain the stemness of adult stem cells in a quiescent state. When tissue is injured, the surrounding microenvironment actively signals stem cells to promote either self-renewal or differentiation to form new tissues. The niches include cell–matrix, cell–protein, protein–matrix, cell–cell interactions, hypoxia, and metabolism. Among these niche factors, cell–matrix interactions play a key role in prompting cell adhesion, migration, proliferation, and differentiation for tissue regeneration. The matrix regulates stem-cell behavior through structural supports, biochemical signaling, growth factor induction, and biomechanical regulation during tissue repair.
Table 2.
Role of ECM in inducing stem-cell fate
| Role | Mechanism(s) | Function(s) |
|---|---|---|
| Structural support | Porosity, mechanical properties, cell–matrix communication | Regulating cell adhesion, growth, differentiation and forming 3D tissue structures [43] |
| Biochemical regulation | Integrins | Regulating cell proliferation, adhesion, migration, differentiation, homing [45,46,49,64] |
| Growth factor regulation | Reservoir, gradients, sequestration, activation, autocrine, paracrine | Regulating growth factor bioavailability dynamically [52]; maintaining stem-cell survival, self-renewal, differentiation [64–66] |
| Biomechanical regulation | ECM topography, microstructure, stiffness, elasticity | Modulating cell shape, tissue elongation, cell–ECM interactions; regulating stem-cell fate [55–57,59,62–64] |
First, ECM provides structural support for cells primarily because of the following: (1) the 3D structure of ECM allows an interconnected porous structure, and (2) the cross-linked fibrillar network and other large molecules provide rich cell-adhesion points [42]. Structural support is essential for cell adhesion, growth, and differentiation [43]. In 2020, Satyam et al. [44] reported a cell-derived ECM platform that could support podocyte proliferation, differentiation, and maintenance of the native phenotype.
With regard to biochemical composition, cells interact with the biochemical composition of ECM through transmembrane receptors. Integrins are the predominant transmembrane receptors on the surface of cells, connecting ECM proteins to the cytoskeleton within cells. They play crucial roles in various cellular activities, such as adhesion, proliferation, migration, differentiation, and homing [45–49]. Various integrin types are associated with the interactions between the cells and ECM, such as integrin α6β1, integrin α9, integrin β1, and integrin αvβ3 [48]. In 2020, Lu et al. [50] reported that integrin β1 knockout inhibits induced pluripotent stem cells (iPSCs)’ adhesion and migration across activated endothelial monolayers. In 2021, Han et al. [51] demonstrated that anti-human integrin β1 antibody could specifically target human iPSCs and differentiate into various lineages in a mouse model.
Furthermore, ECM proteins can bind and regulate growth-factor bioavailability, serving as a growth-factor reservoir. ECM proteins such as FN, collagens, and PGs alone or combined with heparin sulfate can connect to various growth factors, such as fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF) [52]. Compared with unbounded growth factors, binding with ECM can potentiate their bioactivity. The phenomenon has already been observed in HGF, bone morphogenic protein (BMP)-2 and −4, acidic FGF, and insulin like growth factor (IGF)-1 [42,53]. ECM can also serve as microanatomic compartments. For example, due to the restrictions of basement membrane, asymmetric sequestration of bioactive factors occurs [52]. Thus, decellularized ECM having specific interactions with growth factors may generate dynamic and functional niches. In 2019, Ullah et al. [54] reported that replenishing human kidney ECM with VEGF results in more efficient differentiation of human iPSCs into endothelial cells (ECs).
Biomechanical factors including physical and mechanical forces can modulate the topography and microstructure of ECM in the local stem-cell microenvironment. Biomechanical factor changes can lead to variations in stem-cell shape and geometry. The microstructure of substrates could reportedly affect ECM protein binding [55,56]. Additionally, ECM stiffness has been identified as an important element in determining stem-cell fate in terms of lineage commitment [57,58] and self-renewal capacity [59]. For mesenchymal stem cells (MSCs), increased substrate stiffness enhances the osteogenic differentiation of MSCs [60,61], whereas soft matrix is inclined to induce chondrogenesis and adipogenesis [3]. ECM elasticity is another factor. In 2018, Hirata et al. [62] reported that the cardiac differentiation of iPSCs prefers highly elastic substrates in vitro. In 2020, Muncie et al. [63] demonstrated that substrates recapitulating embryo elasticity could promote human embryonic stem cells (ESCs) selforganization.
Recently, we have developed 3D human cell-based systems to replace the use of two-dimensional (2D) cell culture or animals for studying renal cytotoxicity [64]. To induce human urine-derived stem cells into renal tubular epithelial cells in 3D organoid culture, decellularized porcine kidney ECM is used as a culture supplement. Their results demonstrated that the levels of renal injury markers (CYP2E1 and KIM-1) in 3D organoids significantly increase in response to nephrotoxic agents (acetone and cisplatin). This 3D culture system with human stem cells and kidney-tissue ECM offers an alternative approach to renal-cytotoxicity testing [64].
Preparation of dECM
Decellularization is a bioengineering technology used to isolate ECM scaffold from the cells inhabiting it. The ECM scaffold product possesses bioactive molecules from native tissue, which can be used for tissue regeneration and disease remodeling. The goal of ECM decellularization is to retain ECM compounds and structure and remove xenogeneic cell compounds, thereby avoiding immunoreaction. Thus, assessing changes quantitatively and qualitatively in ECM is critical. ECM can also be mediated by certain enzymes, which are responsible for ECM degradation after implantation in vitro, such as MMPs. Currently, commercially used ECM scaffolds are applied in wide-ranging bioengineering applications and are typically divided into C-ECM and TS-ECM (Table 3).
Table 3.
Methodology of decellularized tissue or cell-derived ECM
| Agents/techniques | Mode of action | Effects on ECM |
|---|---|---|
| Physical treatments | ||
| Freeze and dry | Xenogeneic cellular compounds can be washed away after microscopic ice crystals disrupt cell membrane | Disrupt or fracture ECM fibers [92–94] |
| Mechanical-shaking force | Shaking action promotes cell debris removal from matrix | Disrupt ECM structure and clean up the cellular fragments [95–97] |
| NTIRE | Electrical pulse disrupts cellular membranes | Can disrupt ECM [98,99] |
| scCO2 | Deeply penetrates into tissues and solubilizes non-polar molecules | Can disrupt ECM when the system is rapidly depressurized [81] |
| Chemical treatments | ||
| Acids and bases | Disrupts both intracellular organelles and cell membranes | Break down collagen and GAGs and denature proteins or growth factors [95,100] |
| Ionic detergents | Solubilizes plasma membranes and nuclear membranes | Denature proteins via damaging bonds between proteins [82,101,102] |
| Non-ionic detergents | Disrupts bonds between lipids and between lipids and proteins | Beneficial to keep the ECM intact, may disrupt ultrastructure and GAGs [83,101,102] |
| Enzymatic treatments | ||
| Trypsin | Cleaves cell adhesion from ECM | Extended exposure can destroy the structure of ECM, remove fibronectin, laminin, elastin, GAG [103–105] |
| Dispase | Cleaves collagen IV and fibronectin | Extended exposure can destroy the ultrastructure of ECM [95,106] |
| Nuclease (DNase and RNase) | Degrades nucleic acids | Hard to remove, may induce immune reaction [107–109] |
| FBS (serum containing DNase and RNase) | Retains bioactive proteins, degrades remaining DNA/RNA | Can minimize the loss of major bioactive proteins, decrease xenogeneic immune response [86–88] |
| Combined methodologies | ||
| Shaking action + FBS | Optimizes approaches to remove xenogeneic cellular compounds by maintaining bioactive proteins and ECM structure |
ECM, extracellular matrix; GAGs, glycosaminoglycans; NTIRE, non-thermal irreversible electroporation; scCO2, supercritical carbon dioxide; FBS, fetal bovine serum.
Decellularization of cell-derived ECM
With various available treatments for decellularization, the careful monitoring of the combinations of physical, chemical, and enzymatic treatments is essential for the retention of the biochemical, biological, and biophysical properties of ECM [12]. Each of these methods may inflict damage to the structure and components of ECM, but no unified criteria exist for decellularization. Physical decellularization methods may be sufficiently harsh to alter ECM protein structures (e.g., collagen) and mechanical properties [67–70]. Chemical methods may break the connections between DNA and proteins, destroy the ultrastructure and growth factors, and denature ECM proteins [3,67,71–75]. Enzymes such as collagenase, lipase, trypsin, dispase, thermolysin, and nucleases [76,77] can remove cell residue or undesirable ECM components with high specificity. However, one limitation of enzymatic treatment is incomplete cell removal and impairment of recellularization [76]. Enzymatic treatments are insufficient for cell removal alone, so they are often combined with chemical detergents. Specific decellularization methods need to be optimized according to specific cell types, cell density, and ECM thickness [76]. Decellularization treatments are introduced systematically in the following section.
Decellularization of tissue-specific ECM
In TS-ECM, many decellularization methods are designed to remove all cellular components [78,79]. The ideal procedure is to lyse cells and then wash away the cellular compounds from the tissue while retaining the ECM components and bioactive molecules. Thus, TS-ECM products retain natural ECM properties to form bioengineered tissues. After decellularization, the xenogeneic ECM scaffold could be recellularized with stem or progenitor cells, which differentiate into the original cell types in the tissue. Given their diverse applications for tissue regeneration, decellularization techniques must be tailored and integrated to meet the requirements for specific tissues. Decellularization methods that have been investigated include physical, chemical, and enzymatic treatments. Although some are commonly used, the optimal combination for decellularization depends on the tissue’s origin, characteristics, and intended use [76]. As for perfusion and immersion decellularization techniques applied to organs or tissues, they are applicable for tissues with extensive vasculature.
Physical treatments
The most common physical methods used for decellularization are to lyse or break the cell membrane or remove cells from the tissue matrix through temperature changes, mechanical force, and non-thermal irreversible electroporation (NTIRE). The mechanism involved in temperature methods is rapid freeze and thaw. After cell lysis, liquefied chemicals are used to treat the tissue. The purpose of this step is to degrade and wash out undesirable components. Temperature methods retain the ECM physical structure and are most suitable for strong and thick tissues. Mechanical-shaking force is commonly applied to organs with natural planes of dissection, such as the urinary bladder and the small intestine [80]. NTIRE is another alternative to lyse cells by using electrical pulses, which can disrupt the plasma membrane. However, NTIRE technology is suitable only for small tissues.
Interest in supercritical fluid technology to decellularize tissues is also growing. Supercritical carbon dioxide (scCO2) easily penetrates into biological tissues, thereby facilitating the removal of structural components of cellular membranes (lipids). The main advantages of this protocol are the significant reduction in processing time and the sterilizing effects. Nevertheless, the high pressure in a reactor can lead to the rupture of cells with subsequent removal of cellular fragments when the system is rapidly depressurized [81].
Prevalent chemical treatments
The appropriate chemical detergents are selected based on the tissue’s/organ’s thickness, ECM composition, and intended use. The prevalent chemical detergents used for decellularization include acids, bases, ionic detergents, and non-ionic detergents.
Acids and bases are used for solubilizing cellular cytoplasmic components and removing nucleic acids, including RNA and DNA. These chemicals can effectively disrupt both intracellular organelles, cell membranes, and some important molecules, including GAGs. Ionic detergents are used for effectively solubilizing plasma membranes and nuclear membranes by breaking protein–protein interactions [82]. Sodium dodecyl sulfate (SDS) is commonly used because it can effectively lyse cells while not damaging ECM significantly. Right after the cell membranes are lysed by SDS, the genetic contents are degraded by endonucleases and exonucleases. Non-ionic detergents disrupt lipid–lipid and lipid–protein interactions but leave protein–protein interactions intact. Triton X-100 is the most widely used non-ionic detergent [83].
Enzymatic treatments
Enzyme methods are used to destroy attachments between nucleic acid bonds. They interact with cells via adjacent proteins or other components of the cells. Collagenase, lipase, trypsin, dispase, thermolysin, and nuclease have been used to remove cells [76]. Serum has also been successfully used for decellularization due to the existence of nucleases [30].
Collagenase is appropriate for producing ECM scaffolds only when unbroken collagen structures are not required. Lipase is applicable when generating decellularized skin scaffolds. The function of lipase acids in the decellularization of skin dermis is degreasing and breaking the bonds among lipidized cells. Trypsin, a kind of serine protease, is also a common enzymatic agent for decellularization. Dispase is effective in separating undesired cells from ECM scaffold for its use in preventing cell aggregation. However, enzymes such as dispase and thermolysin are ineffective for removing cells inside tissues; they are more effective in combination with mechanical abrasion for complete cell removal [84]. Nucleases including DNase and RNase are often used for the cleavage of nucleic acids. Thus, nucleases are usually used to remove nucleic acids after cell lysis with physical pressure and chemical detergents [85].
Serum is commonly used in cell-culture systems because it contains many essential components that are beneficial for cell growth and propagation. The most extensively used serum is fetal bovine serum (FBS). Serum also contains serum nucleases, which can degrade the DNA and RNA remaining after cell lysis. Utilizing serum in decellularization methods has two extraordinary advantages: (1) retaining bioactive molecules in ECM compared with other reagents for decellularization [86]; and (2) degrading the DNA and RNA remaining after cell lysis, which can potentially induce immune responses [86–88].
In summary, the optimal decellularization approach is to minimize the loss of major bioactive matrix components and the xenogeneic immune responses simultaneously [30,80]. Single or combined decellularization methods are applied to achieve optimal efficiency according to the features of specific tissues and organs.
Handling of decellularized scaffolds
Decellularization yields multiple kinds of decellularized scaffolds, which can be further recellularized for in vitro and in vivo studies. Decellularized scaffolds are deemed the final products if the original ECM architecture is well retained [89]. Furthermore, decellularized C-ECM could be used in either its original format, or it can be fragmented, ground, or solubilized. Either 2D ECM sheets or complicated 3D structures comprising 3D scaffolds can be produced from these formats [12]. In other cases, post-processing techniques are needed to produce various products and thus meet research and clinical requirements, including the lyophilization, milling, and digestion of ECM, resulting in an injectable hydrogel [90]. It can be further cross-linked with genipin or glutaraldehyde to enhance the integrity [91].
Applications of dECM
Considering the desired functions of ECM in mediating cellular behaviors, dECM is extensively used as a coating agent in 2D or 3D scaffolds [110]. Its utility in tissue regeneration and stem-cell lineage induction has now been widely examined among different tissues and organs. Based on the ECM source, we discuss the applications of C-ECM and TS-ECM separately.
Cell-derived ECM
C-ECM is commonly used as a coating on biomaterial surfaces, but more sophisticated approaches exist. For example, the synthesis products of C-ECM can serve as 2D substrates for engineering tissues de novo or facilitating wound healing and regeneration [111]. According to different applications, C-ECM can be used as a biomaterial to regenerate tissues or promote cell-lineage commitment [111].
Compared with TS-ECM, an ideal scaffold material in tissue engineering, C-ECM is normally considered an in vitro niche, in which primary cells and MSCs can be rejuvenated to maintain their proliferation and differentiation capacity [112–114]. For instance, C-ECM has been demonstrated to refresh tissue-specific stem cells such as synovium-derived stem cells (SDSCs) [115–122], bone marrow-derived MSCs (BMSCs) [123–125], umbilical cord-derived MSCs (UCMSCs) [126,127], infrapatellar fat pad-derived stem cells (IPFSCs) [128–130], ESCs [131], periodontal ligament stem cells [132], and neural progenitor cells [133]. C-ECM also refreshes primary cells such as chondrocytes [134,135], nucleus pulposus cells [136,137], and hepatic cells [138] in proliferation and redifferentiation capacities (Table 4). This rejuvenation effect of C-ECM primarily occurs thorough anti-inflammation and antioxidation [121,122,126,135,139], which can reverse senescent stem cells and primary cells [127].
Table 4.
Applications of cell-derived ECM for in vitro tissue formation and in vivo tissue repairing
| Application | ECM types | Cell types and animal models | Outcomes |
|---|---|---|---|
| Tissue regeneration | |||
| Cartilage tissue | Porcine SDSCs | Porcine SDSCs In vitro and in vivo (13 minipigs) | Enhancing SDSCs’ expansion, chondrogenic potential, and repair of cartilage defects [139] |
| Human adult vs. fetal SDSCs | Human adult SDSCs | Promoting adult SDSCs’ chondrogenic capacity by fetal ECM [140] | |
| Human fetal MSCs | Human adult MSCs | Promoting adult MSCs’ proliferation, multipotency, and stemness [141] | |
| Porcine chondrocytes vs. rabbit BMSCs | Rabbit chondrocytes | Supporting attachment and proliferation of chondrocytes [142] | |
| Porcine SDSCs | Porcine chondrocytes | Delaying chondrocyte dedifferentiation and enhanced redifferentiation [134] | |
| Porcine SDSCs vs. NPCs vs. SDSCs/NPCs | Porcine SDSCs | Guiding SDSCs’ differentiation toward the NP lineage [137] | |
| Porcine SDSCs | Porcine NPCs | Rejuvenating NPCs in proliferation and redifferentiation capacity [136] | |
| Bone tissue | Mouse BMSCs | Mouse BMSCs In vitro and in vivo (nude mice) | Enhancing colony formation ability and retaining stemness [143] |
| Human BMSCs | Human BMSCs In vitro and in vivo (nude mice) | Stimulating MSCs’ expansion and preserving their properties [144] | |
| Nerve tissue | Rat Schwann cells | Rat dorsal root ganglion neurons | Improving axonal growth of dorsal root ganglion neurons [145] |
| Lineage commitment | |||
| ESC differentiation | Murine ESCs line | Undifferentiated murine ESCs | Boosting early differentiation of ESCs [131] |
| Osteogenic differentiation | Rat osteoblasts | Human MSCs | Inducing osteogenic differentiation [146] |
| Human BMSCs | Human BMSCs | Enhancing osteogenesis [124,125] | |
| Human BMSCs | Human BMSCs | Further enhancing proliferation and osteogenesis when combined with melatonin [123] | |
| Human USCs | Human BMSCs (passage 8) | Recharging BMSCs’ capacity in endochondral bone formation [125] | |
| Human UCMSCs | Human UCMSCs | Enhancing UCMSCs’ osteogenic differentiation by protecting from H2O2 induced senescence [127] | |
| Chondrogenic differentiation | Rabbit articular chondrocytes | Human MSCs | Guiding chondrogenic differentiation [146] |
| Porcine SDSCs | Porcine SDSCs | Promoting SDSCs’ proliferation and chondrogenic potential [115] | |
| Porcine | Porcine SDSCs | Maximizing SDSCs’ proliferation while maintaining chondrogenic potential when combined with FGF2 and low oxygen [116] | |
| Human fetal SDSCs | Human fetal SDSCs | Enhancing fetal SDSCs’ chondrogenic potential [118] | |
| Human adult vs. fetal SDSCs | Human fetal SDSCs | Enhancing SDSCs’ proliferation and chondrogenic capacity in a pellet culture under hypoxia [117] | |
| Passage 5 vs. 15 human IPFSCs | Passage 15 human IPFSCs | Promoting IPFSCs’ proliferation and chondrogenic potential by C-ECM deposited by passage 5 cells [130] | |
| Human adult SDSCs | Human adult SDSCs | Enhancing SDSCs’ chondrogenic potential compared with those in ECM [121] | |
| Porcine IPFSCs vs. SDSCs | Porcine IPFSCs | Enhancing IPFSCs’ proliferation and chondrogenic potential in both ECM groups [128] | |
| Hepatic differentiation | Human liver progenitor HepaRG | Human DE cells | Aiding hepatic differentiation [138] |
SDSC, synovium-derived stem cell; MSC, mesenchymal stem cell; BMSC, bone marrow-derived mesenchymal stem cell; NPC,nucleus pulposus cell; BM, bone marrow; ESC, embryonic stem cell; USC, urine-derived stem cell; UCMSC, umbilical cord-derived mesenchymal stem cell; IPFSC, infrapatellar fat pad-derived stem cell; DE, definitive endoderm.
To explore the underlying mechanisms, adult human SDSCs are grown on C-ECM deposited by adult stem cells with varied chondrogenic capacity, including SDSCs (strong), adipose-derived stem cells (ADSCs; weak), and urine-derived stem cells (USCs; none), as well as C-ECM deposited by dermal fibroblasts (a non-stem-cell control) [119]. Despite the fact that expansion on C-ECM yields a large quantity of adult SDSCs with higher chondrogenic capacity than those on tissue-culture plastic (TCP), expansion on C-ECM deposited by SDSCs (with stronger chondrogenic capacity) yields SDSCs with less chondrogenic potential than those from other C-ECM groups. Intriguingly, SDSCs grown on C-ECM deposited by USCs display the highest expression of chondrogenic marker genes, aggrecan and type II collagen, which may be associated with the highest expression of basement membrane proteins. Furthermore, one basement membrane component, FN, has been evaluated in a recent study for its effect on the proliferation and differentiation capacity of stem cells by using CRISPR/CAS9-generated FN-knockout (FN1-KO) in human IPFSCs [129]. Wang et al. [129] found that FN1-KO promotes the proliferative capacity of human IPFSCs; however, this capacity is reversed during expansion on C-ECM generated by FN1-KO IPFSCs. The importance of FN in chondrogenic and adipogenic differentiation is also indicated in the FN1-KO IPFSCs and FN– matrix microenvironment.
Another interesting study is to assess the influence of C-ECM expansion and immortalization on stem-cell proliferation and differentiation [130]. Wang et al. [130] found that human IPFSCs transduced with SV40 large T antigen (SV40LT) yields an increase in proliferation and adipogenic capacity but a decrease in chondrogenic potential. Interestingly, expansion on C-ECM generated by SV40LT transduced cells yields human IPFSCs with enhanced proliferation and chondrogenic potential but decreased adipogenic capacity. This outcome has been demonstrated to be highly relevant to the expression and distribution of basement membrane proteins.
Tissue-specific ECM
Despite similar ECM composition among different tissues and organs, subtle differences in function, ratio, architecture, and stiffness of ECM can affect cellular interactions in determining cell fate [147]. Unlike C-ECM, which can refresh tissue-specific and non-tissue-specific stem/progenitor cells and primary cells, TS-ECM tends to function as a tissue-specific scaffold for stem/progenitor cells and primary cells in most cases [26]. Even without specific differentiation media, stem or progenitor cells still possess specific cell-lineage differentiation capacity based on particular interactions between cells and ECM [148]. Thus, compared with regular TCPs or natural scaffold such as collagens, TS-ECM is superior in maintaining [149] and guiding [150] stem-cell differentiation.
Depending on their application, TS-ECM products are generally divided by different organs (bone, articular cartilage, skeletal muscle, skin, and urinary bladder), different systems (musculoskeletal system, urinary system, and digestive system), or different germ layers (endoderm, mesoderm, and ectoderm). To address differences and its superiority to C-ECM, we classify TS-ECM products into four categories, namely, cell-culture supplements, cell sheets, tubular structures, and 3D structures according to different TS-ECM characteristics and applications (Table 5).
Table 5.
Applications of tissue-specific ECM in in vitro tissue construction or in vivo tissue regeneration
| Application | ECM type | Seeded cell types | Culture condition(s) | Outcomes |
|---|---|---|---|---|
| In vitro 3D cultures | ||||
| Powder substrates | Acellular rat skeletal muscle ECM; acellular rat liver ECM; acellular swine skin ECM | Rat muscle cells; HepG2; human foreskin cells | In vitro | Promoting cell proliferation and differentiation [147] |
| Hydrogel substrates | Acellular skeletal muscle ECM combined with hyaluronan-based hydrogel and heparin | MPCs | In vitro | Promoting MPCs’ proliferation and differentiation [30] |
| Cell sheet tissue regeneration | ||||
| Skin (dermis) | Acellular human dermal ECM, allogeneic | None | In vivo (14 patients) [161]; in vivo (2 patients) [163] | Reducing scar and contracture [161,163] |
| Cornea | Acellular porcine cornea ECM, xenogeneic | None | In vivo (10 chinchilla bastard rabbits) [164]; in vivo (six eyes of rabbits) [165] | Biocompatible with the host’s epithelium [164,165] |
| Tubular organ regeneration | ||||
| Blood vessels | Acellular porcine aorta, xenogeneic | Human ECs and myofibroblasts | In vivo (5 Lewis rats) | Successfully implanted subcutaneously in a rat model [176] |
| Acellular bovine pericardial ECM combined with poly propylene fumarate, xenogeneic | None | In vitro and in vivo (2 Lewis nude rats) | Remaining patent for two weeks in rat model [178] | |
| Esophagus | Acellular porcine SIS, xenogeneic | None | In vivo (5 patients) | Promoting reconstruction of functional esophageal mucosa in patients [180] |
| Acellular porcine SIS | Porcine BMSCs | In vitro | Meeting clinical-grade criteria, promising for clinical use [184] | |
| Bladder | Acellular porcine SIS, xenogeneic | None, or seeded with dog UCs and SMCs | In vitro and in vivo (22 dogs) | Not achieving the desired bladder regeneration resulting in a subtotal cystectomy model as in the 40% cystectomy model [185] |
| Acellular porcine SIS cross-linked with procyanidins, xenogeneic | None | In vitro and in vivo (48 New Zealand white rabbits) | Promoting in situ tissue regrowth and regeneration of rabbit bladder [187] | |
| 3D organ regeneration | ||||
| Liver | Acellular human liver ECM, allogeneic | hUVECs, hFLCs | In vitro | Decellularizing a whole liver organ for liver regeneration in vitro [201] |
| Acellular human liver ECM, xenogeneic | LX2, Sk-Hep-1, HepG2 | In vitro and in vivo (6 C57BL/6J mice) | Showing excellent viability, motility, proliferation and remodeling of the ECM in a mouse model [204] | |
| Lung | Acellular adult rat lung ECM, allogeneic | Neonatal rat lung epithelial cells | In vitro and in vivo (344 rats) | Engineered lungs participated in gas exchange in a rat model [85] |
| Acellular porcine lung ECM, xenogeneic | Human airway epithelial progenitor cells | In vitro and in vivo (3 pigs) | Demonstrating the feasibility of engineering of viable lung scaffolds in a porcine model [208] | |
| Kidney | Perfusion decellularization of rat kidney and mounted in a whole-organ bioreactor, autologous | hUVECs, rat NKCs | In vitro and in vivo (68 Sprague-Dawley rats) | The resulting grafts produced rudimentary urine in an orthotopic transplantation model [210] |
ECM, extracellular matrix; MPC, skeletal muscle precursor cell; SIS, small intestine submucosa; EC, endothelial cell; BMSC, bone marrow-derived mesenchymal stem cell; UC, urothelial cell; SMC, smooth muscle cell; HepG2, human hepatocarcinoma cell line; hUVEC, human umbilical vein endothelial cell; hFLC, human fetal liver cell; LX2, human cell line hepatic stellate cell; Sk-Hep-1, human cell line hepatocellular carcinoma; NKC, neonatal kidney cell.
TS-ECM as supplements for in vitro 3D culture constructs
In vitro models aim to mimic the composition, ratio, and function of native tissues as closely as possible [151]. TS-ECM compounds could play a vital role in developing a proper in vitro cell-culture system. Compared with universal ECM such as collagen, TS-ECM can provide desirable cell–substrate interactions [147]. These interactions benefit cell proliferation and cellular functions, such as the differentiation capacity of stem or progenitor cells. Here, we focus on two post-processing products of TS-ECM for cell culture in vitro: powder and hydrogel. 3D matrix hydrogels often feature a soft, tissue-like stiffness and mimic the ECM that is naturally present in tissues. Using 3D ECM for cell-culture models presents several benefits as it enhances cell attachment and enables proper carrying of gases, nutrients, peptides, and proteins to the targeted cells, which promotes cell survival, proliferation, migration, and differentiation.
Tissue-like 3D cultures provide a promising tool to study the pathological changes to in vitro microenvironments. Pathogens such as viruses face varying conditions in vivo; however, suitable 3D tissue environments that impact pathogen spread need to be established. Recent studies [152] have developed tissue-like 3D cultures combining quantification of virus replication with imaging to study single-cell and cell-population dynamics. Investigators have analyzed human immunodeficiency virus-1 (HIV-1) spread between primary human CD4 T-lymphocytes using collagen as a tissue-like 3D model through computation technology. This study demonstrates that 3D environmental constructs restrict infection via cell-free virions but promote cell-associated HIV-1 transmission. Experimental validation identifies cell motility and density as essential determinants of the efficacy and mode of HIV-1 spread in 3D culture. 3D tissue constructs represent an adaptable method for the quantitative time-resolved analyses of HIV replication, spread, and interactions under in vitro 3D conditions [152].
The separation of ECM from tissues followed by decellularization and other processes (e.g., milling, pulverizing, lyophilizing, and freezing) are typical steps for producing ECM powder. TS-ECM powder derived from skin, muscle, and liver can be used as coating substrates for promoting targeted cell proliferation and maintaining the cell phenotype of the three cell types [147]. TS-ECM hydrogel is made using solubilized enzymatic procedures [153], which retain the full biochemical complexity of native tissue. Recent efforts have focused on recapitulating a wide variety of physiochemical cues of native ECM [154]. Our studies have demonstrated that synthetic skeletal muscle ECM (mECM) hydrogel, a combination of mECM, HA-based hydrogel, and heparin (HA-Hep), significantly improves the proliferation and differentiation of skeletal muscle precursor cells (MPCs) [30,87,88,155,156]. Additionally, TS-ECM from skin [155], liver [155,157,158], and kidney [64,159] efficiently induces tissue-specific stem cells to differentiate into dermal cells, hepatocytes, and renal cells, respectively, in 2D or 3D cultures.
TS-ECM based biomaterials in the bioengineering field have developed from simply coating cell-culture substrates to native ECM-mimicking scaffold design, aiming at recapitulating the exact dynamics, composition, and structure of native ECM [160]. Based on the different morphologies and topographical structures of TS-ECM, the applications can be further divided as cell-sheet tissue regeneration, tubular organ regeneration, and 3D tissue regeneration.
TS-ECM as cell sheet for tissue regeneration
Xenogeneic TS-ECM scaffolds, conveniently obtained using low-cost procedures, are typically fabricated as single-planar ECM sheets used for 2D tissue regeneration, such as skin (dermis) [161–163], cornea [164,165], and urethra mucosa [166,167]. Decellularized small intestinal submucosa (SIS) (Fig. 2) [168], bladder submucosa, and dermal matrix show promising results as inductive substrates for repairing full-thickness burns and postburn scar contractures [161,163,169]. Furthermore, decellularized porcine corneas using high hydrostatic pressurization show excellent optical properties without prompting an immune reaction when implanted into rabbit corneas [170].
Fig. 2.
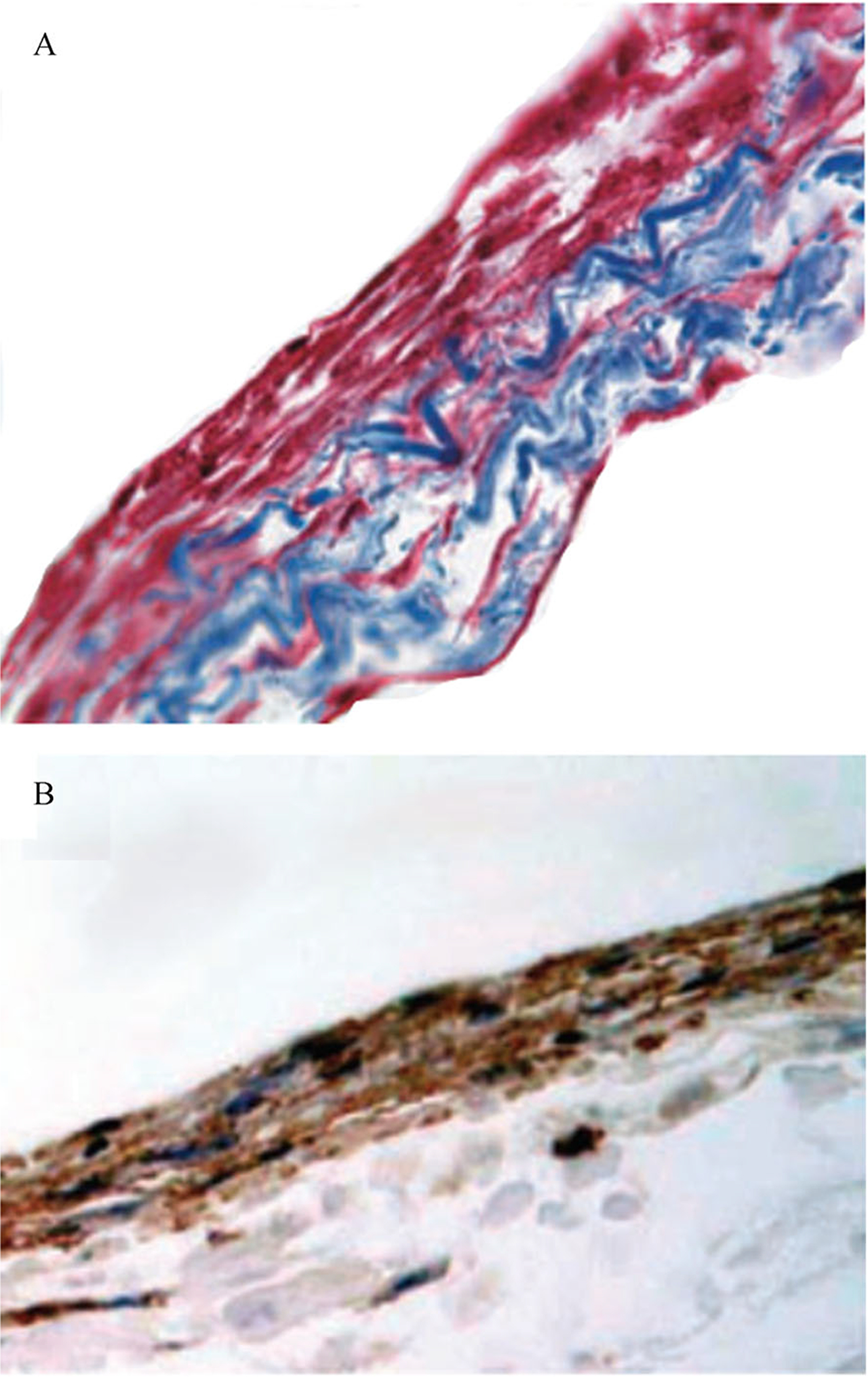
Cell-seeded decellularized small intestine submucosa scaffolds. (A) Masson trichrome staining of canine bone marrow stromal stem cells (red) seeded on SIS scaffolds (blue). (B) Immunohistochemistry staining of α-smooth muscle actin of bone marrow stromal cells (Brown). The photomicrograph of cell-seeded SIS scaffolds is adapted from BJU International [168] with permission.
TS-ECM for tubular organ regeneration
TS-ECM materials can be made into tubular scaffolds, which confer certain potential advantages, such as improved function or performance. Tubular TS-ECM can be used to regenerate blood vessels [171–178], esophagus [179–184], bladder [185–187], urethra [188,189], ureter [190], urinary conduit [191], bowel [192], and vagina [193].
SIS is one of the best established and most widely applied biomaterials [194]. Since it was reported for the first time in 1966 as a vascular substitute for replacing part of the aorta or vena cava in dog models [171–173], extensive research has been performed in the field. SIS-based scaffolds show good graft patency in small-diameter grafts [195]. However, they are observed to have a deficiency in forming intima, thickening media, and dilating grafts with large diameter [174,175]. Subsequently, decellularized vessels are demonstrated as another vascular scaffold. In 2000, acellular aorta scaffold seeded with human myofibroblasts and ECs showed great success following implantation in a rat model [176]. In 2008, the decellularization and recellularization of a whole heart was shown as a functional solid organ for the first time [196]. Large- and small-diameter vascular substitutes are produced from this process, after which the vascular tree could be recapitulated by relining vascular cells [177]. A recent study has reported that the integration of pericardial dECM and poly(propylene fumarate) has robust mechanical properties, adequate re-endothelialization, and tissuegrowth capacity in vivo [178].
Research on esophageal-tissue engineering has undergone rapid development in recent years. In 2000, Badylak et al. [179] successfully repaired esophageal defects in a dog model using acellular porcine SIS or urinary bladder submucosa. In 2011, Badylak et al. [180] first reported that xenogeneic ECM derived from porcine SIS promotes functional esophageal mucosa reconstruction for patients with endoscopic resection. In the same year, Clough et al. [181] reported that acellular porcine SIS matrix successfully repairs traumatic cervical esophageal perforation. In 2014, Syed et al. [182] reported that SIS could be consistently and reliably made into tubular scaffolds with good mechanical properties for esophageal-tissue engineering. In 2018, Luc et al. [183] reported a short biologic scaffold comprising decellularized esophageal matrix in a pig model, mimicking native esophagus in in vitro and in vivo characteristics. In 2019, a clinical-grade acellular matrix study reported an esophagus decellularization process, retaining native esophageal ECM structural, biochemical, and biomechanical properties without cytotoxicity, thereby meeting clinical-grade criteria and showing promise for clinical use [184].
Urinary-tissue regeneration is anatomically divided into urinary bladder, urethra, ureter, and urinary-conduit regeneration. Application of SIS for urinary-bladder reconstruction is extensively investigated. In 1995, Kropp et al. [186] reported that SIS could promote bladder regeneration in a rat model. In 2005, Zhang et al. [168] confirmed the result that SIS is a promising graft for regenerating the urinary bladder in a dog model (Fig. 3). Nowadays, natural porous polymer scaffolds are produced for bladder-bioengineering applications. In 2020, Zhang et al. [187] reported that SIS cross-linked with procyanidins could rapidly promote in situ tissue regrowth and regeneration of the bladder. As for urethral regeneration, since Kropp et al. [188] reported that SIS grafts for urethroplasty promote rabbit urethral regeneration in 1998, research on urethra regeneration has grown remarkably. To date, compared with synthetic scaffolds, tubular scaffolds derived from decellularized tissues can undergo subsequent remodeling with no inflammatory response in vivo. Matrix can be derived from SIS, dermal matrix, corpus spongiosum matrix (CSM), or bladder submucosa matrix (BSM). Among these matrices, acellular CSM and BSM seem to be the most appropriate scaffolds for urethra bioengineering because they possess molecular composition and mechanical and structural characteristics similar to those of native low urinary tract tissue [189]. Similarly, tubular scaffolds applied in ureteral regeneration are produced from decellularized native-tissue specimens such as SIS, amniotic membrane, ureter, blood vessels, or bladder tissue [190]. As for constructing artificial urinary conduits, the regeneration of the urinary conduit is studied primarily in animal models, and only one registered clinical trial has examined the clinical use of artificial urinary-conduit construction (unpublished data) [191].
Fig. 3.
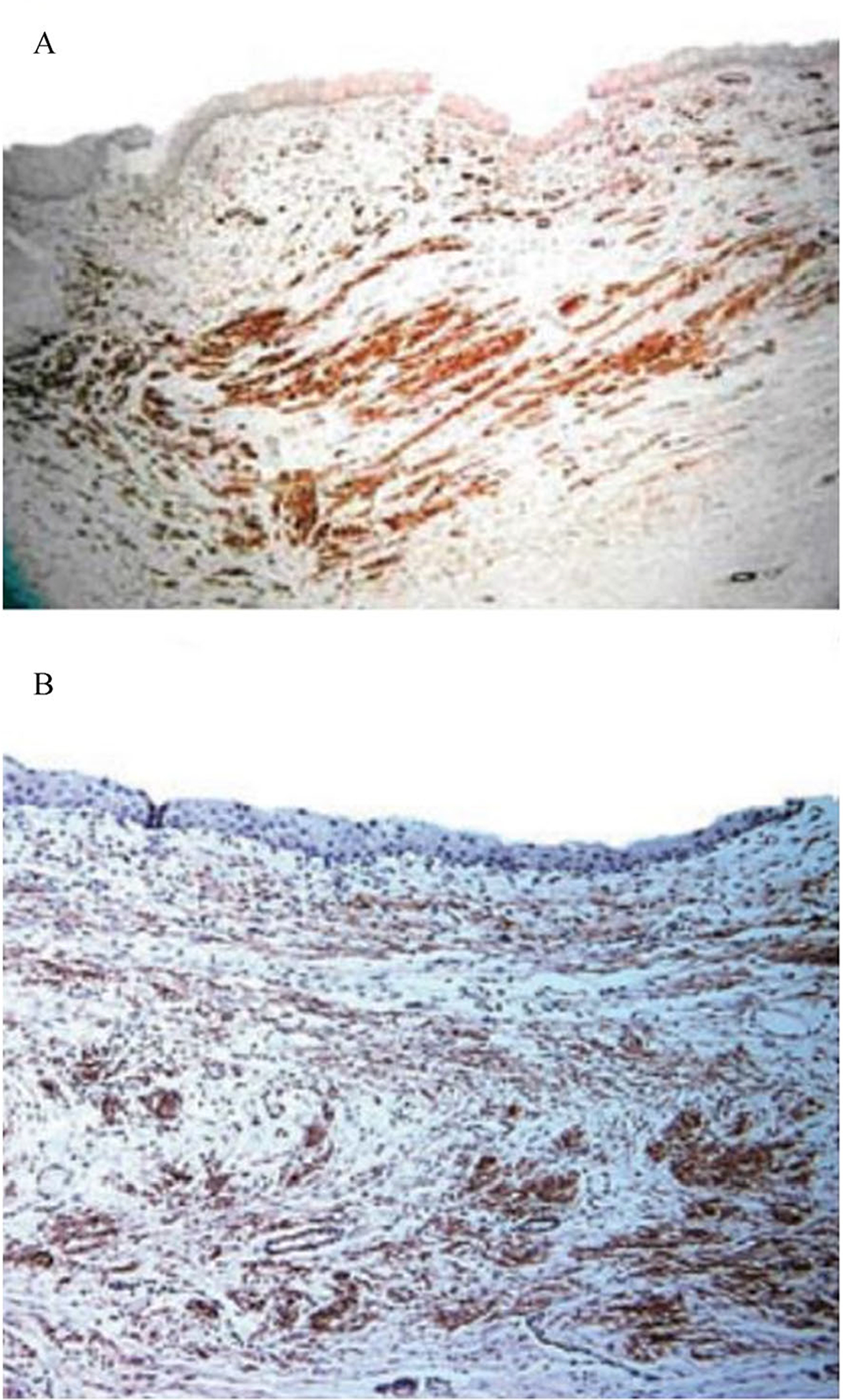
Bone marrow stromal cells-seeded decellularized extracellular matrix promoted in vivo bladder tissue regeneration. Both autologous bone marrow stromal cells-seeded (A) and bladder cells-seeded SIS scaffolds (B) expressed α-smooth muscle actin 10 weeks after transplantation in a canine model following partial cystectomy, assessed by immunohistochemistry staining. The images are adapted from BJU International [168] with permission.
Similar to urinary-conduit regeneration, research on bowel and vagina regeneration is also primarily performed in animals, such as rat [192] and porcine models [193]. However, graft shrinkage and scar-tissue formation are often observed after in vivo implantation. Apparently, keeping the lumen open with physical support is critical for tubular or hollow organ-tissue regeneration. For cell-seeded tissue, a promptly established blood network is required for the survival of implanted cells in the host [185]. Clearly, maintaining cell viability within ECM and preventing graft contraction after implantation require further investigation.
TS-ECM for multicellular-organism regeneration in vivo
Multicellular-organism regeneration requires a 3D framework to provide structural integrity and denote functional tissue boundaries, thereby delineating specific microenvironments [197]. Accordingly, the decellularization of whole tissues and organs provides scaffolds with tissue-specific 3D microarchitecture, serving as templates for whole-organ engineering [160]. The basic strategy for transplantable human-organ generation involves the venous perfusion decellularization of human or animal organs. The resulting product is a 3D framework with intact vasculature. Subsequently, the 3D scaffold is maintained in a bioreactor system to mimic the physiologic conditions of specific organs, such as electrical conduction, pressure gradients, pH, temperature, and oxygen concentration [198]. Next, the recellularization of 3D ECM scaffold proceeds by seeding appropriate cell types in a concentration that matches that for native cell distribution. The achievement of successful perfusion decellularization was first demonstrated on a whole rat heart in 2008 [198], followed by the liver, kidney, and lungs [199].
Several studies have reported the decellularization of liver tissue from animals [199–201]. The 3D ECM framework obtained from liver tissue has been proven to retain excellent functionality of multiple liver-cell types to grow in vitro [202,203]. In 2011, Baptista et al. decellularized a whole cadaveric liver organ by perfusing detergent through the native-liver vascular network, fabricating a natural ECM scaffold for liver regeneration in vitro [201]. In 2015, Mazza et al. [204] decellularized a whole human liver and successfully assessed in vivo quality and biocompatibility. Later, in 2017, Verstegen et al. [205] conducted a clinical series performing the decellularization process in whole liver. They generated a mild nondestructive decellularization protocol by using perfusion through the hepatic artery and the portal vein [205]. This protocol removes cellular DNA and RNA completely and is effective for generating constructs from whole human liver. These constructs contain ECM components, and the architecture of the liver is maintained. Above all, the utilization of artificial hepatic scaffold for liver bioengineering is gaining remarkable success. However, recellularization can be further improved using innovations of more desired bioreactors to better replicate native liver.
The goal of bioengineered lungs is to rehabilitate the architecture and functionality of the two seeding routes, the vasculature and the airway [199]. In vivo gas exchange is the primary outcome for evaluating the efficiency of artificial lungs. Initially in 2010, Petersen et al. [85] demonstrated the feasibility of recellularized artificial lungs based on a rat-transplantation model. In 2011, recellularized lungs transplanted orthotopically in rats partially restored respiratory function [206,207]. In porcine models in 2017, transplanted artificial lungs promoted gas exchange [208]. However, insufficient vascular barrier function and increased thrombogenicity resulted in graft failure [208]. Functional lung regeneration still has a long way to go even though remarkable achievements have been made. To build higher-level function, optimizing the recellularization and maturation of the grafts is necessary. Moreover, experiments based on large animal models need to be performed for preclinical trials before translation to human trials.
The two primary functions of kidneys are to maintain fluid balance and filter harmful substances, which are vital for human physiologic function. For patients with endstage renal diseases, kidney transplant is deemed the firstline treatment [209]. In the kidneys, various successful decellularization and recellularization strategies have been developed. For example, rat kidneys could produce dilute urine after recellularization and culture under perfusion [210]. However, although a piece of tissue like the structure of renal components is reconstructed in vitro, the function of renal tissue with a nephron structure has not yet been determined in vivo [211]. Moreover, the current techniques still have distinct limitations in precise cell arrangement, reconstruction of an entire vascular system, and a continuous urinary-collection system. These limitations impede obtaining complete and functional wholekidney organs. Additional studies need to be conducted prior to clinical applications.
Mechanisms for 3D tissue regeneration
Signaling pathways play crucial roles in substantial cellular functions (cell survival, self-renewal, attachment, proliferation, and differentiation) and tissue regeneration. Understanding the underlying signaling pathways is vital for 3D tissue regenerative repair. Key signaling pathways are involved in tissue regeneration in different systems (Table 6). These signaling pathways regulate stem-cell differentiation and 3D tissue regeneration in a complex cross-talk manner.
Table 6.
Mechanisms for 3D tissue regeneration
| Function | Involved signaling pathway | Cell-matrix interaction related with genes and proteins |
|---|---|---|
| Musculoskeletal system | ||
| Osteogenesis | BMP/TGFβ | Mesenchymal progenitors-BMP2-deficient mice [212], BMP4-deficient mice [213], BMP7-deficient mice [214] |
| Wnt | Primary osteoprogenitors in Axin2LacZ/LacZ mice-Wnt protein [215] Fracture callus tissues-PTH [216] Mesenchymal skeletal cells-peptide ligand with high affinity integrin (CRRETAWAC) [217] |
|
| Notch | MSCs-Notch ligand (Jag1) [218–220] | |
| Chondrogenesis | Wnt/β-catenin | Mesenchymal progenitors-ablation of β-catenin in mesenchymal condensations [221] Micromass of MSCs-protein kinase C inhibitor (PMA), p38 kinase inhibitor (SB203580) [222] |
| TGFβ/Smad | FSTL1 KO MSCs-exogenous recombinant FSTL1 [223] Chondrocytes-Adamtsl2 KO growth plate [224] |
|
| BMP | MSC pellets-BMP inhibitor (dorsomorphin) [225] | |
| BMP/TGFβ | hACs and hMSCs-BMP-2, TGFβ1 [226] SDSCs-BMP-2, TGFβ1 (dexamethasone absent) [227] |
|
| IHH | Chondrocytes-PPR−/− wild-type chimeric mice vs. Ihh−/−PPR−/− wild-type chimeric mice [228] BMSCs-IHH, SHH [229] |
|
| Skeletal myogenesis | Wnt | Adult muscle stem cells-combining APC and β-catenin siRNAs [230] Satellite cells-Islr cKO mice [231] |
| Wnt/IGF | Satellite cell-like reserve myoblasts-GSK-3 inhibitor (LiCl or SB216763), insulin [232] | |
| Notch | Adult muscle stem cells-COLV depleted mice (compound Tg: Pax7-CreERT2; Col5a1flox/flox; R26mTmG(Col5a1 cKO)), CALCR ligand (Elcatonin) injection [233] Satellite cells-Syndecan-3 ablation [234] |
|
| Nervous system | ||
| Neurogenesis in CNC | PI3K/AKT/mTOR | Cerebral organoids-mTOR activators (INSR, ITGB8, IFNAR1) and repressors (PTEN) [235] |
| Notch | Neuronal progenitor cells-NOTCH2NL [236] hSpS spheroids-Notch inhibitor (DAPT) [237] |
|
| Wnt/FGF | mESCs-FGF/Wnt agonist (CHIR)/RA [238] | |
| TGFβ/Shh/Wnt | Astrocytes-TGFβ, Shh, and Wnt activators [239] | |
| Neurogenesis in PNS | c-Myc-TERT | Sensory axon-p53 inhibitor (PFTα), p53 activator (Tenovin-6) [240] |
| Circulatory system | ||
| Cardiomyogenesis | Wnt | Cardiac organoids-Wnt agonist (CHIR) [241–243], WNT inhibitor (IWP2) [243] |
| TGFβ | Cardiac organoids-TGFβ receptor inhibitor (e.g., SB431542) or overexpression of TGFβ receptor negative form [244,245] | |
| BMP | NKX2–5+CD31+ endocardial-like cells from hPSCs-BMP4, CHIR/BMP10, VEGF/BMP10 [246] | |
| Angiogenesis | Notch | Vascular organoids-Notch inhibitor (DAPT), Notch ligands (Dll4, Notch3) [247] |
| Wnt/VEGF-A | hPSCs aggregates-3D collagen I-matrigel gel driven by Wnt agonist (CHIR), BMP-4, VEGF-A, FGF-2 subsequently [248] | |
| Digestive system | ||
| Stomach tissue reconstruction | Wnt | Lgr5+ stem cells-matrigel containing Wnt activator (R-spondin1), Wnt3A [249] |
| Axin2+/Lgr5− stem cells-Wnt activator (R-spondin3) [250] | ||
| Intestine tissue reconstruction | Wnt | Lgr5+ ISCs-Wnt activator (R-spondin1), Wnt ligands [251–253] |
| Wnt/Notch | Lgr5+ ISCs-Wnt inhibitor (IWP-2)/Lgr5+ ISCs-Notch inhibitor (DAPT) [254] | |
| Notch | ISCs-Notch ligands driven by transient Yap1 activation [255] | |
| Hepatogenesis | Wnt | Lgr5+ stem cells-matrigel containing EGF, Wnt activator (R-spondin1) [256] Lgr5+ stem cells-HGF/Wnt activator (R-spondin1) [257] |
| Hedgehog | Hepatocytes and ductular cells-Hh ligands [258] Stellate cells-JNK1 [259] |
|
| Urinary system | ||
| Nephrogenesis | Wnt | Lgr5+ stem cells-Wnt receptor (Lgr5) [260] hPSCs-Wnt agonist (CHIR), Wnt inhibitor (DAPT) [261] |
| Wnt, FGF | hPSCs-Wnt agonist (CHIR), FGF9 [262,263] | |
| Urothelium regeneration | Hedgehog/Wnt | Stromal cells and epithelial cells in bladder-Shh-blocking antibody/stromal cells and epithelial cells-inactivation of essential component of Wnt pathway (Ctnnb1) [264] |
| Hedgehog | Long-term bladder organoids-smoothened agonist (SAG), Hh inhibitor (vismodegib), genetic manipulation [265] | |
| Wnt/Notch | Urothelial organoids-Wnt agonist (CHIR)/urothelial organoids-Notch inhibitor (DBZ) [266] | |
| Reproductive system | ||
| Fallopian tube and oviduct tissue reconstruction | Wnt/Notch | Fallopian tube organoids-Wnt modulators (Wnt3a, R-spondin1, EGF, FGF10), TGFβ inhibitor (ALK4/5), BMP inhibitor (Noggin)/fallopian tube organoids-Notch inhibitor (DBZ) [267] |
| Fallopian tube organoids-Wnt antagonist (PKF118–310)/fallopian tube organoids-Notch inhibitor (DBZ) [268] | ||
| Endometrium | Wnt | Endometrial organoids-Wnt activator (R-spondin1), Wnt inhibitor (IWP2), WNT3A, WNT7A, EGF, Noggin [269] |
| Endometrial organoids-WNT3A, Wnt activator (R-spondin1), EGF, Noggin [270] | ||
| Vagina tissue reconstruction | Wnt | Vaginal organoids-EGF, TGFb/Alk inhibitor (A83-01), ROCK inhibitor (Y-27632), PALL Corporation (Ultraserum-G) [271] |
| Prostate tissue reconstruction | Notch | Prostate organoids-Notch inhibitor (DAPT) [272] |
hAC, human articular chondrocyte; hMSC, human mesenchymal stem cell; SDSC, synovial-derived stem cell; IHH, Indian Hedgehog; PPR, PTH/PTHrP receptor; BMSC, bone marrow-derived mesenchymal stem cell; CNS, central nervous system; PNS, peripheral nervous system; hSpS, hindbrain/cervical spinal cord; mESC, mouse embryonic stem cell; ISC, intestinal stem cell;Hh, Hedgehog; hPSC, human pluripotent stem cell.
Recently, the Hippo signaling pathway YAP/TAZ has been shown to play a pivotal role in regulating 3D tissue regeneration as a new signaling pathway [273]. The core of the Hippo pathway is defined as a serine/threonine kinase cascade, comprising mammal Ste20-like kinase 1 (MST1) and MST2, Salvador 1 (SAV1), MOB1A, and MOB1B, large tumor suppressor kinase 1 (LATS1) and LATS2, the transcriptional co-activators Yes-associated protein (YAP), and transcriptional co-activator with PDZ binding motif (TAZ) [274]. The Hippo pathway is regulated by external changes of stem-cell niche factors, such as mechanical stress and cell–ECM interaction [274]. The effects of these upstream signals are mediated by receptors embedded in the cytoplasm membrane, such as integrin complex (Fig. 4). After the cells sense the signals, the Hippo pathway is regulated by an intracellular network, rather than through dedicated receptors. Thus, following injury, the Hippo pathway can act as a universal pathway to regulate stem-cell behaviors for initiating tissue regeneration [273]. The Hippo pathway regulates stem-cell attachment, proliferation, self-renewal, and differentiation, such as ESCs [275], iPSCs [276,277], and MSCs [278], which are important for tissue regeneration. To date, it is reported to be involved in the regeneration of multiple organs, such as intestine [279], liver [280], skin [281], heart [241,282], and nervous system [283]. However, the downstream effects are closely associated with tumor development [284], thereby increasing the challenge in targeting the Hippo pathway for tissue regeneration.
Fig. 4.
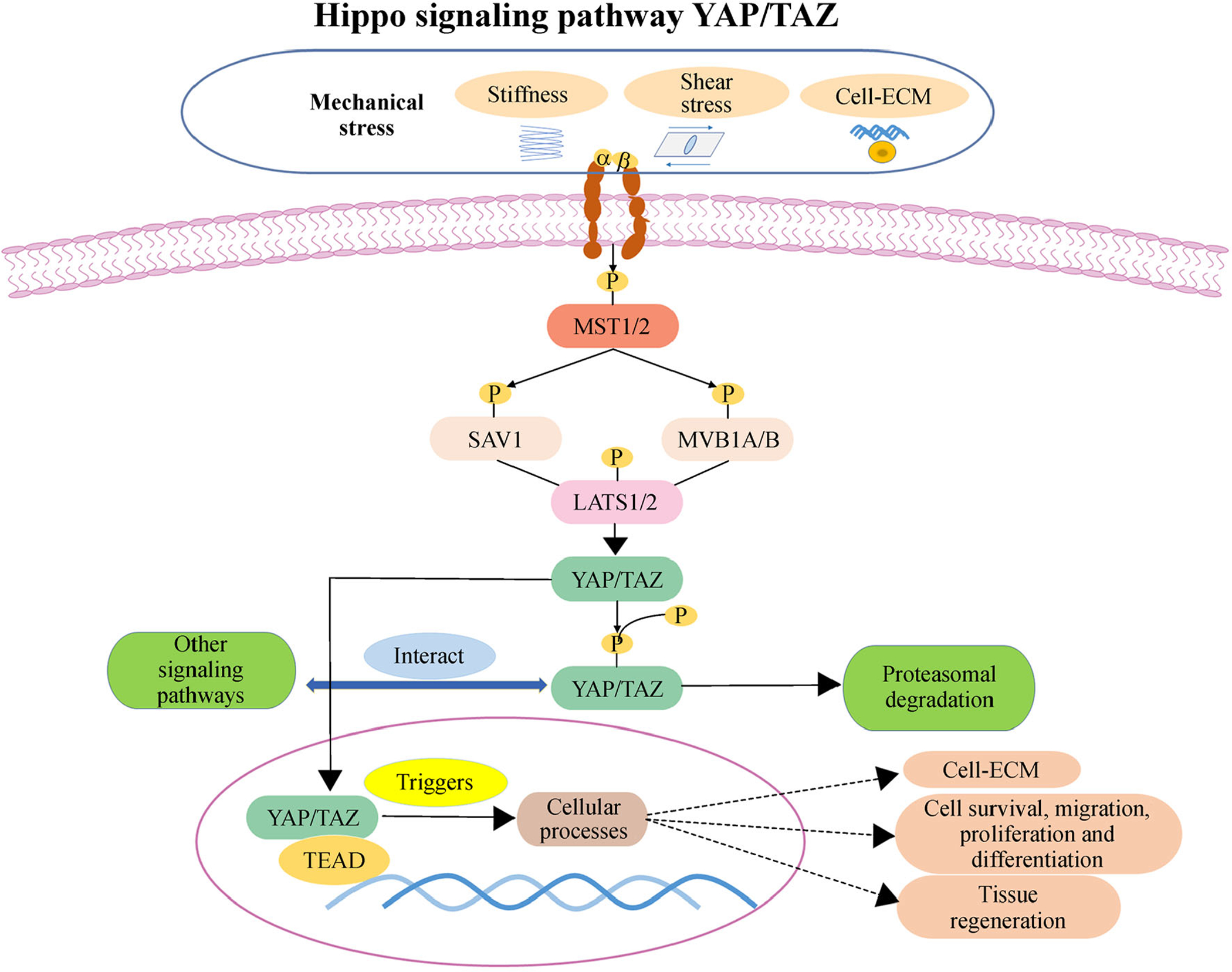
Hippo signaling pathway YAP/TAZ for regulating cell behaviors and tissue regeneration. The Hippo pathway is regulated by an intracellular network relaying a multitude of external inputs. Mechanical stress and cell-extracellular matrix (ECM) adhesion changes can regulate the Hippo pathway through integrin signaling. Activation of the Hippo pathway is associated with the phosphorylation of the core Hippo pathway kinases, including mammal Ste20-like kinase 1 (MST1) and MST2, Salvador 1 (SAV1), MOB1A and MOB1B, large tumor suppressor kinase 1 (LATS1) and LATS2, the transcriptional co-activators Yes-associated protein (YAP) and transcriptional co-activator with PDZ binding motif (TAZ), which leads to proteasomal degradation. Conversely, when the Hippo kinase cascade is not activated, unphosphorylated YAP/TAZ binding with TEAD transcription factor can activate specific genes, regulating ECM remodeling, cellular behaviors (cell attachment, proliferation, migration, and differentiation) and tissue regeneration.
Challenges and future directions
Tissue-derived ECM is an elemental part of the body’s tissues, so it is critical to mimic its properties to develop 3D organoid models in vitro for drug screening, cell therapy, or disease modeling. Hydrogels such as collagen and matrigel are universal products extensively used as substrates for 3D cell cultures. However, the need for more special gels requires the development of various tissue gels. As the porosity, permeability, and mechanical characteristics of different gels vary, the natural origin of the ECM of specific tissues or organs needs to be recapitulated when these ECM gels are designed. TS-ECM compounds also need to be further characterized, controlled, and standardized to prevent variability in either C-ECM or TS-ECM.
For tissue repair in the body, ECM plays an important role in wound healing. As a complex physiologic reaction in response to trauma, would healing involves cellular and ECM events, biochemical reactions, growth factors, and cytokines. The goal for wound healing is scar-free restoration with less tissue shrinkage. Various possibilities have rendered ECM-based scaffolding technologies a turning point in regenerative medicine. To date, animal models have demonstrated that delayed collagen-deposition paired ECM remodeling is one of the traits for scarless wound healing [285]. However, some challenges exist for preclinical animal models, such as low reproducibility, ethical problems, and poor translation to humans. Moreover, the most prominent challenge is the inconsistency between healthy ECM scaffolds and the dysfunctional matrix that is the result of injuries. Dysfunctional matrix includes decreased or excessive ECM compounds [286], often accompanied with a change in soluble factors, such as transforming growth factor β [287] and cross-linking enzymes [288]. A proteomic study has also revealed that the composition of normal and pathological ECM exhibits a completely different profile [286]. Considering this finding, whether ECM scaffolds can provide the correct cues to regulate cell behaviors on pathological tissues is still unclear. To close the gap in knowledge, pathological ECM remodeling and genetically engineered ECM scaffolds offer two alternatives by improving the function and biocompatibility of ECM.
ECM remodeling is a healing process that offers promising therapeutic opportunities for many diseases [5]. Implanted ECM scaffold with a bioactive molecular and porous microstructure can enhance wound healing. For example, the immobilization of signaling molecules on the porous surface of scaffolds can promote cell proliferation, differentiation, and cell–matrix adhesion [289,290]. Selecting a specific enzyme to enhance tissue remodeling is important. One study has shown that curcumin treatment could accelerate wound healing by suppressing MMP-9 in a mouse model [291]. Moreover, attempts to genetically engineer ECM have achieved preliminary success in animal models. ECM sheets and hydrogels generated from porcine, which is alpha-gal deficient (with reduced immune rejection), show that 3D-generated transected anterior cruciate ligament can form in a goat model [292]. As TS-ECMs of different tissues share a common set of proteins, the role of individual ECM components in the unique functions of tissues and the healing process still needs further investigation. A robust and extensive proteomic analysis of TS-ECM components is critical to illustrate the tissue regeneration process induced by TS-ECM. In summary, a pro-regenerative matrix combined with the ECM remodeling of pathological tissues may bring us one step closer to scar-free tissue regeneration. TS-ECM in tissue repair could bring us closer to scarless wound healing.
In conclusion, mimicking the microenvironment of original tissues, TS-ECM and C-ECM possess remarkable promise for developing in vitro 3D culture systems and cell-based therapy. Tissue bioengineering in organoid constructions or 3D culture models offers a novel platform to study diseases and test new drugs. dECM products also provide therapeutic alternatives for the repair of injured or pathological tissues during tissue reconstruction. Compared with C-ECM, emerging evidence suggests that TS-ECM as a scaffold needs to be improved due to its unique biochemical, biological, and biophysical properties. This review highlights the physiologic roles of ECM in 3D organoid formation and tissue repair and presents the currently recognized applications of C-ECM and TS-ECM in modulating cellular construction development and organ-healing processes following tissue injury. To date, TS-ECM products have advanced to several formats such as powder, hydrogel, cell sheet, and decellularized tissue and organ for in vitro 3D structure culture models. Inevitably, tissue repair for wound healing will be refined in future applications.
The past few decades have witnessed substantial progress in TS-ECM or C-ECM developments. However, major hurdles remain in understanding the accurate and specific key ECM proteins and the ratio of these molecules for cell proliferation and targeted cell differentiation for 3D organoid culture and tissue repair. Thus, further basic research and preclinical testing are necessary before clinical translation.
Acknowledgements
We thank Ms. Suzanne Danley (Department of Orthopaedics, West Virginia University) for editing the manuscript. This work was partially supported by Research Grants from the National Institutes of Health (No. 1R01AR067747) to Ming Pei and by NIH/NIAID (Nos. R21AI152832 and R03AI165170) to Yuanyuan Zhang.
Footnotes
Compliance with ethics guidelines
Chuanqi Liu, Ming Pei, Qingfeng Li, and Yuanyuan Zhang declare that they have no conflict of interest. This manuscript is a review article and does not involve a research protocol requiring approval by the relevant institutional review board or ethics committee.
References
- 1.Prewitz MC, Seib FP, von Bonin M, Friedrichs J, Stißel A, Niehage C, Müller K, Anastassiadis K, Waskow C, Hoflack B, Bornhäuser M, Werner C. Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nat Methods 2013; 10(8): 788–794 [DOI] [PubMed] [Google Scholar]
- 2.Hynes RO. The extracellular matrix: not just pretty fibrils. Science 2009; 326(5957): 1216–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sart S, Jeske R, Chen X, Ma T, Li Y. Engineering stem cell-derived extracellular matrices: decellularization, characterization, and biological function. Tissue Eng Part B Rev 2020; 26(5): 402–422 [DOI] [PubMed] [Google Scholar]
- 4.Sart S, Agathos SN, Li Y. Engineering stem cell fate with biochemical and biomechanical properties of microcarriers. Biotechnol Prog 2013; 29(6): 1354–1366 [DOI] [PubMed] [Google Scholar]
- 5.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014; 15(12): 786–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jin J, Saiding Q, Wang X, Qin M, Xiang Y, Cheng R, Cui W, Chen X. Rapid extracellular matrix remodeling via gene-electrospun fibers as a “Patch” for tissue regeneration. Adv Funct Mater 2021; 31(15): 2009879 [Google Scholar]
- 7.Correa D, Hesse E, Seriwatanachai D, Kiviranta R, Saito H, Yamana K, Neff L, Atfi A, Coillard L, Sitara D, Maeda Y, Warming S, Jenkins NA, Copeland NG, Horne WC, Lanske B, Baron R. Zfp521 is a target gene and key effector of parathyroid hormone-related peptide signaling in growth plate chondrocytes. Dev Cell 2010; 19(4): 533–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Chang J, Wu C. Bioactive inorganic/organic nanocomposites for wound healing. Appl Mater Today 2018; 11: 308–319 [Google Scholar]
- 9.Mano JF, Silva GA, Azevedo HS, Malafaya PB, Sousa RA, Silva SS, Boesel LF, Oliveira JM, Santos TC, Marques AP, Neves NM, Reis RL. Natural origin biodegradable systems in tissue engineering and regenerative medicine: present status and some moving trends. J R Soc Interface 2007; 4(17): 999–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu M, Li W, Dong X, Yuan X, Midgley AC, Chang H, Wang Y, Wang H, Wang K, Ma PX, Wang H, Kong D. In vivo engineered extracellular matrix scaffolds with instructive niches for oriented tissue regeneration. Nat Commun 2019; 10(1): 4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Xiao Y, Liu C. The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem Rev 2017; 117(5): 4376–4421 [DOI] [PubMed] [Google Scholar]
- 12.Zhang W, Zhu Y, Li J, Guo Q, Peng J, Liu S, Yang J, Wang Y. Cell-derived extracellular matrix: basic characteristics and current applications in orthopedic tissue engineering. Tissue Eng Part B Rev 2016; 22(3): 193–207 [DOI] [PubMed] [Google Scholar]
- 13.Smoak MM, Hogan KJ, Grande-Allen KJ, Mikos AG. Bioinspired electrospun dECM scaffolds guide cell growth and control the formation of myotubes. Sci Adv 2021; 7(20): eabg4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang J, Park JY, Gao G, Cho DW. Biomaterials-based 3D cell printing for next-generation therapeutics and diagnostics. Biomaterials 2018; 156: 88–106 [DOI] [PubMed] [Google Scholar]
- 15.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol 2014; 32(8): 773–785 [DOI] [PubMed] [Google Scholar]
- 16.Kim BS, Das S, Jang J, Cho DW. Decellularized extracellular matrix-based bioinks for engineering tissue- and organ-specific microenvironments. Chem Rev 2020; 120(19): 10608–10661 [DOI] [PubMed] [Google Scholar]
- 17.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 2014; 5(1): 3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Wang J, Qian D, Chen L, Mo X, Wang L, Wang Y, Cui W. Electrospun fibrous sponge via short fiber for mimicking 3D ECM. J Nanobiotechnology 2021; 19(1): 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kishan AP, Cosgriff-Hernandez EM. Recent advancements in electrospinning design for tissue engineering applications: a review. J Biomed Mater Res A 2017; 105(10): 2892–2905 [DOI] [PubMed] [Google Scholar]
- 20.Su Y, Shi Y, Stolow MA, Shi YB. Thyroid hormone induces apoptosis in primary cell cultures of tadpole intestine: cell type specificity and effects of extracellular matrix. J Cell Biol 1997; 139(6): 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon-Assmann P, Kedinger M, De Arcangelis A, Rousseau V, Simo P. Extracellular matrix components in intestinal development. Experientia 1995; 51(9–10): 883–900 [DOI] [PubMed] [Google Scholar]
- 22.Mahoney ZX, Stappenbeck TS, Miner JH. Laminin α 5 influences the architecture of the mouse small intestine mucosa. J Cell Sci 2008; 121(15): 2493–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HY, Nelson CM. Extracellular matrix and cytoskeletal dynamics during branching morphogenesis. Organogenesis 2012; 8(2): 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci 2010; 123(24): 4195–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhen G, Cao X. Targeting TGFβ signaling in subchondral bone and articular cartilage homeostasis. Trends Pharmacol Sci 2014; 35(5): 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng CW, Solorio LD, Alsberg E. Decellularized tissue and cell-derived extracellular matrices as scaffolds for orthopaedic tissue engineering. Biotechnol Adv 2014; 32(2): 462–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Özbek S, Balasubramanian PG, Chiquet-Ehrismann R, Tucker RP, Adams JC. The evolution of extracellular matrix. Mol Biol Cell 2010; 21(24): 4300–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel G, Tonon T, Scornet D, Cock JM, Kloareg B. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytol 2010; 188(1): 82–97 [DOI] [PubMed] [Google Scholar]
- 29.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev 2016; 97: 4–27 [DOI] [PubMed] [Google Scholar]
- 30.Li L, Liu G, Timashev P, Sun XS, Criswell T, Atala A, Zhang Y. Biofabrication of tissue-specific extracellular matrix proteins to enhance the expansion and differentiation of skeletal muscle progenitor cells. Appl Phys Rev 2019; 6(2): 021309 [Google Scholar]
- 31.Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol 2011; 3(1): a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LeBleu VS, Macdonald B, Kalluri R. Structure and function of basement membranes. Exp Biol Med (Maywood) 2007; 232(9): 1121–1129 [DOI] [PubMed] [Google Scholar]
- 33.Wagenseil JE, Mecham RP. New insights into elastic fiber assembly. Birth Defects Res C Embryo Today 2007; 81(4): 229–240 [DOI] [PubMed] [Google Scholar]
- 34.Kjellén L, Lindahl U. Specificity of glycosaminoglycan-protein interactions. Curr Opin Struct Biol 2018; 50: 101–108 [DOI] [PubMed] [Google Scholar]
- 35.Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J 1993; 7(13): 1233–1241 [PubMed] [Google Scholar]
- 36.Niklason LE. Understanding the extracellular matrix to enhance stem cell-based tissue regeneration. Cell Stem Cell 2018; 22(3): 302–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avnur Z, Geiger B. The removal of extracellular fibronectin from areas of cell-substrate contact. Cell 1981; 25(1): 121–132 [DOI] [PubMed] [Google Scholar]
- 38.Vasvani S, Kulkarni P, Rawtani D. Hyaluronic acid: a review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromol 2020; 151: 1012–1029 [DOI] [PubMed] [Google Scholar]
- 39.Vigetti D, Viola M, Karousou E, Deleonibus S, Karamanou K, De Luca G, Passi A. Epigenetics in extracellular matrix remodeling and hyaluronan metabolism. FEBS J 2014; 281(22): 4980–4992 [DOI] [PubMed] [Google Scholar]
- 40.Huleihel L, Hussey GS, Naranjo JD, Zhang L, Dziki JL, Turner NJ, Stolz DB, Badylak SF. Matrix-bound nanovesicles within ECM bioscaffolds. Sci Adv 2016; 2(6): e1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nelson CM, Bissell MJ. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu Rev Cell Dev Biol 2006; 22(1): 287–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei W, Li J, Chen S, Chen M, Xie Q, Sun H, Ruan J, Zhou H, Bi X, Zhuang A, You Z, Gu P, Fan X. In vitro osteogenic induction of bone marrow mesenchymal stem cells with a decellularized matrix derived from human adipose stem cells and in vivo implantation for bone regeneration. J Mater Chem B Mater Biol Med 2017; 5(13): 2468–2482 [DOI] [PubMed] [Google Scholar]
- 43.Choudhury D, Tun HW, Wang T, Naing MW. Organ-derived decellularized extracellular matrix: a game changer for bioink manufacturing? Trends Biotechnol 2018; 36(8): 787–805 [DOI] [PubMed] [Google Scholar]
- 44.Satyam A, Tsokos MG, Tresback JS, Zeugolis DI, Tsokos GC. Cell derived extracellular matrix-rich biomimetic substrate supports podocyte proliferation, differentiation and maintenance of native phenotype. Adv Funct Mater 2020; 30(44): 1908752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Legate KR, Wickström SA, Fässler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev 2009; 23(4): 397–418 [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Gautam A, Yang J, Qiu L, Melkoumian Z, Weber J, Telukuntla L, Srivastava R, Whiteley EM, Brandenberger R. Differentiation of oligodendrocyte progenitor cells from human embryonic stem cells on vitronectin-derived synthetic peptide acrylate surface. Stem Cells Dev 2013; 22(10): 1497–1505 [DOI] [PubMed] [Google Scholar]
- 47.Mathews S, Bhonde R, Gupta PK, Totey S. Extracellular matrix protein mediated regulation of the osteoblast differentiation of bone marrow derived human mesenchymal stem cells. Differentiation 2012; 84(2): 185–192 [DOI] [PubMed] [Google Scholar]
- 48.Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fässler R, Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on β1-integrin. Cell Stem Cell 2008; 3(5): 533–542 [DOI] [PubMed] [Google Scholar]
- 49.Brafman DA, Phung C, Kumar N, Willert K. Regulation of endodermal differentiation of human embryonic stem cells through integrin-ECM interactions. Cell Death Differ 2013; 20(3): 369–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu M, Xue R, Wang P, Wang X, Tian X, Liu Y, Wang S, Cui A, Xie J, Le L, Zhao M, Quan J, Li N, Meng D, Wang X, Sun N, Chen AF, Xiang M, Chen S. Induced pluripotent stem cells attenuate chronic allogeneic vasculopathy in an integrin beta-1-dependent manner. Am J Transplant 2020; 20(10): 2755–2767 [DOI] [PubMed] [Google Scholar]
- 51.Han S, Kang B, Son HY, Choi Y, Shin MK, Park J, Min JK, Park D, Lim EK, Huh YM, Haam S. In vivo monitoring platform of transplanted human stem cells using magnetic resonance imaging. Biosens Bioelectron 2021; 178: 113039. [DOI] [PubMed] [Google Scholar]
- 52.Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol 2012; 24(5): 645–651 [DOI] [PubMed] [Google Scholar]
- 53.Ghaedi M, Duan Y, Zern MA, Revzin A. Hepatic differentiation of human embryonic stem cells on growth factor-containing surfaces. J Tissue Eng Regen Med 2014; 8(11): 886–895 [DOI] [PubMed] [Google Scholar]
- 54.Ullah I, Abu-Dawud R, Busch JF, Rabien A, Erguen B, Fischer I, Reinke P, Kurtz A. VEGF-supplemented extracellular matrix is sufficient to induce endothelial differentiation of human iPSC. Biomaterials 2019; 216: 119283. [DOI] [PubMed] [Google Scholar]
- 55.Giamblanco N, Martines E, Marletta G. Laminin adsorption on nanostructures: switching the molecular orientation by local curvature changes. Langmuir 2013; 29(26): 8335–8342 [DOI] [PubMed] [Google Scholar]
- 56.González-García C, Sousa SR, Moratal D, Rico P, Salmerón-Sánchez M. Effect of nanoscale topography on fibronectin adsorption, focal adhesion size and matrix organisation. Colloids Surf B Biointerfaces 2010; 77(2): 181–190 [DOI] [PubMed] [Google Scholar]
- 57.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell 2006; 126(4): 677–689 [DOI] [PubMed] [Google Scholar]
- 58.Sthanam LK, Barai A, Rastogi A, Mistari VK, Maria A, Kauthale R, Gatne M, Sen S. Biophysical regulation of mouse embryonic stem cell fate and genomic integrity by feeder derived matrices. Biomaterials 2017; 119: 9–22 [DOI] [PubMed] [Google Scholar]
- 59.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 2010; 329(5995): 1078–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gobaa S, Hoehnel S, Roccio M, Negro A, Kobel S, Lutolf MP. Artificial niche microarrays for probing single stem cell fate in high throughput. Nat Methods 2011; 8(11): 949–955 [DOI] [PubMed] [Google Scholar]
- 61.Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 2011; 26(4): 730–738 [DOI] [PubMed] [Google Scholar]
- 62.Hirata M, Yamaoka T. Effect of stem cell niche elasticity/ECM protein on the self-beating cardiomyocyte differentiation of induced pluripotent stem (iPS) cells at different stages. Acta Biomater 2018; 65: 44–52 [DOI] [PubMed] [Google Scholar]
- 63.Muncie JM, Ayad NME, Lakins JN, Xue X, Fu J, Weaver VM. Mechanical tension promotes formation of gastrulation-like nodes and patterns mesoderm specification in human embryonic stem cells. Dev Cell 2020; 55(6): 679–694.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guo H, Deng N, Dou L, Ding H, Criswell T, Atala A, Furdui CM, Zhang Y. 3-D human renal tubular organoids generated from urine-derived stem cells for nephrotoxicity screening. ACS Biomater Sci Eng 2020; 6(12): 6701–6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 2008; 132(4): 598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: stem cells and their niche. Cell 2004; 116(6): 769–778 [DOI] [PubMed] [Google Scholar]
- 67.Badylak SF, Taylor D, Uygun K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng 2011; 13(1): 27–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ngangan AV, McDevitt TC. Acellularization of embryoid bodies via physical disruption methods. Biomaterials 2009; 30(6): 1143–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hirata M, Yamaoka T. Hepatocytic differentiation of iPS cells on decellularized liver tissue. J Artif Organs 2017; 20(4): 318–325 [DOI] [PubMed] [Google Scholar]
- 70.Lu H, Hoshiba T, Kawazoe N, Chen G. Autologous extracellular matrix scaffolds for tissue engineering. Biomaterials 2011; 32(10): 2489–2499 [DOI] [PubMed] [Google Scholar]
- 71.Nair R, Shukla S, McDevitt TC. Acellular matrices derived from differentiating embryonic stem cells. J Biomed Mater Res A 2008; 87A(4): 1075–1085 [DOI] [PubMed] [Google Scholar]
- 72.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med 2010; 16(8): 927–933 [DOI] [PubMed] [Google Scholar]
- 73.Reing JE, Brown BN, Daly KA, Freund JM, Gilbert TW, Hsiong SX, Huber A, Kullas KE, Tottey S, Wolf MT, Badylak SF. The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 2010; 31(33): 8626–8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haas R, Culp LA. Binding of fibronectin to gelatin and heparin: effect of surface denaturation and detergents. FEBS Lett 1984; 174(2): 279–283 [DOI] [PubMed] [Google Scholar]
- 75.Sart S, Ma T, Li Y. Extracellular matrices decellularized from embryonic stem cells maintained their structure and signaling specificity. Tissue Eng Part A 2014; 20(1–2): 54–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials 2011; 32(12): 3233–3243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parmaksiz M, Elçin AE, Elçin YM. Decellularized cell culture ECMs act as cell differentiation inducers. Stem Cell Rev Rep 2020; 16(3): 569–584 [DOI] [PubMed] [Google Scholar]
- 78.Freytes DO, Stoner RM, Badylak SF. Uniaxial and biaxial properties of terminally sterilized porcine urinary bladder matrix scaffolds. J Biomed Mater Res B Appl Biomater 2008; 84B(2): 408–414 [DOI] [PubMed] [Google Scholar]
- 79.Yang B, Zhang Y, Zhou L, Sun Z, Zheng J, Chen Y, Dai Y. Development of a porcine bladder acellular matrix with wellpreserved extracellular bioactive factors for tissue engineering. Tissue Eng Part C Methods 2010; 16(5): 1201–1211 [DOI] [PubMed] [Google Scholar]
- 80.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials 2006; 27(19): 3675–3683 [DOI] [PubMed] [Google Scholar]
- 81.Gafarova ER, Grebenik EA, Lazhko AE, Frolova AA, Kuryanova AS, Kurkov AV, Bazhanov IA, Kapomba BS, Kosheleva NV, Novikov IA, Shekhter AB, Golubeva EN, Soloviova AB, Timashev PS. Evaluation of supercritical CO2-assisted protocols in a model of ovine aortic root decellularization. Molecules 2020; 25(17): 3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 2004; 1666(1–2): 105–117 [DOI] [PubMed] [Google Scholar]
- 83.Grauss RW, Hazekamp MG, van Vliet S, Gittenberger-de Groot AC, DeRuiter MC. Decellularization of rat aortic valve allografts reduces leaflet destruction and extracellular matrix remodeling. J Thorac Cardiovasc Surg 2003; 126(6): 2003–2010 [DOI] [PubMed] [Google Scholar]
- 84.Hopkinson A, Shanmuganathan VA, Gray T, Yeung AM, Lowe J, James DK, Dua HS. Optimization of amniotic membrane (AM) denuding for tissue engineering. Tissue Eng Part C Methods 2008; 14(4): 371–381 [DOI] [PubMed] [Google Scholar]
- 85.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, Gavrilov K, Yi T, Zhuang ZW, Breuer C, Herzog E, Niklason LE. Tissue-engineered lungs for in vivo implantation. Science 2010; 329(5991): 538–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gui L, Chan SA, Breuer CK, Niklason LE. Novel utilization of serum in tissue decellularization. Tissue Eng Part C Methods 2010; 16(2): 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang D, Zhang Y, Zhang Y, Yi H, Wang Z, Wu R, He D, Wei G, Wei S, Hu Y, Deng J, Criswell T, Yoo J, Zhou Y, Atala A. Tissue-specific extracellular matrix enhances skeletal muscle precursor cell expansion and differentiation for potential application in cell therapy. Tissue Eng Part A 2017; 23(15–16): 784–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yi H, Forsythe S, He Y, Liu Q, Xiong G, Wei S, Li G, Atala A, Skardal A, Zhang Y. Tissue-specific extracellular matrix promotes myogenic differentiation of human muscle progenitor cells on gelatin and heparin conjugated alginate hydrogels. Acta Biomater 2017; 62: 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buckenmeyer MJ, Meder TJ, Prest TA, Brown BN. Decellularization techniques and their applications for the repair and regeneration of the nervous system. Methods 2020; 171: 41–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crapo PM, Medberry CJ, Reing JE, Tottey S, van der Merwe Y, Jones KE, Badylak SF. Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 2012; 33(13): 3539–3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang T, Ren XJ, Tang JL, Yin H, Wang KJ, Zhou CL. Preparation and characterization of genipin-crosslinked rat acellular spinal cord scaffolds. Mater Sci Eng C 2013; 33(6): 3514–3521 [DOI] [PubMed] [Google Scholar]
- 92.Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF. Macrophage phenotype and remodeling outcomes in response to biologic scaffolds with and without a cellular component. Biomaterials 2009; 30(8): 1482–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods 2010; 16(3): 525–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, Winston S, Wang J, Walls S, Nichols JE. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A 2010; 16(8): 2565–2580 [DOI] [PubMed] [Google Scholar]
- 95.Prasertsung I, Kanokpanont S, Bunaprasert T, Thanakit V, Damrongsakkul S. Development of acellular dermis from porcine skin using periodic pressurized technique. J Biomed Mater Res B Appl Biomater 2008; 85B(1): 210–219 [DOI] [PubMed] [Google Scholar]
- 96.Montoya CV, McFetridge PS. Preparation of ex vivo-based biomaterials using convective flow decellularization. Tissue Eng Part C Methods 2009; 15(2): 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bolland F, Korossis S, Wilshaw SP, Ingham E, Fisher J, Kearney JN, Southgate J. Development and characterisation of a full-thickness acellular porcine bladder matrix for tissue engineering. Biomaterials 2007; 28(6): 1061–1070 [DOI] [PubMed] [Google Scholar]
- 98.Sano MB, Neal RE 2nd, Garcia PA, Gerber D, Robertson J, Davalos RV. Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed Eng Online 2010; 9(1): 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Phillips M, Maor E, Rubinsky B. Nonthermal irreversible electroporation for tissue decellularization. J Biomech Eng 2010; 132(9): 091003. [DOI] [PubMed] [Google Scholar]
- 100.Dong X, Wei X, Yi W, Gu C, Kang X, Liu Y, Li Q, Yi D. RGDmodified acellular bovine pericardium as a bioprosthetic scaffold for tissue engineering. J Mater Sci Mater Med 2009; 20(11): 2327–2336 [DOI] [PubMed] [Google Scholar]
- 101.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med 2010; 16(7): 814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nakayama KH, Batchelder CA, Lee CI, Tarantal AF. Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Eng Part A 2010; 16(7): 2207–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McFetridge PS, Daniel JW, Bodamyali T, Horrocks M, Chaudhuri JB. Preparation of porcine carotid arteries for vascular tissue engineering applications. J Biomed Mater Res A 2004; 70A(2): 224–234 [DOI] [PubMed] [Google Scholar]
- 104.Teebken OE, Bader A, Steinhoff G, Haverich A. Tissue engineering of vascular grafts: human cell seeding of decellularised porcine matrix. Eur J Vasc Endovasc Surg 2000; 19(4): 381–386 [DOI] [PubMed] [Google Scholar]
- 105.Gamba PG, Conconi MT, Lo Piccolo R, Zara G, Spinazzi R, Parnigotto PP. Experimental abdominal wall defect repaired with acellular matrix. Pediatr Surg Int 2002; 18(5–6): 327–331 [DOI] [PubMed] [Google Scholar]
- 106.Chen RN, Ho HO, Tsai YT, Sheu MT. Process development of an acellular dermal matrix (ADM) for biomedical applications. Biomaterials 2004; 25(13): 2679–2686 [DOI] [PubMed] [Google Scholar]
- 107.Rieder E, Kasimir MT, Silberhumer G, Seebacher G, Wolner E, Simon P, Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J Thorac Cardiovasc Surg 2004; 127(2): 399–405 [DOI] [PubMed] [Google Scholar]
- 108.Dahl SLM, Koh J, Prabhakar V, Niklason LE. Decellularized native and engineered arterial scaffolds for transplantation. Cell Transplant 2003; 12(6): 659–666 [DOI] [PubMed] [Google Scholar]
- 109.Woods T, Gratzer PF. Effectiveness of three extraction techniques in the development of a decellularized bone-anterior cruciate ligament-bone graft. Biomaterials 2005; 26(35): 7339–7349 [DOI] [PubMed] [Google Scholar]
- 110.Shakouri-Motlagh A, O’Connor AJ, Brennecke SP, Kalionis B, Heath DE. Native and solubilized decellularized extracellular matrix: a critical assessment of their potential for improving the expansion of mesenchymal stem cells. Acta Biomater 2017; 55: 1–12 [DOI] [PubMed] [Google Scholar]
- 111.Assunção M, Dehghan-Baniani D, Yiu CHK, Später T, Beyer S, Blocki A. Cell-derived extracellular matrix for tissue engineering and regenerative medicine. Front Bioeng Biotechnol 2020; 8: 602009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pei M, Li JT, Shoukry M, Zhang Y. A review of decellularized stem cell matrix: a novel cell expansion system for cartilage tissue engineering. Eur Cell Mater 2011; 22: 333–343 [DOI] [PubMed] [Google Scholar]
- 113.Pei M. Environmental preconditioning rejuvenates adult stem cells’ proliferation and chondrogenic potential. Biomaterials 2017; 117: 10–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sun Y, Yan L, Chen S, Pei M. Functionality of decellularized matrix in cartilage regeneration: a comparison of tissue versus cell sources. Acta Biomater 2018; 74: 56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.He F, Chen X, Pei M. Reconstruction of an in vitro tissue-specific microenvironment to rejuvenate synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A 2009; 15(12): 3809–3821 [DOI] [PubMed] [Google Scholar]
- 116.Li J, Pei M. Optimization of an in vitro three-dimensional microenvironment to reprogram synovium-derived stem cells for cartilage tissue engineering. Tissue Eng Part A 2011; 17(5–6): 703–712 [DOI] [PubMed] [Google Scholar]
- 117.Li J, He F, Pei M. Creation of an in vitro microenvironment to enhance human fetal synovium-derived stem cell chondrogenesis. Cell Tissue Res 2011; 345(3): 357–365 [DOI] [PubMed] [Google Scholar]
- 118.Li J, He F, Pei M. Chondrogenic priming of human fetal synovium-derived stem cells in an adult stem cell matrix microenvironment. Genes Dis 2015; 2(4): 337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li J, Narayanan K, Zhang Y, Hill RC, He F, Hansen KC, Pei M. Role of lineage-specific matrix in stem cell chondrogenesis. Biomaterials 2020; 231: 119681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pei M, Zhang Y, Li J, Chen D. Antioxidation of decellularized stem cell matrix promotes human synovium-derived stem cell-based chondrogenesis. Stem Cells Dev 2013; 22(6): 889–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y, Li J, Davis ME, Pei M. Delineation of in vitro chondrogenesis of human synovial stem cells following preconditioning using decellularized matrix. Acta Biomater 2015; 20: 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang Y, Pizzute T, Li J, He F, Pei M. sb203580 preconditioning recharges matrix-expanded human adult stem cells for chondrogenesis in an inflammatory environment—a feasible approach for autologous stem cell based osteoarthritic cartilage repair. Biomaterials 2015; 64: 88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.He F, Liu X, Xiong K, Chen S, Zhou L, Cui W, Pan G, Luo ZP, Pei M, Gong Y. Extracellular matrix modulates the biological effects of melatonin in mesenchymal stem cells. J Endocrinol 2014; 223(2): 167–180 [DOI] [PubMed] [Google Scholar]
- 124.Pei M, He F, Kish VL. Expansion on extracellular matrix deposited by human bone marrow stromal cells facilitates stem cell proliferation and tissue-specific lineage potential. Tissue Eng Part A 2011; 17(23–24): 3067–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pei M, Li J, Zhang Y, Liu G, Wei L, Zhang Y. Expansion on a matrix deposited by nonchondrogenic urine stem cells strengthens the chondrogenic capacity of repeated-passage bone marrow stromal cells. Cell Tissue Res 2014; 356(2): 391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu X, Zhou L, Chen X, Liu T, Pan G, Cui W, Li M, Luo ZP, Pei M, Yang H, Gong Y, He F. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater Sci Eng C 2016; 61: 437–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou L, Chen X, Liu T, Zhu C, Si M, Jargstorf J, Li M, Pan G, Gong Y, Luo ZP, Yang H, Pei M, He F. SIRT1-dependent antisenescence effects of cell-deposited matrix on human umbilical cord mesenchymal stem cells. J Tissue Eng Regen Med 2018; 12(2): e1008–e1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He F, Pei M. Extracellular matrix enhances differentiation of adipose stem cells from infrapatellar fat pad toward chondrogenesis. J Tissue Eng Regen Med 2013; 7(1): 73–84 [DOI] [PubMed] [Google Scholar]
- 129.Wang Y, Fu Y, Yan Z, Zhang XB, Pei M. Impact of fibronectin knockout on proliferation and differentiation of human infrapatellar fat pad-derived stem cells. Front Bioeng Biotechnol 2019; 7: 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang Y, Hu G, Hill RC, Dzieciatkowska M, Hansen KC, Zhang XB, Yan Z, Pei M. Matrix reverses immortalization-mediated stem cell fate determination. Biomaterials 2021; 265: 120387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goh SK, Olsen P, Banerjee I. Extracellular matrix aggregates from differentiating embryoid bodies as a scaffold to support ESC proliferation and differentiation. PLoS One 2013; 8(4): e61856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xiong X, Yang X, Dai H, Feng G, Zhang Y, Zhou J, Zhou W. Extracellular matrix derived from human urine-derived stem cells enhances the expansion, adhesion, spreading, and differentiation of human periodontal ligament stem cells. Stem Cell Res Ther 2019; 10(1): 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hoshiba T, Sugano Y, Yokoyama N. Murine neural stem cell (NSC) line, MEB5-derived decellularized matrix as an in vitro extracellular matrix model in NSC niche. Chem Lett 2018; 47(12): 1498–1501 [Google Scholar]
- 134.Pei M, He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Physiol 2012; 227(5): 2163–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yan J, Chen X, Pu C, Zhao Y, Liu X, Liu T, Pan G, Lin J, Pei M, Yang H, He F. Synovium stem cell-derived matrix enhances antiinflammatory properties of rabbit articular chondrocytes via the SIRT1 pathway. Mater Sci Eng C 2020; 106: 110286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He F, Pei M. Rejuvenation of nucleus pulposus cells using extracellular matrix deposited by synovium-derived stem cells. Spine 2012; 37(6): 459–469 [DOI] [PubMed] [Google Scholar]
- 137.Pei M, Shoukry M, Li J, Daffner SD, France JC, Emery SE. Modulation of in vitro microenvironment facilitates synovium-derived stem cell-based nucleus pulposus tissue regeneration. Spine 2012; 37(18): 1538–1547 [DOI] [PubMed] [Google Scholar]
- 138.Kanninen LK, Porola P, Niklander J, Malinen MM, Corlu A, Guguen-Guillouzo C, Urtti A, Yliperttula ML, Lou YR. Hepatic differentiation of human pluripotent stem cells on human liver progenitor HepaRG-derived acellular matrix. Exp Cell Res 2016; 341(2): 207–217 [DOI] [PubMed] [Google Scholar]
- 139.Pei M, He F, Li J, Tidwell JE, Jones AC, McDonough EB. Repair of large animal partial-thickness cartilage defects through intraarticular injection of matrix-rejuvenated synovium-derived stem cells. Tissue Eng Part A 2013; 19(9–10): 1144–1154 [DOI] [PubMed] [Google Scholar]
- 140.Li J, Hansen KC, Zhang Y, Dong C, Dinu CZ, Dzieciatkowska M, Pei M. Rejuvenation of chondrogenic potential in a young stem cell microenvironment. Biomaterials 2014; 35(2): 642–653 [DOI] [PubMed] [Google Scholar]
- 141.Ng CP, Sharif AR, Heath DE, Chow JW, Zhang CB, Chan-Park MB, Hammond PT, Chan JK, Griffith LG. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials 2014; 35(13): 4046–4057 [DOI] [PubMed] [Google Scholar]
- 142.Xu Y, Xu GY, Tang C, Wei B, Pei X, Gui JC, Min BH, Jin CZ, Wang LM. Preparation and characterization of bone marrow mesenchymal stem cell-derived extracellular matrix scaffolds. J Biomed Mater Res B Appl Biomater 2015; 103(3): 670–678 [DOI] [PubMed] [Google Scholar]
- 143.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res 2007; 22(12): 1943–1956 [DOI] [PubMed] [Google Scholar]
- 144.Lai Y, Sun Y, Skinner CM, Son EL, Lu Z, Tuan RS, Jilka RL, Ling J, Chen XD. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cells Dev 2010; 19(7): 1095–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gu Y, Zhu J, Xue C, Li Z, Ding F, Yang Y, Gu X. Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 2014; 35(7): 2253–2263 [DOI] [PubMed] [Google Scholar]
- 146.Kwon SH, Lee TJ, Park J, Hwang JE, Jin M, Jang HK, Hwang NS, Kim BS. Modulation of BMP-2-induced chondrogenic versus osteogenic differentiation of human mesenchymal stem cells by cell-specific extracellular matrices. Tissue Eng Part A 2013; 19(1–2): 49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009; 30(23–24): 4021–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Thibault RA, Scott Baggett L, Mikos AG, Kasper FK. Osteogenic differentiation of mesenchymal stem cells on pregenerated extracellular matrix scaffolds in the absence of osteogenic cell culture supplements. Tissue Eng Part A 2010; 16(2): 431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.De Waele J, Reekmans K, Daans J, Goossens H, Berneman Z, Ponsaerts P. 3D culture of murine neural stem cells on decellularized mouse brain sections. Biomaterials 2015; 41: 122–131 [DOI] [PubMed] [Google Scholar]
- 150.Navarro-Tableros V, Herrera Sanchez MB, Figliolini F, Romagnoli R, Tetta C, Camussi G. Recellularization of rat liver scaffolds by human liver stem cells. Tissue Eng Part A 2015; 21(11–12): 1929–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yao Q, Zheng YW, Lan QH, Kou L, Xu HL, Zhao YZ. Recent development and biomedical applications of decellularized extracellular matrix biomaterials. Mater Sci Eng C 2019; 104: 109942. [DOI] [PubMed] [Google Scholar]
- 152.Imle A, Kumberger P, Schnellbächer ND, Fehr J, Carrillo-Bustamante P, Ales J, Schmidt P, Ritter C, Godinez WJ, Müller B, Rohr K, Hamprecht FA, Schwarz US, Graw F, Fackler OT. Experimental and computational analyses reveal that environmental restrictions shape HIV-1 spread in 3D cultures. Nat Commun 2019; 10(1): 2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Saldin LT, Cramer MC, Velankar SS, White LJ, Badylak SF. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater 2017; 49: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.González-Díaz EC, Varghese S. Hydrogels as extracellular matrix analogs. Gels 2016; 2(3): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zhang Y, He Y, Bharadwaj S, Hammam N, Carnagey K, Myers R, Atala A, Van Dyke M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009; 30(23–24): 4021–4028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yi H, S. F, Zhang Y, Skardal A. Bio-functionalized alginate hydrogels for improved cell-matrix interactions and growth factor sequestration kinetics. Tissue Eng Part A 2015; 21(Suppl 1): S187 [Google Scholar]
- 157.Skardal A, Smith L, Bharadwaj S, Atala A, Soker S, Zhang Y. Tissue specific synthetic ECM hydrogels for 3-D in vitro maintenance of hepatocyte function. Biomaterials 2012; 33(18): 4565–4575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lang R, Stern MM, Smith L, Liu Y, Bharadwaj S, Liu G, Baptista PM, Bergman CR, Soker S, Yoo JJ, Atala A, Zhang Y. Three-dimensional culture of hepatocytes on porcine liver tissue-derived extracellular matrix. Biomaterials 2011; 32(29): 7042–7052 [DOI] [PubMed] [Google Scholar]
- 159.Xiong G, Tang W, Zhang D, He D, Wei G, Atala A, Liang XJ, Bleyer AJ, Bleyer ME, Yu J, Aloi JA, Ma JX, Furdui CM, Zhang Y. Impaired regeneration potential in urinary stem cells diagnosed from the patients with diabetic nephropathy. Theranostics 2019; 9(14): 4221–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hussey GS, Dziki JL, Badylak SF. Extracellular matrix-based materials for regenerative medicine. Nat Rev Mater 2018; 3(7): 159–173 [Google Scholar]
- 161.Juhasz I, Kiss B, Lukacs L, Erdei I, Peter Z, Remenyik E. Long-term followup of dermal substitution with acellular dermal implant in burns and postburn scar corrections. Dermatol Res Pract 2010; 2010(1): 210150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Landsman AS, Cook J, Cook E, Landsman AR, Garrett P, Yoon J, Kirkwood A, Desman E. A retrospective clinical study of 188 consecutive patients to examine the effectiveness of a biologically active cryopreserved human skin allograft (TheraSkin®) on the treatment of diabetic foot ulcers and venous leg ulcers. Foot Ankle Spec 2011; 4(1): 29–41 [DOI] [PubMed] [Google Scholar]
- 163.Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995; 21(4): 243–248 [DOI] [PubMed] [Google Scholar]
- 164.Yoeruek E, Bayyoud T, Maurus C, Hofmann J, Spitzer MS, Bartz-Schmidt KU, Szurman P. Reconstruction of corneal stroma with decellularized porcine xenografts in a rabbit model. Acta Ophthalmol 2012; 90(3): e206–e210 [DOI] [PubMed] [Google Scholar]
- 165.Hashimoto Y, Hattori S, Sasaki S, Honda T, Kimura T, Funamoto S, Kobayashi H, Kishida A. Ultrastructural analysis of the decellularized cornea after interlamellar keratoplasty and microkeratome-assisted anterior lamellar keratoplasty in a rabbit model. Sci Rep 2016; 6(1): 27734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu Y, Ma W, Liu B, Wang Y, Chu J, Xiong G, Shen L, Long C, Lin T, He D, Butnaru D, Alexey L, Zhang Y, Zhang D, Wei G. Urethral reconstruction with autologous urine-derived stem cells seeded in three-dimensional porous small intestinal submucosa in a rabbit model. Stem Cell Res Ther 2017; 8(1): 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Y, Liu G, Kropp BP. Re-epithelialization of demucosalized stomach patch with tissue-engineered urothelial mucosa combined with Botox A in bladder augmentation. BJU Int 2012; 110(2b): E106–E112 [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y, Lin HK, Frimberger D, Epstein RB, Kropp BP. Growth of bone marrow stromal cells on small intestinal submucosa: an alternative cell source for tissue engineered bladder. BJU Int 2005; 96(7): 1120–1125 [DOI] [PubMed] [Google Scholar]
- 169.Nherera LM, Romanelli M, Trueman P, Dini V. An overview of clinical and health economic evidence regarding porcine small intestine submucosa extracellular matrix in the management of chronic wounds and burns. Ostomy Wound Manage 2017; 63(12): 38–47 [PubMed] [Google Scholar]
- 170.Hashimoto Y, Funamoto S, Sasaki S, Honda T, Hattori S, Nam K, Kimura T, Mochizuki M, Fujisato T, Kobayashi H, Kishida A. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010; 31(14): 3941–3948 [DOI] [PubMed] [Google Scholar]
- 171.Matsumoto T, Holmes RHO, Burdick CO, Heisterkamp CA 3rd, O’Connell TJ Jr. Replacement of large veins with free inverted segments of small bowel: autografts of submucosal membrane in dogs and clinical use. Ann Surg 1966; 164(5): 845–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Matsumoto T, Holmes RH, Burdick CO, Heisterkamp CA 3rd, O’Connell TJ Jr. The fate of the inverted segment of small bowel used for the replacement of major veins. Surgery 1966; 60(3): 739–743 [PubMed] [Google Scholar]
- 173.Matsumoto T, Holmes RH, Burdick CO, Metzger JF, Heisterkamp CA 3rd, O’Connell TJ Jr. A study of inverted intestinal graft in the major veins. Angiology 1966; 17(11): 842–850 [DOI] [PubMed] [Google Scholar]
- 174.Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res 1989; 47(1): 74–80 [DOI] [PubMed] [Google Scholar]
- 175.Hiles MC, Badylak SF, Lantz GC, Kokini K, Geddes LA, Morff RJ. Mechanical properties of xenogeneic small-intestinal submucosa when used as an aortic graft in the dog. J Biomed Mater Res 1995; 29(7): 883–891 [DOI] [PubMed] [Google Scholar]
- 176.Bader A, Steinhoff G, Strobl K, Schilling T, Brandes G, Mertsching H, Tsikas D, Froelich J, Haverich A. Engineering of human vascular aortic tissue based on a xenogeneic starter matrix. Transplantation 2000; 70(1): 7–14 [PubMed] [Google Scholar]
- 177.Taylor DA, Sampaio LC, Ferdous Z, Gobin AS, Taite LJ. Decellularized matrices in regenerative medicine. Acta Biomater 2018; 74: 74–89 [DOI] [PubMed] [Google Scholar]
- 178.Kimicata M, Allbritton-King JD, Navarro J, Santoro M, Inoue T, Hibino N, Fisher JP. Assessment of decellularized pericardial extracellular matrix and poly(propylene fumarate) biohybrid for small-diameter vascular graft applications. Acta Biomater 2020; 110: 68–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Badylak S, Meurling S, Chen M, Spievack A, Simmons-Byrd A. Resorbable bioscaffold for esophageal repair in a dog model. J Pediatr Surg 2000; 35(7): 1097–1103 [DOI] [PubMed] [Google Scholar]
- 180.Badylak SF, Hoppo T, Nieponice A, Gilbert TW, Davison JM, Jobe BA. Esophageal preservation in five male patients after endoscopic inner-layer circumferential resection in the setting of superficial cancer: a regenerative medicine approach with a biologic scaffold. Tissue Eng Part A 2011; 17(11–12): 1643–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Clough A, Ball J, Smith GS, Leibman S. Porcine small intestine submucosa matrix (Surgisis) for esophageal perforation. Ann Thorac Surg 2011; 91(2): e99–e100 [DOI] [PubMed] [Google Scholar]
- 182.Syed O, Walters NJ, Day RM, Kim HW, Knowles JC. Evaluation of decellularization protocols for production of tubular small intestine submucosa scaffolds for use in oesophageal tissue engineering. Acta Biomater 2014; 10(12): 5043–5054 [DOI] [PubMed] [Google Scholar]
- 183.Luc G, Charles G, Gronnier C, Cabau M, Kalisky C, Meulle M, Bareille R, Roques S, Couraud L, Rannou J, Bordenave L, Collet D, Durand M. Decellularized and matured esophageal scaffold for circumferential esophagus replacement: proof of concept in a pig model. Biomaterials 2018; 175: 1–18 [DOI] [PubMed] [Google Scholar]
- 184.Arakelian L, Caille C, Faivre L, Corté L, Bruneval P, Shamdani S, Flageollet C, Albanese P, Domet T, Jarraya M, Setterblad N, Kellouche S, Larghero J, Cattan P, Vanneaux V. A clinical-grade acellular matrix for esophageal replacement. J Tissue Eng Regen Med 2019; 13(12): 2191–2203 [DOI] [PubMed] [Google Scholar]
- 185.Zhang Y, Frimberger D, Cheng EY, Lin HK, Kropp BP. Challenges in a larger bladder replacement with cell-seeded and unseeded small intestinal submucosa grafts in a subtotal cystectomy model. BJU Int 2006; 98(5): 1100–1105 [DOI] [PubMed] [Google Scholar]
- 186.Kropp BP, Eppley BL, Prevel CD, Rippy MK, Harruff RC, Badylak SF, Adams MC, Rink RC, Keating MA. Experimental assessment of small intestinal submucosa as a bladder wall substitute. Urology 1995; 46(3): 396–400 [DOI] [PubMed] [Google Scholar]
- 187.Zhang XZ, Jiang YL, Hu JG, Zhao LM, Chen QZ, Liang Y, Zhang Y, Lei XX, Wang R, Lei Y, Zhang QY, Li-Ling J, Xie HQ. Procyanidins-crosslinked small intestine submucosa: a bladder patch promotes smooth muscle regeneration and bladder function restoration in a rabbit model. Bioact Mater 2021; 6(6): 1827–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kropp BP, Ludlow JK, Spicer D, Rippy MK, Badylak SF, Adams MC, Keating MA, Rink RC, Birhle R, Thor KB. Rabbit urethral regeneration using small intestinal submucosa onlay grafts. Urology 1998; 52(1): 138–142 [DOI] [PubMed] [Google Scholar]
- 189.Davis NF, Callanan A, McGuire BB, Flood HD, McGloughlin TM. Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue-engineered extracellular matrices. Urology 2011; 77(4): 1007.e1–1007.e7 [DOI] [PubMed] [Google Scholar]
- 190.Janke HP, de Jonge PKJD, Feitz WFJ, Oosterwijk E. Reconstruction strategies of the ureter and urinary diversion using tissue engineering approaches. Tissue Eng Part B Rev 2019; 25(3): 237–248 [DOI] [PubMed] [Google Scholar]
- 191.Adamowicz J, Van Breda SV, Kloskowski T, Juszczak K, Pokrywczynska M, Drewa T. Constructing artificial urinary conduits: current capabilities and future potential. Expert Rev Med Devices 2019; 16(2): 135–144 [DOI] [PubMed] [Google Scholar]
- 192.Totonelli G, Maghsoudlou P, Garriboli M, Riegler J, Orlando G, Burns AJ, Sebire NJ, Smith VV, Fishman JM, Ghionzoli M, Turmaine M, Birchall MA, Atala A, Soker S, Lythgoe MF, Seifalian A, Pierro A, Eaton S, De Coppi P. A rat decellularized small bowel scaffold that preserves villus-crypt architecture for intestinal regeneration. Biomaterials 2012; 33(12): 3401–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Arunkalaivanan AS, Barrington JW. Randomized trial of porcine dermal sling (Pelvicol implant) vs. tension-free vaginal tape (TVT) in the surgical treatment of stress incontinence: a questionnairebased study. Int Urogynecol J Pelvic Floor Dysfunct 2003; 14(1): 17–23 [DOI] [PubMed] [Google Scholar]
- 194.Andrée B, Bär A, Haverich A, Hilfiker A. Small intestinal submucosa segments as matrix for tissue engineering: review. Tissue Eng Part B Rev 2013; 19(4): 279–291 [DOI] [PubMed] [Google Scholar]
- 195.Roeder RA, Lantz GC, Geddes LA. Mechanical remodeling of small-intestine submucosa small-diameter vascular grafts—a preliminary report. Biomed Instrum Technol 2001; 35(2): 110–120 [PubMed] [Google Scholar]
- 196.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 2008; 14(2): 213–221 [DOI] [PubMed] [Google Scholar]
- 197.Mecham RP. Overview of extracellular matrix. Current Protocols in Cell Biology 2012; Chapter 10: Unit 10.11 [DOI] [PubMed] [Google Scholar]
- 198.Plunkett N, O’Brien FJ. Bioreactors in tissue engineering. Technol Health Care 2011; 19(1): 55–69 [DOI] [PubMed] [Google Scholar]
- 199.Scarritt ME, Pashos NC, Bunnell BA. A review of cellularization strategies for tissue engineering of whole organs. Front Bioeng Biotechnol 2015; 3: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nari GA, Mariana C, Romina C, Laura R, Gustavo J, Ricardo T, Salvatierra NA. Preparation of a three-dimensional extracellular matrix by decellularization of rabbit livers. Rev Esp Enferm Dig 2013; 105(3): 138–143 [DOI] [PubMed] [Google Scholar]
- 201.Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 2011; 53(2): 604–617 [DOI] [PubMed] [Google Scholar]
- 202.Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, Costello MJ, Cardinale V, Wauthier E, Barbier C, Gerber DA, Alvaro D, Reid LM. Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 2011; 53(1): 293–305 [DOI] [PubMed] [Google Scholar]
- 203.Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H, Reing J, Gramignoli R, Komori J, Ross M, Nagaya M, Lagasse E, Stolz D, Strom SC, Fox IJ, Badylak SF. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods 2011; 17(6): 677–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Mazza G, Rombouts K, Rennie Hall A, Urbani L, Vinh Luong T, Al-Akkad W, Longato L, Brown D, Maghsoudlou P, Dhillon AP, Fuller B, Davidson B, Moore K, Dhar D, De Coppi P, Malago M, Pinzani M. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep 2015; 5(1): 13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Verstegen MMA, Willemse J, van den Hoek S, Kremers GJ, Luider TM, van Huizen NA, Willemssen FEJA, Metselaar HJ, IJzermans JNM, van der Laan LJW, de Jonge J. Decellularization of whole human liver grafts using controlled perfusion for transplantable organ bioscaffolds. Stem Cells Dev 2017; 26(18): 1304–1315 [DOI] [PubMed] [Google Scholar]
- 206.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, Ott HC. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg 2011; 92(3): 998–1006 [DOI] [PubMed] [Google Scholar]
- 207.Petersen TH, Calle EA, Colehour MB, Niklason LE. Bioreactor for the long-term culture of lung tissue. Cell Transplant 2011; 20(7): 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhou H, Kitano K, Ren X, Rajab TK, Wu M, Gilpin SE, Wu T, Baugh L, Black LD, Mathisen DJ, Ott HC. Bioengineering human lung grafts on porcine matrix. Ann Surg 2018; 267(3): 590–598 [DOI] [PubMed] [Google Scholar]
- 209.Zambon JP, Ko IK, Abolbashari M, Huling J, Clouse C, Kim TH, Smith C, Atala A, Yoo JJ. Comparative analysis of two porcine kidney decellularization methods for maintenance of functional vascular architectures. Acta Biomater 2018; 75: 226–234 [DOI] [PubMed] [Google Scholar]
- 210.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 2013; 19(5): 646–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mandrycky C, Phong K, Zheng Y. Tissue engineering toward organ-specific regeneration and disease modeling. MRS Commun 2017; 7(3): 332–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 2006; 38(12): 1424–1429 [DOI] [PubMed] [Google Scholar]
- 213.Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am 2008; 90(Suppl 1): 14–18 [DOI] [PubMed] [Google Scholar]
- 214.Tsuji K, Cox K, Gamer L, Graf D, Economides A, Rosen V. Conditional deletion of BMP7 from the limb skeleton does not affect bone formation or fracture repair. J Orthop Res 2010; 28(3): 384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. Wnt proteins promote bone regeneration. Sci Transl Med 2010; 2(29): 29ra30. [DOI] [PubMed] [Google Scholar]
- 216.Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Toben D, Jacobsen KA, Al-Sebaei MO, Song M, Trackman PC, Morgan EF, Gerstenfeld LC, Barnes GL. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res 2007; 22(12): 1903–1912 [DOI] [PubMed] [Google Scholar]
- 217.Saidak Z, Le Henaff C, Azzi S, Marty C, Da Nascimento S, Sonnet P, Marie PJ. Wnt/β-catenin signaling mediates osteoblast differentiation triggered by peptide-induced α5β1 integrin priming in mesenchymal skeletal cells. J Biol Chem 2015; 290(11): 6903–6912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhu F, Sweetwyne MT, Hankenson KD. PKCδ is required for Jagged-1 induction of human mesenchymal stem cell osteogenic differentiation. Stem Cells 2013; 31(6): 1181–1192 [DOI] [PubMed] [Google Scholar]
- 219.Dishowitz MI, Zhu F, Sundararaghavan HG, Ifkovits JL, Burdick JA, Hankenson KD. Jagged1 immobilization to an osteoconductive polymer activates the Notch signaling pathway and induces osteogenesis. J Biomed Mater Res A 2014; 102(5): 1558–1567 [DOI] [PubMed] [Google Scholar]
- 220.Tian Y, Xu Y, Xue T, Chen L, Shi B, Shu B, Xie C, Max Morandi M, Jaeblon T, Marymont JV, Dong Y. Notch activation enhances mesenchymal stem cell sheet osteogenic potential by inhibition of cellular senescence. Cell Death Dis 2017; 8(2): e2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Day TF, Guo X, Garrett-Beal L, Yang Y. Wnt/β-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell 2005; 8(5): 739–750 [DOI] [PubMed] [Google Scholar]
- 222.Ryu JH, Kim SJ, Kim SH, Oh CD, Hwang SG, Chun CH, Oh SH, Seong JK, Huh TL, Chun JS. Regulation of the chondrocyte phenotype by beta-catenin. Development 2002; 129(23): 5541–5550 [DOI] [PubMed] [Google Scholar]
- 223.Chaly Y, Blair HC, Smith SM, Bushnell DS, Marinov AD, Campfield BT, Hirsch R. Follistatin-like protein 1 regulates chondrocyte proliferation and chondrogenic differentiation of mesenchymal stem cells. Ann Rheum Dis 2015; 74(7): 1467–1473 [DOI] [PubMed] [Google Scholar]
- 224.Delhon L, Mahaut C, Goudin N, Gaudas E, Piquand K, Le Goff W, Cormier-Daire V, Le Goff C. Impairment of chondrogenesis and microfibrillar network in Adamtsl2 deficiency. FASEB J 2019; 33(2): 2707–2718 [DOI] [PubMed] [Google Scholar]
- 225.Fischer J, Knoch N, Sims T, Rosshirt N, Richter W. Timedependent contribution of BMP, FGF, IGF, and HH signaling to the proliferation of mesenchymal stroma cells during chondrogenesis. J Cell Physiol 2018; 233(11): 8962–8970 [DOI] [PubMed] [Google Scholar]
- 226.Murphy MK, Huey DJ, Hu JC, Athanasiou KA. TGF-β1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem Cells 2015; 33(3): 762–773 [DOI] [PubMed] [Google Scholar]
- 227.Kovermann NJ, Basoli V, Della Bella E, Alini M, Lischer C, Schmal H, Kubosch EJ, Stoddart MJ. BMP2 and TGF-β cooperate differently during synovial-derived stem-cell chondrogenesis in a dexamethasone-dependent manner. Cells 2019; 8(6): 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chung UI, Schipani E, McMahon AP, Kronenberg HM. Indian hedgehog couples chondrogenesis to osteogenesis in endochondral bone development. J Clin Invest 2001; 107(3): 295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen L, Liu G, Li W, Wu X. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells following transfection with Indian hedgehog and sonic hedgehog using a rotary cell culture system. Cell Mol Biol Lett 2019; 24(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Parisi A, Lacour F, Giordani L, Colnot S, Maire P, Le Grand F. APC is required for muscle stem cell proliferation and skeletal muscle tissue repair. J Cell Biol 2015; 210(5): 717–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhang K, Zhang Y, Gu L, Lan M, Liu C, Wang M, Su Y, Ge M, Wang T, Yu Y, Liu C, Li L, Li Q, Zhao Y, Yu Z, Wang F, Li N, Meng Q. Islr regulates canonical Wnt signaling-mediated skeletal muscle regeneration by stabilizing Dishevelled-2 and preventing autophagy. Nat Commun 2018; 9(1): 5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rochat A, Fernandez A, Vandromme M, Molès JP, Bouschet T, Carnac G, Lamb NJ. Insulin and wnt1 pathways cooperate to induce reserve cell activation in differentiation and myotube hypertrophy. Mol Biol Cell 2004; 15(10): 4544–4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Baghdadi MB, Castel D, Machado L, Fukada SI, Birk DE, Relaix F, Tajbakhsh S, Mourikis P. Reciprocal signalling by Notch-Collagen V-CALCR retains muscle stem cells in their niche. Nature 2018; 557(7707): 714–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pisconti A, Cornelison DD, Olguín HC, Antwine TL, Olwin BB. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J Cell Biol 2010; 190(3): 427–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Pollen AA, Bhaduri A, Andrews MG, Nowakowski TJ, Meyerson OS, Mostajo-Radji MA, Di Lullo E, Alvarado B, Bedolli M, Dougherty ML, Fiddes IT, Kronenberg ZN, Shuga J, Leyrat AA, West JA, Bershteyn M, Lowe CB, Pavlovic BJ, Salama SR, Haussler D, Eichler EE, Kriegstein AR. Establishing cerebral organoids as models of human-specific brain evolution. Cell 2019; 176(4): 743–756.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fiddes IT, Lodewijk GA, Mooring M, Bosworth CM, Ewing AD, Mantalas GL, Novak AM, van den Bout A, Bishara A, Rosenkrantz JL, Lorig-Roach R, Field AR, Haeussler M, Russo L, Bhaduri A, Nowakowski TJ, Pollen AA, Dougherty ML, Nuttle X, Addor MC, Zwolinski S, Katzman S, Kriegstein A, Eichler EE, Salama SR, Jacobs FMJ, Haussler D. Human-specific NOTCH2NL genes affect notch signaling and cortical neurogenesis. Cell 2018; 173(6): 1356–1369.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Andersen J, Revah O, Miura Y, Thom N, Amin ND, Kelley KW, Singh M, Chen X, Thete MV, Walczak EM, Vogel H, Fan HC, Paşca SP. Generation of functional human 3D cortico-motor assembloids. Cell 2020;183(7):1913–1929.e1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol 2014; 12(8): e1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Rivetti di Val Cervo P, Romanov RA, Spigolon G, Masini D, Martín-Montañez E, Toledo EM, La Manno G, Feyder M, Pifl C, Ng YH, Sánchez SP, Linnarsson S, Wernig M, Harkany T, Fisone G, Arenas E. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson’s disease model. Nat Biotechnol 2017; 35(5): 444–452 [DOI] [PubMed] [Google Scholar]
- 240.Ma JJ, Ju X, Xu RJ, Wang WH, Luo ZP, Liu CM, Yang L, Li B, Chen JQ, Meng B, Yang HL, Zhou FQ, Saijilafu. Telomerase reverse transcriptase and p53 regulate mammalian peripheral nervous system and CNS axon regeneration downstream of c-Myc. J Neurosci 2019; 39(46): 9107–9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proc Natl Acad Sci USA 2017; 114(40): E8372–E8381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Titmarsh DM, Glass NR, Mills RJ, Hidalgo A, Wolvetang EJ, Porrello ER, Hudson JE, Cooper-White JJ. Induction of human iPSC-derived cardiomyocyte proliferation revealed by combinatorial screening in high density microbioreactor arrays. Sci Rep 2016; 6(1): 24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Drakhlis L, Biswanath S, Farr CM, Lupanow V, Teske J, Ritzenhoff K, Franke A, Manstein F, Bolesani E, Kempf H, Liebscher S, Schenke-Layland K, Hegermann J, Nolte L, Meyer H, de la Roche J, Thiemann S, Wahl-Schott C, Martin U, Zweigerdt R. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol 2021; 39(6): 737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kodo K, Ong SG, Jahanbani F, Termglinchan V, Hirono K, InanlooRahatloo K, Ebert AD, Shukla P, Abilez OJ, Churko JM, Karakikes I, Jung G, Ichida F, Wu SM, Snyder MP, Bernstein D, Wu JC. iPSC-derived cardiomyocytes reveal abnormal TGF-β signalling in left ventricular non-compaction cardiomyopathy. Nat Cell Biol 2016; 18(10): 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of cell cycle to stimulate adult cardiomyocyte proliferation and cardiac regeneration. Cell 2018; 173(1): 104–116.e12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Mikryukov AA, Mazine A, Wei B, Yang D, Miao Y, Gu M, Keller GM. BMP10 signaling promotes the development of endocardial cells from human pluripotent stem cell-derived cardiovascular progenitors. Cell Stem Cell 2021; 28(1): 96–111.e7 [DOI] [PubMed] [Google Scholar]
- 247.Wimmer RA, Leopoldi A, Aichinger M, Wick N, Hantusch B, Novatchkova M, Taubenschmid J, Hämmerle M, Esk C, Bagley JA, Lindenhofer D, Chen G, Boehm M, Agu CA, Yang F, Fu B, Zuber J, Knoblich JA, Kerjaschki D, Penninger JM. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019; 565(7740): 505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wimmer RA, Leopoldi A, Aichinger M, Kerjaschki D, Penninger JM. Generation of blood vessel organoids from human pluripotent stem cells. Nat Protoc 2019; 14(11): 3082–3100 [DOI] [PubMed] [Google Scholar]
- 249.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, Danenberg E, van den Brink S, Korving J, Abo A, Peters PJ, Wright N, Poulsom R, Clevers H. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010; 6(1): 25–36 [DOI] [PubMed] [Google Scholar]
- 250.Sigal M, Logan CY, Kapalczynska M, Mollenkopf HJ, Berger H, Wiedenmann B, Nusse R, Amieva MR, Meyer TF. Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 2017; 548(7668): 451–455 [DOI] [PubMed] [Google Scholar]
- 251.Yan KS, Janda CY, Chang J, Zheng GXY, Larkin KA, Luca VC, Chia LA, Mah AT, Han A, Terry JM, Ootani A, Roelf K, Lee M, Yuan J, Li X, Bolen CR, Wilhelmy J, Davies PS, Ueno H, von Furstenberg RJ, Belgrader P, Ziraldo SB, Ordonez H, Henning SJ, Wong MH, Snyder MP, Weissman IL, Hsueh AJ, Mikkelsen TS, Garcia KC, Kuo CJ. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature 2017; 545(7653): 238–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kim KA, Kakitani M, Zhao J, Oshima T, Tang T, Binnerts M, Liu Y, Boyle B, Park E, Emtage P, Funk WD, Tomizuka K. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 2005; 309(5738): 1256–1259 [DOI] [PubMed] [Google Scholar]
- 253.Schuijers J, Junker JP, Mokry M, Hatzis P, Koo BK, Sasselli V, van der Flier LG, Cuppen E, van Oudenaarden A, Clevers H. Ascl2 acts as an R-spondin/Wnt-responsive switch to control stemness in intestinal crypts. Cell Stem Cell 2015; 16(2): 158–170 [DOI] [PubMed] [Google Scholar]
- 254.Yin X, Farin HF, van Es JH, Clevers H, Langer R, Karp JM. Nicheindependent high-purity cultures of Lgr5+ intestinal stem cells and their progeny. Nat Methods 2014; 11(1): 106–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Serra D, Mayr U, Boni A, Lukonin I, Rempfler M, Challet Meylan L, Stadler MB, Strnad P, Papasaikas P, Vischi D, Waldt A, Roma G, Liberali P. Self-organization and symmetry breaking in intestinal organoid development. Nature 2019; 569(7754): 66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, Haft A, Vries RG, Grompe M, Clevers H. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013; 494(7436): 247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lin Y, Fang ZP, Liu HJ, Wang LJ, Cheng Z, Tang N, Li T, Liu T, Han HX, Cao G, Liang L, Ding YQ, Zhou WJ. HGF/R-spondin1 rescues liver dysfunction through the induction of Lgr5+ liver stem cells. Nat Commun 2017; 8(1): 1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M, Choi SS, Diehl AM. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology 2010; 51(5): 1712–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Langiewicz M, Graf R, Humar B, Clavien PA. JNK1 induces hedgehog signaling from stellate cells to accelerate liver regeneration in mice. J Hepatol 2018; 69(3): 666–675 [DOI] [PubMed] [Google Scholar]
- 260.Barker N, Rookmaaker MB, Kujala P, Ng A, Leushacke M, Snippert H, van de Wetering M, Tan S, Van Es JH, Huch M, Poulsom R, Verhaar MC, Peters PJ, Clevers H. Lgr5+ve stem/progenitor cells contribute to nephron formation during kidney development. Cell Rep 2012; 2(3): 540–552 [DOI] [PubMed] [Google Scholar]
- 261.Low JH, Li P, Chew EGY, Zhou B, Suzuki K, Zhang T, Lian MM, Liu M, Aizawa E, Rodriguez Esteban C, Yong KSM, Chen Q, Campistol JM, Fang M, Khor CC, Foo JN, Izpisua Belmonte JC, Xia Y. Generation of human PSC-derived kidney organoids with patterned nephron segments and a de novo vascular network. Cell Stem Cell 2019; 25(3): 373–387.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell 2018; 23(6): 869–881.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Forbes TA, Howden SE, Lawlor K, Phipson B, Maksimovic J, Hale L, Wilson S, Quinlan C, Ho G, Holman K, Bennetts B, Crawford J, Trnka P, Oshlack A, Patel C, Mallett A, Simons C, Little MH. Patient-iPSC-derived kidney organoids show functional validation of a ciliopathic renal phenotype and reveal underlying pathogenetic mechanisms. Am J Hum Genet 2018; 102(5): 816–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 2011; 472(7341): 110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kim E, Choi S, Kang B, Kong J, Kim Y, Yoon WH, Lee HR, Kim S, Kim HM, Lee H, Yang C, Lee YJ, Kang M, Roh TY, Jung S, Kim S, Ku JH, Shin K. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 2020; 588(7839): 664–669 [DOI] [PubMed] [Google Scholar]
- 266.Santos CP, Lapi E, Martínez de Villarreal J, Álvaro-Espinosa L, Fernández-Barral A, Barbáchano A, Domínguez O, Laughney AM, Megías D, Muñoz A, Real FX. Urothelial organoids originating from Cd49fhigh mouse stem cells display Notchdependent differentiation capacity. Nat Commun 2019; 10(1): 4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kessler M, Hoffmann K, Brinkmann V, Thieck O, Jackisch S, Toelle B, Berger H, Mollenkopf HJ, Mangler M, Sehouli J, Fotopoulou C, Meyer TF. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun 2015; 6(1): 8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Xie Y, Park ES, Xiang D, Li Z. Long-term organoid culture reveals enrichment of organoid-forming epithelial cells in the fimbrial portion of mouse fallopian tube. Stem Cell Res (Amst) 2018; 32: 51–60 [DOI] [PubMed] [Google Scholar]
- 269.Boretto M, Maenhoudt N, Luo X, Hennes A, Boeckx B, Bui B, Heremans R, Perneel L, Kobayashi H, Van Zundert I, Brems H, Cox B, Ferrante M, Uji-I H, Koh KP, D’Hooghe T, Vanhie A, Vergote I, Meuleman C, Tomassetti C, Lambrechts D, Vriens J, Timmerman D, Vankelecom H. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol 2019; 21(8): 1041–1051 [DOI] [PubMed] [Google Scholar]
- 270.Boretto M, Cox B, Noben M, Hendriks N, Fassbender A, Roose H, Amant F, Timmerman D, Tomassetti C, Vanhie A, Meuleman C, Ferrante M, Vankelecom H. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development 2017; 144(10): 1775–1786 [DOI] [PubMed] [Google Scholar]
- 271.Ali A, Syed SM, Jamaluddin MFB, Colino-Sanguino Y, Gallego-Ortega D, Tanwar PS. Cell lineage tracing identifies hormoneregulated and Wnt-responsive vaginal epithelial stem cells. Cell Rep 2020; 30(5): 1463–1477.e7 [DOI] [PubMed] [Google Scholar]
- 272.Zhang B, Ci X, Tao R, Ni JJ, Xuan X, King JL, Xia S, Li Y, Frierson HF, Lee DK, Xu J, Osunkoya AO, Dong JT. Klf5 acetylation regulates luminal differentiation of basal progenitors in prostate development and regeneration. Nat Commun 2020; 11(1): 997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wang Y, Yu A, Yu FX. The Hippo pathway in tissue homeostasis and regeneration. Protein Cell 2017; 8(5): 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol 2019; 20(4): 211–226 [DOI] [PubMed] [Google Scholar]
- 275.Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, Ding S, Guan KL. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev 2010; 24(11): 1106–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Qin H, Blaschke K, Wei G, Ohi Y, Blouin L, Qi Z, Yu J, Yeh RF, Hebrok M, Ramalho-Santos M. Transcriptional analysis of pluripotency reveals the Hippo pathway as a barrier to reprogramming. Hum Mol Genet 2012; 21(9): 2054–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Qin H, Hejna M, Liu Y, Percharde M, Wossidlo M, Blouin L, Durruthy-Durruthy J, Wong P, Qi Z, Yu J, Qi LS, Sebastiano V, Song JS, Ramalho-Santos M. YAP induces human naive pluripotency. Cell Rep 2016; 14(10): 2301–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Heng BC, Zhang X, Aubel D, Bai Y, Li X, Wei Y, Fussenegger M, Deng X. Role of YAP/TAZ in cell lineage fate determination and related signaling pathways. Front Cell Dev Biol 2020; 8: 735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Hong AW, Meng Z, Guan KL. The Hippo pathway in intestinal regeneration and disease. Nat Rev Gastroenterol Hepatol 2016; 13(6): 324–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Driskill JH, Pan D. The Hippo pathway in liver homeostasis and pathophysiology. Annu Rev Pathol 2021; 16(1): 299–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, Boeing S, Wculek SK, Cordero J, Tan EH, Ridgway R, Brunton VG, Sahai E, Gerhardt H, Behrens A, Malanchi I, Sansom OJ, Thompson BJ. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016; 143(10): 1674–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Wang J, Liu S, Heallen T, Martin JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol 2018; 15(11): 672–684 [DOI] [PubMed] [Google Scholar]
- 283.He X, Zhang L, Queme LF, Liu X, Lu A, Waclaw RR, Dong X, Zhou W, Kidd G, Yoon SO, Buonanno A, Rubin JB, Xin M, Nave KA, Trapp BD, Jankowski MP, Lu QR. A histone deacetylase 3-dependent pathway delimits peripheral myelin growth and functional regeneration. Nat Med 2018; 24(3): 338–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zanconato F, Cordenonsi M, Piccolo S. YAP/TAZ at the roots of cancer. Cancer Cell 2016; 29(6): 783–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Erickson JR, Echeverri K. Learning from regeneration research organisms: the circuitous road to scar free wound healing. Dev Biol 2018; 433(2): 144–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Taha IN, Naba A. Exploring the extracellular matrix in health and disease using proteomics. Essays Biochem 2019; 63(3): 417–432 [DOI] [PubMed] [Google Scholar]
- 287.Doyle JJ, Gerber EE, Dietz HC. Matrix-dependent perturbation of TGFβ signaling and disease. FEBS Lett 2012; 586(14): 2003–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Argyropoulos AJ, Robichaud P, Balimunkwe RM, Fisher GJ, Hammerberg C, Yan Y, Quan T. Alterations of dermal connective tissue collagen in diabetes: molecular basis of aged-appearing skin. PLoS One 2016; 11(4): e0153806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Vigneswari S, Chai JM, Kamarudin KH, Amirul AA, Focarete ML, Ramakrishna S. Elucidating the surface functionality of biomimetic RGD peptides immobilized on nano-P(3HB-co-4HB) for H9c2 myoblast cell proliferation. Front Bioeng Biotechnol 2020; 8: 567693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Masaeli E, Nasr-Esfahani MH. An in vivo evaluation of induced chondrogenesis by decellularized extracellular matrix particles. J Biomed Mater Res A 2021; 109(5): 627–636 [DOI] [PubMed] [Google Scholar]
- 291.Yen YH, Pu CM, Liu CW, Chen YC, Chen YC, Liang CJ, Hsieh JH, Huang HF, Chen YL. Curcumin accelerates cutaneous wound healing via multiple biological actions: the involvement of TNF-α, MMP-9, α-SMA, and collagen. Int Wound J 2018; 15(4): 605–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Fisher MB, Liang R, Jung HJ, Kim KE, Zamarra G, Almarza AJ, McMahon PJ, Woo SL. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc 2012; 20(7): 1357–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]


