Graphical Abstract
Keywords: Helicobacter pylori, treatment, eradication therapy, antibiotics, susceptibility testing, culture, next generation sequencing
Introduction
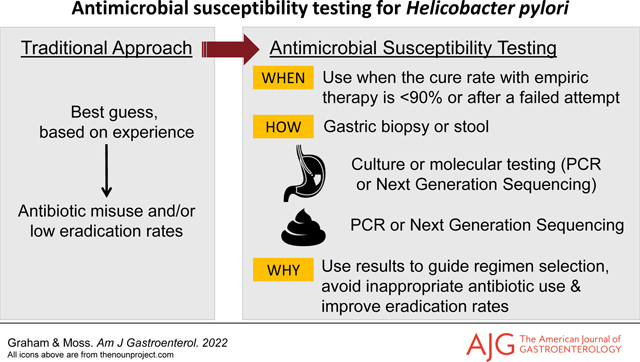
H. pylori gastritis is now recognized as an important transmissible infectious disease involving the stomach (1). Susceptibility testing for H. pylori has become widely available in the United States allowing for full utilization of susceptibility-based antimicrobial therapy (Table 1). To reliably achieve high cure rates one should only use optimized regimens defined as consistently achieving high cure rates (e.g., ≥95%) in adherent patients with susceptible infections (2, 3). Optimized treatment regimens are shown in Table 2 (4). These are best used as susceptibility-based therapies. The decision to use an optimized regimen empirically should be based on knowledge of the local susceptibility patterns as well as monitoring treatment outcomes to ensure that the regimen remains highly effective locally (2, 3). Effectiveness must be evaluated in near real-time by routinely obtaining post treatment test-of-cure results which serves to both validate the clinician’s current practices and informs when a change to a more effective treatment regimen is required. A decline in cure rates would then prompt clinicians to obtain susceptibility data until a new proven locally effective regimen was identified, which could then be used empirically. Ideally, test-of-cure data should be shared to inform local/regional data regarding treatment effectiveness (2, 3). Formal reporting mechanism to do this have yet to be established in the United States.
Table 1.
Where to obtain H. pylori susceptibility testing in the United States
Table 2:
Currently available and effective H. pylori therapies in the United States.
| Empiric Therapies | |
|---|---|
| Bismuth quadruple therapy Bismuth subsalicylate q.i.d. 14 days |
Bismuth (e.g., PeptoBismol®) 2 tablets or 2 capsules q.i.d. 30 min before meals, tetracycline HCl 500 mg and metronidazole 500 mg 30 minutes after meals q.i.d. plus a PPI, 30 minutes b.i.d. before breakfast and with the evening meal (see PPI recommendations below) |
| Pylera®. 3-in1 formulation of bismuth quadruple therapy with bismuth citrate) metronidazole and tetracycline 14-days | Give combination tablets 4 times daily (with meals and at bedtime) plus a PPI 30 minutes before breakfast (see PPI recommendations below) If the pharmacist will only dispense a 10-day supply, utilize 10 days or consider using 14-day generic bismuth quadruple therapy instead (see above) |
| Rifabutin triple therapy. 14-days | Rifabutin 150 mg b.i.d. 30 after breakfast and the evening meal, amoxicillin 1 gram t.i.d. 30 after breakfast, the evening meal, and bedtime plus 40 mg of esomeprazole or rabeprazole 30 minutes before breakfast and the evening meal. (see PPI recommendations below). |
| Talicia® 3-in 1 formulation of rifabutin/amoxicillin/omeprazole triple therapy. 14-days | 4 capsules t.id., as directed by the package insert |
| Therapies only effective as susceptibility-based therapy Do not use empirically unless proven to cure >90% locally | |
| Clarithromycin triple therapy. 14-days |
Clarithromycin 500 mg b.i.d., amoxicillin 1 gram b.i.d. 30 minutes after meal plus a PPI b.i.d. 30 minutes before breakfast and the evening meal (see PPI recommendations below)** |
| Metronidazole triple therapy. 14-days |
Metronidazole 500 mg b.i.d., amoxicillin 1 gram b.i.d., 30 minutes after meal plus a PPI b.i.d. 30 minutes before breakfast and the evening meal (see PPI recommendations below) |
| Levofloxacin triple therapy. 14-days* |
Levofloxacin 500 mg in a.m., amoxicillin 1 gram b.i.d., 30 minutes after meal plus a PPI b.i.d. 30 minutes before breakfast and the evening meal (see PPI recommendations below) |
| PPI should preferably be a PPI which is minimally affected by CYP2C19 metabolism (i.e., rabeprazole or esomeprazole) and at least 20 mg per dose (preferably 40 mg) of rabeprazole or esomeprazole b.i.d. | |
| Regimens that include at least one antibiotic that offers no therapeutic benefit and serve to increase global antimicrobial resistance include: concomitant, hybrid, reverse hybrid, sequential therapies and vonoprazan clarithromycin and amoxicillin triple therapy 3. | |
Abbreviations: HCL = hydrochloride, mg = milligram, q.i.d. = 4 times daily, b.i.d. = two time daily, t.i.d. = three times daily, PPI = proton pump inhibitor, a.m. = in the morning.
The FDA recommends fluoroquinolones be used as a last choice because of the risk of serious side effects. Table adapted from reference (4), with permission
Currently successful empiric therapies most often utilize antibiotics for which resistance is rare (e.g., tetracycline, amoxicillin and rifabutin and furazolidone where available) (Table 2). Metronidazole is also used in bismuth quadruple therapy as resistance can often be overcome by increasing the dose 1,500 to 2,000 mg/day and the duration to 14 days (5–8). Currently suitable empiric regimens include bismuth quadruple therapy and rifabutin triple therapy (Table 2). Theoretically, proton pump inhibitor (PPI) or vonoprazan plus amoxicillin dual therapy are candidates for an empiric first-line choice, especially in Asia, but results with such dual therapy have not reached consistently high cure rates in western countries. We predict that after optimization for use in western countries, vonoprazan dual therapy is likely to become a preferred therapy. Importantly, obtaining high cure rates requires that patients adhere to the details of the regimen (Table 3). Patient education cannot be over-emphasized (Table 4).
Table 3:
Guidelines for prescribing successful H. pylori therapies
| Take an antibiotic use history and if available review prior prescriptions to identify the antibiotics where resistance is likely. |
| Prescribe only therapies that are proven to be effective locally (i.e., cure rates ≥90%) or preferably highly effective locally (i.e., cure rates of ≥95%). |
| The rules of thumb regarding therapy include: only use antibiotics to which the organism is susceptible. Antibiotic doses and dosing frequency are based on local results. A duration of 14-days is best. Esomeprazole or rabeprazole 40 mg b.i.d. are preferred as they are more potent and minimally affected by CYP2C19 metabolism. |
| Do not prescribe clarithromycin, metronidazole, or levofloxacin for H. pylori infections unless susceptibility has been confirmed. The exceptions are use of metronidazole in bismuth quadruple therapy, and confirmed excellent outcomes locally with these triple therapies. |
| Quinolones (e.g., levofloxacin) have recently been associated with severe long term side effects and should not be prescribed unless a) susceptibility is confirmed and b) no other options are available. |
| Resistance to tetracycline, amoxicillin and rifabutin are still rare |
| Therapies that contain unneeded antibiotics (e.g., concomitant, sequential, hybrid, reverse hybrid and vonoprazan clarithromycin triple therapies). should not be prescribed as the unneeded antibiotic (most often clarithromycin) unnecessarily contributes to increased global antimicrobial resistance |
| Perform Test-of-Cure after every treatment to provide continuing feedback regarding current effectiveness. |
| Share Test-of-Cure results with partners and colleagues so as to contribute to the local and regional experience regarding which H. pylori therapies are locally effective vs. ineffective. |
| Successful use of an empiric therapy is critically dependent on monitoring its effectiveness and the willingness to abandon an empiric therapy if its effectiveness declines. |
| Susceptibility data must be coupled with optimized therapy to achieve its full potential |
Table 4.
Methods to enhance the effectiveness of H. pylori therapy
| Take a detailed medical and antibiotic use history and provide adequate time for office visits. |
| Explain in simplistic terms the effects of the infection on the stomach, the potential outcomes of the infection, and how cure of the infection results in healing of the damage, prevention of ulcers and ulcer recurrences, and greatly reducing the risk of gastric cancer. |
| Provide a clear written description of the complexities of the regimen chosen and the necessity for adherence to the full treatment schedule |
| Provide a clear written description of the medications and plan for dosing and, if possible, providing appropriate containers (pill boxes or blister packs) arranged according to the dosing plans in relation to meals and bedtime. |
| Emphasize that the medications are taken concurrently for the full 14-day period and to not start to take the medications until all of the drugs have been received. |
| Describe the adverse effects which are commonly expected as a consequence of the treatment, such as feeling unwell with nausea, headaches, taste disturbances, loose or dark stool. etc.. |
| Provide written instructions in a language that can be read and understood for patients where English is not their first language. |
| To ensure adherence, provide a contact available after hours and weekends that can answer questions. |
| Monitor adherence by discussion and by pill counting during treatment if necessary. |
| A test-of-cure by breath or stool antigen /PCR test should be done 4 or more weeks after therapy and off PPIs for at least 2 weeks to ensure cure and provide feedback on the local effectiveness of the therapy utilized. H2 receptor antagonists can be substituted during the PPI abstinence period. |
| Test-of-cure results should be shared with colleagues and institutions locally to provide information regarding local susceptibility patterns. |
Adapted from reference (13), with permission
How to order/obtain susceptibility testing for H pylori.
As noted in Table 1, susceptibility testing is currently available from a number of diagnostic laboratories making it possibly to restrict the prescription of clarithromycin, levofloxacin or metronidazole triple therapies to confirmed susceptible infections. It now behooves clinicians to explore how to add H. pylori susceptibility testing to their practices. An email or phone call to one of the listed facilities generally leads to a rapid response that includes a requisition and collection instructions. We suggest ordering susceptibility testing for the 6 commonly used antibiotics: amoxicillin, metronidazole, tetracycline, levofloxacin, and clarithromycin and rifabutin (note rifampin is different than rifabutin) when sending samples.
Culture and susceptibility testing is available using fresh or frozen gastric biopsies. Molecular testing can be performed on the same material and, in addition, on the formalin-fixed gastric biopsies that had been used for histology. Importantly, molecular testing can also be performed on stools.
Gastric biopsies:
One should take at least two biopsies, preferably using large cup forceps, one from the corpus and one from the antrum which can be placed in the same bottle. If the culture is positive, susceptibility testing is reflexively done and reported. Traditionally, gastric biopsies have been placed in a transport media [e.g., Brucella broth with 20% glycerol or Portagerm pylori®, (bioMerieux, Durham, North Carolina) or an equivalent] and shipped overnight. However, Quest, LabCorp and AURP require the samples in saline which generally results in a reduced proportion of cases with successful growth. Currently, only Microbiology Specialists Inc. provides Brucella broth with 20% glycerol for transport. The sample in the Brucella broth is immediately frozen, preferably by a quick freeze at −70° to −80°C (although −20°C will suffice), and shipped overnight on dry ice. Kept frozen, the sample will be stable for up to a week before culturing. If maintained in a −70° to −80°C freezer the sample will remain viable for months, if not years, and can be shipped on dry ice when convenient.
The alternative to culture is susceptibility testing using molecular methods such as Next Generation Sequencing (NGS) (American Molecular Laboratories or AML). AML provides specimen containers with a preservative medium that can either be sent immediately for H. pylori diagnosis (PyloriDx™) and, if positive, the specimen will reflect to susceptibility testing. Alternately, the protected preserved specimen can be retained 1 to 2 weeks at room temperature and only sent for susceptibility testing if the histology is positive or equivocal.
Formalin fixed gastric biopsies:
AML offers NGS susceptibility testing using the formalin fixed gastric tissue blocks (sections can be sent although care must be taken not to contaminate them while sectioning) (9, 10).
Stools:
Susceptibility testing using stools has the advantage of obviating the need for endoscopy and gastric biopsy (11). AML offers an as a reflex option for stool antigen testing using NGS to reflexively test stool for the 6 commonly prescribed antibiotics. Mayo Clinic Laboratories offers reflex stool testing for clarithromycin resistance.
The effect of susceptibility testing on endoscopy practices.
Although primary susceptibility-based therapy is a good option, we anticipate use of a mixed empiric and susceptibility-based approach, depending on the local success of first-line empiric therapies and prior treatment attempts (Figure 1). Whenever H. pylori is suspected at upper gastrointestinal endoscopy biopsies can be collected for susceptibility and either immediately sent for H. pylori diagnosis with susceptibility testing for positive tests, or else the specimen can be retained until the histology is reported, and then shipped for testing. Successful culture is dependent on the handling and shipment of the biopsies. If the culture of known infected samples is <80% successful, we recommend switching to molecular testing with NGS (e.g., AML) or to a laboratory that provides Brucella broth with glycerol as transport.
Figure 1.
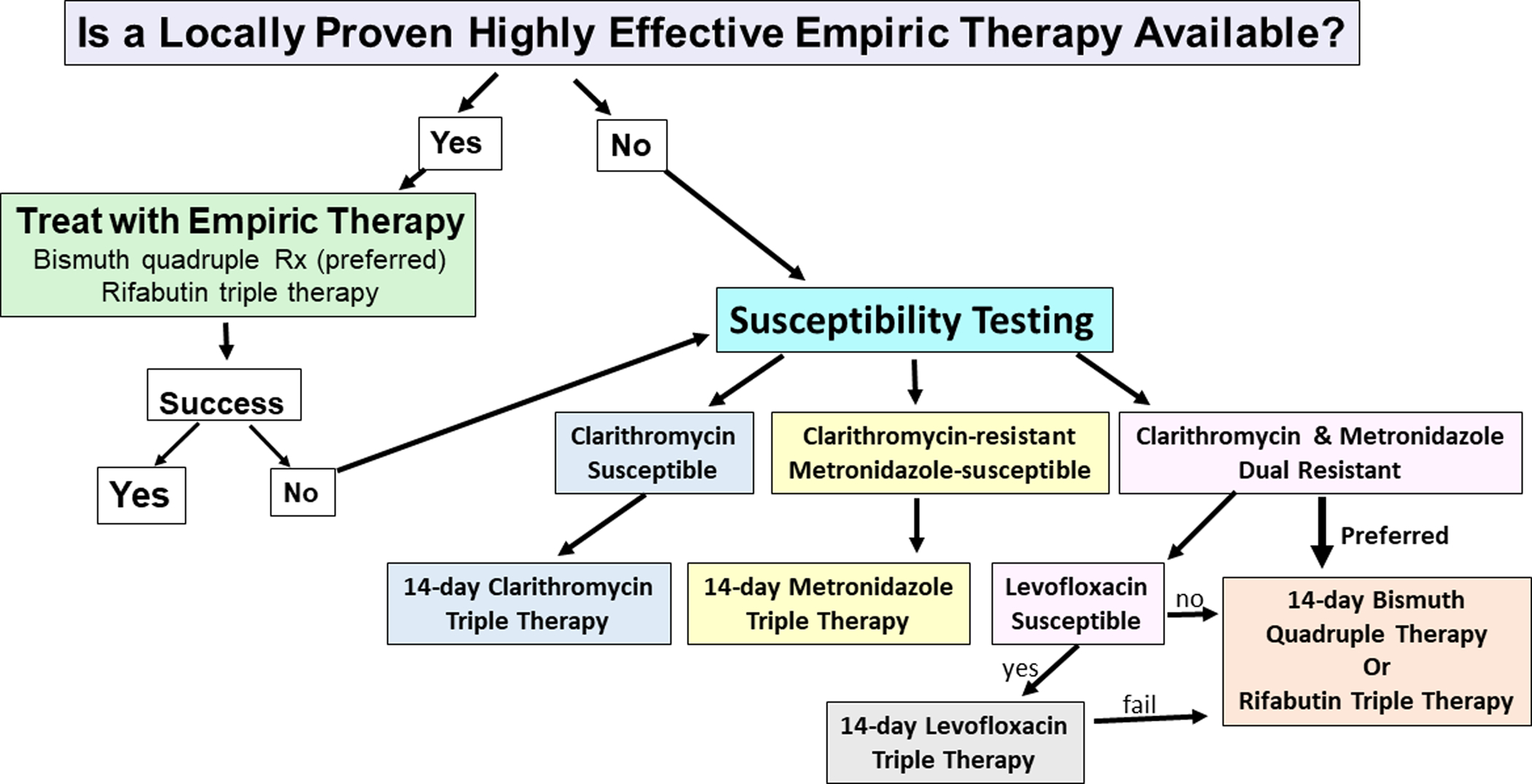
Proposed algorithm for selection of H. pylori regimen based upon knowledge of the results of empiric first-line therapies, and the results of susceptibility testing
Summary
The widespread availability of H. pylori susceptibility testing now renders the low cure-rate empiric therapies (e.g., triple or quadruple therapies containing clarithromycin, metronidazole or levofloxacin) obsolete. The exception is the use of high dose metronidazole in 14-day bismuth quadruple therapy. Susceptibility-based therapy, when combined with optimized regimens, will reliably achieve high cure rates, provided the patient is well-advised and motivated to be adherent with the regimen (Table 4). Proper utilization of susceptibility testing requires attention to the details of gastric specimen collection.
Susceptibility-based therapy can be used for initial therapy. Alternately, an empiric therapy first strategy can be used if the empiric therapy has been confirmed to be highly effective locally. Both susceptibility or empiric therapy first strategies require self-monitoring using a test-of-cure to provide feedback to confirm continuing success. Use of an empiric therapy first approach must be based on willingness to abandon empiric therapy if its effectiveness declines. Patients with a history of prior treatment failure should receive susceptibility-based therapy. It is important to note that clarithromycin, metronidazole and levofloxacin triple therapies are highly effective when used as susceptibility-based therapy (12). Finally, the recent FDA warning about serious long term side effects of quinolone therapy dictate that fluoroquinolones only be used as susceptibility-based therapies to the patient experiencing only risks.
Acknowledgements:
The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. The authors thank Dr. William Chey for his valuable comments.
Funding:
Dr Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grant DK56338 which funds the Texas Medical Center Digestive Diseases Center.
Competing interests:
Dr. Graham is a consultant for RedHill Biopharma and Phathom Pharmaceuticals regarding novel H. pylori therapies and has received research support for culture of Helicobacter pylori. He is also a consultant for DiaSorin regarding H. pylori diagnostics and with Otsuka Japan regarding novel breath tests. He has ongoing collaborative research projects with American Molecular Laboratories regarding molecular diagnostics for H. pylori. He was the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease.
Dr. Moss is a consultant for Takeda, has served on advisory boards for RedHill Biopharma and Phathom Pharmaceuticals regarding novel H. pylori therapies, and has received research support from American Molecular Laboratories regarding molecular diagnostics for H. pylori.
References
- 1.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015;64:1353–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham DY. Transitioning of Helicobacter pylori therapy from trial and error to antimicrobial stewardship. Antibiotics (Basel) 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham DY, Liou JM. Primer for Development of Guidelines for Helicobacter pylori Therapy Using Antimicrobial Stewardship. Clin Gastroenterol Hepatol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YC, Dore MP, Graham DY. Diagnosis and Treatment of Helicobacter pylori Infection. Annu.Rev.Med 2022;73:4.1–.12. [DOI] [PubMed] [Google Scholar]
- 5.Graham DY, Dore MP, Lu H. Understanding treatment guidelines with bismuth and non-bismuth quadruple Helicobacter pylori eradication therapies. Expert Rev. Anti Infect Ther 2018;16:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment. Pharmacol. Ther 2007;26:343–357. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: A randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol 2016;111:1736–1742. [DOI] [PubMed] [Google Scholar]
- 8.Roghani HS, Massarrat S, Pahlewanzadeh MR, et al. Effect of two different doses of metronidazole and tetracycline in bismuth triple therapy on eradication of Helicobacter pylori and its resistant strains. Eur. J Gastroenterol Hepatol 1999;11:709–712. [DOI] [PubMed] [Google Scholar]
- 9.Argueta EA, Alsamman MA, Moss SF, et al. Impact of Antimicrobial Resistance Rates on Eradication of Helicobacter pylori in a US Population. Gastroenterology 2021;160:2181–2183 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulten KG, Genta RM, Kalfus IN, et al. Comparison of Culture With Antibiogram to Next-Generation Sequencing Using Bacterial Isolates and Formalin-Fixed, Paraffin-Embedded Gastric Biopsies. Gastroenterology 2021;(in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss SF, Atsawarungruang A, Dang L, et al. Profiling H. pylori Antibiotic Resistance through Sequencing Stool Samples: A Direct Comparison with Gastric Biopsies. Am J Gastroenterol 2021;(in press). [Google Scholar]
- 12.Chen Q, Long X, Ji Y, et al. Randomised controlled trial: susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment Pharmacol Ther 2019;49:1385–1394. [DOI] [PubMed] [Google Scholar]
- 13.Shiotani A, Roy P, Lu H, et al. Helicobacter pylori diagnosis and therapy in the era of antimicrobial stewardship. Therap Adv Gastroenterol 2021. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]


