Abstract
Over the past decade, the use of immune checkpoint inhibitors (ICIs) has expanded across a wide spectrum of oncology indications. Immune-related adverse events (irAEs) from ICIs represent a significant source of morbidity, and in rare instances, can lead to treatment-related mortality. There are significant opportunities to better identify patients at increased risk for immune-related toxicity, diagnose irAEs more accurately and earlier in their course, and develop more individualized therapeutic strategies once complications arise. Clinical characteristics, germline and somatic genetic features, microbiome composition, and circulating biomarkers have all been associated with higher risk of developing irAEs in retrospective series. Many of these data suggest that both anti-tumor and anti-host ICI-associated immune reactions may be driven by common features of either the tumor or the patient’s pre-existing immune milieu. While irAE diagnosis is currently based on clinical history, exclusion of alternative etiologies, and sometimes pathologic confirmation, novel blood-based and radiographic assays are in development to identify these complications more precisely. Anecdotal reports and small case series have highlighted the potential role of targeted immunomodulatory agents to treat irAEs, though further prospective investigation is needed to evaluate more rigorously their use in these settings. In this review, we highlight the current state of knowledge about predicting, diagnosing, and treating irAEs with a translational focus and discuss emerging strategies which aim to improve each of these domains.
Introduction
Since the initial regulatory approval of the first anti-CTLA-4 antibody ipilimumab for metastatic melanoma in 2011,(1) the use of additional immune checkpoint inhibitors (ICIs) including nivolumab, pembrolizumab, durvalumab, atezolizumab, avelumab, dostarlimab, and cemiplimab, has expanded across a wide spectrum of oncology indications.(2) Treatment-associated immune-related adverse events (irAEs) from ICIs represent a significant source of morbidity. IrAEs can affect any organ system, though the gastrointestinal tract, endocrine glands, skin, and liver are most commonly affected.(3) While most irAEs can be successfully treated with steroids and other immunomodulatory agents, rare, severe, irAEs such as myocarditis have been associated with mortality rates as high as 24%.(4) Severe irAEs occur more frequently when ICIs are combined; trials of the combination of nivolumab and ipilimumab in melanoma, renal cell carcinoma, and non-small cell lung cancer (NSCLC) have reported Grade 3-4 irAEs in 32-59% of patients.(5–7) The rate of severe irAEs is lower with single-agent blockade of programmed cell death-1 (PD-1) or programmed death ligand-1 (PD-L1), with a recent meta-analysis identifying Grade 3-4 irAEs in 14% of 20,128 patients treated with PD-1/PD-L1 monotherapy across 125 trials.(8) Interestingly, emerging combinations of PD-1/PD-L1 inhibitors with agents targeting novel checkpoints (e.g. LAG3, TIGIT) do not appear to cause the same rates of immune-related toxicity as combination PD-1 and CTLA-4 blockade, (9,10), though rates of irAEs are still higher than with PD-1 monotherapy.
As the administration of ICIs in routine clinical practice continues to increase, improved management of associated toxicities has major implications for patient outcomes. There are significant opportunities to better identify patients at increased risk for immune-related toxicity, diagnose irAEs more accurately and earlier in their course, and develop more individualized therapeutic strategies once complications arise. Other consensus reviews discuss recommendations for specific irAE management, including those from the American Society of Clinical Oncology (ASCO),(11) National Comprehensive Cancer Network (NCCN),(12) Society for Immunotherapy of Cancer (SITC),(13) and European Society for Medical Oncology (ESMO).(14) As such, we highlight the current state of knowledge about predicting, diagnosing, and treating irAEs with a translational focus and discuss emerging strategies which aim to improve each of these domains.
Prediction of irAE development in high-risk patients
Immune-related adverse events affect patients in markedly heterogeneous ways. Methods to identify patients who may be at greatest risk for developing irAEs are actively being investigated. Several clinical parameters as well as exploratory biomarkers have been associated with greater toxicity, though none has yet been prospectively validated. Published studies on factors related to prediction of irAEs are summarized in Table 1.
Table 1.
Published studies on factors related to prediction of immune-related adverse events (irAEs).
| Factors Associated with irAEs | Cited References |
|---|---|
| Pre-existing autoimmune disease | van der Kooij et al. (15), Fountzilas et al. (16), Alexander et al. (17), Abu-Sbeih et al. (18), Abdel-Wahab et al. (19), Michailidou et al. (20), Yeung et al. (21), Abdel-Wahab et al. (22), Cappelli et al. (24), Stamatouli et al. (25), Toi et al. (26), de Moel et al. (27) |
| Sex and body mass index | Valpione et al. (29), Guzman-Prado et al. (30), Shah et al. (31), Young et al. (32) |
| Response to ICI | Giacomo et al. (33), Das et al. (34), Xing et al. (35), Jing et al. (36), Haratani et al. (39), Wang et al. (40), Bomze et al. (41) |
| Circulating cytokines and immune cells | Valpione et al. (29), Tarhini et al. (42), Lim et al. (43), Tyan et al. (44), Eun et al. (45), Pavan et al. (46), Chu et al. (47) |
| Inherited genetic variants | Queirolo et al. (48), Bins et al. (49) |
| Microbiome | Andrews et al. (51), Dubin et al. (52) |
Pre-existing autoimmune disease
Since irAEs represent the aberrant activation of the immune system against normal non-malignant tissues, patients with underlying autoimmune disease have been identified as potentially being at greater risk of developing irAEs. As in unselected populations, rates of irAEs among patients with autoimmune disease, are higher with combined anti-CTLA-4/PD-1 combination therapy and anti-CTLA-4 monotherapy than with anti-PD-1 monotherapy.(15) Some irAEs in patients with autoimmune disease affect the organs previously involved in their underlying autoimmune disorder. Retrospective analyses of patients with autoimmune diseases treated with commercially available ICIs have identified rates of autoimmune disease flare ranging from 28 to 60%.(16,17) In a retrospective multicenter analysis of 102 patients with underlying inflammatory bowel disease (IBD) treated with ICIs, gastrointestinal adverse events occurred in 41% of these patients compared to 11% of patients treated at the same centers without histories of IBD.(18) irAEs can also affect new organ sites that were unaffected by autoimmunity prior to ICI therapy. A systematic review of 123 patients with a variety of autoimmune diseases treated with CTLA-4 and/or PD-1 blockade demonstrated exacerbations of existing autoimmune diseases in 41% of patients, de novo irAEs in 25% of patients, and both in 9% of patients.(19) A separate retrospective analysis of 470 patients treated with ICIs identified an association between both personal history (adjusted odds ratio (OR) 2.57, p = 0.001) and family history (adjusted OR 5.98, p < 0.001) of autoimmune disease and the development of any irAE.(20)
An association between autoimmune disease and irAEs, however, has not been universally identified in all published patient cohorts—a retrospective analysis of 417 patients treated with ICIs did not identify any association between underlying autoimmune disease and irAE incidence or severity.(21) In interpreting these conflicting data, it is important to consider whether patients with less severe manifestations of autoimmune disease or longer periods of quiescence may be more likely to be considered for ICI therapy in the standard-of care setting.
Mechanistically, recently identified germline genetic features have been suggestive of shared biological pathways between the development of irAEs and autoimmune disease. An exploratory sequencing study of 89 patients with melanoma that received ICIs identified 30 variants or single-nucleotide polymorphisms that were associated with an increased or decreased risk of developing irAEs; nine of the identified SNPs mapped to eight genes that have been implicated in autoimmune disease.(22) Germline inheritance of human leukocyte antigen (HLA) DRB1 shared epitope alleles are a known risk factor for the development of rheumatoid arthritis,(23) and rates of HLA-DRB1 shared epitope alleles were recently found to be higher in patients that developed ICI-related arthritis compared to healthy controls (62% v. 41%; OR 2.3, p = 0.04).(24) The HLA-DR4 allele has similarly been associated with ICI-induced type 1 diabetes.(25)
Circulating biomarkers have also suggested mechanistic links between autoimmune disease and irAEs. In a cohort study examining the incidence of irAEs in 137 patients with NSCLC treated with nivolumab or pembrolizumab monotherapy, pre-treatment autoantibodies (including antinuclear antibody, antithyroglobulin, and antithyroid peroxidase) and positive rheumatoid factor (>15 IU/mL) were associated with higher rates of irAEs (p = 0.002, p = 0.006, respectively).(26) In a separate cohort of 60 patients with melanoma treated with ipilimumab followed by PD-1 blockade, 7 of 11 (54.6%) patients with antithyroid antibodies after ipilimumab developed thyroid dysfunction with anti-PD1 therapy versus 7 of 49 (14.3%) patients without antibodies (OR, 9.96; 95% CI, 1.94–51.1).(27) These findings suggest that ICI administration may push a population of patients with sub-clinical autoimmunity toward clinically significant autoimmune events. Though PD-1 blockade is thought to exert its anti-cancer effect primarily though effector T cell activation, autoantibody-producing B cell populations may also express PD-1 and can be activated with PD-1 inhibitors.(28) Further investigation is needed to better elucidate the role of both B and T cell dependent autoimmunity in the development of irAEs. In addition to a history of autoimmune disease, sex(29) and body mass index (BMI)(30) have been identified as risk factors for the development of irAEs, though these associations have not been demonstrated consistently in other patient cohorts. (31,32).
Response to ICI therapy
Additionally, observational and translational data have suggested a potential association between tumor response to ICI and increased risk of developing irAEs. While an early analysis of patients with melanoma treated though the ipilimumab expanded access program did not identify a relationship between progression-free survival and irAE incidence,(33) several subsequent studies have linked radiographic tumor response with irAE incidence.(34) A meta-analysis of 7,936 patients with advanced solid tumors across 48 clinical trials treated with ICIs identified a correlation between the objective response rate (ORR) to nivolumab with the incidence of dermatologic (p < 0.001), gastrointestinal (p = 0.006), and endocrine (P < 0.001) irAEs, but not hepatic, pulmonary, and renal irAEs. In the same study, the ORR of combined nivolumab + ipilimumab correlated with incidence of skin (p = 0.04) and gastrointestinal irAEs (p = 0.02).(35)
Similarly, a pharmacoepidemiologic study of irAE reports across 18,706 patients in the FDA Adverse Events Reporting System (FAERS) receiving PD-1/PD-L1 checkpoint blockade showed a marginal association between irAE frequency and the ORR across tumor types (Rs = 0.44, p = 0.049).(36) This same analysis identified a bivariate model of lymphocyte cytosolic protein 1 (LCP1) and adenosine diphosphate dependent glucokinase (ADPGK) expression across tumors as predictive of irAE incidence (Rs = 0.91); both of these genes have been associated with T-cell activation in other contexts.(37,38) There are more limited available data linking irAE incidence to response or survival within a single disease entity; for example, in a population of 134 patients with NSCLC, Haratani et al. demonstrated irAEs within six weeks were positively associated with improved survival outcome, with hazard ratios of 0.525 (p = 0.03) for PFS and 0.282 (95% CI, 0.101 to 0.667; P = .003) for OS.(39) A similar association between ICI-related diarrhea and improved OS has also been reported.(40) Of note, the relationship between irAEs and clinical outcomes could be confounded by time-dependent biases (i.e., an irAE may appear to be associated with more favorable OS if the irAE takes time to develop, while patients who progress or die early may not have sufficient time to develop an irAE). Landmark analyses, such as those used by Haratani et al., aim to minimize these biases by using only irAEs documented in a fixed time period to distinguish clinical cohorts and limiting survival analysis to patients who are either alive (for OS analyses) or without progression (for PFS analyses) at the conclusion of that time period.
A number of tissue and circulating biomarkers recently associated with irAEs share similarities with biomarkers associated with tumor response to ICI therapy. Comparing multiple tumor types, higher tumor mutational burden(41) and a bivariate model of CD8+ T cells and T-cell receptor diversity have been associated with higher rates of irAEs.(36) Among a cohort of 470 patients with a variety of solid tumors treated with ICI therapy, higher baseline ALC, > 2.6 k/ul (adjusted OR: 4.30), absolute monocyte count > 0.29 k/ul (adjusted OR: 2.34) and platelet count > 145 k/ul (adjusted OR: 2.23) were also associated with a higher incidence of irAEs.(20)
Circulating cytokines and immune cells
The role of cytokines in ICI response and toxicity remains less well characterized, but some studies have linked pre-treatment cytokine levels with the development of irAEs. In a prospective series of 140 patients with metastatic melanoma treated with ipilimumab, low baseline interleukin (IL)-6 serum levels were associated with higher rates of irAEs (OR = 2.84, p = 0.007).(29) Conversely, higher pre-treatment serum IL-17 levels were associated with development of Grade 3+ colitis in 29 patients with melanoma treated with peri-operative ipilimumab (p = 0.02).(42) Lim et al integrated measurements of 11 pre-treatment and early-treatment cytokines (G-CSF, GM-CSF, fractalkine, FGF-2, IFNa2, IL12p70, IL1a, IL1B, IL1RA, IL2, and IL13) into a CYTOX score to distinguish patients with melanoma that experienced severe toxicity with PD-1 monotherapy or combined PD-1/CTLA-4 therapy. This score was then validated in a separate cohort of 49 patients with melanoma with area under the curve of 0.68 (pre-treatment; p = 0.037) and 0.70 (early treatment; p = 0.017).(43) Notably, a separate analysis of pre-treatment cytokines in 52 patients with melanoma that experienced irAEs—including eight of the cytokines included in the CYTOX score—did not discern patients that experienced Grade 1-2 irAEs from those with Grade 3-4 irAEs (i.e., severe toxicity).(44)
In addition to cytokines, ratios of circulating immune cells have also been associated with the development of irAEs. In a single-institutional study of 391 patients treated with pembrolizumab for a variety of oncologic indications, the risk of irAEs was significantly lower in patients that had a baseline neutrophil-to-lymphocyte ratio of 3 or greater compared to those with a ratio less than 3 (OR = 0.37, 95% CI 0.17–0.81, p = 0.012).(45) This association was also observed in a separate analysis of 184 patients with NSCLC treated with anti-PD-1 monotherapy.(46) Baseline circulating absolute eosinophils greater than 0.125 × 109 cells/L were also associated with higher rates of ICI-associated pneumonitis (27.7% v. 9.8%, p < 0.001) in a retrospective analysis of 300 patients with advanced NSCLC.(47)
Inherited genetic variants
There also has been interest in identifying germline genetic variants associated with irAEs, beyond those associated with autoimmune disease. The single-nucleotide polymorphism (SNP) CTLA-4 -1661A>G was associated with an increased risk of endocrine irAEs in a cohort of 173 patients with melanoma treated with ipilimumab. (48) A similar analysis of 96 patients with NSCLC treated with nivolumab identified an association between the SNP PDCD1 804C>T and decreased incidence of irAEs (OR 0.4; 95% CI 0.2–1.0; p = 0.039), but this finding was not confirmed in a validation cohort.(49)
Microbiome & future directions
Recently, the role of the gut microbiome in anti-cancer immunity has emerged as an area of active research,(50) and the microbiome has similarly been implicated in the development of irAEs. Profiling of gut microbiota using 16s RNA sequencing demonstrated a significantly higher abundance of Bacteroides intestinalis (p = 0.009) and Intestinibacter bartlettii (p = 0.009) in patients with melanoma and toxicity from combined PD-1 and CTLA-4 blockade, compared to those who did not experience irAEs.(51) Interestingly, in a separately published cohort of 34 melanoma patients treated with ipilimumab, higher stool abundance of the Bacteroidetes phylum (which includes Bacteroides as well as other commensal genera) was associated with resistance to ICI-induced colitis.(52)
All of the above analyses have generated hypotheses that have yet to be validated in prospective clinical trials, and no reported trial has adapted ICI therapy regimens based on pre-treatment risk of irAEs. An ongoing single-center prospective trial at University of Colorado Denver (NCT 03409016) is collecting blood samples at pre-treatment and three on-treatment time points to identify biomarkers that may predict ICI-related immunotherapy toxicity. There remains a significant opportunity to better discern which patients may be at highest risk for irAEs, and to individualize therapy based on this risk. Identification of highest-risk patients may be particularly important for trials of preventative interventions, as concomitant budesonide was ineffective for prevention of ipilimumab-associated colitis in a randomized controlled trial in an unselected population of patients with melanoma.(53) Several NIH-sponsored “Cancer Moonshot” U01 studies are prospectively assessing biomarkers of irAEs and exploring mitigation strategies for toxicities such as colitis and dermatitis.
Diagnosis of IrAEs
In addition to predicting which patients may be at highest risk for developing toxicity prior to initiating ICI therapy, early and accurate diagnosis of irAEs may provide clinicians opportunities to prevent hospitalizations or interruptions to anti-cancer therapy. From a research standpoint, standardized diagnostic criteria for irAEs may facilitate the aggregation of irAE data across clinical trials and real-world data.(54)
Recently published clinical practice guidelines from ASCO,(11) NCCN,(12) SITC,(13) and ESMO(14) provide broad diagnostic guidelines for a variety of irAE types. Generally, exclusion of alternate (namely infectious) etiologies is pursued in parallel with holding immunotherapy and/or beginning immunosuppression; pathologic confirmation is sometimes but not always obtained. To improve diagnostic accuracy, multiple groups have explored novel biomarkers that could eventually be translated to practice.
While pre-treatment biomarkers have been used to identify patients at highest risk for irAEs, analysis of on-treatment blood samples have identified dynamic changes in circulating immune parameters that coincide with or precede the development of irAEs. If validated in prospective patient cohorts, it is possible that these pre-treatment or on-treatment biomarkers could facilitate early diagnosis of irAEs before other clinical, laboratory, or radiographic findings appear. In a phase II study of 27 prostate cancer patients treated with ipilimumab and androgen deprivation therapy, clonal expansion of 55 or more T cell clones in the peripheral blood preceded development of Grade 2-3 ipilimumab-induced toxicities.(55) This finding was confirmed in a second cohort of 11 prostate cancer patients by the same authors. Changes in circulating cytokines, RNA transcripts, and autoantibodies have also been correlated with toxicity. Among 52 patients with melanoma treated with ipilimumab, IL-17 levels rose in patients with colitis and decreased with symptom resolution(56). Similarly, serum IL-6 levels were noted to rise in a small series of patients with malignant melanoma and nivolumab-associated psoriasiform dermatitis, while it did not rise in patients who did not experience any irAEs.(57)
In a separate analysis of 360 patients treated with the anti-CTLA-4 antibody tremelimumab, an on-treatment RNA gene expression signature in peripheral blood was associated with treatment-associated diarrhea/colitis.(58) While most published reports do not correlate tissue-specific biomarkers with clinical patterns of ICI-related toxicity, Tahir et al. identified dynamic changes in anti-GNAL and anti-ITM2B antibodies (both reactive to antigens expressed in pituitary epithelium) in patients that experienced ICI-related hypophysitis as well as increases in anti-CD74 (expressed in lung and other tissues) in patients that experienced ICI-related pneumonitis.(59)
Ongoing translational trials will provide opportunity for further identification of potential diagnostic biomarkers. The Alliance A151804 trial (NCT04242095) opened in January 2020 and is prospectively collecting tissue, blood, and stool samples from patients experiencing Grade 3-4 adverse events. The Autoimmune Events Resulting from Systemic Modulation by Immuno-Therapy (AEROSMITH) trial led by the Parker Institute for Cancer Immunotherapy will collect longitudinal clinical and peripheral blood samples on up to 1,600 patients with a goal of elucidating mechanisms underlying irAE development. Additionally, a single-center study at Universitair Ziekenhuis Leuven in Belgium (NCT04807127) will prospectively apply single cell RNA- and TCR-sequencing on up to 5,000 single cells collected from broncheoalveolar lavage in patients experiencing pneumonitis.
In addition to circulating and tissue-based biomarkers, radiographic imaging also plays an important role in the noninvasive diagnosis of irAEs. Computerized tomography (CT) is commonly used to evaluate a variety of irAEs, and bowel wall thickening on CT was shown to be highly predictive (positive predictive value 96%) of biopsy-proven ICI-related colitis.(60) There are no pathognomonic radiographic findings that correlate with ICI-related pneumonitis--among 20 patients with solid tumors that developed ICI-related pneumonitis, a variety of radiographic patterns were identified but cryptogenic organizing pneumonia (COP) was most common.(61) A separate series of 27 patients treated at Memorial Sloan Kettering Cancer Center also demonstrated variable radiographic patterns, with ground glass opacities being the most common.(62)
In the future, advances in imaging technologies may improve the diagnosis of irAEs. For instance, a machine learning algorithm was able to distinguish between radiographic patterns of ICI and radiation pneumonitis (AUC 0.76)(63). Additionally, new positron emission tomography (PET) imaging modalities which image CD8+ T cells and granzyme B may be useful in non-invasively identifying organs affected by irAEs, as shown in a mouse model.(64,65)
Emerging Treatment Strategies for IrAEs
Current irAE treatment guidelines from ASCO, ESMO, SITC, and the NCCN(11–14) are largely based on expert opinion, as there has been limited prospective clinical investigation in this setting to date. In many cases, management involves cessation of ICI therapy with or without initiation of topical, oral, or parenteral steroids depending on the organ involved and severity of the irAE. The best initial steroid dose and duration of steroid treatment have not been prospectively studied, raising questions as to whether the conventional one milligram per kilogram steroid dose for significant irAEs included in most consensus guidelines is really needed. There are also significant opportunities to develop novel therapies both in steroid-refractory settings (occurring, for example, in up to 18% of patients with ICI-related pneumonitis(66)) as well as with the goal to initially spare high doses of steroids, as systemic steroids themselves can be associated with many short- and long-term sequelae, and may negatively impact the efficacy of ICI therapy.(67,68)
Based on longstanding experience from the treatment of inflammatory bowel disease,(69) TNF-alpha blockade in steroid-refractory ICI-related enterocolitis is among the most well established targeted therapy in the management of irAEs, with 81% efficacy in steroid-refractory colitis observed in a recent meta-analysis.(70) The addition of the anti-TNF monoclonal antibody infliximab has also been associated with a shorter time to symptom resolution (3 v. 9 days, p < 0.001) despite higher-grade colitis in infliximab-treated patients.(71) However, when rigorously assessing response rates in a multicenter review of 127 patients with steroid-refractory ICI-related enterocolitis treated infliximab, only 51% of patients achieved steroid-free remission at 26 weeks. Though the primary endpoint in this study mandated complete resolution of diarrhea and may underrepresent the number of patients that derived clinical benefit, this result highlights the need for rigorous, prospective studies and exploration of novel targets for irAE treatment (72) (Figure 1).
Figure 1.
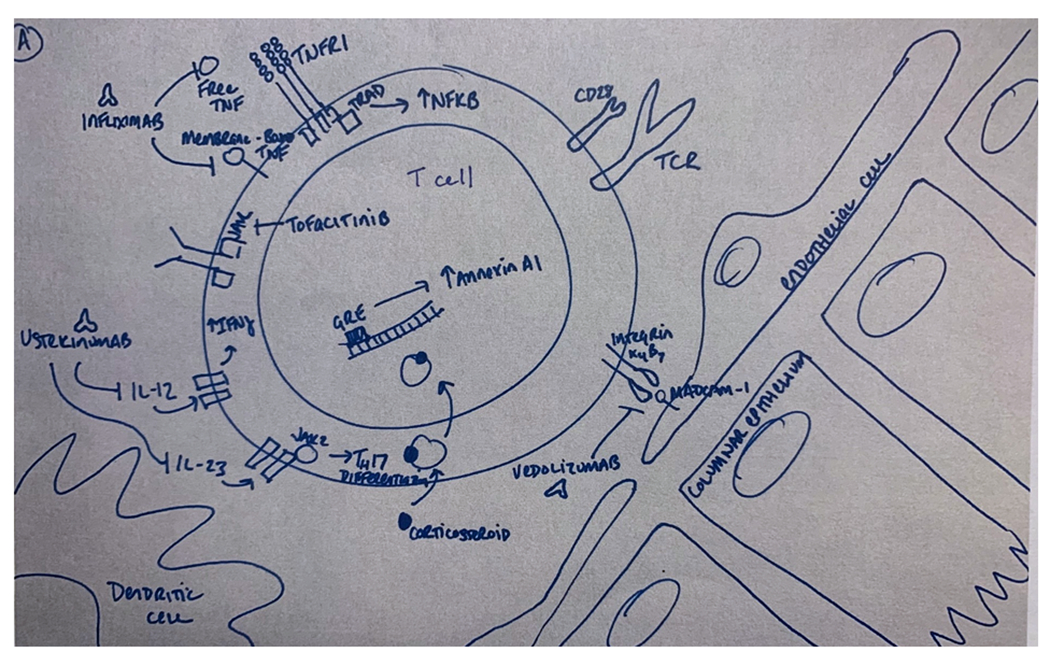
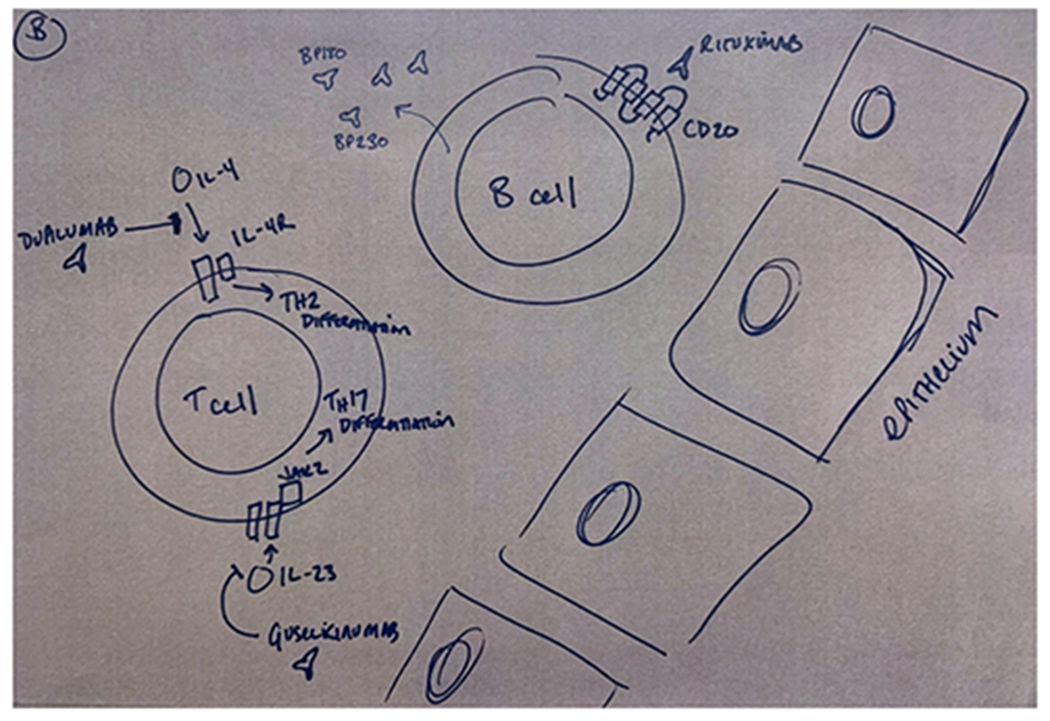
Mechanisms of selected immunosuppressive agents reported in the treatment of gastrointestinal (A) and cutaneous (B) immune-related adverse events (irAEs). Monoclonal antibodies developed for other inflammatory indications have been used in the steroid-refractory setting. Examples include infliximab (anti-TNF), dupilimumab (anti-IL-4), guselkinumab (anti-IL-23), and ustekinumab (anti IL-12/23). Small molecules such as tofacitinib (anti-JAK1/3) have also been used.
More recently, investigators have studied the co-administration of TNF blockade in combination with immune checkpoint blockade. In a preclinical Rag2−/−Il2rg−/−mouse model adoptively transferred with human mononuclear cells, prophylactic TNF blockade with combined CTLA-4 and PD-1 blockade reduced colitis without compromising immune-mediated control of xenografted colon tumors.(73) A series of five patients treated at Massachusetts General Hospital with concurrent infliximab and ICI following ICI-related colitis facilitated steroid tapering without impairing tumor control.(74) Additionally, the ongoing TICIMEL trial (NCT03293784) is a Ph 1b study evaluating the safety and tolerability of treating metastatic melanoma with combination nivolumab and ipilimumab with either infliximab or certolizumab.(75) Early results of 14 treated patients showed treatment was well-tolerated, and all seven evaluable patients in the certolizumab arm had an objective radiographic response (four partial responses and three compete responses).(76) More mature data from this trial along with larger studies will be needed to determine if anti-TNF agents can reduce the incidence of irAEs without compromising anti-tumor outcomes, as was suggested by one registry study.(77)
Other targeted immunomodulatory agents have demonstrated efficacy in treating steroid-refractory ICI-related enterocolitis in published case reports and case series. The best studied is the anti-α4β7 integrin monoclonal antibody vedolizumab,(78,79), with the notable advantage of being a gut-selective agent with theoretically less interference with systemic anti-tumor immune response. Other agents that have been effective in several reports include the JAK inhibitor tofacitinib,(80) and the anti-IL12/23 monoclonal antibody ustekinumab.(81) While these reports are suggestive of a variety of treatment strategies for ICI-related enterocolitis, further prospective evaluation of these agents is needed to clarify their optimal use in routine clinical practice.
ICI-associated hepatic injury occurs less commonly than ICI-related colitis, but can be life-threatening in some cases. Mycophenolate mofetil has been shown to be safe and effective in the steroid-refractory setting.(82) Infliximab has not been tested in this setting due to concerns for hepatic toxicity, and is generally not used in patients with colitis and concomitant elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST). However, one retrospective study of 56 patients treated with infliximab for a variety of steroid-refractory irAEs did not identify any significant differences in pre- and post-treatment AST and ALT. Also, one patient in this series was treated with infliximab for steroid-refractory ICI-associated hepatitis and was noted to have a complete recovery with no additional liver toxicity. (83)
There are more limited data available regarding novel immunomodulatory therapies for non-gastrointestinal irAEs. Though preclinical animal models for irAEs represent a major unmet research need, Wei and colleagues developed a genetic knockout mouse model that recapitulated ICI-related myocarditis and was ameliorated by treatment with abatacept.(84) Other reported data are predominantly clinical case series: in a single-institution series of 26 patients with steroid-refractory or steroid-resistant pneumonitis, addition of a second immunomodulatory agent (anti-TNF or mycophenolate) was associated with durable response in 10 patients (38%), including three with a complete response allowing for discontinuation of all immunosuppressants.(85) Treatment with the anti-IL-6 monoclonal antibody tocilizumab was associated with clinical benefit in 21 of 22 patients with advanced melanoma and a variety of irAEs (20 patients) or pre-existing autoimmune disorders (two patients). Circulating IL-6 was elevated in 12 of 13 patients with evaluable levels prior to tocilizumab administration.(86) A separate analysis of 34 patients treated with tocilizumab for nivolumab-associated steroid-refractory irAEs reported a clinical improvement in 27 of 34 evaluable patients (79%).(87)In a multicenter series of 285 patients with cutaneous irAEs, seven patients with steroid-refractory cutaneous irAEs were treated with biologics.(88) Three patients received the anti-CD20 antibody rituximab, two patients received the anti-IL-4 receptor alpha antibody dupilumab, one patient received ustekinumab, and one patient received the anti-IL-23a subunit antibody guselkumab. All seven patients experienced moderate to significant improvement in their cutaneous symptoms, though their presenting rashes were variable in morphology (e.g., bullous pemphigoid-like, psoriaform, eczematous). Three patients with radiographic tumor responses prior to adding a biologic maintained these responses after addition of the biologic. Successful treatment of pembrolizumab-induced psoriasiform dermatologic toxicity with the anti IL-17a monoclonal antibody has also been reported separately.(89)
More anecdotal data sets have highlighted the utility of targeted immunosuppressive agents in less common immune-related toxicities. These include tofacitinib and tocilizumab in ICI-associated arthritis,(90,91) infliximab in refractory ICI-related pericarditis,(92) and infliximab in ICI-associated acute tubular interstitial nephritis.(93)
Given the emerging role of the gut microbiome in immune-related toxicity, fecal microbiota transplant (FMT) has also been studied in the management of irAEs. Wang et al. reported the successful treatment of ICI-related colitis refractory to steroids, infliximab, and vedolizumab in two patients with FMT.(94) In both patients, there was a relative increase in CD4+ FoxP3+ T regulatory cells in comparison to other T cell lineages. There were also significant changes in the bacterial flora in both patients following FMT, but no single bacterial family was predominant in both instances. Several ongoing trials are prospectively assessing the role of FMT in treatment of ICI-related colitis (NCT04883762, NCT04038619, NCT03819296). In the ongoing Canadian PERFORM trial (NCT04163289), prophylactic FMT is being examined as tool to reduce the risk of colitis in patients with renal cell carcinoma receiving combination ipilimumab/nivolumab.
Given the rapid adoption of ICIs across multiple oncology indications, adept management of ICI-associated toxicity will have a major impact on a growing population of patients with cancer in the coming years. While the management of irAEs to date has not been based on randomized clinical trial data, clinical experiences to date have generated translational insights that may inform future advances in the prediction, diagnosis, and management of these adverse events.
Funding sources:
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 (D. M. Faleck and M. A. Postow).
Conflicts of interest:
J.W.S. reports no conflicts of interest. D.M.F reports consulting fees from Kaleido Biosciences. M.A.P reports consulting fees from BMS, Merck, Array BioPharma, Novartis, Incyte, NewLink Genetics, Aduro, Eisai, Pfizer; honoraria from BMS and Merck; institutional support from RGenix, Infinity, BMS, Merck, Array BioPharma, Novartis, AstraZeneca
Sources
- 1.Ledford H Melanoma drug wins US approval. Nature 2011;471(7340):561- doi 10.1038/471561a. [DOI] [PubMed] [Google Scholar]
- 2.Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Network Open 2019;2(5):e192535–e doi 10.1001/jamanetworkopen.2019.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378(2):158–68 doi 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Infante N, Ramirez-Flores YA, Castillo EC, Lozano O, Garcia-Rivas G, Torre-Amione G. Cardiotoxicity associated with immune checkpoint inhibitor therapy: a meta-analysis. Eur J Heart Fail 2021. doi 10.1002/ejhf.2289. [DOI] [PubMed] [Google Scholar]
- 5.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. New England Journal of Medicine 2019;381(21):2020–31 doi 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018;378(14):1277–90 doi 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. New England Journal of Medicine 2019;381(16):1535–46 doi 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncology 2019;5(7):1008–19 doi 10.1001/jamaoncol.2019.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipson EJ, Tawbi HA-H, Schadendorf D, Ascierto PA, Matamala L, Gutiérrez EC, et al. Relatlimab (RELA) plus nivolumab (NIVO) versus NIVO in first-line advanced melanoma: Primary phase III results from RELATIVITY-047 (CA224-047). Journal of Clinical Oncology 2021;39(15_suppl):9503- doi 10.1200/JCO.2021.39.15_suppl.9503. [DOI] [Google Scholar]
- 10.Rodriguez-Abreu D, Johnson ML, Hussein MA, Cobo M, Patel AJ, Secen NM, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). Journal of Clinical Oncology 2020;38(15_suppl):9503- doi 10.1200/JCO.2020.38.15_suppl.9503. [DOI] [Google Scholar]
- 11.Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36(17):1714–68 doi 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Network NCC 2021 July 31, 2021. Management of Immunotherapy-Related Toxicities (Version 03.2021). <https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf>. July 31, 2021.
- 13.Brahmer JR, Abu-Sbeih H, Ascierto PA, Brufsky J, Cappelli LC, Cortazar FB, et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. Journal for ImmunoTherapy of Cancer 2021;9(6):e002435 doi 10.1136/jitc-2021-002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28(suppl_4):iv119–iv42 doi 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 15.van der Kooij MK, Suijkerbuijk KPM, Aarts MJB, van den Berkmortel F, Blank CU, Boers-Sonderen MJ, et al. Safety and Efficacy of Checkpoint Inhibition in Patients With Melanoma and Preexisting Autoimmune Disease : A Cohort Study. Ann Intern Med 2021;174(5):641–8 doi 10.7326/m20-3419. [DOI] [PubMed] [Google Scholar]
- 16.Fountzilas E, Lampaki S, Koliou GA, Koumarianou A, Levva S, Vagionas A, et al. Real-world safety and efficacy data of immunotherapy in patients with cancer and autoimmune disease: the experience of the Hellenic Cooperative Oncology Group. Cancer Immunol Immunother 2021. doi 10.1007/s00262-021-02985-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander S, Swami U, Kaur A, Gao Y, Fatima M, Ginn MM, et al. Safety of immune checkpoint inhibitors in patients with cancer and pre-existing autoimmune disease. Ann Transl Med 2021;9(12):1033 doi 10.21037/atm-20-8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abu-Sbeih H, Faleck DM, Ricciuti B, Mendelsohn RB, Naqash AR, Cohen JV, et al. Immune Checkpoint Inhibitor Therapy in Patients With Preexisting Inflammatory Bowel Disease. Journal of Clinical Oncology 2020;38(6):576–83 doi 10.1200/jco.19.01674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann Intern Med 2018;168(2):121–30 doi 10.7326/m17-2073. [DOI] [PubMed] [Google Scholar]
- 20.Michailidou D, Khaki AR, Morelli MP, Diamantopoulos L, Singh N, Grivas P. Association of blood biomarkers and autoimmunity with immune related adverse events in patients with cancer treated with immune checkpoint inhibitors. Scientific Reports 2021;11(1):9029 doi 10.1038/s41598-021-88307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung C, Kartolo A, Holstead R, Moffat GT, Hanna L, Hopman W, et al. Safety and Clinical Outcomes of Immune Checkpoint Inhibitors in Patients With Cancer and Preexisting Autoimmune Diseases. J Immunother 2021. doi 10.1097/cji.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Wahab N, Diab A, Yu RK, Futreal A, Criswell LA, Tayar JH, et al. Genetic determinants of immune-related adverse events in patients with melanoma receiving immune checkpoint inhibitors. Cancer Immunol Immunother 2021;70(7):1939–49 doi 10.1007/s00262-020-02797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. an approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis & Rheumatism 1987;30(11):1205–13 doi 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 24.Cappelli LC, Dorak MT, Bettinotti MP, Bingham CO, Shah AA. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology (Oxford) 2019;58(3):476–80 doi 10.1093/rheumatology/key358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamatouli AM, Quandt Z, Perdigoto AL, Clark PL, Kluger H, Weiss SA, et al. Collateral Damage: Insulin-Dependent Diabetes Induced With Checkpoint Inhibitors. Diabetes 2018;67(8):1471–80 doi 10.2337/dbi18-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toi Y, Sugawara S, Sugisaka J, Ono H, Kawashima Y, Aiba T, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2019;5(3):376–83 doi 10.1001/jamaoncol.2018.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Moel EC, Rozeman EA, Kapiteijn EH, Verdegaal EME, Grummels A, Bakker JA, et al. Autoantibody Development under Treatment with Immune-Checkpoint Inhibitors. Cancer Immunol Res 2019;7(1):6–11 doi 10.1158/2326-6066.CIR-18-0245. [DOI] [PubMed] [Google Scholar]
- 28.Thibult ML, Mamessier E, Gertner-Dardenne J, Pastor S, Just-Landi S, Xerri L, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol 2013;25(2):129–37 doi 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 29.Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, et al. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J Transl Med 2018;16(1):94 doi 10.1186/s12967-018-1467-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzman-Prado Y, Ben Shimol J, Samson O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: a systematic review and meta-analysis. Cancer Immunol Immunother 2021;70(1):89–100 doi 10.1007/s00262-020-02663-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah KP, Song H, Ye F, Moslehi JJ, Balko JM, Salem JE, et al. Demographic Factors Associated with Toxicity in Patients Treated with Anti-Programmed Cell Death-1 Therapy. Cancer Immunol Res 2020;8(7):851–5 doi 10.1158/2326-6066.CIR-19-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young AC, Quach HT, Song H, Davis EJ, Moslehi JJ, Ye F, et al. Impact of body composition on outcomes from anti-PD1 +/− anti-CTLA-4 treatment in melanoma. J Immunother Cancer 2020;8(2) doi 10.1136/jitc-2020-000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giacomo AMD, Grimaldi AM, Ascierto PA, Queirolo P, Vecchio MD, Ridolfi R, et al. Correlation between efficacy and toxicity in pts with pretreated advanced melanoma treated within the Italian cohort of the ipilimumab expanded access programme (EAP). Journal of Clinical Oncology 2013;31(15_suppl):9065- doi 10.1200/jco.2013.31.15_suppl.9065. [DOI] [Google Scholar]
- 34.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7(1):306 doi 10.1186/s40425-019-0805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, et al. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: a systematic review and meta-analysis. Journal for ImmunoTherapy of Cancer 2019;7(1):341 doi 10.1186/s40425-019-0779-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jing Y, Liu J, Ye Y, Pan L, Deng H, Wang Y, et al. Multi-omics prediction of immune-related adverse events during checkpoint immunotherapy. Nat Commun 2020;11(1):4946 doi 10.1038/s41467-020-18742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wabnitz GH, Kocher T, Lohneis P, Stober C, Konstandin MH, Funk B, et al. Costimulation induced phosphorylation of L-plastin facilitates surface transport of the T cell activation molecules CD69 and CD25. Eur J Immunol 2007;37(3):649–62 doi 10.1002/eji.200636320. [DOI] [PubMed] [Google Scholar]
- 38.Kaminski MM, Sauer SW, Kaminski M, Opp S, Ruppert T, Grigaravicius P, et al. T cell activation is driven by an ADP-dependent glucokinase linking enhanced glycolysis with mitochondrial reactive oxygen species generation. Cell Rep 2012;2(5):1300–15 doi 10.1016/j.celrep.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K, Kato R, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 2018;4(3):374–8 doi 10.1001/jamaoncol.2017.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Abu-Sbeih H, Mao E, Ali N, Ali FS, Qiao W, et al. Immune-checkpoint inhibitor-induced diarrhea and colitis in patients with advanced malignancies: retrospective review at MD Anderson. J Immunother Cancer 2018;6(1):37 doi 10.1186/s40425-018-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bomze D, Hasan Ali O, Bate A, Flatz L. Association Between Immune-Related Adverse Events During Anti-PD-1 Therapy and Tumor Mutational Burden. JAMA Oncol 2019;5(11):1633–5 doi 10.1001/jamaoncol.2019.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarhini AA, Zahoor H, Lin Y, Malhotra U, Sander C, Butterfield LH, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. Journal for ImmunoTherapy of Cancer 2015;3(1):39 doi 10.1186/s40425-015-0081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lim SY, Lee JH, Gide TN, Menzies AM, Guminski A, Carlino MS, et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1–Based Immunotherapy. Clinical Cancer Research 2019;25(5):1557–63 doi 10.1158/1078-0432.Ccr-18-2795. [DOI] [PubMed] [Google Scholar]
- 44.Tyan K, Baginska J, Brainard M, Giobbie-Hurder A, Severgnini M, Manos M, et al. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer Immunol Immunother 2021;70(8):2209–21 doi 10.1007/s00262-021-02855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eun Y, Kim IY, Sun JM, Lee J, Cha HS, Koh EM, et al. Risk factors for immune-related adverse events associated with anti-PD-1 pembrolizumab. Sci Rep 2019;9(1):14039 doi 10.1038/s41598-019-50574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral Blood Markers Identify Risk of Immune-Related Toxicity in Advanced Non-Small Cell Lung Cancer Treated with Immune-Checkpoint Inhibitors. Oncologist 2019;24(8):1128–36 doi 10.1634/theoncologist.2018-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chu X, Zhao J, Zhou J, Zhou F, Jiang T, Jiang S, et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer 2020;150:76–82 doi 10.1016/j.lungcan.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 48.Queirolo P, Dozin B, Morabito A, Banelli B, Carosio R, Fontana V, et al. CTLA-4 gene variant -1661A>G may predict the onset of endocrine adverse events in metastatic melanoma patients treated with ipilimumab. Eur J Cancer 2018;97:59–61 doi 10.1016/j.ejca.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 49.Bins S, Basak EA, El Bouazzaoui S, Koolen SLW, Oomen-de Hoop E, van der Leest CH, et al. Association between single-nucleotide polymorphisms and adverse events in nivolumab-treated non-small cell lung cancer patients. Br J Cancer 2018;118(10):1296–301 doi 10.1038/s41416-018-0074-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018;359(6371):97–103 doi 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen W- S, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nature Medicine 2021. doi 10.1038/s41591-021-01406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nature Communications 2016;7(1):10391 doi 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A Randomized, Double-Blind, Placebo-Controlled, Phase II Study Comparing the Tolerability and Efficacy of Ipilimumab Administered with or without Prophylactic Budesonide in Patients with Unresectable Stage III or IV Melanoma. Clinical Cancer Research 2009;15(17):5591–8 doi 10.1158/1078-0432.Ccr-09-1024. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds KL, Arora S, Elayavilli RK, Louv WC, Schaller TH, Khandelwal A, et al. Immune-related adverse events associated with immune checkpoint inhibitors: a call to action for collecting and sharing clinical trial and real-world data. Journal for ImmunoTherapy of Cancer 2021;9(7):e002896 doi 10.1136/jitc-2021-002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subudhi SK, Aparicio A, Gao J, Zurita AJ, Araujo JC, Logothetis CJ, et al. Clonal expansion of CD8 T cells in the systemic circulation precedes development of ipilimumab-induced toxicities. Proc Natl Acad Sci U S A 2016;113(42):11919–24 doi 10.1073/pnas.1611421113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Callahan MK, Yang A, Tandon S, Xu Y, Subudhi SK, Roman RA, et al. Evaluation of serum IL-17 levels during ipilimumab therapy: Correlation with colitis. Journal of Clinical Oncology 2011;29(15_suppl):2505- doi 10.1200/jco.2011.29.15_suppl.2505. [DOI] [Google Scholar]
- 57.Tanaka R, Okiyama N, Okune M, Ishitsuka Y, Watanabe R, Furuta J, et al. Serum level of interleukin-6 is increased in nivolumab-associated psoriasiform dermatitis and tumor necrosis factor-α is a biomarker of nivolumab recativity. J Dermatol Sci 2017;86(1):71–3 doi 10.1016/j.jdermsci.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 58.Friedlander P, Wood K, Wassmann K, Christenfeld AM, Bhardwaj N, Oh WK. A whole-blood RNA transcript-based gene signature is associated with the development of CTLA-4 blockade-related diarrhea in patients with advanced melanoma treated with the checkpoint inhibitor tremelimumab. Journal for ImmunoTherapy of Cancer 2018;6(1):90 doi 10.1186/s40425-018-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tahir SA, Gao J, Miura Y, Blando J, Tidwell RSS, Zhao H, et al. Autoimmune antibodies correlate with immune checkpoint therapy-induced toxicities. Proc Natl Acad Sci U S A 2019;116(44):22246–51 doi 10.1073/pnas.1908079116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia-Neuer M, Marmarelis ME, Jangi SR, Luke JJ, Ibrahim N, Davis M, et al. Diagnostic Comparison of CT Scans and Colonoscopy for Immune-Related Colitis in Ipilimumab-Treated Advanced Melanoma Patients. Cancer Immunology Research 2017;5(4):286–91 doi 10.1158/2326-6066.Cir-16-0302. [DOI] [PubMed] [Google Scholar]
- 61.Nishino M, Ramaiya NH, Awad MM, Sholl LM, Maattala JA, Taibi M, et al. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin Cancer Res 2016;22(24):6051–60 doi 10.1158/1078-0432.CCR-16-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Naidoo J, Wang X, Woo KM, Iyriboz T, Halpenny D, Cunningham J, et al. Pneumonitis in Patients Treated With Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. Journal of Clinical Oncology 2017;35(7):709–17 doi 10.1200/jco.2016.68.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen X, Sheikh K, Nakajima E, Lin CT, Lee J, Hu C, et al. Radiation versus Immune Checkpoint Inhibitor Associated Pneumonitis: Distinct Radiologic Morphologies. Oncologist 2021. doi 10.1002/onco.13900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ferreira CA, Heidari P, Ataeinia B, Sinevici N, Sise ME, Colvin RB, et al. Non-Invasive Detection of Immunotherapy-induced Adverse Events. Clinical Cancer Research 2021:clincanres.4641.2020 doi 10.1158/1078-0432.Ccr-20-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pandit-Taskar N, Postow MA, Hellmann MD, Harding JJ, Barker CA, O’Donoghue JA, et al. First-in-Humans Imaging with (89)Zr-Df-IAB22M2C Anti-CD8 Minibody in Patients with Solid Malignancies: Preliminary Pharmacokinetics, Biodistribution, and Lesion Targeting. J Nucl Med 2020;61(4):512–9 doi 10.2967/jnumed.119.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balaji A, Hsu M, Lin CT, Feliciano J, Marrone K, Brahmer JR, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J Immunother Cancer 2021;9(1) doi 10.1136/jitc-2020-001731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bai X, Hu J, Betof Warner A, Quach HT, Cann CG, Zhang MZ, et al. Early use of high-dose-glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clinical Cancer Research 2021:clincanres.1283.2021. doi 10.1158/1078-0432.Ccr-21-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer 2018;124(18):3706–14 doi 10.1002/cncr.31629. [DOI] [PubMed] [Google Scholar]
- 69.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353(23):2462–76 doi 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 70.Ibraheim H, Baillie S, Samaan MA, Abu-Sbeih H, Wang Y, Talley NJ, et al. Systematic review with meta-analysis: effectiveness of anti-inflammatory therapy in immune checkpoint inhibitor-induced enterocolitis. Alimentary Pharmacology & Therapeutics 2020;52(9):1432–52 doi 10.1111/apt.15998. [DOI] [PubMed] [Google Scholar]
- 71.Johnson DH, Zobniw CM, Trinh VA, Ma J, Bassett RL Jr., Abdel-Wahab N, et al. Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J Immunother Cancer 2018;6(1):103 doi 10.1186/s40425-018-0412-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alexander JL, Ibraheim H, Sheth B, Little J, Khan MS, Richards C, et al. Clinical outcomes of patients with corticosteroid refractory immune checkpoint inhibitor-induced enterocolitis treated with infliximab. J Immunother Cancer 2021;9(7) doi 10.1136/jitc-2021-002742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V, et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019;569(7756):428–32 doi 10.1038/s41586-019-1162-y. [DOI] [PubMed] [Google Scholar]
- 74.Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent therapy with immune checkpoint inhibitors and TNFalpha blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer 2019;7(1):226 doi 10.1186/s40425-019-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montfort A, Dufau C, Colacios C, Andrieu-Abadie N, Levade T, Filleron T, et al. Anti-TNF, a magic bullet in cancer immunotherapy? Journal for ImmunoTherapy of Cancer 2019;7(1):303 doi 10.1186/s40425-019-0802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Montfort A, Filleron T, Virazels M, Dufau C, Milhès J, Pagès C, et al. Combining Nivolumab and Ipilimumab with Infliximab or Certolizumab in Patients with Advanced Melanoma: First Results of a Phase Ib Clinical Trial. Clinical Cancer Research 2021;27(4):1037–47 doi 10.1158/1078-0432.Ccr-20-3449. [DOI] [PubMed] [Google Scholar]
- 77.Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel F, van den Eertwegh AJM, et al. Association of Anti-TNF with Decreased Survival in Steroid Refractory Ipilimumab and Anti-PD1-Treated Patients in the Dutch Melanoma Treatment Registry. Clin Cancer Res 2020;26(9):2268–74 doi 10.1158/1078-0432.Ccr-19-3322. [DOI] [PubMed] [Google Scholar]
- 78.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother 2017;66(5):581–92 doi 10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abu-Sbeih H, Ali FS, Alsaadi D, Jennings J, Luo W, Gong Z, et al. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor–induced colitis: a multi-center study. Journal for ImmunoTherapy of Cancer 2018;6(1):142 doi 10.1186/s40425-018-0461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esfahani K, Hudson M, Batist G. Tofacitinib for Refractory Immune-Related Colitis from PD-1 Therapy. New England Journal of Medicine 2020;382(24):2374–5 doi 10.1056/NEJMc2002527. [DOI] [PubMed] [Google Scholar]
- 81.Thomas AS, Ma W, Wang Y. Ustekinumab for Refractory Colitis Associated with Immune Checkpoint Inhibitors. New England Journal of Medicine 2021;384(6):581–3 doi 10.1056/NEJMc2031717. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka R, Fujisawa Y, Sae I, Maruyama H, Ito S, Hasegawa N, et al. Severe hepatitis arising from ipilimumab administration, following melanoma treatment with nivolumab. Jpn J Clin Oncol 2017;47(2):175–8 doi 10.1093/jjco/hyw167. [DOI] [PubMed] [Google Scholar]
- 83.Araujo DV, Muniz TP, Yang A, Keshavarzi S, Sorotsky H, Butler MO, et al. Real World Outcomes and Hepatotoxicity of Infliximab in the Treatment of Steroid-Refractory Immune-Related Adverse Events. Curr Oncol 2021;28(3):2173–9 doi 10.3390/curroncol28030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei SC, Meijers WC, Axelrod ML, Anang NAS, Screever EM, Wescott EC, et al. A Genetic Mouse Model Recapitulates Immune Checkpoint Inhibitor-Associated Myocarditis and Supports a Mechanism-Based Therapeutic Intervention. Cancer Discov 2021;11(3):614–25 doi 10.1158/2159-8290.CD-20-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Beattie J, Rizvi H, Fuentes P, Luo J, Schoenfeld A, Lin I-H, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. Journal for ImmunoTherapy of Cancer 2021;9(2):e001884 doi 10.1136/jitc-2020-001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dimitriou F, Hogan S, Menzies AM, Dummer R, Long GV. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur J Cancer 2021;157:214–24 doi 10.1016/j.ejca.2021.08.031. [DOI] [PubMed] [Google Scholar]
- 87.Stroud CR, Hegde A, Cherry C, Naqash AR, Sharma N, Addepalli S, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract 2019;25(3):551–7 doi 10.1177/1078155217745144. [DOI] [PubMed] [Google Scholar]
- 88.Phillips GS, Wu J, Hellmann MD, Postow MA, Rizvi NA, Freites-Martinez A, et al. Treatment Outcomes of Immune-Related Cutaneous Adverse Events. Journal of Clinical Oncology 2019;37(30):2746–58 doi 10.1200/jco.18.02141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson D, Patel AB, Uemura MI, Trinh VA, Jackson N, Zobniw CM, et al. IL17A Blockade Successfully Treated Psoriasiform Dermatologic Toxicity from Immunotherapy. Cancer Immunology Research 2019;7(6):860–5 doi 10.1158/2326-6066.Cir-18-0682. [DOI] [PubMed] [Google Scholar]
- 90.Murray K, Floudas A, Murray C, Fabre A, Crown J, Fearon U, et al. First use of tofacitinib to treat an immune checkpoint inhibitor-induced arthritis. BMJ Case Rep 2021;14(2) doi 10.1136/bcr-2020-238851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis 2017;76(12):2061–4 doi 10.1136/annrheumdis-2017-211560. [DOI] [PubMed] [Google Scholar]
- 92.Moriyama S, Fukata M, Tatsumoto R, Kono M. Refractory constrictive pericarditis caused by an immune checkpoint inhibitor properly managed with infliximab: a case report. Eur Heart J Case Rep 2021;5(1):ytab002 doi 10.1093/ehjcr/ytab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin JS, Mamlouk O, Selamet U, Tchakarov A, Glass WF, Sheth RA, et al. Infliximab for the treatment of patients with checkpoint inhibitor-associated acute tubular interstitial nephritis. Oncoimmunology 2021;10(1):1877415 doi 10.1080/2162402x.2021.1877415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, DuPont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med 2018;24(12):1804–8 doi 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


