Abstract
The entorhinal cortex is the site of some of the earliest pathological changes in Alzheimer’s disease, including neuronal, synaptic and volumetric loss. Specifically, the lateral entorhinal cortex shows significant accumulation of tau neurofibrillary tangles in the amnestic mild cognitive impairment (aMCI) phase of Alzheimer’s disease. Although decreased entorhinal cortex activation has been observed in patients with aMCI in the context of impaired memory function, it remains unclear if functional changes in the entorhinal cortex can be localized to the lateral or medial entorhinal cortex. To assess subregion specific changes in the lateral and medial entorhinal cortex, patients with aMCI and healthy aged-matched control participants underwent high-resolution structural and functional magnetic resonance imaging. Patients with aMCI showed significantly reduced volume, and decreased activation localized to the lateral entorhinal cortex but not the medial entorhinal cortex. These results show that structural and functional changes associated with impaired memory function differentially engage the lateral entorhinal cortex in patients with aMCI, consistent with the locus of early disease related pathology.
Keywords: Amnestic Mild Cognitive Impairment, Functional Neuroimaging, Entorhinal Cortex
1. Introduction
Amnestic mild cognitive impairment (aMCI) is defined by memory function that is worse than would be expected for a person’s age (Petersen, 2004) and is associated with both structural and functional changes in medial temporal lobe structures (Bai et al., 2009; Trivedi et al., 2011). Studies using functional magnetic resonance imaging (fMRI) have reported elevated hippocampal activation in the context of impaired memory function in patients in this transitional stage between healthy aging and a clinical diagnosis of Alzheimer’s Disease (AD) (Dickerson et al., 2005; Dickerson et al., 2004; Hämäläinen et al., 2007; Miller et al., 2007). High-resolution fMRI studies have further localized this increased hippocampal activation specifically to the dentate gyrus / CA3 (DG/CA3) subregion of the hippocampus using a three-judgment memory task designed to tax pattern separation, a function ascribed to the granule cells of the dentate gyrus to reduce mnemonic interference by encoding distinctive representations for similar input patterns (Bakker et al., 2008; Yassa et al., 2010). Longitudinal follow up of patients with aMCI has shown that hippocampal hyperactivation predicts subsequent cognitive decline and progression to dementia (Huijbers et al., 2015; Miller et al., 2007) and as such, is recognized as a characteristic feature of the aMCI phase of AD (for a review, see Ewers et al., 2011)
The primary source of neocortical inputs to the DG/CA3 regions of the hippocampal formation derives from the layer 2 neurons of the entorhinal cortex (Amaral & Witter, 1989; Canto et al., 2008), which are the site of some of the earliest pathological changes in AD including accumulation of tau neurofibrillary tangles (Braak and Braak, 1991; Lace et al., 2009) and frank neuronal degeneration and loss (Gómez-Isla et al., 1996; Hoesen et al., 1991; Kordower et al., 2001; Price et al., 2001). Early atrophy of the entorhinal cortex can also be detected by in vivo structural magnetic resonance imaging (MRI) studies of patients with MCI and AD showing substantial reductions in entorhinal cortex volume, paralleling neuronal loss (Dickerson et al., 2001; Killiany et al., 2000), and correlated with impaired cognitive function in these patients (Juottonen et al., 1998; Paola et al., 2007; Soldan et al., 2015).
More recently, interest in the topography of AD progression affecting the entorhinal cortex has emerged with mounting evidence suggesting that the lateral entorhinal cortex (including transentorhinal cortex) may be affected earlier in the disease trajectory. Studies examining tau neurofibrillary tangles, a characteristic feature of AD pathology, suggest that tau accumulation is observed in the lateral entorhinal cortex prior to being observed in the medial entorhinal cortex in postmortem studies of human subjects (Braak and Braak, 1995). Longitudinal in vivo structural neuroimaging studies showed a similar pattern with atrophy following a lateral-caudal to medial-rostral gradient in entorhinal cortex in patients with MCI (Tward et al., 2017). These findings are consistent with evidence of early functional changes in a metabolic imaging study of preclinical patients as well as in a tau-APP mouse model of AD (Khan et al., 2014). Although, hypoactivation of the entorhinal cortex in the context of hippocampal hyperactivity and associated memory impairment has been observed in patients with aMCI (Bakker et al., 2012, 2015; Yassa et al., 2010), it remains unclear if this hypoactivation is regionally localized in the entorhinal cortex.
In this study, we utilized high-resolution structural and functional MRI methods to directly address this question and assess the topography of structural and functional changes in the lateral and medial entorhinal cortex in patients with amnestic mild cognitive impairment when compared to cognitively normal older adults. Patients with aMCI in this study showed both reduced volume and reduced activation specifically localized to the lateral entorhinal cortex in the context of both impaired performance on a three-judgement memory task and hippocampal hyperactivity localized to the DG/CA3 region (previously reported in Tran et al., 2017). In contrast, no significant differences in task related activation were observed in the medial entorhinal cortex when compared to age-matched control subjects. These findings show that structural and functional changes associated with impaired memory function affect the lateral entorhinal cortex in patients with aMCI, consistent with the locus of early disease-related AD pathology.
2. Methods
2.1. Participants
Patients with aMCI and cognitively normal age-matched control participants between the age of 50 and 85 were recruited from ongoing studies of aging and memory impairment at Johns Hopkins. All participants completed medical, psychiatric and neurological evaluations. Participants were excluded if they had current neurological or psychiatric disorders, major head trauma, or a history of substance abuse or dependencies. In addition, all participants completed a neuropsychological assessment including the Mini Mental Status Exam (Folstein et al., 1975), the Buschke Selective Reminding Test (Buschke & Fuld, 1974), the Verbal Paired Associative subtest of the Wechsler Memory Scale (Wechsler, 1945) and the Benton Visual Retention Test (Benton, 1974) to examine levels of cognitive performance. All patients met criteria for aMCI as defined by Petersen (2004) including: 1) a global Clinical Dementia Rating scale (CDR; Morris, 1993) score of 0.5 with a sum of boxes score not exceeding 2.5; 2) a reported memory complaint confirmed by an informant; 3) impaired memory performance on neuropsychological testing (e.g. 1.5 standard deviations below the norm) and; 4) no decline in activity of daily living. All cognitively normal age-matched control subjects had a global CDR score of 0, endorsed no memory complaint, and scored within the norm on neuropsychological assessment. None of the participants in the study met criteria for dementia. The study protocol was approved by the Institutional Review Board of the Johns Hopkins Medical Institutions and all participants provided written informed consent.
After excluding 3 participants from analysis due to task performance that was inadequate for analysis of the fMRI data (above 20% no response rate) or excessive in-scanner motion (more than 8 volumes with more than 3mm of translation or 3mm of rotation), 42 patients with aMCI and 35 cognitively normal age-matched controls were selected from the study sample such that all patients with aMCI were included and the aMCI and control groups were, on average, equivalent for age, education and distribution of sex. This sample was previously used to provide the results reported in Tran et al. (2017) and data from 32 aMCI patients and 20 control participants included here, also contributed to results previously described in Bakker et al. (2012; 2015).
2.2. fMRI task
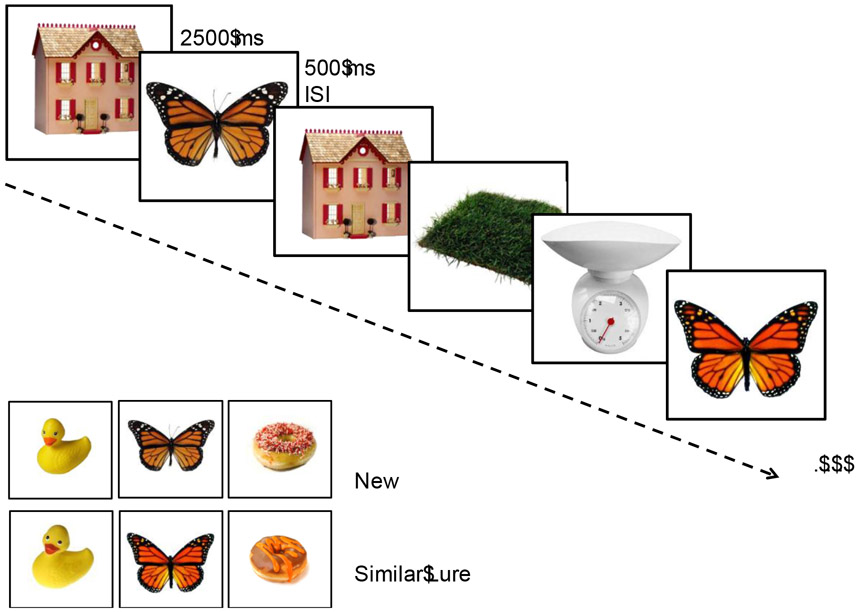
The fMRI task, previously described in detail (Bakker et al., 2008; 2012; Kirwan & Stark, 2007), is a memory task designed to assess computational functions thought to be mediated by the structures of the medial temporal lobe and critical for episodic memory function. For this task, participants viewed 768 stimuli of common objects. These stimuli included 384 single unrelated pictures of objects used as foils, 96 pairs in which an identical image was repeated, referred to as repeats, and 96 pairs consisted of highly similar but not identical stimuli referred to as lures. Participants completed the task over eight runs with 96 stimuli per run. Stimuli were presented for 2500 ms with a 500 ms inter-stimulus-interval consisting of a blank screen. Trials were presented in a pseudo-random order, with lures or repeated stimuli presented within 30 trials of its pair. The task was presented as a 3-alternative forced choice task, with participants asked to judge each stimulus as ‘new’, ‘old’ or ‘similar’ but not identical to previously seen stimuli (Figure 1). An item is correctly judged as “new” if the item was seen for the first time in the context of the task, and “old” if the item was a repetition of a previously seen item. Of critical interest is the participants’ response to the lure trials. The lure trials are correctly called “similar” if the item resembles a previously seen item in the task but is not exactly the same. Performance on these critical lure trials has been associated with hippocampal DG/CA3 functioning and provides a sensitive measure to detect memory impairment in older adults and patients with aMCI (Bakker et al., 2015; Stark et al., 2013).
Figure 1.
Sample stimuli and timing of the memory task. Participants were asked to judge if each item was ‘old’, ‘similar’ or ‘new’. Recognition of the similar lure items served as the critical trials thought to depend on the integrity of functions dependent on the hippocampal DG/CA3 network based on input from the entorhinal cortex. In prior studies, older adults (Yassa et al, 2010) and patients with aMCI (Bakker et al., 2012; 2015; Tran et al., 2017) show reduced accuracy on these critical trials.
Data was collected using an Apple Macintosh laptop computer running MATLAB software (The Mathworks, Natick, MA) and a Cedrus RB-610 response box. Participants were given a five-minute practice task outside of the scanner to become familiar with the task.
2.3. MRI acquisition
Data was acquired on a 3 Tesla Philips scanner equipped with a SENSE parallel imaging head coil and higher order shims to compensate for local field distortions located at the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute on the Johns Hopkins Medical campus. Functional images were collected using a T2*-weighted echo planar single shot pulse, an echo time of 30 ms and a flip angle of 70, an in-plane resolution of 1.5 x 1.5 mm, a TR of 1.5 seconds, and an acquisition matrix of 64 x 64. Functional volumes consisted of 19 slices oriented along the principal axis of the hippocampus covering the medial temporal lobe bilaterally. Structural images were acquired using a T1-weighted sequence with 231 oblique slices, a 0.65 mm isotropic resolution and a field of view of 240 mm.
2.4. Structural MRI analysis
Medial temporal lobe structures and hippocampal sub regions were manually segmented using methods previously described in (Bakker et al., 2008; 2012, 2015; Kirwan & Stark, 2007). Segmented regions include the bilateral entorhinal cortex, perirhinal cortex, parahippocampal cortex and temporopolar cortices using the landmarks described by Insausti (Insausti et al., 1998). Given the known vulnerability of the transentorhinal cortex to AD pathology, the lateral boundary of the entorhinal cortex was extended to include the transentorhinal cortex by as defined by Braak and Braak (Braak and Braak 1991; 1995) and referred to as area 35 by others (see Ding and Van Hoesen, 2010), recognizing the depth of the collateral sulcus as a factor in the lateral boundary at either the fundus (regular) or at the midpoint of the medial bank of the collateral sulcus (deep) (Insausti, 1998). Segmentations of the CA1, DG/CA3, and subiculum regions of the hippocampus followed coronal landmarks described by Duvernoy (Duvernoy et al., 2005). T.T. completed all manual segmentations which were reviewed by A.B. Both were blinded to the diagnostic group.
Initial affine registration was used to transfer the subject’s anatomical images to the Talairach coordinate system (Tournoux and Talairach, 1988). Using both the segmentation-label based anatomical information and the T1 grayscale image, Advanced Normalization Tools (ANTS) (Avants et al., 2009) was used to calculate the 3D vector field transformation needed to align the individual subject ROIs to a template modal model of the ROIs based on the entire sample by equally weighting the segmentation and the T1 grayscale data. This procedure greatly diminishes the alignment error in cross subject alignment for model template creation by taking into account individual anatomical variations (Kirwan and Stark, 2007).
The modal template was used to manually delineate the lateral and medial entorhinal cortex by dividing the segmentation of the entorhinal cortex into a medial and a lateral component. The boundary between the medial and lateral entorhinal cortex was defined by drawing vertical line from the most medial inferior point of the white matter forming the medial bank of the collateral sulcus to the inferior edge of the entorhinal cortex (see supplementary Figure 1). The segmentations of the lateral and medial entorhinal cortex delineated on the modal template were then back projected to the participants’ individual anatomical scans using the 3D vector field transformation generated in the alignment process. The resulting segmentation of lateral and medial entorhinal cortex in individual subject space was then used to obtain the volume of the lateral and medial entorhinal cortex for each participant in the sample.
2.5. fMRI subject-level analysis
Data analysis was performed using the Analysis of Functional Neuroimages (AFNI) software package (Cox, 1996). Functional images were co-registered to correct for slice time acquisition differences and head motion using a three-dimensional registration algorithm creating motion vectors used to remove volumes in which a significant head motion occurred (more than 3mm of translation or 3mm of rotation) as well as the previous and subsequent TR from further analysis.
Functional runs were concatenated and six vectors were defined to model the different trial types: (1) repeats subsequently called “old”, (2) lures subsequently called “similar”, (3) lures subsequently called “old”, (4) repeats called “old”, (5) lures called “similar” and (6) lures called old. Remaining response types (misses, false alarms) were modeled but not included in further analyses. The full set of vectors was used to model each participant’s data using a deconvolution approach that was based on a general linear regression treating the single foil presentations that were correctly rejected as a non-zero baseline against all other conditions. The resulting statistical fit coefficient maps represent the difference in activity between each of the trial types and the baseline for a given time point for a given voxel. The sum of fit coefficients over the length of the hemodynamic response (~3 – 12 seconds after the onset of the trial) was taken as the model’s estimate of the response to each trial type. The statistical maps were then smoothed using a Gaussian kernel of 3 mm to account for variations in individual functional anatomy. The 3D vector fields for each individual obtained from the structural image registration was then applied to the smoothed concatenated fit coefficient maps warping the individual subject results to the common template space.
2.6. Statistical analysis
Age, education, neuropsychological and functional assessment scores and resulting measures were compared between groups using independent sample Welch’s t-tests.
The primary objective of the study was to examine lateral and medial entorhinal activation in patients with aMCI compared to cognitively normal control participants. Voxel selection was based on a two-way ANOVA with trial type (6 trial vectors) and group status (aMCI and control) as fixed factors and subject as a random factor nested within group. A voxel threshold of p = 0.05 was used on the overall model F-statistic in combination with a spatial extent threshold of 15 voxels to select areas of task-related activation. The cluster threshold was based on simulations using the updated 3dClustSim (AFNI version 19.2.10 'Claudius'; Cox et al., 2017; Forman et al., 1995). Briefly, this method avoids false positives by simulating noise-only data within the a priori region of interest. Using a mask of the entorhinal cortex, cluster sizes that occurred with a global false positive rate of less than 0.05 across 10000 simulations were considered statistically significant resulting in a cluster threshold of 15 voxels for the entorhinal cortex.
Observed areas of activation were then combined with the anatomical segmentations of the lateral and medial entorhinal cortex in order to include only voxels within our areas of interest. This hybrid functional/anatomical analysis resulted in clusters of voxels where activity varied systematically across trial types within each of the anatomical regions of interest. Voxels within each functional/anatomical region of interest were then collapsed and mean activation for each trial type within each region of interest was calculated for further analysis.
3. Results
3.1. Behavioral Results
Demographics and neuropsychological test performance for all study participants is shown in Table 1. Patients with aMCI and cognitively normal control participants did not differ in age or education. Patients with aMCI showed significantly impaired performance compared to controls (p < 0.05) on the Mini Mental Status Exam and all neuropsychological assessments of memory function. On the three-judgement in-scanner memory task, patients with aMCI incorrectly identified the critical lure items as ‘old’ more often and gave relatively fewer correct responses of ‘similar’ when compared to the control participants as previously reported (Controls vs. aMCI by Old vs. Similar: F(1,77) = 18.35, p < 0.001; Tran et al., 2017). This pattern of results is consistent with the changes in integrity of functions dependent on the entorhinal cortex and hippocampal network observed in patients with aMCI.
Table 1:
Demographics and clinical characterization of healthy controls and aMCI participants.
| Characteristic | Controls | aMCI | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-value | |
| Subjects | 35 | 42 | - | ||
| Sex (M/F) | 18/17 | 23/19 | |||
| Age (years) | 69.0 (52 – 81) | 7.73 | 71.5 (55.0 - 85.0) | 7.3 | 0.41 |
| Education (years) | 16.4 (12.0 – 20.0) | 2.6 | 15.5 (11.0 – 20.0) | 2.8 | 0.18 |
| CDR | 0 | 0.5 | - | ||
| CDR Sum of Boxes | 0.01 (0.0 – 0.5) | 0.08 | 1.0 | 0.5 | < 0.001 |
| MMSE | 28.6 (24.0 –30.0) | 1.4 | 26.1 (20.0 – 30.0) | 2.3 | < 0.001 |
| Wechsler LM Delayed Recall | 31.4 (19.0 – 43.0) | 6.7 | 17 (5.0 – 33.0) | 7.6 | < 0.001 |
| BSRT Delayed Recall | 8.6 (4.0 –12.0) | 2.2 | 3.3 (0.0 – 8.0) | 1.9 | < 0.001 |
| Wechsler VPA Delayed Recall | 7.3 (3.0 – 8.0) | 1.2 | 3.9 (0.0 – 8.0) | 2.6 | < 0.001 |
| Benton Visual Retention Test | 6.1 (3.0 – 9.0) | 1.6 | 4.5 (2.0 – 7.0) | 1.2 | < 0.001 |
CDR: Clinical Dementia Rating; MMSE: Mini Mental Status Exam; LM: Logical Memory Paragraph Recall; BSRT: Buschke Selective Reminding Test; VPA: Verbal Paired Associates. Values provide mean (range) and standard deviations (SD). P-values are based on independent samples t-tests comparing aMCI and control subjects.
3.2. Structural Imaging Results
Analysis of the volumetric data was adjusted for variations in head size by calculating the proportion of the volume of the regions of interest to total intracranial volume for each participant (Du et al., 2003; Nordenskjöld et al., 2013). Total intercranial volume was calculated using SIENAX (Smith et al., 2001, 2002), part of FSL (Smith et al., 2004). Compared to control participants, patients with aMCI showed a significantly decreased volume in the left lateral entorhinal cortex (t(72.40) = 2.80, p < 0.01, d = 0.64) and right lateral entorhinal cortex (t(74.56) = 2.70, p < 0.01, d = 0.61) (Figure 2). The difference in volume between aMCI patients and controls showed a trend towards significance in the left medial entorhinal cortex (t(74.25) = 1.89, p = 0.06, d = 0.432) and the right medial entorhinal cortex (t(74.82) = 1.94, p = 0.06, d = 0.44). A two-way ANOVA showed a significant interaction between entorhinal cortex subregion (lateral vs. medial) and group (aMCI vs. age-matched control) (F(3,225) = 2.95, p = 0.03) suggesting the difference in volume is more specific to the lateral entorhinal cortex.
Figure 2.
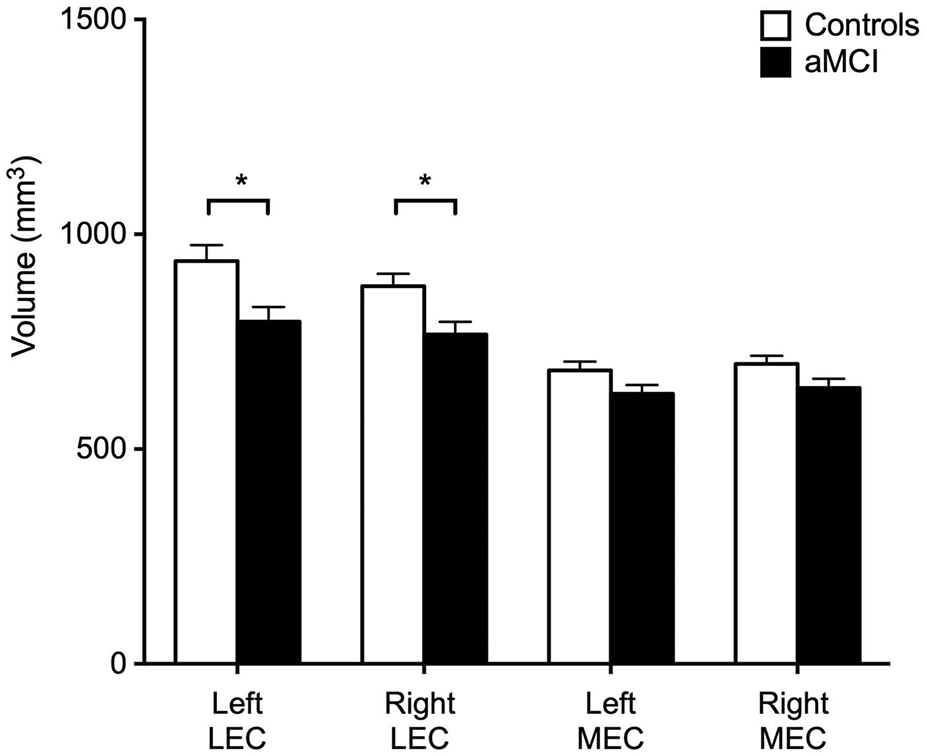
Decreased volume in patients with aMCI is observed in the lateral entorhinal cortex. Patients with aMCI showed a significantly decreased volume in both the left and right lateral entorhinal cortex when compared to controls. Patients with aMCI did not show a significant difference in the volume of both the left and right medial entorhinal cortex, although the differences showed a trend towards significance. *p < 0.01.
3.3. Functional Imaging Results
Functional image analysis resulted in three clusters of blood oxygenation level dependent (BOLD) activation localized to the left lateral entorhinal cortex, left medial entorhinal cortex and the right lateral entorhinal cortex (Figure 3). In the left and right lateral entorhinal cortex patients with aMCI showed significantly decreased activation during the critical lure trials correctly called similar when compared to aged-matched controls (t(74.32) = 1.99, p = 0.03; t(66.17) = 2.13, p = 0.04 respectively; Figure 4). In contrast, in the left medial entorhinal cortex activation during the critical lure trials did not differ between patients with aMCI and control participants (t(57.51) = 1.29, p = 0.20). Although most voxels in the cluster of activation observed in the right entorhinal cortex localized to the lateral entorhinal cortex, a portion of the voxel in this cluster extended into the medial entorhinal cortex. To confirm that hypoactivation is localized to the lateral entorhinal cortex in this study, this cluster of activation was split in to a lateral and a medial component. Activation in the medial component of the cluster did not significantly differ between aMCI patients and control participants (t(68) = 1.33, p = 0.19). However, in the lateral component of the cluster patients with aMCI showed significantly decreased activation compared to control participants (t(68) = 2.02, p = 0.047).
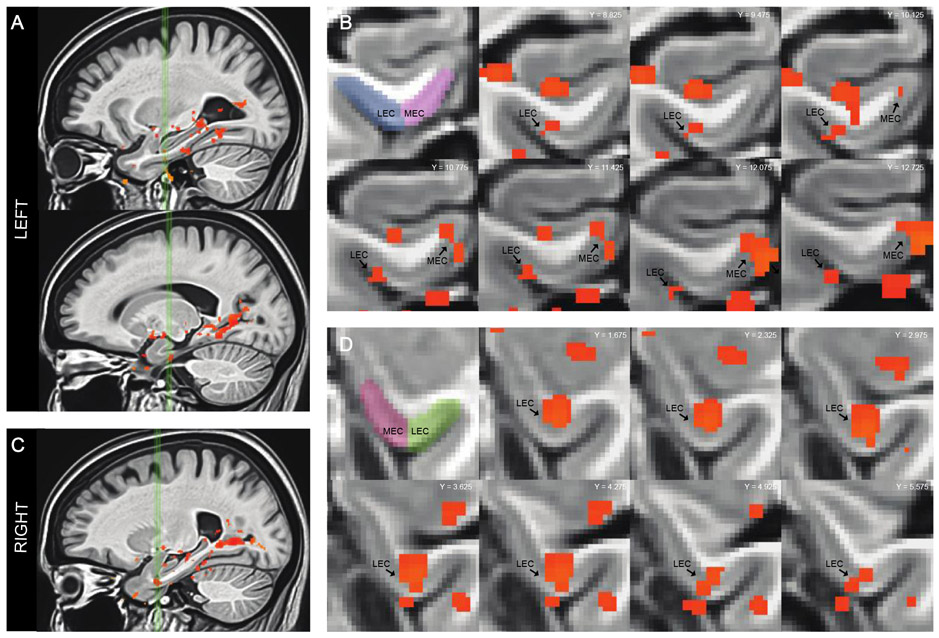
Figure 3.
Statistical maps based on the F-statistic of the overall model of the data shows areas where activity varied systematically across trial types within each anatomical region of interest in the medial temporal lobe. A. Sagittal view of the left medial temporal lobe. Green vertical lines identify slices through the left lateral entorhinal cortex (top) and the left medial entorhinal cortex (bottom). B. Coronal slices show statistical maps of the extent of task related activation in both the left lateral entorhinal cortex and medial entorhinal cortex. C. Sagittal view of the right medial temporal lobe. Green vertical lines identify slices through the right lateral entorhinal cortex. D. Coronal slices show statistical maps of the extent of task related activation in the right lateral entorhinal cortex.
Figure 4.
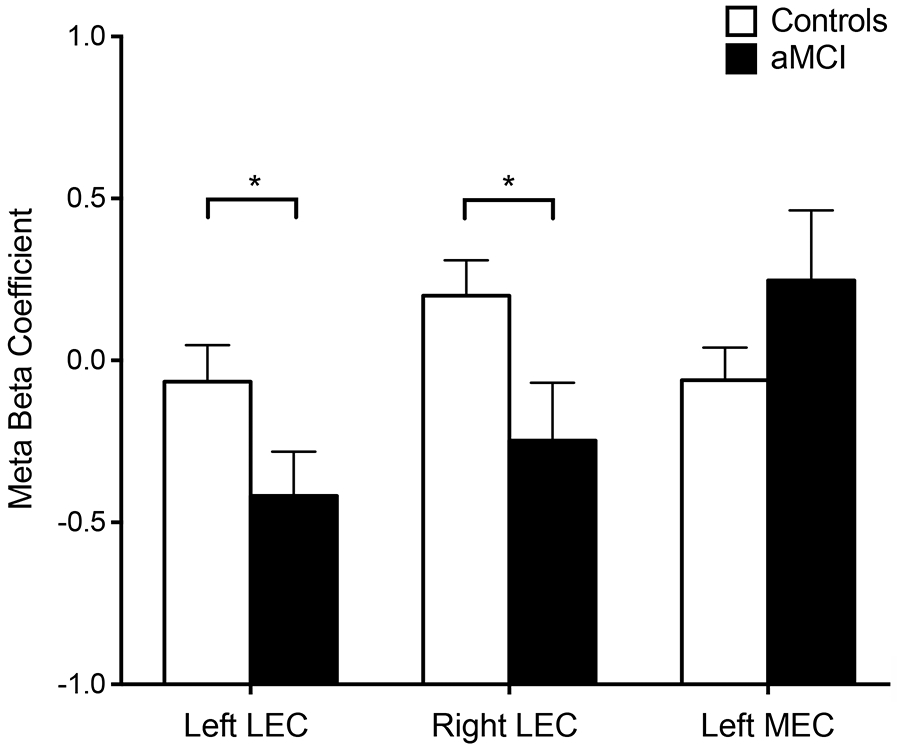
Decreased functional activation is observed in the lateral entorhinal cortex in patients with aMCI. Patients with aMCI showed decreased activation during lure trials called ‘similar’ in the left and right lateral entorhinal cortex in the context of impairment memory performance compared to control participants. No difference in activation was observed in the left medial entorhinal cortex between patients and control participants. *p<0.05.
Activation in the left lateral entorhinal cortex showed a significant correlation with behavioral performance on the memory task defined by the proportion correctly answered lure trials in patients with aMCI (F(1,40) = 6.03, p = 0.02, R2 = 0.13). This relationship was not significant in age-matched control participants (F(1, 35) = 2.53, p = 0.12, R2 = 0.07). Activation in the left medial entorhinal cortex was not correlated with behavioral performance in aMCI patients (F(1,40) = 0.002, p = 0.97), R2 < 0.001) or in age-matched control participants (F(1,35) = 1.27, p = 0.27, R2 = 0.03). Activation in the right lateral entorhinal cortex also was not correlated with behavioral performance in patients with aMCI (F(1,40) = 0.27, p = 0.61, R2 = 0.006) or control participants (F(1,35) = 0.10, p = 0.75, R2 = 0.003). Behavioral performance on the memory task was not significantly correlated with volume of the lateral entorhinal cortex in patients with aMCI (left: R2 < 0.01, F(1,40) =0.03, p = 0.87; right: R2 < 0.01, F(1,40) = 0.001, p = 0.93) or in age-matched control participants (left: R2 = 0.07, F(1,33) =2.465, p = 0.13; right: R2 = 0.03, F(1,33), p = 0.38). Behavioral performance on the memory task was also not correlated with volume of the medial entorhinal cortex in aMCI patients (left: R2 < 0.01, F(1,40) = 0.22, p = 0.64; right: R2 < 0.01, F(1,40) < 0.01, p = 0.95) or in age-matched control participants (left: R2 = 0.06, F(1,33) = 2.25, p = 0.14; right: R2 =0.11, F(1,33) = 3.86, p = 0.06). Finally, activation in the lateral entorhinal cortex was not significantly correlated with volume of this region in patients with aMCI (left: R2 = 0.001, F(1,40) = 0.06, p = 0.80; right: R2 = 0.04, F(1,40) = 1.56, p = 0.20) or in age-matched control participants (left: R2 = 0.10, F(1,33) = 3.83, p = 0.06; right: R2 = 0.05, F(1,33) = 1.58, p = 0.22). Activation in the left medial entorhinal cortex was not similarly not correlated with volume of this region in patients with aMCI (R2 < .01, F(1,40) = 0.40, p = 0.53) but showed a significant correlation in age-matched control participants (R2 = 0.13, F(1,33) = 4.76, p = 0.04).
To examine potential effects of signal drop-out in the lateral and medial entorhinal cortex, the temporal signal to noise ratio was calculated by dividing the average signal across all runs by the standard deviation of the noise. The temporal signal to noise ratio did not differ between patients with aMCI and age-matched control participants in the lateral entorhinal cortex (t(75) = 0.65, p = 0.52) or the medial entorhinal cortex (t(75) = 0.34, p = 0.73). When examining the difference between lateral and medial entorhinal cortex by group in a 2-way ANOVA, there was a significant effect of the region (F(1,75) = 45,09, p < 0.001) with an increased temporal signal to noise ratio in the lateral entorhinal cortex (M = 7.52, SD = 2.07) compared to the medial entorhinal cortex (M = 6.81, SD = 2.02). There was no significant interaction between participant and region (F(1,75) = 1.69, p = 0.20).
4. Discussion
This study aimed to assess whether decreased activation of the entorhinal cortex previously observed in aMCI patients (Bakker et al., 2012, 2015; Yassa et al., 2010) can be localized to the lateral versus the medial entorhinal cortex and to assess the structural volume of those subdivisions in the same patients. Patients with aMCI showed significantly decreased volume of the lateral entorhinal cortex bilaterally compared to age-matched controls while volume differences in the medial entorhinal cortex showed only a trend towards significance. Additionally, patients with aMCI showed significantly reduced activation limited to the lateral entorhinal cortex during critical memory trials in the three-judgment recognition task when compared to healthy control participants. Lateral and medial entorhinal cortex volume was not associated with task-related activation in either the aMCI of control groups and did therefore not contribute to observed activation differences between the groups. Finally, activity in the left lateral entorhinal cortex was positively correlated with task performance showing that higher activation was associated with better lure discrimination. The current findings extend the prior reports of decreased entorhinal cortex activation in patients with aMCI that was reliably observed in the left hemisphere (Bakker et al., 2012; 2015; Yassa et al, 2010). The focus here on anatomical localization within the entorhinal cortex revealed sensitivity to structural and functional alterations in lateral entorhinal cortex in both hemispheres. These results indicate that decreased activation of the entorhinal cortex is reliably localized to the lateral entorhinal cortex together with evidence for loss of structural integrity in patients with aMCI_and associated with the memory impairment observed in these patients.
A localization of structural and functional alterations in lateral entorhinal cortex is consistent with animal studies showing signatures in the lateral entorhinal cortex in age-related memory impairment. In an outbred rodent model well-characterized for individual differences in age-related memory impairment, glutamatergic cells, that form the origin of the perforant path projection from the lateral entorhinal cortex to the hippocampus, show reduced expression of reelin, a glycoprotein involved in learning and synaptic plasticity (Stranahan et al., 2011). This reduced expression was not observed in aged unimpaired rats and no age- or cognitive-related loss of reelin was observed in the medial entorhinal cortex. More recently, a corresponding finding of reelin reduction in the entorhinal cortex was observed in aged rhesus monkeys individually characterized for memory impairment (Long et al., 2020). Reduced numbers of reelin-positive neurons, predominantly in the lateral subdivision of the entorhinal region, were observed in aged cognitively impaired monkeys relative to both young and unimpaired aged cohorts, which did not differ from one another. Furthermore, reduced expression of reelin in memory-impaired aged rodents was observed in conjunction with increased tau phosphorylation, also localized to the lateral entorhinal cortex (Stranahan et al., 2011), suggesting that aging could provide a selective vulnerability for AD related pathology in this area.
Biomarker evidence of AD pathology was not collected in the current study, potentially resulting in a limited difference in pathology accumulation and associated differences in task-related activation between the groups. Although measures of amyloid or tau accumulation were not avaiable, the reduced volume and task related activation of the lateral entorhinal cortex were observed in the context of hippocampal hyperactivation previously reported in this sample of patients with aMCI (Tran et al., 2017). Multiple human fMRI studies have reported dysfunctional hippocampal hyperactivation localized to the DG/CA3 regions together with decreased activation of the entorhinal cortex (Bakker et al., 2012, 2015; Yassa et al., 2010). In the context of disease pathology, Cho et al. (2016) reported that increased 18F-AV-1451 binding, a tau PET tracer used for in vivo imaging, showed an increase in aMCI predominantly in the entorhinal cortex, whereas an increase in other MTL regions and cortex was observed in AD patients with dementia.
Subsequent studies in elderly humans have shown a strong relationship between the emergence of hippocampal hyperactivity even in clinically normal aging with tau measures determined in CSF sampling and tau PET imaging (Adams et al., 2021; Berron et al., 2019; Huijbers et al., 2019; Maass et al., 2018). That relationship is interesting in light of autopsy studies that have reported a localization of tangles composed of phosphorylated tau occurring in approximately half of those over age 60 in particularly the transentorhinal cortex (Braak Stage 1; Braak and Braak, 1995). Recent evidence that neural activity drives the spread of tau in the medial temporal lobe (Wu et al., 2016) suggests that hippocampal hyperactivity concurrent with the spread of tau to cortical regions occurs in early disease. The directional relationship between hippocampal hyperactivity and AD pathology in those clinical studies is suggested by the occurrence of augmented hippocampal neural activity in the absence of AD neurodegenerative disease across species, occurring in aged memory-impaired rodents and rhesus monkeys in the CA3/dentate gyrus (Simkin et al., 2015; Thomé et al., 2016; Wilson et al., 2005), regions that receive their major cortical input from the layer 2 neurons of entorhinal cortex with high phenotypic expression of reelin. In animal models of AD, reduced reelin expression has been demonstrated and reported to accelerate amyloid-plaque formation and tau pathology in the medial temporal lobe (Chin et al., 2007; Kocherhans et al., 2010). Thus, the loss of reelin in layer 2 entorhinal neurons in the lateral entorhinal region during aging itself could also play an early initiating role in AD progression (Long et al., 2020; Stranahan et al., 2011).
4.1. Anatomical divisions of the lateral and medial entorhinal
In addition to differential engagement in age-related changes associated with prodromal AD, the lateral and medial entorhinal cortex also have distinct cytoarchitecture and dissociable connectivity patterns. Initial anatomical studies showed that the lateral entorhinal cortex predominantly receives its input from the perirhinal cortex while the medial entorhinal cortex receives its input predominantly from the parahippocampal cortex (Canto et al., 2008; Insausti et al., 1998; Witter et al., 1988; Witter and Amaral, 1991). However, emerging evidence shows that the lateral entorhinal cortex also receives input from the parahippocampal cortex, suggesting that the lateral entorhinal cortex may receive multimodal information and likely plays a role in a number of memory processes (Doan et al., 2019; Nilssen et al., 2019). A preferential connectivity pattern has also been observed in-vivo using high-resolution fMRI approaches in human subjects. Several groups have reported that the anterior-lateral entorhinal cortex shows preferential functional connectivity with the perirhinal cortex while the posterior-medial entorhinal cortex showed preferential connectivity with the parahippocampal gyrus (Maass et al., 2015; Schröder et al., 2015). In addition, the medial and lateral entorhinal cortex showed differential connectivity with an anterior proximal and posterior distal gradient in the subiculum suggesting that the medial and lateral entorhinal cortex mediate processing pathways through the hippocampal formation. Emerging evidence from rodent, primate and human studies also suggest a functional distinction for these medial and lateral pathways. There appears to be a preference for allocentric spatial information in the parahippocampal – medial entorhinal cortex – proximal subiculum pathway while in contrast the perirhinal – lateral entorhinal - distal subiculum pathway demonstrates limited spatial specificity but strong involvement in processing of items and events among other contextual information (Davachi, 2006; Deshmukh et al., 2012; Knierim, et al., 2015; Lee et al., 2020). Consistent with episodic memory impairments that define the early stages of AD, there appears to be a selective bias toward impairment in processing of items and events in the lateral entorhinal cortex in humans (Berron et al., 2018, 2019; Reagh et al., 2016, 2018; but see and Fernandez-Baizan et al., 2020).
4.2. Functional role of the lateral and medial entorhinal cortex
Given a possible functional dissociation observed in the current investigation, it is important to acknowledge that the findings in this study were observed in the context of a task designed to tax hippocampal function using objects of varying similarity. Although the results are consistent with earlier reports of hypoactivation in the entorhinal cortex together with AD-related structural and functional changes in the lateral entorhinal cortex, the task stimuli employed in this study do not contain any spatial or contextual information and the task is not designed to engage all aspects of the proposed functions of the lateral and medial entorhinal cortex. Therefore, the absence of an observed difference in activation in the medial entorhinal cortex could be the result of a lack of sensitivity of the task in the spatial domain. Recent studies have employed tasks designed to contrast object identity processing (Olsen et al., 2017; Reagh et al., 2016; 2018; Stark et al., 2013; 2015) or spatial or context processing (Reagh et al., 2016; 2018; Tran et al., 2021) aiming to differentially task the contribution of the subregions of the entorhinal cortex. Additional studies using such task conditions and particularly the conjunctive encoding of an object and it’s position within a scene to assess their contribution to normal memory function and memory impairments observed due to AD would be informative. Notwithstanding the content of test materials in the current study, differences in the lateral and medial structural integrity and the relationship between lateral entorhinal cortex activation and lure discrimination reported here are of interest.
4.3. Functional role of the lateral and medial entorhinal cortex in aMCI
The landmarks employed here based on boundary definitions provided by Insausti (Insausti 1998; 2005) and inclusion of part of the perirhinal cortex, referred to as transentorhinal cortex (Braak and Braak, 1995) or area 35 (Ding and Van Hoesen, 2010) laterally given its known vulnerability to AD pathology, provide a reasonable demarcation to ascribe the observed functional clusters of activation to a medial or lateral component of the entorhinal cortex. By contrast, an alternative approach is provided in the study by Maass and colleagues (2015), in which functional connectivity was used to provide an anterolateral to posterior medial dissociation in the entorhinal cortex. This anterolateral to posterior medial dissociation provides a distinction in entorhinal subregions similar to an observed progression of anterior-lateral to posterior-medial atrophy observed by Tward et al. (2017) using longitudinal diffeomorphic mapping of structural MRI scans in patients with aMCI. These findings suggest that in addition to a lateral-medial distinction of the entorhinal cortex, a dissociation in the anterior-posterior axis must also be considered. However, establishing such dissociations based on functional connectivity approaches in aMCI patients is likely problematic given the altered functional connectivity patterns due to accumulation of pathology in these patients while manual segmentation approaches could introduce bias due to the anatomical differences between these populations. Alternative approaches based on objective detailed anatomical landmarks or projection methods using high-resolution atlases must therefore be considered and a consensus of landmarks and terminology based on both anatomical and functional studies is needed to compare the results across studies and further assess the functional significance of lateral entorhinal cortex dysfunction in patients with amnestic mild cognitive impairment.
Conclusion
AD related tau pathology is initially observed in the transentorhinal cortex, a region located between the perirhinal and entorhinal cortices in autopsy specimens from elderly individuals (Braak and Braak, 1995). This region is included in what is defined here as the lateral entorhinal cortex. In subsequent Braak stage II, tau extends further to largely encompass the entorhinal cortex progressing from lateral entorhinal cortex to medial entorhinal cortex, consistent with increased tau binding in vivo in patients with aMCI relative to healthy older adults (Cho et al., 2016). These findings have been further extended by Tward et al. (2017), which shows the longitudinal progression of atrophy in the entorhinal cortex also follows a lateral to medial gradient as well as an anterior to posterior progression. The findings reported here are consistent with these reports showing that structural and functional changes differentially engage the lateral entorhinal cortex in patients with aMCI beyond what is observed in healthy aged controls. Future studies employing longitudinal assessments of regional tau accumulation, in the context of functional changes in the anterolateral and posterior medial entorhinal cortex, will be critical in assessing the role of the entorhinal cortex in the progression of AD related memory impairment
Supplementary Material
Highlights.
Patients with aMCI show decreased lateral entorhinal cortex volume relative to controls.
Patients with aMCI show decreased fMRI activation in lateral entorhinal cortex relative to controls.
Selective involvement of the lateral entorhinal cortex is consistent with tau pathology.
Acknowledgements
We would like to thank the staff of the F.M. Kirby Center for Functional Brain Imaging for their assistance with data collection and Marilyn Albert, George Rebok and Vyash Puliyadi for helpful comments and discussion on previous drafts of this manuscript. This work was supported by NIH grants RC2AG036419, RO1AG048349 and P50AG05146 and the Phyllis F. Albstein Fund. T.T. is supported by a NIA T32 training grant and a National Defense Science and Engineering Graduate Fellowship (NDSEG) grant awarded by the DoD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a.
Financial Support
This work was supported by NIH grants RC2AG036419, RO1AG048349 and P50AG05146 and the Phyllis F. Albstein Fund. T.T. is supported by a NIA T32 training grant and a National Defense Science and Engineering Graduate Fellowship (NDSEG) grant awarded by the DoD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Credit Author Statement
Tammy Tran: Conceptualization, Investigation, Formal analysis, Writing – Original draft preparation. Caroline Speck: Data curation. Michela Gallagher: Conceptualization, Writing – Review and Editing, Funding acquisition. Arnold Bakker: Supervision, Writing – Review and Editing, Funding acquisition.
Declaration of Competing Interest
M.G. is the founder of AgeneBio. M.G. and A.B. are inventors on Johns Hopkins University intellectual property with patents pending and licensed to AgeneBio. M.G. consults for the company and owns company stock, which is subject to certain restrictions under University policy. M.G. and A.B’s role in the current study was in compliance with the conflict of interest policies of the Johns Hopkins School of Medicine.
Submission to Neurobiology of Aging
The current manuscript has not been previously published elsewhere and nor is it under consideration for publication elsewhere. All authors listed have reviewed the contents of the manuscript and approved the current submission to Neurobiology of Aging. The authors can further confirm that data collected for this manuscript has been in compliance with the Institutional Review Board of Johns Hopkins University – School of Medicine as described in the manuscript.
References
- Adams JN, Maass A, Berron D, Harrison TM, Baker SL, Thomas WP, Stanfill M, & Jagust WJ (2021). Reduced repetition suppression in aging is driven by tau-related hyperactivity in medial temporal lobe. The Journal of Neuroscience, 41, 3917–3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, & Witter MP (1989). The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience, 31(3), 571–591. 10.1016/0306-4522(89)90424-7 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison N, & Song G (2009). Advanced normalization tools (ANTS). Insight j, 2, 1–35. [Google Scholar]
- Bai F, Zhang Z, Watson DR, Yu H, Shi Y, Yuan Y, Zang Y, Zhu C, & Qian Y (2009). Abnormal Functional Connectivity of Hippocampus During Episodic Memory Retrieval Processing Network in Amnestic Mild Cognitive Impairment. Biological Psychiatry, 65(11), 951–958. 10.1016/j.biopsych.2008.10.017 [DOI] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, & Stark CEL (2008). Pattern Separation in the Human Hippocampal CA3 and Dentate Gyrus. Science, 319(5870), 1640–1642. 10.1126/science.1152882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker Arnold, Albert MS, Krauss G, Speck CL, & Gallagher M (2015). Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. NeuroImage: Clinical, 7, 688–698. 10.1016/j.nicl.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker Arnold, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, & Gallagher M (2012). Reduction of Hippocampal Hyperactivity Improves Cognition in Amnestic Mild Cognitive Impairment. Neuron, 74(3), 467–474. 10.1016/j.neuron.2012.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton AL (1974). Visual retention test. Psychological Corporation. [Google Scholar]
- Berron D, Cardenas-Blanco A, Bittner D, Metzger CD, Spottke A, Heneka MT, Fliessbach K, Schneider A, Teipel SJ, Wagner M, Speck O, Jessen F, & Düzel E (2019). Higher CSF Tau Levels Are Related to Hippocampal Hyperactivity and Object Mnemonic Discrimination in Older Adults. Journal of Neuroscience, 39(44), 8788–8797. 10.1523/JNEUROSCI.1279-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron D, Neumann K, Maass A, Schütze H, Fliessbach K, Kiven V, Jessen F, Sauvage M, Kumaran D, & Düzel E (2018). Age-related functional changes in domain-specific medial temporal lobe pathways. Neurobiology of Aging, 65, 86–97. 10.1016/j.neurobiolaging.2017.12.030 [DOI] [PubMed] [Google Scholar]
- Braak H, & Braak E (1991). Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica, 82(4), 239–259. 10.1007/BF00308809 [DOI] [PubMed] [Google Scholar]
- Braak Heiko, & Braak E (1995). Staging of alzheimer’s disease-related neurofibrillary changes. Neurobiology of Aging, 16(3), 271–278. 10.1016/0197-4580(95)00021-6 [DOI] [PubMed] [Google Scholar]
- Buschke H, & Fuld PA (1974). Evaluating storage, retention, and retrieval in disordered memory and learning. Neurology, 24(11), 1019–1025. [DOI] [PubMed] [Google Scholar]
- Canto CB, Wouterlood FG, & Witter MP (2008). What Does the Anatomical Organization of the Entorhinal Cortex Tell Us? Neural Plasticity, 2008, 1–18. 10.1155/2008/381243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Massaro CM, Palop JJ, Thwin MT, Yu G-Q, Bien-Ly N, Bender A, & Mucke L, (2007). Reelin Depletion in the Entorhinal Cortex of Human Amyloid Precursor Protein Transgenic Mice and Humans with Alzheimer’s Disease. Journal of Neuroscience, 27(11), 2727–2733. 10.1523/JNEUROSCI.3758-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Choi JY, Hwang MS, Lee JH, Kim YJ, Lee HM, … & Lee MS (2016). Tau PET in Alzheimer disease and mild cognitive impairment. Neurology, 87(4), 375–383. 10.1212/WNL.0000000000002892 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research, 29(3), 162–173. 10.1006/cbmr.1996.0014 [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RJB, & Taylor PA (2017). FMRI clustering and false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 114(17). 10.1073/pnas.1614961114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L (2006). Item, context and relational episodic encoding in humans. Current opinion in neurobiology, 16(6), 693–700. 10.1016/j.conb.2006.10.012 [DOI] [PubMed] [Google Scholar]
- Deshmukh SS, Johnson JL, & Knierim JJ (2012). Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: comparison with lateral entorhinal cortex. Hippocampus, 22(10), 2045–2058. 10.1002/hipo.22046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC , Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, & Beckett LA (2001). MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiology of Aging, 8. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, & Sperling RA (2005). Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology, 65(3), 404–411. 10.1212/01.wnl.0000171450.97464.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson Bradford C., Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, & Sperling RA (2004). Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology, 56(1), 27–35. 10.1002/ana.20163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S-L, Van Hoesen GW Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Hum. Brain Mapp. 2010;31:1359–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Zhu XP, Jagust WJ, Miller BL, Reed BR, Kramer JH, Mungas D, Yaffe K, Chui HC, & Weiner MW (2003). Atrophy rates of entorhinal cortex in AD and normal aging. Neurology, 60(3), 481–486. 10.1212/01.WNL.0000044400.11317.EC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doan TP, Lagartos-Donate MJ, Nilssen ES, Ohara S, & Witter MP (2019). Convergent Projections from Perirhinal and Postrhinal Cortices Suggest a Multisensory Nature of Lateral, but Not Medial, Entorhinal Cortex. Cell Reports, 29(3), 617–627.e7. 10.1016/j.celrep.2019.09.005 [DOI] [PubMed] [Google Scholar]
- Duvernoy HM (2005). The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Springer Science & Business Media. [Google Scholar]
- Ewers M, Frisoni GB, Teipel SJ, Grinberg LT, Amaro E, Heinsen H, Thompson PM, & Hampel H (2011). Staging Alzheimer’s disease progression with multimodality neuroimaging. Progress in Neurobiology, 95(4), 535–546. 10.1016/j.pneurobio.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Baizan C, Arias JL, & Mendez M (2020). Spatial memory assessment reveals age-related differences in egocentric and allocentric memory performance. Behavioral Brain Research, 388, 112646. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, & McHugh PR (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, & Noll DC (1995). Improved Assessment of Significant Activation in Functional Magnetic Resonance Imaging (fMRI): Use of a Cluster-Size Threshold. Magnetic Resonance in Medicine, 33(5), 636–647. 10.1002/mrm.1910330508 [DOI] [PubMed] [Google Scholar]
- Gómez-Isla T, Price JL, Jr DWM, Morris JC, Growdon JH, & Hyman BT (1996). Profound Loss of Layer II Entorhinal Cortex Neurons Occurs in Very Mild Alzheimer’s Disease. Journal of Neuroscience, 16(14), 4491–4500. 10.1523/JNEUROSCI.16-14-04491.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A, Pihlajamäki M, Tanila H, Hänninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, & Soininen H (2007). Increased fMRI responses during encoding in mild cognitive impairment. Neurobiology of Aging, 28(12), 1889–1903. 10.1016/j.neurobiolaging.2006.08.008 [DOI] [PubMed] [Google Scholar]
- Hoesen GW van, Hyman BT, & Damasio AR (1991). Entorhinal cortex pathology in Alzheimer’s disease. Hippocampus, 1(1), 1–8. 10.1002/hipo.450010102 [DOI] [PubMed] [Google Scholar]
- Huijbers W, Mormino EC, Schultz AP, Wigman S, Ward AM, Larvie M, Amariglio RE, Marshall GA, Rentz DM, Johnson KA, & Sperling RA (2015). Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain, 138(4), 1023–1035. 10.1093/brain/awv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Schultz AP, Papp KV, LaPoint MR, Hanseeuw B, Chhatwal JP, Hedden T, Johnson KA, & Sperling RA (2019). Tau Accumulation in Clinically Normal Older Adults Is Associated with Hippocampal Hyperactivity. Journal of Neuroscience, 39(3), 548–556. 10.1523/JNEUROSCI.1397-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, & Pitkanen A (1998). MR Volumetric Analysis of the Human Entorhinal, Perirhinal, and Temporopolar Cortices. 13. [PMC free article] [PubMed] [Google Scholar]
- Juottonen K, Laakso MP, Insausti R, Lehtovirta M, Pitkänen A, Partanen K, & Soininen H (1998). Volumes of the Entorhinal and Perirhinal Cortices in Alzheimer’s Disease. Neurobiology of Aging, 19(1), 15–22. 10.1016/S0197-4580(98)00007-4 [DOI] [PubMed] [Google Scholar]
- Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, Mayeux R, Duff KE, & Small SA (2014). Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nature Neuroscience, 17(2), 304–311. 10.1038/nn.3606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killiany RJ, Gomez-Isla T, Moss M, Kikinis R, Sandor T, Jolesz F, Tanzi R, Jones K, Hyman BT, & Albert MS (2000). Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Annals of Neurology, 47(4), 430–439. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, & Stark CEL (2007). Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning & Memory, 14(9), 625–633. 10.1101/lm.663507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ (2015). From the GPS to HM: Place cells, grid cells, and memory. Hippocampus, 25(6), 719–725. 10.1002/hipo.22453 [DOI] [PubMed] [Google Scholar]
- Kocherhans S, Madhusudan A, Doehner J, Breu KS, Nitsch RM, Fritschy J-M, & Knuesel I (2010). Reduced Reelin Expression Accelerates Amyloid-β Plaque Formation and Tau Pathology in Transgenic Alzheimer’s Disease Mice. Journal of Neuroscience, 30(27), 9228–9240. 10.1523/JNEUROSCI.0418-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, & Mufson EJ (2001). Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Annals of Neurology, 49(2), 202–213. [DOI] [PubMed] [Google Scholar]
- Lace G, Savva GM, Forster G, de Silva R, Brayne C, Matthews FE, Barclay JJ, Dakin L, Ince PG, & Wharton SB (2009). Hippocampal tau pathology is related to neuroanatomical connections: An ageing population-based study. Brain, 132(5), 1324–1334. 10.1093/brain/awp059 [DOI] [PubMed] [Google Scholar]
- Lee H, Goodsmith D, & Knierem JJ (2020). Parallel processing streams in the hippocampus. Curr. Opinion in Neurobiology, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JM, Perez EJ, Roberts JA, Roberts MT, & Rapp PR (2020). Reelin in the Years: Decline in the number of reelin immunoreactive neurons in layer II of the entorhinal cortex in aged monkeys with memory impairment. Neurobiology of Aging, 87, 132–137. 10.1016/j.neurobiolaging.2019.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Berron D, Libby LA, Ranganath C, & Düzel E (2015). Functional subregions of the human entorhinal cortex. ELife, 4. 10.7554/eLife.06426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, Baker SL, Rabinovici GD, & Jagust WJ (2018). Entorhinal Tau Pathology, Episodic Memory Decline, and Neurodegeneration in Aging. The Journal of Neuroscience, 38(3), 530–543. 10.1523/JNEUROSCI.2028-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, & Dickerson BC (2007). Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. Journal of Neurology, Neurosurgery & Psychiatry, 79(6), 630–635. 10.1136/jnnp.2007.124149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 10.1212/wnl.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Nilssen ES, Doan TP, Nigro MJ, Ohara S, & Witter MP (2019). Neurons and networks in the entorhinal cortex: A reappraisal of the lateral and medial entorhinal subdivisions mediating parallel cortical pathways. Hippocampus. 10.1002/hipo.23145 [DOI] [PubMed] [Google Scholar]
- Nordenskjöld NR, Malmberg F, Larsson E-M, Simmons A, Brooks SJ, Lind L, Ahlström H, Johansson L, & Kullberg J (2013). Intracranial volume estimated with commonly used methods could introduce bias in studies including brain volume measurements. NeuroImage, 83, 355–360. 10.1016/j.neuroimage.2013.06.068 [DOI] [PubMed] [Google Scholar]
- Paola M, Macaluso E, Carlesimo GA, Tomaiuolo F, Worsley KJ, Fadda L, & Caltagirone C (2007). Episodic memory impairment in patients with Alzheimer’s disease is correlated with entorhinal cortex atrophy: A voxel-based morphometry study. Journal of Neurology, 254(6), 774–781. 10.1007/s00415-006-0435-1 [DOI] [PubMed] [Google Scholar]
- Petersen RC (2004). Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine, 256(3), 183–194. 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, & Morris JC (2001). Neuron Number in the Entorhinal Cortex and CA1 in Preclinical Alzheimer Disease. Archives of Neurology, 58(9), 1395. 10.1001/archneur.58.9.1395 [DOI] [PubMed] [Google Scholar]
- Reagh ZM, Ho HD, Leal SL, Noche JA, Chun A, Murray EA, & Yassa MA (2016). Greater loss of object than spatial mnemonic discrimination in aged adults: SELECTIVE OBJECT MEMORY DEFICITS IN AGING. Hippocampus, 26(4), 417–422. 10.1002/hipo.22562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagh ZM, Noche JA, Tustison NJ, Delisle D, Murray EA, & Yassa MA (2018). Functional Imbalance of Anterolateral Entorhinal Cortex and Hippocampal Dentate/CA3 Underlies Age-Related Object Pattern Separation Deficits. Neuron, 97(5), 1187–1198.e4. 10.1016/j.neuron.2018.01.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder T, Haak KV, Zaragoza Jimenez NI, Beckmann CF, & Doeller CF (2015). Functional topography of the human entorhinal cortex. ELife, 4. 10.7554/eLife.06738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin D, Hattori S, Ybarra N, Musial TF, Buss EW, Richter H, Oh MM, Nicholson DA, & Disterhoft JF (2015). Aging-Related Hyperexcitability in CA3 Pyramidal Neurons Is Mediated by Enhanced A-Type K+ Channel Function and Expression. Journal of Neuroscience, 35(38), 13206–13218. 10.1523/JNEUROSCI.0193-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, De Stefano N, Jenkinson M, & Matthews PM (2001). Normalized Accurate Measurement of Longitudinal Brain Change. Journal of Computer Assisted Tomography, 25(3), 466–475. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, & Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23, S208–S219. 10.1016/j.neuroimage.2004.07.051 [DOI] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, & De Stefano N (2002). Accurate, Robust, and Automated Longitudinal and Cross-Sectional Brain Change Analysis. NeuroImage, 17(1), 479–489. 10.1006/nimg.2002.1040 [DOI] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Lu Y, Wang M-C, Selnes O, Albert M, Brown T, Ratnanather JT, Younes L, Miller MI, & The BIOCARD Research Team. (2015). Relationship of medial temporal lobe atrophy, APOE genotype, and cognitive reserve in preclinical Alzheimer’s disease: Atrophy in Preclinical Alzheimer’s Disease. Human Brain Mapping, 36(7), 2826–2841. 10.1002/hbm.22810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Lacy JW, & Stark CEL (2013). A task to assess behavioral pattern separation (BPS) in humans: Data from healthy aging and mild cognitive impairment. Neuropsychologia, 51(12), 2442–2449. 10.1016/j.neuropsychologia.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Salas-Vega S, Jiam NT, & Gallagher M (2011). Interference with reelin signaling in the lateral entorhinal cortex impairs spatial memory. Neurobiology of Learning and Memory, 96(2), 150–155. 10.1016/j.nlm.2011.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomé A, Gray DT, Erickson CA, Lipa P, & Barnes CA (2016). Memory impairment in aged primates is associated with region-specific network dysfunction. Molecular Psychiatry, 21(9), 1257–1262. 10.1038/mp.2015.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournoux T, & Talairach J (1988). Co-planar stereotaxic atlas of the human brain. Stuggart, Germany: Theime. [Google Scholar]
- Tran TT, Speck CL, Pisupati A, Gallagher M, & Bakker A (2017). Increased hippocampal activation in ApoE-4 carriers and non-carriers with amnestic mild cognitive impairment. NeuroImage: Clinical, 13, 237–245. 10.1016/j.nicl.2016.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MA, Stoub TR, Murphy CM, George S, deToledo-Morrell L, Shah RC, Whitfield-Gabrieli S, Gabrieli JDE, & Stebbins GT (2011). Entorhinal cortex volume is associated with episodic memory related brain activation in normal aging and amnesic mild cognitive impairment. Brain Imaging and Behavior, 5(2), 126–136. 10.1007/s11682-011-9117-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tward DJ, Sicat CS, Brown T, Bakker A, Gallagher M, Albert M, & Miller M (2017). Entorhinal and transentorhinal atrophy in mild cognitive impairment using longitudinal diffeomorphometry. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 9, 41–50. 10.1016/j.dadm.2017.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1945). Wechsler memory scale. Psychological Corporation [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, &Tanila H (2005). Age-Associated Alterations of Hippocampal Place Cells Are Subregion Specific. Journal of Neuroscience, 25(29), 6877–6886. 10.1523/JNEUROSCI.1744-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, & Amaral DG (1991). Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. The Journal of Comparative Neurology, 307(3), 437–459. 10.1002/cne.903070308 [DOI] [PubMed] [Google Scholar]
- Witter MP, Griffioen AW, Jorritsma-Byham B, & Krijnen JLM (1988). Entorhinal projections to the hippocampal CA1 region in the rat: An underestimated pathway. Neuroscience Letters, 85(2), 193–198. 10.1016/0304-3940(88)90350-3 [DOI] [PubMed] [Google Scholar]
- Wu JW, Hussaini SA, Bastille IM, Rodriguez GA, Mrejeru A, Rilett K, Sanders DW, Cook C, Fu H, Boonen RACM, Herman M, Nahmani E, Emrani S, Figueroa YH, Diamond MI, Clelland CL, Wray S, & Duff KE (2016). Neuronal activity enhances tau propagation and tau pathology in vivo. Nature Neuroscience, 19(8), 1085–1092. 10.1038/nn.4328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, & Stark CEL (2010). Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proceedings of the National Academy of Sciences, 107(28), 12687–12691. 10.1073/pnas.1002113107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.