To the Editor:
Although asthma is often associated with cytokines typically produced by Th2 cells, the “T2 paradigm” is unable to explain the full spectrum of asthma severity; indeed, “severe” asthmatics often display inflammatory profiles consistent with mixed T2/T17 inflammation.1 Using a mouse model of asthma, we have shown that while severe asthma-like disease is associated with expansion of both Th2 and Th17 cells, increased production of Th17-associated cytokines are accompanied by increased measures of IL-17A-induced cellular activation (intracellular signalling, IL-17A-induced gene expression) in lung structural cells.2 These findings suggest that intrinsic differences in IL-17A responsiveness may contribute to the development of more severe asthma independently of changes in IL-17A production. The goals of the current study were to identify clinical factors associated with IL-17A responsiveness in children with asthma, and determine if IL-17A responsiveness differs in nasal epithelial cells from children with mild, moderate and severe asthma.
Following IRB approval by Cincinnati Children’s Hospital Medical Center Institutional Review Board, youth (5–19 years old) enrolled in the Pediatric Environmental Exposure Study (PEES)3 with physician-diagnosed asthma, and currently taking asthma medications, were invited for a follow-up research visit. Participants taking nasal steroids in the past 30 days were ineligible. Other medication usage was not restricted but was considered when categorizing asthma severity. Research participants provided consent/assent for the described studies, and participants and parents completed surveys to assess exposure to tobacco smoke (primary smoker, secondhand smoke (SHS) exposure, or early life maternal smoking (<5 years)), respiratory symptom score (RSS - frequency of wheeze, shortness of breath, cough, and chest tightness over the past 4 weeks), level of asthma control (childhood Asthma Control Test (ACT)), and medication usage/treatment step. Blood was drawn to quantify serum IgE levels and assess peripheral eosinophil and neutrophil counts. Spirometry was performed to assess lung function at time of appointment. Current asthma severity (mild, moderate, severe) was determined after consideration of spirometry outcomes, symptom scores, ACT scores, medication usage, and frequency and severity of exacerbations according to ATS guidelines4. Air pollutant exposure was estimated based on mean concentration of elemental carbon attributable to traffic (ECAT) exposure at birth and current address3. Nasal epithelial cells (NECs) were collected from beneath the inferior turbinate using a cytology brush according to published techniques5. Importantly cells were cultured for 1–4 weeks including 2 passages to limit effects of in vivo exposures (environmental, medications) on cellular readouts. To measure IL-17A responsiveness, passaged NECs were plated in 24 well plates, allowed to reach confluence and then treated with medium alone or IL-17A (100 ng/ml) for 18–20 hours. Production of the IL-17A-induced chemokine, CXCL1 (GROα), was assessed in supernatants by ELISA. CXCL1 production was chosen as the major endpoint due to its relevance in recruitment of neutrophils. IL-17A stimulated CXCL1 released from NECs was measurable in all participants (1.5 - >750-fold induction; Fig 1A).
Figure 1:
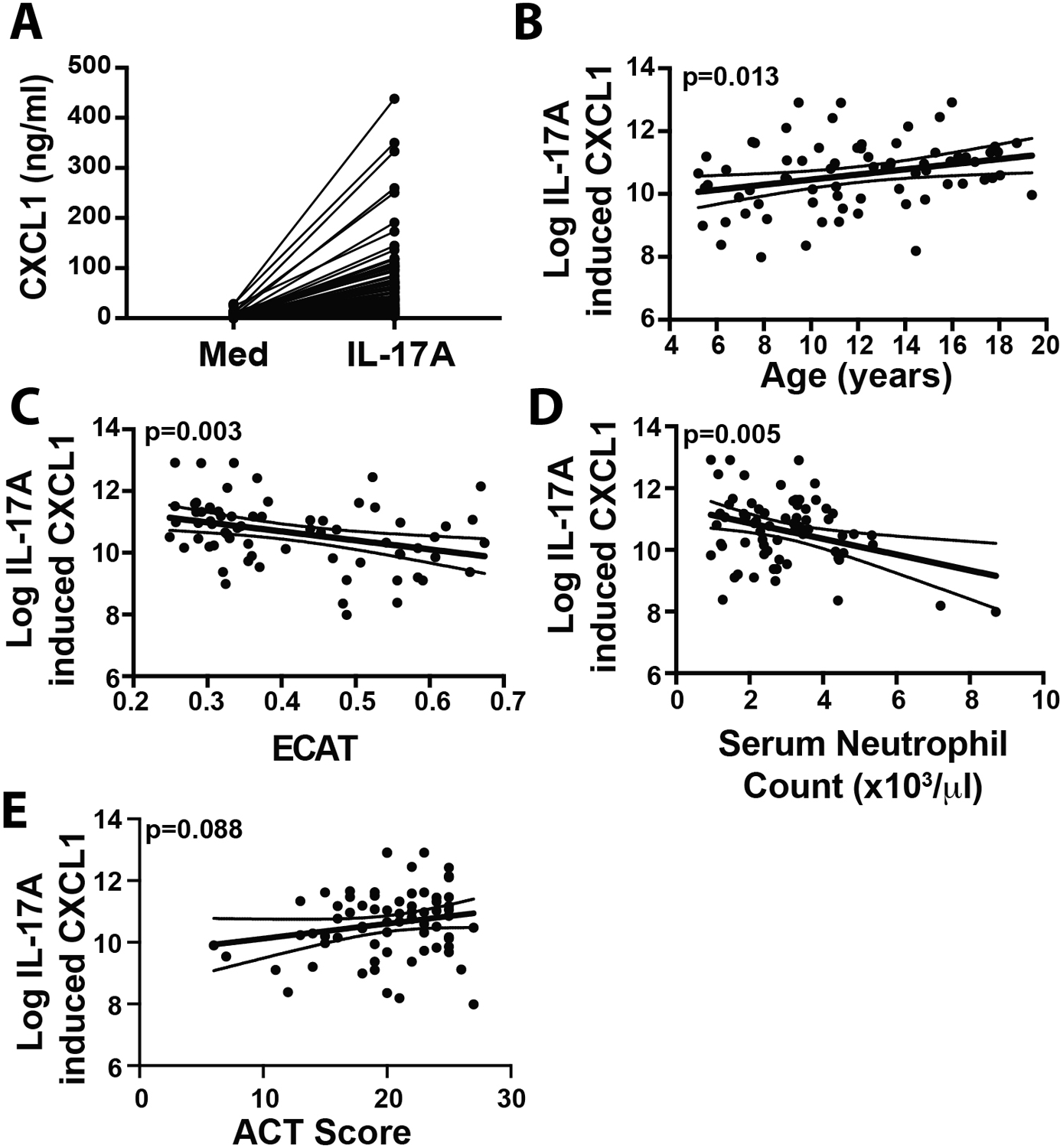
IL-17A-induced epithelial cell CXCL1 production is associated with age, asthma control, pollution exposure and circulating neutrophil levels in a pediatric asthma population. Nasal epithelial cells from children with asthma were stimulated with IL-17A, and CXCL1 production was assessed by ELISA (A). Strong unadjusted associations between Log IL-17A-induced CXCL1 production and age (B), asthma control (ACT Score) (C) estimated current exposure to Environmental Carbon Attributable to Traffic (ECAT) levels (D) and serum neutrophil count (X103/ul) (E) were observed. Solid line indicates fitted curve. Dotted lines indicate 95% confidence limits.
To determine if IL-17A-induced CXCL1 production by NECs varied with severity, children with mild (n=19), moderate (n=30) or severe asthma (n=21) were identified. One subject did not have the asthma severity defined but was included for analyses on factors other than asthma severity. Among the three severity groups, no differences were observed in age, sex, race, peripheral eosinophils or neutrophils, smoking or ECAT levels (information available in the following repository: https://doi.org/10.5281/zenodo.5706344). Total IgE levels were significantly higher in moderate (median: 222 IU/ml (IQR:77–645)) and severe (402 IU/ml (IQR:122–826)) asthmatics compared to those with mild disease (69.5 IU/ml; (IQR:39–160)) (p=0.004). ACT scores were significantly lower in individuals with severe asthma (median: 19.0 (IQR:16.0–21.0)) compared to those with moderate (20.5 (IQR:16.0–24.0)) or mild (23.0 (IQR:20.4–24.8)) asthma. % predicted FEV1 was also significantly lower in severe asthmatics (mean: 81.95 (SD:22.0)) compared to moderate (mean: 93.8 (SD:16.1)) and mild asthmatics (mean: 92.8 (SD:14.2)). IL-17A-induced CXCL1 production (CXCL1 concentration in IL-17A-stimulated cultures minus CXCL1 concentration in medium-stimulated cultures) was natural log transformed and used as the primary outcome variable in statistical analyses. Univariate linear regression analysis was performed to examine the association of IL-17A-induced CXCL1 production with each clinical parameter. Neither asthma severity (p=0.608) nor symptom severity (p=0.273) were significantly associated with IL-17A-induced CXCL1 production (Table 1). IL-17A-induced CXCL1 production was significantly associated with age (β=0.082; p=0.013, Fig 1B), current ECAT levels (β=−2.918; p=0.003, Fig 1C), serum neutrophil count (β=−0.255, p=0.005, Fig 1D), and demonstrated a trend towards association with ACT score (β=0.048, p=0.088, Fig 1E)(Table 1). No associations were observed with sex, race, total IgE, % predicted FEV1, blood eosinophils, symptom severity, SHS exposure, or pregnancy ECAT (Table 1). To adjust for potential confounders, a multivariate linear regression was performed with all factors except for the pregnancy ECAT which had much higher missing rate (information available in the following repository: https://doi.org/10.5281/zenodo.5706344). Age (p=0.004), current ECAT (p=0.005), ACT score (p=0.015) and blood neutrophil count (p=0.002) remained significantly associated with IL-17-induced CXCL1 production.
Table 1:
Unadjusted Associations with IL-17A Induced CXCL1 production in Primary Human Nasal Epithelial Cells‡
| N | β coefficient | SE | p-value | |
|---|---|---|---|---|
| Age (years) | 71 | 0.082 | 0.032 | 0.013 |
| Sex | 71 | 0.385 | 0.284 | 0.177 |
| Race¶ | 71 | 0.165 | 0.283 | 0.559 |
| Total IgE | 68 | 0.0001 | 0.0001 | 0.580 |
| % Predicted FEV1 | 71 | 0.007 | 0.007 | 0.341 |
| Blood Eosinophils (%) | 68 | 0.026 | 0.038 | 0.502 |
| ACT Score | 71 | 0.048 | 0.028 | 0.088 |
| Current ECAT§ | 68 | −2.918 | 0.966 | 0.003 |
| Pregnancy ECAT‡‡ | 53 | 0.231 | 0.869 | 0.791 |
| Early Maternal Smoking¶¶ | 70 | −0.188 | 0.275 | 0.494 |
| Current SHS§§ | 71 | −0.452 | 0.274 | 0.103 |
| Primary Smoker | 71 | 0.633 | 0.402 | 0.118 |
| Blood Neutrophil Count (×103/μl) | 68 | −0.255 | 0.088 | 0.005 |
| Symptom Severity‡‡‡ | 71 | 0.273 | ||
| Severe vs Mild | 0.098 | 0.323 | ||
| Moderate vs Mild | 0.496 | 0.342 | ||
| Asthma Severity¶¶¶ | 70 | 0.608 | ||
| Severe vs Mild | 0.235 | 0.343 | ||
| Moderate vs Mild | −0.068 | 0.317 |
IL-17A-induced CXCL1 production was defined as concentration of CXCL1 in IL-17A-stimulated cultures minus concentration of CXCL1 in media control cultures. This was Log transformed to better approximate a normal distribution. The association between IL-17A-induced CXCL1 production and indicated variables was tested using linear regression. Number of measurements available (N), regression coefficient (β coefficient), Standard Error (SE) and p value are indicated.
Race was defined as black vs. non-black (self-reported).
Current ECAT exposure was determined using the geocode of the children’s current address.
Pregnancy elemental carbon attributable to traffic (ECAT) exposure was determined using the geocode of home address during pregnancy.
Early maternal smoking was defined as maternal smoking during pregnancy, or during the first 5 years of life.
Current SHS was defined as presence of mother, father or other individuals living in the same household described as anything other than a nonsmoker.
Symptom score severity is based on the frequency of respiratory symptoms queried (cough, wheeze, shortness of breath and chest tightness) over the past 4 weeks: Mild (never), Moderate (1–2 days/week); Severe (>2 days/week)
Asthma severity was determined by a physician using published criteria. % predicted of forced expiratory volume in 1 second (FEV1) was also independently evaluated.
Subsequently, we examined associations with IL-17A-induced CXCL1 production included in our model adjusting for asthma severity, and parameters demonstrating significant (p-value <0.05) (age, ACT score, current ECAT, and serum neutrophil count). In this reduced model, age, ACT score, current ECAT, and serum neutrophil count remained significantly associated with IL-17A-induced CXCL1 production (data not shown), while asthma severity showed marginal association (p=0.105). However due to significant collinearity between asthma severity and ACT score (p=0.002), we opted to finalize the model with only age, ACT score, current ECAT and serum neutrophil count. In the final model, effects on IL-17A-induced CXCL1 production remained unchanged for age (β=0.093; p=0.001), ACT score (β=0.054; p=0.026), current ECAT (β=−2.760, p=0.001), and serum neutrophil count (β=−0.273, p<0.001). Age, ACT score, current ECAT, and serum neutrophil count explained the total variance in IL-17A-induced CXCL1 production by 11%, 5%, 11%, and 12% respectively. No co-linearity between these four factors was observed (all Variance Inflation Factors (VIF)=1.0, Pearson correlation coefficient ranges 0.04–0.16), suggesting that increased responsiveness to IL-17A is independently associated with each of these variables.
To our knowledge, this represents the first attempt to determine if nasal epithelial cell responsiveness to IL-17A is associated with asthma-relevant clinical parameters, or asthma severity. Andersson et al. did examine neutrophil recruitment and IL-17RA expression in the bronchial epithelium in a pediatric cohort with severe treatment refractory asthma (STRA)6, reporting variable intraepithelial neutrophil accumulation in the airways of children with STRA. Importantly, those with evidence of increased subepithelial cell neutrophil recruitment had less severe asthma, displaying higher % predicted FEV1, good asthma control (as indicated by higher ACT score), and required lower corticosteroid doses to maintain asthma control. Our study supports these observations, demonstrating a significant association between IL-17A-induced epithelial cell production of CXCL1, a potent neutrophil chemoattractant, and good asthma control (higher ACT scores). Further, our observation of an inverse correlation between capacity for IL-17A-induced CXCL1 production by NECs, and blood neutrophil count is consistent with the hypothesis that high local (tissue) production of CXCL1 is driving robust neutrophil recruitment out of the blood and into the tissues. Finally, we observed an inverse association between IL-17A-induced epithelial cell production of CXCL1 and ECAT exposure, an exposure associated with severe asthma in our cohort7. Collectively, these results suggest a potentially asthma-protective role for IL-17A-induced neutrophil accumulation in the lungs of children with asthma.
Andersson et al. also demonstrated that IL-17A-induced CXCL1 production by bronchial epithelial cells from children with STRA was significantly elevated compared to bronchial epithelial cells from non-asthmatic donors6. Our study was specifically designed to test the hypothesis that IL-17A responsiveness would vary in children with mild, moderate, or severe allergic asthma and thus a non-asthmatic control group was not included. However, our results demonstrate that increased IL-17A-induced CXCL1 production is comparable in children with different asthma severities, suggesting that increased epithelial cell responsiveness to IL-17A may be a general marker of asthma pathogenesis and not be a marker of severe asthma as proposed by Andersson et al. Although an alternative interpretation - that nasal epithelial cells do not phenocopy lower airway epithelial cells - cannot be completely ruled out, we arrived at a similar conclusion regarding a protective role for IL-17A in pediatric asthma as Andersson et al. using our methodology. To us, this suggests that nasal sampling does provide a reasonable proxy for more invasive bronchial brushings, or biopsies, as proposed elsewhere8.
In contrast to the protective role of IL-17A suggested by the results of this study, we9, and others10 have published data demonstrating a pathogenic effect of IL-17A in allergic asthma. We ascribe this to our previously described IL-17A-mediated enhancement of IL-13-induced gene expression9. Importantly, studies linking IL-17A with more severe asthma, and increased recruitment of neutrophils in the airways of severe asthmatics were carried out in adults whereas studies suggesting a protective role for IL-17A were performed in children6. The reason for a potential shift in the pathogenic versus protective role of IL-17A in asthma pathogenesis in children and adults is unclear. However, it is interesting to note that in our previous study in the same study population, we noted an inverse association between age and IL-13 responsiveness9 which contrasts with the positive association between age and IL-17A responsiveness observed here. It is possible that the balance of IL-13 and IL-17A receptor-derived signaling, and variations in this balance at different ages impacts the influence of IL-17A on asthma outcomes. Nonetheless, the dichotomous role of IL-17A as a potentially pathogenic or protective cytokine in asthma is intriguing. More work is needed to elucidate the pathogenic versus protective functions of IL-17A in allergic asthma in various patients and stages of disease.
There are a few caveats to our study which must also be considered. As our study was focused on identifying factors that contribute to overall asthma severity, it did not include a non-asthmatic control group, and we recognize that our cohort is predominantly urban, African-American, with low socioeconomic status, and high relative levels of ECAT exposure. Although children with asthma often suffer from other allergic diseases, (atopic dermatitis, allergic rhinitis), given the association between IL-17A responsiveness and asthma specific influences (pollution exposure, ACT score), but not markers of a general atopic state (total IgE, blood eosinophilia) it is more likely that IL-17A responsiveness is associated with asthma rather than the general atopic disease. Additionally, the observed marginal association between IL-17A responsiveness and asthma severity suggests that IL-17A responsiveness may contribute to altered asthma severity in addition to other factors, especially ACT score. Although multiple parameters were considered in assigning severity (lung function, medication usage, exacerbation history, symptomology, clinical measures), according to a published framework, it is unclear how stable this classification is over time, and whether this observation would be observed if samples from these same participants were collected at different times. Importantly, as our participants with moderate and severe disease still displayed robust IgE responses, this suggests that these participants were not “T2 low” asthmatics, and that any influences of IL-17A responsiveness on asthma severity also occur in individuals with robust T2-associated responses. Nonetheless, the current study suggests that IL-17A plays a complex role in regulating the development of allergic asthma. Future studies to examine the link between IL-17A production (and responsiveness) in asthmatics across the spectrum of age, racial distribution, pollutant exposure levels, and severity are warranted.
Key Messages:
IL-17A-induced CXCL1 is associated with good asthma control, low peripheral neutrophils, and low pollution exposure
These associations suggest that high epithelial cell IL-17A-responsiveness may be protective in children with asthma
These studies suggest that IL-17A may differentially impact asthma pathogenesis in adults and children
Funding:
This study was funded by NHLBI R01 HL122300 (IPL) and NIEHS R21 ES016830 (MBK).
Footnotes
Conflict of Interest:
AL is currently employed by Amgen, in a role unrelated to the submitted work. The other authors report no conflicts of interest.
Ethical Approval Statement
The research was conducted ethically following the World Medical Association Declaration of Helsinki. All subject provided their informed consent/assent and the study was approved the Cincinnati Children’s Hospital Medical Center Institutional Review Board (IRB #2008–0711).
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
- 1.Chesne J, Braza F, Mahay G, Brouard S, Aronica M, Magnan A. IL-17 in severe asthma. Where do we stand? Am J Respir Crit Care Med. 2014;190(10):1094–1101. [DOI] [PubMed] [Google Scholar]
- 2.Lajoie S, Lewkowich IP, Suzuki Y, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nat Immunol. 2010;11(10):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McAlees JW, Baker T, Kaur D, et al. Age and early maternal smoking contribute to epithelial cell IL-13 responsiveness in a pediatric asthma population. Allergy. 2019;74(12):2485–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–138. [DOI] [PubMed] [Google Scholar]
- 5.Ulm A, Mayhew CN, Debley J, Khurana Hershey GK, Ji H. Cultivate Primary Nasal Epithelial Cells from Children and Reprogram into Induced Pluripotent Stem Cells. J Vis Exp. 2016(109). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson CK, Adams A, Nagakumar P, et al. Intraepithelial neutrophils in pediatric severe asthma are associated with better lung function. J Allergy Clin Immunol. 2017;139(6):1819–1829 e1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandt EB, Kovacic MB, Lee GB, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132(5):1194–1204 e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDougall CM, Blaylock MG, Douglas JG, Brooker RJ, Helms PJ, Walsh GM. Nasal epithelial cells as surrogates for bronchial epithelial cells in airway inflammation studies. Am J Respir Cell Mol Biol. 2008;39(5):560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall SL, Baker T, Lajoie S, et al. IL-17A enhances IL-13 activity by enhancing IL-13-induced signal transducer and activator of transcription 6 activation. J Allergy Clin Immunol. 2017;139(2):462–471 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irvin C, Zafar I, Good J, et al. Increased frequency of dual-positive TH2/TH17 cells in bronchoalveolar lavage fluid characterizes a population of patients with severe asthma. J Allergy Clin Immunol. 2014;134(5):1175–1186 e1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


