Abstract
Aberrant gait biomechanics following anterior cruciate ligament reconstruction (ACLR) likely contribute to post-traumatic osteoarthritis (PTOA) development. Gait biomechanics are typically assessed overground, but the use of instrumented/force-measuring treadmills is increasingly common. The purpose of this study was to compare gait biomechanics overground and on an instrumented treadmill in individuals with ACLR and healthy controls. Twenty-four individuals with ACLR and 24 healthy controls completed overground and gait biomechanics assessments. Biomechanical outcomes included peak vertical ground reaction force (vGRF), internal knee extension (KEM) and abduction (KAM) moments, and knee flexion (KFA) and adduction angles; KFA at heel strike; knee flexion displacement; and inter-limb symmetry for each outcome. Peak KEM (P < 0.001, 95%CI [−0.016, −0.007 xBW*Ht]) and vGRF (P < 0.001, 95%CI [−0.09. −0.03 xBW]) were significantly less symmetrical in the ACLR group compared to the control group on the treadmill but not overground. Additionally, peak KEM was smaller in the ACLR limb compared to the contralateral limb both overground (P = 0.005, 95%CI [−0.010, −0.001 xBW*Ht]) and on the treadmill (P < 0.001, 95%CI [−0.015, −0.007 xBW*Ht]), but this difference was 1.8x larger on the treadmill compared to overground. Peak KFA (P = 0.001, 95%CI [−4.2, −1.2°]) and vGRF (P < 0.001, 95%CI [−0.07, −0.03 xBW]) were smaller in the ACLR limb on the treadmill but not overground. These findings suggest aberrant gait biomechanics are exacerbated during treadmill walking post-ACLR and that evaluating kinematics and kinetics on instrumented treadmills may be valuable for assessing risk factors of PTOA development.
Keywords: Biomechanics, Gait, Treadmill, PTOA
Introduction
Anterior cruciate ligament (ACL) injury occurs in roughly 250,000 individuals in the United States annually (Griffin et al., 2006). Though most patients undergo surgical reconstruction (ACLR) (Sanders et al., 2016), this approach does not mitigate post-traumatic osteoarthritis (PTOA) risk (Harris et al., 2017; Luc et al., 2014). While PTOA development is likely multifactorial, altered gait biomechanics are considered driving factors in PTOA pathogenesis (Khandha et al., 2017; Wellsandt et al., 2016).
Gait biomechanics are typically evaluated during level, overground walking. Under these conditions, individuals with ACLR display smaller sagittal plane knee moments and angles compared to controls and the contralateral limb (Hart et al., 2016; Kaur et al., 2016; Slater et al., 2017). Ambiguity exists regarding the frontal plane knee moment, as individuals with ACLR reportedly display smaller (Patterson et al., 2014; Slater et al., 2017), larger (Butler et al., 2009), or equivalent (Hall et al., 2012; Varma et al., 2014) values compared to controls. Additionally, smaller vertical ground reaction forces (vGRF) have been reported in the ACLR limb in symptomatic patients compared to those who are asymptomatic (Pietrosimone et al., 2018). Furthermore, aberrant overground gait biomechanics during weight acceptance (e.g. smaller vGRF and sagittal plane moments and angles, and greater inter-limb loading asymmetry) have been associated with PTOA development and poor knee joint health following ACLR (Khandha et al., 2017; Pfeiffer et al., 2019; Pietrosimone et al., 2017, 2016; Wellsandt et al., 2016).
Instrumented/force-measuring treadmills have been increasingly used to evaluate gait biomechanics (Alton et al., 1998; Christensen et al., 2018; Dewig et al., 2021; Goetschius et al., 2018; Malatesta et al., 2017; Shabani et al., 2015) in lieu of overground assessments. Instrumented treadmills have several advantages including requirement of a smaller capture space, objective control of gait speed, and continuous data collection during ambulation. Conversely, overground assessments more closely resemble real-world ambulation. While treadmill gait differs from overground gait in healthy individuals (Alton et al., 1998; Lee and Hidler, 2008), we are unaware of studies comparing overground and treadmill gait biomechanics in individuals with ACLR. Of particular importance, the sagittal plane knee moment, an outcome linked to PTOA pathogenesis, is smaller during treadmill walking compared to overground (Lee and Hidler, 2008). As such, treadmill walking might mask aberrant gait biomechanics in individuals with ACLR, potentially misinforming clinical intervention and PTOA risk. Therefore, the purpose of this study was to compare overground and treadmill gait biomechanics between individuals with and without ACLR. We hypothesized that differences in gait biomechanics (individual limbs and inter-limb symmetry) between individuals with ACLR and healthy controls would be larger overground compared to treadmill gait. Similarly, we hypothesized that differences between the involved and uninvolved limbs in the ACLR cohort would be larger overground compared to the treadmill.
Methods
Experimental Design & Subjects
A priori power analysis indicated that 7-17 subjects were necessary to identify significant differences in the sagittal plane knee moment and angle during overground walking between those with and without ACLR (power = 0.80, α = 0.05, Cohen’s d = 1.00 – 1.77) using a cross-sectional case-control design (Lewek et al., 2002). We enrolled 24 individuals with a history of ACLR and 24 uninjured controls to ensure adequate power and account for the addition of a second gait condition (treadmill). Individuals with ACLR were required to have 1) undergone primary, unilateral ACLR 6-12 months prior to testing, 2) no history of other ACL injury, 3) been cleared for unrestricted physical activity, and 4) not been diagnosed with knee arthritis or fracture at the time of ACL injury. Control subjects were required to have no previous history of lower extremity surgery, and all subjects were between the ages of 18-35 years, had no lower extremity injury within the past 6 months or neurological disorder, and were not currently pregnant. Subject demographics are provided in Table 1. The study was approved by the university’s Institutional Review Board and all subjects provided written informed consent.
Table 1:
Subject Demographics
| ACLR (n = 24) | CONT (n = 24) | P value | |
|---|---|---|---|
| Age (yrs) | 20.8 ± 3.5 | 21.8 ± 3.8 | 0.79 |
| Height (m) | 1.71 ± 0.09 | 1.72 ± 0.08 | 0.66 |
| Mass (kg) | 73.3 ± 14.7 | 69.7 ± 13.6 | 0.38 |
| Body Mass Index (kg/m2) | 25.0 ± 3.4 | 23.6 ± 4.7 | 0.30 |
| Gait speed (m/s) | 1.27 ± 0.10 | 1.22 ± 0.14 | 0.05* |
| Post-Op (weeks) | 36 ± 6 | N/A | |
| Sex | 11 M, 13 W | 7 M, 17 W |
Gait Biomechanics Assessment
Subjects completed five overground walking trials through infrared timing gates to determine self-selected speed. To create a common segment-linkage model for the treadmill and overground conditions, 29 retroreflective markers were placed bilaterally on the heads of the 1st and 5th metatarsals, lateral malleoli, medial malleoli, calcanei, tibial crests, medial femoral epicondyles, lateral femoral epicondyles, mid thighs, anterior superior iliac spines, greater trochanters, posterior superior iliac spines, and acromion processes. Individual markers were placed at the L4/L5 interspace, coccyx and sternum (Blackburn et al., 2020; Pfeiffer et al., 2018). A static calibration trial was completed prior to each condition to estimate joint centers and align the laboratory and segment coordinate systems with the subject facing forward, feet shoulder width apart, and arms abducted 90°. Hip joint centers were calculated via the Bell method (Bell et al., 1989), while knee and ankle joint centers were estimated as the midpoint between the medial and lateral femoral epicondyles and malleoli, respectively. Markers on the medial femoral epicondyles and malleoli were then removed to permit unrestricted gait. Subjects wore their own athletic shoes and first completed the overground condition which was immediately followed by the treadmill condition.
Subjects completed five overground trials over force plates (Bertec Corp, Columbus, OH) embedded in a 6m walkway. Three-dimensional marker trajectories were sampled at 120 Hz using a 10-camera motion capture system (Vicon Motion System, Oxford, UK) while force plate data were sampled at 1,200 Hz. Subjects took at least 3 steps prior to striking the first force plate and at least 2 steps following contact with the second force plate. Trials were successful when contact was made with each foot independently on a separate force plate, gait speed was ±5% of the preferred speed, and there were no visible alterations (e.g. stutter-step).
Subjects then walked for 5 minutes on a split-belt instrumented treadmill (Bertec Corp, Columbus, OH). The first 3.5 minutes were an acclimation period (Garcia et al., 2021; Richards et al., 2018) while data were captured during the final 90s. Participants walked at the preferred overground speed while three-dimensional marker trajectories were sampled at 120 Hz using an 8-camera motion capture system (Qualisys Miqus, Göteborg, Sweden) and force plate data were sampled at 1,200 Hz.
We calculated the linear and angular orientations between five stationary markers spaced fixed distances relative to each other with both motion capture systems to quantify between-system measurement discrepancies. Errors in linear distances ranged 2-4mm and angular error was ~ 0.7°.
Data Processing
All data was processed using Visual3D (C-Motion Inc., Germantown, MD) utilizing identical Link Model Based computations. The stance phase was defined as the interval from heelstrike (vGRF > 20 N) to toeoff (vGRF < 20 N). Kinetic and kinematic data were lowpass filtered at 10 Hz (4th order Butterworth). Euler angles were calculated as motion of the shank relative to the thigh utilizing a sagittal-frontal-transverse sequence. Standard inverse dynamics procedures were employed to combine kinetic, kinematic, and anthropometric data to derive net internal joint moments. Ground reaction forces were normalized to body weight (xBW), while joint moments were normalized to the product of body weight and height (xBW*Ht).
Gait variables were assessed during the first 50% of the stance phase (Khandha et al., 2017; Pietrosimone et al., 2017, 2016) and included the peak vGRF, peak internal knee extension (KEM) and abduction (KAM) moments, peak knee flexion (KFA) and adduction angles, knee flexion angle at heel strike, and knee flexion displacement (i.e. peak – heel strike). Peak KEM and KAM represented negative values as defined by our angular conventions, but were expressed as positive values for ease of interpretation (i.e. larger positive values represent larger biomechanical phenomena). Outcomes were averaged over the five overground trials and the first five steps following the 3.5 minute acclimation period on the treadmill.
In the ACLR group, the involved limb was defined as the ACLR limb. In the control group, the involved limb was determined via a random number generator to produce the same proportion of left and right limbs as the ACLR limbs. Inter-limb symmetry was calculated for all outcomes by subtracting the contralateral limb from the involved limb (involved – contralateral), with 0 representing perfect symmetry and positive values indicating larger values in the involved limb (Khandha et al., 2017).
Statistical Analyses
Statistical significance was established a priori as P ≤ 0.05. All data were screened for normality via the Shapiro-Wilk test and visual inspection of histograms. Gait biomechanics in the involved limb were compared across groups and conditions by separate 2x2 repeated-measures ANCOVA controlling for gait speed. Similarly, inter-limb symmetry was compared across groups and conditions utilizing separate 2x2 repeated-measures ANCOVA controlling for gait speed. Gait biomechanics were also compared between limbs and conditions in the ACLR group via separate 2x2 repeated-measures ANOVA. Significant models were evaluated post hoc via paired-samples t-tests to compare overground vs. treadmill within each group or limb, and between limbs for each condition for the ACLR-specific models. Additional post hoc analyses comparing biomechanical outcomes between groups for each condition were conducted using one-way ANCOVA controlling for gait speed. All post hoc tests were evaluated via Bonferroni-adjusted alpha levels (0.05/4 = 0.0125) and reported with the 95% confidence interval of the difference (95%CI) calculated as overground-treadmill, ACLR-control, and ACLR-contralateral, respectively.
Results
Between-group Comparisons
There were no group main effects for any outcome (P = 0.187 – 0.959; Table 2). However, there was a significant group x condition interaction effect for KEM (P = 0.013). Post hoc evaluation indicated a significantly larger KEM overground compared to the treadmill for both the ACLR (P < 0.001, 95%CI [0.005, 0.011 xBW*Ht]) and control (P = 0.006, 95%CI [0.001, 0.007 xBW*Ht]) groups, but no differences between groups for the overground (P = 0.738, 95%CI [−0.007, 0.005 xBW*Ht]) or treadmill (P = 0.066, 95%CI [−0.012, 0.000 xBW*Ht]) conditions. There were no other significant group x condition interaction effects (peak KAM [P = 0.953], peak KFA [P = 0.558], KFA at heel strike [P = 0.690], peak knee adduction angle [P = 0.574], knee flexion displacement [P = 0.877], vGRF [P = 0.704]).
Table 2:
Between-group Comparisons: Statistical Results (P-value)
| Condition Effect | Group Effect | Condition*Group Effect | |
|---|---|---|---|
| Peak KEM | 0.021* | 0.256 | 0.013* |
| Peak KAM | 0.995 | 0.378 | 0.953 |
| Peak KFA | 0.066 | 0.656 | 0.558 |
| Knee Flexion Displacement | 0.012* | 0.959 | 0.877 |
| Knee Flexion Angle at Heelstrike | 0.450 | 0.488 | 0.690 |
| Peak Knee Adduction Angle | 0.226 | 0.561 | 0.574 |
| Peak vGRF | 0.051 | 0.187 | 0.704 |
Indicates significant result (P < 0.05)
There were significant condition main effects (Table 2), with KEM being larger overground compared to the treadmill (P = 0.021, 95%CI [0.004, 0.008 xBW*Ht]) and knee flexion displacement being smaller overground compared to the treadmill (P = 0.012, 95%CI [−3.0, −1.7°]). There were no other condition effects (peak KAM [P = 0.995], peak KFA [P = 0.066], KFA at heel strike [P = 0.450], peak knee adduction angle [P = 0.226], vGRF [P = 0.051]). Group means and standard deviations are listed in Table 3. Respective waveforms time-normalized to 101 data points are provided in Figure 4 A-E, with internal moments expressed in the original angular conventions.
Table 3:
Discrete Variables for ACLR and Control groups (Mean ± SD)
| ACLR Limb | Contralateral Limb | CONT | |
|---|---|---|---|
| Overground | |||
| KEM (xBW*Ht) | 0.021 ± 0.01 | 0.027 ± 0.01 | 0.021 ± 0.1 |
| KAM (xBW*Ht) | 0.024 ± 0.01 | 0.026 ± 0.01 | 0.025 ± 0.01 |
| Peak KFA (°) | 8.5 ± 4.7 | 9.95 ± 4.7 | 8.7 ± 5.5 |
| Knee flexion displacement (°) | 12.9 ± 3.4 | 15.0 ± 3.4 | 13.0 ± 3.8 |
| Knee Flexion Angle at Heelstrike (°) | −4.4 ± 3.4 | −5.1 ± 3.5 | −3.9 ± 3.7 |
| Knee Adduction Angle (°) | 0.3 ± 3.1 | 0.5 ± 3.7 | 0.3 ± 3.2 |
| vGRF (xBW) | 1.08 ± 0.06 | 1.10 ± 0.06 | 1.09 ± 0.06 |
| Treadmill | |||
| KEM (xBW*Ht) | 0.013 ± 0.01 | 0.024 ± 0.01 | 0.017 ± 0.01 |
| KAM (xBW*Ht) | 0.024 ± 0.01 | 0.026 ± 0.01 | 0.024 ± 0.01 |
| Peak KFA (°) | 9.7 ± 5.0 | 12.4 ± 4.9 | 10.0 ± 5.3 |
| Knee Flexion Displacement (°) | 15.5 ± 3.0 | 17.9 ± 3.5 | 15.1 ± 3.1 |
| Knee Flexion Angle at Heelstrike (°) | −5.8 ± 4.0 | −5.3 ± 3.3 | −5.1 ± 3.8 |
| Knee Adduction Angle (°) | −0.1 ± 3.1 | 1.0 ± 2.5 | 0.3 ± 3.2 |
| vGRF (xBW) | 1.08 ± 0.06 | 1.13 ± 0.07 | 1.09 ± 0.05 |
Figure 4A.
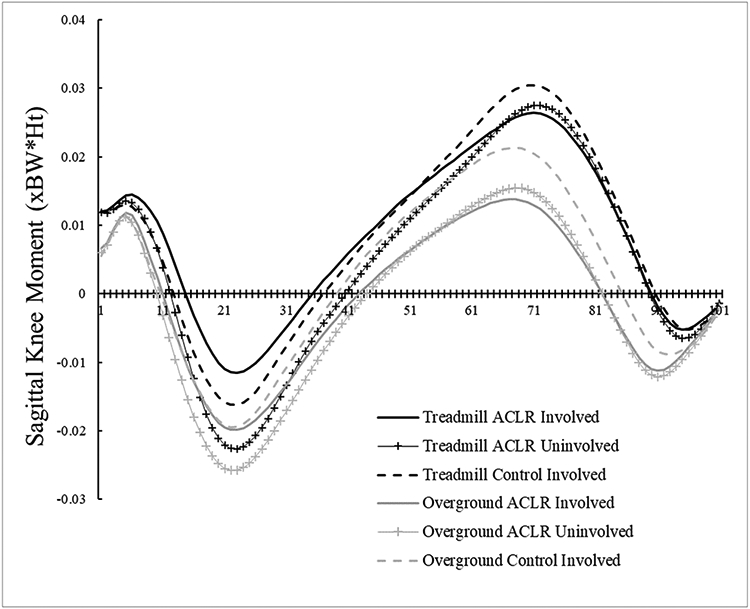
Internal sagittal plane knee moment time-normalized waveforms. Negative values indicate internal extension moments, while positive values indicate internal flexion moments.
Figure 4E.
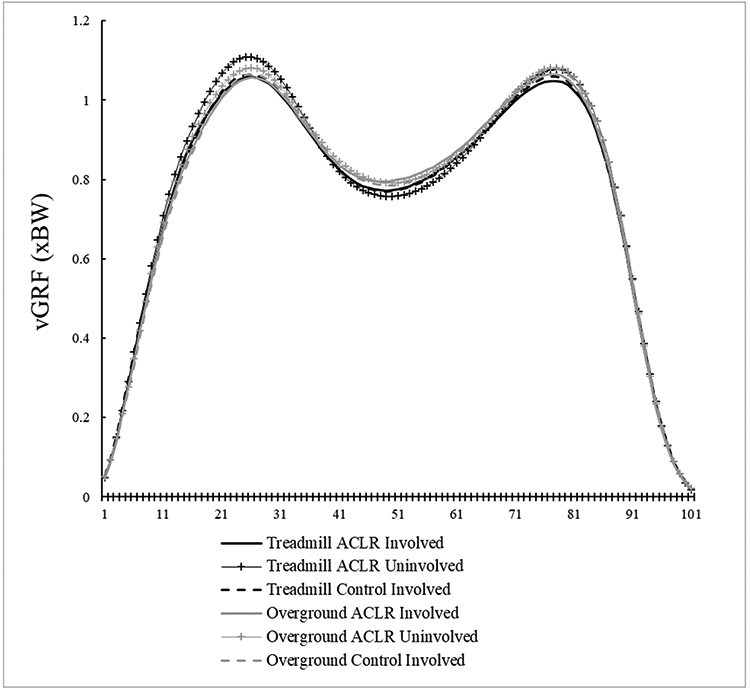
Vertical Ground Reaction Force (vGRF) time-normalized waveforms.
Between-limb Comparisons (ACLR cohort only)
There were significant limb x condition interaction effects for peak KEM (P = 0.005; Figure 1), peak KFA (P = 0.024; Figure 2), and peak vGRF (P = 0.005; Figure 3). Post hoc analyses indicated smaller KEM in the ACLR limb compared to the contralateral limb in both the overground (P = 0.005, 95%CI [−0.010, −0.001 xBW*Ht]) and treadmill (P < 0.001, 95%CI [−0.015, −0.007 xBW*Ht]) conditions. Additionally, KEM was significantly larger overground compared to the treadmill in the ACLR limb (P < 0.001, 95%CI [0.005. 0.011 xBW*Ht]) but was not different between conditions for the contralateral limb (P = 0.150, 95%CI [−0.001, 0.007 xBW*Ht]). KFA was significantly smaller in the ACLR limb compared to the contralateral limb in the treadmill condition (P = 0.001, 95%CI [−4.2, −1.2°]), but not overground (P = 0.101, 95%CI [−3.1, 0.3°]). Smaller KFAs were observed in overground condition compared to the treadmill in both the ACLR (P = 0.012, 95%CI [−2.0. −0.3°]) and contralateral limbs (P = 0.001, 95%CI [−3.7, −1.2°]). Post hoc analyses also indicated smaller vGRF in the ACLR limb compared to the contralateral limb during the treadmill condition (P < 0.001, 95%CI [−0.07, −0.03 xBW]), but not overground (P = 0.016 [Bonferroni-adjusted critical α = 0.0125], 95%CI [−0.04, 0.00 xBW]). Finally, vGRF was smaller in the contralateral limb overground compared to the treadmill (P = 0.002, 95%CI [−0.05, −0.01 xBW]), but there was no difference between conditions in the ACLR limb (P = 0.798, 95%CI [−0.02, 0.01 xBW]). There were no other significant interaction effects (peak KAM [P = 0.808], knee flexion angle at heelstrike [P = 0.056], peak knee adduction angle [P = 0.347], knee flexion displacement [P = 0.604]).
Figure 1.
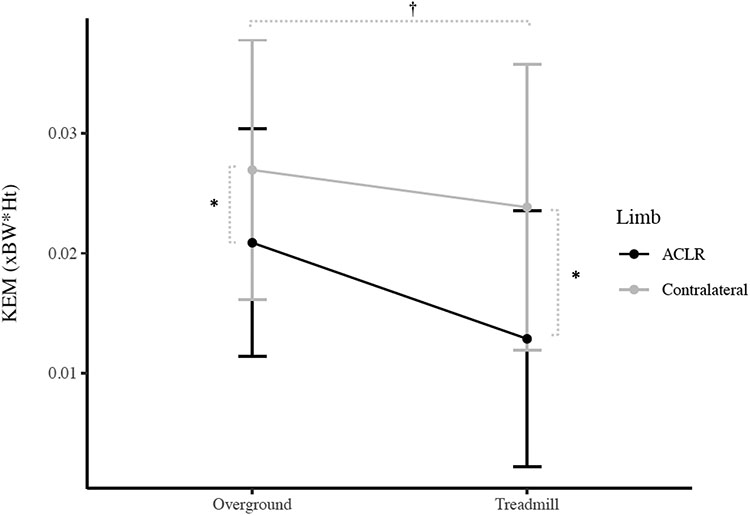
Peak internal knee extension moment (KEM) Condition x Limb Interaction (Mean ± 1 SD)
* Indicates significant difference between the ACLR limb and contralateral limb
† Indicates significant difference between the overground and treadmill condition in the ACLR limb
Figure 2.
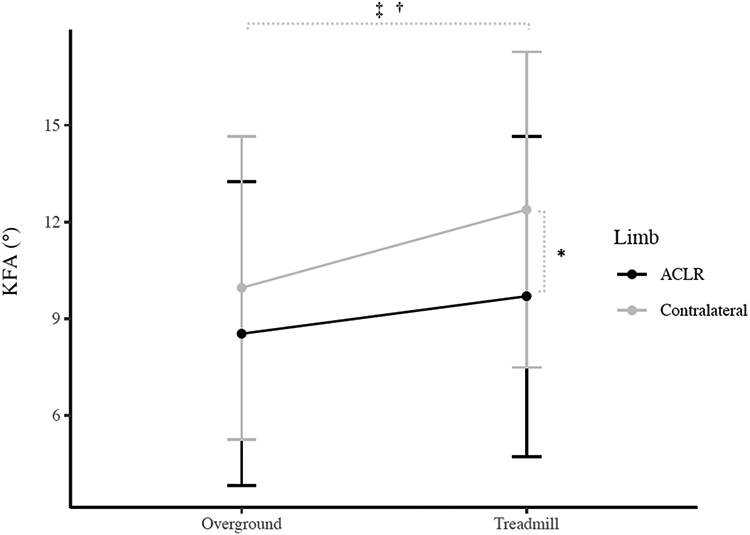
Peak knee flexion angle (KFA) Condition x Limb Interaction (Mean ± 1 SD)
* Indicates significant difference between the ACLR limb and contralateral limb
† Indicates significant difference between the overground and treadmill condition in the ACLR limb
‡ Indicates significant difference between the overground and treadmill condition in the contralateral limb
Figure 3.
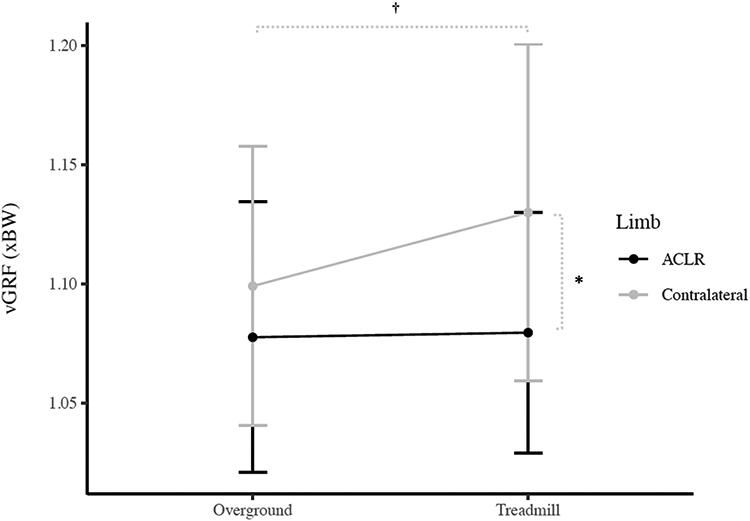
Peak vertical ground reaction force (vGRF) Condition x Limb Interaction (Mean ± 1 SD)
* Indicates significant difference between the ACLR limb and contralateral limb
† Indicates significant difference between the overground and treadmill condition in the contralateral limb
Condition main effects were identified with peak KEM being larger overground (P = 0.001) while peak KFA (P = 0.001), KFA at heel strike (P = 0.012), knee flexion displacement (P < 0.001), and peak vGRF (P = 0.022) were smaller overground. There were no condition main effects for peak KAM (P = 0.278) or peak knee adduction angle (P = 0.949). Significant limb main effects were also observed, with peak KEM (P < 0.001), KFA (P = 0.011), knee flexion displacement (P = 0.001), and peak vGRF (P < 0.001) all being smaller in the ACLR limb. There were no limb main effects for peak KAM (P = 0.283), KFA at heelstrike (P = 0.812), or peak adduction angle (P = 0.299).
Inter-limb Symmetry Comparisons
There were significant group x condition interaction effects for peak KEM (P = 0.002) and peak vGRF (P = 0.018). Post hoc evaluation revealed significantly more symmetrical peak KEM (P = 0.005, 95%CI [0.002, 0.008 xBW*Ht]) and vGRF (P = 0.005, 95%CI [0.01, 0.05 xBW]) in the ACLR group overground compared to the treadmill, but no difference between conditions in the control group inter-limb symmetry for peak KEM (P = 0.178, 95%CI [−0.005, 0.001 xBW*Ht]) or vGRF (P = 0.404, 95%CI [−0.04, 0.02 xBW]). The peak KEM was significantly less symmetrical in the ACLR group than the control group during treadmill gait (P < 0.001, 95%CI [−0.016, −0.007 xBW*Ht]), but not overground (P = 0.058, 95%CI [−0.009, 0.000 xBW*Ht]). Similarly, peak vGRF was significantly less symmetrical in the ACLR group than the control group during the treadmill condition (P < 0.001, 95%CI [−0.09. −0.03 xBW]), but not overground (P = 0.093, 95%CI [−0.04, 0.003 xBW]). There were no other significant group x condition interaction effects (peak KAM [P = 0.519], peak KFA [P = 0.095], KFA at heelstrike [P = 0.250], peak knee adduction angle [P = 0.411], knee flexion displacement [P = 0.938]).
There were no condition main effects for the inter-limb symmetry values for any variable (peak KEM [P = 0.589], peak KAM [P = 0.673], peak KFA [P = 0.761], KFA at heelstrike [P = 0.733], knee flexion displacement [P = 0.353], peak knee adduction angle [P = 0.051], peak vGRF [P = 0.882]). There were, however, significant group main effects with peak KEM (P < 0.001), peak knee flexion angle (P = 0.009), knee flexion displacement (P = 0.003), and peak vGRF (P < 0.001) all being less symmetrical in the ACLR group. No group effects existed for peak KAM inter-limb symmetry (P = 0.698), KFA at heelstrike (P = 0.529), or peak adduction angle (P = 0.736).
Discussion
Differences in gait biomechanics between the overground and treadmill conditions were generally consistent with previous reports in healthy controls (Alton et al., 1998; Lee and Hidler, 2008). Lee and Hilder (2008) reported larger sagittal plane joint moments in healthy individuals overground compared to treadmill walking but no difference in frontal plane moments. Similarly, we observed larger KEM overground in both groups combined (i.e. condition main effect), but no effects were observed for KAM. We also observed greater knee flexion displacement in both groups combined and a trend for a greater peak KFA on the treadmill in both groups combined (P = 0.066), similar to the findings of Alton et al. (1998) in healthy males. The absence of differences in peak vGRF between the overground and treadmill in the groups combined is similar to the findings of Lee and Hilder (2008) in healthy individuals. As sagittal plane moments are typically smaller following ACLR and have been associated with PTOA development (Dewig et al., 2021; Goetschius et al., 2018; Hart et al., 2010; Khandha et al., 2017; Slater et al., 2017), it is imperative to recognize that differing gait assessment conditions may elucidate dissimilar results.
Contrary to our hypothesis, differences in gait biomechanics between the ACLR group and controls, and between limbs in the ACLR group, were generally greater on the treadmill compared to overground rather than being masked. Peak KEM was more asymmetrical in the ACLR group compared to controls on the treadmill, but this phenomenon was not present overground. Additionally, peak KEM in the ACLR group was more asymmetrical on the treadmill compared to overground, but did not differ between conditions in the control group. Similarly, peak KEM was smaller in the ACLR limb compared to the contralateral limb in both gait conditions, but this between-limb difference was 1.8x larger on the treadmill compared to overground. The peak vGRF was more asymmetrical in the ACLR group compared to controls on the treadmill, but this group difference was not present overground. Additionally, peak vGRF in the ACLR group was 2.4x more asymmetrical on the treadmill compared to overground, but did not differ between conditions in the control group. Furthermore, the between-limb difference in peak KFA in the ACLR group was 1.8x larger on the treadmill compared to overground.
These findings suggest treadmill walking may elucidate aberrant gait biomechanics patterns that are not observed overground. Additionally, the ACLR group demonstrated gait patterns consistent with PTOA development, irrespective of condition, but these patterns were exacerbated on the treadmill. Notably, participants in the ACLR group were cleared for full sport/exercise participation, but still demonstrated aberrant gait biomechanics.
It is possible that treadmill walking elucidates a variant gait pattern that is not employed overground and that individuals with ACLR may be unable to adequately adjust to these unique biomechanical demands. Moraiti et al. (2010) reported greater variability in the knee flexion-extension time series in the ACLR limb compared to healthy controls during treadmill walking. While not evaluated in this study, the presence of persistent quadriceps dysfunction has been associated with aberrant gait biomechanics such as smaller sagittal plane moments and angles following ACLR (Lewek et al., 2002). As those with ACLR demonstrated smaller KEM in their involved limb compared to the contralateral limb in both conditions, and smaller peak KFA vGRF in the treadmill condition, it is possible that altered quadriceps function precipitated these aberrant gait biomechanics. Overground gait requires quadriceps activity during the stance phase both for body support and propulsion as the center of mass translates over the stationary base of support. In contrast, treadmills effectively pull the stance limb posteriorly, thus minimizing the propulsive demands placed on the quadriceps. The reduced demands placed on the quadriceps (and the hip and ankle extensors) likely leads to off-loading of the ACLR limb as evidenced by smaller vGRF and KEM consistent with the “stiffened knee pattern” that has been associated with poor knee joint health following ACLR (Khandha et al., 2017; Pfeiffer et al., 2019; Wellsandt et al., 2016). Although knee flexion angles are generally larger during treadmill gait compared to overground, the ACLR limb demonstrates significantly smaller KFA compared to the contralateral limb during treadmill gait thus, differences in knee kinematics may also be exacerbated on the treadmill. Khandha et al. (2017) reported that the peak KFA was 5° smaller in individuals with ACLR who developed PTOA within 5 years compared to those who did not develop PTOA. Additionally, Di Stasi et al. (Di Stasi et al., 2013) reported a minimal clinically important difference (MCID) in peak KFA of 3° in healthy individuals. These data collectively suggest that the 2.7° difference in peak KFA that we observed between limbs in the ACLR group on the treadmill may be clinically meaningful. Additionally, the 0.011 xBW*Ht difference in KEM in the ACLR group between limbs during treadmill gait is larger than the MCID reported in Di Stasi et al (2013) (0.003 xBW*Ht). These findings also suggest that treadmill gait may be an effective approach for identifying aberrant gait biomechanics not observed via traditional overground assessments.
Limitations
This study should be evaluated in context of its limitations. Participants walked at their preferred overground speed for both gait conditions. However, preferred gait speed may differ on treadmills (Malatesta et al., 2017), thus potentially limiting generalizability. Moreover, all individuals in the ACLR group received patellar tendon autografts, potentially limiting application to patients with other graft types. However, previous research has demonstrated that differences do not exist during overground walking between individuals with patellar tendon and hamstring autografts (Johnston et al., 2019). We also did not match the control and ACLR groups with respect to sex. However, we repeated our analyses using sex as a covariate and found no changes to the group, condition, or group x condition effects for any outcome. While our use of two different motion capture systems for the gait conditions introduced systematic error, these linear and angular errors were on the order of 4mm and 0.7°, respectively, and were smaller than the observed statistical differences. Additionally, we only evaluated peak variables, thus future research should evaluate differences in time-series waveforms. For example, the knee adduction angle waveforms are visually dissimilar, but it should be noted that the total range of motion throughout the stance phase for all groups was small (~ 5 degrees), thus likely obscuring any potential discrete differences. Lastly, this study did not include ACLR patients during the early post-operative rehabilitative stages or more than 12 months post-ACLR, thus it is unclear if the findings are applicable to different post-ACLR intervals.
Conclusion
Aberrant gait biomechanics associated with PTOA development existed in the ACLR group compared to controls and contralateral limbs and were exacerbated in the treadmill condition. Thus, instrumented treadmills may further elicit aberrant gait biomechanics following ACLR. Future research is necessary to determine if similar results are obtained in individuals further removed from ACLR who have presumably resumed “normal” gait patterns.
Figure 4B.
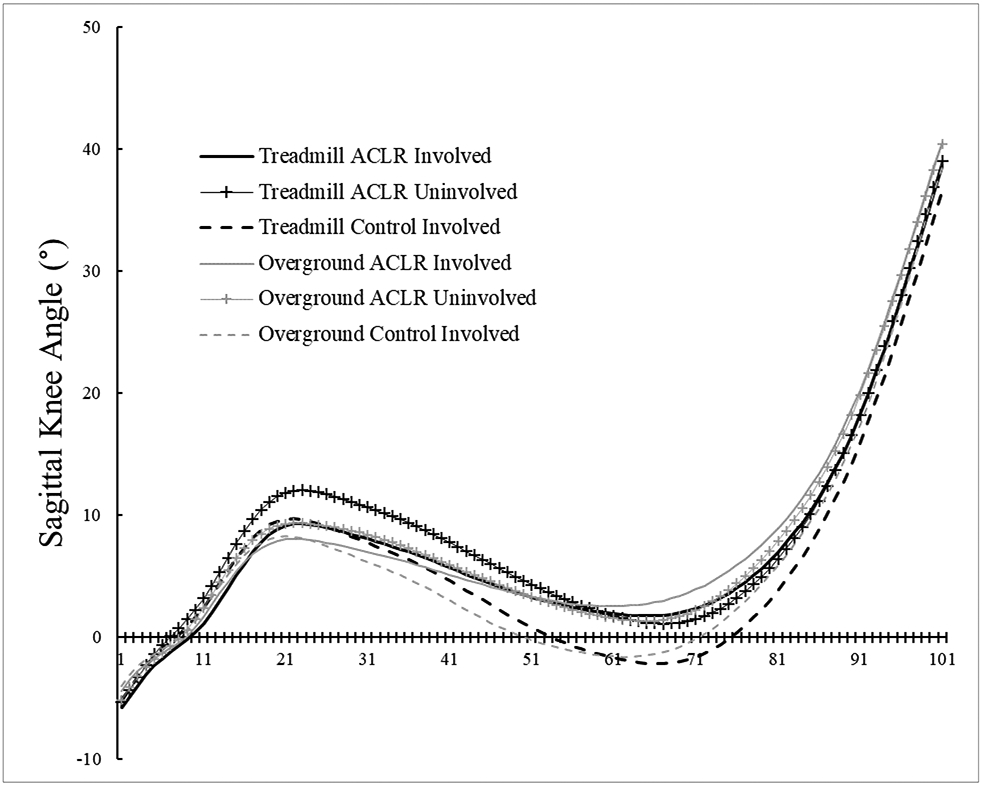
Sagittal plane knee angle time-normalized waveforms. Negative values indicate knee extension, while positive values indicate knee flexion
Figure 4C.
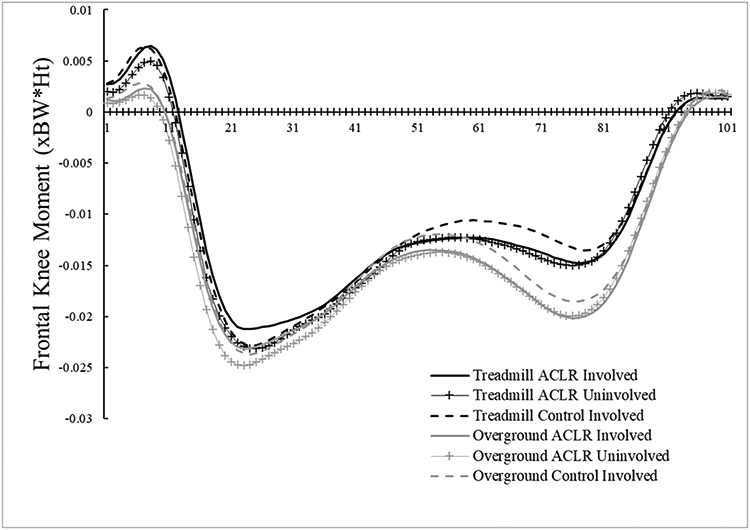
Internal frontal plane knee moment time-normalized waveforms. Negative values indicate internal abduction moments, while positive values indicate internal adduction moments.
Figure 4D.
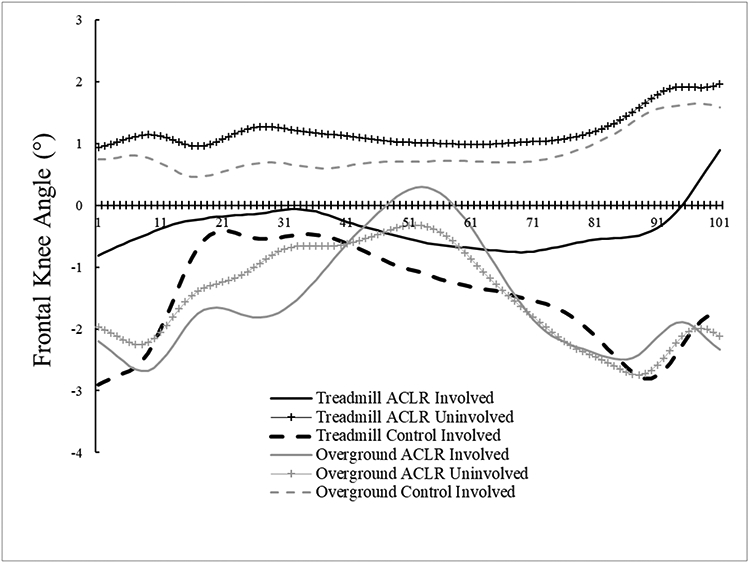
Frontal plane knee angle time-normalized waveforms. Negative values indicate knee abduction, while positive values indicate knee adduction.
Acknowledgements
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number (1R21AR074094-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
References
- Alton F, Baldey L, Caplan S, Morrissey MC, 1998. A kinematic comparison of overground and treadmill walking. Clin. Biomech. (Bristol, Avon) 13, 434–440. 10.1016/s0268-0033(98)00012-6 [DOI] [PubMed] [Google Scholar]
- Bell AL, Brand RA, Pedersen DR, 1989. Prediction of hip joint centre location from external landmarks. Hum. Mov. Sci 8, 3–16. [Google Scholar]
- Blackburn T, Padua DA, Pietrosimone B, Schwartz TA, Spang JT, Goodwin JS, Dewig DR, Johnston CD, 2020. Vibration improves gait biomechanics linked to posttraumatic knee osteoarthritis following anterior cruciate ligament injury. J. Orthop. Res 10.1002/jor.24821 [DOI] [PubMed] [Google Scholar]
- Butler RJ, Minick KI, Ferber R, Underwood F, 2009. Gait mechanics after ACL reconstruction: Implications for the early onset of knee osteoarthritis. Br. J. Sports Med 43, 366–370. 10.1136/bjsm.2008.052522 [DOI] [PubMed] [Google Scholar]
- Christensen JC, LaStayo PC, Mizner RL, Marcus RL, Pelt CE, Stoddard GJ, Foreman KB, 2018. Joint mechanical asymmetries during low- and high-demand mobility tasks: Comparison between total knee arthroplasty and healthy-matched peers. Gait Posture 60, 104–110. 10.1016/j.gaitpost.2017.11.017 [DOI] [PubMed] [Google Scholar]
- Dewig DR, Johnston CD, Pietrosimone B, Blackburn JT, 2021. Long-term gait biomechanics in level, uphill, and downhill conditions following anterior cruciate ligament reconstruction. Clin. Biomech. (Bristol, Avon) 84, 105345. 10.1016/j.clinbiomech.2021.105345 [DOI] [PubMed] [Google Scholar]
- Di Stasi SL, Logerstedt D, Gardinier ES, Snyder-Mackler L, 2013. Gait patterns differ between ACL-reconstructed athletes who pass return-to-sport criteria and those who fail. Am. J. Sports Med 41, 1310–1318. 10.1177/0363546513482718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SA, Brown SR, Koje M, Krishnan C, Palmieri-Smith RM, 2021. Gait asymmetries are exacerbated at faster walking speeds in individuals with acute anterior cruciate ligament reconstruction. J. Orthop. Res 10.1002/jor.25117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschius J, Hertel J, Saliba S, Brockmeier SF, Hart JM, 2018. Gait Biomechanics in ACL Reconstructed Knees at Different Time Frames Post-surgery, Medicine & Science in Sports & Exercise. 10.1249/MSS.0000000000001693 [DOI] [PubMed] [Google Scholar]
- Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, DeMaio M, Dick RW, Engebretsen L, Garrett WE, Hannafin JA, Hewett TE, Huston LJ, Ireland ML, Johnson RJ, Lephart S, Mandelbaum BR, Mann BJ, Marks PH, Marshall SW, Myklebust G, Noyes FR, Powers C, Shields C, Shultz SJ, Silvers H, Slauterbeck J, Taylor DC, Teitz CC, Wojtys EM, Yu B, 2006. Understanding and preventing noncontact anterior cruciate ligament injuries: A review of the Hunt Valley II Meeting, January 2005. Am. J. Sports Med 34, 1512–1532. 10.1177/0363546506286866 [DOI] [PubMed] [Google Scholar]
- Hall M, Stevermer CA, Gillette JC, 2012. Gait analysis post anterior cruciate ligament reconstruction: knee osteoarthritis perspective. Gait Posture 36, 56–60. 10.1016/j.gaitpost.2012.01.003 [DOI] [PubMed] [Google Scholar]
- Harris KP, Driban JB, Sitler MR, Cattano NM, Balasubramanian E, Hootman JM, 2017. Tibiofemoral Osteoarthritis After Surgical or Nonsurgical Treatment of Anterior Cruciate Ligament Rupture: A Systematic Review. J. Athl. Train 52, 507–517. 10.4085/1062-6050-49.3.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart HF, Culvenor AG, Collins NJ, Ackland DC, Cowan SM, Machotka Z, Crossley KM, 2016. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br. J. Sports Med 50, 597–612. 10.1136/bjsports-2015-094797 [DOI] [PubMed] [Google Scholar]
- Hart JM, Ko J-WK, Konold T, Pietrosimone B, 2010. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin. Biomech. (Bristol, Avon) 25, 277–283. 10.1016/j.clinbiomech.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Johnston CD, Goodwin JS, Spang JT, Pietrosimone B, Blackburn JT, 2019. Gait biomechanics in individuals with patellar tendon and hamstring tendon anterior cruciate ligament reconstruction grafts. J. Biomech 82, 103–108. 10.1016/j.jbiomech.2018.10.011 [DOI] [PubMed] [Google Scholar]
- Kaur M, Ribeiro DC, Theis JC, Webster KE, Sole G, 2016. Movement Patterns of the Knee During Gait Following ACL Reconstruction: A Systematic Review and Meta-Analysis. Sport. Med 46, 1869–1895. 10.1007/s40279-016-0510-4 [DOI] [PubMed] [Google Scholar]
- Khandha A, Manal K, Wellsandt E, Capin J, Snyder-Mackler L, Buchanan TS, 2017. Gait mechanics in those with/without medial compartment knee osteoarthritis 5 years after anterior cruciate ligament reconstruction. J. Orthop. Res 35, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Hidler J, 2008. Biomechanics of overground vs. treadmill walking in healthy individuals. J. Appl. Physiol 104, 747–755. 10.1152/japplphysiol.01380.2006 [DOI] [PubMed] [Google Scholar]
- Lewek M, Rudolph K, Axe M, Snyder-Mackler L, 2002. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin. Biomech. (Bristol, Avon) 17, 56–63. [DOI] [PubMed] [Google Scholar]
- Luc B, Gribble PA, Pietrosimone BG, 2014. Osteoarthritis prevalence following anterior cruciate ligament reconstruction: A systematic review and numbers-needed-to-treat analysis. J. Athl. Train 49, 806–819. 10.4085/1062-6050-49.3.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malatesta D, Canepa M, Menendez Fernandez A, 2017. The effect of treadmill and overground walking on preferred walking speed and gait kinematics in healthy, physically active older adults. Eur. J. Appl. Physiol 117, 1833–1843. 10.1007/s00421-017-3672-3 [DOI] [PubMed] [Google Scholar]
- Moraiti CO, Stergiou N, Vasiliadis HS, Motsis E, Georgoulis A, 2010. Anterior cruciate ligament reconstruction results in alterations in gait variability. Gait Posture 32, 169–175. 10.1016/j.gaitpost.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Patterson MR, Delahunt E, Caulfield B, 2014. Peak knee adduction moment during gait in anterior cruciate ligament reconstructed females. Clin. Biomech 29, 138–142. 10.1016/j.clinbiomech.2013.11.021 [DOI] [PubMed] [Google Scholar]
- Pfeiffer SJ, Blackburn JT, Luc-Harkey B, Harkey MS, Stanley LE, Frank B, Padua D, Marshall SW, Spang JT, Pietrosimone B, 2018. Peak knee biomechanics and limb symmetry following unilateral anterior cruciate ligament reconstruction: Associations of walking gait and jump-landing outcomes. Clin. Biomech 53, 79–85. 10.1016/j.clinbiomech.2018.01.020 [DOI] [PubMed] [Google Scholar]
- Pfeiffer SJ, Spang J, Nissman D, Lalush D, Wallace K, Harkey MS, Pietrosimone LS, Schmitz R, Schwartz T, Blackburn T, Pietrosimone B, 2019. Gait Mechanics and T1rho MRI of Tibiofemoral Cartilage 6 Months after ACL Reconstruction. Med. Sci. Sports Exerc 51, 630–639. 10.1249/MSS.0000000000001834 [DOI] [PubMed] [Google Scholar]
- Pietrosimone B, Blackburn JT, Harkey MS, Luc BA, Hackney AC, Padua DA, Driban JB, Spang JT, Jordan JM, 2016. Greater Mechanical Loading During Walking Is Associated With Less Collagen Turnover in Individuals With Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med 44, 425–432. 10.1177/0363546515618380 [DOI] [PubMed] [Google Scholar]
- Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, Luc-Harkey BA, Ulici V, Marshall SW, Jordan JM, Spang JT, 2017. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J. Orthop. Res 35, 2288–2297. 10.1002/jor.23534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosimone B, Seeley MK, Johnston C, Pfeiffer SJ, Spang JT, Troy Blackburn J, 2018. Walking Ground Reaction Force Post-ACL Reconstruction: Analysis of Time and Symptoms. Med. Sci. Sports Exerc 10.1249/MSS.0000000000001776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards RE, van den Noort JC, van der Esch M, Booij MJ, Harlaar J, 2018. Effect of real-time biofeedback on peak knee adduction moment in patients with medial knee osteoarthritis: Is direct feedback effective? Clin. Biomech. (Bristol, Avon) 57, 150–158. 10.1016/j.clinbiomech.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Sanders TL, Maradit Kremers H, Bryan AJ, Larson DR, Dahm DL, Levy BA, Stuart MJ, Krych AJ, 2016. Incidence of Anterior Cruciate Ligament Tears and Reconstruction: A 21-Year Population-Based Study. Am. J. Sports Med 44, 1502–1507. 10.1177/0363546516629944 [DOI] [PubMed] [Google Scholar]
- Shabani B, Bytyqi D, Lustig S, Cheze L, Bytyqi C, Neyret P, 2015. Gait knee kinematics after ACL reconstruction: 3D assessment. Int. Orthop 39, 1187–1193. 10.1007/s00264-014-2643-0 [DOI] [PubMed] [Google Scholar]
- Slater LV, Hart JM, Kelly AR, Kuenze CM, 2017. Progressive Changes in Walking Kinematics and Kinetics After Anterior Cruciate Ligament Injury and Reconstruction: A Review and Meta-Analysis. J. Athl. Train 52, 1062–6050.52.6.06. 10.4085/1062-6050.52.6.06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma RK, Duffell LD, Nathwani D, McGregor AH, 2014. Knee moments of anterior cruciate ligament reconstructed and control participants during normal and inclined walking. BMJ Open 4, 1–7. 10.1136/bmjopen-2013-004753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellsandt E, Gardinier ES, Manal K, Axe MJ, Buchanan TS, Snyder-Mackler L, 2016. Decreased Knee Joint Loading Associated With Early Knee Osteoarthritis After Anterior Cruciate Ligament Injury. Am. J. Sports Med 44, 143–151. 10.1177/0363546515608475 [DOI] [PMC free article] [PubMed] [Google Scholar]


