Abstract
Natural antibodies are primarily produced by B-1 cells and are essential for protection against Streptococcus pneumoniae. The incidence and mortality rate for pneumococcal infection increases dramatically after age 65, disproportionately affecting males in both human and murine systems. To date, there is a significant gap in our understanding of the relationship among sex, aging, natural IgM efficacy, and natural IgM repertoire. Our investigation demonstrates the protective capacity of serum IgM against pneumococcal infection is maintained in IgM obtained from aged female mice but absent in IgM from aged male mice. To understand this difference in protective capacity, we examined serum immunoglobulin, discovering that the protective change was not associated with shifts in levels of phosphorylcholine (PC) nor PPS3-specific IgM. Interestingly, we observed aged females have an increase in the total number of CD5+ B-1 cells, higher serum IL-5 levels, and a larger percentage of aged female CD5+ B-1 cells expressed CD86 as compared to aged males. Furthermore, single-cell IgM repertoire analysis from peritoneal PC+, splenic PC+, and bone marrow CD5+ B-1 cell subsets demonstrated greater diversity with age and a higher level of germline status in female mice than previously observed in studies of aged male mice. Aged female CD5+ B-1 cells also expressed higher levels of transcripts associated with cell activity and self-renewal, such as Nanog and Hmga2. Together, these data indicate females maintain a more diverse and active CD5+ B-1 cell pool and natural IgM repertoire, which has implications for sex-related susceptibility to infection and disease.
Introduction
Natural antibodies are polyreactive, low-affinity immunoglobulins of varying isotypes, present prior to encountering cognate antigen thereby, providing the first line of defense against infection (1, 2). In mice, 80–90% of natural IgM is produced by B-1 cells (2–4), a phenotypically and functionally distinct subset of B cells (5). Beyond protection from infection, B-1 cell-derived natural antibodies provide many essential immune system functions including regulation of B cell development (6–8), selection of the B cell repertoire (7, 9), clearance of apoptotic debris (1), protection against atherosclerosis (10, 11), and allergic suppression (12). Notably, B-1 cell natural IgM is essential for protection against Streptococcus pneumoniae (13).
The incidence and mortality rate for pneumococcal infection increases dramatically in people over 65 (14). Since 1983, vaccination with the 23-valent pneumococcal polysaccharide (PPSV23) is recommended for protection against pneumococcal infection in those aged 65 and over (15), yet in 2017 the rate of death from pneumococcal infection was 8x greater in people over the age of 65 (14). While those over the age of 65 produce similar post-vaccination antibody titers compared to young adults (under 45), the antibodies produced are less effective at clearing bacteria (16–18). Significantly, there is greater incidence and susceptibility of males to pneumococcal infection in both human and murine systems (19, 20). Furthermore, antibody response to pneumococcal vaccination differs between males and females (21). Notably, natural IgM plays a role in B cell repertoire selection (7, 9) and in T cell-independent and dependent IgG responses where studies show reduced IgG levels after immunization (6, 9, 22–24) or infection (22–24) in the absence of natural IgM.
B-1 cell-derived natural IgM effectively clears S. pneumoniae infections by use of its unique repertoire, which recognizes discrete microbial cell wall determinates such as phosphorylcholine (PC, principal cell wall antigen of S. pneumoniae) (25, 26). During VDJ recombination of the B cell receptor, the enzyme terminal deoxytransferase (TdT) inserts non-template-encoded N nucleotides (N-region additions) to the V-D and D-J junctions. CD5+ B-1 cells originate mainly during fetal life, when TdT is not expressed, and persist throughout adult life primarily by self-renewal (4, 5, 27). Consequentially, antigen receptor diversification is limited in CD5+ B-1 cells, resulting in germline-like IgM due to minimal insertion of N-additions and little somatic hypermutation (28, 29). The prototypical CD5+ B-1 anti-PC antibody, T15, lacks N-additions and is highly protective against S. pneumoniae infection (30, 31). Mice expressing TdT constitutively are unable to produce germline-like antibody, and when vaccinated with heat-killed S. pneumoniae generate an anti-PC response; however, these anti-PC antibodies containing abundant N-additions are not protective against S. pneumoniae infection (32). These studies highlight the importance of germline-like antibody structure for providing protection against infection.
While B-1 cell derived natural antibodies are effective at providing protection against pneumococcal infection, it is not understood how this protection is influenced by advancing age in the context of biological sex. Since pneumococcal infection still poses significant challenges for prevention and treatment in those over 65, and sex-based differences in outcomes are well documented (19, 20), it is critical to understand how B-1 cell-derived natural IgM changes with age in the context of sex. Numerous studies have demonstrated that pituitary hormones and estrogen can affect B cell development (33–35), B cell maturation, and/or selection (36–38). Estrogen, in particular, has been shown to block B cell development during adult but not fetal life (39). Furthermore, it was recently demonstrated that production of natural antibodies protective against E. coli infection depends upon estrogen (40). We have previously shown that the protective capacity of natural serum IgM diminishes with advancing age in male mice, and the germline status of CD5+ B-1 cell-derived natural IgM in male mice declines with age (41), a shift that depends on the specificity of the IgM and location of the CD5+ B-1 cell (peritoneal cavity versus spleen) (42). Furthermore, we have shown the age-related changes in germline status of natural IgM are a consequence of selection pressures acting upon the peritoneal CD5+ B-1 cell pool over time (41). Examining this information in sum, we hypothesized the female environment might differentially affect natural antibodies with advanced age. Using a mouse model system to examine age and sex variables, our results demonstrate significant sex-based differences in the protective capacity and structure of CD5+ B-1 cell derived IgM, CD5+ B-1 cell numbers, and gene expression. Our study greatly extends the understanding of how natural antibodies and the essential innate B cell subset are influenced by sex during advancing age.
Materials and Methods
Mice
Male and female BALB/cByJ mice were obtained from The Jackson Laboratory at 6–8 weeks of age and aged in our vivarium. Male and female CB17-SCID mice were obtained from The Jackson Laboratory at 6–8 weeks of age and bred within our vivarium for use at 3–4 months of age. Mice were housed at 5 mice per cage with a 12-hour light/ 12-hour dark cycle and ad libitum access to water and food. Mice were cared for and handled in accordance with the Guide for the Care and Use of Laboratory Animals, National Institutes of Health, and institutional guidelines. All animal studies were approved by the institutional IACUC committee.
Cell Purification and Flow Cytometry
Peritoneal lavage and spleen removals were performed on euthanized mice. Spleens were homogenized using the Miltenyi gentleMACS dissociator and then passed through a 70-um cell strainer. All samples were treated with RBC lysis buffer for 2 minutes (Lonza), subsequently diluted with HBSS with 2.5% FBS, and then centrifuged at 1200rpm for 10 minutes. The cells were resuspended in HBSS with 2.5% FBS, stained with immunofluorescent antibodies, and then analyzed on a Fortessa SORP flow cytometer or Influx cell sorter (BD Biosciences) with gating on live cells by forward side scatter and/or Aqua Live/Dead stain (Invitrogen). Images were constructed with FlowJo 10.0 software (BD Biosciences). The following antibodies were obtained from BD Pharmingen: CD19 (clone ID3), CD43 (clone S7), B220/CD45 (clone RA3–6B2), CD23 (clone B3B4), CD5 (clone 53–7.3), CD80 (clone 16–10A), CD86 (clone GL1), CD25 (clone PC61), PDL2 (clone TY25). For PC staining the following were used: PE-Cy7 labeled PC-BSA and FITC labeled BSA used at 10ug/ml (PC+BSA− CD5+ B-1 cells were used for sorting).
Single Cell Sequencing and Analysis
Peritoneal washout cells, splenocytes, and bone marrow cells were obtained from BALB/c-ByJ mice at the indicated age and stained with fluorescence labeled antibodies. CD5+ B-1 cell populations were single-cell sorted using an Influx cell sorter (BD Biosciences) into a 96-well plate containing lysis buffer (RNaseOut, 5X Buffer, DTT, IgePAL, carrier RNA, Invitorgen). Post-sort re-analysis of CD5+ B-1 cell populations showed them to be ≥98% pure. To obtain cDNA, a 20μl reverse transcription reaction was run per well using the SuperScript III enzyme and random hexamers (Invitrogen). We then performed a semi-nested PCR reaction to amplify the VDJ region of the heavy chain as previously described (42). The PCR products were run on the Qiagen Qiaxcel. PCR products were sequenced (Genewiz) using the MsVHE primer. Sequences were analyzed using an online sequence analysis tool, IMGT/HighV-Quest (43).
Total Ig, Antigen Specific Ig, and IL-5 Serum Analysis
Serum was collected from individual BALB/c-ByJ naïve mice at the time of euthanasia at the ages indicated. The serum was analyzed for total IgM, IgG, or IgA by ELISA according to the manufacturer’s instructions (Bethyl Laboratories). IgM and IgA specific PC and PPS3 serum levels were measured by coating 96-well plates with PC-BSA (Biosearch Technologies) or PPS3 (American Type Culture Collection) at 5 μg/ml in 1X PBS, as previously described (41, 44). Specifically, IgM or IgA standards were included on each plate and PC / PPS3 specific antibody levels were interpreted as volume equivalent of the IgM or IgA standards, as described in (44). Therefore, the amount of PC or PPS3 specific IgM or IgA is calculated relative to an IgM or IgA standard, respectively. IL-5 was measured using a chemiluminescence-based assay from Meso Scale Discovery (MSD; Gaithersburg, MD). Analyses were done using a QuickPlex SQ 120 instrument (MSD), DISCOVERY WORKBENCH 5.1 software (MSD), and GraphPad Prism. Only samples were used in which the calculated concentration CV < 12.
Pneumococcal Infection
Serum was obtained from naïve 3-, 18-, 23-, and 24-month-old BALB/c-ByJ mice at the time of euthanasia. The serum samples obtained from these mice were depleted of IgG using protein G (Santa Cruz Biotechnology). Each serum sample from each aged mouse was kept separate and not pooled. Serum samples from young (3-month-old) mice were pooled. After depletion of IgG, the amount of IgM present in each sample was assessed by ELISA. Each sample to be injected was adjusted to 120 ug of total IgM in 400 μl. CB17-SCID mice were first injected i.p. with IgG-depleted serum samples containing 120 ug of IgM. Four hours after receiving the serum IgM, mice were injected i.p. with 60 CFU of Streptococcus pneumoniae strain WU2. The mice were then monitored for survival over the next 10 days.
The infection experiment was performed with whole bacteria WU2, a type 3 strain of strain of Streptococcus pneumoniae. WU2 was kindly provided by Dr. David E. Briles (University of Alabama at Birmingham). This strain was grown in Todd-Hewitt broth supplemented with 0.5% yeast extract and once it reached mid-log phase of growth was frozen as glycerol stocks at −70°C. Frozen stocks were kept at −70°C for 2 weeks before determining CFUs by enumerating colony growth on blood agar plates (BAP). At the time of infection, bacteria were diluted in sterile PBS and the number of CFUs was reconfirmed.
Gene Expression Analysis
The sex-dependent changes in gene expression associated with aging were assessed in CD5+ B-1 cells isolated from the peritoneal cavity of young (3–4-month-old) and aged (18–24-month-old) males and females by using the Qiagen RT2 Profiler PCR array and qPCR (Taqman). CD5+ B-1 cells were sorted, washed, and resuspended in RNAprotect (Qiagen). Total RNA was isolated from the cells using the RNeasy Plus Micro Kit (Qiagen) in accordance with the manufacture’s protocol. The quantity and purity of extracted RNA was determined using Qubit RNA HS assay (Thermofisher) and Qubit RNA IQ assay (Thermofisher). Only samples of sufficiently high quality and purity were used for the RT2 Profiler array and qPCR.
Estrogen Receptor Signaling RT2 Profiler PCR Array
The Qiagen RT2 Profiler PCR array for the mouse estrogen receptor signaling profiles the expression of 84 genes implicated with estrogen receptor cofactors and other interacting proteins. An equal amount of isolated CD5+ B-1 RNA was used to generate cDNA using the RT2 PCR array first strand kit in accordance with the manufacturer’s protocol (Qiagen). The synthesized cDNA was mixed with RT master mix (RT2 SYBR Green; Qiagen) in accordance with the supplier’s protocol and pipetted into the 96-well PCR array plate which contains 5 housekeeping genes (B2M, Actb, Gusb, Gapdh, and Hsp90ab1) and PCR controls. The array reactions were performed using the QuantStudio 3 (Applied Biosystems) with an initial denaturation step at 95°C for 10 minutes, followed by 40 cycles of denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute. Melting curve stages were from 60° to 95°C, performed according to the manufacturer’s instructions.
The Ct values for each sample were set automatically by the thermal cycler according to the amplification curves. The baseline and threshold values were set manually as recommended by the RT2 Profiler PCR array manual. Results were evaluated by the RT2 Profiler PCR Array Data Analysis version 5.1. Expression data obtained were normalized to the average Ct value of the five housekeeping genes included in the array to minimize functional biases and analyzed as relative expression (2dCt) with at least three animals per age/sex group. A heat map of gene expression was generated using Heatmapper (45) with the clustering method set as the average linkage and Euclidean distance measurement applied. Variations in CD5+ B-1 gene expression in each group are shown as normalized relative gene expression with red being lowest expression and green being highest expression.
Gene Expression using qPCR
qPCR analyses were conducted to examine differential gene expression. TaqMan assays were used for the following six genes (Life Technologies): Ers1 (Mm00433149), Esr2 (Mm00599821), Irgm1 (Mm00492596), Hmga2 (Mm04183367), Nanog (Mm01617762), and Ikzf1 (Mm01187877). Efficiencies of all assays were determined to be between 90–100%. Assays were run in the TaqMan Fast Advanced Master Mix (2X; Applied Biosystems) with a 2 min hold at 50°C for optimal UNG activity followed by a 2 min hold at 95°C for polymerase activation with a 40-cycle PCR with a 1 second denaturation at 95°C followed by a 20 second anneal/extend at 60°C using the QuantStudio 3. The endogenous reference genes (IDT DNA), ActB (Mm.PT.39a.22214849) and B2M (Mm.PT.39a.22214835), were used as internal references to calculate the relative gene expression for each group by replacing the single reference gene Ct in each calculation with an averaged Ct-value from the two reference genes from all individual mice (2−ΔCt). Inclusion of multiple reference genes greatly stabilizes gene expression calculations (46).
Statistical Analysis
Statistical analyses were performed using Prism (Version 9.0). All statistical analyses used are indicated in each figure legend. The outlier test was performed on all data sets using Prism’s ROUT method of identifying outliers. Outliers were removed when detected by Prism’s ROUT method using the coefficient Q set at 1%.
Results
Protective capacity of natural IgM against pneumococcal infection differs in aged male mice versus aged female mice.
While aging alters the protective capacity of natural antibody against pneumococcal infection in aged male mice (41), age-related changes in natural IgM antibody have not been elucidated for aged female mice. To address the anti-microbial function of natural IgM antibody in female mice with age, we examined serum samples from pooled 3-month-old male (n=13), pooled 3-month-old female (n=16), individual 18–24-month-old male (n=12), and individual 18–24-month-old female (n=16) mice. Serum samples were completely depleted of IgG using protein G, and IgM content verified. CB17-SCID mice were then injected with PBS or 120 μg of serum IgM from either young or aged mice 4 hours prior to infection with 60 CFU of Streptococcus pneumoniae (WU2 strain). Kaplan-Meier analysis of SCID mice receiving 3-month-old male or female serum IgM was significantly longer (p<0.0001) than survival of SCID mice not receiving serum (Figure 1). In this system, serum IgM from 18–24-month-old males was significantly less protective than serum IgM from 3-month-old males (p=0.0004) with no significant difference from SCID mice not receiving serum. In contrast, survival of SCID animals receiving serum IgM from 18–24-month-old females was comparable to those with serum IgM from 3-month-old females and was significantly longer than survival of SCID mice receiving no serum (p<0.0001). While there was a significant survival difference between SCID mice receiving serum IgM from 18–24-month-old female mice versus 18–24-month-old male mice (p=0.0070), there was no significant difference in survival with serum IgM from 3-month-old female versus 3-month-old male mice. Thus, in contrast to protection of natural IgM from young male and female mice, natural IgM from aged males provides no defense against pneumococcal infection. Surprisingly, natural IgM from aged females continues to provide protection against pneumococcal infection throughout old age, suggesting an age-associated loss of natural antibody-mediated anti-microbial activity in males but not females.
Figure 1: Serum IgM protection against pneumococcal infection differs when obtained from aged female mice versus aged male mice.

Serum samples were obtained from 3- or 18–24-month-old male or female BALB/c-ByJ mice at time of euthanasia. Samples were depleted of IgG by protein G clearance. An equal quantity of serum IgM (120μg) was injected in a total volume of 400 μl (i.p.) into CB17-SCID mice from either 3-month or 18–24-month-old serum samples, with no serum as a control group. Four hours post injection, the CB17-SCID mice were injected (i.p.) with 60 CFU of Streptococcus pneumoniae WU2 strain. The number of individual young or aged serum donors into the equal number of CB17-SCID mice included: 13 from 3-month-old male BALB/c-ByJ mice, 12 from 18–24-month-old male BALB/c-ByJ mice, 16 from 3-month-old female BALB/c-ByJ mice, and 16 mice from 18–24-month-old female BALB/c-ByJ mice. Statistical analysis was performed using the log rank test: no serum vs. young male, p<0.0001; no serum vs. young female, p<0.0001; no serum vs. aged female, p=0.0003; young male vs. aged male, p=0.0004; aged male vs. aged female, p=0.0070. Results shown are an average of 3 independent experiments.
Serum anti-PC and anti-PPS3 levels do not explain the difference in anti-microbial activity of natural IgM between aged males and females.
To understand the difference in anti-pneumococcal activity in natural IgM from old male and female mice, we first examined serum samples for PC and PPS3 specific IgM. Initially, sera from young adult (3-month) and aged adult (18–23-month) mice were assessed for total IgM, IgG, and IgA levels by ELISA. We found the total amount of serum IgM (Figure 2A) was significantly higher in aged females as compared to young females (p=0.0005). Aged males also displayed an increase in the amount of total IgM; however, this change was not significantly different as in females. Interestingly, both total serum IgG (Figure 2B) and IgA (Figure 2C) levels were significantly higher in both male and female aged mice (p<0.0001).
Figure 2: Serum Ig from aged male and female mice is significantly different from young male and female serum.
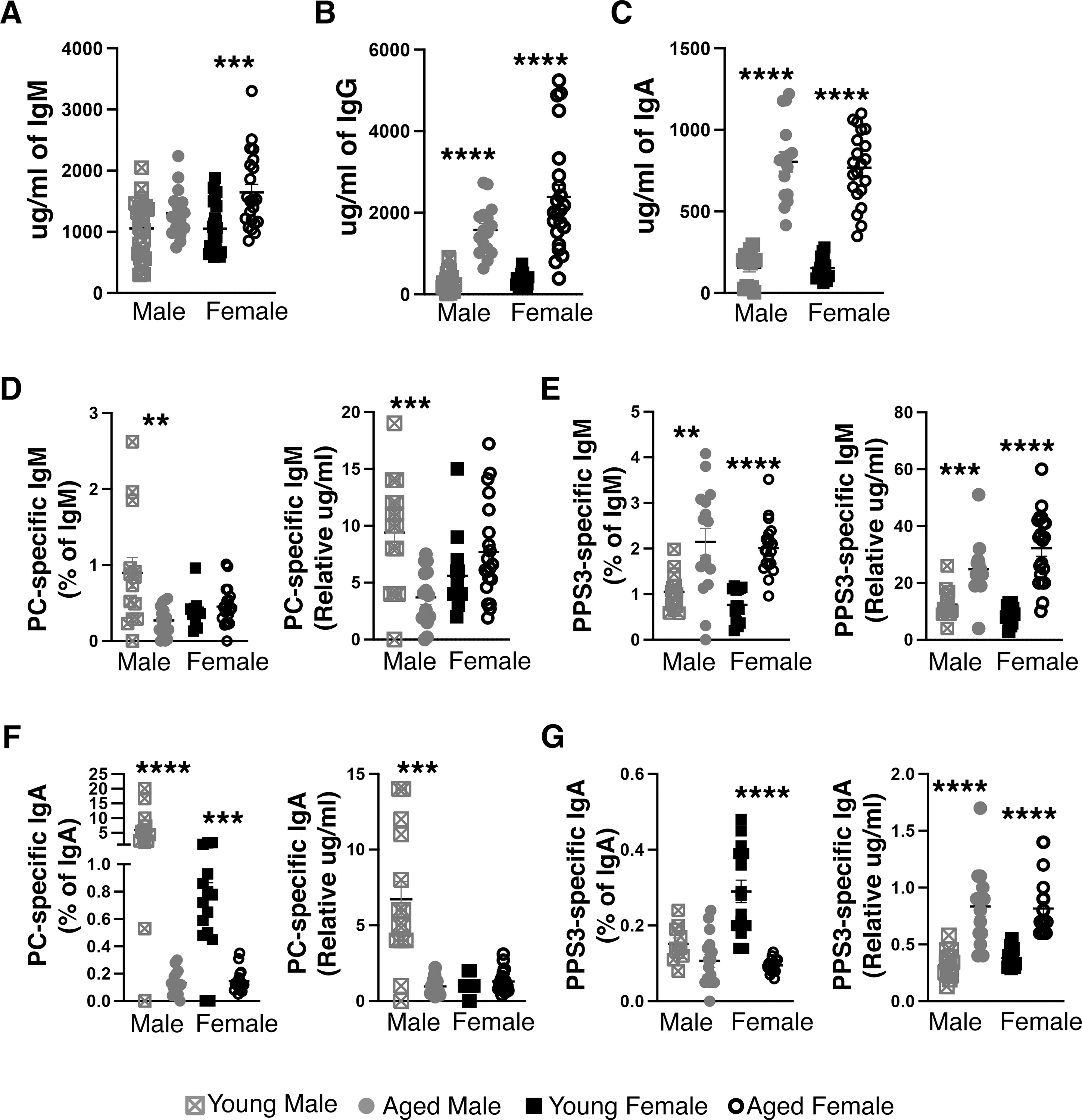
Serum samples were obtained from 3- or 18–24-month-old male or female BALB/c-ByJ mice at time of euthanasia and analyzed for (A) total IgM, (B) total IgG, (C) total IgA, (D) PC-specific IgM (E) PPS3 specific IgM, (F) PC specific IgA, and (G) PPS3 specific IgA. The amount of PC and PPS3 specific IgM was calculated relative to either an IgM or IgA standard curve. Results are displayed either as the relative amount (calculated based on an IgM or IgA standard) or as a percent of total IgM or IgA (using the relative amount and the calculated amount of total Ig from each sample). Grey squares represent young male mice (n=12), grey circles represent aged male mice (n=21), black squares represent young female mice (n=14), and open black circles represent aged female mice (n=16). Values are displayed as the mean (±SEM) of individual mouse serum samples obtained from 5 independent experiments. Statistics were performed using unpaired, two-tailed Mann-Whitney test.
Next, the same serum samples were assessed for PC (Figure 2D) and PPS3 specific IgM (Figure 2E). In the absence of a standard for these specificities, we utilized an IgM standard for relative quantification as previously described (41, 44). Since the total amount of IgM is different between groups, we present the data in two ways: 1) as an amount of PC or PPS3 specific IgM relative to an IgM standard, and 2) a percent of total IgM (using the relative amount of PC or PPS3 and the total amount of IgM from the sample). As we previously reported, the amount of PC specific IgM was significantly decreased in aged males ((41) and Figure 2D). However, the amount of PC specific IgM was not significantly different in young versus aged females (Figure 2D). In contrast, the level of PPS3 specific IgM was significantly increased in both aged males (p=0.0037, percent; p=0.0002, relative amount) and aged females (p<0.0001) (Figure 2E) as compared to young male and female mice, respectively. As protein G depletion does not remove IgA, we assessed the levels of PC and PPS3 specific IgA as well. When examining PC specific serum IgA as a percent of total IgA, it is significantly lower in both aged male and aged female mice as compared to young male and female mice respectively (p<0.0001, p=0.0002) (Figure 2F). However, this decrease in PC specific IgA is not seen in aged females when examining the relative amount of PC specific IgA (Figure 2F). When examining PPS3 specific serum IgA as a percent of total IgA, it is significantly lower only in aged females as compared to young females (p<0.0001), and young males displayed significantly lower levels of PPS3 specific IgA than young females (p=0.0008) (Figure 2G). In contrast, when examining PPS3 specific IgA levels as a relative amount, the level of PPS3 specific IgA was significantly increased in both aged males (p<0.0001) and aged females (p<0.0001) as compared to young male and female mice, respectively (Figure 2G). When considering PPS3 specific IgA, the relatively low amount of PPS3 specific IgA as compared to PPS3 specific IgM should be noted. Serum IgM from aged male mice failed to protect against infection (Figure 1) even though the levels of anti-PC specific IgM in aged males do not differ from young or aged female mice (Figure 2D), nor did the levels of anti-PPS3 specific IgM in aged males differ from the levels observed in aged females (Figure 2E). Furthermore, neither PC (Figure 2F) or PPS3-specific (Figure 2G) IgA levels differed between aged male or female mice.
Together, these results demonstrate a significant loss of the protective capacity of natural serum IgM in aged male mice but no change in protective capacity in aged female mice, which is not accounted for by a change in the level of PC or PPS3 specific IgM nor PC or PPS3 specific IgA. These results raise the possibility of other differential changes between males and females with age, which may result in the preservation of protective natural antibody into old age in females but not males. To investigate what affects this change in natural antibody, we next examined the percent and number of natural antibody producing B-1 cells in male and female mice.
Aged female mice have more peritoneal and splenic CD5+ B-1 cells than aged male mice.
Most circulating natural IgM is derived from B-1 cells (2–4), which arise early in life and persist into adulthood via self-renewal (4, 27, 47). B-1 cells are found in the peritoneal cavity, pleural cavity, spleen, bone marrow, lymph nodes, intestinal lamina propria, lung parenchyma, and blood (reviewed in (5)). Although the frequency of B-1 cells is greatest in the peritoneal cavity, B-1 cells residing in the spleen and bone marrow are the major sources of protective natural antibody (48, 49). B-1 cells were originally identified by their expression of CD5 and were further characterized by surface expression of IgMhigh, IgDlow, CD19high, B220low, CD23−, and CD43+ (50–52), which contrasts with the surface phenotype of follicular B-2 cells: CD5−, IgMlow, IgDhigh, CD19+, B220+, CD23+, and CD43−. Later, an additional population of B-1 cells was identified sharing the characteristics of CD5+ B-1 but lacking CD5 expression (53). Recently, it was shown CD5+ B-1 cells lose CD5 expression upon TLR activation and CD5− B-1 cells comprise the largest proportion of antibody secreting B-1 cells (49, 54). However, it is currently not possible to distinguish CD5− B-1 cells from B2 cell derived plasmablasts without the use of a chimeric system as they lack phenotypic differences (54). Therefore, to begin to understand the difference observed in natural antibody effectiveness between aged male and female mice in a natural healthy aged setting, we examined CD5+ B-1 cells, which represent the available natural antibody repertoire.
We determined the number and percent of peritoneal and splenic CD5+ B-1 cells from male and female mice in the young and aged. As previously published, our data here confirms aged males have significantly fewer peritoneal and splenic CD5+ B-1 cells as compared to young males in both percentage and numbers ((42) and Figure 3A–F). Aged females have significantly higher frequency and number of peritoneal and splenic CD5+ B-1 cells as compared to aged males (Figure 3A–F). Young females have significantly higher numbers of peritoneal and splenic CD5+ B-1 cells as compared to young males (Figure 3A–F). The total number of peritoneal CD5+ B-1 cells is significantly higher in aged females as compared to young females (Figure 3C). The percent (Figure 3E) and number (Figure 3F) of splenic CD5+ B-1 is significantly higher in aged females as compared to young females. Upon examination of BM CD5+ B-1 cells, the only significant difference observed was an increase in the number of total bone marrow cells in young female mice as compared to young male mice (Figure 3G). Since it has been shown CD5− B-1 cells make up a large proportion of antibody secreting B-1 cells (49, 54), we examined CD5−B220loCD19+CD23− B cells (we refrain from calling these B-1 cells since they cannot be distinguished from B-2 cell derived plasmablasts). We find aged females have significantly higher numbers of peritoneal and splenic CD5− B cells as compared to young females and aged males. Aged males also have significantly higher numbers of peritoneal CD5− B cells as compared to young males (Supplemental Figure 1). Overall, these results demonstrate female mice sustain higher percent and numbers of both splenic and peritoneal CD5+ B-1 cells in the aged.
Figure 3: Number of CD5+ B-1 cells differs in male versus female mice.

CD5+ B-1 cells examined in the young (3-month-old) and aged (18–24-month-old), male and female, mice were assesed for percent and number. (A) The total number of peritoneal cavity cells, (B) the percent of live peritoneal lymphocytes staining postitive for CD5+ B-1 cells (B220loCD5+CD19hiCD23−), (C) total number of peritoneal CD5+ B-1 cells, (D) the total number of splenocytes, (E) the percent of live splenocytes staining postitive for CD5+ B-1 cells (B220loCD5+CD19hiCD23−), (F) total number of splenic CD5+ B-1 cells, (G) the total number of bone marrow cells, (H) the percent of live bone marrow cells staining positive for CD5+ B-1 cells (IgM+IgDloCD5+CD19hiCD43+), and (I) total number of bone marrow CD5+ B-1 cells. Grey squares represent young male mice, grey circles represent aged male mice, black squares represent young female mice, and open black circles represent aged female mice. Results are based on 6 independent experiments. Values are displayed as the mean (±SEM) of individual mouse serum samples. Statistics used: Mann-Whitney test. (J) IL-5 was measured in the serum of BALB/c-ByJ mice using the MESO V-plex kit against a standard curve. Concentrations (pg/uL) were then averaged for each sex and age group (n=5 old male, n=6 young male, n=9 old female, n=10 young female) and a two-way ANOVA was used to calculate p-value for the group. Asterisks for p values: *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
CD5+ B-1 cell maintenance has been shown to be dependent upon IL-5 (55). Interestingly, IL-4, IL-5, and IL-6 levels have been reported to be unchanged or increased in the aged whereas, IL-2 and IL-3 levels display an age-associated decline (56); however, sex was not considered in these studies. Therefore, we examined the serum of aged male and female BALB/c-ByJ mice for IL-5. Our results demonstrate males express less circulating IL-5 than females, regardless of age (p=0.0085, Figure 3J). Young males were found to express 3.38pg/uL IL-5 while young females expressed 6.641pg/uL. Interestingly, as the males aged, expression levels decreased to 1.5pg/uL while aging females increased expression of IL-5 to 7.49pg/uL (Figure 3J). These results suggest the maintenance of female CD5+ B-1 cells with age could in part be due to the increase in serum IL-5 levels observed in the serum of aged females. It has been shown PD-L2 on CD5+ B-1 cells blocks IL-5 production by T cells and in turn limits natural antibody production by CD5+ B-1 cells (57). Since it is possible aging differentially affects cell surface marker expression, we next examined surface markers uniquely expressed by naïve CD5+ B-1 cells.
Female but not male mice display differences in CD5+ B-1 cell surface marker expression in the aged.
Unlike conventional naïve splenic B2 cells, peritoneal and splenic CD5+ B-1 cells have unique surface expression of CD80, CD86, CD25, and PDL2 in the absence of stimulation (58–61). We examined the expression of these surface antigens on peritoneal and splenic CD5+ B-1 cells from male and female mice by flow cytometry in the young and aged. There were no significant differences in the percent expression of CD80 (Figure 4B and 4F) nor PDL2 (Figure 4D and 4H) in male or female in either the young or aged. It is possible the increased serum IL-5 we observed in aged female mice (Figure 3J) is not influenced by PD-L2 expression in aged CD5+ B-1 cells. Others have shown the IL-5 regulating CD5+ B-1 cell maintenance and development can be derived from cells other than T cells (55), and this non-T cell derived IL-5 could then regulate aged female CD5+ B-1 cells. We found the percent of CD86 expression was significantly higher on aged female peritoneal CD5+ B-1 cells than on young female peritoneal CD5+ B-1 cells (68% +/−4.5 in aged versus 44% +/−1.8 in young, p<0.0001, Figure 4A). The percent of CD86 expression was also significantly higher on aged female splenic CD5+ B-1 cells than on young female splenic CD5+ B-1 cells (83% +/−2.5 in aged versus 63% +/−1.6 in young, p<0.0001, Figure 4E). Conversely, the percent of CD25 expression was significantly lower on aged female peritoneal CD5+ B-1 cells than on young female peritoneal CD5+ B-1 cells (11% +/−1.5 in aged versus 25% +/−1.3 in young, p<0.0001, Figure 4C). Yet, the percent of CD25 expression was significantly higher on aged female splenic CD5+ B-1 cells than on young female splenic CD5+ B-1 cells (44% +/−4.8 in aged versus 13% +/−1.1 in young, p<0.0001, Figure 4G). There were no significant changes in CD86, CD80, CD25, or PDL2 on peritoneal or splenic CD5+ B-1 cells obtained from male mice with age. These results demonstrate differential effects of aging upon the expression of CD86, CD80, CD25, and PDL2 on CD5+ B-1 cells between males and females with age. Increased levels of CD86 and CD25 could have implications for activation status and/or self-renewal capabilities (61, 62) in aged females versus aged males.
Figure 4: Surface CD86, CD80, CD25, and PDL2 expression in male and female mice with age.

Peritoneal (A-D) and splenic (E-H) naïve CD5+ B-1 cells obtained from young (3-month-old) and aged (18–24-month-old) male and female mice were assesed for expression of CD86 (A and E), CD80 (B and F), CD25 (C and G), and PDL2 (D and H) by flow cytometry. Grey squares represent young male mice, grey circles represent aged male mice, black squares represent young female mice, and open black circles represent aged female mice. Results are based on 3 (male) and 5 (female) independent experiments. Values are displayed as the mean (±SEM) of individual mouse serum samples. Statistics used: Mann-Whitney test.
Considering aged females maintain protective natural antibodies, have an increased number of CD5+ B-1 cells, show evidence of increased activation status, and have serum antibody levels that point to a qualitative (natural antibody structure) rather than quantitative (abundance) difference in natural antibody producing cells as compared to males, we hypothesized the aged female repertoire of anti-phosphorylcholine specific CD5+ B-1 cells might be distinct from that of aged males.
Structure of natural IgM from peritoneal and splenic PC specific CD5+ B-1 cells changes with age in female mice.
Phosphorylcholine (PC) specific CD5+ B-1 cells (CD19+B220loCD5+CD23−PC+) were obtained by single cell sorting from either the peritoneal cavity or spleen of young and aged female mice. The sorting strategy is shown in Supplemental Figure 2. The heavy chain of these single cells was examined for variable (VH), diversity (DH), and joining (JH) gene segment use as well as germline status. We observed many significant differences in VH, DH, and JH use between young and aged females (Figure 5A, 5B, 5C), which are summarized in detail in Supplemental Table 1. The germline-like structure of peritoneal and splenic PC+ CD5+ B-1 cells changes with age (Figure 5D). We find a significant decrease in the number of sequences lacking N-additions at both junctions (germline-like) with age in both peritoneal PC+ CD5+ B-1 cells (32% in aged versus 76% in young, p<0.0001, x2, 2x2) and splenic PC+ CD5+ B-1 cells (50% in aged versus 65% in young, p=0.0006, x2, 2x2). Interestingly, there is no significant difference in the number of peritoneal PC+ CD5+ B-1 cell sequences lacking N-additions between aged female mice (32%) as compared to aged male mice (30%); however, there is a significant difference in the number of splenic PC+ CD5+ B-1 cell sequences lacking N-additions between aged female mice (50%) as compared to aged splenic male mice (15%) (p<0.0001, x2, 2x2). These results are shown in Figure 5D and are intriguing as B-1 cells residing in the spleen and bone marrow are the major sources of protective natural antibody while peritoneal cavity B-1 cells rapidly respond to inflammatory stimuli (63, 64).
Figure 5: Repertoire analysis of natural IgM from peritoneal and splenic PC+ CD5+ B-1 cells in adult female young and aged mice.

PC+ CD5+ B-1 cells were single cell sorted from peritoneal cavity or spleen of 3- and 18–26-month-old female BALB/c-ByJ mice. The VH region was amplified and sequenced as detailed in Materials and Methods. (A) The percent of VH gene segment usage. (B) The percent of DH gene segment usage. (C) The percent of JH gene segment usage. (D) The percent of sequences containing zero N-additions (light grey bars) or 1 or more N-additions (dark grey hashed bars) at both junctions is shown with replicate sequences included in the analysis. For direct comparison of N-additions, PC+ CD5+ B-1 cells were single cell sorted from peritoneal cavity or spleen of young and aged male BALB/c-ByJ mice (as previously published (41, 42)). (E) The percent of VH gene segment usage within the replicate sequences. (F) Distribution of replicate CDR-H3 sequences in the young and aged (number in the middle represents the number of replicates within the population). Each color represents a unique CDR-H3 amino acid sequence. Results are based on 2 independent experiments with sequences combined from each independent experiment (n=5 for 3-month-old mice, n=7 for 18–26-month-old mice). Statistics used: 2x2 chi-square test.
The shifts we observed in peritoneal and splenic VH use in female mice is due to an increase in replicate sequences, meaning sequences with the exact same CDR-H3 (same VH, DH, JH, N-additions, and P-insertions). We refer to these sequences as replicates instead of clones because we have previously observed diversity in light chain use with these replicates (42). Of the 264 total sequences from young peritoneal PC+ CD5+ B-1 cells, 184 were replicate sequences (70%). Of the 270 total sequences from aged peritoneal PC+ CD5+ B-1 cells, 233 were replicate sequences (83%). Of the 190 total sequences from young splenic PC+ CD5+ B-1 cells, 72 were replicate sequences (38%). Of the 390 total sequences from aged splenic PC+ CD5+ B-1 cells, 304 were replicate sequences (78%). Of the replicates observed in aged peritoneal PC+ CD5+ B-1 cells VH2, VH5, and VH7 were the most abundantly used whereas, VH1 and VH5 were the most abundantly used in aged splenic PC+ CD5+ B-1 cells (Figure 5E). Furthermore, we observe an increase in diversity (unique CDR-H3 sequences) within the replicate sequences in aged female peritoneal and splenic PC+ CD5+ B-1 cells (Figure 5F). We previously did not observe such significant numbers of replicate sequences in PC+ CD5+ B-1 cells from aged males (41, 42). Figure 6A–D shows the most utilized CDR-H3 sequences in peritoneal and splenic PC+ CD5+ B-1 cells obtained from young and aged female and male (41, 42) mice. Interestingly, the T15 idiotype (ARDYYGSSYWFDV) is found only in replicate sequences within young female peritoneal and splenic PC+ CD5+ B-1 cell subsets. The PC / PtC cross-reactive CDR-H3 (MRYGNYWYFDV) (65) is found only in aged female splenic PC+ CD5+ B-1 cells.
Figure 6: Significant differences in female versus male peritoneal and splenic PC+ CD5+ B-1 cell IgM VH and CDR-H3 use.

PC+ CD5+ B-1 cells were single cell sorted from peritoneal cavity or spleen of young and aged female BALB/c-ByJ mice (as presented in Figure 5). For direct comparison, PC+ CD5+ B-1 cells were single cell sorted from peritoneal cavity or spleen of young and aged male BALB/c-ByJ mice (as previously published (41, 42)). (A-D) Comparison of the most frequently utilized CDR-H3 sequences of PC+ CD5+ B-1 cells from young and aged male and female mice. (E-H) Significant differences in VH use between PC+ CD5+ B-1 cells obtained from young and aged male and female mice.
Our previously published data in male mice demonstrated much less change with age in peritoneal PC+ CD5+ B-1 cells as only VH2 and VH11 utilization increased with age (41), and no age-related change in VH use with age in splenic PC+ CD5+ B-1 cells from male mice (42). In contrast, both peritoneal and splenic PC+ CD5+ B-1 cells from young and aged female mice display numerous differences in VH usage as compared to young and aged males. These differences are summarized in Figure 6E–H. Notably, in peritoneal PC+ CD5+ B-1 cells aged females have significantly more VH5 and VH7 usage whereas, males display more VH1 and VH3 usage. In the splenic PC+ CD5+ B-1 cells, females display more VH5 usage whereas males display more VH2 usage. These findings are intriguing as VH5 is the most JH proximal VH gene segment and is most frequently utilized during fetal life (66–69), possibly suggesting maintenance of more fetal-like CD5+ B-1 cells in aged female than aged males.
Sequence analysis of natural IgM from bone marrow CD5+ B-1 cells demonstrates changes with age.
Most of natural serum IgM (80–90%) is derived directly from B-1 cells resident to the spleen and bone marrow (BM) (48, 49). While numerous studies have examined the repertoire of splenic B-1 cells (29, 41, 42, 65, 70, 71), the natural antibody repertoire of BM CD5+ B-1 cells from healthy young and aged mice has yet to be analyzed. Therefore, we examined the variable (VH), diversity (DH), and joining (JH), gene segments of the heavy chain as well as germline status from single CD5+ B-1 cells (IgMhiIgDlo/-CD19+CD43+CD5+) obtained from the bone marrow of young and aged female and male mice (Figure 7). The sorting strategy is shown in Supplemental Figure 3. Aged female BM CD5+ B-1 cells used VH3 (16% versus 10%), VH12 (3% versus 0%), and VH14 (11% versus 3%) more frequently than young female BM CD5+ B-1 cells whereas, the young female BM CD5+ B-1 cells used VH1 (41% versus 30%) and VH2 (23% versus 16%) more frequently than aged female BM CD5+ B-1 cells. Aged male BM CD5+ B-1 cells used VH14 (6% versus 3%) more frequently than young male BM CD5+ B-1 cells. These results are shown in Figure 7A with statistical analyses. Interestingly, as observed in other CD5+ B-1 cell subsets, male mice show less differences with age in terms of VH usage than female mice. Aged female mice utilized VH11 (2% vs. 0%, p=0.0079), VH12 (3% vs. 0%, p=0.0033), and VH14 (11% vs. 6%, p=0.0266) more frequently than aged male mice, whereas there were no significant differences in VH usage between young males and young females.
Figure 7: Repertoire analysis of natural IgM from bone marrow CD5+ B-1 cells in young and aged adult male and female mice.

CD5+ B-1 cells were single cell sorted from the bone marrow of 3- and 16–21-month-old male mice and 3- and 19–24-month-old female BALB/c-ByJ mice. The VH region was amplified and sequenced as detailed in the Materials and Methods section. (A) The percent of VH gene segment usage. (B) The percent of DH gene segment usage. (C) The percent of JH gene segment usage. (D) The percent of sequences containing zero N-additions (light grey bars) or 1 or more N-additions (dark grey hashed bars) at both junctions is shown with replicate sequences included in the analysis. (E) The percent of VH gene segment usage within the replicate sequences is displayed. (F) Distribution of replicate CDR-H3 sequences in the young and aged (number in the middle represents the number of replicates within the population). Each color represents a unique CDR-H3 amino acid sequence. For male mice results are based on 4 independent experiments with sequences combined from each independent experiment (n=12 for 3-month-old male mice, n=11 for 16–21-month-old male mice). For female mice, results are based on 3 independent experiments with sequences combined from each independent experiment (n=12 for 3-month-old female mice, n=12 for 19–24-month-old mice). Statistics used: 2x2 chi-square test.
Examination of the DH and JH genes show significant differences in utilization in the aged versus young BM CD5+ B-1 cell populations. Aged female BM CD5+ B-1 cells used DH4 (14% versus 8%) and DH6 (3% versus 0%) more frequently than young female BM CD5+ B-1 cells. Aged male BM CD5+ B-1 cells no significant differences in DH use. These results are shown in Figure 7B with statistical analyses. Aged female BM CD5+ B-1 cells used JH1 (21% versus 12%) more frequently than young female BM CD5+ B-1 cells whereas, the young female BM CD5+ B-1 cells used JH4 (32% versus 22%) more frequently than aged female BM CD5+ B-1 cells. Aged male BM CD5+ B-1 cells used JH1 (18% versus 10%) more frequently than young male BM CD5+ B-1 cells. These results are shown in Figure 7C with statistical analyses.
The germline-like structure of female but not male BM CD5+ B-1 cells changes with age (Figure 7D). We find a significant increase in the number of sequences lacking N-additions at both junctions (germline-like) with age in female BM CD5+ B-1 cells (30% in aged versus 18% in young, p=0.0011, x2, 2x2). Male BM CD5+ B-1 cells show no difference with age in sequences lacking N-additions at both junctions (17% in aged versus 20% in young). Interestingly, there is no significant difference in the number of BM CD5+ B-1 cell sequences lacking N-additions between young female mice (18%) as compared to young male mice (20%); however, there is a significant difference in the number of BM CD5+ B-1 cell sequences lacking N-additions between aged female mice (30%) as compared to aged male mice (17%) (p<0.0001, x2, 2x2). These results are shown in Figure 7D. Together these results demonstrate significant differences in the repertoire of BM CD5+ B-1 cells obtained from male and female mice in young and aged.
Both male and female mice display replicate sequences in BM CD5+ B-1 cells in young and aged. Of the 234 total sequences from young female BM CD5+ B-1 cells, 5 were replicate sequences (2%). Of the 322 total sequences from aged female BM CD5+ B-1 cells, 102 were replicate sequences (32%). Of the 295 total sequences from young male BM CD5+ B-1 cells, 4 were replicate sequences (1%). Of the 452 total sequences from aged male BM CD5+ B-1 cells, 44 were replicate sequences (10%). Of the replicates observed in aged female BM CD5+ B-1 cells VH1, VH3, and VH14 were the most abundantly used VH gene segment whereas, VH3 and VH10 were the most abundantly used VH gene segments in aged male BM CD5+ B-1 cells (Figure 7E). Overall, BM CD5+ B-1 cells from young male and female mice (Figure 7F) have less diversity within the replicate CDR-H3 sequences than peritoneal and splenic CD5+ B-1 cell subsets (Figure 5F). Furthermore, we observe an increase in diversity (unique CDR-H3 sequences) within the replicate sequences in aged female and male BM CD5+ B-1 cells; however, there is less diversity observed within the BM CD5+ B-1 cell CDR-H3 replicate sequences of male mice than female mice (Figure 7F). Figure 8 shows the most utilized CDR-H3 sequences in male and female BM CD5+ B-1 cells obtained from young and aged female and male mice. In young BM CD5+ B-1 cells, the most frequently used CDR-H3 utilizes VH7 in both males and females whereas, in aged BM CD5+ B-1 cells the most frequently used CDR-H3 utilizes VH3 in both males and females (Figure 8).
Figure 8: Significant differences in female versus male bone marrow CD5+ B-1 cell IgM CDR-H3 use.

CD5+ B-1 cells were single cell sorted from bone marrow of young and aged female and male BALB/c-ByJ mice (as presented in Figure 7). (A) Comparison of the most frequently utilized CDR-H3 sequences of CD5+ B-1 cells from aged female mice. (B) Comparison of the most frequently utilized CDR-H3 sequences of CD5+ B-1 cells from aged male mice.
Together these results demonstrate significant changes in the repertoire of both peritoneal and splenic CD5+ PC-specific B-1 cell IgM as well as bone marrow CD5+ B-1 cell IgM obtained from aged female mice as compared to young female mice. Furthermore, these differences observed in female mice differ from previously published results examining PC-specific natural IgM from aged male mice as compared to young male mice (41, 42). Importantly, these results suggest differential maintenance of CD5+ B-1 cell specificities between aged males and females over time. Therefore, we further examined the CDR-H3 region of these female antibodies.
Hydrophobicity and amino acid content of the CDR-H3 loop changes with age and sex.
The CDR-H3 region of an antibody is the central point for antigen contact and the most variable in structure. Therefore, properties of amino acids comprising the CDR-H3 have the most influence on antigen interaction with antibody. Previous studies have shown the CDR-H3 of autoreactive antibodies is more charged than the CDR-H3 of non-autoreactive antibodies (72, 73). Since we identified IgM structure differences with age and sex in peritoneal and splenic PC+ CD5+ B-1 cells as well as bone marrow CD5+ B-1 cells, we examined the hydrophobicity of these CD5+ B-1 cell subsets to determine if there are significant changes within the CDR-H3 region. Using the Kyte-Doolittle scale, we calculated the average hydrophobicity of each CDR-H3 loop. Our results demonstrate the CDR-H3 loop of female peritoneal PC+ CD5+ B-1 cell IgM decreases in charge (increases in hydrophobicity) with age (−0.37 +/−0.2 in young versus −0.28 +/−0.01 in aged, p<0.0001) (Figure 9A). Conversely, the CDR-H3 loop of female PC+ splenic CD5+ B-1 cells did not change with age (Figure 9A). We have previously shown the same for male peritoneal and splenic PC+ CD5+ B-1 cells (41, 42); however, comparing males and females we find both peritoneal and splenic PC+ CD5+ B-1 cell IgM from young females is more charged than young males and only the peritoneal PC+ CD5+ B-1 cell IgM from aged females is more charged than aged males. Examining the hydrophobicity of BM CD5+ B-1 cells from male and female mice with age (Figure 9B), we find the CDR-H3 loop of female BM CD5+ B-1 cell IgM increases in charge with age (−0.17 +/−0.02 in young versus −0.20 +/−0.02 in aged, p<0.0386). The CDR-H3 loop of male BM CD5+ B-1 cell IgM does not change with age. These results are interesting in that females seem to retain more highly charged CDR-H3s, which are indicative of autoantibodies and females have a higher incidence of autoimmunity (72, 73).
Figure 9: CDR-H3 hydrophobicity and amino acid changes with age and sex.

Peritoneal or splenic PC+ CD5+ B-1 cell were single cell sorted from female BALB/c-ByJ mice as indicated in Figure 5. Bone marrow CD5+ B-1 cells were single cell sorted from female and male BALB/c-ByJ mice as indicated in Figure 7. The VH region was amplified and sequenced as detailed in the Materials and Methods section. (A) The average charge of the CDR-H3 loop region of IgM from peritoneal or splenic PC+ CD5+ B-1 cells. (B) The average charge of the CDR-H3 loop region of IgM from bone marrow CD5+ B-1 cells. (C-F) The percent of each amino acid used within the CDR-H3 was determined for each CD5+ B-1 cell subset as indicated. Results are based on sequences obtained from experiments performed in Figures 5 and Figure 7 (see figures 5 and 7 for number of animals and independent experiment number). Statistics used: Mann-Whitney test for (A and B) and Chi-square for (C-F).
The hydrophobicity of the CDR-H3 is influenced by DH use, JH use, N-additions, and/or amino acid content (73). Mature murine B cells display predominance of tyrosine and glycine within the CDR-H3 region (74, 75), and changes of the amino acid content within this region can result in decreased B cell development, decreased antibody production, and an increase in infection susceptibility (76–78). Interestingly, we find many differences in amino acid content within the CDR-H3 region of PC+ CD5+ B-1 cell IgM between young and aged females (Figure 9B and 9C). Our analysis demonstrates a predominance of tyrosine and glycine in IgM from both peritoneal and splenic PC+ CD5+ B-1 cells as well as BM CD5+ B-1 cells in males and females from both young and aged mice (Figure 9C–F). We observe a significant decrease in both tyrosine and glycine in the splenic PC+ CD5+ B-1 cell IgM CDR-H3 region (Figure 9D). We also observe significant increases in arginine in both the peritoneal and splenic PC+ CD5+ B-1 cell IgM CDR-H3 region (Figure 9C–D), which is associated with an increase in autoreactive antibodies ((72, 79, 80); whereas, arginine use in female BM CD5+ B-1 cells does not change with age but decreases in aged males (Figure 9E–F). These results uncover differences in hydrophobicity and amino acid content of the CDR-H3 loop in female CD5+ B-1 cell populations with age and sex.
Together, the changes observed in aged females as compared to young males and young females in terms of increased cell number, increased activation status, increase repertoire diversity, and changes in CDR-H3 hydrophobicity / amino acid content suggest females retain active maintenance of a diverse pool of CD5+ B-1 cells over time. The mechanism of how this might occur is unclear, although increased levels of IL-5 in aged females hints at a possible mechanism. We initiated examination of this question through gene expression analysis of young and aged, male and female CD5+ B-1 cells.
Changes in CD5+ B-1 cell gene expression associated with age and sex.
Since we observed sex-specific changes in aged CD5+ B-1 cells, in particular aged females retaining protective antibodies, we examined CD5+ B-1 cells for expression of genes related to estrogen signaling to determine whether sex has a differential effect upon gene expression in the context of age. We sorted peritoneal CD5+ B-1 cells from young and aged male and female mice and analyzed differentially expressed genes (DEGs) using the RT2 Profiler PCR Array for genes associated with estrogen receptor signaling. While all genes included in this array are associated with estrogen signaling, many of these genes are not exclusively regulated by estrogen and have roles in numerous signaling pathways. To better understand the gene expression profiles from the RT2 Profiler array, relative gene expression was determined using the 2ΔCt method. The gene expression was then normalized and heat mapped, where high expression is shown in green and low expression in shown in red (Figure 10A). Hierarchical clustering of the groups shows primary linkage between gene expression profiles of young females and young males (Figure 10A). Interestingly, the next linkage group is that of the aged females, leaving the aged male gene most distinct. As expected, these data indicate genes associated with estrogen signaling are differentially expressed with sex; however, unexpectedly, these data indicate aged females maintained a CD5+ B-1 signaling niche that was more comparable to young male and female CD5+ B-1 cells than aged males.
Figure 10: Estrogen-related gene expression profiles in male and female mice with age.

RT2 profiler array for estrogen related signaling genes was performed on peritoneal CD5+ B-1 cells isolated by sorting from young (3-month-old, n=4) or aged (18–24-month-old, n=4) female or male BALB/c-ByJ mice. (A) Relative gene expression (2−ΔCt) was calculated for each gene in the array as compared to five housekeeping genes using the RT2 Profiler Array Data Analysis. Relative gene expression was then normalized using GraphPad Prism and heat mapped; high levels of gene expression are in green and low levels of gene expression are in red. Clustering analysis between groups was perfomed with average linkage and Euclidean measurement method. (B,C) qPCR was performed using TaqMan assays with cDNA from peritoneal CD5+ B-1 cells (n=4 individual mice for each group run in duplicate); assays for Esr1, Irgm1, Ikzf1, Hmga2, Nanog, and were all run and relative gene expression was calculated and plotted for each individual sex and age group. Young and aged samples were obtained from 2 independent experiments. (D) Graphical overview of how Hmga2 and Nanog relate to B-1 cell biology. The details of this figure are reviewed in the discussion section.
To validate the RT2 profiler array, qPCR analysis was performed. Interestingly, the RT2 profiler array indicated aged females upregulate estrogen receptor α (Esr1); this trend was validated with qPCR showing a large increase in Esr1 expression in the aged female CD5+ B-1 cells compared to that of any other group. Conversely, Esr2, estrogen receptor β, levels were shown to be highest in young males however, overall expression of Esr2 is much lower than Esr1 (Figure 10B). Notably, it was previously shown Esr2 deficiency had no effect on the levels of natural antibody against E. coli whereas, Esr1 deficiency led to lower levels of natural antibody against E. coli (40).
Since we observed an increase in B-1 cells in female mice with age whereas, male B-1 cells decreased in numbers with age, we examined expression of four genes related to B cell development, proliferation, and/or self-renewal (Figure 10C). IKAROS family zinc finger 1 (Ikzf1), which promotes the transition from pro-B cell to pre-B cell by promoting pre-BCR signaling and migration (81), was shown to decrease in both aged populations, but expression is largely absent in the aged male CD5+ B-1 pool in contrast to the aged female CD5+ B-1 pool. This absence in males is intriguing as it has been suggested Ikaros plays a role in controlling clonal expansion of B-1 cells possibly leading to CLL (82), which has a greater incidence in males than females. Immunity-related GTPase family M protein 1 (Irgm1) was shown to decrease in old males. Irgm1 is required for defense against various intracellular pathogens (83–86) and plays a role in HSC proliferation and response to stimuli (87). High mobility group AT-hook 2 (Hmga2), a transcription factor associated with ERα (88, 89), was shown to be upregulated in aged females compared to other groups. Hmga2 is a developmental regulator of stem cell self-renewal in mice and has been shown to be important in the aging stem cell (90, 91). Lastly, Nanog, a gene associated with embryonic stem cell renewal (92) was highly expressed in aged females as compared to all other age/sex groups. These results are displayed in Figure 10C. Hmga2 and Nanog are particularly interesting as they may have curious connections to B-1 cell biology (Figure 10D, as reviewed in the discussion). Taken together, these data indicate aged male CD5+ B-1 cells decrease expression of genes associated with stem cell proliferation (Irgm1) and/or self-renewal (Hmga2 and Nanog) while aged female CD5+ B-1 cells maintain or increase expression comparable to that of young CD5+ B-1 cells.
Discussion
Reports have demonstrated greater incidence and susceptibility of males to streptococcal infection in both human and murine systems (19, 20). Importantly, it has been shown that estrogen can affect B cell development (35, 39), B cell maturation and/or selection (36, 38), and is required for natural antibodies protective against E. coli infection (40). Herein we demonstrate natural IgM from female mice maintains protective capacity against pneumococcal infection with age (Figure 1), in stark contrast to natural IgM from aged male mice (41). This difference in protective capacity of serum IgM between aged female and male mice is not due to quantitative differences in serum Ig levels (Figure 2). Therefore, we assessed whether the cells secreting natural antibodies were influenced by sex in the aged. Although, CD5+ B-1 cells have long been thought to be the primary cells contributing to natural serum Ig, recent studies have shown B-1 cells lacking CD5 comprise the largest proportion of B-1 cells secreting antibody and importantly, CD5 expression is lost upon TLR stimulation (49, 54). Therefore, CD5+ B-1 cells might be viewed as holding the available natural antibody repertoire and CD5− B-1 cells as having the actual natural antibody repertoire. Currently, there is no way to distinguish CD5− B2 cell-derived plasmablasts from CD5− B-1 cells without a chimeric system, which limits options for study of these cells in a natural unmanipulated healthy aging environment. Based on this recent literature and limitation, we examined whether CD5+ B-1 cells, which are capable of producing natural serum antibody (4) and contribute most of the available natural antibody repertoire, exhibit sex related differences in the context of age.
Herein we find female mice display an increase in the number of peritoneal and splenic CD5+ B-1 cells with age as compared to male mice. Even in young mice, females have an increased number of peritoneal and splenic CD5+ B-1 cells (Figure 3). These results suggest an increase in expansion of female CD5+ B-1 cells with age. While expansion of CD5+ B-1 cells with age has been previously reported, these studies were performed in autoimmune prone mice (93); whereas herein, the mice are BALB/c-ByJ mice. Upon examination of the female young and aged repertoire, we find female mice maintain greater repertoire diversity with age and a higher level of structurally germline-like natural antibody as compared to our previously published results for aged male mice (Figure 5, Figure 7 and (41, 42)). Notably, both peritoneal and splenic PC-specific CD5+ B-1 cell IgM from aged females displayed more VH5 (J7183) use than PC-specific CD5+ B-1 cell IgM from aged males (Figure 6). VH5 is the most J-proximal VH gene segment and is most frequently utilized during fetal life (66–69). Frequent use of VH5 along with sustained germline-like Ig status in aged females raises the possibility that, in contrast to aged males, aged females encourage survival (and possibly expansion) of fetal-derived CD5+ B-1 cells over time, and the female environment may support the maintenance and selection of self-renewing fetal-derived CD5+ B-1 cells more so than the male environment.
CD5+ B-1 cell antibody production and maintenance are dependent upon IL-5 (55). Herein, we find serum IL-5 levels are significantly increased in aged females as compared to aged males (Figure 3J) with a significant decrease in aged males as compared to young males. While the increase in serum IL-5 correlates with a rise (females) or decline (males) in total CD5+ B-1 cell numbers with age, this increase in serum IL-5 does not correlate with serum Ig levels (Figure 2), which suggests IL-5 may play a role in maintenance of CD5+ B-1 cells with age but not antibody production. Production of IL-5 from T cells has been shown to be dependent upon PD-L2 expression on CD5+ B-1 cells (57). We did not observe a difference in surface PD-L2 expression on CD5+ B-1 cells from young or aged, male, or female mice (Figure 4). However, we do find a significant increase in CD86 surface expression on aged female peritoneal and splenic CD5+ B-1 cells (Figure 4), suggesting an increased active state. CD86 and CD80 are constitutively expressed on CD5+ B-1 cells (58). Blocking CD86 reduces CD5+ B-1 cells’ ability to present antigen, partially reduces CD5+ B-1 cell mediated Th17 generation, and increases CD5+ B-1 cell mediated Treg generation (59, 94). Therefore, alterations in CD86 with age could have significant consequences upon essential CD5+ B-1 cell mediated immune functions, but further studies are needed to elucidate the role of CD86 in aged female CD5+ B-1 cells. Furthermore, CD86 is expressed on hematopoietic stem cells (HSCs) and indicates lymphopoietic potential (62); CD5+ B-1 cells are maintained throughout adult life mainly through their ability to self-renew (4, 5, 27), an ability shared by HSCs. Therefore, in addition to activation, it is possible CD5+ B-1 cells’ self-renewal ability is differentially affected over time in the female versus male environment.
Since the data presented herein demonstrate sex-based differences in CD5+ B-1 cells over time, we examined whether gene expression changes in relation to sex and/or age in peritoneal CD5+ B-1 cells. We found aged females increased or retained significant expression levels of Hmga2 and Nanog, which are key genes involved in stem cell self-renewal and have possible connections to B-1 cells biology. Hmga2 is a an architectural transcription factor, which is important for regulation of stem cell self-renewal (90, 95) as well as promotion of cell proliferation through activation of the estrogen receptor alpha (88, 89). Hmga2 expression is regulated by Lin28/Let-7 and controls self-renewal through repression of Ink4a-Arf (91, 95), which inhibit cell cycle (96). Interesting, ectopic expression of Lin28b in adult HSC resulted in preferential reconstitution of CD5+ B-1 cells and other innate-like lymphocytes, which was shown to be mediated via Let-7 control of Arid3a; however, this ectopic expression of Lin28b did not produce the normal CD5+ B-1 cell repertoire (97, 98). Bmi1 also represses expression of Ink4a-Arf and is required for CD5+ B-1 cell self-renewal (99). Interestingly, age-related changes in Let-7 and Hmga2 expression have been shown to result in a decline in neural stem cell function (90). The transcription factor Nanog enhances self-renewal and aids in maintaining a stem-like state in embryonic stem (ES) cells (100, 101), and can enhance pSTAT3 activation downstream of the cytokine leukemia inhibitory factor (LIF) (102). LIF also maintains self-renewal of ES cells (103). Expression levels of Nanog and Hmga2 are much lower in aged male as compared to aged female CD5+ B-1 cells. Thus, female CD5+ B-1 cells, in particular aged female CD5+ B-1 cells, appear to maintain expression of a suite of genes involved in stem cell function and cellular self-renewal (see Figure 10D for summary).
Peritoneal CD5+ B-1 cells expressing CD25 have been shown to constitutively express pSTAT3, express LIF receptor, and respond to LIF (61) (summarized in Figure 10D). Herein, we find the percent of CD25+ CD5+ B-1 cells decreases in the peritoneal cavity of aged females, whereas the population increases in the spleen of aged females (Figure 4). CD25 on CD5+ B-1 cells does not mediate signaling in response to IL-2 due to the lack of CD122 (IL-2R beta chain) but instead, reflects the continual chronic B cell receptor signaling observed in these unique cells (61, 94). It is possible CD25+ CD5+ B-1 cells relocate from the peritoneal cavity to the spleen due to differential activation in the aged females. Numerous studies have demonstrated higher innate and adaptive immune responses in females as compared to males, with higher levels of TLR expression / activity and cytokine production (reviewed in (104)). Such studies suggest the female environment could play a role in increasing the activation state of CD5+ B-1 cells. Differential activation within the peritoneal cavity could lead to relocation to the spleen, as it has been shown CD5+ B-1 cell TLR activation leads to downregulation of CD9 and subsequent migration to the spleen (105). Such changes in location of CD5+ B-1 cells could have consequences upon selection of the CD5+ B-1 cell pool over time. We have previously demonstrated selection of the CD5+ B-1 cell repertoire into old age is dependent upon antigen specificity as well as location (peritoneal cavity versus spleen) (42). In addition, more recent studies have demonstrated TLR activation leads to downregulation of CD5 on CD5+ B-1 cells and CD5− B-1 cells make up a large proportion of antibody secreting B-1 cells (49, 54). We examined CD5− B cells and found aged females have increased numbers (Supplemental Figure 1); future studies will be needed to fully elucidate the how aging affects CD5− B-1 cells and the relationship to CD5+ B-1 cells in the aged setting. Thus, our results and the results of others suggests the nature of the female environment may impact the selection of CD5+ B-1 cells (and possibly CD5− B-1 cells) and/or maintain a fetal CD5+ B-1 cell population through self-renewal over time.
The results presented herein show for the first-time natural antibody from females retains its protective capacity against pneumococcal infection into old age whereas natural antibody from aged males does not preserve its protective capacity. This sex-based difference in natural antibody protection is not merely due to differing levels of serum antibody, we demonstrate cells capable of producing natural antibody, CD5+ B-1 cells, display numerous sex-related changes with age (summarized in the graphical abstract). Differences seen in aged female mice as compared to aged male mice include increased CD5+ B-1 cell numbers, surface CD86 and CD25 expression, distinct repertoires alterations, and increased Nanog and Hmg2a expression. These changes in CD5+ B-1 cells suggest that, in contrast to aged male CD5+ B-1 cells, aged female CD5+ B-1 cells may retain a higher level of self-renewal capacity into old age. While our results begin to provide clues as to the mechanism of how CD5+ B-1 cells are maintained throughout old age, further investigation is required to fully understand the distinct mechanism of maintenance over time. Together, the differences observed have implications for susceptibility to pneumococcal infection and/or other diseases common to the aged. Our study greatly extends the understanding of how this essential innate B cell subset is influenced by sex during advancing age.
Supplementary Material
Key Points:
Aged female, not male, mice maintain protective anti-pneumococcal natural antibody.
CD5+ B-1 cells from aged female and male mice differ in repertoire.
Sex influences CD5+ B-1 cells’ number, phenotype, and gene expression.
Acknowledgements
We would like to thank Dr. Thomas L. Rothstein for his thoughtful and insightful review of the manuscript.
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI154539. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ehrenstein MR, and Notley CA 2010. The importance of natural IgM: scavenger, protector and regulator. Nat Rev Immunol 10: 778–786. [DOI] [PubMed] [Google Scholar]
- 2.Blandino R, and Baumgarth N 2019. Secreted IgM: New tricks for an old molecule. J Leukoc Biol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forster I, and Rajewsky K 1987. Expansion and functional activity of Ly-1+ B cells upon transfer of peritoneal cells into allotype-congenic, newborn mice. Eur J Immunol 17: 521–528. [DOI] [PubMed] [Google Scholar]
- 4.Lalor PA, Herzenberg LA, Adams S, and Stall AM 1989. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol 19: 507–513. [DOI] [PubMed] [Google Scholar]
- 5.Baumgarth N 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11: 34–46. [DOI] [PubMed] [Google Scholar]
- 6.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, and Chen J 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 160: 4776–4787. [PubMed] [Google Scholar]
- 7.Nguyen TT, Elsner RA, and Baumgarth N 2015. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol 194: 1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsiantoulas D, Kiss M, Bartolini-Gritti B, Bergthaler A, Mallat Z, Jumaa H, and Binder CJ 2017. Secreted IgM deficiency leads to increased BCR signaling that results in abnormal splenic B cell development. Sci Rep 7: 3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ehrenstein MR, Cook HT, and Neuberger MS 2000. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J Exp Med 191: 1253–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weksler ME, Pawelec G, and Franceschi C 2009. Immune therapy for age-related diseases. Trends Immunol 30: 344–350. [DOI] [PubMed] [Google Scholar]
- 11.Binder CJ, and Silverman GJ 2005. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin Immunopathol 26: 385–404. [DOI] [PubMed] [Google Scholar]
- 12.Kearney JF, Patel P, Stefanov EK, and King RG 2015. Natural antibody repertoires: development and functional role in inhibiting allergic airway disease. Annu Rev Immunol 33: 475–504. [DOI] [PubMed] [Google Scholar]
- 13.Haas KM, Poe JC, Steeber DA, and Tedder TF 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23: 7–18. [DOI] [PubMed] [Google Scholar]
- 14.Prevention, C. f. D. C. a. 2017. Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pneumoniae, 2017
- 15.1997. Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 46: 1–24. [PubMed] [Google Scholar]
- 16.Jackson LA, Neuzil KM, Yu O, Benson P, Barlow WE, Adams AL, Hanson CA, Mahoney LD, Shay DK, and Thompson WW 2003. Effectiveness of pneumococcal polysaccharide vaccine in older adults. N Engl J Med 348: 1747–1755. [DOI] [PubMed] [Google Scholar]
- 17.Ochoa-Gondar O, Vila-Corcoles A, Ansa X, Rodriguez-Blanco T, Salsench E, de Diego C, Raga X, Gomez F, Valdivieso E, Fuentes C, and Palacios L 2008. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN-65 study. Vaccine 26: 1955–1962. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone J, Eurich DT, Minhas JK, Marrie TJ, and Majumdar SR 2010. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis 51: 15–22. [DOI] [PubMed] [Google Scholar]
- 19.Gutierrez F, Masia M, Mirete C, Soldan B, Rodriguez JC, Padilla S, Hernandez I, Royo G, and Martin-Hidalgo A 2006. The influence of age and gender on the population-based incidence of community-acquired pneumonia caused by different microbial pathogens. J Infect 53: 166–174. [DOI] [PubMed] [Google Scholar]
- 20.Kadioglu A, Cuppone AM, Trappetti C, List T, Spreafico A, Pozzi G, Andrew PW, and Oggioni MR 2011. Sex-based differences in susceptibility to respiratory and systemic pneumococcal disease in mice. J Infect Dis 204: 1971–1979. [DOI] [PubMed] [Google Scholar]
- 21.Fink AL, and Klein SL 2015. Sex and Gender Impact Immune Responses to Vaccines Among the Elderly. Physiology (Bethesda) 30: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopf M, Abel B, Gallimore A, Carroll M, and Bachmann MF 2002. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med 8: 373–378. [DOI] [PubMed] [Google Scholar]
- 23.Baumgarth N, Herman OC, Jager GC, Brown LE, Herzenberg LA, and Chen J 2000. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med 192: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen TT, Klasener K, Zurn C, Castillo PA, Brust-Mascher I, Imai DM, Bevins CL, Reardon C, Reth M, and Baumgarth N 2017. The IgM receptor FcmuR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol 18: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masmoudi H, Mota-Santos T, Huetz F, Coutinho A, and Cazenave PA 1990. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol 2: 515–520. [DOI] [PubMed] [Google Scholar]
- 26.Arnold LW, and Haughton G 1992. Autoantibodies to phosphatidylcholine. The murine antibromelain RBC response. Ann N Y Acad Sci 651: 354–359. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa K, Hardy RR, Stall AM, and Herzenberg LA 1986. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol 16: 1313–1316. [DOI] [PubMed] [Google Scholar]
- 28.Feeney AJ 1990. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med 172: 1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantor AB, Merrill CE, Herzenberg LA, and Hillson JL 1997. An unbiased analysis of V(H)-D-J(H) sequences from B-1a, B-1b, and conventional B cells. J Immunol 158: 1175–1186. [PubMed] [Google Scholar]
- 30.Briles DE, Forman C, Hudak S, and Claflin JL 1982. Anti-phosphorylcholine antibodies of the T15 idiotype are optimally protective against Streptococcus pneumoniae. J Exp Med 156: 1177–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benedict CL, Gilfillan S, Thai TH, and Kearney JF 2000. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev 175: 150–157. [PubMed] [Google Scholar]
- 32.Benedict CL, and Kearney JF 1999. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity 10: 607–617. [DOI] [PubMed] [Google Scholar]
- 33.Foster MP, Montecino-Rodriguez E, and Dorshkind K 1999. Proliferation of bone marrow pro-B cells is dependent on stimulation by the pituitary/thyroid axis. J Immunol 163: 5883–5890. [PubMed] [Google Scholar]
- 34.Montecino-Rodriguez E, Clark R, Johnson A, Collins L, and Dorshkind K 1996. Defective B cell development in Snell dwarf (dw/dw) mice can be corrected by thyroxine treatment. J Immunol 157: 3334–3340. [PubMed] [Google Scholar]
- 35.Cohen-Solal JF, Jeganathan V, Hill L, Kawabata D, Rodriguez-Pinto D, Grimaldi C, and Diamond B 2008. Hormonal regulation of B-cell function and systemic lupus erythematosus. Lupus 17: 528–532. [DOI] [PubMed] [Google Scholar]
- 36.Hill L, Jeganathan V, Chinnasamy P, Grimaldi C, and Diamond B 2011. Differential roles of estrogen receptors alpha and beta in control of B-cell maturation and selection. Mol Med 17: 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe K, Iwatani Y, Hidaka Y, Watanabe M, and Amino N 1995. Long-term effects of thyroid hormone on lymphocyte subsets in spleens and thymuses of mice. Endocr J 42: 661–668. [DOI] [PubMed] [Google Scholar]
- 38.Medina KL, Garrett KP, Thompson LF, Rossi MI, Payne KJ, and Kincade PW 2001. Identification of very early lymphoid precursors in bone marrow and their regulation by estrogen. Nat Immunol 2: 718–724. [DOI] [PubMed] [Google Scholar]
- 39.Igarashi H, Kouro T, Yokota T, Comp PC, and Kincade PW 2001. Age and stage dependency of estrogen receptor expression by lymphocyte precursors. Proc Natl Acad Sci U S A 98: 15131–15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng Z, Surewaard BGJ, Wong CHY, Guettler C, Petri B, Burkhard R, Wyss M, Le Moual H, Devinney R, Thompson GC, Blackwood J, Joffe AR, McCoy KD, Jenne CN, and Kubes P 2018. Sex-hormone-driven innate antibodies protect females and infants against EPEC infection. Nat Immunol 19: 1100–1111. [DOI] [PubMed] [Google Scholar]
- 41.Holodick NE, Vizconde T, Hopkins TJ, and Rothstein TL 2016. Age-Related Decline in Natural IgM Function: Diversification and Selection of the B-1a Cell Pool with Age. J Immunol 196: 4348–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji NR, T.L.; Holodick NE 2020. Antigen Receptor Specificity and Cell Location Influence the Diversification and Selection of the B-1a Cell Pool with Age. J Immunol In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alamyar E, Duroux P, Lefranc MP, and Giudicelli V 2012. IMGT((R)) tools for the nucleotide analysis of immunoglobulin (IG) and T cell receptor (TR) V-(D)-J repertoires, polymorphisms, and IG mutations: IMGT/V-QUEST and IMGT/HighV-QUEST for NGS. Methods Mol Biol 882: 569–604. [DOI] [PubMed] [Google Scholar]
- 44.Shriner AK, Liu H, Sun G, Guimond M, and Alugupalli KR IL-7-dependent B lymphocytes are essential for the anti-polysaccharide response and protective immunity to Streptococcus pneumoniae. J Immunol 185: 525–531. [DOI] [PubMed] [Google Scholar]
- 45.Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A, and Wishart DS 2016. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res 44: W147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riedel G, Rudrich U, Fekete-Drimusz N, Manns MP, Vondran FW, and Bock M 2014. An extended DeltaCT-method facilitating normalisation with multiple reference genes suited for quantitative RT-PCR analyses of human hepatocyte-like cells. PLoS One 9: e93031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayakawa K, Hardy RR, and Herzenberg LA 1985. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 161: 1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi YS, Dieter JA, Rothaeusler K, Luo Z, and Baumgarth N 2012. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol 42: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savage HP, Yenson VM, Sawhney SS, Mousseau BJ, Lund FE, and Baumgarth N 2017. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med 214: 2777–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baumgarth N 2004. B-cell immunophenotyping. Methods Cell Biol 75: 643–662. [DOI] [PubMed] [Google Scholar]
- 51.Hayakawa K, Hardy RR, Parks DR, and Herzenberg LA 1983. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med 157: 202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hayakawa K, Hardy RR, Honda M, Herzenberg LA, Steinberg AD, and Herzenberg LA 1984. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A 81: 2494–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kantor AB, Stall AM, Adams S, and Herzenberg LA 1992. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A 89: 3320–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savage HP, Klasener K, Smith FL, Luo Z, Reth M, and Baumgarth N 2019. TLR induces reorganization of the IgM-BCR complex regulating murine B-1 cell responses to infections. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moon BG, Takaki S, Miyake K, and Takatsu K 2004. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol 172: 6020–6029. [DOI] [PubMed] [Google Scholar]
- 56.Weksler ME 1993. Immune senescence and adrenal steroids: immune dysregulation and the action of dehydroepiandrosterone (DHEA) in old animals. Eur J Clin Pharmacol 45 Suppl 1: S21–23; discussion S43–24. [DOI] [PubMed] [Google Scholar]
- 57.McKay JT, Haro MA, Daly CA, Yammani RD, Pang B, Swords WE, and Haas KM 2017. PD-L2 Regulates B-1 Cell Antibody Production against Phosphorylcholine through an IL-5-Dependent Mechanism. J Immunol 199: 2020–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tumang JR, Hastings WD, Bai C, and Rothstein TL 2004. Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur J Immunol 34: 2158–2167. [DOI] [PubMed] [Google Scholar]
- 59.Zhong X, Gao W, Degauque N, Bai C, Lu Y, Kenny J, Oukka M, Strom TB, and Rothstein TL 2007. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol 37: 2400–2404. [DOI] [PubMed] [Google Scholar]
- 60.Zhong X, Tumang JR, Gao W, Bai C, and Rothstein TL 2007. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol 37: 2405–2410. [DOI] [PubMed] [Google Scholar]
- 61.Tumang JR, Holodick NE, Vizconde TC, Kaku H, Frances R, and Rothstein TL 2011. A CD25(−) positive population of activated B1 cells expresses LIFR and responds to LIF. Front Immunol 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimazu T, Iida R, Zhang Q, Welner RS, Medina KL, Alberola-Lla J, and Kincade PW 2012. CD86 is expressed on murine hematopoietic stem cells and denotes lymphopoietic potential. Blood 119: 4889–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savage HP, and Baumgarth N 2015. Characteristics of natural antibody-secreting cells. Ann N Y Acad Sci 1362: 132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumgarth N, Waffarn EE, and Nguyen TT 2015. Natural and induced B-1 cell immunity to infections raises questions of nature versus nurture. Ann N Y Acad Sci 1362: 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prohaska TA, Que X, Diehl CJ, Hendrikx S, Chang MW, Jepsen K, Glass CK, Benner C, and Witztum JL 2018. Massively Parallel Sequencing of Peritoneal and Splenic B Cell Repertoires Highlights Unique Properties of B-1 Cell Antibodies. J Immunol 200: 1702–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Freitas AA, Lembezat MP, and Coutinho A 1989. Expression of antibody V-regions is genetically and developmentally controlled and modulated by the B lymphocyte environment. Int Immunol 1: 342–354. [DOI] [PubMed] [Google Scholar]
- 67.Marshall AJ, Wu GE, and Paige GJ 1996. Frequency of VH81x usage during B cell development: initial decline in usage is independent of Ig heavy chain cell surface expression. J Immunol 156: 2077–2084. [PubMed] [Google Scholar]
- 68.Malynn BA, Yancopoulos GD, Barth JE, Bona CA, and Alt FW 1990. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med 171: 843–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kirkham PM, and Schroeder HW Jr. 1994. Antibody structure and the evolution of immunoglobulin V gene segments. Semin Immunol 6: 347–360. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Wang C, Yang Q, Kantor AB, Chu H, Ghosn EE, Qin G, Mazmanian SK, Han J, and Herzenberg LA 2015. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife 4: e09083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holodick NE, Vizconde T, and Rothstein TL 2014. Splenic B-1a Cells Expressing CD138 Spontaneously Secrete Large Amounts of Immunoglobulin in Naive Mice. Front Immunol 5: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnan MR, Jou NT, and Marion TN 1996. Correlation between the amino acid position of arginine in VH-CDR3 and specificity for native DNA among autoimmune antibodies. J Immunol 157: 2430–2439. [PubMed] [Google Scholar]
- 73.Khass M, Vale AM, Burrows PD, and Schroeder HW Jr. 2018. The sequences encoded by immunoglobulin diversity (DH ) gene segments play key roles in controlling B-cell development, antigen-binding site diversity, and antibody production. Immunol Rev 284: 106–119. [DOI] [PubMed] [Google Scholar]
- 74.Tonegawa S 1983. Somatic generation of antibody diversity. Nature 302: 575–581. [DOI] [PubMed] [Google Scholar]
- 75.Zemlin M, Klinger M, Link J, Zemlin C, Bauer K, Engler JA, Schroeder HW Jr., and Kirkham PM 2003. Expressed murine and human CDR-H3 intervals of equal length exhibit distinct repertoires that differ in their amino acid composition and predicted range of structures. J Mol Biol 334: 733–749. [DOI] [PubMed] [Google Scholar]
- 76.Ippolito GC, Schelonka RL, Zemlin M, Ivanov II, Kobayashi R, Zemlin C, Gartland GL, Nitschke L, Pelkonen J, Fujihashi K, Rajewsky K, and Schroeder HW Jr. 2006. Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J Exp Med 203: 1567–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nguyen HH, Zemlin M, Ivanov II, Andrasi J, Zemlin C, Vu HL, Schelonka R, Schroeder HW Jr., and Mestecky J 2007. Heterosubtypic immunity to influenza A virus infection requires a properly diversified antibody repertoire. J Virol 81: 9331–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schelonka RL, Zemlin M, Kobayashi R, Ippolito GC, Zhuang Y, Gartland GL, Szalai A, Fujihashi K, Rajewsky K, and Schroeder HW Jr. 2008. Preferential use of DH reading frame 2 alters B cell development and antigen-specific antibody production. J Immunol 181: 8409–8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Radic MZ, Mackle J, Erikson J, Mol C, Anderson WF, and Weigert M 1993. Residues that mediate DNA binding of autoimmune antibodies. J Immunol 150: 4966–4977. [PubMed] [Google Scholar]
- 80.Silva-Sanchez A, Liu CR, Vale AM, Khass M, Kapoor P, Elgavish A, Ivanov II, Ippolito GC, Schelonka RL, Schoeb TR, Burrows PD, and Schroeder HW Jr. 2015. Violation of an evolutionarily conserved immunoglobulin diversity gene sequence preference promotes production of dsDNA-specific IgG antibodies. PLoS One 10: e0118171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schwickert TA, Tagoh H, Gultekin S, Dakic A, Axelsson E, Minnich M, Ebert A, Werner B, Roth M, Cimmino L, Dickins RA, Zuber J, Jaritz M, and Busslinger M 2014. Stage-specific control of early B cell development by the transcription factor Ikaros. Nat Immunol 15: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oliveira VC, Lacerda MP, Moraes BBM, Gomes CP, Maricato JT, Souza OF, Schenkman S, Pesquero JB, Moretti NS, Rodrigues CA, and Popi AF 2019. Deregulation of Ikaros expression in B-1 cells: New insights in the malignant transformation to chronic lymphocytic leukemia. J Leukoc Biol 106: 581–594. [DOI] [PubMed] [Google Scholar]
- 83.Collazo CM, Yap GS, Sempowski GD, Lusby KC, Tessarollo L, Vande Woude GF, Sher A, and Taylor GA 2001. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J Exp Med 194: 181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feng CG, Collazo-Custodio CM, Eckhaus M, Hieny S, Belkaid Y, Elkins K, Jankovic D, Taylor GA, and Sher A 2004. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J Immunol 172: 1163–1168. [DOI] [PubMed] [Google Scholar]
- 85.MacMicking JD, Taylor GA, and McKinney JD 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science 302: 654–659. [DOI] [PubMed] [Google Scholar]
- 86.Santiago HC, Feng CG, Bafica A, Roffe E, Arantes RM, Cheever A, Taylor G, Vieira LQ, Aliberti J, Gazzinelli RT, and Sher A 2005. Mice deficient in LRG-47 display enhanced susceptibility to Trypanosoma cruzi infection associated with defective hemopoiesis and intracellular control of parasite growth. J Immunol 175: 8165–8172. [DOI] [PubMed] [Google Scholar]
- 87.Feng CG, Weksberg DC, Taylor GA, Sher A, and Goodell MA 2008. The p47 GTPase Lrg-47 (Irgm1) links host defense and hematopoietic stem cell proliferation. Cell Stem Cell 2: 83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu B, Chen G, He Q, Liu M, Gao K, Cai B, Qu J, Lin S, Geng A, Li S, Wang K, Mao Z, Wan X, and Yan Q 2020. An HMGA2-p62-ERalpha axis regulates uterine leiomyomas proliferation. FASEB J 34: 10966–10983. [DOI] [PubMed] [Google Scholar]
- 89.Wang C, Zhang T, Wang K, Zhang S, Sun Q, and Yang X 2021. ER-alpha36 Promotes the Malignant Progression of Cervical Cancer Mediated by Estrogen via HMGA2. Front Oncol 11: 712849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishino J, Kim I, Chada K, and Morrison SJ 2008. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell 135: 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hammond SM, and Sharpless NE 2008. HMGA2, microRNAs, and stem cell aging. Cell 135: 1013–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torres J, and Watt FM 2008. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFkappaB and cooperating with Stat3. Nat Cell Biol 10: 194–201. [DOI] [PubMed] [Google Scholar]
- 93.Stall AM, Farinas MC, Tarlinton DM, Lalor PA, Herzenberg LA, Strober S, and Herzenberg LA 1988. Ly-1 B-cell clones similar to human chronic lymphocytic leukemias routinely develop in older normal mice and young autoimmune (New Zealand Black-related) animals. Proc Natl Acad Sci U S A 85: 7312–7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holodick NE, Tumang JR, and Rothstein TL 2009. Continual signaling is responsible for constitutive ERK phosphorylation in B-1a cells. Mol Immunol 46: 3029–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Copley MR, Babovic S, Benz C, Knapp DJ, Beer PA, Kent DG, Wohrer S, Treloar DQ, Day C, Rowe K, Mader H, Kuchenbauer F, Humphries RK, and Eaves CJ 2013. The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat Cell Biol 15: 916–925. [DOI] [PubMed] [Google Scholar]
- 96.Kim WY, and Sharpless NE 2006. The regulation of INK4/ARF in cancer and aging. Cell 127: 265–275. [DOI] [PubMed] [Google Scholar]
- 97.Zhou Y, Li YS, Bandi SR, Tang L, Shinton SA, Hayakawa K, and Hardy RR 2015. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J Exp Med 212: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, and Muljo SA 2012. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 335: 1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kobayashi M, Lin Y, Mishra A, Shelly C, Gao R, Reeh CW, Wang PZ, Xi R, Liu Y, Wenzel P, Ghosn E, Liu Y, and Yoshimoto M 2020. Bmi1 Maintains the Self-Renewal Property of Innate-like B Lymphocytes. J Immunol 204: 3262–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J, Levasseur DN, and Orkin SH 2008. Requirement of Nanog dimerization for stem cell self-renewal and pluripotency. Proc Natl Acad Sci U S A 105: 6326–6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heurtier V, Owens N, Gonzalez I, Mueller F, Proux C, Mornico D, Clerc P, Dubois A, and Navarro P 2019. The molecular logic of Nanog-induced self-renewal in mouse embryonic stem cells. Nat Commun 10: 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stuart HT, van Oosten AL, Radzisheuskaya A, Martello G, Miller A, Dietmann S, Nichols J, and Silva JC 2014. NANOG amplifies STAT3 activation and they synergistically induce the naive pluripotent program. Curr Biol 24: 340–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cartwright P, McLean C, Sheppard A, Rivett D, Jones K, and Dalton S 2005. LIF/STAT3 controls ES cell self-renewal and pluripotency by a Myc-dependent mechanism. Development 132: 885–896. [DOI] [PubMed] [Google Scholar]
- 104.Klein SL, and Flanagan KL 2016. Sex differences in immune responses. Nat Rev Immunol 16: 626–638. [DOI] [PubMed] [Google Scholar]
- 105.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, and Fagarasan S 2006. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med 203: 2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


