Abstract
Background:
Abdominal emergencies in neonates require surgical management in almost all cases and complications may include bowel perforation, sepsis, shock, and even death. Radiological imaging has become a very important aid in the clinical setting as it shortens time to diagnosis.
Objective:
The objective of this review is to discuss the more prevalent neonatal gastrointestinal emergencies, review appropriate imaging options, and illustrate common radiological presentations of these entities.
Conclusion:
Despite advancements in imaging techniques, it is important to keep in mind that neonates have a higher susceptibility to the adverse effects of ionizing radiation, and therefore radiography and ultrasonography remain the main diagnostic modalities for ruling out the diseases with the worst prognosis. Other modalities (fluoroscopy, computed tomography, and magnetic resonance imaging) may have limited use in very specific conditions. All providers in an emergency department should be familiar with the basic radiological findings that may indicate a gastrointestinal emergency, especially in health institutions that do not have 24-h radiologist coverage.
Keywords: Gastrointestinal emergencies, Abdominal emergencies, Radiology, Neonatal imaging, Pediatric radiology
1. Introduction
Gastrointestinal emergencies in neonates (infants < 1 month old) require rapid diagnosis and treatment to optimize outcomes. Clinicians, including pediatricians, emergency physicians, critical care physicians, and generalists, as well as radiologists, must be familiar with appropriate imaging protocols for these entities and their presentations on multiple imaging modalities. Further complicating diagnosis is the goal of limiting ionizing radiation exposure, which, while necessary for all patients, is particularly important for the neonatal and pediatric population [1]. The objective of this review is to discuss neonatal abdominal emergencies, review appropriate imaging options, and illustrate common radiological presentations of these entities.
2. Methods
In this narrative, pictorial review, we conducted a literature search of the most common neonatal abdominal/gastrointestinal etiologies considered as emergencies, based on the need for prompt surgery or imminent risk of complications. PubMed, Embase, and ScienceDirect were explored. We used the following terms: “neonatal abdominal emergencies,” “neonatal abdominal surgery,” “duodenal atresia,” “jejunal atresia,” “ileal atresia,” “colonic atresia,” “Hirschsprung disease,” “midgut volvulus,” “inguinal hernia,” hypertrophic pyloric stenosis,” “intussusception,” and “necrotizing enterocolitis.” No time or language filters were set. We included articles that evaluated the performance of the different imaging modalities in diagnosing the selected pathologies in children younger than 1 month of age; conditions were divided into upper and lower depending on the location of the pathology. Our Institutional Review Board approved the use of images of cases that were selected to illustrate each condition.
3. Gastrointestinal Emergencies
3.1. Upper gastrointestinal tract
3.1.1. Hypertrophic pyloric stenosis
Hypertrophic pyloric stenosis (HPS) results from the thickening of the muscular layer of the pylorus and its failure to relax. It manifests between 3 and 6 weeks after birth with symptoms of gastric outlet obstruction, including classic projectile nonbilious emesis (occurring in 3 per 1000 live births) and associated hypochloremic alkalosis [2]. Prompt pyloromyotomy is the standard of treatment in the United States. If allowed to progress, a palpable pyloric mass, or “olive,” may be felt on physical examination [3].
Limited information in diagnosing pyloric stenosis is apparent on radiographs, but these may demonstrate a distended stomach and a paucity of intestinal gas (Fig. 1). Ultrasound (US) has outstanding sensitivity and specificity for diagnosing HPS. Real-time evaluation of the pylorus allows for exclusion of other pathologies, such as pylorospasm. HPS is characterized by a thickened wall (more than 3 mm) and elongated (more than 14 mm) antropylorus. However, pylorus measurements may vary based on age and weight [3, 4]. In contrast to pylorospasm, the pylorus will not open after extended (usually 15 min) observation. While US is the best modality for depicting HPS, the condition may also be discovered during upper gastrointestinal fluoroscopic examination of a vomiting infant (Fig. 1). The “double-track” sign and hyper or retrograde peristalsis may be observed fluoroscopically. A thin line of contrast may extend through the narrowed pyloric channel representing the “string” sign [5]. Computed tomography (CT) and magnetic resonance imaging (MRI) do not have a primary role in diagnosing HPS.
Fig. 1. Hypertrophic pyloric stenosis.
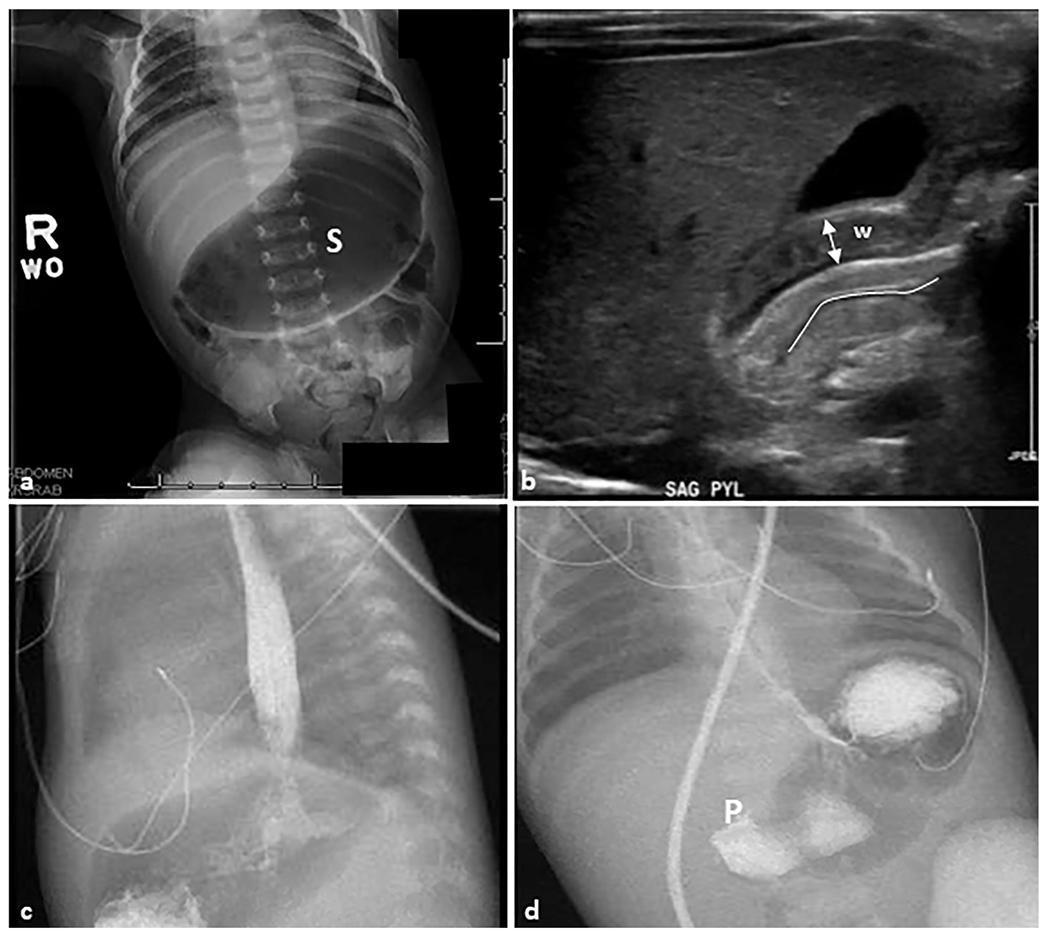
A 28-day-old female patient presented with non-bilious emesis and abdominal distension. (a) Anteroposterior abdominal radiograph demonstrating a markedly distended gas-filled stomach (S) without signs of perforation. (b) Sagittal grayscale ultrasound image reveals a thickening of the pyloric muscle wall (W) that measures 4 mm (double-headed arrow). The pyloric channel is elongated and measures 19 mm (line) and the pylorus does not open on dynamic imaging. (c, d) Sagittal and anteroposterior view contrast upper GI series show contrast crosses the lower esophageal sphincter into the air-distended stomach. There is no contrast passage across the pylorus (P), which is consistent with the previous findings.
3.1.2. Duodenal atresia
Duodenal atresia is a congenital anomaly resulting from failure of intestinal recanalization of the duodenal lumen during early embryogenesis, which results in a blind-ending duodenum. It can be associated with cardiac abnormalities and trisomy 21 and is classified as an intrinsic cause of duodenal obstruction [6]. Antenatal diagnosis through fetal US is made in approximately half of all cases [7]. Clinical manifestations include bilious vomiting within hours of birth and little or absent abdominal distention, although bilious vomiting may not occur when atresia is proximal to the biliary ducts. Other conditions such as annular pancreas and duodenal duplication cyst may mimic duodenal atresia and this often triggers similar clinical investigation [8, 9].
Conventional abdominal radiography represents the appropriate first step for suspected duodenal atresia. The presence of the “double-bubble” sign, a combination of dilated stomach and proximal duodenum with an absence of distal bowel gas, is classic for this diagnosis (Fig. 2). Antenatal US can provide early diagnosis and postnatal ultrasound can also confirm the diagnosis. On US, findings of duodenal atresia may include a dilated, fluid-filled stomach or sonographic double-bubble sign (Fig. 2), noting that a fluid-filled, dilated stomach and duodenum may represent either an intrinsic or extrinsic obstruction [10]. In duodenal atresia, there is typically a normal anatomic relationship between the superior mesenteric artery and superior mesenteric vein. Prenatally, polyhydramnios is a commonly associated secondary finding although this may not be detected until later in gestation.
Fig. 2. Duodenal atresia.
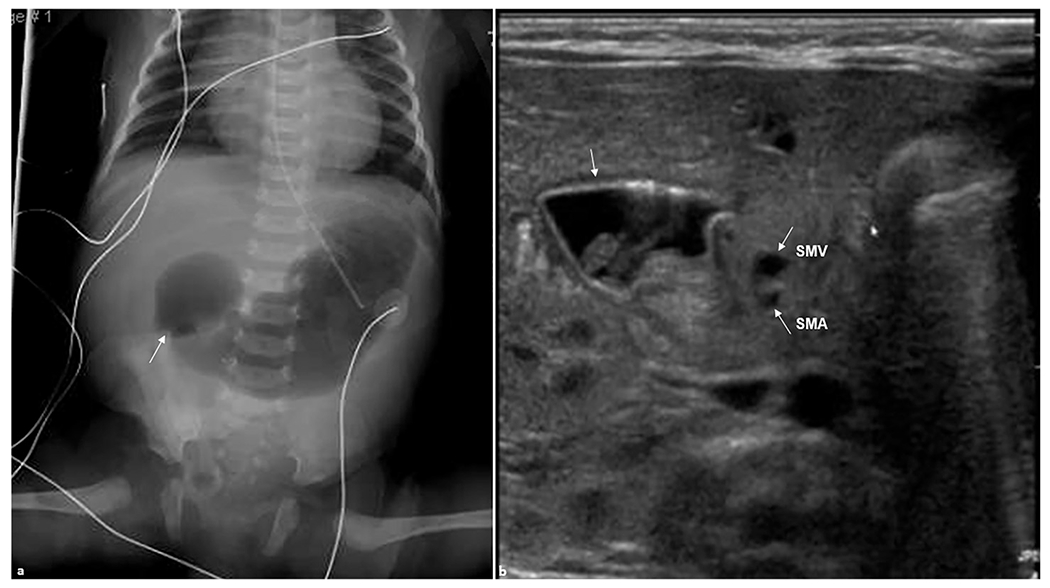
A 1-day-old female patient presented with abdominal distension. (a) Anteroposterior abdominal radiograph demonstrating gas-distended stomach and proximal duodenum (arrow) with no bowel gas seen distally. (b) Transverse real-time ultrasound examination revealed apparent dilation of the stomach and duodenal bulb (arrow) consistent with duodenal atresia. Superior mesenteric artery (SMA) and superior mesenteric vein (SMV) are shown (arrows). SMV is slightly to the left than expected of its normal location likely due to the mass effect from dilated duodenum.
Some differential diagnoses can be contemplated in the setting of duodenal obstruction [7]. Intrinsic causes of obstruction include duodenal stenosis, with milder symptoms than duodenal atresia, and duodenal webs, which are generated by an intraluminal diaphragm or diverticulum. They occur most frequently in the second part of the duodenum, and the web can be complete or perforated. Unlike duodenal atresia, distal bowel gas may be present in duodenal webs due to partial passage of intraluminal gas. Duodenal stenosis will present as an incomplete obstruction of the duodenum lumen [9]. On the other hand, extrinsic causes include annular pancreas and duodenal duplication cyst, among others. Annular pancreas occurs when there is a failure of the ventral bud to rotate behind the second portion of the duodenum. This generates a ring-like structure that may cause obstructive symptoms within the first weeks of life [11]. Additionally, duodenal duplication cyst is generally located in the first or second duodenal portions and may produce obstructive symptoms in neonates; however, this entity is rare and its clinical presentation would depend on its location and its contiguity with adjacent structures. US is considered the initial diagnostic tool for assessing both aforementioned conditions [12].
While contrast fluoroscopy is not commonly indicated for the diagnosis of duodenal atresia, it may be performed to confirm the obstruction or to help differentiate duodenal atresia from other conditions, such as midgut volvulus, that may require emergency surgery. CT is not recommended for evaluation of duodenal atresia as the diagnosis can be made with modalities that use lower doses of ionizing radiation and do not require sedation or intravenous contrast administration. MRI is not used for diagnosing duodenal atresia.
3.1.3. Midgut volvulus
During embryogenesis, growth of the bowel in the first trimester outpaces expansion of the abdominal cavity, causing it to protrude into the umbilical cord, where it rotates 90° counterclockwise before entering the abdominal cavity, at which time it undergoes an additional 180-degree rotation. Errors of rotation during these steps, which can be classified based on their rotation configuration (commonly, there is an incomplete counter-clockwise rotation), produce intestinal malrotation, placing the patient at risk for midgut volvulus, an abdominal emergency [13, 14]. Volvulus leads to bowel obstruction and ischemia secondary to twisting of bowel and the mesenteric blood supply. Symptoms including bilious emesis and irritability are seen most often during the first few weeks after birth. Emergent surgical interventions (e.g., Ladd’s procedure) must be performed to prevent advanced intestinal ischemia and circulatory collapse. Complications include sepsis, short bowel syndrome, and death [15].
Conventional abdominal radiographs may reveal dilation of the stomach and proximal duodenum with air-fluid levels and a paucity of intestinal gas; however, a normal bowel gas pattern does not exclude volvulus (Fig. 3). US can show evidence of malrotation, such as abnormal relation of the superior mesenteric artery and the superior mesenteric vein. In normal bowel rotation, the superior mesenteric artery is to the left of the superior mesenteric vein, a relationship that can be reversed in malrotation. Retromesenteric localization of the third portion of the duodenum, vessel malposition, and “whirlpool sign” are suggestive of volvulus on US. The “whirlpool sign,” a clockwise swirling of mesenteric vessels, has high sensitivity and specificity for the diagnosis of midgut volvulus (Fig. 4). Sequelae of prenatal midgut volvulus, such as fetal ascites, bowel dilation, or meconium peritonitis, can be identified by fetal US [16].
Fig. 3. Midgut volvulus.
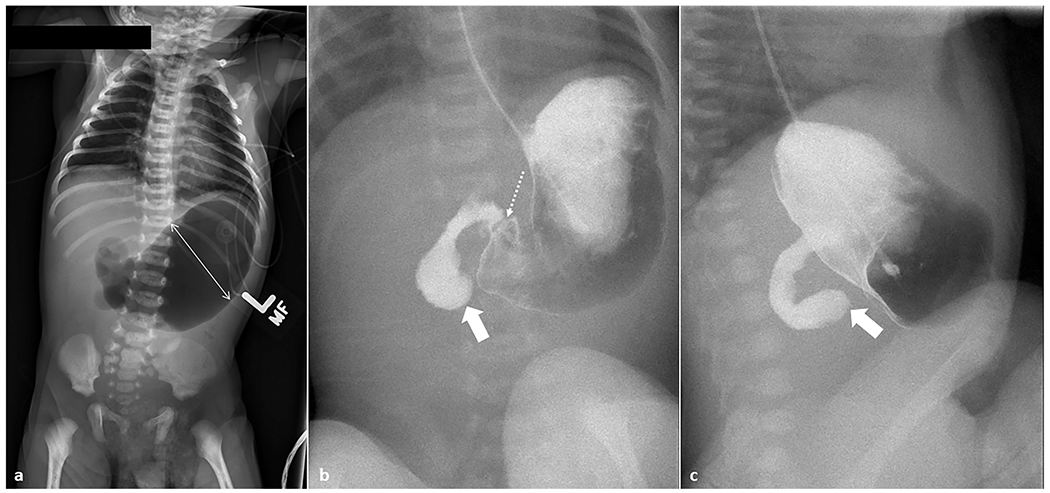
A 4-day-old male, full-term neonate presenting with bilious emesis. (a) Anteroposterior chest and abdomen radiograph (“babygram”) shows high-grade proximal bowel obstruction. The stomach and proximal duodenum are markedly distended (double-headed arrow); there is a paucity of bowel gas in the remainder of the abdomen. (b, c) Anteroposterior and lateral upper GI series show a distended stomach with contrast passing unobstructed across the pylorus (dotted arrow). Enteric contrast is seen freely progressing into the proximal duodenum with a discrete cut-off and blunted, rounded appearance of the opacified duodenum (arrows).
Fig. 4. Midgut volvulus.
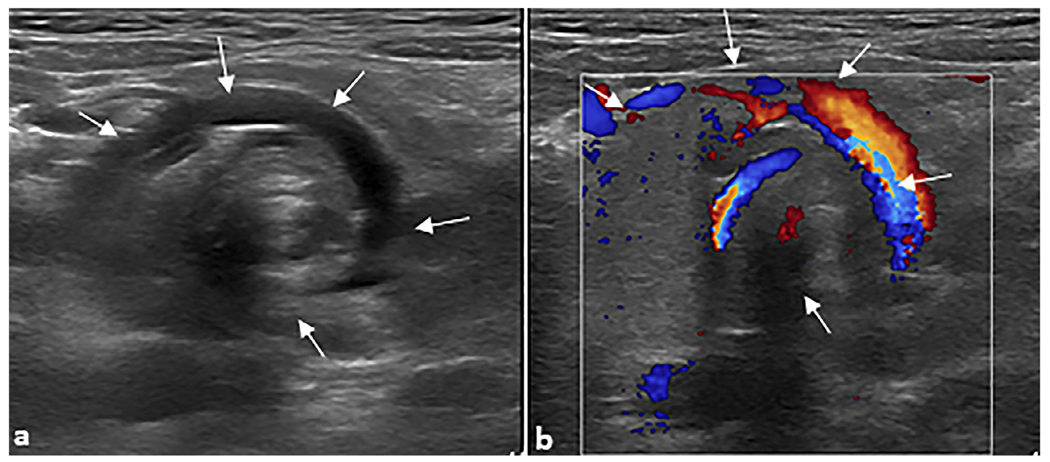
A 3-year-old female patient presented to the emergency department with intermittent abdominal pain. Transverse grayscale ultrasound without (a) and with (b) color Doppler was performed, revealing the “whirlpool” sign of midgut volvulus (arrows).
Fluoroscopic upper GI series is the standard method for definitive diagnosis (Fig. 5). Its sensitivity ranges from 93% to 100% [17]. Under normal conditions, the duodenojejunal junction (DJJ) should be located crossing to the left of the spine and rising to the level of the duodenal bulb. A true lateral fluoroscopic image can confirm retroperitoneal course. When there is incomplete rotation, the DJJ does not cross the midline, remaining on the right. “Corkscrew” or “coiled spring” appearances of the proximal small bowel are often described [13, 15]. The “bird-beaked” appearance can be present at the level of the obstruction due to luminal narrowing (Fig. 4). Regarding barium enema, if malrotation is present, the cecum could be displaced into the right upper quadrant or the left lower quadrant. CT and MR are not recommended for initial imaging evaluation of midgut volvulus, but may incidentally detect the twisting mesentery when not suspected clinically (Fig. 5).
Fig. 5. Midgut volvulus.
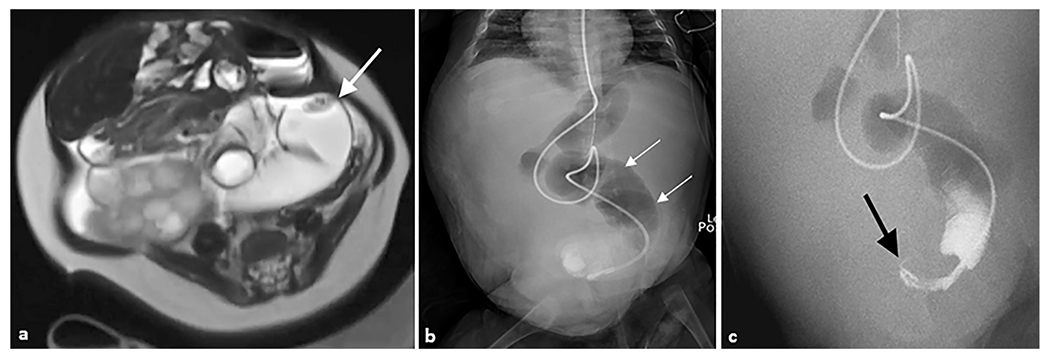
A 4-month-old female patient with a history of Beckwith–Wiedemann syndrome presented with a distended abdomen. (a) Axial T2-weighted magnetic resonance image of the abdomen demonstrates a distended loop of bowel in the left abdomen (arrow) with an apparent twist in the mesentery. (b) Anteroposterior abdominal radiograph demonstrating multiple dilated loops of bowel with an enteric tube terminating in the pelvis. (c) Coronal upper GI series with contrast passed through the enteric feeding tube and revealed a “bird’s beak” sign (arrow), indicating twist around mesenteric axis. Subsequent exploratory laparotomy revealed jejunal volvulus.
3.1.4. Jejuno-ileal atresia
Jejuno-ileal atresias are secondary to intrauterine vascular flow insults occurring between the ligament of Treitz and the ileocecal valve. Overall, jejunal atresias are more common than ileal atresias and can be associated with gastroschisis and cystic fibrosis. Patients with jejuno-ileal atresia may present with bilious emesis, abdominal distention, and failure to pass meconium; however, abdominal distention is less common if the atresia occurs proximally, which means that clinical presentation can vary depending on the level of atresia, whether it is complete or partial, and the gestational age at which the event leading to atresia occurred. One third of these patients are premature, and the diagnosis is usually made in the context of the first hospitalization of neonates presenting with intestinal obstruction [18]. Prenatal diagnosis provides an opportunity for antenatal counseling and delivery and surgical planning [18, 19]. The Grosfeld modification of Louw’s classification divides jejuno-ileal atresias into four types: type I is caused by internal membranes without mesenteric defects, type II manifests as two blind intestinal ends connected by a fibrous cord, type IIIa comprises a mesenteric gap, type IIIb is an “apple peel” atresia, and type IV consists of multiple atretic segments, although all have similar imaging manifestations and distinguishing between the four types is not typically possible. Type II and III with blind ends have higher mortality from deficient peristalsis after anastomosis. Type IIIb accounts for the least common type of jejuno-ileal atresia [18–20]. Short bowel syndrome (SBS) can occur after surgery is performed; types III and IV are more commonly related with this condition [18,19].
Conventional abdominal radiographs may show multiple dilated loops of bowel with or without air–fluid levels. This results in the classic “triple-bubble” sign with dilation of the stomach, duodenum, and jejunum (Fig. 6). Calcifications may indicate prenatal bowel perforation and meconium pseudocyst formation or meconium peritonitis [18]. By US, the presence of polyhydramnios and dilated loops of bowel can raise antenatal suspicion. However, this modality alone does not confirm or exclude the condition (Fig. 6). Fluoroscopic contrast enema can demonstrate narrow-caliber microcolon and reflux of contrast into a collapsed terminal ileum in the case of distal ileal atresia (Fig. 6) [18,21]. Upper GI fluoroscopic study may show abrupt cut-off at any level (Fig. 6). Neither CT nor MRI are used for diagnosing jejuno-ileal atresia.
Fig. 6. Jejuno-ileal atresia.
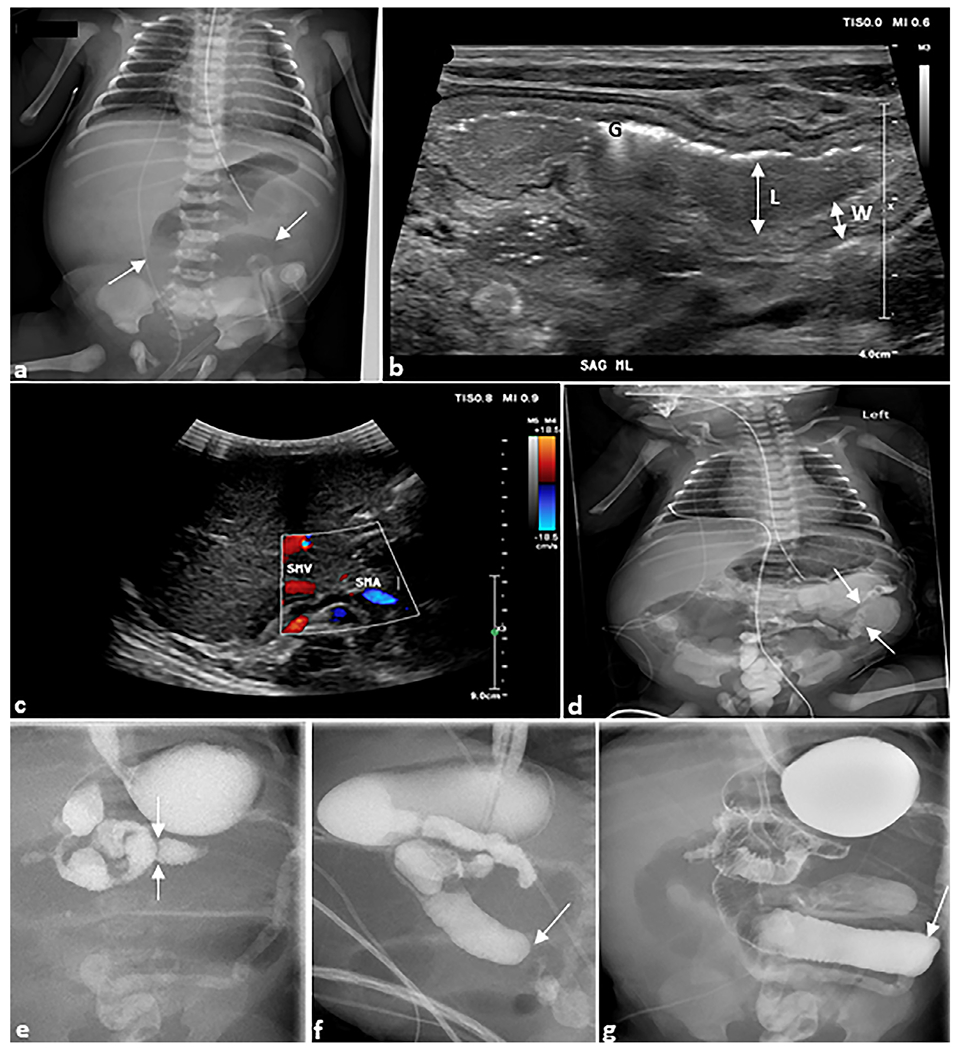
Ex-premature infant. (a) Anteroposterior chest and abdomen radiograph (“babygram”) of a 1-day-old ex-premature infant born to parents with cystic fibrosis presenting with bilious emesis. The radiograph demonstrates dilated bowel loops (arrows). (b) Sagittal grayscale ultrasound image of the midline abdomen shows a dilated (L) loop of bowel with thickened walls (W). Echogenic foci are intraluminal gas (G). (c) Axial grayscale ultrasound of the right upper quadrant with color Doppler demonstrates the superior mesenteric vein and artery in normal position arguing against malrotation with midgut volvulus. (d) Water-soluble contrast enema demonstrates microcolon (arrows). (e) Upper gastrointestinal series demonstrates a normal duodeno-jejunal junction in the left upper quadrant (arrows) followed by abrupt cutoffs of the jejunum (arrows) in (f) sagittal and (g) anteroposterior views. At surgery, this was determined to be jejunal atresia, type IIIB.
3.1.5. Necrotizing enterocolitis
Necrotizing enterocolitis usually presents in premature newborns with extremely low birth weight. This entity predisposes to bowel infection and ischemia resulting in sepsis and hemodynamic instability, and it commonly presents within the first week of life in late preterm infants or up to the fourth week in severely premature infants. Mortality can be as high as 30%. Predisposing factors for mortality include lower birth weight, lower gestational age, history of hypotension, sepsis, and surgical management of the disease, among others. Signs and symptoms are abdominal distention, bilious emesis, and poor feeding. As the disease progresses, fever, lethargy, and blood in stool can also be present. The Bell system of imaging findings has been proposed to standardize the stratification of these patients. Differential considerations given these clinical findings include bowel obstruction and/or perforation [22, 23].
Serial radiographs are used to evaluate disease progression, although images can be normal at early stages of the disease. Intestinal dilation and paucity of bowel gas in the right lower quadrant can be seen, although bowel decompression by enteric sump tube may mask these findings. A very distinctive finding is the presence of pneumatosis intestinalis or pneumatosis coli, gas within the bowel wall visible as rounded lucencies (Fig. 7), most commonly in the right lower quadrant. Portal venous gas may be present [22–24]. If bowel necrosis is present, even without pneumatosis, sentinel loops (fixed bowel loops) may be evident. Surgery is indicated if there are radiographic signs of free intraperitoneal air, such as Rigler’s sign (both sides of the bowel wall are visualized), or the football sign (massive pneumoperitoneum distending the abdominal cavity). Radiographs may be taken in a supine cross-lateral and left lateral decubitus to increase sensitivity for detecting pneumoperitoneum. Normal radiographs do not exclude the disorder [24].
Fig. 7. Necrotizing enterocolitis.
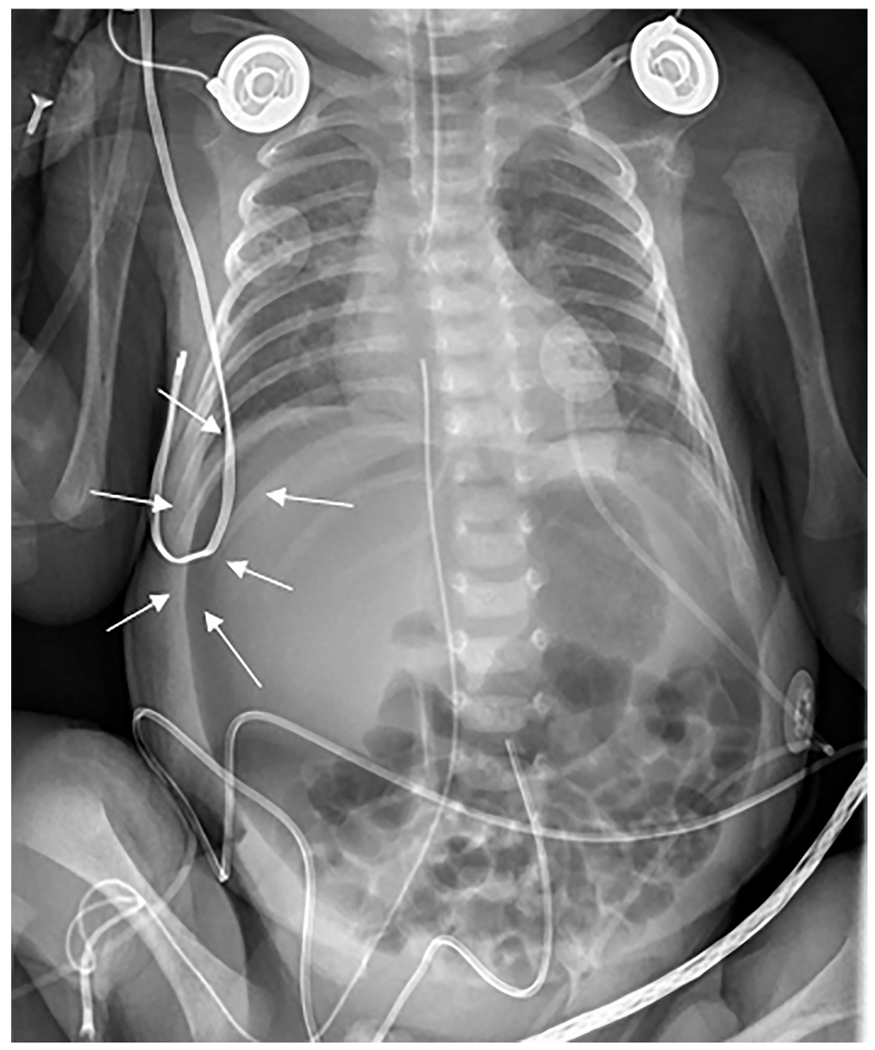
Anteroposterior chest and abdomen radiograph (“babygram”) of a 2-day-old ex-30-week premature infant admitted to the intensive care unit with profound metabolic acidosis and tense abdomen. The radiograph revealed apparent decreased density of the liver (“lucent liver sign”) with a crescentic lucency outlining the liver edge (arrows). Subtle small lucencies were seen overlying multiple bowel loops, most notable in the right lower quadrant. This patient had necrotizing enterocolitis with segmental volvulus and bowel perforation.
US has become fundamental for evaluating early changes in peristalsis, abdominal wall integrity, presence of intramural or portal gas, blood flow, and free fluid/gas in the peritoneal cavity (Fig. 8) [25]. Pneumatosis and portal venous gas can be detected earlier by US as hyperechoic foci with dirty shadowing than by radiographs. Moreover, US can help predict disease severity [26, 27]. Even though this modality has a high specificity for detecting findings in necrotizing enterocolitis, such as intestinal bowel thickening, intramural gas, and vascular alterations, its overall sensitivity is low when compared to plain radiograph [28]. Bowel wall thickness and hyperemia can be detected at early stages of the disease on grayscale and color Doppler ultrasound. The thickening of the valvulae conniventes and their interspaces resemble zebra stripes (Fig. 9) [29]. A decreased diastolic flow rate in the umbilical artery can represent an independent risk factor for poor prognosis [27].
Fig. 8. Necrotizing enterocolitis.
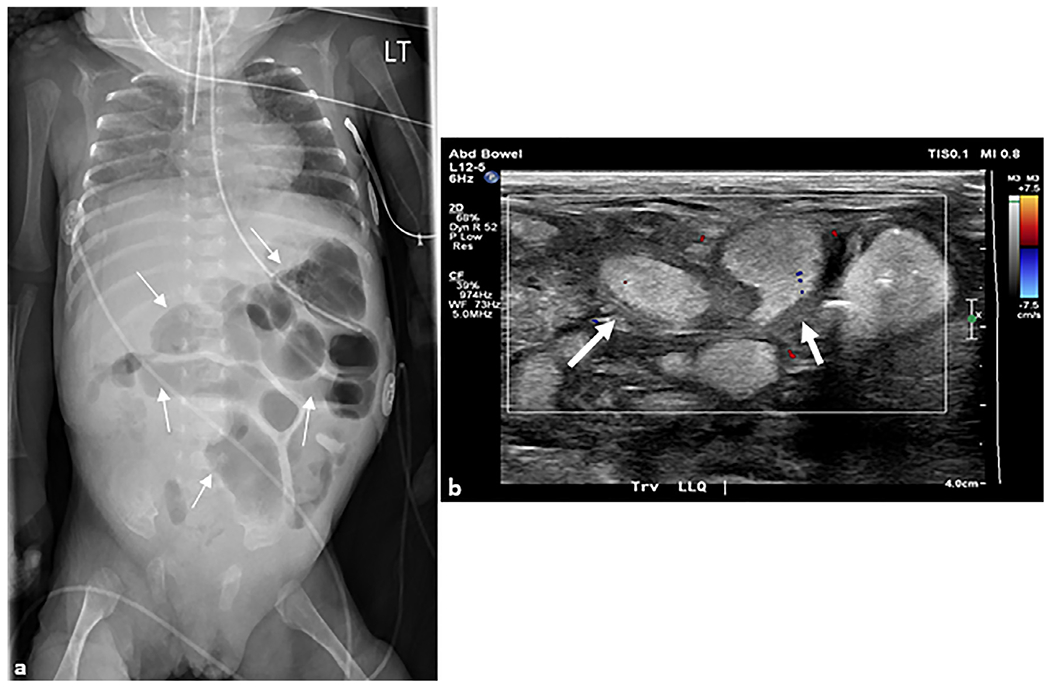
(a) Anteroposterior chest and abdomen radiograph (“babygram”) of a 2-month-old ex-premature infant who presented to the intensive care unit with a tense and distended abdomen. Multiple distended loops of bowel (arrows) are seen in the left abdomen with a paucity of bowel in the right lower quadrant. (b) Transverse grayscale ultrasound image with color Doppler overlay of the right lower quadrant demonstrates multiple loops of distended small bowel containing complex fluid with thickened walls (arrows) and echogenic contents. No color Doppler flow is seen in the bowel wall concerning for ischemia. At surgery, this patient had 22 cm of ischemic bowel.
Fig. 9. Necrotizing enterocolitis.
An 8-day-old premature girl was born at 31 weeks. (a) Sagittal and (b) transverse grayscale ultrasound images show hypoperistaltic bowel loops with areas of wall thickening and mucosal hyperechogenicity consistent with “zebra pattern” (arrows). No discrete pneumatosis or portal venous air is present.
Contrast-enhanced US (CEUS) has been described in aiding in the detection of vascular abnormalities earlier than conventional US, as well as objectively quantifying perfusion kinetics and serves as a promising modality for screening high-risk neonates and following infants diagnosed with necrotizing enterocolitis [30]. CEUS may provide additional information in some cases (Fig. 10); however, further studies are necessary to evaluate their cost-effectiveness [31]. CT and MRI are not recommended for evaluation of necrotizing enterocolitis.
Fig. 10. Necrotizing enterocolitis.
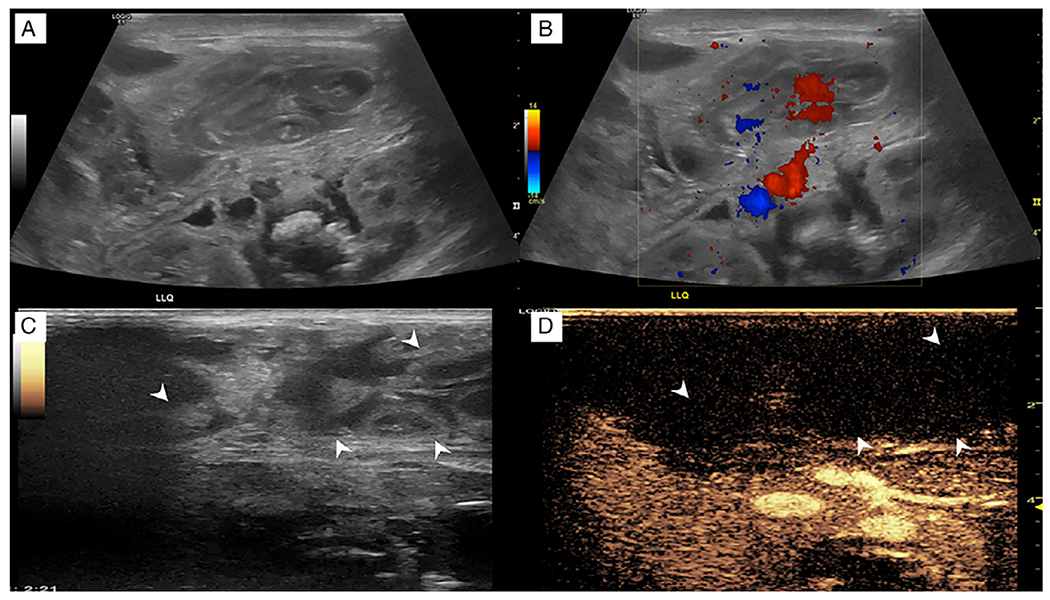
A 1-day-old formerly premature girl was born at 29 weeks with gaseous distention on abdominal radiography. (a) Grayscale ultrasound in the left upper quadrant shows multiple dilated loops of bowel with wall thickening and hypoperistalsis to aperistalsis in real time. (b) Corresponding color Doppler image shows apparent flow in the mesentery but no appreciable flow in the bowel. However, the interpretation was limited by pulsatile motion from the patient’s high-frequency oscillator. (c, d) Dual-screen contrast-enhanced ultrasound displays loops of bowel (arrowheads) in the right upper quadrant that do not enhance (arrowheads) regardless of the high-frequency oscillator. (Images reprinted from Benjamin J et al. [31] with permission).
3.2. Lower gastrointestinal tract
3.2.1. Inguinal hernia
Inguinal hernias occur when abdominal viscera protrude through a weakening or abnormal opening of the lower anterior abdominal wall. They are more common on the right side (because of delayed descent of the right testicle compared to the left), in males, and in premature infants. If incarcerated or eventually strangulated, these can lead to death or considerable morbidity including gonadal dysfunction or intestinal obstruction, stricture, necrosis, and perforation [32]. As such, diagnosis and surgical intervention should be prompt.
Indirect inguinal hernias, due to a congenitally patent processus vaginalis, are the most common inguinal hernia in children, and pass through the deep and superficial inguinal rings, lateral to the inferior epigastric vessels. Although clinical examination is sufficient for diagnosis and making decisions on surgical interventions in most cases, US may add value when suspecting incarcerated or strangulated hernias [33]. Inguinal hernias may contain bowel, the appendix, and/or gonadal tissue (testes and ovaries). Non-obliteration of processus vaginalis in males can lead to either an indirect scrotal hernia or a congenital communicating hydrocele. Funicular or encysted hydroceles can also appear. Similarly, incomplete obliteration of the canal of Nuck in females can lead to inguinolabial swelling, hernia, and hydrocele into the labia majora [34].
Plain abdominal radiographs will poorly demonstrate the soft tissue components of the hernia, but air in bowel loops may be seen in abnormal locations, such as overlying the scrotum (Fig. 11). After clinical examination, US is the modality of choice for assessment of inguinal hernias, even though a normal US may not rule out a persistent processus vaginalis (Fig. 11) [34]. The anatomical landmarks around the inguinal canal should be used for systematic examination from the deep to the superficial ring. Changes in patient positioning and maneuvers that increase intra-abdominal pressure can facilitate visualization of hernias, such as making the patients cry reactively by preventing their free movement or by removing their feeding bottle. Color Doppler US helps identify strangulated hernias in which Doppler flow in vascular structures will be decreased or absent. Manual reduction by the radiologist can be attempted in US for incarcerated hernias provided there is no peritonitis [32].
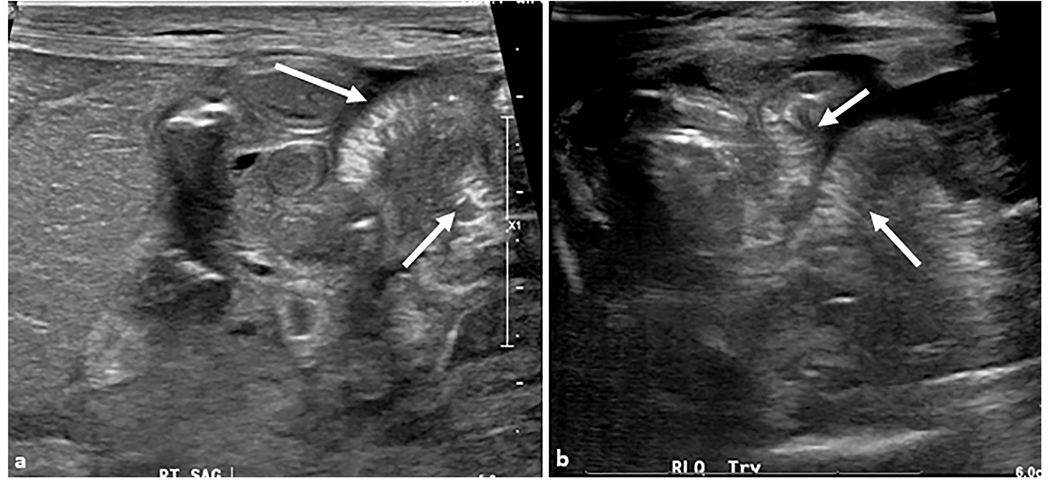
Fig. 11. Inguinal hernia.
A 14-day-old male patient presented with vomiting and right scrotal mass. (a) Cross-table lateral abdominal radiograph showing mildly dilated loops of small bowel with multiple air–fluid levels concerning for mechanical obstruction. (b, c) Transverse grayscale ultrasound examination of the right inguinal region shows a right inguinal hernia containing multiple loops of small bowel (arrow). The herniated bowel is hyperperistaltic with mild wall thickening. Motility and inflammation indicate viable bowel but urgent intervention is required to prevent incarceration and ischemia.
3.2.2. Intussusception
This condition is defined as an invagination of a proximal intestinal segment into the distal intestine. Although most cases occur in infants, there have been reported cases of this condition in neonates, accounting for 0.3% of all cases of intussusceptions. Symptoms include lethargy, colicky abdominal pain, vomiting, and bloody mucous stool. Most cases present at the ileocolic junction and are idiopathic. Respiratory distress syndrome and patent ductus arteriosus may be associated with intussusception in neonates [15, 35].
A soft-tissue mass with paucity of bowel gas in the right upper quadrant may be seen in plain abdominal radiographs; however, there is evidence of poor sensitivity and specificity on this condition. This modality could be useful to exclude perforation by presence of free air in the abdominal cavity. In fact, some institutions require radiographs prior to fluoroscopic reduction [15]. US is the gold standard for detecting intussusceptions, especially in neonates, since it has high sensitivity (96.6–100%) and specificity (88–100%) [36]. Moreover, no ionizing radiation is used. The “target sign” seen by US contains the layers of the small and large bowel and is represented as alternating concentric structures in the transverse plane. The small bowel is relatively hypoechoic but layered, the surrounding fat is hyperechoic, and the external large bowel is also hypoechoic but also layered (Fig. 12). Longitudinally, there would be hypoechoic large bowel surrounding hyperechoic fat and a hypoechoic small bowel intussusceptum, also known as “sandwich-like” appearance. Presence of free peritoneal air is indicative of perforation, usually accompanied by necrotic bowel appearance, which requires surgical intervention [37, 15]. Fluoroscopic contrast enema can be both diagnostic and therapeutic in older children, but not recommended in preterm neonates due to the ileo-ileal location of the intussusceptions and the higher risk of complications, such as intestinal perforation or metabolic disturbances. Insufflation of air into the colon can reduce the intussusception [35]. Enema is contraindicated if perforation and peritonitis are suspected. CT and MRI are not recommended for evaluation of intussusception, but it may be detected incidentally on CT or MRI performed for other diagnostic purposes
Fig. 12. Intussusception.
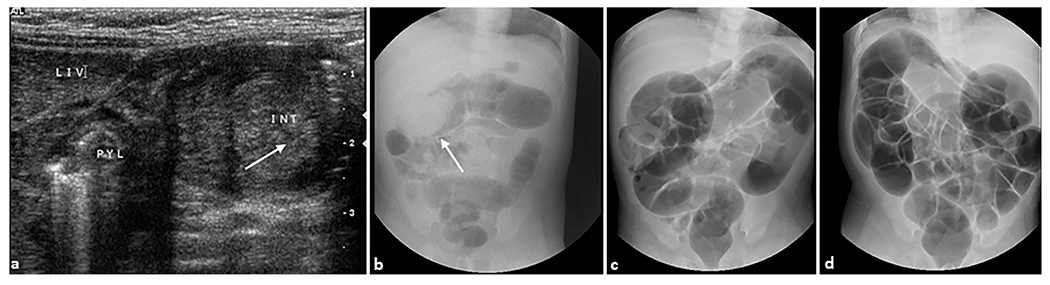
A 1-month-old female infant presented with bloody stools and abdominal distension. (a) Transverse grayscale ultrasound showed an elongated mass containing concentric layers of different echogenicity in the mid upper abdomen, consistent with ileocolic intussusception (arrow). The mass is medial to the liver and pylorus. Therapeutic air enema was performed. (b) A preliminary spot radiograph of the abdomen shows a soft tissue density (arrow) in the mid upper abdomen thought to represent the intussusceptum/intussuscepiens complex. At the first attempt the intussusception was easily reduced to the level of the ileocecal valve. During the second attempt (c), the intussusception was completely reduced past the ileocecal valve, with air entering the terminal ileum and distal small bowel. At the end of the evaluation (d), there was no radiographic evidence of intussusception.
3.2.3. Hirschsprung disease
Also called “congenital aganglionic megacolon,” Hirschsprung’s disease (HD) is a congenital condition in which neural crest cells do not migrate to the submucosal and myenteric plexuses, resulting in functional obstruction; patients with trisomy 21 and neurocristopathy syndromes are at higher risk of presenting with this condition [38]. Up to 20% of neonates with bowel obstruction have HD, and the archetypal presentation is abdominal distension and failure to pass meconium beyond the first 24 h of life. Aganglionic regions are usually present in the recto-sigmoid colon, with approximately 5% of cases involving the entire colon [39, 40]. Definitive diagnosis is made with a biopsy demonstrating absence of ganglion cells in the colonic intramural tissue. Neonatal bowel perforation is a severe complication if HD is left untreated [41].
Plain abdominal radiography is the preferred initial imaging modality for initial evaluation of HD. Gaseous distension and air-fluid levels proximal to the aganglionic segment with absence of rectal gas are observed; the best approach for this image is prone cross-table lateral radiographs to visualize the gas within the anti-dependent distal colon and rectum (Fig. 13). Routine second-trimester prenatal US is neither sensitive nor specific in the antenatal diagnosis of HD and a normal fetal anatomic survey does not exclude the diagnosis [42]. Theoretically, fetal US could reveal a caliber change at the junction of ganglionic and aganglionic segments that can be identified in the late second trimester and has also been described in fetal MRI [43]. US in HD can show echogenic bowel reflecting meconium from colonic hypo-peristalsis. Other findings include increased fetal abdominal distension, as measured by the abdominal circumference measurement [40, 43]. Fluoroscopic contrast enema can help identify the transition zone between ganglionic and aganglionic segments [44]. The primary fluoroscopic finding is a rectosigmoid ratio of less than 1 in infants with HD. Baad et al. demonstrated that neonatal contrast enema had a moderate specificity (87.7%) and low sensitivity (65.5%) for HD [45]. A “saw-toothed” appearance of the aganglionic segment’s mucosa secondary to impaired peristalsis can also be seen (Fig. 13). CT is not recommended for evaluation of HD. MRI is not commonly used for diagnosing HD. However, fetal MRI could identify decreased caliber of the colon in certain cases [43].
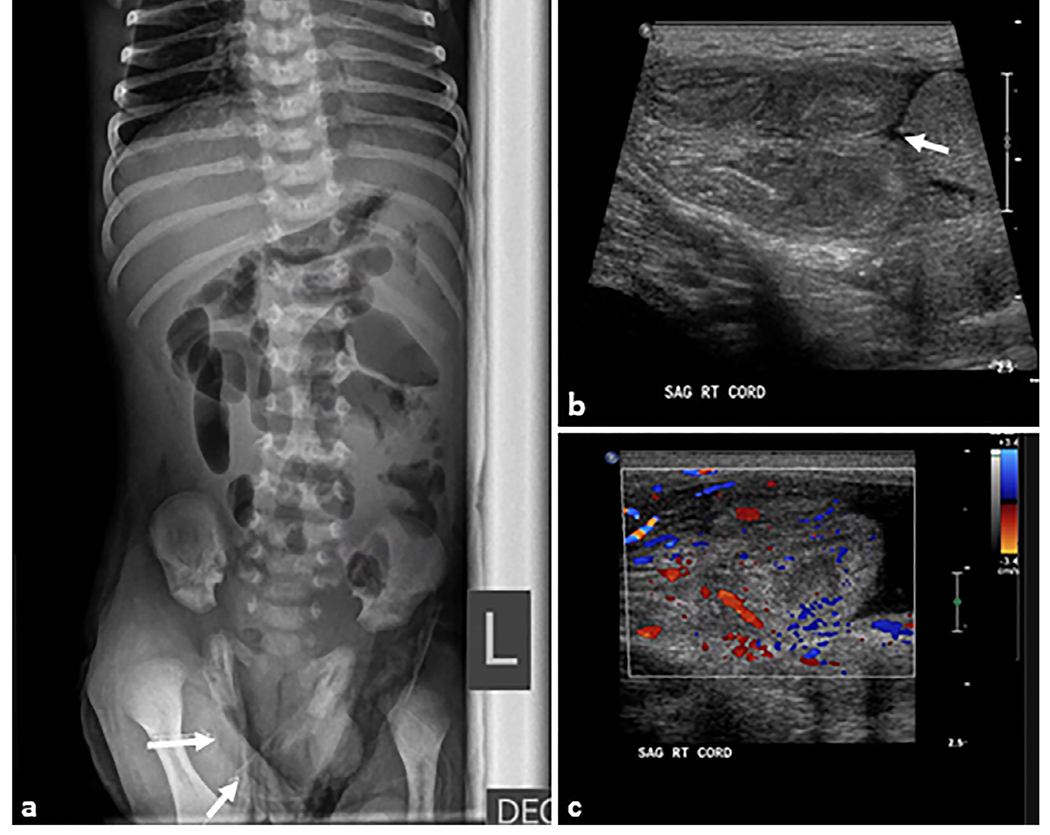
Fig. 13. Hirschsprung disease.
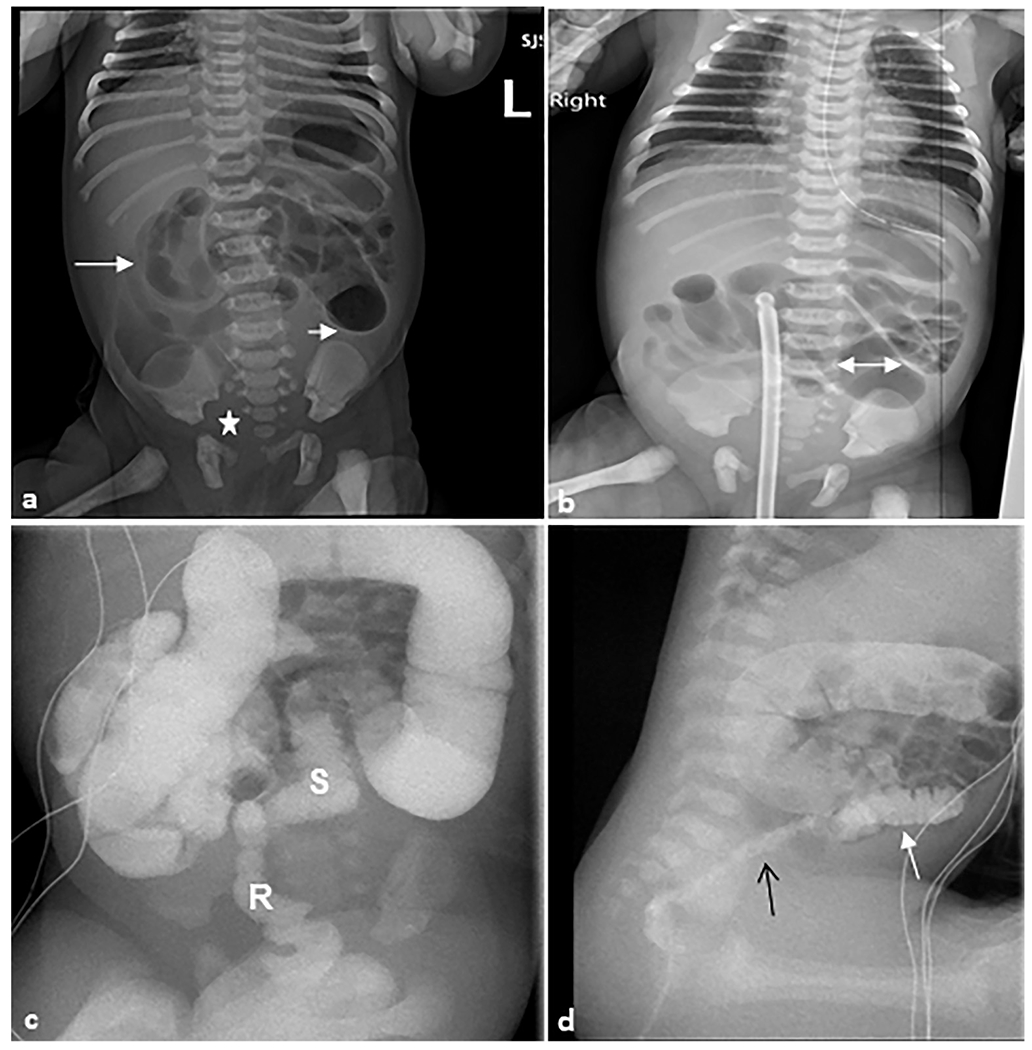
A 1-day-old female patient who presented with a distended abdomen and emesis. Rectal biopsy confirmed Hirschsprung disease. (a) Anteroposterior abdominal radiograph demonstrating multiple loops of air distended bowel (arrows) with paucity of bowel gas within the pelvis (star). (b) There are dilated proximal bowel loops with the maximal diameter of 2.7 cm (double-headed arrow) with featureless appearance of bowel loops in the right upper quadrant. (c) Fluoroscopic contrast enema demonstrating the sigmoid colon and rectum are small (S) in caliber up to the descending colon-sigmoid colon junction. A rectal catheter (R) is present with tip to the right of L2–L3. (d) Sagittal fluoroscopic image demonstrating an abnormal rectosigmoid ratio (<1) with the rectum (black arrow) of much smaller caliber than the sigmoid colon (white arrow) and upstream colon.
Differential diagnoses include: colonic atresia; small left colon syndrome; meconium plug syndrome, in which the rectosigmoid ratio would be greater than 1; and meconium ileus, more common in patients with cystic fibrosis. These entities can present with symptoms of bowel obstruction and can therefore be considered as abdominal emergencies. Small left colon syndrome in neonates can occur due to maternal diabetes and is described as a short-term functional condition due to poorly developed myenteric ganglion cells, demonstrating a reduction in descending colon caliber with an abrupt transition zone on abdominal radiographs or fluoroscopy [46]. Meconium plug syndrome is the most common cause of failure to pass meconium beyond 48 h after birth and is generally mild. Filling defects in the distal colon/rectum can be seen in contrast enema studies, which are also therapeutic [47]. Meconium ileus includes the colon and the distal small bowel. Clinical evaluation includes examination of meconium consistency, which would appear inspissated. Complications of this condition include perforation and peritonitis [48].
3.2.4. Colonic atresia
Colonic atresia is an uncommon cause of intestinal obstruction in neonates. The right colon is more frequently affected than the left [49]. It is secondary to both intrauterine extrinsic mesenteric vascular and intraluminal obstruction and is often associated with other GI pathologies, such as small intestine atresia, gastroschisis, omphalocele, and extra-abdominal malformations. Common clinical manifestations include abdominal distension and failure to pass meconium [50].
Conventional abdominal radiographs will show multiple distended bowel loops and air-fluid levels within the large bowel. US is not typically used for this diagnosis but could be useful for diagnosis of colonic atresia if further explored. In addition, US can identify lesions as alternate causes of obstruction, such as meconium plug. Fluoroscopic contrast enema is preferred for identifying the anatomical configuration of the colon (Fig. 14) [51, 52]. A narrow colon will be seen to the level of the atresia without filling of the bowel proximal to the obstruction. If the distal colon loops back on itself, this produces the “hook sign.” This sign would account for a mesenteric defect associated with the colonic atresia [53]. Neither CT nor MRI are used for diagnosing colonic atresia.
Fig. 14. Colonic atresia.
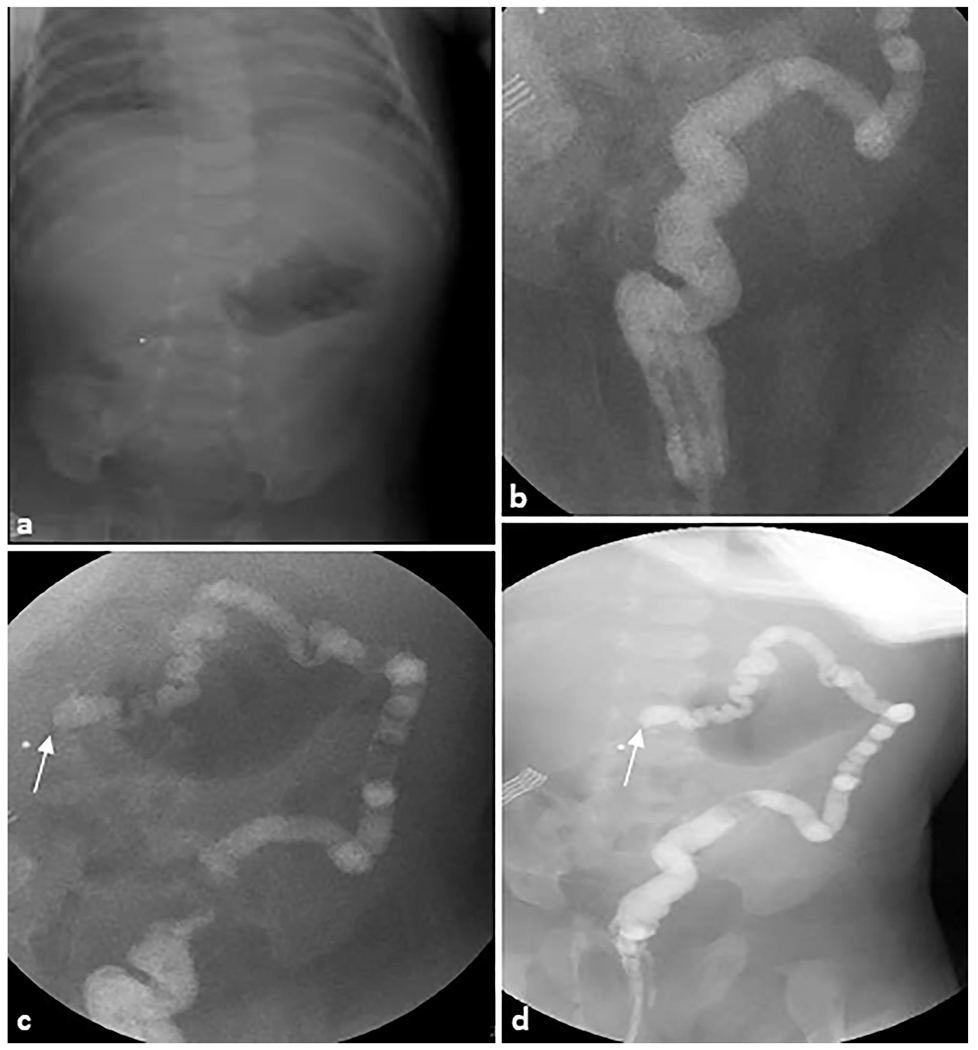
A 30-day-old female patient was evaluated for status post failed ileocolonic anastomosis due to intestinal atresia. (a) Scout radiograph demonstrates an unremarkable bowel gas pattern. (b–d) Fluoroscopic contrast enema demonstrates contrast refluxing into the rectum, sigmoid, descending, and distal transverse colon, which is small in caliber. There is abrupt termination of the contrast column in the mid transverse colon (arrows), highly suggestive of an area of colonic atresia.
4. Discussion
Abdominal pain, distension, and vomiting are frequent problems in neonatal emergency services. Contemporary management of abdominal emergencies requires an expeditious radiological examination to determine the need for surgical intervention. Therefore, familiarity with the appropriate imaging protocols for evaluating these symptoms is a critical skill for clinicians (Table 1). Conventional radiographs are a common and proper choice for initial evaluation of abdominal distension in the neonatal population in the acute setting due to their relatively low radiation dose, portability, and speed of acquisition; the preferred technique for imaging acquisition is an anteroposterior view with a supine patient during full inspiration; lateral decubitus and cross-table projections can be useful for evaluating presence of free gas when suspecting perforation [54–56]. Some diseases have distinctive imaging findings on radiography and require no additional workup, whereas other entities overlap in their radiographic findings. In the latter case, radiography often guides the next imaging step, which is frequently upper GI or enema fluoroscopy. CT is rarely needed in neonatal abdominal emergencies. However, it may be useful in characterization of masses or for abdominal emergencies in older infants and children.
Table 1.
Abdominal emergency versus imaging modality of choice according to existing literature.
| Entity vs. Imaging modality of choice | Plain abdominal X-ray | Ultrasound | Upper GI series (fluoroscopy) | Contrast enema (fluoroscopy) | Computed tomography | Magnetic resonance imaging |
|---|---|---|---|---|---|---|
| Hypertrophic pyloric stenosis | Not commonly indicated but sometimes useful | Preferred modality | Not commonly indicated but sometimes useful | Not recommended | Not recommended | Not recommended |
| Duodenal atresia | Preferred modality | Useful in most cases* | Not commonly indicated but sometimes useful | Not commonly indicated but sometimes useful | Not recommended | Not recommended |
| Midgut volvulus | Useful in most cases | Useful in most cases | Preferred modality | Not commonly indicated but sometimes useful | Not commonly indicated but sometimes useful | Not commonly indicated but sometimes useful |
| Jejuno-ileal atresia | Preferred modality | Useful in most cases* | Useful in most cases | Useful in most cases | Not recommended | Not recommended |
| Necrotizing enterocolitis | Preferred modality | Useful in most cases | Not recommended | Not recommended | Not recommended | Not recommended |
| Inguinal hernia | Not commonly indicated but sometimes useful | Preferred modality | Not recommended | Not recommended | Not commonly indicated but sometimes useful | Not commonly indicated but sometimes useful |
| Intussusception | Not commonly indicated but sometimes useful | Preferred modality | Not recommended | Useful in most cases+ | Not recommended | Not recommended |
| Hirschsprung disease | Preferred modality | Not commonly indicated but sometimes useful* | Not recommended | Useful in most cases | Not recommended | Not commonly indicated but sometimes useful* |
| Colonic atresia | Useful in most cases | Not commonly indicated but sometimes useful | Not recommended | Preferred modality | Not recommended | Not recommended |
Can be performed antenatally and aid in diagnosis
Can be diagnostic and therapeutic.
US, while dependent on sonographer skill and limitations in the setting of gas-distended bowel, is a valuable modality in evaluating the neonatal abdomen. US enables the acquisition of images in any orientation, does not use ionizing radiation, can be performed and analyzed in real-time at the bedside, and is less expensive than most other cross-sectional imaging techniques [57]. MRI can be considered but has limited utility in some clinical entities and with its higher cost, long acquisition times, and susceptibility to motion artifacts is not routinely used in the context of abdominal emergencies in children [58]. Detailed diagnostic algorithms for approaching neonates with suspected GI obstruction have been proposed and can help the clinician make stepwise decisions [59, 60].
5. Conclusion
Neonatal abdominal emergencies frequently demand surgical intervention; therefore, a rapid and precise diagnosis should be made after arrival at the emergency department or detection in the newborn nursery. Clinicians must be aware of which image modality is useful for confirming a suspected condition and should have basic knowledge of how to interpret those studies. Plain abdominal radiography is still the gold standard for several illnesses, although US has emerged as an important technique given its availability as a bedside modality that does not require ionizing radiation or sedation.
Abbreviations:
- HPS
Hypertrophic Pyloric Stenosis
- US
Ultrasound
- MRI
Magnetic Resonance Imaging
- CT
Computed Tomography
- SBS
Short Bowel Syndrome
- HD
Hirschsprung’s Disease
- CEUS
Contrast-Enhanced Ultrasound
- DJJ
Duodenojejunal Junction
Footnotes
Conflict of interest statement
None declared.
References
- [1].Louie JP. Essential diagnosis of abdominal emergencies in the first year of life. Emerg Med Clin North Am 2007;25:1009–40 vi. [DOI] [PubMed] [Google Scholar]
- [2].Ezomike UO, Ekenze SO, Amah CC, et al. Infantile hypertrophic pyloric stenosis - our experience and challenges in a developing country. Afr J Paediatr Surg 2018;15:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Costa Dias S, Swinson S, Torrão H, et al. Hypertrophic pyloric stenosis: tips and tricks for ultrasound diagnosis. Insights Imaging 2012;3:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Argyropoulou MI, Hadjigeorgi CG, Kiortsis DN. Antro-pyloric canal values from early prematurity to full-term gestational age: an ultrasound study. Pediatr Radiol 1998;28:933–6. [DOI] [PubMed] [Google Scholar]
- [5].Hiorns MP. Gastrointestinal tract imaging in children: current techniques. Pediatr Radiol 2011;41:42–54. [DOI] [PubMed] [Google Scholar]
- [6].Al-Salem AH. Congenital intrinsic duodenal obstruction: a review of 35 cases. Ann Saudi Med 2007;27:289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choudhry MS, Rahman N, Boyd P, et al. Duodenal atresia: associated anomalies, prenatal diagnosis and outcome. Pediatr Surg Int 2009;25:727–30. [DOI] [PubMed] [Google Scholar]
- [8].Bethell GS, Long AM, Knight M, et al. One-year outcomes of congenital duodenal obstruction: a population-based study. J Pediatr Gastroenterol Nutr 2021;72:239–43. [DOI] [PubMed] [Google Scholar]
- [9].Sigmon DF, Eovaldi BJ, Cohen HL. Duodenal atresia and stenosis. Treasure Island (FL): StatPearls Publishing; 2021. StatPearls. [PubMed] [Google Scholar]
- [10].Bishop JC, McCormick B, Johnson CT, et al. The double bubble sign: duodenal atresia and associated genetic etiologies. Fetal Diagn Ther 2020;47:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yigiter M, Yildiz A, Firinci B, et al. Annular pancreas in children: a decade of experience. Eurasian J Med 2010;42:116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ramallo Varela S, Casal Beloy I, Lema Carril A, et al. [Neonatal pyloroduodenal duplication cyst. A case report]. An Sist Sanit Navar 2021;44:463–8. [DOI] [PubMed] [Google Scholar]
- [13].Langer JC. Intestinal rotation abnormalities and midgut volvulus. Surg Clin North Am 2017;97:147–59. [DOI] [PubMed] [Google Scholar]
- [14].Nguyen HN, Sammer MBK, Ditzler MG, et al. Transition to ultrasound as the first-line imaging modality for midgut volvulus: keys to a successful roll-out. Pediatr Radiol 2021;51:506–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Naffaa L, Barakat A, Baassiri A, et al. Imaging acute non-traumatic abdominal pathologies in pediatric patients: a pictorial review. J Radiol Case Rep 2019;13:29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen D, Tam KH, Zhang Y, et al. Prenatal diagnosis of midgut volvulus with jejunal atresia by ultrasonography. J Obstet Gynaecol Res 2020;46:1203–6. [DOI] [PubMed] [Google Scholar]
- [17].Marine MB, Karmazyn B. Imaging of malrotation in the neonate. Semin Ultrasound CT MR 2014;35:555–70. [DOI] [PubMed] [Google Scholar]
- [18].Adams SD, Stanton MP. Malrotation and intestinal atresias. Early Hum Dev 2014;90:921–5. [DOI] [PubMed] [Google Scholar]
- [19].Osuchukwu OO, Rentea RM. Ileal atresia. Treasure Island (FL): StatPearls Publishing; 2021. StatPearls. [PubMed] [Google Scholar]
- [20].Grosfeld JL, Ballantine TV, Shoemaker R. Operative mangement of intestinal atresia and stenosis based on pathologic findings. J Pediatr Surg.1979;14:368–75. [DOI] [PubMed] [Google Scholar]
- [21].Virgone C, D’antonio F, Khalil A, et al. Accuracy of prenatal ultrasound in detecting jejunal and ileal atresia: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2015;45:523–9. [DOI] [PubMed] [Google Scholar]
- [22].Neu J, Walker WA. Necrotizing enterocolitis. N Engl J Med 2011;364:255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wertheimer F, Arcinue R, Niklas V. Necrotizing enterocolitis: enhancing awareness for the general practitioner. Pediatr Rev 2019;40:517–27. [DOI] [PubMed] [Google Scholar]
- [24].Knell J, Han SM, Jaksic T, et al. Current status of necrotizing enterocolitis. Curr Probl Surg 2019;56:11–38. [DOI] [PubMed] [Google Scholar]
- [25].Hwang M, Tierradentro-García LO, Dennis RA, et al. The role of ultrasound in necrotizing enterocolitis. Pediatr Radiol 2021. Oct Online ahead of print. doi: 10.1007/s00247-021-05187-5. [DOI] [PubMed] [Google Scholar]
- [26].D’Angelo G, Impellizzeri P, Marseglia L, et al. Current status of laboratory and imaging diagnosis of neonatal necrotizing enterocolitis. Ital J Pediatr 2018;44:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Deeg KH. Sonographic and Doppler sonographic diagnosis of necrotizing enterocolitis in preterm infants and newborns. Ultraschall Med 2019;40:292–318. [DOI] [PubMed] [Google Scholar]
- [28].Cuna AC, Lee JC, Robinson AL, et al. Bowel ultrasound for the diagnosis of necrotizing enterocolitis: a meta-analysis. Ultrasound Q 2018;34:113–8. [DOI] [PubMed] [Google Scholar]
- [29].Fonseca EKUN Ponte MPTR, Sameshima YT. Zebra pattern” in necrotizing enterocolitis. Abdom Radiol (NY) 2017;42:2776–7. [DOI] [PubMed] [Google Scholar]
- [30].Al-Hamad S, Hackam DJ, Goldstein SD, et al. Contrast-enhanced ultrasound and near-infrared spectroscopy of the neonatal bowel: novel, bedside, noninvasive, and radiation-free imaging for early detection of necrotizing enterocolitis. Am J Perinatol 2018;35:1358–65. [DOI] [PubMed] [Google Scholar]
- [31].Benjamin JL, Dennis R, White S, et al. Improved diagnostic sensitivity of bowel disease of prematurity on contrast-enhanced ultrasound. J Ultrasound Med 2020;39:1031–6. [DOI] [PubMed] [Google Scholar]
- [32].Sameshima YT, Yamanari MGI, Silva MA, et al. The challenging sonographic inguinal canal evaluation in neonates and children: an update of differential diagnoses. Pediatr Radiol 2017;47:461–72. [DOI] [PubMed] [Google Scholar]
- [33].Dreuning KMA, Ten Broeke CEM, Twisk JWR, et al. Diagnostic accuracy of preoperative ultrasonography in predicting contralateral inguinal hernia in children: a systematic review and meta-analysis. Eur Radiol 2019;29:866–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rafailidis V, Varelas S, Apostolopoulou F, et al. Nonobliteration of the processus vaginalis: sonography of related abnormalities in children. J Ultrasound Med 2016;35:805–18. [DOI] [PubMed] [Google Scholar]
- [35].Prakash A, Doshi B, Singh S, et al. Intussusception in a premature neonate: a rare and often misdiagnosed clinical entity. Afr J Paediatr Surg 2015;12:82–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ramsey KW, Halm BM. Diagnosis of intussusception using bedside ultrasound by a pediatric resident in the emergency department. Hawaii J Med Public Health 2014;73:58–60. [PMC free article] [PubMed] [Google Scholar]
- [37].Bartocci M, Fabrizi G, Valente I, et al. Intussusception in childhood: role of sonography on diagnosis and treatment. J Ultrasound 2015;18:205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Langer JC. Hirschsprung disease. Curr Opin Pediatr 2013;25:368–74. [DOI] [PubMed] [Google Scholar]
- [39].Bhatnagar SN. Hirschsprung’s disease in newborns. J Neonatal Surg 2013;2:51. [PMC free article] [PubMed] [Google Scholar]
- [40].Takahashi H, Matsubara D, Ono S, et al. Novel ultrasound finding of a fetus with Hirschsprung’s disease: a caliber change sign. Eur J Obstet Gynecol Reprod Biol 2017;215:259–60. [DOI] [PubMed] [Google Scholar]
- [41].Das K, Mohanty S. Hirschsprung disease - current diagnosis and management. Indian J Pediatr 2017;84:618–23. [DOI] [PubMed] [Google Scholar]
- [42].Jakobson-Setton A, Weissmann-Brenner A, Achiron R, et al. Retrospective analysis of prenatal ultrasound of children with Hirschsprung disease. Prenat Diagn 2015;35:699–702. [DOI] [PubMed] [Google Scholar]
- [43].Meyers ML, Crombleholme T. Prenatal MRI diagnosis of hirschsprung’s disease at 29 weeks’ gestational age in a fetus with heterotaxy and polysplenia syndrome. Fetal Diagn Ther 2016;40:235–40. [DOI] [PubMed] [Google Scholar]
- [44].Zhou JL, Zhu XC, Fang YL, et al. Radiological feature of skip-segment Hirschsprung’s disease. Arch Dis Child Fetal Neonatal Ed 2019;104:F616. [DOI] [PubMed] [Google Scholar]
- [45].Baad M, Delgado J, Dayneka JS, et al. Diagnostic performance and role of the contrast enema for low intestinal obstruction in neonates. Pediatr Surg Int 2020;36:1093–101. [DOI] [PubMed] [Google Scholar]
- [46].Wood K, Jinadatha A, Agrawal K, et al. Neonatal small left colon syndrome (NSLCS): rare but important complication in an infant of diabetic mother. BMJ Case Rep 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Loening-Baucke V, Kimura K. Failure to pass meconium: diagnosing neonatal intestinal obstruction. Am Fam Physician 1999;60:2043–50. [PubMed] [Google Scholar]
- [48].Vinocur DN, Lee EY, Eisenberg RL. Neonatal intestinal obstruction. AJR Am J Roentgenol 2012;198:W1–10. [DOI] [PubMed] [Google Scholar]
- [49].Etensel B, Temir G, Karkiner A, et al. Atresia of the colon. J Pediatr Surg 2005;40:1258–68. [DOI] [PubMed] [Google Scholar]
- [50].El-Asmar KM, Abdel-Latif M, El-Kassaby AHA, et al. Colonic atresia: association with other anomalies. J Neonatal Surg 2016;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pasto ME, Deiling JM, O’Hara AE, et al. Neonatal colonic atresia: ultrasound findings. Pediatr Radiol 1984;14:346–8. [DOI] [PubMed] [Google Scholar]
- [52].Winters WD, Weinberger E, Hatch EI. Atresia of the colon in neonates: radiographic findings. AJR Am J Roentgenol 1992;159:1273–6. [DOI] [PubMed] [Google Scholar]
- [53].Selke AC, Jona JZ. The hook sign in type 3 congenital colonic atresia. AJR Am J Roentgenol 1978;131:350–1. [DOI] [PubMed] [Google Scholar]
- [54].Carroll AG, Kavanagh RG, Ni Leidhin C, et al. Comparative effectiveness of imaging modalities for the diagnosis of intestinal obstruction in neonates and infants:: a critically appraised topic. Acad Radiol 2016;23:559–68. [DOI] [PubMed] [Google Scholar]
- [55].John SD. Imaging of acute abdominal emergencies in infants and children. Curr Probl Diagn Radiol 2000;29:141–79. [DOI] [PubMed] [Google Scholar]
- [56].van Heurn LWE, Pakarinen MP, Wester T. Contemporary management of abdominal surgical emergencies in infants and children. Br J Surg 2014;101:e24–33. [DOI] [PubMed] [Google Scholar]
- [57].Vasavada P Ultrasound evaluation of acute abdominal emergencies in infants and children. Radiol Clin North Am 2004;42:445–56. [DOI] [PubMed] [Google Scholar]
- [58].Pedrosa I, Rofsky NM. MR imaging in abdominal emergencies. Radiol Clin North Am 2003;41:1243–73. [DOI] [PubMed] [Google Scholar]
- [59].Maxfield CM, Bartz BH, Shaffer JL. A pattern-based approach to bowel obstruction in the newborn. Pediatr Radiol 2013;43:318–29. [DOI] [PubMed] [Google Scholar]
- [60].Tsitsiou Y, Calle-Toro JS, Zouvani A, et al. Diagnostic decision-making tool for imaging term neonatal bowel obstruction. Clin Radiol 2021;76:163–71. [DOI] [PubMed] [Google Scholar]


