Abstract
Arterioles maintain blow flow by adjusting their diameter in response to changes in local blood pressure. In this process called the myogenic response, a vascular smooth muscle mechanosensor controls tone predominantly through altering the membrane potential. In general, myogenic responses occur slowly (minutes). In the heart and skeletal muscle, however, tone is activated rapidly (tens of seconds) and terminated by brief (100 ms) arterial constrictions. Previously, we identified extensive expression of TRPV1 in the smooth muscle of arterioles supplying skeletal muscle, heart and fat. Here we reveal a critical role for TRPV1 in the rapid myogenic tone of these tissues. TRPV1 antagonists dilated skeletal muscle arterioles in vitro and in vivo, increased coronary flow in isolated hearts, and transiently decreased blood pressure. All of these pharmacologic effects were abolished by genetic disruption of TRPV1. Stretch of isolated vascular smooth muscle cells or raised intravascular pressure in arteries triggered Ca2+ signaling and vasoconstriction. The majority of these stretch-responses were TRPV1-mediated, with the remaining tone being inhibited by the TRPM4 antagonist, 9-phenantrol. Notably, tone developed more quickly in arteries from wild-type compared with TRPV1-null mice. Furthermore, the immediate vasodilation following brief constriction of arterioles depended on TRPV1, consistent with a rapid deactivation of TRPV1. Pharmacologic experiments revealed that membrane stretch activates phospholipase C/protein kinase C signaling combined with heat to activate TRPV1, and in turn, L-type Ca2+ channels. These results suggest a critical role, for TRPV1 in the dynamic regulation of myogenic tone and blood flow in the heart and skeletal muscle.
Keywords: TRPV1, vascular smooth muscle, myogenic tone, capsaicin, blood pressure, Ion Channels/Membrane Transport, Vascular biology
Graphical Abstract
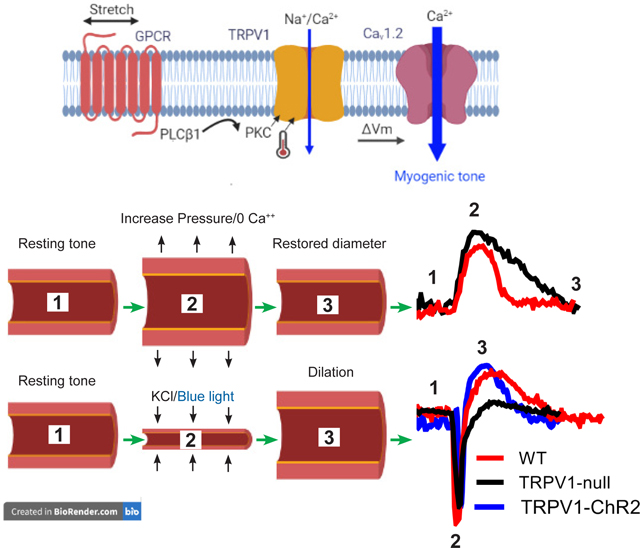
TRPV1 functions as a transduction channel for myogenic tone. Stretch of arteriolar myocytes activates TRPV1 via a PLC/PKC pathway and temperature. In turn, the depolarizing current through TRPV1 activates the L-type Ca2+ channel (CaV1.2) to trigger cell shortening. Moreover, TRPV1 enables dynamic control of tone as revealed by experiments with TRPV1-deficient arteries. Upon arterial stretch the restoration of vessel diameter is significantly slower in the absence of TRPV1. Similarly, the immediate vasodilation following a brief constriction is also TRPV1 dependent. Thus, rapid activation and deactivation of TRPV1 is proposed to underlie these dynamic changes, contributing to the temporal control of blood flow in skeletal muscle and the heart.
Introduction
Arterioles must deliver blood to tissues within a narrow pressure range to enable adequate perfusion without damaging capillaries. These vessels possess the intrinsic capacity to sense changes in local blood pressure and adjust their caliber to stabilize blood flow. This autoregulatory property, known as “the myogenic response” (Bayliss, 1902), helps to maintain tissue perfusion despite fluctuations in blood pressure. Although an intrinsic property of most arterioles, the kinetics of myogenic tone are especially rapid in skeletal muscle (Hill et al., 1990) and the heart (Mosher et al., 1964; Muller et al., 1993), tissues that receive phasic blood flow and large increases in their blood supply going from rest to exercise. Fast myogenic tone in coronary and skeletal muscle arterioles may favor uniform spatiotemporal perfusion (Davis, 2012; Goodwill et al., 2017). Further, the rapid release of myogenic tone upon initial skeletal muscle contraction, augments blood flow to improve the transition to work (Clifford, 2007; Davis, 2012). Significantly, impaired or diminished myogenic tone is a pathophysiological feature that accompanies diabetes, obesity (Hodnett & Hester, 2007), coronary artery disease (Duncker et al., 2015), hypertrophic cardiomyopathy (Petersen et al., 2002) and ageing (Lott et al., 2004; Ghosh et al., 2015). All these conditions are associated with declining cardiac vasodilator reserve and exercise performance.
Although Bayliss described the myogenic effect in skeletal muscle more than a century ago (Bayliss, 1902), details of the molecular components are only just emerging. Mechano-activated ion channels, such as Piezo1 are present in vascular smooth muscle cells but do not contribute to normal myogenic tone (Retailleau et al., 2015). Rather, several studies support a role for G-protein coupled receptors (GPCRs) in mechanosensation (Zou et al., 2004; Mederos y Schnitzler et al., 2008) and as vascular smooth muscle mechanosensors (Mederos y Schnitzler et al., 2008; Gonzales et al., 2014; Hong et al., 2016). These GPCRs couple to either a G12/G13 Rho kinase-dependent pathway (Chennupati et al., 2019) or a Gq/G11 dependent phospholipase C (PLC) pathway (Osol et al., 1993; Matsumoto et al., 1995). PLC-mediated signaling activates transduction channels and ultimately voltage-gated Ca2+ entry to trigger smooth muscle contraction (Knot & Nelson, 1998; Earley & Brayden, 2015). The identity of the transduction channels is unclear but Transient Receptor Potential (TRP) channels are potential candidates; TRPC6 (Welsh et al., 2002) and TRPM4 (Earley et al., 2004; Reading & Brayden, 2007) channels are implicated in the tone of cerebral arterioles, while TRPP1 is essential for myogenic tone in large-diameter, primary arteries (Bulley et al., 2018). Therefore, these findings support the existence of distinct, tissue-specific molecular pathways for regulating myogenic tone.
TRPV1 is an ion channel highly expressed in sensory ganglia with important roles in transducing temperature, pain and itch sensory modalities. Heat activates TRPV1, as well as capsaicin, the pungent component of chilli peppers, protons and a variety of endogenous lipid metabolites (Caterina et al., 1997; Caterina & Julius, 2001; Bang et al., 2010). TRPV1 senses and integrates these physical and chemical stimuli via distinct domains that are allosterically coupled to channel gating (Tominaga et al., 1998; Brauchi et al., 2004; Matta & Ahern, 2007). Notably, TRPV1 has been identified in isolated segments of the vasculature (Lizanecz et al., 2006; Kark et al., 2008; Cavanaugh et al., 2011; Czikora et al., 2012; Czikora et al., 2013; Phan et al., 2016). Recently, we performed functional mapping of TRPV1 throughout the mouse circulation. This analysis revealed extensive TRPV1 expression in the vascular smooth muscle of arterioles in the skeletal muscle, heart and adipose tissues (Phan et al., 2020). Furthermore, we found that activation of arteriolar TRPV1 channels by capsaicin or the endogenous lipid, LPA, markedly increased vascular tone and blood pressure (Phan et al., 2020). Of note, the restricted expression of TRPV1 in small arterioles of skeletal muscle matches the classical studies mapping myogenic tone in the limb and skeletal muscle arterial tree (Uchida & Bohr, 1969). Here using in vitro and in vivo approaches we reveal that TRPV1 mediates the majority of myogenic tone in skeletal muscle and coronary arteries. Further, we reveal that TRPV1 is critical for rapid and dynamic changes in tone in these vessels.
Methods
Ethical approval:
All procedures were approved by Georgetown University, IACUC Protocol Numbers: 2016–1310 and 2018–0033; George Washington University, IACUC number A202, and University of Debrecen, Ethics Committee on Animal Research: 2/2013/DEMÁB and 4–1/2019/DEMÁB. All efforts were made to minimize the number and suffering of the animals used in this study.
Animals:
Wistar and Sprague-Dawley rats (250–450 g) and C57Bl6 mice (25–30 g) were housed at 24–25°C and had ad libitum access to a standard laboratory chow and water.
Mouse lines:
The TRPV1PLAP-nlacZ mice (Jackson Laboratory) were developed by Allan Bausbaum and colleagues (UCSF) to express human placental alkaline phosphatase (PLAP) and nuclear lacZ under the control of the TRPV1 promoter (Cavanaugh et al., 2011). The targeting construct contains an IRES-PLAP-IRES-nlacZ cassette immediately 3’ of the TRPV1 stop codon, which permits the transcription and translation of PLAP and nlacZ in cells expressing TRPV1 without disrupting the TRPV1 coding region. The TRPV1-Cre transgenic mouse line (donated by Dr. Mark Hoon, NIH) was created using a BAC transgene containing the entire TRPV1 gene/promoter (50 kbp of upstream DNA) and IRES-Cre-recombinase (Mishra et al., 2011). Importantly, Cre expression in this mouse faithfully corresponds with the expression of endogenous TRPV1. The TRPV1-Cre (hemizygous) mice were crossed with ChR2/tdTomato mice (The Jackson Laboratory) to generate TRPV1-Cre:ChR2/tdTomato. TRPV1-null mice were purchased from The Jackson Laboratory.
mRNA analysis:
Mice were anesthetized with isoflurane (4% in 100% O2) and euthanized by perfusing the heart with ice-cold PBS (0.1 M, pH 7.3). Radial branch arteries were isolated. RNA was extracted and purified using the RNAqueous Micro Kit (Invitrogen). First-strand cDNA synthesis was performed using SuperScript III Reverse Transcriptase (Invitrogen) with the supplied oligo(dT)20 primer. The resulting cDNA was used as a template for PCR amplification. For comparative analysis of mRNA in WT versus TRPV1-null mice, data were initially normalized to GAPDH and subsequently each data point was normalized to the mean of the WT values.
Arterial smooth muscle (ASM) cell isolation:
Adult mice (C57BL/6J) were euthanised by CO2 /decapitation. Radial branch and cerebellar branch arteries were washed in Mg2+-based physiological saline solution (Mg-PSS) containing 5 mM KCl, 140 mM NaCl, 2 mM MgCl2, 10 mM Hepes, and 10 mM glucose (pH 7.3). Arteries were initially digested in papain (0.6 mg/ml) (Worthington) and dithioerythritol (1 mg/ml) in Mg-PSS at 37°C for 15 min, followed by a 15-min incubation at 37°C in type II collagenase (1.0 mg/ml) (Worthington) in Mg-PSS. The digested arteries were washed three times in ice-cold Mg-PSS solution and incubated on ice for 30 min. After this incubation period, vessels were triturated to liberate smooth muscle cells and stored in ice-cold Mg-PSS before use. Smooth muscle cells adhered loosely to glass coverslips and were studied within 6 hours of isolation.
Ca2+ imaging:
ASM cells and arteries were respectively loaded with 5 μM and 10 μM Fluo-4-AM (Invitrogen, Thermo Fisher Scientific) in a buffer solution containing 140 mM NaCl, 4 mM KCl, 1 mM MgCl2, 1.2 mM CaCl2, 10 mM HEPES, and 5 mM glucose (pH 7.3). Temperature was maintained at 32–33°C using a heated microscope stage (Tokai Hit). The bath temperature was verified by a thermistor probe (Warner instruments). ASM cells and arteries were imaged with 10X and 20X objectives using a Nikon TE2000 microscope with an excitation filter of 480 ± 15 nm and an emission filter of 535 ± 25 nm. The images were captured by a Retiga 3000 digital camera (QImaging) and analysis was performed offline using ImageJ.
Ex vivo artery physiology:
Mice and rats were euthanized (CO2/decapitation). Skeletal muscle arteries (radial artery branch, artery #18, subscapular branch artery #14, see Fig. 6G, (Phan et al., 2020)) were isolated and cannulated with glass micropipettes, and secured with monofilament threads. In some experiments arteries were denuded of endothelium by passing 1 ml of air followed by 1 ml of PSS through the lumen. Effective removal of the endothelium was confirmed by the absence of dilation of the arteries to ACh. The pipette and bathing PSS solution containing (in mM): 125 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 4 D-glucose, and 2 CaCl2,) was aerated with a gas mixture consisting of 95% O2, 5% CO2 to maintain pH (pH 7.4). To induce maximal dilation, arteries were perfused with a PSS solution containing 0 CaCl2, 0.4 mM EGTA and 100 μM sodium nitroprusside (SNP). Arterioles were mounted in a single vessel chamber (Living Systems Instrumentation) and placed on a heated imaging stage (Tokai Hit) to maintain bath temperature between 34–35°C, while intraluminal pressure was maintained by a Pressure Control Station (Stratagene). Arteries were viewed with a 10X objective using a Nikon TE2000 microscope and recorded by a digital camera (Retiga 3000, QImaging). The arteriole inner diameter was measured at several locations along each arteriole using the NIH-ImageJ software’s edge-detection plug-in (Diameter) (Fischer et al., 2010). The software automatically detects the distance between edges (by resampling a total of five times from adjacent pixels) yielding a continuous read-out ±SD of a vessel’s inner diameter.
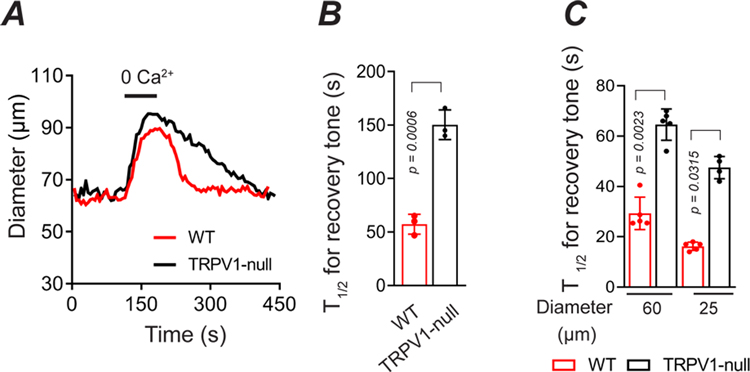
Figure 6. TRPV1 mediates rapid myogenic tone in vivo.
A and B, Development of tone in radial muscle branch arteries after a maximal dilation (0 Ca2+/EGTA) in WT and TRPV1-null mice (n = 3 mice, 3 arteries, unpaired t-test). C, The time course for recovery of tone after a reactive vasodilation response to 6 s application of KCl (40 mM), in small and medium-diameter arterioles from WT and TRPV1-null mice (n = 3 mice, 5 arteries, nested t-test).
Intravital imaging:
Intravital imaging was performed in radial artery branches (about 60 μm in diameter, artery #18 in Fig 6G, (Phan et al., 2020). Mice were restrained by grasping the skin at the nape of the neck and anesthetized with urethane (1.2 g/kg/IP). The adequacy of anesthesia was confirmed by the absence of pedal and corneal reflexes. The forelimb was shaved and an incision was made. The skin and underlying muscle tissue were reflected to expose the brachial-radial artery junction. Both in WT and TRPV1-null mice, the arteries were visualized with a Zeiss stereomicroscope and illuminated with a low power blue light (using a standard GFP filter cube) exploiting the differential auto-fluorescence between tissue and blood. In TRPV1-Cre:ChR2 mice, arteries were visualized with low power visible irradiation and stimulated with blue light. The exposed arteries were locally perfused (using a 250 μm cannula connected to a valve-controlled gravity-fed perfusion system) with preheated buffer described for Ca2+ imaging. The surface tissue temperature (34–35°C) was measured via a thermistor (Warner Instruments) that was positioned next to the artery. KCl (40 mM) was applied for a duration of 6–40 s to constrict arteries and evoke a reactive vasodilation. Arteries were challenged with buffer without Ca2+ and with 1 mM EGTA to measure the passive diameter. The arteriole diameter (inner) was measured using ImageJ as described above for the ex vivo vessels. After the recordings, the mice were euthanized (CO2/decapitation).
Coronary flow measurements:
Sprague–Dawley rats (male, 300–350 g) were placed in a deep surgical plane of anesthesia by isoflurane inhalation (4% in 100% O2), confirmed by lack of pedal reflex. The heart was then exposed via thoracotomy, quickly excised and rinsed in a bath of ice-cold perfusate. The aorta was rapidly cannulated then flushed with 500 units of heparin mixed with the perfusate that contained (in mM): 118 NaCl, 4.7 KCl, 1.25 CaCl2, 0.57 MgSO4, 1.17 KH2PO4, 25 NaHCO3, and 6 glucose. Hearts were then transferred to a retrograde perfusion system that delivered oxygenated (gassed with 95%O2-5%CO2) perfusate to the aorta at constant pressure (70mmHg) and 37±1°C. Coronary flow was measured using a tubing flowsensor (Transonic Systems) placed above the aortic cannula and was continuously acquired with the ECG using a PowerLab system (ADInstruments). BCTC was added to the perfusate reservoir. Data were analyzed off–line and the integral of coronary flow was calculated between the time of injection and the onset of the hyperemia response.
Sensory nerve ablation:
Neonate TRPV1PLAP-nlacZ mice were anesthetized briefly (5 min) with isoflurane (4–5% in 100% O2) and treated with resiniferatoxin (50 ug/kg s.c.) at postnatal days 2 and 5. Animals were allowed to recover in a warm environment (30°C, 2 hr) to minimize any hypothermic effects. This dose of resiniferatoxin causes profound sensory nerve desensitization/block and mice exhibited no outward signs of distress in recovery, and there was no disruption to dam-pup interactions. At 8–12 weeks, mice were either euthanized for tissue collection, or used for BP measurements followed immediately by euthanasia (CO2/decapitation). Sensory nerve ablation was confirmed by measuring nuclear LacZ staining of DRG ganglia (Phan et al., 2020), and by an absence of a nocifensive response to capsaicin administration. Mice received an intraplantar injection of capsaicin (50 ng, 0.01% EtOH) and the time spent shaking, licking or biting the injected paw was measured over 3 minutes. WT mice exhibited ~40 s pain-related behavior.
Systemic blood pressure recording:
The experiments were performed in anesthetized mice (urethane 1.2–1.5 g/kg/IP) and rats (thiopental 50 mg/kg/IP; supplemented by 5 mg/kg/IV if needed). After anesthesia, mice or rats underwent cannulation of the carotid artery and jugular vein as follows:
Surgical preparation in the mouse:
After the depth of anesthesia was confirmed by lack of pedal and corneal reflexes, mice were intubated via the trachea after tracheotomy to maintain an open airway and to institute artificial respiration when necessary. Next, the left carotid artery and the right jugular vein were cannulated with a Millar catheter (1F SPR-1000) and a polyethylene tubing (PE-10), respectively, for monitoring arterial blood pressure and for systemic (intravenous) infusion of drugs. To monitor heart rate, a three-point needle electrode-assembly representing Lead II of the electrocardiogram (ECG) was attached subcutaneously to the right and left forelimbs along with a reference electrode to the left hindlimb. Both the Millar catheter and the ECG assembly were coupled to a PowerLab data acquisition system (ADInstruments). Before vessel cannulation, the adjacent left cervical vagus was carefully isolated from the left carotid artery. Body temperature was monitored by a digital rectal thermometer and maintained at 37 ± 1°C with an infrared heat lamp. After the study, mice were euthanized (CO2/decapitation).
Conscious blood pressure recordings:
RTX-treated mice (8–12 weeks) were anesthetized with isoflurane (2–4%) and surgically implanted with in-dwelling jugular catheters (Instech labs., USA). The adequacy of anesthesia was confirmed by the absence of pedal and corneal reflexes. Post-surgically mice were administered carprofen (one dose of 5mg/kg s.c.) and placed in a cotton-filled cage until ambulatory. After 72 h, the animals were prepared for BP recording. BP was measured by tail-cuff plethysmography (Coda6, Kent Scientific, USA) performed before and immediately after the infusion of drugs. At the end of the study, the mice were euthanized (CO2/decapitation).
Surgical preparation in the rat:
Before the commencement of surgical interventions, the depth of anaesthesia was checked by squeezing the tip of the rat’s tail. If no response to this challenge was observed, the animal was fixed to a plastic foil-covered polystyrene plate by strings and tapes. The animal was placed in a supine position and the collar region was shaved by a razor. A midline incision was made to expose the trachea, carotid arteries, and the jugular vein. Similar to the mouse, following intubation of the trachea, the left carotid artery, and jugular vein were cannulated with a polyethylene tubing (PE50) to monitor blood pressure and infuse drugs, respectively. Blood pressure (and ECG) was continuously recorded via a pressure transducer connected to the Haemosys hemodynamic system (Experimetria, Budapest, Hungary). The ECG was recorded from the extremities of the animal using hypodermic metal needles inserted subcutaneously per the Einthoven method (I, II, III leads). As in the mouse, heart rate was determined from lead II of the ECG recordings, and body core temperature was maintained at 37±1 °C with a temperature-controlled infrared heating lamp. During the experiment, the depth of anesthesia was checked regularly and if necessary was supplemented by an intravenous dose of 5 mg/kg Thiopental. After the study, the animals were euthanized (CO2/decapitation).
Drug administration:
Intravenous infusion of drugs was initiated only when a stable baseline of blood pressure and heart rate was present. This was also the case when drugs were re-administered. Final drug solutions contained: capsaicin (saline with 0.4% EtOH), LPA (saline with 0.6% EtOH).
Chemicals:
Capsaicin, resiniferatoxin, 4-(3-Chloro-2-pyridinyl)-N-[4-(1,1-dimethylethyl)phenyl]-1-piperazinecarboxamide (BCTC) and N-[4-[[6-[4-(Trifluoromethyl)phenyl]-4-pyrimidinyl]oxy]-2-benzothiazolyl]acetamide (AMG517) were purchased from Tocris Bioscience or Adooq Bioscience and stock solutions were prepared in EtOH. Lysophosphatidic acid (LPA) C18:1 was purchased from Cayman Chemical. Unless otherwise indicated, all other chemicals were obtained from Sigma–Aldrich.
Statistical analysis:
Data were analyzed using Prism (GraphPad Software, La Jolla, CA) and are expressed as means + SD. Unless otherwise stated, statistical significance was evaluated using t-test and one-way ANOVA with treatment interactions assessed by Tukey’s post hoc multiple comparisons test. A P value of < 0.05 was considered statistically significant.
Results
TRPV1 contributes to resting tone in skeletal muscle and coronary arterioles
Previously, we demonstrated prominent TRPV1 expression in arterioles supplying skeletal muscle, heart and adipose tissues (Phan et al., 2020). To identify potential physiological roles for TRPV1 in regulating vascular tone we tested the effects of the highly specific TRPV1 antagonists, BCTC and AMG517. Notably, BCTC (1 μM) or AMG517 (10 μM) treatment dilated pressurized arterioles isolated from mouse skeletal muscle by ~35% (P = 0.008 and P= 0.0016, Fig. 1A–D). Importantly and in contrast, the antagonists did not affect the diameter of arteries obtained from TRPV1-null mice (P>0.9999, Fig. 1A–D). Moreover, BCTC did not alter the tone of non-TRPV1-expressing mesenteric arteries (Fig. 1E and F). Taken together, these findings indicate that the dilatory effects of TRPV1 antagonists are mediated by on-target pharmacologic inhibition of TRPV1. Next, to test for a contribution of TRPV1 to in vivo vascular tone, we performed intravital imaging of skeletal muscle feed arterioles (~60 μM diameter) supplying the forelimb radial muscles (Fig. 2). Here, we locally perfused BCTC while maintaining the tissue temperature at 34±0.5°C. Similar to the results in isolated arterioles, BCTC dilated in vivo arterioles in WT but not in TRPV1-null mice (Fig. 2A and B). The dilation to BCTC exhibited a concentration-dependent relationship with a peak dilatory effect ~70% of that produced by a zero Ca2+ solution (Fig. 2C and D). Further, BCTC had a greater dilatory effect in small diameter (~25 μm) compared with medium diameter (~60 μm) arterioles (P = 0.002, Fig. 2E), in agreement with the larger resting tone in smaller arterioles (Fig 2F).
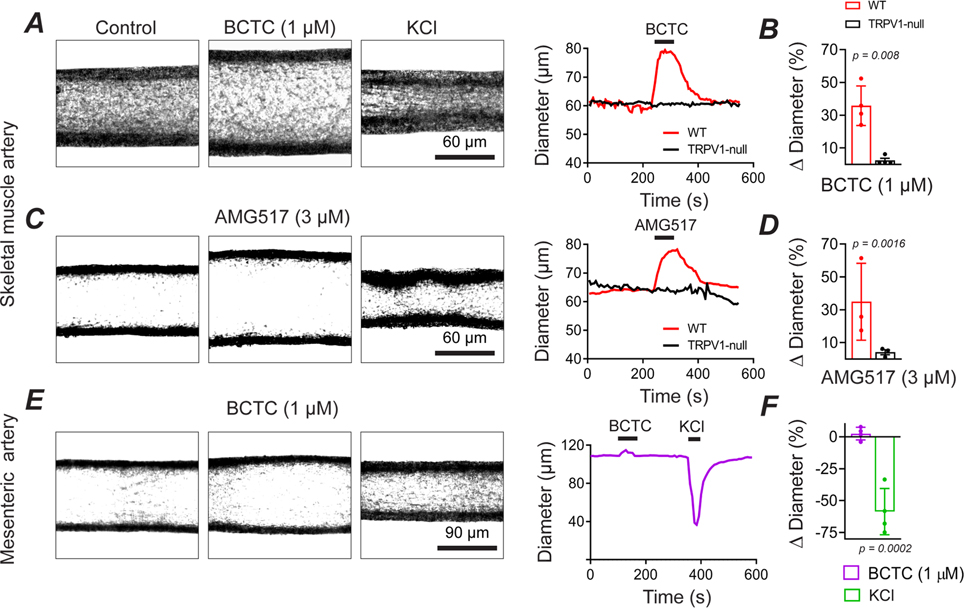
Figure 1. TRPV1 antagonists dilate isolated skeletal muscle arterioles.
A and B, BCTC (1 μM) dilates isolated, pressurized (60 mmHg) skeletal muscle arteries from wild-type mice (n = 3 mice, 5 arteries) but not TRPV1-null mice (n = 3 mice, 4 arteries, nested T-test). The response to 40 mM KCl is shown for reference. C and D, AMG517 (3 μM) selectively dilates arteries from WT but not TRPV1-null mice (n = 3 mice, 3 arteries, unpaired t-test). E and F, BCTC fails to dilate mesenteric arteries from WT mice (n = 4 arteries, 4 mice, unpaired t-test, P = 0.764).
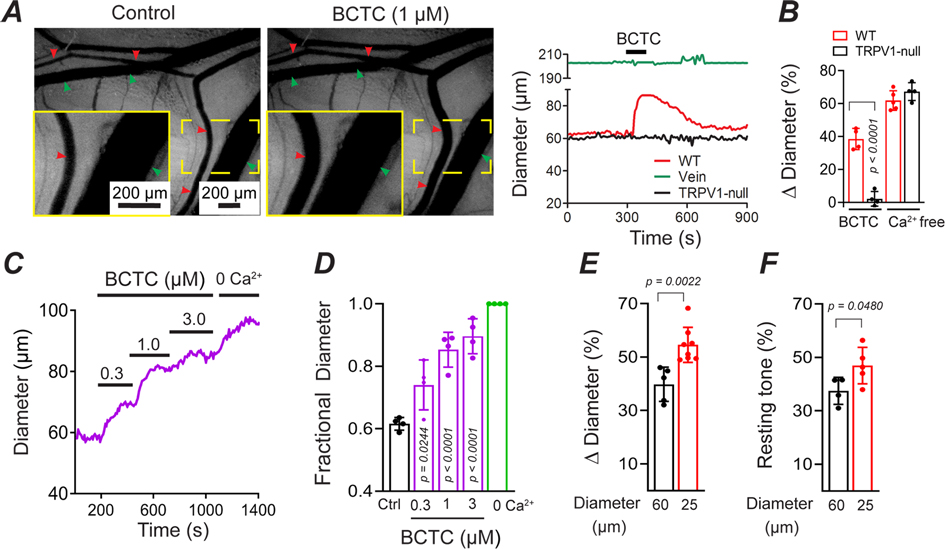
Figure 2. TRPV1 antagonists dilate skeletal muscle arterioles in vivo.
A, Intravital imaging show that BCTC (1 μM) dilates in vivo radial branch arterioles (red arrows) without affecting arterioles in TRPV1- null mice or veins (green arrows). B, Mean arteriole dilation evoked by BCTC in WT and TRPV1-null mice (n = 5 mice, 5 arteries) and maximal dilation with zero Ca2+/EGTA (unpaired t-test). C and D, Concentration-dependent dilation by BCTC normalized to zero Ca2+/EGTA (n = 4 arteries, 4 mice, one-way ANOVA Tukey’s Multi. comp.). E BCTC-evoked dilation in small (~25 μm, n = 8 arteries, 5 mice) and medium (~60 μm, n = 5 arteries, 4 mice) diameter arteries (unpaired t-test). F, Resting tone in small (n = 5 arteries, 4 mice) and medium (n = 4 arteries, 4 mice) diameter arteries (unpaired t-test).
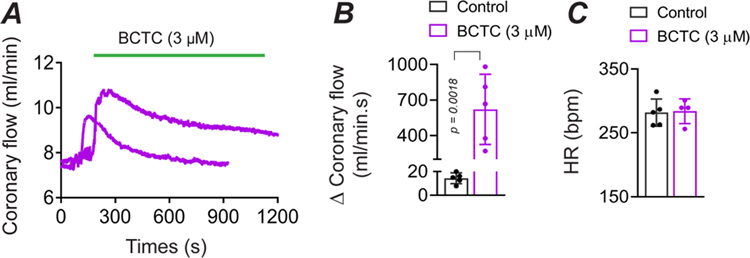
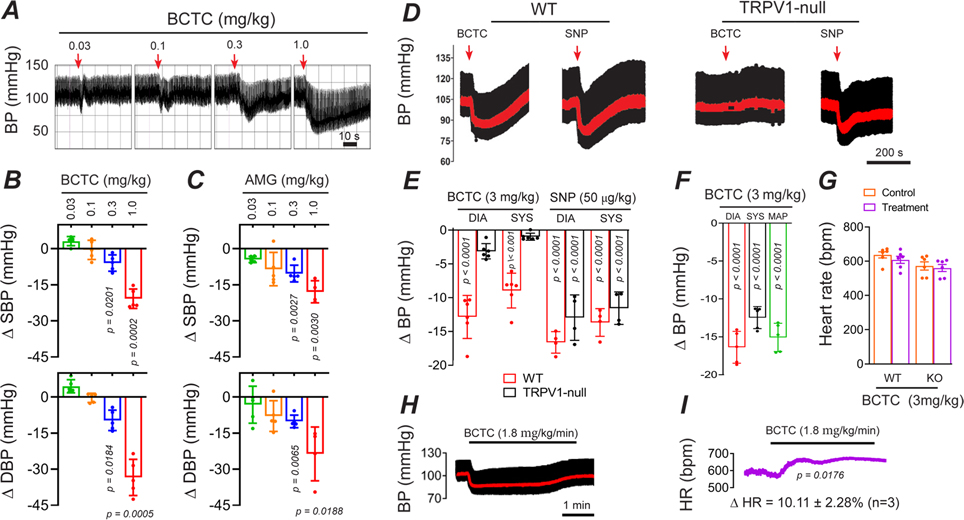
Next, we tested for the presence of TRPV1-dependent myogenic tone in the heart. Previously, we identified prominent TRPV1 expression in small-diameter, sub-epicardial arterioles (<120 μm diameter) of the ventricular myocardium (Phan et al., 2020), and we therefore tested whether TRPV1 contributes to tone in the coronary circulation. Indeed, inline infusion of BCTC into the isolated rat hearts (3 μM final concentration) markedly increased coronary flow by ~600 ml/min.s (P = 0.0018, Fig. 3A and B), that partially recovered over a 15-minute period. The elevated coronary perfusion occurred without alterations in heart rate (Fig. 3C). Thus, we conclude that TRPV1 contributes significantly to coronary myogenic tone.
Figure 3. BCTC increases coronary perfusion.
A, BCTC infusion into the coronary circulation increases coronary perfusion in an isolated rat heart. B, Mean changes in perfusion evoked by BCTC (measured over 13 to 17 minutes, n = 5, unpaired t-test), C, BCTC does not affect the heart rate (n = 5, unpaired t-test, P = 0.874842).
TRPV1 antagonists transiently decrease systemic blood pressure
Since skeletal muscle arterioles contribute significantly to systemic BP we tested whether inhibition of TRPV1-dependent tone would alter BP. In rats, bolus IV administration of BCTC dose-dependently decreased BP by up to 25 mmHg (Fig. 4A and B). AMG517 also significantly decreased BP (Fig. 4C). In mice, an IV infusion (10 s duration) of BCTC similarly decreased BP by ~15 mm Hg, comparable in magnitude to sodium nitroprusside (50μg/kg, Fig. 4D and E). In contrast, BCTC did not change BP in TRPV1-null mice. Furthermore, we tested conscious mice that were treated as neonates with resiniferatoxin to permanently ablate TRPV1-expressing sensory neurons while preserving TRPV1 expression in arteries (Phan et al., 2020). In these mice, we observed equivalent BP responses to BCTC (~15 mmHg, Fig. 4F), ruling out side effects of anesthesia and revealing an arterial-delimited effect. The peak responses to BCTC occurred without any significant changes in heart rate (Fig. 4G), however during prolonged (5 min) infusion of BCTC the BP recovered to baseline accompanied by an increase in heart rate (P = 0.0176, Fig. 4H and I).
Figure 4. BCTC transiently decreases systemic blood pressure.
A-C, Blood pressure (BP) changes in rats during bolus IV BCTC or AMG517 (n = 5, one-way ANOVA Tukey’s Multi. comp.). D and E, BP changes in WT and TRPV1-null mice in response to IV infusion (20 s) of BCTC (n = 6) and sodium nitroprusside (n = 4, unpaired t-test). Mean arterial pressure is shown in red. F, BP changes in response to IV BCTC in conscious, sensory nerve-ablated mice (n = 5, unpaired t-test). G Heart-rate changes in WT and TRPV1-null mice (n = 6, unpaired t-test) in response to IV BCTC. H and I, Representative changes in BP and heart-rate in response to prolonged (4 minute) infusion of BCTC.
TRPV1 enables rapid myogenic responses
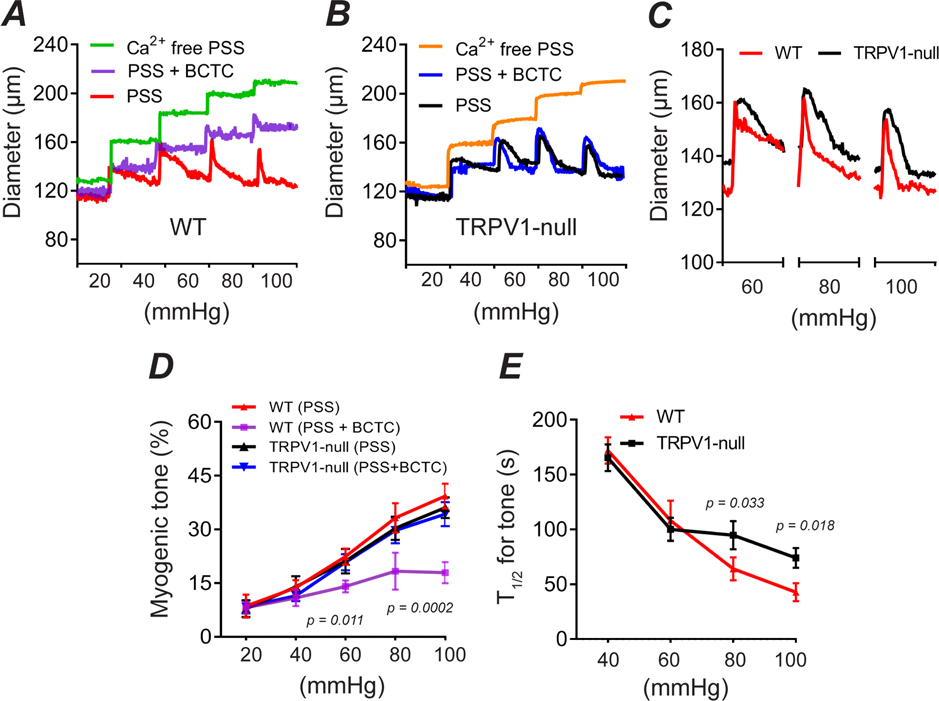
The dilation of skeletal muscle and coronary arterioles by TRPV1 antagonists suggests a role for TRPV1 in the regulation of myogenic tone in these tissues. To explore this hypothesis directly, we measured the responses of isolated skeletal muscle feed arterioles (~120μm) to changing intraluminal pressure (20 – 100 mmHg). Step increases in pressure immediately increased the arterial diameter followed by reflex constrictions (reflecting developing myogenic tone) that became progressively faster at higher pressure levels (Fig. 5A, D, E). Notably, BCTC markedly inhibited these myogenic constrictions by greater than 50% (P < 0.01). In contrast, BCTC did not alter pressure-evoked responses in arteries isolated from TRPV1-null mice (P=0.99, Figs. 5B and D). Moreover, while arteries from TRPV1-null mice exhibited an unchanged final tone (recorded after 5 min), at pressures greater than 60 mmHg the development of tone was significantly slower compared with WT arteries (P < 0.05, Fig. 5B–E). The difference between pharmacologic and genetic disruption of TRPV1 may reflect developmental compensation in the TRPV1-null mice.
Figure 5. TRPV1 mediates rapid myogenic tone in vitro.
A-C, Pressure-diameter relationship in isolated skeletal muscle arterioles from WT and TRPV1-null mice under control (PSS), 3 μM BCTC or 0 Ca2+/EGTA/SNP. Bath temperature was 35°C. D and E, Myogenic tone and kinetics for development of tone (T1/2) versus intraluminal pressure (n = 3 mice, 3 arteries, one-way ANOVA Tukey’s Multi. comp.).
To further examine the kinetics of myogenic tone in small (~25 μm) and medium (~60 μm)-diameter arterioles that highly express TRPV1, we used intravital imaging. After medium-diameter arterioles in vivo were dilated with a zero Ca2+ buffer, the tone returned 3-times faster (P = 0.0006) in wild-type compared with TRPV1-null mice (Fig. 6A and B). Similarly, the recovery of tone after a brief vasodilation was more 2-fold faster in wild-type mice; Figure 6C show that genetic disruption of TRPV1 increased the T1/2 for tone from 16 s to 48 s in small diameter arterioles (P = 0.0315) and from 29 s to 65 s in medium diameter arterioles (P = 0.0023, Fig. 6C). Thus, pharmacological or genetic disruption of TRPV1 impairs the magnitude and/or rate of development of myogenic tone.
TRPM4 contributes to myogenic tone of skeletal muscle arterioles
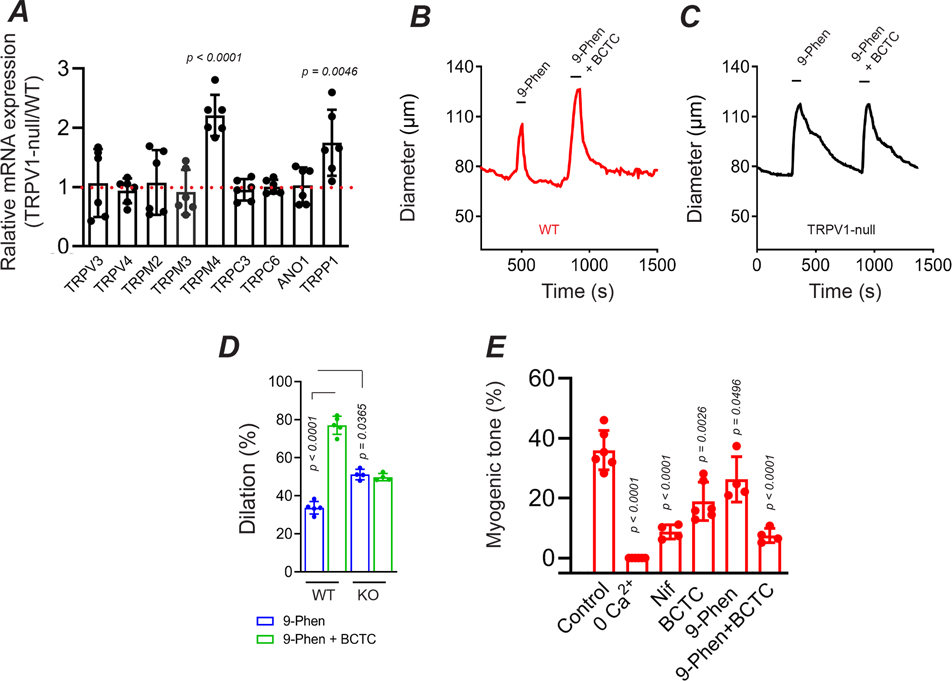
Previous studies have showed that TRP channels regulate myogenic tone via depolarization-induced activation of the L-type Ca2+ channel, CaV1.2 (Knot & Nelson, 1998; Earley & Brayden, 2015). Indeed, we found that the CaV1.2 antagonist, nifedipine, inhibited ~80% of tone in skeletal muscle arterioles (Fig. 7E). Further, nifedipine treatment prevented the BCTC-evoked dilation consistent with TRPV1 signaling entirely via the CaV1.2 pathway. Analysis of the respective relaxation to nifedipine and BCTC revealed that TRPV1 signaling contributes approximately two-thirds of the CaV1.2 dependent tone (Fig. 7E). To identify the TRPV1-independent component of CaV1.2 -mediated tone, we screened WT and TRPV1-null arteries for mRNA expression of candidate channels with known expression in arteries and/or thermosensitivity; TRPC3 & 6, TRPM2, 3 & 4, TRPP1 and anoctamin1. This analysis (Fig.7A) showed that genetic disruption of TRPV1 led to a significant increase in TRPM4 (P<0.0001) and TRPP1 (P= 0.0043) expression. Interestingly, previous studies have demonstrated a role for TRPM4 in the myogenic tone of cerebral arterioles (Earley et al., 2004; Reading & Brayden, 2007), while a recent study showed that TRPP1 contributes to the modest tone found in primary arteries (Bulley et al., 2018). To test a role for TRPM4 in skeletal muscle we employed the antagonist 9-phenanthrol. Treatment with 9-phenanthrol dilated arteries and reduced tone by ~40% (Fig. 7B – D). Further, co-treatment with 9-phenanthrol and BCTC inhibited tone by 80%, to the same extent as nifedipine (Fig. 7). Notably, 9-phenanthrol produced a significantly greater dilation in arteries from TRPV1-KO compared with WT mice (Figs. 7B–D, P=0.036), an effect consistent with the increased expression of TRPM4 following genetic disruption of TRPV1. This compensatory change, however, did not restore the kinetic properties observed in WT mice, and therefore, TRPV1 appears critical for the fast development of tone.
Figure 7. TRPM4 contributes to non TRPV1-dependent myogenic tone.
A, Relative mRNA expression for candidate ion channels in TRPV1-null compared with WT skeletal muscle arteries (n = 6 mice, unpaired t-test). B-D, Arterial dilation evoked by 9-phenanthrol (30 μM) and 9-phenanthrol plus BCTC in WT and TRPV1-null mice (n = 4 mice, 4 arteries, unpaired t-test). E, Summary of myogenic tone in arteries from WT mice: control (n = 6), 0 Ca2+ (n = 6), nifedipine (5 μM, n = 4), BCTC (3 μM, n = 6), 9-phenanthrol (30 μM, n = 4), BCTC plus 9-phenanthrol (n = 4, unpaired t-test).
TRPV1 enables rapid reactive vasodilation
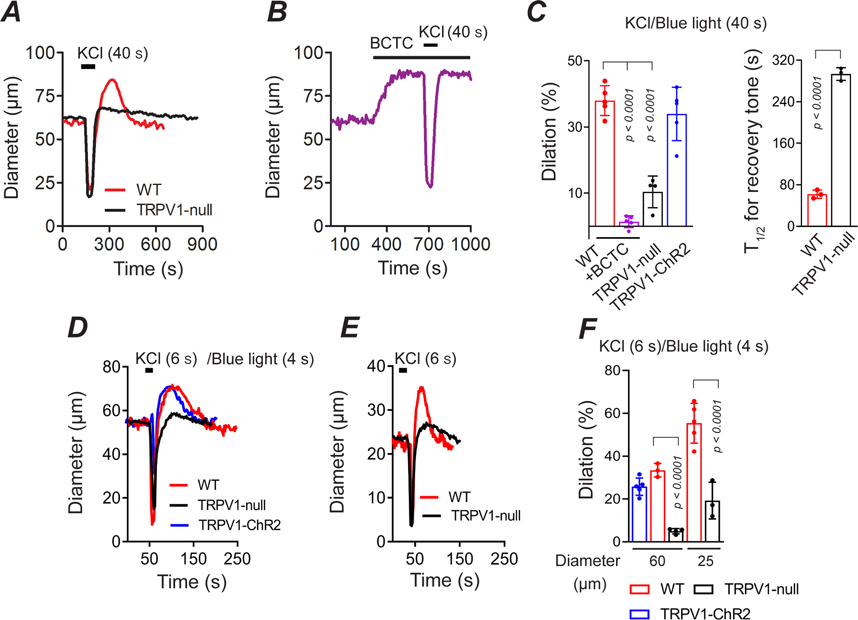
At the onset of physical activity, skeletal muscle arterioles immediately dilate (Clifford, 2007), and this phenomenon may involve a relaxation of myogenic tone (Davis, 2012). We therefore tested whether TRPV1 contributes to this process by measuring the dynamic changes in the diameter of skeletal muscle arterioles in vivo in response to vasoconstrictive stimuli. We found that local application of KCl (40 s) in wild-type mice or blue light (40 s) in TRPV1-Cre:ChR2 mice constricted arteries followed by a rebound dilation of approximately 35% (Fig. 8A and C). Notably, BCTC fully inhibited the post-KCl dilation (Fig. 8B and C). Furthermore, disruption of Trpv1 gene expression markedly suppressed (by >70%) the amplitude of the dilation (Fig. 8A and C; P < 0.001). Hyperemia following a 40 s arterial constriction may reflect both myogenic and metabolic pathways. However, we observed similar arterial dilations after brief (4 – 6 s) constrictions evoked by either KCl or blue-light pulses (Fig. 8D–F), supporting a primary role for a myogenic mechanism (Davis, 2012). Additionally, the magnitude of the vasodilation was approximately two-fold greater in small diameter (~25 μm) compared to medium diameter (~60μm) arterioles (Fig. 8D–F). These data suggest that upon arterial constriction TRPV1 is rapidly deactivated or inhibited to promote a rebound vasodilation. Subsequently, after a brief period of hyper-perfusion, TRPV1 reactivates to restore myogenic tone.
Figure 8. TRPV1 is critical for reactive vasodilation.
A-C, Arterial dilation following 40 s constriction evoked by KCl (40 mM) or blue light (TRPV1-ChR2 mice) and the reemergence of tone. BCTC and disruption of the TRPV1 gene inhibits the magnitude of the dilation and rate of recovery of tone (WT, n = 5 mice, 5 arteries; WT+BCTC, n = 5 mice, 5 arteries; TRPV1-null, n = 4 mice, 4 arteries; TRPV1-ChR2, n = 5 mice, 5 arteries, unpaired t-test). D-F, Dilation of medium- (~60 μm) and small (~25 μm) diameter arteries in response to 4 s blue light (TRPV1-ChR2 mice) or 6 s application of 40 mM KCl (WT, 60 μm, 3 mice, 3 arteries; 25 μm, 5 mice, 5 arteries; TRPV1-null, 60 and 25 μm, n = 4 mice, 4 arteries; TRPV1-ChR2, n = 5 mice, 5 arteries, unpaired t-test).
A PLC/PKC pathway underlies stretch-evoked activation of TRPV1 in vascular smooth muscle
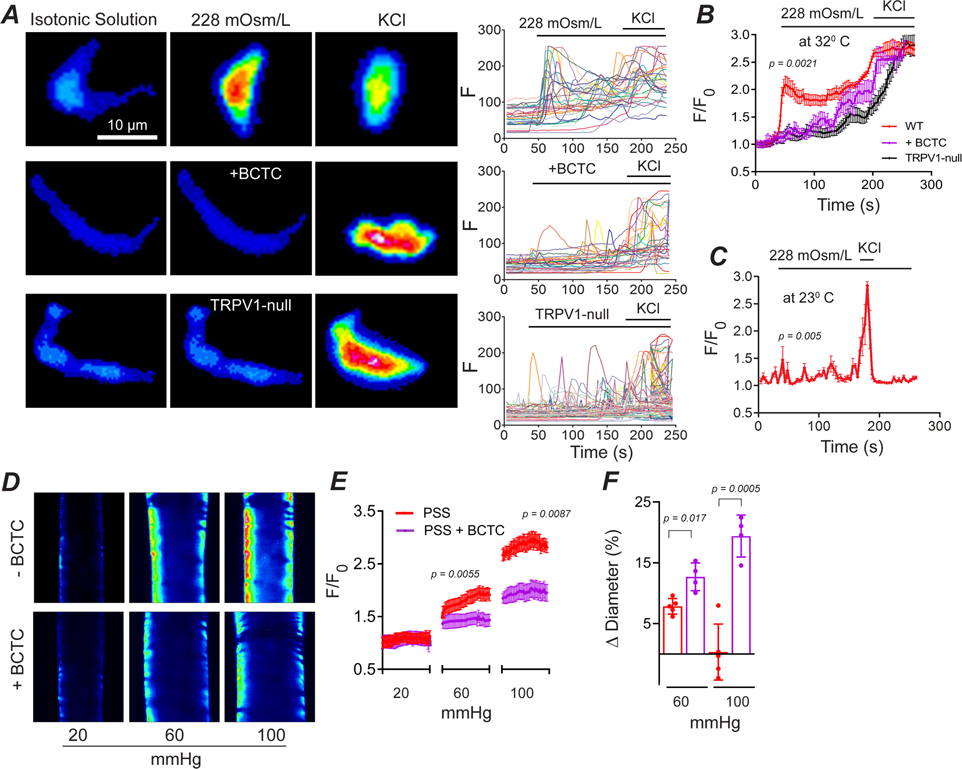
To explore whether stretch-mediated activation of TRPV1 is cell autonomous, we studied mechano-sensing in isolated ASM cells at 32°C. Consistent with an earlier report (Mederos y Schnitzler et al., 2008), we found that hypo-osmotic stretch rapidly increased intracellular [Ca2+] in ASM cells isolated from wild-type mice (Fig. 9A and B). In contrast, stretch evoked Ca2+ signals were significantly slower and smaller in ASM cells treated either with BCTC (P= 0.0021) or isolated from TRPV1-null mice (P= 0.0047). Furthermore, reducing the temperature from 32°C to 23°C abolished stretch-evoked Ca2+ signaling in ASM cells (Fig. 9C, P= 0.005).
Figure 9. Stretch of arterial smooth muscle cells activates TRPV1 in a heat-dependent manner.
A-C, Ca2+ signaling evoked by a hypo-osmotic solution in ASM cells isolated from wild-type (n = 47 cells, 3 mice), wild-type plus BCTC (n = 35 cells, 3 mice, nested t-test) and TRPV1-null (n = 30 cells, 3 mice, nested t-test) mice. Both, BCTC (1 μM), and reducing temperature from 32°C to 23°C, inhibits the stretch-evoked response (n = 28 cells, 3 mice, nested t-test). D and E, Intraluminal pressure-evoked Ca2+ increases in arteries denuded of endothelium are blocked by BCTC (3 μM; control, n = 51 cells, 4 mice, nested t-test, and BCTC, n = 29 cells, 4 mice, nested t-test). F, Changes in diameter of denuded arteries with or without BCTC (n = 4 arteries, 4 mice, nested t-test).
Osmotic stimuli are not perfect surrogates for mechanotransduction, therefore, to test the effects of physiological stretch we performed Ca2+ imaging in isolated pressurized arteries denuded of the endothelium. Under control conditions, increasing intraluminal pressure (from 20 to 60 and 100 mm Hg) triggered graded increases in Ca2+ accompanied by an unchanged vessel diameter (Fig. 9D and E). In contrast, BCTC inhibited the pressure-evoked rise in Ca2+ leading to vessel dilation (Fig. 9D and E). These results confirm that membrane stretch of vascular smooth muscle cells rapidly activates TRPV1.
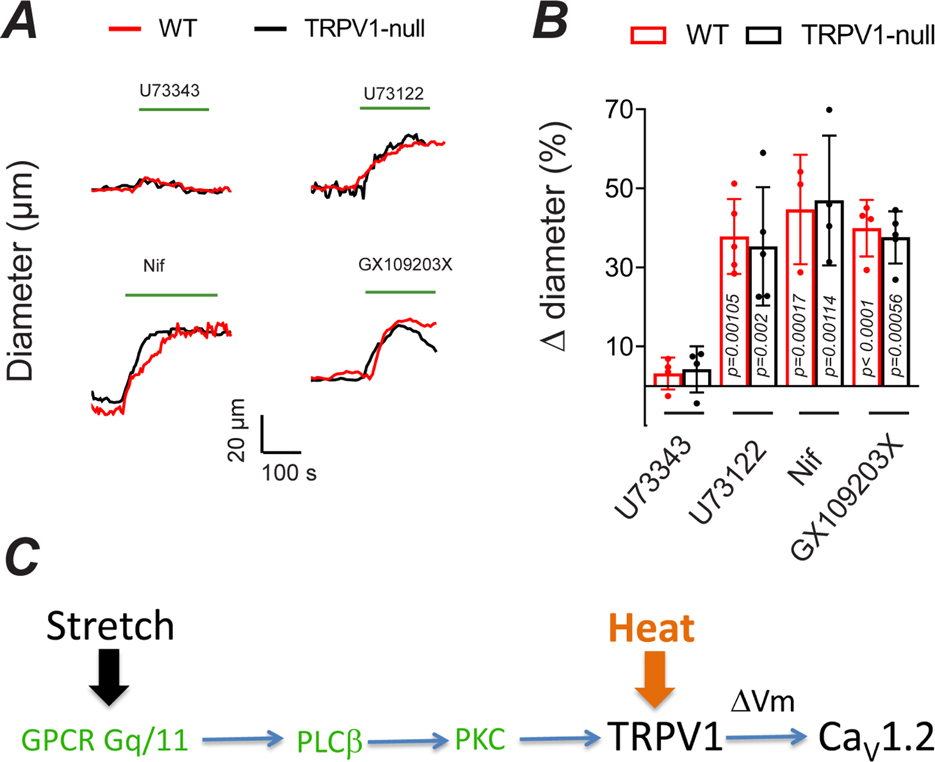
Next, we explored the underlying mechanism for stretch-induced activation of TRPV1. Although not intrinsically mechanosensitive, TRPV1 is activated or sensitized by PLC-dependent signaling (Premkumar & Ahern, 2000; Chuang et al., 2001; Tominaga et al., 2001). Indeed, both PLCβ and PLCγ isoforms are implicated in membrane stretch and the generation of myogenic tone (Osol et al., 1993; Matsumoto et al., 1995; Gonzales et al., 2014). We found that the PLC inhibitor, U73122, but not the inactive analogue, U73343, decreased arterial tone to a similar extent as BCTC (Fig. 10). PLC cleaves PIP2 to form diacylglycerol (DAG). In turn, DAG can activate/sensitize TRPV1 channels via PKC-dependent phosphorylation (Premkumar & Ahern, 2000; Vellani et al., 2001; Numazaki et al., 2002). We found that the PKC antagonist, GX109203X, inhibited the development of tone in these arterioles to the same extent as inhibition of PLC. This result agrees with earlier studies demonstrating a critical role for PKC in the myogenic tone of skeletal muscle arterioles (Hill et al., 1990; Korzick et al., 2004; Hong et al., 2016). Pharmacologic blockade of PLC and PKC similarly suppressed myogenic tone in TRPV1-null arteries (Fig. 10B). Collectively, these results support a signaling pathway whereby membrane stretch engages a PLC/PKC pathway to activate TRPV1 (Fig. 10C). TRPM4, which is also activated by PLC/PKC (Guinamard et al., 2002; Nilius et al., 2005; Earley et al., 2007) may contribute to tone and compensate for the genetic disruption of TRPV1. In turn, the resultant depolarization activates CaV1.2 to trigger muscle contraction.
Figure 10. Arterial stretch activates TRPV1 in a PLC/PKC dependent manner.
A and B, Changes in the diameter of pressurized (80 mm Hg, 34–35°C) skeletal muscle arterioles from WT and TRPV1-null mice treated with the PLC inhibitor, U73122 (10 μM, n = 4), the inactive analog, U73343 (10 μM, n = 4), nifedipine (5 μM, n = 3) and the PKC inhibitor, GF 109203X (n = 4, nested one-way ANOVA), C, Proposed signaling cascade for the generation of TRPV1-dependent myogenic tone in skeletal muscle arterioles.
Discussion
Myogenic tone is a fundamental auto-regulatory property of arterioles that enables precise, local control of tissue perfusion. Although, fairly ubiquitous throughout the circulation there are differences in the properties of myogenic tone among tissues. Notably, the generation of myogenic tone is markedly faster in skeletal muscle (Hill et al., 1990) and heart (Mosher et al., 1964; Muller et al., 1993) compared with the brain (Osol et al., 1993; Welsh et al., 2002) and mesentery (Blodow et al., 2014). The underlying mechanisms for these differences are unclear. Our data show that TRPV1, localized in skeletal muscle, coronary and adipose arteries, participates in the regulation of myogenic tone. Several lines of evidence support this conclusion. Administration of the selective TRPV1 pharmacologic inhibitors, BCTC and AMG517, dilated arterioles in vitro and in vivo, decreased blood pressure and increased coronary perfusion. The depressor effect of TRPV1 antagonists was transient due to an increase in HR. This cardiovascular compensation and/or inadequate dosage may explain why BP changes were not reported after oral administration of TRPV1 antagonists in humans and animals (Chizh et al., 2007; Gavva et al., 2007). Importantly, the antagonists failed to elicit responses in arteries from TRPV1-null mice or non-TRPV1-expressing mesenteric arteries, confirming a selective action at TRPV1. Furthermore, BCTC inhibited the constriction of isolated arterioles in response to raised intraluminal pressure, and arterial smooth muscle cells in response to stretch. We found that myogenic tone in skeletal muscle arterioles (120 μm diameter) was largely sensitive to nifedipine (75–80%), indicating an important role for CaV1.2 and in agreement with earlier findings (Knot & Nelson, 1998; Mauban et al., 2013). BCTC blocked ~two-thirds of this nifedipine-sensitive tone, while the TRPM4 antagonist, 9-phenanthrol inhibited the remaining fraction. In contrast to pharmacologic blockade, genetic disruption of TRPV1 did not alter the steady-state tone measured 5 minutes after a pressure step. Developmental compensation by other ion channels in these constitutive TRPV1 knock-out mice may explain this discrepancy. Indeed, we found that expression of TRPM4 and TRPP1 increased significantly in arteries of TRPV1-null mice. Further, 9-phenanthrol elicited a greater inhibitory effect in TRPV1-null arteries consistent with the larger fraction of TRPM4-dependent tone. Although these data should be interpreted cautiously; 9-phenanthrol may not be perfectly selective for TRPM4 (Burris et al., 2015), the results support the hypothesis that TRPM4 mediates the TRPV1-independent tone in WT arteries, and that up-regulation of TRPM4 expression partly compensates for genetic disruption of TRPV1. Potentially other TRP channels, including TRPP1, may also contribute to restore steady-state tone in TRPV1-null mice.
A key finding of this study is that TRPV1 regulates the rate of myogenic tone. Although TRPV1-deficient arteries exhibited unchanged steady-state tone, the development of tone was slowed by ~2–3-fold compared with wild-type arteries. This difference was most pronounced in the smallest diameter arterioles studied (25 μm diameter) where disruption of TRPV1 expression increased the T1/2 for the development of tone from ~15 to 45 s. The rapid tone in WT arteries agrees with earlier reports. For example, raised intraluminal pressure in third-order cremaster muscle arterioles arterioles (~10–15 μm diameter) triggered a decrease in diameter within 10 s (Hill et al., 1990). Similarly, rapid myogenic responses are evident in isolated coronary arterioles (Muller et al., 1993) and in the beating dog heart (Mosher et al., 1964), where increases in perfusion pressure triggered autoregulation of coronary flow within 10 s. Although we can’t exclude contribution of other factors, our data suggest that the abundant expression of TRPV1 in skeletal muscle and coronary arterioles significantly contributes to the rapid myogenic tone of these tissues. In addition to controlling the onset of myogenic tone, our data reveal a critical role for TRPV1 in the rapid cessation of tone. Skeletal muscle arterioles characteristically dilate at the beginning of muscle contractions (Clifford, 2007). Indeed, using intravital imaging we observed prominent vasodilation following arteriole constrictions of duration from 4 to 40 s. Notably, BCTC completely occluded this reactive vasodilation. Furthermore, genetic disruption of TRPV1 inhibited greater than 70% of the response demonstrating minimal developmental compensation. Metabolic factors may underlie reactive hyperemia particularly with long duration vessel occlusion. However, previous studies have shown that even very brief (100 ms) muscle contractions elicit vasodilation of skeletal muscle arterioles in situ (Sinkler et al., 2016). Further, a rapid-onset vasodilation occurs in isolated arteries (Clifford et al., 2006) and in the human forearm (Kirby et al., 2007) in response to intermittent raised extravascular pressure. Thus, rapid-onset vasodilation may purely reflect a response to mechanical compression of the vessel. Importantly, our findings suggest that disruption of myogenic tone via, deactivation of TRPV1, is a critical component of this process.
How does TRPV1, not recognized as intrinsically mechanosensitive, participate in arterial mechano-signaling? Gq/G11 GPCRs are candidate cellular mechanosensors and are implicated in the detection of intraluminal stretch (Mederos y Schnitzler et al., 2008). In skeletal muscle arterioles, the type 1a angiotensin receptor (AT1aR) contributes to the generation of myogenic tone, signaling via a PLC-PKC pathway (Hong et al., 2016). Indeed, we found a key role for PLC/PKC signaling; pharmacological antagonists of these proteins inhibited myogenic tone by 75–80%. Notably, this same fraction of myogenic tone was sensitive to nifedipine. Thus, consistent with earlier reports (Hill et al., 1990; Korzick et al., 2004), PKC signaling appears to underlie the majority of the myogenic tone of skeletal muscle arterioles, linking membrane stretch to the activation of voltage–gated CaV1.2 channels. Our data support TRPV1 as a key intermediary in this signaling pathway, with a smaller contribution from TRPM4, a channel also stimulated by PKC (Guinamard et al., 2002; Nilius et al., 2005; Earley et al., 2007). Thus, PKC stimulation of TRPV1, and to a lesser extent TRPM4, mediates depolarization-induced activation of CaV1.2. It should be noted, however, that our experiments with pharmacologic PKC inhibitors can’t exclude the participation of other PKC-regulated proteins. Notably, PKC-dependent phosphorylation sensitizes TRPV1 to various stimuli including heat, reducing the temperature threshold substantially from 42°C to 32°C (Sugiura et al., 2002). We found that stretch of arteriolar myocytes activated Ca2+ signaling in a temperature dependent manner; responses were only evident when the temperature was raised from 23°C to 32°C. Further, stretch-induced Ca2+ responses at 32°C were inhibited by BCTC, consistent with activation of TRPV1 via sensitization to heat. These results also agree with the earlier observations that myogenic tone is temperature dependent; vessels from cremaster muscle or cheek pouch are relaxed at room temperature and tone only develops when tissue are warmed to the physiological range of 34–36°C (Duling et al., 1981; Davis & Sikes, 1990).
Multimodal gating of TRPV1 may explain the rapid kinetics of tone. The distinct chemical and physical sensor domains in TRPV1 are allosterically coupled to channel gating (Tominaga et al., 1998; Brauchi et al., 2004; Voets et al., 2004; Matta & Ahern, 2007). Thus, PKC in combination with temperature and other stimuli may underlie rapid activation of TRPV1 and changes in myogenic tone. Conversely, a fall in intraluminal pressure and de-phosphorylation of TRPV1 would rapidly decrease TRPV1 activity and reduce tone. Previously, we showed that Ca2+ permeability through TRPV1 activated by high concentrations of capsaicin is sufficient to constrict arterioles (Phan et al., 2020). Here we found that nifedipine completely inhibits myogenic tone, negating a significant contribution of Ca2+ entry through TRPV1. Presumably, this reflects weak activation (low channel open probability, Po) of TRPV1 during stretch signaling compared with the strong activation evoked by saturating concentrations of capsaicin (high Po of ~0.8)(Premkumar et al., 2002; Hui et al., 2003). Moreover, the relative level of TRPV1 expression in vascular smooth muscle is low, ~10% of sensory neurons (Phan et al., 2020), and therefore a high Po of these channels would likely be required to raise myoplasmic Ca2+ sufficiently to initiate contraction.
A prevailing hypothesis is that myogenic autoregulation holds arteries in a partly constricted state thereby facilitating bi-directional changes in vessel caliber and blood flow. Indeed, its suppression with ageing (Lott et al., 2004; Ghosh et al., 2015) and in diabetes/cardiovascular disease (Petersen et al., 2002; Hodnett & Hester, 2007; Duncker et al., 2015) underscores an important role for the myogenic response in cardiac and exercise performance. Our data reveal that TRPV1 speeds both the development and removal of myogenic tone and thus aids in the dynamic regulation of perfusion in skeletal muscle and the heart. This property may be advantageous in these tissues that experience both large and fast fluctuations in blood pressure. Further, our data reveal a critical role for TRPV1 in rapid reactive vasodilation, providing a molecular mechanism for how skeletal muscle contractions at the onset of activity immediately increase tissue blood flow. We propose that TRPV1 may enable a similar process in myocardial perfusion as our data show that TRPV1 contributes significantly to coronary myogenic tone.
Supplementary Material
Key points summary:
We explored the physiological role of TRPV1 in vascular smooth muscle. TRPV1 antagonists dilated skeletal muscle arterioles both ex vivo and in vivo, increased coronary perfusion and decreased systemic blood pressure.
Stretch of arteriolar myocytes and increases in intraluminal pressure in arteries triggered rapid Ca2+ signaling and vasoconstriction respectively. Pharmacologic and/or genetic disruption of TRPV1 significantly inhibited the magnitude and rate of these responses. Furthermore, disrupting TRPV1 blunted the rapid vasodilation evoked by arterial constriction.
Pharmacological experiments identified key roles for phospholipase C and protein kinase C, combined with temperature, in TRPV1-dependent arterial tone.
These results show that TRPV1 in arteriolar myocytes dynamically regulates myogenic tone and blood flow in the heart and skeletal muscle.
Funding:
This study was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R01 HL155979 (G.A) and R01 HL146169 (M.K), the National Institute of Diabetes and Digestive and Kidney Diseases under award U01 DK101040 (G.A.), the Hungarian Research Fund (OTKA K116940 to RP and AT) and by the GINOP-2.3.2-15-2016-00043 and GINOP-2.3.2-15-2016-00050 grants (to AT). The project is co-financed by the European Union and the European Regional Development Fund. Hajnalka Gulyás was supported by Gedeon Richter Talentum Foundation (Hungary, Budapest 1103, Gyömrői str. 19.–21.)
Biography

Thieu X. Phan received his PhD at University of Bucharest in 2012. Since 2014 he has worked in the group of Dr. Gerard Ahern at Georgetown University Medical Center in Washington DC. Thieu is interested in the functional properties of Transient Receptor Potential (TRP) channels. Currently, he is focused on mapping the expression and function of TRPV1 in artery smooth muscle (ASM) cells, and in particular, how TRPV1 regulates regional blood flow and blood pressure.
Footnotes
Competing Interest Statement: The authors declare no conflict of interest.
Data availability:
All data are included in the manuscript.
References
- Bang S, Yoo S, Oh U & Hwang SW. (2010). Endogenous lipid-derived ligands for sensory TRP ion channels and their pain modulation. Arch Pharm Res 33, 1509–1520. [DOI] [PubMed] [Google Scholar]
- Bayliss WM. (1902). On the local reactions of the arterial wall to changes of internal pressure. The Journal of physiology 28, 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blodow S, Schneider H, Storch U, Wizemann R, Forst A-L, Gudermann T & Mederos y Schnitzler M. (2014). Novel role of mechanosensitive AT1B receptors in myogenic vasoconstriction. Pflugers Archiv : European journal of physiology 466, 1343–1353. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Orio P & Latorre R. (2004). Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci USA 101, 15494–15499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulley S, Fernández-Peña C, Hasan R, Leo MD, Muralidharan P, Mackay CE, Evanson KW, Moreira-Junior L, Mata-Daboin A, Burris SK, Wang Q, Kuruvilla KP & Jaggar JH. (2018). Arterial smooth muscle cell PKD2 (TRPP1) channels regulate systemic blood pressure. eLife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris SK, Wang Q, Bulley S, Neeb ZP & Jaggar JH. (2015). 9-Phenanthrol inhibits recombinant and arterial myocyte TMEM16A channels. British Journal of Pharmacology 172, 2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ & Julius D. (2001). The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24, 487–517. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD & Julius D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, O’Donnell D, Nicoll RA, Shah NM, Julius D & Basbaum AI. (2011). Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J Neurosci 31, 5067–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennupati R, Wirth A, Favre J, Li R, Bonnavion R, Jin Y-J, Wietelmann A, Schweda F, Wettschureck N, Henrion D & Offermanns S. (2019). Myogenic vasoconstriction requires G12/G13 and LARG to maintain local and systemic vascular resistance. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizh BA, O’Donnell MB, Napolitano A, Wang J, Brooke AC, Aylott MC, Bullman JN, Gray EJ, Lai RY, Williams PM & Appleby JM. (2007). The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 132, 132–141. [DOI] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV & Julius D. (2001). Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411, 957–962. [DOI] [PubMed] [Google Scholar]
- Clifford PS. (2007). Skeletal muscle vasodilatation at the onset of exercise. The Journal of Physiology 583, 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PS, Kluess HA, Hamann JJ, Buckwalter JB & Jasperse JL. (2006). Mechanical compression elicits vasodilatation in rat skeletal muscle feed arteries. The Journal of Physiology 572, 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czikora Á, Lizanecz E, Bakó P, Rutkai I, Ruzsnavszky F, Magyar J, Pórszász R, Kark T, Facskó A, Papp Z, Edes I & Tóth A. (2012). Structure-activity relationships of vanilloid receptor agonists for arteriolar TRPV1. British Journal of Pharmacology 165, 1801–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czikora Á, Rutkai I, Pásztor ET, Szalai A, Pórszász R, Boczán J, Édes I, Papp Z & Tóth A. (2013). Different Desensitization Patterns for Sensory and Vascular TRPV1 Populations in the Rat: Expression, Localization and Functional Consequences. PloS one 8, e78184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ. (2012). Perspective: Physiological Role(s) of the Vascular Myogenic Response. Microcirculation 19, 99–114. [DOI] [PubMed] [Google Scholar]
- Davis MJ & Sikes PJ. (1990). Myogenic responses of isolated arterioles: test for a rate-sensitive mechanism. The American journal of physiology 259, H1890–1900. [DOI] [PubMed] [Google Scholar]
- Duling BR, Gore RW, Dacey RG & Damon DN. (1981). Methods for isolation, cannulation, and in vitro study of single microvessels. The American journal of physiology 241, H108–116. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Koller A, Merkus D & Canty JM. (2015). Regulation of coronary blood flow in health and ischemic heart disease. Progress in cardiovascular diseases 57, 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S & Brayden JE. (2015). Transient receptor potential channels in the vasculature. Physiological reviews 95, 645–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Straub SV & Brayden JE. (2007). Protein kinase C regulates vascular myogenic tone through activation of TRPM4. American journal of physiology Heart and circulatory physiology 292, H2613–2622. [DOI] [PubMed] [Google Scholar]
- Earley S, Waldron BJ & Brayden JE. (2004). Critical role for transient receptor potential channel TRPM4 in myogenic constriction of cerebral arteries. Circulation research 95, 922–929. [DOI] [PubMed] [Google Scholar]
- Fischer MJM, Uchida S & Messlinger K. (2010). Measurement of meningeal blood vessel diameter in vivo with a plug-in for ImageJ. Microvascular research 80, 258–266. [DOI] [PubMed] [Google Scholar]
- Gavva NR, Bannon AW, Surapaneni S, Hovland DN Jr., Lehto SG, Gore A, Juan T, Deng H, Han B, Klionsky L, Kuang R, Le A, Tamir R, Wang J, Youngblood B, Zhu D, Norman MH, Magal E, Treanor JJ & Louis JC. (2007). The vanilloid receptor TRPV1 is tonically activated in vivo and involved in body temperature regulation. J Neurosci 27, 3366–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P, Mora Solis FR, Dominguez JM, Spier SA, Donato AJ, Delp MD & Muller-Delp JM. (2015). Exercise training reverses aging-induced impairment of myogenic constriction in skeletal muscle arterioles. Journal of applied physiology (Bethesda, Md : 1985) 118, 904–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales AL, Yang Y, Sullivan MN, Sanders L, Dabertrand F, Hill-Eubanks DC, Nelson MT & Earley S. (2014). A PLCγ1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci Signal 7, ra49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill AG, Dick GM, Kiel AM & Tune JD. (2017). Regulation of Coronary Blood Flow. Comprehensive Physiology 7, 321–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinamard R, Rahmati M, Lenfant J & Bois P. (2002). Characterization of a Ca2+-activated nonselective cation channel during dedifferentiation of cultured rat ventricular cardiomyocytes. The Journal of membrane biology 188, 127–135. [DOI] [PubMed] [Google Scholar]
- Hill MA, Falcone JC & Meininger GA. (1990). Evidence for protein kinase C involvement in arteriolar myogenic reactivity. The American journal of physiology 259, H1586–1594. [DOI] [PubMed] [Google Scholar]
- Hodnett BL & Hester RL. (2007). Regulation of muscle blood flow in obesity. Microcirculation 14, 273–288. [DOI] [PubMed] [Google Scholar]
- Hong K, Zhao G, Hong Z, Sun Z, Yang Y, Clifford PS, Davis MJ, Meininger GA & Hill MA. (2016). Mechanical activation of angiotensin II type 1 receptors causes actin remodelling and myogenic responsiveness in skeletal muscle arterioles. The Journal of Physiology 594, 7027–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K, Liu B & Qin F. (2003). Capsaicin activation of the pain receptor, VR1: multiple open states from both partial and full binding. Biophysical journal 84, 2957–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kark T, Bagi Z, Lizanecz E, Pásztor ET, Erdei N, Czikora A, Papp Z, Edes I, Pórszász R & Tóth A. (2008). Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Molecular pharmacology 73, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Kirby BS, Carlson RE, Markwald RR, Voyles WF & Dinenno FA. (2007). Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. The Journal of Physiology 583, 861–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ & Nelson MT. (1998). Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. The Journal of Physiology 508 (Pt 1), 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzick DH, Laughlin MH & Bowles DK. (2004). Alterations in PKC signaling underlie enhanced myogenic tone in exercise-trained porcine coronary resistance arteries. Journal of applied physiology (Bethesda, Md : 1985) 96, 1425–1432. [DOI] [PubMed] [Google Scholar]
- Lizanecz E, Bagi Z, Pásztor ET, Papp Z, Edes I, Kedei N, Blumberg PM & Tóth A. (2006). Phosphorylation-dependent desensitization by anandamide of vanilloid receptor-1 (TRPV1) function in rat skeletal muscle arterioles and in Chinese hamster ovary cells expressing TRPV1. Molecular pharmacology 69, 1015–1023. [DOI] [PubMed] [Google Scholar]
- Lott MEJ, Herr MD & Sinoway LI. (2004). Effects of age on brachial artery myogenic responses in humans. American journal of physiology Regulatory, integrative and comparative physiology 287, R586–591. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Baron CB & Coburn RF. (1995). Smooth muscle stretch-activated phospholipase C activity. The American journal of physiology 268, C458–465. [DOI] [PubMed] [Google Scholar]
- Matta JA & Ahern GP. (2007). Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol 585, 469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauban JRH, Zacharia J, Zhang J & Wier WG. (2013). Vascular tone and Ca(2+) signaling in murine cremaster muscle arterioles in vivo. Microcirculation 20, 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M & Gudermann T. (2008). Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27, 3092–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Tisel SM, Orestes P, Bhangoo SK & Hoon MA. (2011). TRPV1-lineage neurons are required for thermal sensation. EMBO J 30, 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosher P, Ross J, Mcfate PA & Shaw RF. (1964). Control of coronary blood flow by an autoregulatory mechanism. Circulation research 14, 250–259. [DOI] [PubMed] [Google Scholar]
- Muller JM, Myers PR & Laughlin MH. (1993). Exercise training alters myogenic responses in porcine coronary resistance arteries. Journal of applied physiology (Bethesda, Md : 1985) 75, 2677–2682. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Tang J, Wang C, Owsianik G, Janssens A, Voets T & Zhu MX. (2005). Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. The Journal of biological chemistry 280, 6423–6433. [DOI] [PubMed] [Google Scholar]
- Numazaki M, Tominaga T, Toyooka H & Tominaga M. (2002). Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem 277, 13375–13378. [DOI] [PubMed] [Google Scholar]
- Osol G, Laher I & Kelley M. (1993). Myogenic tone is coupled to phospholipase C and G protein activation in small cerebral arteries. The American journal of physiology 265, H415–420. [DOI] [PubMed] [Google Scholar]
- Petersen HH, Choy J, Stauffer B, Moien-Afshari F, Aalkjaer C, Leinwand L, McManus BM & Laher I. (2002). Coronary artery myogenic response in a genetic model of hypertrophic cardiomyopathy. American journal of physiology Heart and circulatory physiology 283, H2244–2249. [DOI] [PubMed] [Google Scholar]
- Phan TX, Ton HT, Chen Y, Basha ME & Ahern GP. (2016). Sex-dependent expression of TRPV1 in bladder arterioles. American journal of physiology Renal physiology 311, F1063–F1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan TX, Ton HT, Gulyás H, Pórszász R, Tóth A, Russo R, Kay MW, Sahibzada N & Ahern GP. (2020). TRPV1 expressed throughout the arterial circulation regulates vasoconstriction and blood pressure. The Journal of Physiology 598, 5639–5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS, Agarwal S & Steffen D. (2002). Single-channel properties of native and cloned rat vanilloid receptors. The Journal of Physiology 545, 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar LS & Ahern GP. (2000). Induction of vanilloid receptor channel activity by protein kinase C. Nature 408, 985–990. [DOI] [PubMed] [Google Scholar]
- Reading SA & Brayden JE. (2007). Central role of TRPM4 channels in cerebral blood flow regulation. Stroke; a journal of cerebral circulation 38, 2322–2328. [DOI] [PubMed] [Google Scholar]
- Retailleau K, Duprat F, Arhatte M, Ranade SS, Peyronnet R, Martins JR, Jodar M, Moro C, Offermanns S, Feng Y, Demolombe S, Patel A & Honoré E. (2015). Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. CellReports 13, 1161–1171. [DOI] [PubMed] [Google Scholar]
- Sinkler SY, Fernando CA & Segal SS. (2016). Differential α-adrenergic modulation of rapid onset vasodilatation along resistance networks of skeletal muscle in old versus young mice. The Journal of Physiology 594, 6987–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H & Mizumura K. (2002). Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88, 544–548. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI & Julius D. (1998). The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M & Masu M. (2001). Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A 98, 6951–6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida E & Bohr DF. (1969). Myogenic tone in isolated perfused vessels. Occurrence among vascular beds and along vascular trees. Circulation Research 25, 549–555. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB & McNaughton PA. (2001). Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol 534, 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V & Nilius B. (2004). The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430, 748–754. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Morielli AD, Nelson MT & Brayden JE. (2002). Transient receptor potential channels regulate myogenic tone of resistance arteries. Circulation Research 90, 248–250. [DOI] [PubMed] [Google Scholar]
- Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T & Komuro I. (2004). Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nature cell biology 6, 499–506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the manuscript.