Abstract
Oral administration of 2′-deoxy-3′-oxa-4′-thiocytidine (BCH-10652), a nucleoside analog structurally similar to lamivudine (3TC), caused dose-dependent inhibition of viral replication in SCID-hu Thy/Liv mice infected with human immunodeficiency virus type 1 NL4-3 and with an NL4-3 clone containing the M184V mutation in reverse transcriptase that confers resistance to 3TC. These experiments demonstrate the utility of this mouse model for evaluating drug resistance and for performing direct comparisons between antiviral compounds in vivo.
Heterosubstituted 2′,3′-dideoxynucleoside analogs, such as lamivudine (3TC) and 5-fluoro 3TC (FTC), differ from other nucleoside analog reverse transcriptase (RT) inhibitors by having a carbon replaced by a sulfur in the 3′ position (11, 15, 16). Although 3TC is one of the most commonly used nucleoside analogs in first-line combination therapy for human immunodeficiency virus type 1 (HIV-1) infections (9), 3TC monotherapy results in the selection of preexisting 3TC-resistant viral variants and a concomitant rebound in plasma viral load (13, 25). High-level resistance to 3TC is conferred by a single mutation at codon 184 (from methionine to either valine or isoleucine) in the catalytic domain of HIV-1 RT (8, 24, 32), and the M184V substitution increases 50% inhibitory concentrations (IC50s) of 3TC at least 1,000-fold (13, 31).
Concerns about the development of viral resistance to 3TC have spurred the discovery of structurally related nucleosides with activity against HIV-1 isolates containing common 3TC resistance mutations. The nucleoside analog 2′-deoxy-3′-oxa-4′-thiocytidine (dOTC, or BCH-10652) is a racemic mixture of two relatively equipotent enantiomers with antiviral activity against 3TC-resistant clinical HIV-1 isolates (5, 11, 16), and it is currently in clinical development. Although dOTC is structurally related to 3TC, it has an inversion of the oxygen and sulfur in the furanosyl ring and is a mixture of enantiomers with d-sugar [(−)dOTC] and l-sugar [(+)dOTC] configurations, whereas 3TC is the l-sugar racemate only (Fig. 1). In this study, we compared the antiviral activities of 3TC and dOTC against the HIV-1 molecular clone NL4-3 (1) and an NL4-3 clone containing the M184V mutation in RT (NL4-3/M184V) in phytohemagglutinin-activated human peripheral blood mononuclear cells (PBMCs) and in the SCID-hu Thy/Liv mouse model of HIV-1 infection (2, 7, 17, 21, 26, 27).
FIG. 1.
Chemical structures of the enantiomers of dOTC and of 3TC.
Pooled human PBMCs from four to eight donors were inoculated in bulk at a multiplicity of infection of 0.005 by adding 20,000 50% tissue culture infective doses (TCID50s) of HIV-1 to 4 × 106 cells in 2 ml of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, l-glutamine, and 5 U of human lymphocyte interleukin-2 (IL-2) (Boehringer-Mannheim) per ml for 2 h at 37°C. Cells were washed, and 100,000 cells in 50 μl were added to triplicate wells of round-bottom 96-well plates. Wells were treated with 200 μl of eight different concentrations of test agent (in 10-fold increments) or with medium alone, and supernatants were assayed at day 7 for HIV-1 gag p24 by enzyme-linked immunosorbent assay (ELISA) in antibody-coated microplates (DuPont). The IC50 is the concentration of test agent that was calculated (by linear regression) to reduce p24 optical density values by 50%. NL4-3/M184V was highly resistant to 3TC; the mean IC50 for NL4-3/M184V was approximately 1,000-fold higher than that for wild-type NL4-3 (Table 1). In contrast to 3TC, dOTC had virtually identical mean IC50s against the two viruses (9.6 μM for NL4-3/M184V versus 7.2 μM for NL4-3). The M184V mutation in NL4-3 thus confers high-level resistance to 3TC but causes essentially no increase in the IC50 of dOTC. Despite the demonstrable activity of dOTC against NL4-3/M184V, dOTC was 80-fold less potent than 3TC against wild-type NL4-3 in human PBMCs (IC50s, 7.2 versus 0.09 μM). Parallel cellular toxicity determinations in treated uninfected PBMCs incubated with (4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) (20) on day 7 yielded similar mean 50% cytotoxic concentrations (CC50s) for both compounds (830 μM for 3TC and 520 μM for dOTC).
TABLE 1.
In vitro antiviral activities and cytotoxicities of 3TC, dOTC, and AZT in phytohemagglutinin-activated human PBMCs
| Drug | IC50 (μM)a for:
|
CC50 (μM)a | |
|---|---|---|---|
| NL4-3 | NL4-3/M184V | ||
| 3TC | 0.09 ± 0.03 (4) | 85 ± 6.5 (2) | 830 ± 91 (3) |
| dOTC | 7.2 ± 4.2 (3) | 9.6 ± 7.5 (2) | 520 ± 49 (3) |
| AZT | 4.3 ± 2.2 × 10−5 (3) | 2.7 ± 0.3 × 10−5 (2) | 29 ± 11 (3) |
The IC50s and CC50s were calculated from dose-response curves obtained by linear regression of the percent control optical density (for either p24 or MTT) with eight drug concentrations assayed in triplicate wells. Data are presented as means ± standard errors for the numbers of independent experiments shown in parentheses.
The efficacies of dOTC and 3TC against NL4-3 and NL4-3/M184V were evaluated in vivo in the SCID-hu Thy/Liv mouse model of HIV-1 infection. The human thymus implant in this model supports long-term differentiation of human lymphoid and myeloid cells (18, 22) and has been used to study the pathogenic effects of HIV-1 in vivo (3, 6, 7, 12, 14, 29, 30). The model has also been standardized for the preclinical evaluation of antiviral compounds against HIV-1 (10, 23, 28). By use of previously described procedures (23, 27), dOTC or 3TC in 0.5% carboxymethylcellulose was administered to SCID-hu Thy/Liv mice by oral gavage twice daily at various dosage levels, starting 24 h before direct injection of 1,000 TCID50s of either NL4-3 or NL4-3/M184V into each Thy/Liv implant. Each of four independent antiviral evaluations was performed in a cohort of mice implanted with thymus and liver from a single donor (with different cohorts made from different donors). Each dosing group comprised five to seven mice.
After 13 days of daily treatment (i.e., 12 days after virus inoculation), implants were excised and dispersed into single-cell suspensions, and cells were lysed for quantitation of p24 by ELISA and were also stained with antibodies to CD3, CD4, and CD8 for analysis of T-cell subsets by flow cytometry. For quantitation of cell-associated HIV-1 RNA by branched DNA assay, 5 × 106 cells were disrupted with sterile disposable pestles and a cordless motor grinder (Kontes, Vineland, N.J.) in 8 M guanidine HCl with 0.5% sodium N-lauroylsarcosine. The RNA was extracted by adding 5 ml of 100% ethanol containing 20 μg of polyadenylic acid (Sigma) per ml, and each sample was vortexed and pelleted at 12,000 × g for 20 min at 4°C. Supernatants were aspirated to remove DNA, and RNA pellets were washed with 5 ml of 70% ethanol, placed on dry ice, and digested with reagents supplied by the manufacturer (Quantiplex HIV-1 RNA assay 2.0; Chiron Corporation, Emeryville, Calif.). The HIV-1 RNA load in the implant is expressed as copies per 106 implant thymocytes, and the log10 values were used for the calculation of geometric means. The limit of detection was typically 2.3 to 2.6 log10 RNA copies per 106 cells, and the lower-limit value was used for mean calculation of implants with undetectable viral RNA. The Mann-Whitney U test (StatView 5.0; Abacus Concepts, Berkeley, Calif.) was used for statistical analysis of p24 and viral RNA levels in implants, and P values of ≤0.05 were considered statistically significant. The day of implant collection was chosen based on previous studies of NL4-3 replication and thymocyte depletion kinetics in the SCID-hu Thy/Liv model (23); p24 in implants normally reaches half-maximum levels by day 12 in the absence of virus-induced thymocyte depletion. A preliminary SCID-hu experiment with NL4-3/M184V indicated that the mutant virus had replication kinetics in the human thymus similar to those of NL4-3 (data not shown), in contrast to the attenuated viral growth reported for M184V mutants in human PBMC cultures (19).
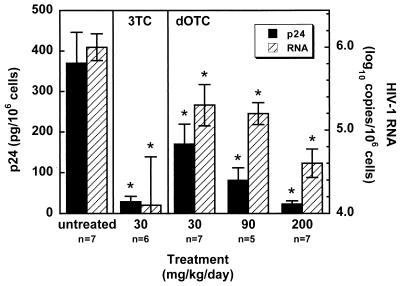
The first experiment was designed to evaluate the antiviral activity of dOTC in mice with wild-type NL4-3-infected Thy/Liv implants. There were statistically significant, dose-dependent reductions in implant p24 and viral RNA levels in mice treated with dOTC at doses of 30, 90, and 200 mg/kg of body weight/day compared to levels in untreated mice (Fig. 2). Treatment with 200 mg of dOTC/kg/day resulted in a 16-fold reduction in p24 and a 25-fold reduction in viral RNA, and these reductions were comparable to those obtained in mice treated with 3TC at 30 mg/kg/day in the same experiment. The in vivo activity of dOTC therefore appears less potent than that of 3TC, as was predicted by the IC50s obtained in NL4-3-infected PBMCs (Table 1).
FIG. 2.
In vivo inhibition of NL4-3 replication by 3TC and dOTC. Shown are levels of p24 (arithmetic means) and viral RNA (geometric means) in implants of mice treated twice daily by oral gavage with either 3TC or dOTC at the indicated dosage levels beginning 24 h before intrathymic inoculation of 1,000 TCID50s of NL4-3 per implant. Implants were collected 12 days after virus inoculation. Data for treated mice (five to seven mice per group) were compared to those for untreated mice, and P values of ≤0.05 by the Mann-Whitney U test (indicated by asterisks) were considered statistically significant. Error bars, standard errors.
In the second experiment, mice with NL4-3/M184V-infected implants were treated with higher doses of either 3TC or dOTC to determine the level of in vivo resistance conferred by the M184V mutation in RT. Treatment with dOTC at 200 and 400 mg/kg/day caused 2- and 6-fold reductions in p24 and 3- and 25-fold reductions in viral RNA, respectively, whereas 3TC at these doses had no detectable activity against the resistant virus (Fig. 3). These data demonstrate that dOTC has activity against 3TC-resistant HIV-1 in vivo.
FIG. 3.
In vivo inhibition of NL4-3/M184V by dOTC but not by 3TC. Shown are levels of p24 and viral RNA in the implants of five to seven mice per group treated with 3TC or dOTC and inoculated with NL4-3/M184V as described for Fig. 2.
A direct comparison of the activities of 3TC and dOTC against NL4-3 and NL4-3/M184V was performed in a third experiment with a large cohort (n = 42) of SCID-hu Thy/Liv mice prepared with tissue from a single donor. In mice with NL4-3-infected implants, 30 mg of 3TC/kg/day produced reductions in implant viral load that were identical to those produced by dOTC at 200 mg/kg/day (Fig. 4), confirming the results we obtained in the first experiment (Fig. 2). In contrast, the same doses of 3TC and dOTC had no statistically significant activity against NL4-3/M184V. Although 200 mg of dOTC/kg/day produced statistically significant reductions in the p24 level in NL4-3/M184V-infected implants in the second experiment (Fig. 3), that was not the case in the third experiment, indicating that the M184V mutant is somewhat less sensitive to dOTC than is wild-type NL4-3.
FIG. 4.
Comparison of the antiviral activities of 3TC and dOTC against NL4-3 and NL4-3/M184V in a single large (n = 42) SCID-hu Thy/Liv mouse cohort. Shown are implant p24 and viral RNA levels in six to seven mice per group treated with 3TC or dOTC and inoculated with either NL4-3 or NL4-3/M184V as described for Fig. 2. Four additional mice were mock inoculated with medium alone.
To confirm further the differential activity of 3TC and dOTC against NL4-3 and NL4-3/M184V, a fourth experiment was performed in a single SCID-hu cohort treated with 3TC or dOTC at the higher dosage level of 400 mg/kg/day. Treatment with high-dose dOTC reduced p24 to undetectable levels and reduced viral RNA levels 630-fold in NL4-3-infected implants but produced less-dramatic reductions (13-fold for p24 and 25-fold for viral RNA) in NL4-3/M184V-infected implants (Fig. 5).
FIG. 5.
In vivo inhibition of NL4-3/M184V by dOTC at a dosage level ineffective for 3TC. Shown are implant p24 and viral RNA levels in four to seven mice per group treated twice daily by oral gavage with 3TC or dOTC and inoculated with either NL4-3 or NL4-3/M184V as described for Fig. 2.
Curiously, we observed statistically significantly higher viral loads in the NL4-3/M184V-infected implants of 3TC-treated mice than in those of untreated mice (Fig. 5). This paradoxical enhancement of NL4-3/M184V replication in the implants of 3TC-treated mice deserves further study and may be related to an in vitro phenomenon reported for zidovudine (AZT)-resistant HIV-1 in which AZT stimulated reverse transcription in cells infected with viruses containing the M41L or T215Y mutation in RT (4), although it has not been reported with 3TC-resistant HIV-1 grown in the presence of 3TC. The enhancement effect was not statistically significant with 3TC at 30 mg/kg/day (Fig. 4) but was significant in one of two experiments with mice treated with 400 mg/kg/day (compare Fig. 3 with Fig. 5).
In summary, dOTC had activity against wild-type NL4-3 both in human PBMCs and in the SCID-hu Thy/Liv model. Although dOTC was less potent than 3TC against NL4-3, dOTC inhibited replication of 3TC-resistant NL4-3/M184V at a dosage level (400 mg/kg/day) that was ineffective for 3TC. Treatment of mice with dOTC for 13 days caused no apparent toxicity and no significant weight loss, no reduction in implant cell numbers or viability (as assessed by forward- and side-scatter characteristics on the flow cytometer), and no perturbations in the percentages of different thymocyte subpopulations (data not shown). These data indicate that dOTC may be an effective agent against 3TC-resistant virus in humans. The experimental protocols also demonstrate the utility and flexibility of the SCID-hu Thy/Liv mouse model in direct comparisons of structurally related antiviral compounds in vivo and in the preclinical evaluation of drug resistance against HIV-1.
Acknowledgments
This work was supported by a contract from NIAID/NIH (N01-AI65309 to J. M. McCune). J. M. McCune is an Elizabeth Glaser Scientist supported by the Elizabeth Glaser Pediatric AIDS Foundation.
We thank Sam Lee and Bobby Benitez for expert technical assistance and Sandra Bridges of DAIDS/NIAID for scientific input.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldrovandi G M, Feuer G, Gao L, Jamieson B, Kristeva M, Chen I S Y, Zack J A. The SCID-hu mouse as a model for HIV-1 infection. Nature. 1993;363:732–736. doi: 10.1038/363732a0. [DOI] [PubMed] [Google Scholar]
- 3.Aldrovandi G M, Gao L, Bristol G, Zack J A. Regions of human immunodeficiency virus type 1 nef required for function in vivo. J Virol. 1998;72:7032–7039. doi: 10.1128/jvi.72.9.7032-7039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arts E J, Quiñones-Mateu M E, Albright J L, Marois J-P, Hough C, Gu Z, Wainberg M A. 3′-Azido-3′-deoxythymidine (AZT) mediates cross-resistance to nucleoside analogs in the case of AZT-resistant human immunodeficiency virus type 1 variants. J Virol. 1998;72:4858–4865. doi: 10.1128/jvi.72.6.4858-4865.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedard J, Taylor D L, Kelly L A, Wood L A, Tyms A S, McCune J M, Stoddart C A, Rando R F. Anti-HIV activity, drug combination, and resistance studies of dOTC (BCH-10652) Antivir Res. 1999;41:A19. . (Abstract 44.) [Google Scholar]
- 6.Berkowitz R D, Alexander S, Bare C, Linquist-Stepps V, Bogan M, Moreno M E, Gibson L, Wieder E D, Kosek J, Stoddart C A, McCune J M. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J Virol. 1998;72:10108–10117. doi: 10.1128/jvi.72.12.10108-10117.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonyhadi M L, Rabin L, Salimi S, Brown D A, Kosek J, McCune J M, Kaneshima H. HIV induces thymus depletion in vivo. Nature. 1993;363:728–732. doi: 10.1038/363728a0. [DOI] [PubMed] [Google Scholar]
- 8.Boucher C A, Cammack N, Schipper P, Schuurman R, Rouse P, Wainberg M A, Cameron J M. High-level resistance to (−) enantiomeric 2′-deoxy-3′-thiacytidine in vitro is due to one amino acid substitution in the catalytic site of human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1993;37:2231–2234. doi: 10.1128/aac.37.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter C C J, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S G, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society—USA Panel. JAMA. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 10.Datema R, Rabin L, Hincenbergs M, Moreno M B, Warren S, Linquist V, Rosenwirth B, Seifert J, McCune J M. Antiviral efficacy in vivo of the anti-human immunodeficiency virus bicyclam SDZ SID 791 (JM 3100), an inhibitor of infectious cell entry. Antimicrob Agents Chemother. 1996;40:750–754. doi: 10.1128/aac.40.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Muys J-M, Gourdeau H, Nguyen-Ba N, Taylor D L, Ahmed P S, Mansour T, Locas C, Richard N, Wainberg M A, Rando R F. Anti-human immunodeficiency virus type 1 activity, intracellular metabolism, and pharmacokinetic evaluation of 2′-deoxy-3′-oxa-4′-thiocytidine. Antimicrob Agents Chemother. 1999;43:1835–1844. doi: 10.1128/aac.43.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaneshima H, Su L, Bonyhadi M L, Connor R I, Ho D D, McCune J M. Rapid-high, syncytium-inducing isolates of human immunodeficiency virus type 1 induce cytopathicity in the human thymus of the SCID-hu mouse. J Virol. 1994;68:8188–8192. doi: 10.1128/jvi.68.12.8188-8192.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavlick M F, Shirasaka T, Kojima E, Pluda J M, Hui F, Jr, Yarchoan R, Mitsuya H. Genotypic and phenotypic characterization of HIV-1 isolated from patients receiving (−)-2′,3′-dideoxy-3′-thiacytidine. Antivir Res. 1995;28:133–146. doi: 10.1016/0166-3542(95)00044-m. [DOI] [PubMed] [Google Scholar]
- 14.Kitchen S G, Zack J A. CXCR4 expression during lymphopoiesis: implications for human immunodeficiency virus type 1 infection of the thymus. J Virol. 1997;71:6928–6934. doi: 10.1128/jvi.71.9.6928-6934.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansour T S, Jin H, Wang W, Dixit D M, Evans C A, Tse H L A, Belleau B, Gillard J W, Hooker E, Ashman C, Cammack N, Salomon H, Belmonte A R, Wainberg M A. Structure-activity relationships among a new class of antiviral heterosubstituted 2′,3′-dideoxynucleoside analogues. Nucleosides Nucleotides. 1995;14:627–635. [Google Scholar]
- 16.Mansour T S, Jin H, Wang W, Hooker E U, Ashman C, Cammack N, Salomon H, Belmonte A R, Wainberg M A. Anti-human immunodeficiency virus and anti-hepatitis-B virus activities and toxicities of the enantiomers of 2′-deoxy-3′-oxa-4′-thiocytidine and their 5-fluoro analogues in vitro. J Med Chem. 1995;38:1–4. doi: 10.1021/jm00001a001. [DOI] [PubMed] [Google Scholar]
- 17.McCune J M. Animal models of HIV-1 disease. Science. 1997;278:2141–2142. doi: 10.1126/science.278.5346.2141. [DOI] [PubMed] [Google Scholar]
- 18.McCune J M, Namikawa R, Kaneshima H, Shultz L D, Lieberman M, Weissman I L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 19.Miller M D, Anton K E, Mulato A S, Lamy P D, Cherrington J M. Human immunodeficiency virus type 1 expressing the lamivudine-associated M184V mutation in reverse transcriptase shows increased susceptibility to adefovir and decreased replication capability in vitro. J Infect Dis. 1999;179:92–100. doi: 10.1086/314560. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Namikawa R, Kaneshima H, Lieberman M, Weissman I L, McCune J M. Infection of the SCID-hu mouse by HIV-1. Science. 1988;242:1684–1686. doi: 10.1126/science.3201256. [DOI] [PubMed] [Google Scholar]
- 22.Namikawa R, Weilbaecher K N, Kaneshima H, Yee E J, McCune J M. Long-term human hematopoiesis in the SCID-hu mouse. J Exp Med. 1990;172:1055–1063. doi: 10.1084/jem.172.4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rabin L, Hincenbergs M, Moreno M B, Warren S, Linquist V, Datema R, Charpiot B, Seifert J, Kaneshima H, McCune J M. Use of standardized SCID-hu Thy/Liv mouse model for preclinical efficacy testing of anti-human immunodeficiency virus type 1 compounds. Antimicrob Agents Chemother. 1996;40:755–762. doi: 10.1128/aac.40.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schinazi R F, Lloyd R M, Jr, Nguyen M-H, Cannon D L, McMillan A, Ilksoy N, Chu C K, Liotta D C, Bazmi H Z, Mellors J W. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob Agents Chemother. 1993;37:875–881. doi: 10.1128/aac.37.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuurman R, Nijhuis M, van Leeuwen R, Schipper P, de Jong D, Collis P, Danner S A, Mulder J, Loveday C, Christopherson C, Kwok S, Sninsky J, Boucher C A B. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC) J Infect Dis. 1995;171:1411–1419. doi: 10.1093/infdis/171.6.1411. [DOI] [PubMed] [Google Scholar]
- 26.Stanley S K, McCune J M, Kaneshima H, Justement J S, Sullivan M, Boone E, Baseler M, Adelsberger J, Bonyhadi M, Orenstein J, Fox C H, Fauci A S. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J Exp Med. 1993;178:1151–1163. doi: 10.1084/jem.178.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoddart C A. The SCID-hu Thy/Liv mouse: an animal model for HIV-1 infection. In: Zak O, Sande M A, editors. Handbook of animal models of infection. London, United Kingdom: Academic Press; 1999. pp. 1069–1076. [Google Scholar]
- 28.Stoddart C A, Rabin L, Hincenbergs M, Moreno M E, Linquist-Stepps V, Leeds J M, Truong L A, Wyatt J R, Ecker D J, McCune J M. Inhibition of human immunodeficiency virus type 1 infection in SCID-hu Thy/Liv mice by the G-quartet-forming oligonucleotide, ISIS 5320. Antimicrob Agents Chemother. 1998;42:2113–2115. doi: 10.1128/aac.42.8.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su L, Kaneshima H, Bonyhadi M L, Lee R, Auten J, Wolf A, Du B, Rabin L, Hahn B H, Terwilliger E, McCune J M. Identification of HIV-1 determinants for replication in vivo. Virology. 1997;227:45–52. doi: 10.1006/viro.1996.8338. [DOI] [PubMed] [Google Scholar]
- 30.Su L, Kaneshima H, Bonyhadi M, Salimi S, Kraft D, Rabin L, McCune J M. HIV-1-induced thymocyte depletion is associated with indirect cytopathicity and infection of progenitor cells in vivo. Immunity. 1995;2:25–36. doi: 10.1016/1074-7613(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 31.Tisdale M, Kemp S D, Parry N R, Larder B A. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:5653–5656. doi: 10.1073/pnas.90.12.5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wainberg M A, Salomon H, Gu Z, Montaner J S, Cooley T P, McCaffrey R, Ruedy J, Hirst H M, Cammack N, Cameron J, Nicholson W. Development of HIV-1 resistance to (−)2′-deoxy-3′-thiacytidine in patients with AIDS or advanced AIDS-related complex. AIDS. 1995;9:351–357. [PubMed] [Google Scholar]