Abstract
Interleukin-22 is a multi-faceted cytokine with both pro- and anti-inflammatory functions that is implicated in multiple pathologies. However, the role of IL-22 in maternal-fetal immunity in late gestation is poorly understood. Herein, we first showed that IL-22+ T cells co-expressing RORγt are enriched at the human maternal-fetal interface of women with preterm labor and birth, which was confirmed by in silico analysis of single-cell RNASeq placental data. T-cell activation leading to preterm birth in mice was preceded by a surge in IL-22 in the maternal circulation and amniotic cavity; yet, intravenous administration of IL-22 in mice did not induce adverse perinatal outcomes. Next, using an ex vivo human system, we showed that IL-22 can cross from the choriodecidua to the intra-amniotic space, where its receptors (Il22ra1, Il10rb, and Il22ra2) are highly expressed by murine gestational and fetal tissues in late pregnancy. Importantly, amniotic fluid concentrations of IL-22 were elevated in women with sterile or microbial intra-amniotic inflammation, suggesting a dual role for this cytokine. The intra-amniotic administration of IL-22 alone shortened gestation and caused neonatal death in mice, the latter involving lung maturation and inflammation. IL-22 plays a role in host response by participating in the intra-amniotic inflammatory milieu preceding Ureaplasma parvum-induced preterm birth in mice, which was rescued by the deficiency of IL-22. Collectively, these data show that IL-22 alone is capable of causing fetal injury leading to neonatal death and can participate in host defense against microbial invasion of the amniotic cavity leading to preterm labor and birth.
INTRODUCTION
IL-22 is a pleiotropic cytokine with both pro- and anti-inflammatory functions (1, 2), whose role varies according to location and microenvironment (3). Indeed, IL-22 has been recognized as a cytokine acting as a double-edged sword in the pathogenesis of many diseases, with respect to either inducing or ameliorating inflammation (4). The pro-inflammatory functions of IL-22 are well-documented in skin and autoimmune disorders such as psoriasis (5, 6) and rheumatoid arthritis (7, 8). Yet, IL-22 also has a protective role in specific compartments such as mucosal tissues or epithelial barriers (9–11). In addition to its bi-dimensional nature, IL-22 is a unique cytokine because it is primarily produced by lymphoid cells but acts on non-immune cells such as epithelial or stromal cells (4). Despite the abundance of literature documenting the role of IL-22 in autoimmune diseases, cancers, and intestinal complications, there is a paucity of information regarding the role of IL-22 in maternal-fetal immunity during late gestation, a vulnerable period in which maternal-fetal immune dysregulation can lead to premature birth and adverse neonatal outcomes (12–14).
Most cases of premature birth are preceded by spontaneous preterm labor (12, 15, 16), a syndrome of multiple pathological processes (17, 18). Of these etiologies, only acute pathological inflammation of the amniotic cavity (i.e. intra-amniotic inflammation) has been causally linked to preterm labor leading to premature birth (19–23). Such a local inflammatory process can be induced by microorganisms invading the amniotic cavity (i.e. microbial intra-amniotic inflammation or intra-amniotic infection) or alarmins released upon cellular stress (i.e. sterile intra-amniotic inflammation) (24–27). In the former, the inflammatory process represents the host response against microbes invading the amniotic cavity (28–33), which leads to preterm labor and birth as an unintended consequence (34, 35). Both microbial products and alarmins are sensed by pattern recognition receptors (PRRs), which are mainly present in innate immune cells (36); therefore, most maternal-fetal immunology research has focused on the role of innate immunity (37–54). Yet, acute inflammation can also be mediated by maternal T cells, whose infiltration is well documented at the maternal-fetal interface (i.e., decidua) (55–62). Indeed, our group has shown that the activation of decidual maternal T cells is associated with the physiological process of labor at term (61–65). Furthermore, we have reported that the premature activation of maternal effector T cells can trigger acute inflammatory mechanisms that lead to preterm labor and birth (66, 67) and that such activation can be non-invasively monitored in the maternal circulation (65, 68). However, the soluble mediators produced by effector and activated T cells at the maternal-fetal interface of women with preterm labor, which may represent a potential therapeutic target for premature birth and its adverse neonatal outcomes, are poorly understood.
Herein, we first aimed to identify the cellular sources of IL-22 at the maternal-fetal interface of women who underwent preterm labor and birth. Using available single-cell RNA sequencing human data, we also explored the expression of IL-22 and its receptors by decidual immune cell types. Next, we investigated whether systemic levels of IL-22 were increased upon in vivo maternal T-cell activation, and whether the intravenous administration of this cytokine alone could induce adverse pregnancy outcomes in mice. In addition, we explored the expression of Il22 and its receptors by gestational (fetal membranes and placenta) and fetal (lung and intestine) tissues in late murine pregnancy as a possible explanation of the elevated concentrations of IL-22 in the amniotic cavity (i.e., fetal compartment) of dams upon in vivo maternal T-cell activation. We also evaluated whether IL-22 can cross from the maternal to the fetal side using an ex vivo model of the human maternal-fetal interface, and measured the concentrations of this cytokine in amniotic fluid from well-characterized subsets of women who underwent spontaneous preterm labor. Such clinical data led us to propose a dual role for IL-22 in maternal-fetal immunity: 1) IL-22 alone is capable of causing fetal tissue injury leading to neonatal death, and 2) IL-22 participates in host defense in microbial invasion of the amniotic cavity leading to preterm birth. To test the former, IL-22 was intra-amniotically injected in mice under ultrasound guidance to evaluate adverse perinatal outcomes, and its effects on tissue injury were determined in the neonatal lung and intestine. To test the latter, the role of IL-22 in host defense against Ureaplasma parvum-induced preterm birth was thoroughly evaluated using wild type and knockout mice.
MATERIALS AND METHODS
Human subjects and clinical specimens
Human placental basal plate (decidua basalis) and chorioamniotic membrane (decidua parietalis) samples were collected within 30 min after delivery at the Detroit Medical Center, Wayne State University, Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS), Detroit, MI. Amniotic fluid samples were retrieved from the Biorepository of the Perinatology Research Branch. The collection and utilization of biological materials for research purposes was approved by the Institutional Review Boards of these institutions. All participating women provided written informed consent. Two separate cohorts of women were used in this study.
The first cohort included women who delivered at term in labor (TIL), at term without labor (TNL), preterm in labor (PTL), or preterm without labor (PTNL) whose placental basal plate and chorioamniotic membranes were collected and used for immunophenotyping (please see clinical definitions below). The demographic and clinical characteristics of the study populations are shown in Table I.
Table I.
Demographic and clinical characteristics of the study population for immunophenotyping of cellular sources of IL-22
| Term not in labor (n=11) | Term in labor (n=30) | Preterm not in labor (n=11) | Preterm in labor (n=26) | p-value | |
|---|---|---|---|---|---|
| Maternal age (years; median [IQR])a | 26 (23–31) | 24 (20.3–25.8) | 31 (27.5–33) | 26 (22.3–28.8) | 0.004 |
| Body mass index (kg/m2; median [IQR])a | 35.1 (29.2–39) | 25.6 (23–31.2) | 34.7 (29.1–35.8) | 31.1 (25.9–40.4)c | 0.02 |
| Primiparityb | 0% (0/11) | 6.7% (2/30) | 0% (0/11) | 23.1% (6/26) | 0.09 |
| Race/ethnicityb | 0.4 | ||||
| African-American | 81.8% (9/11) | 90% (27/30) | 90.9% (10/11) | 76.9% (20/26) | |
| White | 18.2% (2/11) | 3.3% (1/30) | 9.1% (1/11) | 19.2% (5/26) | |
| Other | 0% (0/11) | 6.7% (2/30) | 0% (0/11) | 3.8% (1/26) | |
| Gestational age at delivery (weeks; median [IQR])a | 39.1 (39–39.3) | 39.2 (38.6–40.3) | 33.4 (31–36.4) | 34.6 (33.7–35.9) | <0.001 |
| Birthweight (grams; median [IQR])a | 3370 (3125–3705) | 3188 (2839–3319) | 1730 (1383–2508) | 2233 (1809–2440) | <0.001 |
| Acute maternal inflammatory responseb | |||||
| Stage 1 (Early acute subchorionitis or chorionitis) | 9.1% (1/11) | 33.3% (10/30) | 0% (0/11) | 15.4% (4/26) | 0.07 |
| Stage 2 (Acute chorioamnionitis) | 0% (0/11) | 23.3% (7/30) | 0% (0/11) | 15.4% (4/26) | 0.14 |
| Stage 3 (Necrotizing chorioamnionitis) | 0% (0/11) | 0% (0/30) | 0% (0/11) | 0% (0/26) | 1 |
| Acute fetal inflammatory responseb | |||||
| Stage 1 (Chorionic vasculitis or umbilical phlebitis) | 0% (0/11) | 23.3% (7/30) | 0% (0/11) | 3.8% (1/26) | 0.06 |
| Stage 2 (Umbilical arteritis) | 0% (0/11) | 6.7% (2/30) | 0% (0/11) | 15.4% (4/26) | 0.4 |
| Stage 3 (Necrotizing funisitis) | 0% (0/11) | 0% (0/30) | 0% (0/11) | 0% (0/26) | 1 |
Data are given as median (interquartile range, IQR) and percentage (n/N).
Kruskal-Wallis test,
Fisher’s exact test,
Three missing data.
The second cohort included (i) women with spontaneous preterm labor and intact membranes who delivered at term without intra-amniotic inflammation, and women with preterm labor and intact membranes who delivered preterm who were divided into the following groups: (ii) women without microbial or sterile intra-amniotic inflammation, (iii) women with sterile intra-amniotic inflammation, and (iv) women with microbial intra-amniotic inflammation. Please see clinical definitions below. In this cohort, amniotic fluid samples (the remnant that was not used for clinical determinations) were used for the determination of IL-22. The demographic and clinical characteristics of the study population are shown in Table II.
Table II.
Demographic and clinical characteristics of the study population for measurements of IL-22 in amniotic fluid.
| Term Delivery | Preterm Delivery | ||||
|---|---|---|---|---|---|
| Preterm labor who delivered at term (n=20) | Preterm labor without sterile or microbial intra-amniotic inflammation (n=27) | Preterm labor with sterile intra-amniotic inflammation (n=27) | Preterm labor with microbial intra-amniotic inflammation (n=16) | p-value | |
| Maternal age (years; median [IQR])a | 23 (20.8–25.5) | 22 (19–25.5) | 24 (20.5–27) | 23.5 (20–27) | 0.7 |
| Body mass index (kg/m2; median [IQR])a | 22.7 (20.9–29.4) | 25.3 (21.5–28.9)e | 28.2 (23.2–33.4)e | 23.7 (21.6–31.7)d | 0.2 |
| Primiparityb | 10% (2/20) | 29.6% (8/27) | 56.3% (9/16) | 18.8 (3/16) | 0.2 |
| Race/ethnicityb | 0.6 | ||||
| African-American | 100% (20/20) | 81.5% (22/27) | 88.9% (24/27) | 93.8% (15/16) | |
| White | 0% (0/20) | 11.1% (3/27) | 7.4% (2/27) | 6.2% (1/16) | |
| Other | 0% (0/20) | 7.4% (2/27) | 3.7% (1/27) | 0% (0/16) | |
| Gestational age at amniocentesis (weeks; median [IQR])a | 31.3 (30.7–32.3) | 30.6 (28.5–32.4) | 26.4 (23.8–30.2) | 26.4 (22.5–30.2) | 0.002 |
| IL-6 (ng/mL; median [IQR])a | 0.4 (0.3–0.7) | 0.5 (0.4–1) | 11.2 (5.4–22.9) | 98.9 (26.6–130.1) | <0.001 |
| Amniotic fluid glucose (mg/dl; median [IQR])a | 28.5 (22.8–32) | 27 (20–32.5) | 21 (19–26)d | 10 (1–18)c | <0.001 |
| Amniotic fluid WBC (cells/mm3; median [IQR])a | 0.5 (0–4.3) | 1 (0–3) | 2.5 (0.8–13.3)e | 285 (23–458) | <0.001 |
| Gestational age at delivery (weeks; median [IQR])a | 38.7 (37.4–39) | 34 (31.8–35.8) | 26.7 (24.5–31.3) | 26.4 (22.5–30.3) | <0.001 |
| Cesarean sectionb | 0% (0/20) | 3.7% (1/27) | 22.2% (6/27) | 12.5% (2/16) | 0.047 |
| Birthweight (grams; median [IQR])a | 3049 (2900–3294) | 2190 (1588–2364) | 917 (593–1545) | 985 (458–1261) | <0.001 |
| Acute maternal inflammatory responseb,c | |||||
| Stage 1 (Early acute subchorionitis or chorionitis) | 10.5% (2/19)c | 13% (3/23)f | 29.2% (7/24)e | 13.3 % (2/15)c | 0.5 |
| Stage 2 (Acute chorioamnionitis) | 15.8% (3/19)c | 21.7% (5/23)f | 12.5% (3/24)e | 20% (3/15)c | 0.9 |
| Stage 3 (Necrotizing chorioamnionitis) | 0% (0/19)c | 0% (0/23)f | 16.6% (4/24)e | 66.6% (10/15)c | <0.001 |
| Acute fetal inflammatory responseb,c | |||||
| Stage 1 (Chorionic vasculitis or umbilical phlebitis) | 15.8% (3/19)c | 8.7% (2/23)f | 20.8% (5/24)e | 33.3% (5/15)c | 0.1 |
| Stage 2 (Umbilical arteritis) | 5.3% (1/19)c | 4.3% (1/23)f | 4.2% (1/24)e | 46.7% (7/15)c | <0.001 |
| Stage 3 (Necrotizing funisitis) | 0% (0/19)c | 0% (0/23)f | 0% (0/24)e | 0% (0/15)c | 1.0 |
Data are given as median (interquartile range, IQR) and percentage (n/N).
Kruskal-Wallis test with multiple comparison,
Fisher’s exact test,
One missing datum,
Two missing data,
Three missing data,
Four missing data
Abbreviations: WBC, white blood cells.
Clinical definitions
Gestational age was determined by the date of the last menstrual period and confirmed by the first ultrasound examination or by ultrasound examination alone if the sonographic determination of gestational age was inconsistent with menstrual dating (69). Spontaneous preterm labor was defined as the presence of regular uterine contractions with a frequency of at least two every 10 min and cervical ripening between 20 and 36 (6/7) weeks of gestation. Preterm delivery was defined as birth before 37 weeks of gestation. Term labor was defined by the presence of regular uterine contractions at a frequency of at least two contractions every 10 min with cervical changes resulting in delivery at term (≥37 weeks of gestation). Standard clinical laboratory determinations in amniotic fluid included the evaluation of the white blood cell count (70), glucose concentration (71), Gram stain (72), and microbiological culture of aerobic/anaerobic bacteria and genital mycoplasmas (73). The study group classifications used in this study (second cohort) were determined by combining the presence or absence of microbes together with the evaluation of amniotic fluid IL-6 concentrations as an indicator of intra-amniotic inflammation, as previously established (74). Thus, a positive microbial signal (either by culture/Gram stain or PCR/ESIMS), together with elevated IL-6 (≥2.6 ng/ml), indicates microbial intra-amniotic inflammation (24–26, 75); a negative microbial signal indicated by both culture/Gram stain and PCR/ESI-MS, together with elevated IL-6, indicates sterile intra-amniotic inflammation (24–26, 75); and the absence of microbial signals (indicated by both culture/Gram stain and PCR/ESI-MS), together with low IL-6 levels, indicates no intra-amniotic inflammation. Women with preterm prelabor rupture of membranes (pPROM), multiple gestations, or those who had a fetus with chromosomal and/or sonographic abnormalities were excluded from this study. Maternal and neonatal data were obtained by retrospective clinical chart review.
Placental histopathological examination
Placentas were examined histologically by perinatal pathologists blinded to clinical diagnoses and obstetrical outcomes according to standardized Perinatology Research Branch protocols (76). Briefly, three to nine sections of the placenta were examined, and at least one full-thickness section was taken from the center of the placenta; others were taken randomly from the placental disc. Acute inflammatory lesions of the placenta were diagnosed according to established criteria (77–79).
Human decidual leukocyte isolation
Decidual leukocytes were isolated from the decidual tissue of each study group (Table I) as previously described (80). Briefly, the decidua basalis was collected from the basal plate of the placenta and the decidua parietalis was separated from the chorioamniotic membranes. The decidual tissues were homogenized using a gentleMACS Dissociator (Miltenyi Biotec, San Diego, CA) in StemPro Accutase Cell Dissociation Reagent (Life Technologies, Grand Island, NY). Homogenized tissues were incubated in Accutase for 45 min at 37°C with gentle agitation. Following incubation, tissues were washed in 1X phosphate-buffered saline (PBS; Life Technologies) and filtered through a 100-μm cell strainer (Fisher Scientific, Durham, NC). Cell suspensions were collected and centrifuged at 300 × g for 10 min at 4°C. The decidual mononuclear cells were purified using Ficoll-Paque Plus (GE Healthcare Biosciences, Uppsala, Sweden) following the manufacturer’s instructions. The mononuclear cells were then collected and washed with PBS and immediately used for immunophenotyping.
Immunophenotyping of human decidual IL-22-expressing cells
Mononuclear cell suspensions from decidual tissues were stained with the BD Horizon Fixable Viability Stain 510 dye (BD Biosciences) prior to incubation with extracellular and intracellular monoclonal antibodies (mAbs) (Supplemental Table I). Mononuclear cell suspensions were then washed with FACS staining buffer (catalog no. #554656; BD Biosciences) and incubated with 20 μl of human FcR Blocking Reagent (catalog no. #130-059-901; Miltenyi Biotec) in 80 μl of FACS staining buffer (BD Biosciences) for 10 min at 4°C. The cells were incubated with extracellular fluorochrome-conjugated anti-human mAbs (Supplemental Table I) for 30 min at 4°C in the dark. After extracellular staining, the cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (catalog no. #00-5523-00; eBioscience, San Diego, CA) prior to staining with intracellular and intranuclear antibodies (Supplemental Table I). Finally, stained cells were washed and re-suspended in 0.5 mL of FACS staining buffer and acquired using an LSRFortessa flow cytometer and FACSDiva 6.0 software (BD Biosciences). Leukocyte subsets were gated within the viability gate. Immunophenotyping included the identification of IL-22 expressing cells in the following subsets: neutrophils (CD14−CD15+); monocytes/macrophages (CD15−CD14+); T cells (CD14−CD15−CD19−CD3+); B cells (CD14−CD15−CD3−CD19+); NK cells (CD14−CD15−CD3−CD19−CD56+); and innate lymphoid cells (ILCs; CD14−CD15−CD3−CD19−CD11b−CD56−(Lin−)CD127+). The expression of IL-22 by RORγt+ and CD69+RORγt+ T cells was also reported. Flow cytometry analysis was performed using FlowJo software version 10 (FlowJo, Ashland, OR).
In silico single-cell RNA sequencing (scRNAseq) analysis of the human placental tissues
Publically available scRNAseq data from the decidual tissues (the basal plate and chorioamniotic membranes) of TNL, TIL, and PTL women (n = 3 per group (68)) were used to explore the expression of IL22, IL22RA1, IL10RB, IL22RA2, and RORC. Briefly, raw fastq files were downloaded from previously established resources in NCBI dbGaP phs001886.v1.p1 (68). The fastq files were then aligned using kallisto (81), and bustools (82) summarized the cell/gene transcript counts in a matrix for each sample, using the “lamanno” workflow for scRNAseq. Each sample was then processed using DIEM (83) to eliminate debris and empty droplets. All count data matrices were then normalized and combined using the “NormalizeData,” “FindVariableFeatures,” and “ScaleData” methods implemented in the Seurat package in R (Seurat version 3.1, R version 3.6.1) (84, 85). Afterward, the Seurat “RunPCA” function was applied to obtain the first 50 principal components, and the different batches and locations were integrated and harmonized using the Harmony package in R (86). To label the cells, the Seurat “FindTransferAnchors” and “TransferData” functions were used for each group of locations separately to assign a cell type identity based on our previously labeled data as reference panel (as performed in (68)). Visualization of gene expression for each cell type was performed using the ggplot2 (87) package in R with gene expression values scaled to transcripts per million (TPM) and to the proportion of cells expressing the transcript within a given cell type (88).
Maternal-fetal IL-22 transfer assay
Human chorioamniotic membrane samples were collected from women at term without labor (TNL) who delivered via cesarean section. All experiments were performed under sterile conditions. Chorioamniotic membranes were rinsed carefully in sterile PBS and cut into 5 cm × 5 cm squares. The polyethylene terephthalate membranes of 6-well Falcon cell culture inserts (Corning Life Sciences, Glendale, AZ) were removed and chorioamniotic membrane sections were held in place using orthodontic rubber bands with the choriodecidual side facing upward. The culture inserts with membrane sections were placed into the 6-well culture plates. Then, 1 mL of DMEM supplemented with 10% Fetal Bovine Serum (FBS; ThermoFisher Scientific, Waltham, MA) and 1% penicillin/streptomycin (ThermoFisher Scientific) containing either 6 ng (n = 3), 60 ng (n = 4), or 600 ng (n = 3) of the carrier-free recombinant human IL-22 (rhIL-22; catalog no. #782-IL/CF; R&D Systems, Minneapolis, MN) was gently added on top of the culture insert, and 1 mL of the same media without rhIL-22 was added to the bottom wells of the culture plate. After incubation for 8 h at 37°C with 5% CO2, the supernatants (media) from upper wells (choriodecidua – maternal side) and lower wells (amnion – fetal side) were collected into separate cryovials and stored at −80°C. The IL-22 concentrations from the upper and lower supernatants were determined using the Human IL-22 Quantikine ELISA Kit (R&D systems), according to the manufacturer’s instructions. Assays were read using the SpectraMax iD5 (Molecular Devices, San Jose, CA) and analyte concentrations were calculated with SoftMax Pro 7 (Molecular Devices). The sensitivity of the assay was 2.7 pg/mL. The lower (fetal side) supernatant concentrations were divided by the upper (maternal side) supernatant concentrations to obtain the proportion of IL-22 that was transferred across the chorioamniotic membranes.
Determination of IL-22 concentrations in human amniotic fluid
Human amniotic fluid samples from women with spontaneous preterm labor were retrieved from the Biorepository of the Perinatology Research Branch (Table II). A U-PLEX immunoassay (Meso Scale Discovery, Rockville, MD) was used to measure the concentrations of IL-22 in the human amniotic fluid samples, according to the manufacturer’s instructions. The plate was read using the MESO QuickPlex SQ 120 (Meso Scale Discovery) and analyte concentrations were calculated with Discovery Workbench 4.0 (Meso Scale Discovery). The sensitivity of the IL-22 assay was 0.13 pg/mL.
Mice
C57BL/6 (Stock No. 000664; hereafter referred to as B6 or Il22+/+) and C57BL/6-Il22tm1.1(icre)Stck (Stock No. 027524; hereafter referred to as Il22−/−) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred in the animal care facility at the C.S. Mott Center for Human Growth and Development at Wayne State University (Detroit, MI). All mice were kept under a circadian cycle (light:dark, 12:12 h). Females aged eight to twelve weeks old were bred with males of the same genotype and proven fertility. Female mice were checked daily between 8:00 a.m. and 9:00 a.m. for the appearance of a vaginal plug, indicating 0.5 days post coitum (dpc). Females were then housed separately from the males, their weights were monitored daily, and pregnancy was confirmed by 12.5 dpc by a gain of at least 2 g. Animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Wayne State University (Protocol numbers: A 09-08-12, 07-03-15, 18-03-0584, and 21-04-3506).
Murine model of maternal T-cell activation-induced preterm birth
B6 dams were injected intraperitoneally (i.p.) with 10 μg/200 μL of monoclonal anti-CD3ε Ab (Clone 145-2C11; BD Biosciences) in sterile 1X PBS (Fisher Scientific Chemicals, Fair Lawn, NJ) using a 26-gauge (G) needle on 16.5 dpc as previously described (66, 89). Controls were injected i.p. with 10 μg/200 μL of IgG1 κ isotype control (Clone A19-3; BD Biosciences) in sterile PBS. Mice were euthanized 12 – 16 h post-injection and maternal peripheral blood was collected by cardiac puncture [n = 18 (isytope control) and 19 (anti-CD3ε)]. Amniotic fluid was collected from each amniotic sac with a 26G needle (n = 5 per group). Serum was separated from the maternal blood samples by centrifugation (1,300 × g for 10 min at room temperature) and stored at −20 °C. Amniotic fluid samples were centrifuged at 1,300 × g for 5 at 4°C, and the supernatants were separated and stored at −20°C. The mouse/rat IL-22 Quantikine ELISA kit (R&D systems) was used to measure the concentrations of IL-22 in the amniotic fluid and serum samples, according to the manufacturer’s instructions. The plates were read using the SpectraMax iD5 (Molecular Devices) and analyte concentrations were calculated with SoftMax Pro 7 (Molecular Devices). The sensitivity of the IL-22 assay was 3.2 pg/mL.
Analysis of the basal expression of Il22 and its receptors by the murine fetal tissues
B6 dams were euthanized at 16.5 (n = 8) or 17.5 (n = 8) dpc to harvest the placenta, fetal membranes, fetal lungs, and fetal intestines. The lungs of dams intraperitoneally injected with 10 μg/200μL of LPS (Escherichia coli; 055:B5, Sigma Aldrich, St. Louis, MO) on 16.5 dpc (n = 3) (90) were used as a positive control for Il22 expression. Tissues were collected into RNAlater solution (Thermo Fisher Scientific, Vilnius, Lithuania) and stored at −80°C until RNA isolation.
Ultrasound-guided intra-amniotic injection of IL-22 in mice
B6 dams were anesthetized on 16.5 dpc by inhalation of 2% isoflurane (Aerrane; Baxter Healthcare Corporation, Deerfield, IL; Fluriso™, Isoflurane USP, Vetone Boise, ID) and 1 to 2 liters/min of oxygen in an induction chamber. Anesthesia was maintained with a mixture of 1.5 to 2% isoflurane and 1.5 to 2 liters/min of oxygen. Mice were positioned on a heating pad and stabilized with adhesive tape. Fur was removed from the abdomen of the dams by applying Nair cream (Church & Dwight Co., Inc., Ewing, NJ). Body temperature was maintained in the range of 37 ± 1°C and detected with a rectal probe (VisualSonics, Toronto, Ontario, Canada), and respiratory and heart rates were monitored by electrodes embedded in the heating pad. An ultrasound probe was fixed and mobilized with a mechanical holder, and the transducer was slowly moved towards the abdomen. Syringes were stabilized by a mechanical holder (VisualSonics). Injections were performed in each amniotic sac using a 30G needle (BD PrecisionGlide needle; Becton Dickinson, Franklin Lakes, NJ). Dams were intra-amniotically injected with 1.2 pg/25 μL (n = 6), 70 pg/25 μL (n = 8), or 612 pg/25 μL (n = 8) of carrier-free recombinant mouse IL-22 (rmIL-22; catalog no. #576202; Biolegend, San Diego, CA) into each amniotic sac on 16.5 dpc. Controls were injected with 25 μL of PBS per sac (n = 8). After ultrasound examination, mice were placed under heat lamps for recovery (defined as when the mouse resumed normal activities such as walking and responding), which typically occurred within 10 min after removal from anesthesia.
Intravenous administration of IL-22 in mice
B6 dams were intravenously injected with 689 pg of rmIL-22 in 100 μL of PBS using a 26G needle on 16.5 dpc (n = 8). Controls were injected with 100 μL of PBS alone (n =5). The utilized dose of rmIL-22 was selected based on the plasma concentrations of IL-22 in women with spontaneous preterm labor (91).
Video monitoring of pregnancy and neonatal outcomes induced by IL-22 in mice
Immediately after intra-amniotic or intravenous injection, B6 dams were monitored until delivery using an infrared camera (Sony Corporation, Tokyo, Japan). Gestational length was defined as the time elapsed from the detection of the vaginal plug (0.5 dpc) to the delivery of the first pup. The rate of neonatal mortality at birth was determined for each litter (pups delivered from the same dam) and defined as the proportion of delivered pups found dead among the total litter size. The average of the rates of neonatal mortality per litter was then calculated and plotted for each experimental group. Representative images of neonates (day 0) born to dams injected with rmIL-22 or PBS (controls) are shown. Neonatal weights were recorded at 1, 2, and 3 weeks of age.
Tissue analysis of neonates born to mice intra-amniotically injected with IL-22
B6 dams were intra-amniotically injected with 612pg/25μL per sac of rmIL-22 (n=10) or 25 μL PBS per sac (controls, n=10) on 16.5 dpc, and were monitored until delivery. Neonates born to dams were euthanized on day 0 to harvest the neonatal lung and intestine. Tissues were collected into RNAlater solution and stored at −80°C until RNA isolation. Furthermore, neonatal lungs were snap frozen for preparation of tissue extracts, in which the concentration of surfactant protein A (SP-A) was determined using the Mouse Pulmonary Surfactant-Associated Protein A (SP-A) ELISA Kit (Cusabio, Wuhan, China), following the manufacturer’s instructions. Plates were read using the SpectraMax iD5 (Molecular Devices, San Jose, CA, USA) and analyte concentrations were calculated with the SoftMax Pro 7 (Molecular Devices). The sensitivity of the assay was 0.78 pg/mL.
Murine model of Ureaplasma parvum-induced preterm birth
Ureaplasma parvum was prepared for intra-amniotic injection, as previously described (92). Briefly, Ureaplasma parvum was isolated from women with intra-amniotic infection, aliquoted, and stored at −80°C with 50% glycerol (Teknova, Hollister, CA; with a final concentration of glycerol 25%). The stocks were inoculated in SP4 broth (Hardy Diagnostics, Santa Maria, CA) and cultured at 37°C for 4 to 12 h to reach the exponential phase based on growth rate. After incubation, the bacterial cells were counted by flow cytometry using the Live/Dead BacLight bacterial viability and counting kit (Invitrogen by Thermo Fisher Scientific, Carlsbad, CA). The cells were diluted to a target concentration (1–5 × 104 cells/25 μL) with SP4 broth.
B6 dams were intra-amniotically injected with 1–5 × 104 cells/25 μL of Ureaplasma parvum per sac (n = 10) or 25 μL SP4 broth per sac (controls, n = 10) on 16.5 dpc. The rate of preterm birth induced by Ureaplasma parvum in B6 mice is ~40–50% (92), which is consistently reported herein in Il22+/+ mice. B6 dams were euthanized 16 h post-injection, 4–5 h prior to previously observed times of preterm delivery, for collection of peripheral blood by cardiac puncture. Amniotic fluid was also collected from each amniotic sac with a 26G needle. Serum was separated from the maternal blood samples by centrifugation (1,300 × g for 10 min at room temperature) and stored at −20 °C. Amniotic fluid samples were centrifuged at 1,300 × g for 5 min at 4°C, and the supernatants were separated and stored at −20°C. A ProcartaPlex mouse cytokine and chemokine panel 1A 36-plex (Thermo Fisher Scientific, Vienna, Austria) was used to measure the concentrations of IL-22 in serum and amniotic fluid samples, according to the manufacturer’s instructions. The plates were read using the Luminex FLEXMAP 3D (Luminex, Austin, TX) and analyte concentrations were calculated with xPONENT version 4.2 (Luminex). The sensitivity of the IL-22 assay was 0.24 pg/mL.
Collection of amniotic fluid and fetal tissues from mice intra-amniotically injected with Ureaplasma parvum
B6 dams were intra-amniotically injected with 1 × 104 cells/25 μL of Ureaplasma parvum per sac (n = 8) or 25 μL SP4 broth per sac (controls, n = 8) on 16.5 dpc. Dams were euthanized 16 h post-injection and amniotic fluid was collected from each amniotic sac with a 26G needle. The placenta, fetal membranes, fetal lungs, and fetal intestines were collected into RNAlater solution (Thermo Fisher Scientific) and stored at −80°C until RNA isolation.
Analysis of amniotic fluid from mice intra-amniotically injected with Ureaplasma parvum
The murine amniotic fluid concentrations of several IL-22 pathway-related cytokines were determined using the U-PLEX Biomarker Group 1 (ms) Assay, SECTOR (Meso Scale Discovery), following the manufacturer’s instructions. This assay allowed for the determination of IFN-γ, IL-1β, IL-6, IL-13, IL-17A, IL-17C, IL-17E/IL-25, IL-21, IL-22, and IL-23 concentrations. The plate was read using the MESO QuickPlex SQ 120 (Meso Scale Discovery) and analyte concentrations were calculated with the Discovery Workbench 4.0 (Meso Scale Discovery). The sensitivities of the assays were: 0.16 pg/mL (IFN-γ), 3.1 pg/mL (IL-1β), 4.8 pg/mL (IL-6), 2.7 pg/mL (IL-13), 0.30 pg/mL (IL-17A), 2.3 pg/mL (IL-17C), 1.6 pg/mL (IL-17E/IL-25), 6.5 pg/mL (IL-21), 1.2 pg/mL (IL-22), and 4.9 pg/mL (IL-23).
Cytospin slides of amniotic fluid samples (n = 3) were prepared using Fisherbrand Superfrost microscope slides (Thermo Fisher Scientific) and a Shandon Cytospin 3 cytocentrifuge (Thermo Fisher Scientific) at 800 rpm for 5 min. After fixation with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA), the cytospin slides were rinsed with 1X PBS at room temperature for 2 min. Slides were then permeabilized using 0.25% Triton X-100 (Electron Microscopy Sciences) diluted in 1X PBS for 5 min. Then, rinsed with 1X PBS for 5 min. Non-specific antibody interaction was blocked by using the BlockAid blocking solution (Cat# B10710, Thermo Fisher Scientific) for 1 h at RT. Afterards, the rat anti-mouse F4/80 (Cat# 123140, BioLegend) and rabbit polyclonal IL-22 (Cat# bs-2623R, Bioss, Woburn, MA) antibodies were added and the slides incubated for 60 min. Then, the slides were washed with phosphate-buffered saline with 0.1% Tween-20 (PBST) three times for 10 min each time. Next, the slides were stained with the secondary antibodies goat anti-rat F4/80-Alexa Fluor 594 (Cat# A11007, Thermo Fisher Scientific) and goat anti-rabbit IgG-Alexa Fluor 647 (Cat# A31634, Thermo Fisher Scientific) for 30 min in the dark and washed again three times with PBST for 10 min each time. Then, the slides were stained with the rat anti-mouse Ly-6G Alexa-Fluor 488 antibody (Cat# 127626, BioLegend) and incubated for 60 min. The slides were again washed three times with PBST for 10 min each time, treated with Spectral DAPI (Cat# 2451506, PerkinElmer, Waltham, MA) for 5 min, then washed in PBST for 10 min. Lastly, the ProLong® Diamond Anti-Fade Mountant with DAPI (Thermo Fisher Scientific) was applied to the slide and sealed with a cover slip. The immunofluorescence images were obtained using an All-in-One Fluorescence Microscope BZ-X810 (Keyence, Itasca, IL) and prepared with BZ-X800 Analyzer software v1.1.1.8 (Keyence).
RNA isolation and qRT-PCR
RNA was isolated from RNAlater-collected tissues using the Qiagen RNeasy mini kit (Qiagen, Hilden, Germany), following the manufacturer’s instructions. The purity, concentration, and integrity of the RNA samples were assessed using the NanoDrop 1000 or 8000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA) and the Bioanalyzer 2100 (Agilent Technologies, Wilmington, DE, USA). Complementary (c)DNA was synthesized using SuperScript IV VILO master mix (Invitrogen, Thermo Fisher Scientific Baltics UAB, Vilnius, Lithuania). The gene expression of Il22, Il22ra1, Il10rb, and Il22ra2 was determined by qPCR using ABI 7500 Fast real-time PCR system (Applied Biosystems, Alameda, CA) with commercial TaqMan gene expression assays. cDNA from the maternal lung was used for generating a standard curve to calculate the limit of detection of Il22, Il22ra1, Il10rb, and Il22ra2. Briefly, a standard curve based on duplicate two-fold serial dilutions ranging from 100ng cDNA to 2.4 × 10−4 ng was generated and the performance of the qPCR assay by estimation of its efficiency based on the slope of regression lines was reported as the limit of detection.
Gene expression profiling was performed on the BioMark system for high-throughput qRT-PCR (Fluidigm, San Francisco, CA) using commercial TaqMan gene expression assays (Applied Biosystems, Life Technologies Corporation, Pleasanton, CA). Delta threshold cycle (ΔCt) values were determined using multiple reference genes (Actb, Gusb, Gapdh and Hsp90ab1) averaged within each sample to determine gene expression levels. The −ΔCt values were normalized by calculating the z-score of each gene, and heatmaps were performed using the Subio platform (Subio, Inc, Kagoshima, Japan; https://www.subioplatform.com/).
IL-22 detection in human amniotic fluid leukocytes by immunofluorescence
Cytospin slides of human amniotic fluid samples from women with microbial intra-amniotic inflammation (n = 3, please see clinical definitions above) were prepared using Fisherbrand Superfrost microscope slides and a Shandon Cytospin 3 cytocentrifuge at 800 rpm for 5 min. After fixation with 4% paraformaldehyde, the cytospin slides were rinsed with 1X PBS at room temperature for 2 min. The slides were then permeabilized using 0.25% Triton X-100 diluted in 1X PBS for 5 min and rinsed with 1X PBS for 5 min. Non-specific antibody interaction was blocked by incubating the slides with BlockAid blocking solution for 1 h at room temperature. The mouse anti-human CD14 (Cat# 347490, BD Biosciences) and rabbit IL-22 polyclonal antibody (Cat# bs-2623R, Bioss) were added and the slides were incubated for 60 min at room temperature. The slides were then washed three times for 10 min each time, after which the secondary antibodies goat anti-mouse IgG Alexa Fluor 594 (Cat# A32742, Thermo Fisher Scientific) and goat anti-rabbit IgG Alexa Fluor 488 (Cat# A11034, Thermo Fisher Scientific) were added and the slides were incubated for 30 min. The slides were again washed three times with PBST for 10 min each time. A third primary antibody, mouse anti-human CD15-Alexa Fluor 647 (Cat# 562369, BD Pharmingen) was added and the slides were incubated for 60 min. The slides were washed in PBST three times for 10 min each time. The slides were then incubated with Spectral DAPI for 5 min and washed with PBST for 10 min. Lastly, the ProLong® Diamond Anti-Fade Mountant was applied to the slide and sealed with a cover slip. The immunofluorescence images were obtained using an All-in-One Fluorescence Microscope BZ-X810 and prepared with BZ-X800 Analyzer software v1.1.1.8.
Intra-amniotic injection of Ureaplasma parvum into Il22+/+ and Il22−/− mice
Il22+/+ and Il22−/− dams were intra-amniotically injected with 5 × 104 cells/25 μL of Ureaplasma parvum per sac (n = 10 each genotype) or 25 μL SP4 broth per sac (controls, n = 10 each genotype) on 16.5 dpc. Dams were video monitored to determine rate of preterm birth (preterm birth was defined as delivery occurring before 18.5 dpc, and its rate was represented by the percentage of females delivering preterm among the total number of mice injected) as well as to calculate the gestational length and rate of neonatal mortality, as described above.
Statistics
Statistical analyses were performed using SPSS v19.0 (IBM, Armonk, NY) or GraphPad Prism version 8.0.1 for Windows (GraphPad Software, San Diego, CA). For human demographic data, the group comparisons were performed using Kruskal-Wallis tests for non-normally distributed continuous variables or the Fisher’s exact test for nominal variables. The Mann-Whitney U-test was used to compare differences between term and preterm (human) and control and treatment (mouse) groups. For comparisons of more than two groups, Kruskal-Wallis tests followed by Dunn’s post-hoc test for multiple comparisons were performed. The Mantel-Cox test was utilized when Kaplan-Meier survival curves were plotted and compared. Linear regression tests were performed to determine correlations. A P-value ≤ 0.05 was considered statistically significant.
RESULTS
IL-22 is predominantly expressed by T cells at the maternal-fetal interface of women with preterm labor
The main cellular source of IL-22 belongs to the lymphoid lineage, including both innate and adaptive immune cells such as αβ T cells, γδ T cells, NKT cells, and ILCs (4). Therefore, we first investigated the expression of IL-22 by total T cells, B cells, NK cells, and ILCs at the human maternal-fetal interface. Herein, we use the term human maternal-fetal interface to refer to the decidua basalis and parietalis, which are the site of contact between the uterine endometrium and the placental tissues (Fig. 1A). Figure 1B represents the gating strategy utilized to identify major cell types in the decidual tissues, which are depicted in a t-SNE plot in Fig. 1C. Notably, IL-22+ T cells were more abundant in the decidual tissues of women with preterm labor compared to term labor controls (Fig. 1D). Such T cells co-expressed RORγt, and therefore IL-22+RORγt+ T cells were enriched in the decidual tissues of women with preterm labor (Fig. 1E). Intriguingly, IL-22+ T cells lacked expression of IL-17A, IFNγ, IL-5, and IL-13, while simultaneously expressing moderate levels of CD127 (a marker of T-cell memory (93, 94)) and high levels of CD69 (a marker of early T-cell activation (95, 96)) (Fig. 1F). Indeed, CD69+RORγt+IL-22+ T cells were present in high proportions in the human decidua, suggesting that the majority of such T cells are activated (Fig. S1A). By contrast, IL-22+ B cells, IL-22+ NK cells (cells previously reported in the uterine mucosa and decidua (97, 98)), and IL-22+ ILCs (cells previously reported in the uterine tissues (99–101)) were not significantly altered in the decidual tissues of women with preterm labor compared to term labor controls (Fig. S1B–D). IL-22 can also be produced by myeloid and non-hematopoietic cells such as macrophages, neutrophils, and fibroblasts (4). Macrophages and neutrophils play central roles in host defense in maternal (49, 68, 102) and fetal (28–33, 103, 104) compartments of women with spontaneous preterm labor who were diagnosed with intra-amniotic infection. Therefore, we also determined the expression of IL-22 by neutrophils and macrophages in the decidual tissues. Consistent with the concept that IL-22 is primarily produced by T cells, the expression of this cytokine by decidual macrophages and neutrophils did not differ between women with preterm labor and those with a term pregnancy (Fig. S1E–F). Collectively, these results indicate that the main cellular source of IL-22 at the maternal-fetal interface is T cells, and that such cells are enriched in women with preterm labor. It is worth mentioning that the increase in IL-22+ T cells was not due to differences in gestational age, as no differences in the cell types expressing IL-22 were found between women who delivered preterm and those who delivered at term in the absence of labor (Fig. S2A–H).
FIGURE 1. Immunophenotyping of cellular sources of IL-22 in human decidual tissues.
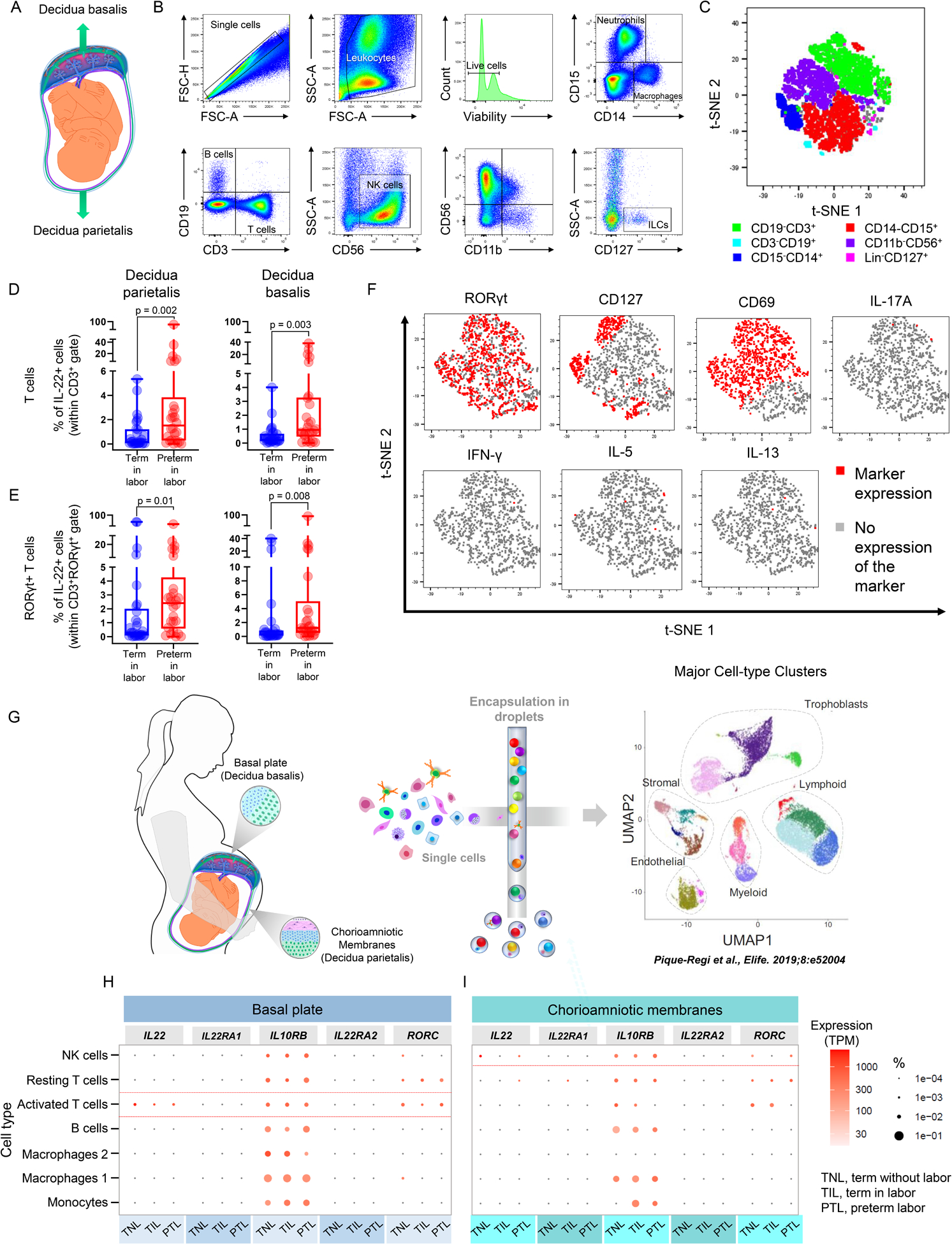
(A) Spatial localization of the human decidua basalis and parietalis. (B) Gating strategy used to identify neutrophils (CD14−CD15+), macrophages (CD15−CD14+), T cells (CD14−CD15−CD19−CD3+), B cells (CD14−CD15−CD3−CD19+); NK cells (CD14−CD15−CD3−CD19−CD56+), and innate lymphoid cells (ILCs; CD14−CD15−CD3−CD19−CD11b−CD56−(Lin−)CD127+) in the human decidua parietalis and basalis. (C) Representative t-SNE plot showing the relative distribution of immune cell populations in the human decidual tissues. (D) Proportions of IL-22+ T cells in the decidua parietalis of women with term labor (TIL, n = 29) or preterm labor (PTL, n = 26) and decidua basalis of women with TIL (n = 30) or PTL (n = 26). (E) Proportions of IL-22+RORγt+ T cells in the decidua parietalis of women with TIL (n = 29) or PTL (n = 26) and decidua basalis of women with TIL (n = 30) or PTL (n = 26). The p-values were determined using Mann-Whitney U-tests. Data are shown as scatter plots with medians, interquartile ranges, and min/max ranges. Demographic and clinical characteristics of the study population are shown in Table I. (F) Representative t-SNE plots showing the relative distribution of RORγt, CD69, CD127, IL-17A, IFNγ, IL-5, and IL-13 expression by IL-22+ T cells in the human decidual tissues. (G) Overview of the single-cell RNA-sequencing (scRNA-seq) of the human decidual tissues showing the major cell types identified in a Uniform Manifold Approximation Plot (UMAP) as reported in (68). Gene expression (scaled to transcripts per million) of IL22, Il22RA1, IL10RB, IL22RA2, and RORC by immune cells in the (H) basal plate and (I) chorioamniotic membranes of women who delivered at term without labor (TNL, n = 3), TIL (n = 3), or PTL (n = 3). Dot size corresponds to the proportion of cells expressing each transcript within a given cell type.
In silico single-cell RNAseq analysis of IL22 expression by immune cells at the human maternal-fetal interface
Next, we explored the unbiased expression of IL22 by decidual immune cells at single-cell resolution. For this, we utilized the previously reported cellular landscape of human placental tissues (basal plate and chorioamniotic membranes) during normal and pathological parturition (68) (Fig. 1G). In silico analysis of this single-cell RNAseq data confirmed our immunophenotyping findings, showing that IL22 is mainly expressed by activated T cells in the decidua basalis that also express RORC in the basal plate (Fig. 1H). NK cells in the decidua parietalis also displayed minimal expression of IL22 and RORC in the chorioamniotic membranes (Fig. 1I). Other immune cells displayed negligible co-expression of IL22 and RORC (Fig. 1H&I). As expected, the transcripts of the activating receptor for IL-22 (IL-22RA1) as well as its binding protein (IL-22RA2), were minimally expressed by decidual immune cells (Fig. 1H&I). In contrast, Il10rb was highly expressed by all decidual immune cells, consistent with its pleiotropic functions (105–107) (Fig. 1H&I). These results support the concept that IL-22 is mainly expressed by T cells at the human maternal-fetal interface.
IL-22 is increased in the maternal circulation upon in vivo T-cell activation in mice
We previously showed that the in vivo activation of T cells induces preterm birth and neonatal mortality in mice in the context of a systemic cytokine storm (66). Given that IL-22 has been implicated in pathologies associated with systemic inflammation (108–110), we next investigated whether this mediator contributes to the cytokine storm resulting from in vivo maternal T-cell activation prior to preterm birth. Dams were intraperitoneally injected with a monoclonal antibody against CD3ε (Fig. 2A), which consistently induces preterm birth in 80–90% of cases as previously reported (66, 89). Circulating levels of IL-22 were increased in dams injected with anti-CD3ε compared to those injected with the isotype (Fig. 2B). Therefore, we next investigated whether the systemic administration of IL-22 alone, at pathophysiological concentrations (91), was sufficient to induce preterm birth and/or adverse neonatal outcomes in mice. The exogenous administration of IL-22 neither shortened gestational length nor induced neonatal mortality in mice (Fig. S3A&B), suggesting that activation of the IL-22 axis in the mother alone is insufficient to induce adverse perinatal outcomes, and that the fetal compartment could be involved. To further explore this concept, we examined the concentrations of IL-22 in the amniotic cavity upon maternal T-cell activation in mice. Amniotic fluid concentrations of IL-22 tended to increase in dams injected with anti-CD3ε compared to those injected with isotype control (3.5-fold increase) (Fig. 2C). These data suggest that maternal T-cell activation causes a surge in systemic IL-22 that may be transferred to the fetal compartment, given that this cytokine is not normally expressed in the fetal tissues (e.g., intestine) in late gestation (111).
FIGURE 2. Maternal T-cell activation induces elevated IL-22 in the maternal circulation and amniotic cavity and the expression of IL22 and its receptors by gestational and fetal tissues in late gestation in mice.
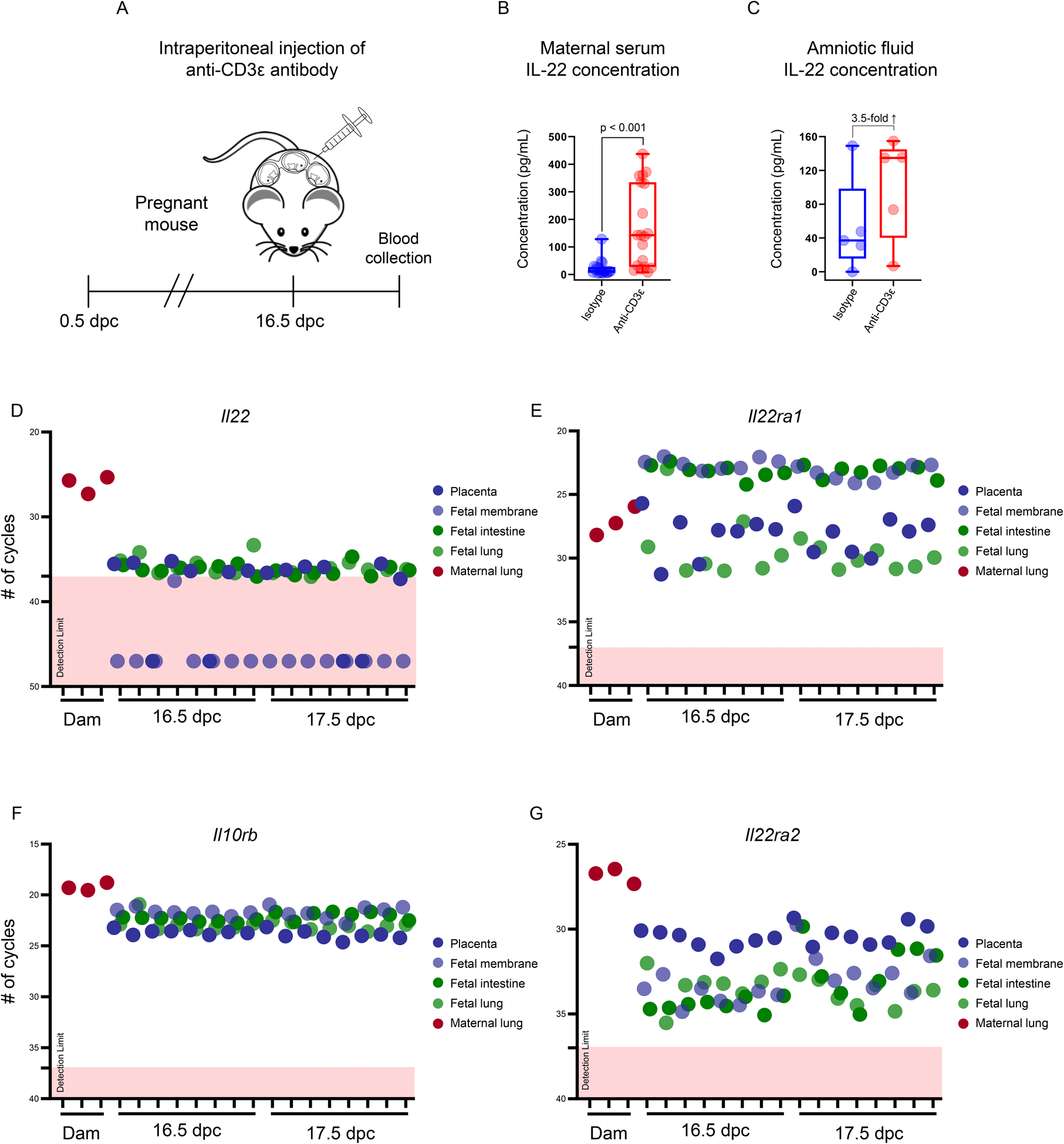
(A) Study design illustrating the intraperitoneal injection of an anti-CD3ε antibody on 16.5 days post coitum (dpc) to induce T-cell activation-induced preterm birth in mice. Maternal serum and amniotic fluid were collected 12 – 16 h after injection for IL-22 determination. (B) Concentrations of IL-22 (pg/mL) in the maternal serum of mice injected with anti-CD3ε (n = 19) or isotype control (n = 18). (C) Concentrations of IL-22 (pg/mL) in the amniotic fluid of mice injected with anti-CD3ε (n = 5) or isotype control (n = 5). The p-values were determined using Mann-Whitney U-tests. Data are shown as scatter plots with medians, interquartile ranges, and min/max ranges. Dot plots representing the expression of (D) Il22, (E) Il22ra1, (F) Il10rb, and (G) Il22ra2 in the murine placenta, fetal membranes, fetal intestine, and fetal lung at 16.5 and 17.5 dpc, as well as the lungs of pregnant mice injected with lipopolysaccharide (positive control) at 17.5 dpc. Limits of detection for each transcript are denoted by dotted lines and pink boxes.
Il22 receptors, but not Il22, are highly expressed in the murine fetal tissues in late gestation
To complement a prior report showing that IL-22 is not expressed by the murine fetal intestine (111), we next determined whether this cytokine was transcribed by gestational tissues (e.g., placenta and fetal membranes) and the fetal lung in mice. Consistently, Il22 was minimally expressed in the fetal intestine, fetal lung, placenta, or fetal membranes in late murine gestation, which contrasted with the high expression of this cytokine by the lungs of pregnant mice undergoing systemic inflammation (Fig. 2D). Yet, the transcripts encoding the primary receptors for IL-22, Il22ra1 and Il10rb, were highly expressed in the fetal and gestational murine tissues (Fig. 2E–F). Similarly, Il22ra2, the transcript encoding the IL-22 binding protein, was also highly expressed by the fetal and gestational murine tissues, with the placenta demonstrating the highest expression (Fig. 2G). Together, these results show that the fetus possesses the machinery for responding to IL-22 signaling in late murine gestation, but does not express this cytokine under physiological conditions.
IL-22 can cross from the maternal to the fetal compartment, where it is associated with intra-amniotic inflammation and preterm labor and birth in humans
The above data suggested to us that the pathological effects of IL-22 may be localized to the fetal compartment rather than the maternal circulation, and that this cytokine may be capable of crossing the human maternal-fetal interface and reaching the amniotic cavity, inducing inflammation and disease. Therefore, we generated a novel ex vivo human system to test this hypothesis (Fig. 3A). IL-22 was capable of crossing from the maternal decidua through the chorioamnion at different doses, suggesting that this maternally-derived cytokine can indeed reach the fetus (Fig. 3B). Next, we evaluated the clinical relevance of increased levels of IL-22 in the human amniotic cavity by investigating the concentrations of this cytokine in different subsets of women with preterm labor. Patients who presented with an episode of preterm labor who delivered at term were considered as controls (Fig. 3C). Notably, amniotic fluid IL-22 concentrations were greater in women with sterile or microbial intra-amniotic inflammation than in those without these clinical conditions who delivered preterm or those who delivered at term (Fig. 3D). In addition, amniotic fluid concentrations of IL-22 tended to increase in women with microbial intra-amniotic inflammation compared to those with sterile intra-amniotic inflammation (Fig. 3D). Together, these data led us to propose that, in the amniotic cavity, IL-22 plays a dual role in maternal-fetal immunity: 1) IL-22 alone is capable of causing fetal tissue injury leading to neonatal death, and 2) IL-22 participates in host defense against microbial invasion leading to preterm birth.
FIGURE 3. IL-22 can cross from the maternal to the fetal compartment and is implicated in intra-amniotic inflammation in women with preterm labor and birth.
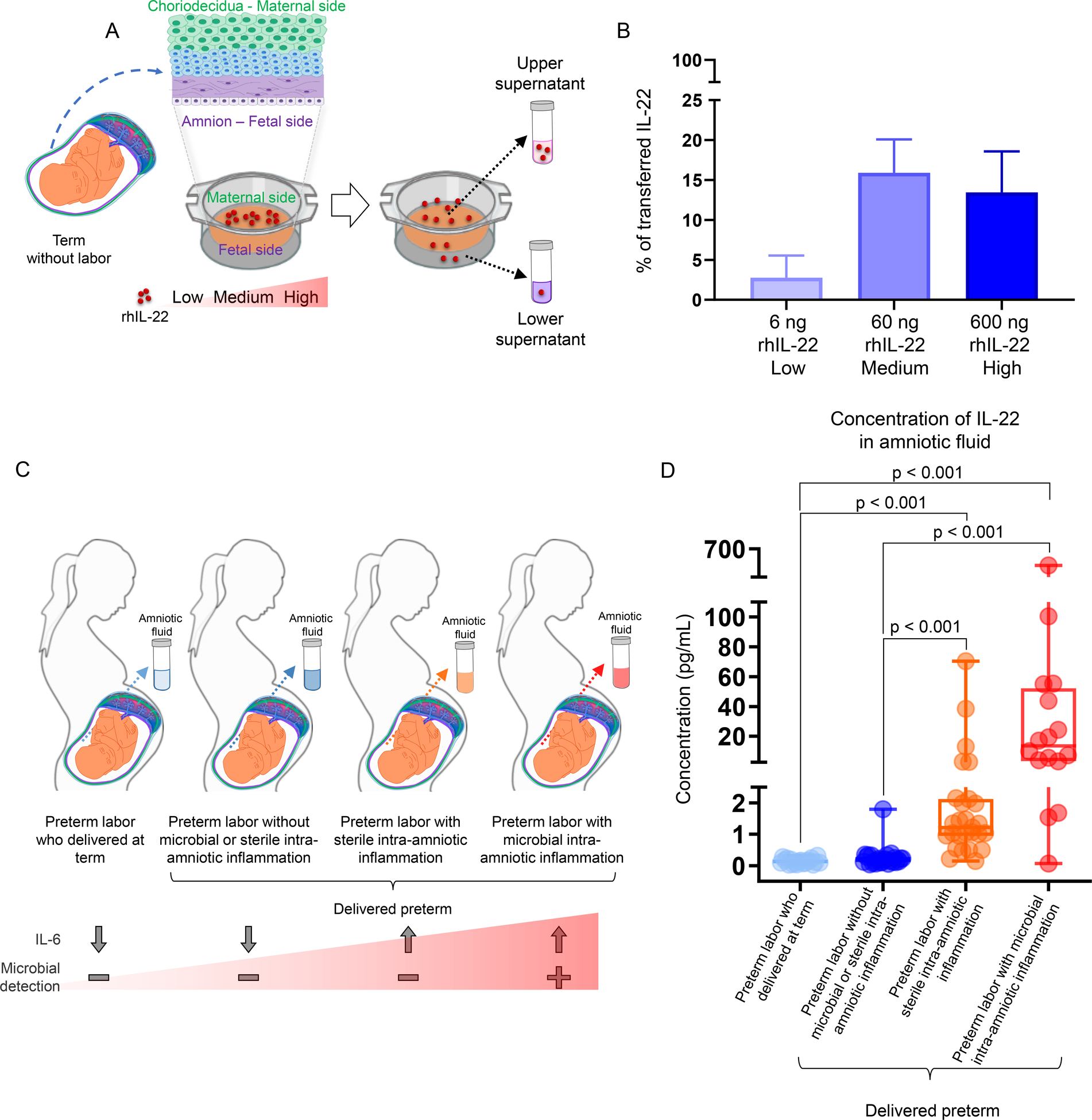
(A) Study design illustrating the ex vivo human model of recombinant human (rh)IL-22 transfer across an explant of chorioamniotic membranes from women without labor at term (TNL). (B) Percent (%) of transferred rhIL-22 at low (6ng; n = 3), medium (60ng; n = 4) and high (600ng; n = 3) concentrations. Data are shown as bar plots (mean ± SEM). BioRender was used to create part of the study design (transwells). (C) Study design illustrating the study groups of women who underwent spontaneous preterm labor and characterization of their intra-amniotic inflammatory and microbiological status and outcomes. (D) Concentrations of IL-22 in amniotic fluid of women with preterm labor who delivered at term (n = 20), preterm labor without microbial or sterile intra-amniotic inflammation who delivered preterm (n = 27), preterm labor with sterile intra-amniotic inflammation who delivered preterm (n = 27), or preterm labor with microbial intra-amniotic inflammation who delivered preterm (n = 16). The p-values were determined using a Kruskal-Wallis test followed by Dunn’s post-hoc test. Data are shown as scatter plots with medians, interquartile ranges, and min/max ranges. Demographic and clinical characteristics of the study population are shown in Table II.
The intra-amniotic administration of IL-22 alone shortens gestational length and induces neonatal death in mice
To establish the role of intra-amniotic IL-22 in fetal injury leading to neonatal death (Fig. 4A), we performed ultrasound-guided intra-amniotic injection of this cytokine in mice (Fig. 4B) at pathophysiological concentrations found in women with intra-amniotic inflammation leading to preterm birth (Fig. 3D). The intra-amniotic administration of IL-22 shortened gestational length (Fig. 4C); however, this reduction did not reach statistical significance. Sample size calculations revealed that 46 animals per group would be required to reach statistical significance with a confidence level of 0.95 and power of 0.9. Regardless, neonates born to mice intra-amniotically injected with 612 pg of IL-22 showed a 50% increase in mortality compared to those born to controls, which was statistically significant (Fig. 4D). Neonates born to control mice appeared healthy compared to those born to IL-22-injected dams, of which half were hypoxic and ultimately died (Fig. 4E). Neonatal weights at weeks 1, 2, and 3 were not significantly different among the groups (Fig. 4F–H). Taken together, these results provide mechanistic evidence that elevated concentrations of IL-22 alone in the amniotic cavity shorten gestational length and, more importantly, induce neonatal death in mice.
FIGURE 4. Intra-amniotic administration of IL-22 shortens gestational length and causes neonatal death in mice.
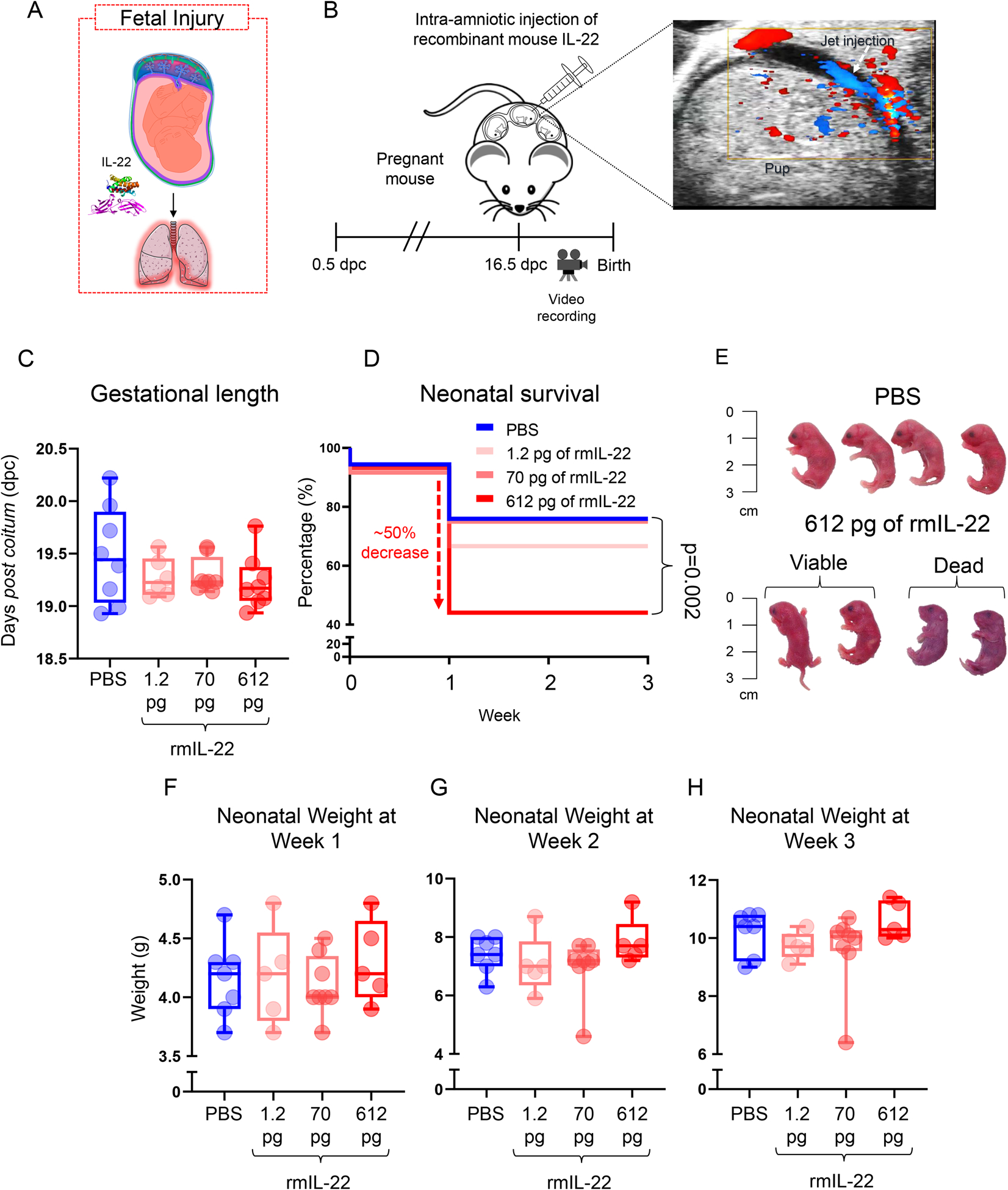
(A) In the absence of microbial invasion of the amniotic cavity, increased IL-22 could lead to fetal injury. (B) Mice received an ultrasound-guided intra-amniotic injection of recombinant mouse (rm)IL-22 on 16.5 days post coitum (dpc) and were monitored until delivery. Color Doppler was used to identify the injection jet sign to confirm successful intra-amniotic injection. (C) Gestational length (dpc) of mice that received an intra-amniotic injection of rmIL-22 at 1.2 (pink; n = 8), 70 (dark pink; n = 6), or 612 (red; n =8) pg/amniotic sac or PBS (blue; n = 8) as vehicle control. (D) Kaplan-Meier survival curves representing survival at weeks 1, 2, and 3 of neonates born to mice that received intra-amniotic injection of rmIL-22 at 1.2 (pink; n =42), 70 (dark pink; n = 60), or 612 (red; n = 59) pg/amniotic sac or PBS (blue; n = 54). P-values were determined using the Mantel-Cox test. (E) Representative images of neonates born to mice that received intra-amniotic injection of PBS (top row) or rmIL-22 (612 pg/amniotic sac) (bottom row). (F-H) Weights at weeks 1, 2, and 3 of neonates born to mice that received intra-amniotic injection of rmIL-22 at 1.2 (pink; n = 5 litters), 70 (dark pink; n = 8 litters), or 612 (red; n = 5 litters) pg/amniotic sac or PBS (blue; n = 7 litters). Each dot corresponds to the mean weight of a litter of pups. Data are shown as scatter plots with means.
Intra-amniotic injection of IL-22 alone induces lung injury in the murine offspring
IL-22 has been previously shown to induce lung inflammation (112, 113). Therefore, to investigate the mechanisms whereby intra-amniotic IL-22 causes neonatal death, we first examined whether this cytokine induces a Respiratory Distress Syndrome (RDS)-like phenomenon, which is a common clinical complication of premature neonates (114, 115). As a readout of RDS, we measured SP-A, a molecule produced in the lung by type II alveolar cells that is essential for maintaining a patent airway (116, 117), in the murine neonatal lungs (Fig 5A). The intra-amniotic injection of IL-22 in mice significantly reduced the concentrations of SP-A (Fig. 5B), suggesting that this cytokine impairs lung maturation in the offspring. Next, we examined the expression of key markers of inflammation (transcripts related to RDS, necrotizing enterocolitis, the IL-22 pathway, etc.) in the neonatal lungs (Fig. 5C) and intestine (Fig. 5D) to evaluate the effects of IL-22 in these murine tissues. IL-22 significantly upregulated the expression of Il33, a pro-inflammatory cytokine implicated in the pathogenesis of preterm birth and neonatal mortality induced by alarmins (27, 118, 119), in both the murine neonatal lung (Fig. 5E) and intestine (Fig. 5F). Furthermore, IL-22 upregulated the transcription of Defb1, a defensin that is increased in the amniotic cavity of women with intra-amniotic inflammation (120, 121), in the murine neonatal lung (Fig. 5E). Moreover, IL-22 upregulated the expression of the gene encoding for IL-22RA1 in the murine neonatal lung (Fig. 5E) and tended to decrease the expression of the gene encoding for the binding protein (Fig. 5C), suggesting that this cytokine orchestrates its engagement in neonatal life. Consistently, the expression of Il22ra2 is significantly reduced in the murine neonatal intestine upon IL-22 administration (Fig. 5F). In addition, IL-22 downregulated the transcription of several inflammatory mediators in the murine neonatal lung such as Ifng, Ccl5, Tslp, Il27, and aryl hydrocarbon receptor (Ahr).
FIGURE 5. Intra-amniotic administration of IL-22 in mice induces lung injury and inflammation in the offspring.
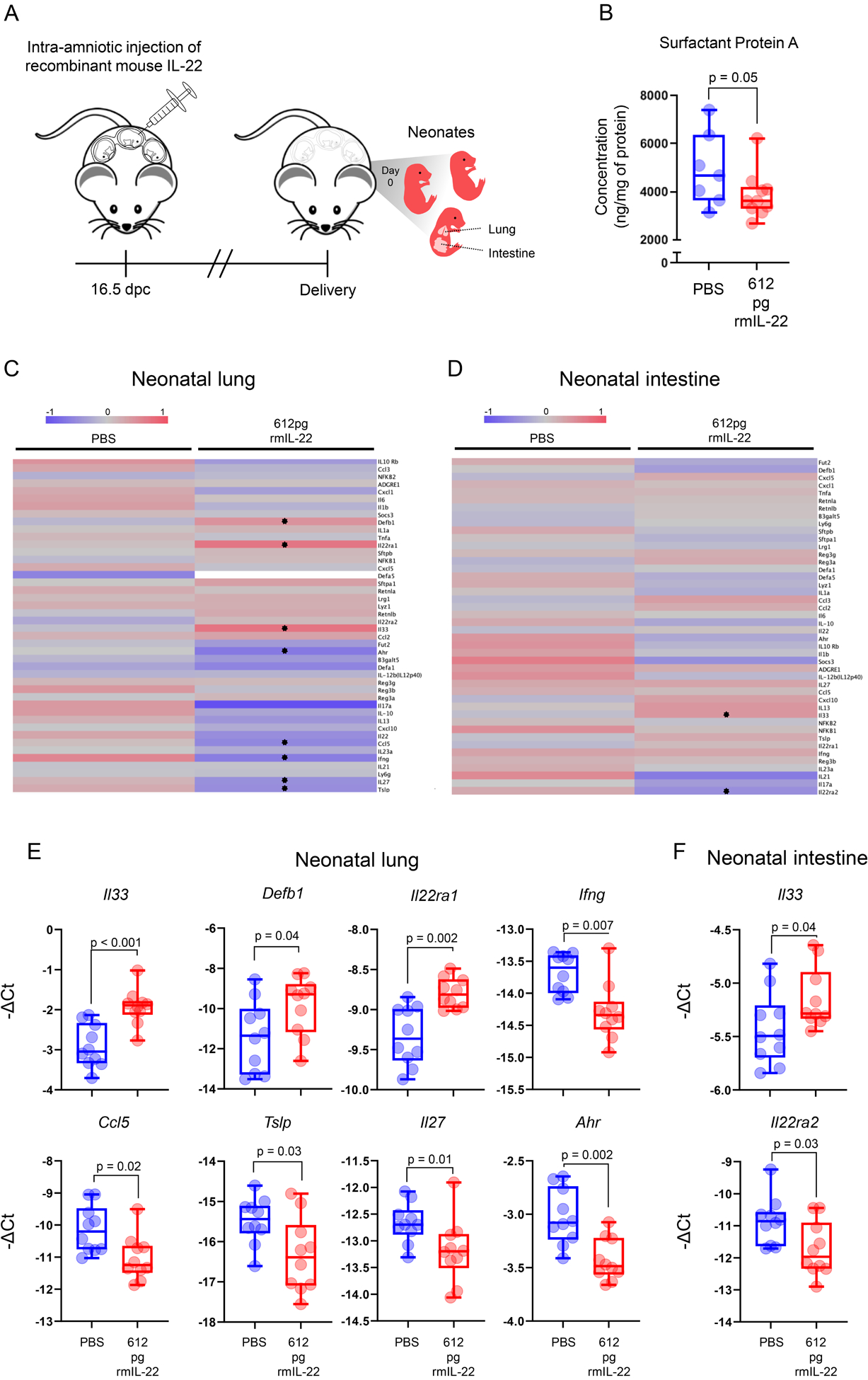
(A) Mice received an ultrasound-guided intra-amniotic injection of recombinant mouse (rm)IL-22 (612 pg/amniotic sac) (n = 10) or PBS (vehicle control; n = 7 – 10) on 16.5 days post coitum (dpc) and were monitored until delivery, after which the neonatal lung and intestine were collected. (B) Surfactant Protein A concentrations (ng/mg of protein) in the lungs of neonates born to mice intra-amniotically injected with PBS (n = 7) or rmIL-22 (n = 10). P-value was determined using a Mann-Whitney U-test. Heatmap representations showing inflammatory gene expression in the (C) lungs and (D) intestines of neonates born to mice intra-amniotically injected with PBS (n = 10) or rmIL-22 (n = 10). Red indicates upregulated expression and blue indicates downregulated expression. Stars indicate significant differentially expressed genes. (E) Expression of Il33, Defb1, Il22ra1, Ifng, Ccl5, Tslp, Il27, and Ahr and in the murine neonatal lung. (F) Expression of Il33 and Il22ra2 in the murine neonatal intestine. P-values were determined using Mann-Whitney U-tests.
Collectively, these data suggest that the mechanisms whereby IL-22 induces neonatal death in mice primarily involve impaired maturation and dysregulation of inflammatory cascades in the neonatal lung.
IL-22 participates in host defense induced by microbial invasion of the amniotic cavity in both mice and humans
During intra-amniotic infection, the inflammatory process represents the host response against microbes invading the amniotic cavity (28–33), which leads to preterm labor and birth as an unintended consequence (34, 35). To establish the role of IL-22 in host defense against microbial invasion of the amniotic cavity leading to preterm birth (Fig. 6A), we utilized a previously established murine model of preterm birth induced by Ureaplasma parvum (92), the most common microorganism found in women with microbial intra-amniotic inflammation (73, 122–125). Amniotic fluid concentrations of IL-22 were significantly increased in dams injected with Ureaplasma parvum compared to those injected with control medium (Fig. 6B). Yet, the intra-amniotic injection of Ureaplasma parvum in mice did not increase the systemic concentrations of IL-22 (Fig. S3C). To appreciate the role of our cytokine of interest in the local host response to Ureaplasma parvum, we determined the correlation between IL-22 concentrations and the concentrations of pro-inflammatory cytokines as well as the expression of IL-22 by infiltrating leukocytes in the murine amniotic cavity, both of which are clinical manifestations of intra-amniotic infection (27, 30–32, 70, 104, 119, 126–128). Consistent with a role for IL-22 in host response, the concentrations of this cytokine were positively correlated with IL-6 (the clinical marker of intra-amniotic inflammation in humans (74, 129)), as well as other pro-inflammatory cytokines such as IL-21 and IL-17A (Fig. 6C). Moreover, both amniotic fluid neutrophils and monocytes/macrophages found in mice injected with Ureaplasma parvum expressed IL-22 (Fig. 6D). The translational value of the latter finding was confirmed in human amniotic fluid samples from women with intra-amniotic infection associated with genital mycoplasmas, where neutrophils and monocytes/macrophages expressed IL-22 (Fig. 6E&F). To further examine the sources and sensing of IL-22 in the amniotic cavity upon microbial invasion, the expression of this cytokine and its receptors was explored in the fetal membranes and placentas of Ureaplasma parvum-injected mice. The expression of Il22 was significantly upregulated in the murine fetal membranes (Fig. 7A) and the placenta (Fig. 7B) compared to controls, suggesting that these gestational tissues express this cytokine as part of the host response triggered by genital mycoplasmas invading the amniotic cavity. Interestingly, the murine placenta upregulated the expression of both Il22ra1 and Il10rb (Fig. 7B), suggesting that intra-amniotic infection induced by genital mycoplasmas promotes the sensing of IL-22 by this fetal organ. Lastly, we evaluated the inflammatory status of the murine fetal lung and intestine as a consequence of the host intra-amniotic immune response triggered by microbes. Consistent with the clinical scenario of fetal inflammatory response syndrome induced by intra-amniotic infection (130–132), fetuses of mice injected with Ureaplasma parvum displayed a massive upregulation of pro-inflammatory mediators in the fetal lung and intestine (Fig. 7C–F). Taken together, these data provide a solid role for IL-22 in the host response to Ureaplasma parvum-induced intra-amniotic inflammation preceding preterm birth in both mice and humans.
FIGURE 6. IL-22 is expressed by neutrophils and monocytes/macrophages in the human and murine amniotic cavity upon microbial infection.
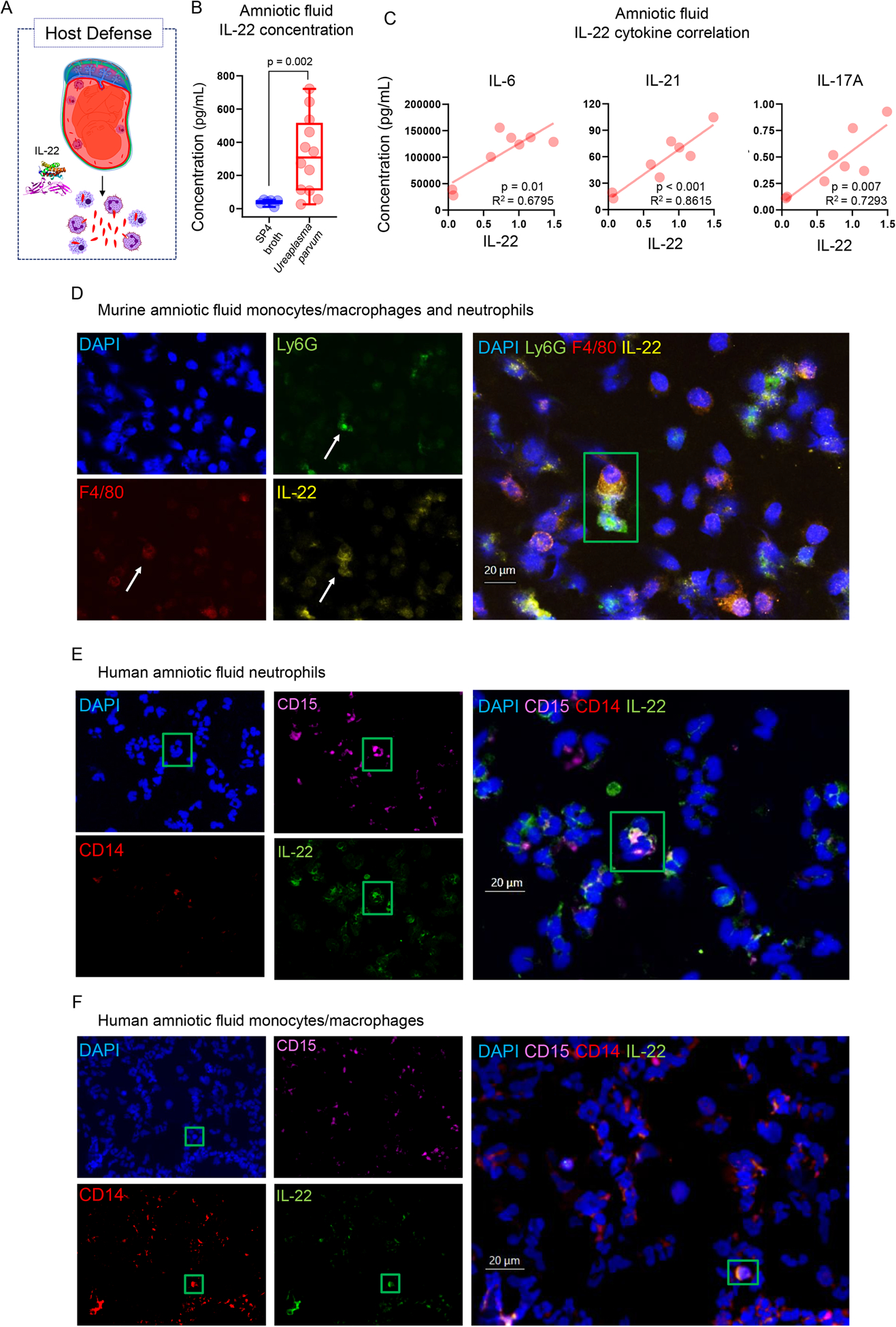
(A) IL-22 participates in the local host immune response to microbial invasion of the amniotic cavity. (B) Concentrations of IL-22 in the amniotic fluid of mice intra-amniotically injected with Ureaplasma parvum (n = 12) or SP4 broth (n = 6). P-value was determined using a Mann-Whitney U-test. (C) Linear regressions showing the correlations between amniotic fluid concentrations of IL-22 and those of IL-6, IL-21, and IL-17A in mice intra-amniotically injected with Ureaplasma parvum (n = 8). (D) Representative immunofluorescence imaging of amniotic fluid neutrophils and monocytes/macrophages from mice intra-amniotically injected with Ureaplasma parvum showing the single and merged expression of DAPI (nuclei, blue), Ly6G (neutrophils, green), F4/80 (monocytes/macrophages, red), and IL-22 (yellow). Representative immunofluorescence imaging of human amniotic fluid (E) neutrophils and (F) monocytes/macrophages obtained from women with intra-amniotic infection showing the single and merged expression of DAPI (blue), CD15 (neutrophils, violet), CD14 (monocytes/macrophages, red), and IL-22 (green). Green boxes correspond to cells of interest. All images taken at 40X magnification. Scale bars indicate 20μm.
FIGURE 7. IL22 participates in host response to Ureaplasma parvum in the placenta, fetal membranes, fetal lung, and fetal intestine in mice.
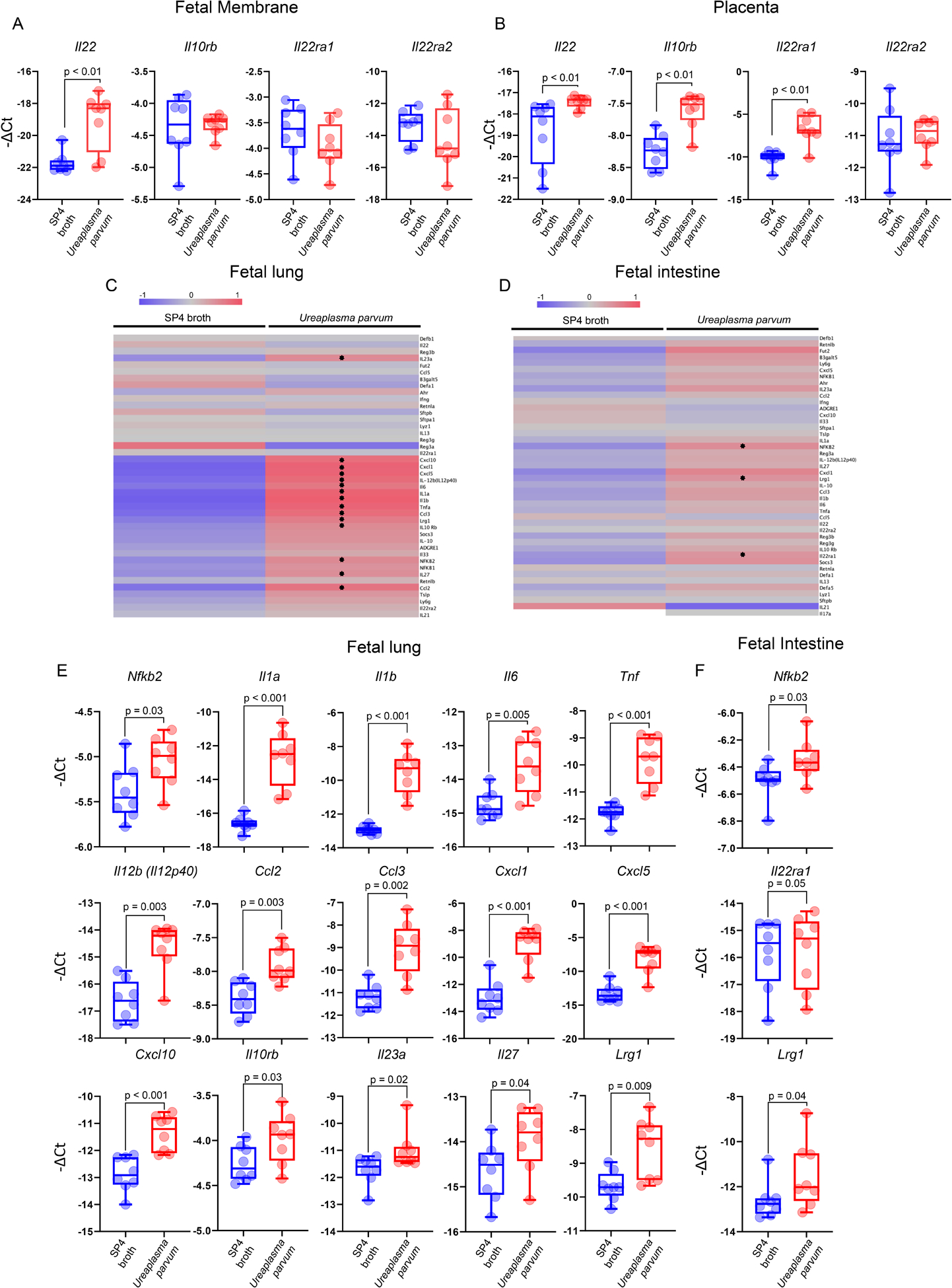
(A) Expression of Il22, Il10rb, Il22ra1, and Il22ra2 in the fetal membranes of mice intra-amniotically injected with Ureaplasma parvum (n = 8) or SP4 broth (n = 8). (B) Expression of Il22, Il10rb, Il22ra1, and Il22ra2 in the placentas of mice intra-amniotically injected with Ureaplasma parvum (n = 8) or SP4 broth (n = 8). Heatmap representations showing inflammatory gene expression in the (C) lung and (D) intestine of fetuses of mice intra-amniotically injected with Ureaplasma parvum (n = 8) or SP4 broth (n = 8). Stars indicate significant differentially expressed genes. (E) Expression of Nfkb2, Il1a, Il1b, Il6, Tnf, Il12b (Il12p40), Ccl2, Ccl3, Cxcl1, Cxcl5, Cxcl10, Il10rb, Il23a, Il27, and Lrg1 in the lung of fetuses of mice intra-amniotically injected with Ureaplasma parvum (n = 8) or SP4 broth (n = 8). (F) Expression of Nfkb2, Il22ra1, and Lrg1 in the intestine of fetuses of mice intra-amniotically injected with Ureaplasma parvum (n = 8) or SP4 broth (n = 8). P-values were determined using Mann-Whitney U-tests.
Il22 deficiency protects against Ureaplasma parvum-induced preterm birth and neonatal mortality
Lastly, to demonstrate causality between IL-22 and adverse perinatal outcomes due to microbial intra-amniotic inflammation, we performed intra-amniotic injection of Ureaplasma parvum in Il22-sufficient and -deficient mice (Fig. 8A). Ureaplasma parvum induced high rates (40%) of preterm birth in Il22+/+ mice, confirming our previously established model of microbial intra-amniotic inflammation-induced preterm birth (92). Notably, Il22−/− mice were protected against preterm birth and delivered later than Il22+/+ dams (Fig. 8B&C). Importantly, although Ureaplasma parvum induced high rates of neonatal mortality in Il22+/+ mice, neonates born to Il22−/− dams experienced minimal mortality rates (Fig. 8D) and thrived up to three weeks of age. This last set of results provides a mechanistic demonstration of a role for IL-22 in the inflammatory response induced by genital mycoplasmas as part of the host defense against infection of the amniotic cavity, which leads to preterm birth and adverse neonatal outcomes as unintended consequences.
FIGURE 8. Il22 deficiency protects against intra-amniotic Ureaplasma parvum-induced preterm birth and neonatal mortality.
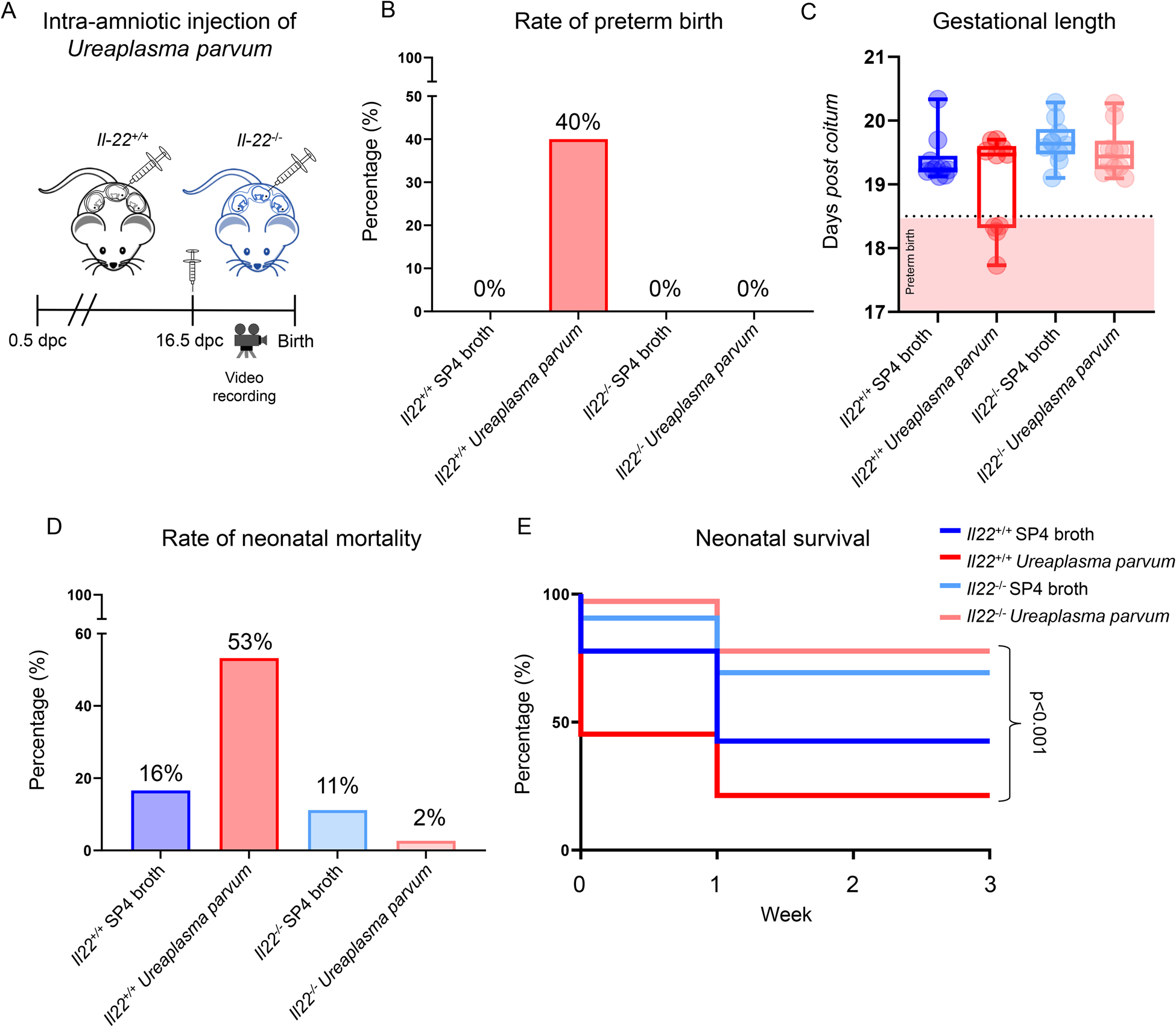
(A) Il22+/+ and Il22−/− mice were intra-amniotically injected with Ureaplasma parvum or SP4 broth (control) on 16.5 days post coitum (dpc) and monitored until delivery. (B) Preterm birth rates of Il22+/+ and Il22−/− mice intra-amniotically injected with Ureaplasma parvum (n = 10 per genotype) or control (n = 10 per genotype). (C) Gestational lengths of Il22+/+ and Il22−/− mice intra-amniotically injected with Ureaplasma parvum or control. Dotted line and pink box represent the cut-off for preterm birth (<18.5 dpc). (D) Mortality rates of neonates born to Il22+/+ and Il22−/− mice intra-amniotically injected with Ureaplasma parvum or control. (E) Kaplan-Meier survival curves representing survival at weeks 1, 2, and 3 of neonates born to Il22+/+ and Il22−/− mice intra-amniotically injected with Ureaplasma parvum or SP4 broth. P-values were determined using the Mantel-Cox test.
DISCUSSION
Herein, we present evidence that IL-22 plays a dual role in maternal-fetal immunity. Specifically, we demonstrated that IL-22+ T cells co-expressing RORγt are enriched at the human maternal-fetal interface of women with preterm labor and birth, as confirmed by in silico analysis of available single-cell RNASeq data of the basal plate. Next, we showed that systemic concentrations of IL-22 were elevated upon maternal T-cell activation leading to preterm birth in mice. Yet, the intravenous administration of IL-22 alone in mice did not induce adverse perinatal outcomes, suggesting that this cytokine must reach the fetal compartment to cause damage. In line with our hypothesis, concentrations of IL-22 were increased in the amniotic cavity upon in vivo maternal T-cell activation where it may be sensed by its receptors (Il22ra1, Il10rb, and Il22ra2), which were highly expressed by gestational and fetal tissues in late murine pregnancy. Next, we confirmed that IL-22 can cross from the maternal (choriodecidua) to the fetal (amniotic cavity) side using an ex vivo human system. Importantly, amniotic fluid concentrations of IL-22 were elevated in women with sterile or microbial intra-amniotic inflammation, leading us to investigate the role of this cytokine in the presence and absence of microbes. A role for IL-22 in fetal tissue injury was evidenced by the intra-amniotic administration of this cytokine alone in mice, which led to shortened gestation and neonatal death, the latter involving impaired lung maturation and tissue inflammation. A role for IL-22 in the host response against microbes invading the amniotic cavity was demonstrated by the participation of this cytokine in the intra-amniotic inflammatory milieu preceding Ureaplasma parvum-induced preterm birth in mice, which was rescued by the deficiency of IL-22. Collectively, these data show that IL-22 alone is capable of causing fetal tissue injury leading to neonatal death and that this cytokine participates in host defense against microbial invasion of the amniotic cavity leading to preterm labor and birth.
T cells of maternal origin are present at the human (56–60, 66, 133–138) and murine (48, 50, 51, 64, 66, 139–141) maternal-fetal interface (67, 142–146). In early pregnancy, antigen-specific and non-specific events are thought to modulate the highly regulated, but limited, recruitment of effector T cells (147), which contribute to the pro-inflammatory milieu at the maternal-fetal interface that is required for successful implantation (148, 149). Such an inflammatory response may be counteracted by the concomitant increase in regulatory T cells that occurs both locally and systemically (150–154) and persists throughout gestation (152). The importance of regulatory T cells in late gestation has been underscored by mechanistic studies showing that the depletion of such cells in early (152, 153, 155–159) and late (160) pregnancy results in pregnancy loss and preterm birth, respectively. Moreover, we have shown that a reduction in regulatory T cells is accompanied by an increased influx of effector T cells at the maternal-fetal interface of women who underwent spontaneous preterm labor (160). These T cells express effector molecules such as granzyme B and perforin that may exert their lytic functions at the maternal-fetal interface (66), thereby inducing tissue damage and chronic decidual inflammation, a hallmark of spontaneous preterm labor (161, 162). Herein, we expand on this concept by demonstrating that a subset of decidual T cells co-express IL-22 and RORγt, and such cells are enriched in women with preterm labor and birth. The expression of such molecules, together with the absence of IL-17A, led us to propose that such decidual T cells belong to the recently described Th22 subset (163–169). Our finding that Th22-like cells are present at the maternal-fetal interface of women with preterm labor is in tandem with a prior study showing that decidual IL-22-expressing T cells are implicated in pregnancy loss (170). Therefore, T cells expressing IL-22 are present at the maternal-fetal interface in early and late pregnancy, where they seem to participate in the mechanisms involved in obstetrical disease.
Consistent with the above concept, we have shown that the in vivo injection of an anti-CD3ε antibody can activate systemic and decidual T cells in mice, resulting in preterm birth and neonatal mortality (66). In the current study, we showed that such activation of T cells is accompanied by elevated concentrations of IL-22 in the murine maternal circulation. In line with this finding, elevated concentrations of IL-22 or numbers of IL-22+ T cells have been reported in systemic inflammatory disorders as well as in host response to pathogens (171–175). Furthermore, IL-22+ T cells are elevated in the circulation of women with preeclampsia (176), a population at increased risk of medically-indicated preterm birth (12, 177). However, the concentrations at which IL-22 displays deleterious effects likely vary according to systemic pathophysiological processes. For example, we report herein that the systemic administration of IL-22, at concentrations reported in women with spontaneous preterm labor (91), is not sufficient to induce preterm birth in mice. Yet, it is plausible that the cytokine storm induced by maternal T-cell activation (66) facilitates the crossing of IL-22 from the maternal to the fetal compartment (i.e., amniotic cavity), as evidenced by the elevated amniotic fluid concentrations of this cytokine in this murine model. Such a concept was confirmed in the current study by demonstrating that maternally-derived IL-22 crosses the human maternal-fetal interface, reaching the fetal compartment. Transfer of cytokines through the placental tissues is not unique to IL-22, since it has been previously demonstrated for IL-8 (178), IL-2 (179), and IL-6 (180, 181).
Interleukin-22 is minimally expressed by murine fetal tissues, as demonstrated herein and previously reported (111). Yet, its canonical receptors are highly expressed by the murine fetal and placental tissues, as shown herein, or by the human placenta (182), suggesting that the fetus can sense IL-22 under pathological conditions. Indeed, here we demonstrate a pathological role for IL-22 in the fetal compartment by showing that the intra-amniotic administration of this cytokine alone shortens gestational length and, more importantly, causes neonatal death in mice. This finding is in tandem with our previous investigation showing that activated fetal T cells, which are capable of producing IL-22 as well as other Th cytokines, are implicated in a subset of preterm labor cases without microbial intra-amniotic inflammation (68). Consistently, the intra-amniotic administration of activated neonatal T cells in mice induced a proportion of preterm births (68), suggesting that the release of multiple T-cell cytokines is necessary to activate the premature cascade of parturition, and provides an explanation as to why IL-22 alone only shortened the gestational length. Nevertheless, intra-amniotic IL-22 was sufficient to induce neonatal death in mice, which could result from the multi-organ pathological effects attributed to this cytokine (4, 183), as evidenced herein by tissue inflammation and impaired lung maturation in neonates.
The indispensable role of IL-22 in host defense mechanisms against infection with bacteria, yeast, and protozoa is well documented (4, 173, 184–187). Specifically, during bacterial infection, this cytokine enhances anti-bacterial defense by epithelial cells and actively participates in the recruitment and activation of immune cells, thus limiting colonization (188–190). Consistently, here we report that IL-22 is part of the intra-amniotic inflammatory response driven by host response against microbes, such as Ureaplasma parvum, in humans and mice. Although we did not observe the direct actions of IL-22 on Ureaplasma parvum, a previous report has shown that IL-22 alone inhibits the growth of Mycobacterium tuberculosis in macrophages (191). The ability of IL-22 to perform such a function with respect to Ureaplasma parvum in the amniotic cavity warrants further investigation. Moreover, we show that fetal innate immune cells infiltrating the amniotic cavity in response to infection (30, 31, 33) are sources of IL-22 in both humans and mice. The mechanisms whereby IL-22 participates in the inflammatory milieu induced by Ureaplasma parvum involve the upregulation of its transcription as well as that of its receptors in the placental tissues, as shown herein. Furthermore, we provide evidence that IL-22 is implicated in the inflammatory pathways leading to preterm birth and fetal injury, given that Il22-deficient mice are resistant to the deleterious effects of Ureaplasma parvum in the amniotic cavity. The mechanisms whereby Il22 deficiency protects against neonatal mortality may involve resolution of the acute fetal inflammatory response syndrome, which is a common complication that can result from microbial invasion of the amniotic cavity and involves massive activation of the innate limb of fetal immunity as part of the host response (130, 132, 192).
In summary, this study provides a previously unrecognized dual role for IL-22 in maternal-fetal immunity. Interleukin-22 is expressed by maternal T cells in the uterine decidua of women with preterm labor and birth. Under pathological circumstances associated with maternal T-cell activation, IL-22 can cross the maternal-fetal interface and reach the amniotic cavity, where it can be sensed by the fetal and gestational tissues, causing fetal injury leading to neonatal death. On the other hand, IL-22 contributes to the host response against microbes invading the amniotic cavity in the context of preterm labor and birth, which involves a fetal inflammatory response leading to neonatal demise. Collectively, these findings shed light on the biology of IL-22 during late gestation and host response mechanisms implicated in intra-amniotic infection resulting in preterm birth, the leading cause of neonatal morbidity and mortality worldwide.
Supplementary Material
KEY POINTS.
IL-22 plays a dual role in the amniotic cavity
IL-22 causes fetal tissue injury leading to neonatal death
IL-22 participates in host defense against infection leading to preterm birth
ACKNOWLEDGMENTS
We would like to thank Dr. Valeria Garcia-Flores for her intellectual input and helpful discussions. We would also like to thank the physicians, nurses, and research assistants from the Center for Advanced Obstetrical Care and Research, Intrapartum Unit, PRB Clinical Laboratory, and PRB Perinatal Translational Science Laboratory (Rona Wang) for their help with collecting and processing samples. Lastly, we would like to thank Dr. Marcelo Farias-Jofre, Chengrui Zou, and David Kracht for their help with the execution of some assays.
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No.HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Non-standard abbreviations:
- dpc
days post coitum
- TIL
term with labor
- TNL
term without labor
- PTL
preterm with labor
- PTNL
preterm without labor
- RORγt
Retinoic Acid Receptor-related orphan receptor γt
Footnotes
DECLARATION OF INTERESTS
The authors declare no potential conflicts of interest.
REFERENCES
- 1.Zenewicz LA, and Flavell RA. 2011. Recent advances in IL-22 biology. Int Immunol 23: 159–163. [DOI] [PubMed] [Google Scholar]
- 2.Saxton RA, Henneberg LT, Calafiore M, Su L, Jude KM, Hanash AM, and Garcia KC. 2021. The tissue protective functions of interleukin-22 can be decoupled from pro-inflammatory actions through structure-based design. Immunity 54: 660–672 e669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutz S, Wang X, and Ouyang W. 2014. The IL-20 subfamily of cytokines--from host defence to tissue homeostasis. Nat Rev Immunol 14: 783–795. [DOI] [PubMed] [Google Scholar]
- 4.Dudakov JA, Hanash AM, and van den Brink MR. 2015. Interleukin-22: immunobiology and pathology. Annu Rev Immunol 33: 747–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma HL, Liang S, Li J, Napierata L, Brown T, Benoit S, Senices M, Gill D, Dunussi-Joannopoulos K, Collins M, Nickerson-Nutter C, Fouser LA, and Young DA. 2008. IL-22 is required for Th17 cell-mediated pathology in a mouse model of psoriasis-like skin inflammation. J Clin Invest 118: 597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lowes MA, Suarez-Farinas M, and Krueger JG. 2014. Immunology of psoriasis. Annu Rev Immunol 32: 227–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, and Nojima Y. 2005. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum 52: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 8.Xie Q, Huang C, and Li J. 2015. Interleukin-22 and rheumatoid arthritis: emerging role in pathogenesis and therapy. Autoimmunity 48: 69–72. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg GF, Fouser LA, and Artis D. 2011. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 12: 383–390. [DOI] [PubMed] [Google Scholar]
- 10.Lo BC, Shin SB, Canals Hernaez D, Refaeli I, Yu HB, Goebeler V, Cait A, Mohn WW, Vallance BA, and McNagny KM. 2019. IL-22 Preserves Gut Epithelial Integrity and Promotes Disease Remission during Chronic Salmonella Infection. J Immunol 202: 956–965. [DOI] [PubMed] [Google Scholar]
- 11.Keir M, Yi Y, Lu T, and Ghilardi N. 2020. The role of IL-22 in intestinal health and disease. J Exp Med 217: e20192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Culhane JF, Iams JD, and Romero R. 2008. Epidemiology and causes of preterm birth. Lancet 371: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, Kinney M, Lawn J, and Born G Too Soon Preterm Birth Action. 2013. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 10 Suppl 1: S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, Lewis C, Rattanakanokchai S, Teng DN, Thinkhamrop J, Watananirun K, Zhang J, Zhou W, and Gulmezoglu AM. 2019. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 7: e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, and Lockwood CJ. 2009. Pathogenesis of Spontaneous Preterm Labor. In Creasy and Resnik’s Maternal-Fetal Medicine: Principles and Practice, Sixth ed. Creasy RK, Resnik R, and Iams J, eds. Elsevier, Philadelphia. 521–543. [Google Scholar]
- 16.Muglia LJ, and Katz M. 2010. The enigma of spontaneous preterm birth. N Engl J Med 362: 529–535. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Dey SK, and Fisher SJ. 2014. Preterm labor: one syndrome, many causes. Science 345: 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barros FC, Papageorghiou AT, Victora CG, Noble JA, Pang R, Iams J, Cheikh Ismail L, Goldenberg RL, Lambert A, Kramer MS, Carvalho M, Conde-Agudelo A, Jaffer YA, Bertino E, Gravett MG, Altman DG, Ohuma EO, Purwar M, Frederick IO, Bhutta ZA, Kennedy SH, Villar J, International F, and Newborn C Growth Consortium for the 21st. 2015. The distribution of clinical phenotypes of preterm birth syndrome: implications for prevention. JAMA Pediatr 169: 220–229. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, and Hobbins JC. 1988. Infection in the pathogenesis of preterm labor. Semin Perinatol 12: 262–279. [PubMed] [Google Scholar]
- 20.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, and Novy MJ. 1994. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 171: 1660–1667. [DOI] [PubMed] [Google Scholar]
- 21.Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Adams Waldorf KM, and Rajagopal L. 2013. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med 210: 1265–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, Rael J, Grove T, Morgan TK, Clewell W, Miller H, Luthy D, Pereira L, Nageotte M, Robilio PA, Fortunato S, Simhan H, Baxter JK, Amon E, Franco A, Trofatter K, and Heyborne K. 2014. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 210: 125.e121–125.e115. [DOI] [PubMed] [Google Scholar]
- 23.McCartney SA, Kapur R, Liggitt HD, Baldessari A, Coleman M, Orvis A, Ogle J, Katz R, Rajagopal L, and Adams Waldorf KM. 2021. Amniotic fluid interleukin 6 and interleukin 8 are superior predictors of fetal lung injury compared with maternal or fetal plasma cytokines or placental histopathology in a nonhuman primate model. Am J Obstet Gynecol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, and Yeo L. 2014. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. Am J Reprod Immunol 71: 330–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, and Yeo L. 2014. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 72: 458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, and Kim YM. 2015. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 28: 1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, Hassan SS, Chaiworapongsa T, and Margolis L. 2015. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 213: 836 e831–836 e818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, Jacques SM, Panaitescu B, Garcia-Flores V, and Hassan SS. 2017. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci 24: 1139–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, Hassan SS, and Panaitescu B. 2017. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, Jacques SM, Hassan SS, Faro J, Alsamsam A, Alhousseini A, Gomez-Roberts H, Panaitescu B, Yeo L, and Maymon E. 2017. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 217: 693.e691–693.e616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Lopez N, Romero R, Leng Y, Xu Y, Slutsky R, Levenson D, Pacora P, Jung E, Panaitescu B, and Hsu CD. 2019. The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection. J Perinat Med 47: 822–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galaz J, Romero R, Xu Y, Miller D, Slutsky R, Levenson D, Hsu CD, and Gomez-Lopez N. 2020. Cellular immune responses in amniotic fluid of women with preterm clinical chorioamnionitis. Inflamm Res 69: 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Lopez N, Romero R, Varrey A, Leng Y, Miller D, Done B, Xu Y, Bhatti G, Motomura K, Gershater M, Pique-Regi R, and Tarca AL. 2021. RNA Sequencing Reveals Diverse Functions of Amniotic Fluid Neutrophils and Monocytes/Macrophages in Intra-Amniotic Infection. J Innate Immun 13: 63–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, and Hassan S. 2007. The role of inflammation and infection in preterm birth. Semin Reprod Med 25: 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Seong HS, Kim BJ, Jun JK, Romero R, and Yoon BH. 2009. Evidence to support that spontaneous preterm labor is adaptive in nature: neonatal RDS is more common in “indicated” than in “spontaneous” preterm birth. J Perinat Med 37: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi O, and Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140: 805–820. [DOI] [PubMed] [Google Scholar]
- 37.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, and Mor G. 2004. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 191: 1346–1355. [DOI] [PubMed] [Google Scholar]
- 38.Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, Espinoza J, Erez O, Nhan-Chang CL, Than NG, Vaisbuch E, and Hassan SS. 2008. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol 60: 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, and Hassan SS. 2008. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 21: 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, and Mor G. 2010. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol 185: 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ilievski V, and Hirsch E. 2010. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod 83: 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abrahams VM 2011. The role of the Nod-like receptor family in trophoblast innate immune responses. J Reprod Immunol 88: 112–117. [DOI] [PubMed] [Google Scholar]
- 43.Romero R, Chaiworapongsa T, Alpay Savasan Z, Xu Y, Hussein Y, Dong Z, Kusanovic JP, Kim CJ, and Hassan SS. 2011. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 24: 1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lappas M 2013. NOD1 and NOD2 regulate proinflammatory and prolabor mediators in human fetal membranes and myometrium via nuclear factor-kappa B. Biol Reprod 89: 14. [DOI] [PubMed] [Google Scholar]
- 45.Jaiswal MK, Agrawal V, Mallers T, Gilman-Sachs A, Hirsch E, and Beaman KD. 2013. Regulation of apoptosis and innate immune stimuli in inflammation-induced preterm labor. J Immunol 191: 5702–5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koga K, Izumi G, Mor G, Fujii T, and Osuga Y. 2014. Toll-like receptors at the maternal-fetal interface in normal pregnancy and pregnancy complications. Am J Reprod Immunol 72: 192–205. [DOI] [PubMed] [Google Scholar]
- 47.Agrawal V, Jaiswal MK, Ilievski V, Beaman KD, Jilling T, and Hirsch E. 2014. Platelet-activating factor: a role in preterm delivery and an essential interaction with Toll-like receptor signaling in mice. Biol Reprod 91: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.St Louis D, Romero R, Plazyo O, Arenas-Hernandez M, Panaitescu B, Xu Y, Milovic T, Xu Z, Bhatti G, Mi QS, Drewlo S, Tarca AL, Hassan SS, and Gomez-Lopez N. 2016. Invariant NKT Cell Activation Induces Late Preterm Birth That Is Attenuated by Rosiglitazone. J Immunol 196: 1044–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, Garcia-Flores V, Hassan SS, Xu Z, Tarca AL, Drewlo S, and Gomez-Lopez N. 2016. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol 196: 2476–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arenas-Hernandez M, Romero R, St Louis D, Hassan SS, Kaye EB, and Gomez-Lopez N. 2016. An imbalance between innate and adaptive immune cells at the maternal-fetal interface occurs prior to endotoxin-induced preterm birth. Cell Mol Immunol 13: 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Schwenkel G, St Louis D, Hassan SS, and Mial TN. 2017. In vivo activation of invariant natural killer T cells induces systemic and local alterations in T-cell subsets prior to preterm birth. Clin Exp Immunol 189: 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musilova I, Andrys C, Krejsek J, Drahosova M, Zednikova B, Pliskova L, Zemlickova H, Jacobsson B, and Kacerovsky M. 2017. Amniotic fluid pentraxins: Potential early markers for identifying intra-amniotic inflammatory complications in preterm pre-labor rupture of membranes. Am J Reprod Immunol. [DOI] [PubMed] [Google Scholar]
- 53.Negishi Y, Shima Y, Takeshita T, and Takahashi H. 2017. Distribution of invariant natural killer T cells and dendritic cells in late pre-term birth without acute chorioamnionitis. Am J Reprod Immunol 77. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y, Romero R, Miller D, Silva P, Panaitescu B, Theis KR, Arif A, Hassan SS, and Gomez-Lopez N. 2018. Innate lymphoid cells at the human maternal-fetal interface in spontaneous preterm labor. Am J Reprod Immunol 79: e12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vince GS, Starkey PM, Jackson MC, Sargent IL, and Redman CW. 1990. Flow cytometric characterisation of cell populations in human pregnancy decidua and isolation of decidual macrophages. J Immunol Methods 132: 181–189. [DOI] [PubMed] [Google Scholar]
- 56.Vargas ML, Sántos JL, Ruiz C, Montes MJ, Alemán P, García-Tortosa C, and García-Olivares E. 1993. Comparison of the proportions of leukocytes in early and term human decidua. Am J Reprod Immunol 29: 135–140. [DOI] [PubMed] [Google Scholar]
- 57.Sindram-Trujillo A, Scherjon S, Kanhai H, Roelen D, and Claas F. 2003. Increased T-cell activation in decidua parietalis compared to decidua basalis in uncomplicated human term pregnancy. Am J Reprod Immunol 49: 261–268. [DOI] [PubMed] [Google Scholar]
- 58.Sindram-Trujillo AP, Scherjon SA, van Hulst-van Miert PP, Kanhai HH, Roelen DL, and Claas FH. 2004. Comparison of decidual leukocytes following spontaneous vaginal delivery and elective cesarean section in uncomplicated human term pregnancy. J Reprod Immunol 62: 125–137. [DOI] [PubMed] [Google Scholar]
- 59.Tilburgs T, Roelen DL, van der Mast BJ, van Schip JJ, Kleijburg C, de Groot-Swings GM, Kanhai HH, Claas FH, and Scherjon SA. 2006. Differential distribution of CD4(+)CD25(bright) and CD8(+)CD28(−) T-cells in decidua and maternal blood during human pregnancy. Placenta 27 Suppl A: S47–53. [DOI] [PubMed] [Google Scholar]
- 60.Taglauer ES, Trikhacheva AS, Slusser JG, and Petroff MG. 2008. Expression and function of PDCD1 at the human maternal-fetal interface. Biol Reprod 79: 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gomez-Lopez N, Vadillo-Perez L, Hernandez-Carbajal A, Godines-Enriquez M, Olson DM, and Vadillo-Ortega F. 2011. Specific inflammatory microenvironments in the zones of the fetal membranes at term delivery. Am J Obstet Gynecol 205: 235 e215–224. [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Lopez N, Vega-Sanchez R, Castillo-Castrejon M, Romero R, Cubeiro-Arreola K, and Vadillo-Ortega F. 2013. Evidence for a role for the adaptive immune response in human term parturition. Am J Reprod Immunol 69: 212–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gomez-Lopez N, Estrada-Gutierrez G, Jimenez-Zamudio L, Vega-Sanchez R, and Vadillo-Ortega F. 2009. Fetal membranes exhibit selective leukocyte chemotaxic activity during human labor. J Reprod Immunol 80: 122–131. [DOI] [PubMed] [Google Scholar]
- 64.Gomez-Lopez N, Olson DM, and Robertson SA. 2016. Interleukin-6 controls uterine Th9 cells and CD8(+) T regulatory cells to accelerate parturition in mice. Immunol Cell Biol 94: 79–89. [DOI] [PubMed] [Google Scholar]
- 65.Tarca AL, Romero R, Xu Z, Gomez-Lopez N, Erez O, Hsu CD, Hassan SS, and Carey VJ. 2019. Targeted expression profiling by RNA-Seq improves detection of cellular dynamics during pregnancy and identifies a role for T cells in term parturition. Sci Rep 9: 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arenas-Hernandez M, Romero R, Xu Y, Panaitescu B, Garcia-Flores V, Miller D, Ahn H, Done B, Hassan SS, Hsu CD, Tarca AL, Sanchez-Torres C, and Gomez-Lopez N. 2019. Effector and Activated T Cells Induce Preterm Labor and Birth That Is Prevented by Treatment with Progesterone. J Immunol 202: 2585–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miller D, Gershater M, Slutsky R, Romero R, and Gomez-Lopez N. 2020. Maternal and fetal T cells in term pregnancy and preterm labor. Cell Mol Immunol 17: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pique-Regi R, Romero R, Tarca AL, Sendler ED, Xu Y, Garcia-Flores V, Leng Y, Luca F, Hassan SS, and Gomez-Lopez N. 2019. Single cell transcriptional signatures of the human placenta in term and preterm parturition. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Romero R, Quintero R, and Brekus C. 1991. Assessment of gestational age. In Abnormal Fetal Growth. Divon MY, ed. Elsevier Science Publishing, New York, NY. 47–65. [Google Scholar]
- 70.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, and Hobbins JC. 1991. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol 165: 821–830. [DOI] [PubMed] [Google Scholar]
- 71.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, and Diamond MP. 1990. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol 163: 968–974. [DOI] [PubMed] [Google Scholar]
- 72.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, and Edberg S. 1988. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol 159: 114–119. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, and Hobbins JC. 1989. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 161: 817–824. [DOI] [PubMed] [Google Scholar]
- 74.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, and Jun JK. 2001. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 185: 1130–1136. [DOI] [PubMed] [Google Scholar]
- 75.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, Ahmed AI, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, and Kim YM. 2015. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 28: 1343–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, Bhatti G, Kim JS, Qureshi F, Jacques SM, Jung EJ, Yeo L, Panaitescu B, Maymon E, Hassan SS, Hsu CD, and Erez O. 2018. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. J Perinat Med 46: 613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Redline RW 2006. Inflammatory responses in the placenta and umbilical cord. Semin Fetal Neonatal Med 11: 296–301. [DOI] [PubMed] [Google Scholar]
- 78.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, and Kim YM. 2015. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 213: S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redline RW 2015. Classification of placental lesions. Am J Obstet Gynecol 213: S21–28. [DOI] [PubMed] [Google Scholar]
- 80.Xu Y, Plazyo O, Romero R, Hassan SS, and Gomez-Lopez N. 2015. Isolation of Leukocytes from the Human Maternal-fetal Interface. J Vis Exp: e52863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bray NL, Pimentel H, Melsted P, and Pachter L. 2016. Near-optimal probabilistic RNA-seq quantification. Nat Biotechnol 34: 525–527. [DOI] [PubMed] [Google Scholar]
- 82.Melsted P, Booeshaghi AS, Gao F, Beltrame E, Lu L, Hjorleifsson KE, Gehring J, and Pachter L. 2019. Modular and efficient pre-processing of single-cell RNA-seq. bioRxiv: 673285. [Google Scholar]
- 83.Alvarez M, Rahmani E, Jew B, Garske KM, Miao Z, Benhammou JN, Ye CJ, Pisegna JR, Pietiläinen KH, Halperin E, and Pajukanta P. 2019. Enhancing droplet-based single-nucleus RNA-seq resolution using the semi-supervised machine learning classifier DIEM. bioRxiv: 786285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hafemeister C, and Satija R. 2019. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 20: 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM 3rd, Hao Y, Stoeckius M, Smibert P, and Satija R. 2019. Comprehensive Integration of Single-Cell Data. Cell 177: 1888–1902 e1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korsunsky I, Millard N, Fan J, Slowikowski K, Zhang F, Wei K, Baglaenko Y, Brenner M, Loh PR, and Raychaudhuri S. 2019. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat Methods 16: 1289–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wickham H 2011. ggplot2. WIREs Computational Statistics 3: 180–185. [Google Scholar]
- 88.Booeshaghi AS, and Pachter L. 2021. Normalization of single-cell RNA-seq counts by log(x + 1)* or log(1 + x). Bioinformatics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gomez-Lopez N, Romero R, Arenas-Hernandez M, Ahn H, Panaitescu B, Vadillo-Ortega F, Sanchez-Torres C, Salisbury KS, and Hassan SS. 2016. In vivo T-cell activation by a monoclonal alphaCD3epsilon antibody induces preterm labor and birth. Am J Reprod Immunol 76: 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Garcia-Flores V, Romero R, Miller D, Xu Y, Done B, Veerapaneni C, Leng Y, Arenas-Hernandez M, Khan N, Panaitescu B, Hassan SS, Alvarez-Salas LM, and Gomez-Lopez N. 2018. Inflammation-Induced Adverse Pregnancy and Neonatal Outcomes Can Be Improved by the Immunomodulatory Peptide Exendin-4. Front Immunol 9: 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bersani I, De Carolis MP, Foell D, Weinhage T, Rossi ED, De Carolis S, Rubortone SA, Romagnoli C, and Speer CP. 2015. Interleukin-22: biomarker of maternal and fetal inflammation? Immunol Res 61: 4–10. [DOI] [PubMed] [Google Scholar]
- 92.Motomura K, Romero R, Xu Y, Theis KR, Galaz J, Winters AD, Slutsky R, Garcia-Flores V, Zou C, Levenson D, Para R, Ahmad MM, Miller D, Hsu CD, and Gomez-Lopez N. 2020. Intra-Amniotic Infection with Ureaplasma parvum Causes Preterm Birth and Neonatal Mortality That Are Prevented by Treatment with Clarithromycin. mBio 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, and Ahmed R. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4: 1191–1198. [DOI] [PubMed] [Google Scholar]
- 94.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, and Busch DH. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc Natl Acad Sci U S A 101: 5610–5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hara T, Jung LK, Bjorndahl JM, and Fu SM. 1986. Human T cell activation. III. Rapid induction of a phosphorylated 28 kD/32 kD disulfide-linked early activation antigen (EA 1) by 12-o-tetradecanoyl phorbol-13-acetate, mitogens, and antigens. J Exp Med 164: 1988–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Testi R, Phillips JH, and Lanier LL. 1989. T cell activation via Leu-23 (CD69). J Immunol 143: 1123–1128. [PubMed] [Google Scholar]
- 97.Male V, Hughes T, McClory S, Colucci F, Caligiuri MA, and Moffett A. 2010. Immature NK cells, capable of producing IL-22, are present in human uterine mucosa. J Immunol 185: 3913–3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vacca P, Vitale C, Montaldo E, Conte R, Cantoni C, Fulcheri E, Darretta V, Moretta L, and Mingari MC. 2011. CD34+ hematopoietic precursors are present in human decidua and differentiate into natural killer cells upon interaction with stromal cells. Proc Natl Acad Sci U S A 108: 2402–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vacca P, Montaldo E, Croxatto D, Loiacono F, Canegallo F, Venturini PL, Moretta L, and Mingari MC. 2015. Identification of diverse innate lymphoid cells in human decidua. Mucosal Immunol 8: 254–264. [DOI] [PubMed] [Google Scholar]
- 100.Doisne JM, Balmas E, Boulenouar S, Gaynor LM, Kieckbusch J, Gardner L, Hawkes DA, Barbara CF, Sharkey AM, Brady HJ, Brosens JJ, Moffett A, and Colucci F. 2015. Composition, Development, and Function of Uterine Innate Lymphoid Cells. J Immunol 195: 3937–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Filipovic I, Chiossone L, Vacca P, Hamilton RS, Ingegnere T, Doisne JM, Hawkes DA, Mingari MC, Sharkey AM, Moretta L, and Colucci F. 2018. Molecular definition of group 1 innate lymphoid cells in the mouse uterus. Nat Commun 9: 4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomez-Lopez N, Romero R, Leng Y, Garcia-Flores V, Xu Y, Miller D, and Hassan SS. 2017. Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, Silva P, Faro J, Alhousseini A, Gill N, Hassan SS, and Hsu CD. 2018. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol 79: e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gomez-Lopez N, Romero R, Galaz J, Xu Y, Panaitescu B, Slutsky R, Motomura K, Gill N, Para R, Pacora P, Jung E, and Hsu CD. 2019. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am J Reprod Immunol 82: e13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cai G, Nie X, Zhang W, Wu B, Lin J, Wang H, Jiang C, and Shen Q. 2012. A regulatory role for IL-10 receptor signaling in development and B cell help of T follicular helper cells in mice. J Immunol 189: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 106.Shouval DS, Ouahed J, Biswas A, Goettel JA, Horwitz BH, Klein C, Muise AM, and Snapper SB. 2014. Interleukin 10 receptor signaling: master regulator of intestinal mucosal homeostasis in mice and humans. Adv Immunol 122: 177–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, Mascanfroni ID, Al Adham Z, Lavoie S, Ibourk M, Nguyen DD, Samsom JN, Escher JC, Somech R, Weiss B, Beier R, Conklin LS, Ebens CL, Santos FG, Ferreira AR, Sherlock M, Bhan AK, Muller W, Mora JR, Quintana FJ, Klein C, Muise AM, Horwitz BH, and Snapper SB. 2014. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity 40: 706–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weber GF, Schlautkötter S, Kaiser-Moore S, Altmayr F, Holzmann B, and Weighardt H. 2007. Inhibition of interleukin-22 attenuates bacterial load and organ failure during acute polymicrobial sepsis. Infect Immun 75: 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bingold TM, Ziesché E, Scheller B, Sadik CD, Franck K, Just L, Sartorius S, Wahrmann M, Wissing H, Zwissler B, Pfeilschifter J, and Mühl H. 2010. Interleukin-22 detected in patients with abdominal sepsis. Shock 34: 337–340. [DOI] [PubMed] [Google Scholar]
- 110.Aziz M, Jacob A, Yang WL, Matsuda A, and Wang P. 2013. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol 93: 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mihi B, Gong Q, Nolan LS, Gale SE, Goree M, Hu E, Lanik WE, Rimer JM, Liu V, Parks OB, Lewis AN, Agrawal P, Laury ML, Kumar P, Huang E, Bidani SS, Luke CJ, Kolls JK, and Good M. 2021. Interleukin-22 signaling attenuates necrotizing enterocolitis by promoting epithelial cell regeneration. Cell Rep Med 2: 100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leyva-Castillo JM, Yoon J, and Geha RS. 2019. IL-22 promotes allergic airway inflammation in epicutaneously sensitized mice. J Allergy Clin Immunol 143: 619–630 e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Starkey MR, Plank MW, Casolari P, Papi A, Pavlidis S, Guo Y, Cameron GJM, Haw TJ, Tam A, Obiedat M, Donovan C, Hansbro NG, Nguyen DH, Nair PM, Kim RY, Horvat JC, Kaiko GE, Durum SK, Wark PA, Sin DD, Caramori G, Adcock IM, Foster PS, and Hansbro PM. 2019. IL-22 and its receptors are increased in human and experimental COPD and contribute to pathogenesis. Eur Respir J 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Colin AA, McEvoy C, and Castile RG. 2010. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics 126: 115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, Kennedy KA, Poindexter BB, Finer NN, Ehrenkranz RA, Duara S, Sanchez PJ, O’Shea TM, Goldberg RN, Van Meurs KP, Faix RG, Phelps DL, Frantz ID 3rd, Watterberg KL, Saha S, Das A, Higgins RD, Eunice H Kennedy Shriver National Institute of Child, and N. Human Development Neonatal Research. 2010. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 126: 443–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Whitsett JA, Wert SE, and Weaver TE. 2015. Diseases of pulmonary surfactant homeostasis. Annu Rev Pathol 10: 371–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, and Calfee CS. 2019. Acute respiratory distress syndrome. Nat Rev Dis Primers 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cekmez Y, Cekmez F, Ozkaya E, Pirgon O, Yilmaz Z, Yilmaz EA, Kaya G, Suer N, and Kucukozkan T. 2013. uPAR, IL-33, and ST2 values as a predictor of subclinical chorioamnionitis in preterm premature rupture of membranes. J Interferon Cytokine Res 33: 778–782. [DOI] [PubMed] [Google Scholar]
- 119.Bhatti G, Romero R, Rice GE, Fitzgerald W, Pacora P, Gomez-Lopez N, Kavdia M, Tarca AL, and Margolis L. 2020. Compartmentalized profiling of amniotic fluid cytokines in women with preterm labor. PLoS One 15: e0227881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Erez O, Romero R, Tarca AL, Chaiworapongsa T, Kim YM, Than NG, Vaisbuch E, Draghici S, and Tromp G. 2009. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 22: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Varrey A, Romero R, Panaitescu B, Miller D, Chaiworapongsa T, Patwardhan M, Faro J, Pacora P, Hassan SS, Hsu CD, and Gomez-Lopez N. 2018. Human beta-defensin-1: A natural antimicrobial peptide present in amniotic fluid that is increased in spontaneous preterm labor with intra-amniotic infection. Am J Reprod Immunol 80: e13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yoon BH, Chang JW, and Romero R. 1998. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol 92: 77–82. [DOI] [PubMed] [Google Scholar]
- 123.DiGiulio DB, Romero R, Kusanovic JP, Gómez R, Kim CJ, Seok KS, Gotsch F, Mazaki-Tovi S, Vaisbuch E, Sanders K, Bik EM, Chaiworapongsa T, Oyarzún E, and Relman DA. 2010. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 64: 38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, Gotsch F, Dong Z, Ahmed AI, Shaman M, Lannaman K, Yoon BH, Hassan SS, Kim CJ, Korzeniewski SJ, Yeo L, and Kim YM. 2015. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 43: 19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rittenschober-Bohm J, Waldhoer T, Schulz SM, Pimpel B, Goeral K, Kasper DC, Witt A, and Berger A. 2019. Vaginal Ureaplasma parvum serovars and spontaneous preterm birth. Am J Obstet Gynecol 220: 594 e591–594 e599. [DOI] [PubMed] [Google Scholar]
- 126.Romero R, Avila C, Santhanam U, and Sehgal PB. 1990. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 85: 1392–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, Chaiyasit N, Yeo L, Shaman M, Lannaman K, Cher B, Hassan SS, and Gomez-Lopez N. 2017. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med 45: 523–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Galaz J, Romero R, Slutsky R, Xu Y, Motomura K, Para R, Pacora P, Panaitescu B, Hsu CD, Kacerovsky M, and Gomez-Lopez N. 2020. Cellular immune responses in amniotic fluid of women with preterm prelabor rupture of membranes. J Perinat Med 48: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, Hassan SS, Chaiworapongsa T, and Yeo L. 2016. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med 29: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, and Berry SM. 1998. The fetal inflammatory response syndrome. Am J Obstet Gynecol 179: 194–202. [DOI] [PubMed] [Google Scholar]
- 131.Jung E, Romero R, Yeo L, Diaz-Primera R, Marin-Concha J, Para R, Lopez AM, Pacora P, Gomez-Lopez N, Yoon BH, Kim CJ, Berry SM, and Hsu CD. 2020. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin Fetal Neonatal Med 25: 101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Para R, Romero R, Miller D, Galaz J, Done B, Peyvandipour A, Gershater M, Tao L, Motomura K, Ruden DM, Isherwood J, Jung E, Kanninen T, Pique-Regi R, Tarca AL, and Gomez-Lopez N. 2021. The Distinct Immune Nature of the Fetal Inflammatory Response Syndrome Type I and Type II. Immunohorizons 5: 735–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang SC, Li YH, Piao HL, Hong XW, Zhang D, Xu YY, Tao Y, Wang Y, Yuan MM, Li DJ, and Du MR. 2015. PD-1 and Tim-3 pathways are associated with regulatory CD8+ T-cell function in decidua and maintenance of normal pregnancy. Cell Death Dis 6: e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lissauer D, Kilby MD, and Moss P. 2017. Maternal effector T cells within decidua: The adaptive immune response to pregnancy? Placenta 60: 140–144. [DOI] [PubMed] [Google Scholar]
- 135.Powell RM, Lissauer D, Tamblyn J, Beggs A, Cox P, Moss P, and Kilby MD. 2017. Decidual T Cells Exhibit a Highly Differentiated Phenotype and Demonstrate Potential Fetal Specificity and a Strong Transcriptional Response to IFN. J Immunol 199: 3406–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van der Zwan A, Bi K, Norwitz ER, Crespo  C, Claas FHJ, Strominger JL, and Tilburgs T. 2018. Mixed signature of activation and dysfunction allows human decidual CD8(+) T cells to provide both tolerance and immunity. Proc Natl Acad Sci U S A 115: 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Slutsky R, Romero R, Xu Y, Galaz J, Miller D, Done B, Tarca AL, Gregor S, Hassan SS, Leng Y, and Gomez-Lopez N. 2019. Exhausted and Senescent T Cells at the Maternal-Fetal Interface in Preterm and Term Labor. J Immunol Res 2019: 3128010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Terzieva A, Dimitrova V, Djerov L, Dimitrova P, Zapryanova S, Hristova I, Vangelov I, and Dimova T. 2019. Early Pregnancy Human Decidua is Enriched with Activated, Fully Differentiated and Pro-Inflammatory Gamma/Delta T Cells with Diverse TCR Repertoires. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pinget GV, Corpuz TM, Stolp J, Lousberg EL, Diener KR, Robertson SA, Sprent J, and Webster KE. 2016. The majority of murine γδ T cells at the maternal-fetal interface in pregnancy produce IL-17. Immunol Cell Biol 94: 623–630. [DOI] [PubMed] [Google Scholar]
- 140.Furcron AE, Romero R, Mial TN, Balancio A, Panaitescu B, Hassan SS, Sahi A, Nord C, and Gomez-Lopez N. 2016. Human Chorionic Gonadotropin Has Anti-Inflammatory Effects at the Maternal-Fetal Interface and Prevents Endotoxin-Induced Preterm Birth, but Causes Dystocia and Fetal Compromise in Mice. Biol Reprod 94: 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Levenson D, Romero R, Garcia-Flores V, Miller D, Xu Y, Sahi A, Hassan SS, and Gomez-Lopez N. 2020. The effects of advanced maternal age on T-cell subsets at the maternal-fetal interface prior to term labor and in the offspring: a mouse study. Clin Exp Immunol 201: 58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gomez-Lopez N, Guilbert LJ, and Olson DM. 2010. Invasion of the leukocytes into the fetal-maternal interface during pregnancy. J Leukoc Biol 88: 625–633. [DOI] [PubMed] [Google Scholar]
- 143.Erlebacher A 2013. Immunology of the maternal-fetal interface. Annu Rev Immunol 31: 387–411. [DOI] [PubMed] [Google Scholar]
- 144.Gomez-Lopez N, StLouis D, Lehr MA, Sanchez-Rodriguez EN, and Arenas-Hernandez M. 2014. Immune cells in term and preterm labor. Cell Mol Immunol 11: 571–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bonney EA 2016. Immune Regulation in Pregnancy: A Matter of Perspective? Obstet Gynecol Clin North Am 43: 679–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bonney EA, and Johnson MR. 2019. The role of maternal T cell and macrophage activation in preterm birth: Cause or consequence? Placenta 79: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, and Erlebacher A. 2012. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science 336: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Griffith OW, Chavan AR, Protopapas S, Maziarz J, Romero R, and Wagner GP. 2017. Embryo implantation evolved from an ancestral inflammatory attachment reaction. Proc Natl Acad Sci U S A 114: E6566–e6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chavan AR, Griffith OW, Stadtmauer DJ, Maziarz J, Pavlicev M, Fishman R, Koren L, Romero R, and Wagner GP. 2021. Evolution of Embryo Implantation Was Enabled by the Origin of Decidual Stromal Cells in Eutherian Mammals. Mol Biol Evol 38: 1060–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aluvihare VR, Kallikourdis M, and Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 5: 266–271. [DOI] [PubMed] [Google Scholar]
- 151.Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, and Saito S. 2004. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod 10: 347–353. [DOI] [PubMed] [Google Scholar]
- 152.Rowe JH, Ertelt JM, Xin L, and Way SS. 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, and Rudensky AY. 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robertson SA, Care AS, and Moldenhauer LM. 2018. Regulatory T cells in embryo implantation and the immune response to pregnancy. J Clin Invest 128: 4224–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zenclussen AC, Gerlof K, Zenclussen ML, Sollwedel A, Bertoja AZ, Ritter T, Kotsch K, Leber J, and Volk HD. 2005. Abnormal T-cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy-induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol 166: 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Darrasse-Jèze G, Klatzmann D, Charlotte F, Salomon BL, and Cohen JL. 2006. CD4+CD25+ regulatory/suppressor T cells prevent allogeneic fetus rejection in mice. Immunol Lett 102: 106–109. [DOI] [PubMed] [Google Scholar]
- 157.Kahn DA, and Baltimore D. 2010. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc Natl Acad Sci U S A 107: 9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, and Way SS. 2011. Foxp3(+) regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen T, Darrasse-Jèze G, Bergot AS, Courau T, Churlaud G, Valdivia K, Strominger JL, Ruocco MG, Chaouat G, and Klatzmann D. 2013. Self-specific memory regulatory T cells protect embryos at implantation in mice. J Immunol 191: 2273–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gomez-Lopez N, Arenas-Hernandez M, Romero R, Miller D, Garcia-Flores V, Leng Y, Xu Y, Galaz J, Hassan SS, Hsu CD, Tse H, Sanchez-Torres C, Done B, and Tarca AL. 2020. Regulatory T Cells Play a Role in a Subset of Idiopathic Preterm Labor/Birth and Adverse Neonatal Outcomes. Cell Rep 32: 107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, and Kim JS. 2010. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol 23: 1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim CJ, Romero R, Chaemsaithong P, and Kim JS. 2015. Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 213: S53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, and Guttman-Yassky E. 2009. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 123: 1244–1252 e1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu Y, Yang B, Zhou M, Li L, Zhou H, Zhang J, Chen H, and Wu C. 2009. Memory IL-22-producing CD4+ T cells specific for Candida albicans are present in humans. Eur J Immunol 39: 1472–1479. [DOI] [PubMed] [Google Scholar]
- 165.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, and Sallusto F. 2009. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10: 857–863. [DOI] [PubMed] [Google Scholar]
- 166.Trifari S, Kaplan CD, Tran EH, Crellin NK, and Spits H. 2009. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol 10: 864–871. [DOI] [PubMed] [Google Scholar]
- 167.Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, and Cavani A. 2009. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119: 3573–3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Plank MW, Kaiko GE, Maltby S, Weaver J, Tay HL, Shen W, Wilson MS, Durum SK, and Foster PS. 2017. Th22 Cells Form a Distinct Th Lineage from Th17 Cells In Vitro with Unique Transcriptional Properties and Tbet-Dependent Th1 Plasticity. J Immunol 198: 2182–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lin X, Tawch S, Wong HT, Roy S, Gaudino S, Castillo P, Elsegeiny W, Wakabayashi N, Oury TD, Pociask D, Chen K, McLinskey N, Melville P, Syritsyna O, Coyle P, Good M, Awasthi A, Kolls JK, and Kumar P. 2021. Nrf2 through Aryl Hydrocarbon Receptor Regulates IL-22 Response in CD4(+) T Cells. J Immunol 206: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Logiodice F, Lombardelli L, Kullolli O, Haller H, Maggi E, Rukavina D, and Piccinni MP. 2019. Decidual Interleukin-22-Producing CD4+ T Cells (Th17/Th0/IL-22+ and Th17/Th2/IL-22+, Th2/IL-22+, Th0/IL-22+), Which Also Produce IL-4, Are Involved in the Success of Pregnancy. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wolk K, Witte E, Hoffmann U, Doecke WD, Endesfelder S, Asadullah K, Sterry W, Volk HD, Wittig BM, and Sabat R. 2007. IL-22 induces lipopolysaccharide-binding protein in hepatocytes: a potential systemic role of IL-22 in Crohn’s disease. J Immunol 178: 5973–5981. [DOI] [PubMed] [Google Scholar]
- 172.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, and Blauvelt A. 2010. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol 130: 1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Basu R, O’Quinn DB, Silberger DJ, Schoeb TR, Fouser L, Ouyang W, Hatton RD, and Weaver CT. 2012. Th22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37: 1061–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sakamoto K, Kim YG, Hara H, Kamada N, Caballero-Flores G, Tolosano E, Soares MP, Puente JL, Inohara N, and Nunez G. 2017. IL-22 Controls Iron-Dependent Nutritional Immunity Against Systemic Bacterial Infections. Sci Immunol 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Huggins MA, Sjaastad FV, Pierson M, Kucaba TA, Swanson W, Staley C, Weingarden AR, Jensen IJ, Danahy DB, Badovinac VP, Jameson SC, Vezys V, Masopust D, Khoruts A, Griffith TS, and Hamilton SE. 2019. Microbial Exposure Enhances Immunity to Pathogens Recognized by TLR2 but Increases Susceptibility to Cytokine Storm through TLR4 Sensitization. Cell Rep 28: 1729–1743 e1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang Z, Liu H, Shi Y, Xu N, Wang Y, Li A, and Song W. 2017. Increased circulating Th22 cells correlated with Th17 cells in patients with severe preeclampsia. Hypertens Pregnancy 36: 100–107. [DOI] [PubMed] [Google Scholar]
- 177.Chappell LC, Cluver CA, Kingdom J, and Tong S. 2021. Pre-eclampsia. Lancet 398: 341–354. [DOI] [PubMed] [Google Scholar]
- 178.Reisenberger K, Egarter C, Vogl S, Sternberger B, Kiss H, and Husslein P. 1996. The transfer of interleukin-8 across the human placenta perfused in vitro. Obstet Gynecol 87: 613–616. [DOI] [PubMed] [Google Scholar]
- 179.Ponzio NM, Servatius R, Beck K, Marzouk A, and Kreider T. 2007. Cytokine levels during pregnancy influence immunological profiles and neurobehavioral patterns of the offspring. Ann N Y Acad Sci 1107: 118–128. [DOI] [PubMed] [Google Scholar]
- 180.Zaretsky MV, Alexander JM, Byrd W, and Bawdon RE. 2004. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol 103: 546–550. [DOI] [PubMed] [Google Scholar]
- 181.Dahlgren J, Samuelsson AM, Jansson T, and Holmang A. 2006. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res 60: 147–151. [DOI] [PubMed] [Google Scholar]
- 182.Xu W, Presnell SR, Parrish-Novak J, Kindsvogel W, Jaspers S, Chen Z, Dillon SR, Gao Z, Gilbert T, Madden K, Schlutsmeyer S, Yao L, Whitmore TE, Chandrasekher Y, Grant FJ, Maurer M, Jelinek L, Storey H, Brender T, Hammond A, Topouzis S, Clegg CH, and Foster DC. 2001. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci U S A 98: 9511–9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sabat R, Ouyang W, and Wolk K. 2014. Therapeutic opportunities of the IL-22-IL-22R1 system. Nat Rev Drug Discov 13: 21–38. [DOI] [PubMed] [Google Scholar]
- 184.De Luca A, Zelante T, D’Angelo C, Zagarella S, Fallarino F, Spreca A, Iannitti RG, Bonifazi P, Renauld JC, Bistoni F, Puccetti P, and Romani L. 2010. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol 3: 361–373. [DOI] [PubMed] [Google Scholar]
- 185.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, and Meager A. 2010. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med 207: 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tripathi D, Radhakrishnan RK, Thandi RS, Paidipally P, Devalraju KP, Neela VSK, Tvinnereim AR, Valluri VL, and Vankayalapati R. 2019. IL-22 reduces the mortality of type 2 diabetes mellitus (T2DM) mice infected with Mycobacterium tuberculosis. The Journal of Immunology 202: 181.119–181.119. [Google Scholar]
- 187.Nadeem A, Deshmukh H, Gray J, and Wang T. 2020. Intestinal commensal bacteria promote AT2 self-renewal and AT1 differentiation in an IL-22 dependent fashion and prepare the newborn to fight potentially fatal respiratory pathogens. The Journal of Immunology 204: 225.230–225.230. [Google Scholar]
- 188.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, and Kolls JK. 2008. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med 14: 275–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, Abbas AR, Modrusan Z, Ghilardi N, de Sauvage FJ, and Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14: 282–289. [DOI] [PubMed] [Google Scholar]
- 190.Laurence A, O’Shea JJ, and Watford WT. 2008. Interleukin-22: a sheep in wolf’s clothing. Nat Med 14: 247–249. [DOI] [PubMed] [Google Scholar]
- 191.Dhiman R, Indramohan M, Barnes PF, Nayak RC, Paidipally P, Rao LV, and Vankayalapati R. 2009. IL-22 produced by human NK cells inhibits growth of Mycobacterium tuberculosis by enhancing phagolysosomal fusion. J Immunol 183: 6639–6645. [DOI] [PubMed] [Google Scholar]
- 192.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, Kim JS, Edwin SS, Gomez R, and Draghici S. 2010. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol 63: 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


