Abstract
Background
Air pollution may cause inflammatory and oxidative stress damage to the brain, leading to neurodegenerative disease. The association between air pollution and dementia, and modification by apolipoprotein E genotype 4 (APOE-ε4) has yet to be fully investigated.
Objectives
To examine associations of air pollution with three types of incident dementias (Alzheimer’s disease (AD), frontotemporal dementia (FTD), and vascular dementia (VAD)), and their potential modification by APOE-ε4 genotype.
Methods
The UK Biobank enrolled >500,000 participants (2006–2010) with ongoing follow-up. We used annual averages of air pollution (PM2.5, PM10, PM2.5–10, PM2.5absorbance, NO2, NOX) for 2010 scaled to interquartile ranges (IQR). We included individuals aged ≥ 60 years, with no dementia diagnosis prior to January 1, 2010. Time to incident dementia and follow-up time were reported from baseline (01/01/2010) to last censor event (death, last hospitalization, or loss to follow-up). Cox proportional hazard ratios (HR) and 95% confidence intervals (95% CI) were calculated to estimate the association of air pollutants and incident dementia, and modification of these associations by APOE-ε4.
Results
Our sample included 187,194 individuals (including N= 680 AD, N=377 VAD, N= 63 FTD) with a mean follow-up of 7.04 years. We observed consistent associations of PM2.5 with greater risk of all-cause dementia (HR=1.17, 95% CI: 1.10, 1.24) and AD (HR=1.17, 95% CI: 1.06, 1.29). NO2 was also associated with greater risk of any incident dementia (HR=1.18, 95% CI: 1.10, 1.25), AD (HR=1.15, 95% CI: 1.04, 1.28) and VAD (HR=1.18, 95% CI: 1.03, 1.35). APOE-ε4 did not modify the association between any air pollutants and dementia.
Discussion
PM2.5 and NO2 levels were associated with several types of dementia, and these associations were not modified by APOE-ε4. Findings from the UK Biobank support and extend to other epidemiological evidence for the potential association of air pollutants with detrimental brain health during aging.
Keywords: Air pollution, Particulate matter, Alzheimer’s, Dementia, apolipoprotein E4, Nitrogen oxides
1. Introduction
Air pollution is a major public health concern that has been associated with a wide range of chronic health disorders including cardiovascular (Kaufman et al. 2016; Rajagopalan et al. 2018) and respiratory diseases (Cui et al. 2015; Kurt et al. 2016). Air pollution has been estimated to be responsible for 16% of disease mortality globally (Landrigan et al. 2018). Exposure to air pollution has also been implicated in the rise of dementia among the ageing population (Peters et al. 2019; Power et al. 2016). In 2010, dementia affected more than 35.6 million individuals aged ≥60 years or older worldwide, and the incidence is expected to double by the year 2030 (Prince et al. 2013).
Inhaled air pollutants may lead to an immune response that triggers the induction of several mechanisms including neuroinflammation, glial activation, neurodegeneration, oxidative stress, and vascular damage (Genc et al. 2012). In a study of mice exposed to chronic air pollution (where dosing mimicked human exposure to automobile traffic emissions), fine particulate matter led to brain structural atrophy among the younger and middle-aged mice, in a manner hypothesized to exacerbate the risk of Alzheimer’s disease (AD) (Woodward et al. 2017). From a study of middle-aged individuals with cognitive decline, increasing air pollution levels were correlated with biomarkers for AD, specifically, cerebrospinal fluid neurofilament light (NfL) and amyloid-β (Aβ) deposition in the brain (Alemany et al. 2021). Yet, in a different study that examined autopsy specimens of brain tissue, investigators found no association with long-term PM2.5 exposure and AD neurodegeneration (Shaffer et al. 2021a).
A few large-scale epidemiological studies have also suggested a strong association between air pollution from traffic emissions/fossil fuels and neurodegenerative disease (Carey et al. 2018; Chen et al. 2017b; Jung et al. 2015), including a cross-sectional analysis of occupational exposures in the UK Biobank that found an increased odds of dementia from exposure to PM2.5 (Dimakakou et al. 2020). In systematic reviews of the evidence provided to date that encompass a variety of methodologies and study designs, acute and chronic exposures to ambient air pollutants have been linked to an increasing risk of dementia among the ageing population (Peters et al. 2019; Power et al. 2016; Weuve et al. 2021). However, associations with different types of dementia, and particularly with AD, have not been explored fully (Andersson et al. 2018; Carey et al. 2018; Cerza et al. 2019, Jung et al. 2015; Kioumourtzoglou et al. 2016; Mortamais et al. 2021; Oudin et al. 2016; Oudin et al. 2019; Shaffer et al. 2021b; Wu et al. 2015; Younan et al. 2021), and potential for interactions with the apolipoprotein E (APOE) ε4 allele, a major genetic risk factor for AD, have been examined to a lesser extent.
To date, the majority of epidemiological research on environmental risk factors for neurodegenerative diseases have studied associations with cognitive function. Study findings from Calvin et al. showed that among a subsample of UK Biobank participants that were administered a selection of brief cognitive tests at baseline and followed for 3 to 8 years, cognitive performance successfully predicted incident dementia and AD (2019). In one such study of air pollution in the UK Biobank cohort, researchers reported weak effect sizes in inconsistent directions for every air pollutant (PM2.5, PM10, PM2.5–10, NO2, NOX) across the available cognitive tests (Cullen et al. 2018). In a 3-year follow-up period, no associations with cognitive decline were observed (Cullen et al. 2018). Other studies have reported an inverse relationship with air pollution across a range of cognitive performance measures (Clifford et al. 2016).
APOE-ε4 is the strongest genetic risk factor for late-onset dementia and AD (Corder et al. 1993; Liu et al. 2013). However, as a susceptibility gene, APOE-ε4 does not account for all cases, indicating that other genetic and environmental factors can increase an individual’s risk for dementia. For instance, in experimental studies using knockout (apoE−/−) mice, it was demonstrated that in spite of APOE deficiency, air pollution induced neurotoxicity (Veronesi et al. 2005). Exposure to O3 among older mice also led to memory decline in mice with APOE genotype 3 (apoE-ε3), but not apoE-ε4 (Jung et al. 2015). APOE-ε4 and air particulates may work on the same oxidative stress and inflammation pathways that may jointly induce risk for cognitive decline and dementia risk (Genc et al. 2012). There are few epidemiological studies on the putative effect of joint exposures to APOE-ε4 and air pollution on neurodegenerative diseases (Mortamais et al. 2021; Oudin et al. 2019; Shaffer et al. 2021b; Wu et al. 2015).
Our objective was to examine prospectively whether air pollution (including PM2.5, PM10, PM2.5–10, PM2.5 absorbance (PM2.5abs), NO2, NOX) is associated with incident diagnosis of Alzheimer’s disease (AD), vascular dementia (VAD), frontotemporal dementia (FTD) or all-cause dementia. We also investigated whether the APOE-ε4 haplotype modifies the association between air pollutants and dementia.
2. Materials and Methods
2.1. Study sample & setting
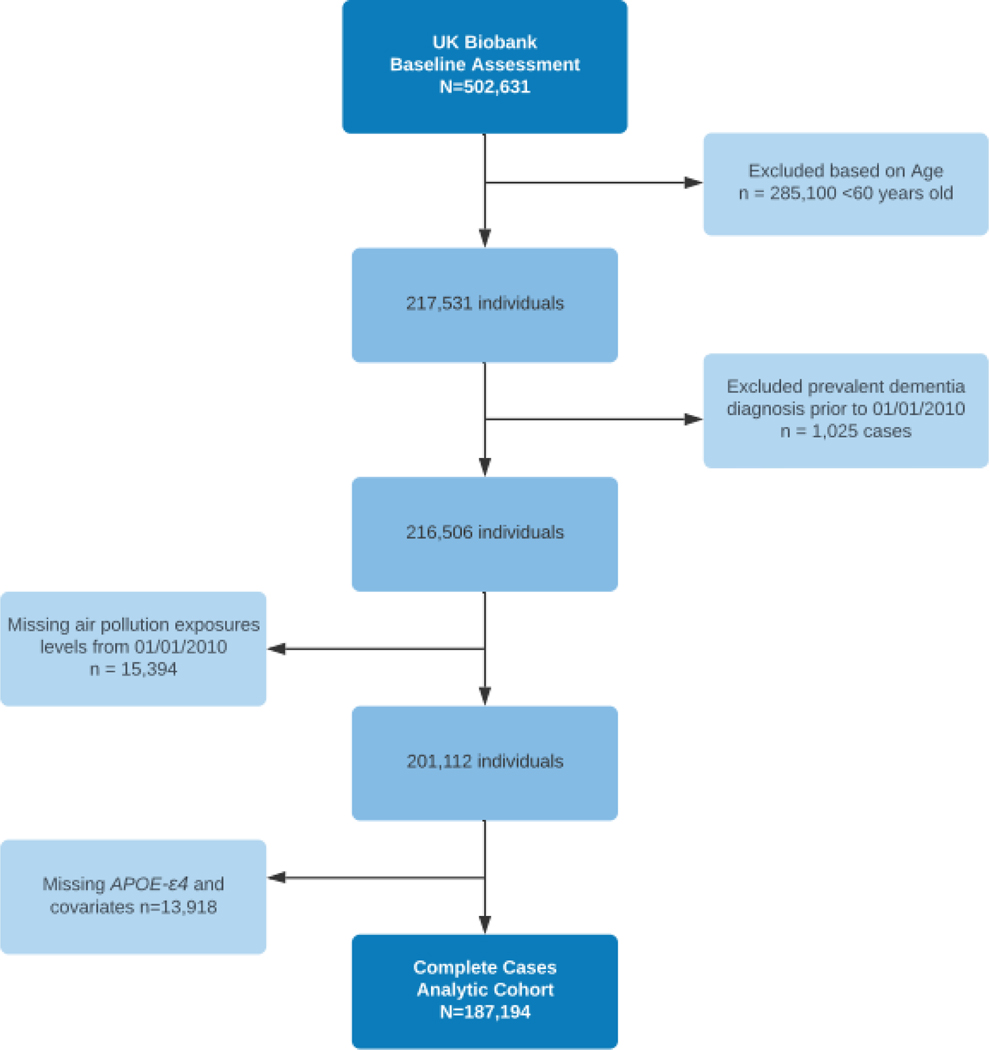
The data for this analysis was provided by the UK Biobank. Over 500,000 individuals from across the United Kingdom have participated in this population-based prospective cohort study. Between 2006 and 2010, individuals aged 40 to 69 years were recruited to participate in 22 research centers in England, Scotland, and Wales (Sudlow et al. 2015). Our study was restricted to individuals aged 60 years and older, at start of follow-up to exclude rare, early-onset of AD/dementia with autosomal dominant mutations, and with available air pollution measures, APOE-ε4 genotype, and without a dementia diagnosis prior to January 1, 2010. Exclusions due to any prevalent dementia diagnosis prior to January 1, 2010, were 1,025 cases, and an additional 29,312 individuals were excluded due to missing covariate and exposure data (Figure 1). All participants provided written informed consent. Ethical approval of the UK Biobank study was given by the North West Multicentre Research Ethics Committee, the National Information Governance Board for Health & Social Care, and the Community Health Index Advisory Group.
Figure 1:
Study Flow Chart for the complete case analysis of incident all-cause dementia, Alzheimer’s disease, vascular dementia, and frontotemporal dementia.
2.2. Exposures
Data on air pollution measures for PM2.5, PM10, PM2.5–10, PM2.5abs, NO2, NOX and traffic variables were provided by the Small Area Health Statistics Unit (http://www.sahsu.org/) as part of the BioSHaRE-EU Environmental Determinants of Health Project (http://www.bioshare.eu/) of which the UK Biobank is a collaborator. Air pollution estimates were calculated at the residential address level for each participant at the pre-determined/assigned follow-up date. Annual average concentrations in 2010 were derived from land-use regression (LUR) models developed for the European Study of Cohorts for Air Pollution Effects (ESCAPE) (https:/www.escapeproject.eu/) (Beelen et al. 2013; Eeftens et al. 2012). LUR models of 2010 air pollutants were completed by ESCAPE monitoring that was conducted from January 26, 2010 through January 18, 2011 to represent the year 2010. Air pollution monitors were placed in 20 areas for particulate matter and 40 areas for nitrogen dioxide/oxide across 20 sites in Europe including London/Oxford and Manchester. ESCAPE estimates for particulates are valid up to 400km from Greater London, but outside this area, the accuracy is unclear. Thus, in the UK Biobank, all addresses that are more than 400km away from Greater London have missing data for the PM measures (number of records: 33,935). Air pollutants were scaled to continuous interquartile ranges (IQR), and quartiles.
2.3. Outcomes
All participants in the UK Biobank consented to release of electronic health records (EHR) from the National Health Service (NHS) that were linked to the UK Biobank database. An algorithm has previously been developed to identify cases of dementia, including AD, VAD and FTD (Wilkinson et al. 2019). Data from the algorithm comes from participant self-reports from site interviews, and from record linkage of International Classification of Diseases codes (ICD 9/10) in death records and hospital admissions by EHR, which were classified as any individual admitted to a hospital who occupied a hospital bed, whether an overnight stay or day case. The algorithm was used to classify individuals as having any type of dementia prior to 2010. This algorithm also allows for a participant to have a diagnosis with more than one type of dementia. If both hospital admission and death register records have ICD 9/10, the earliest recorded code was used. When cases have both a self-report from the baseline visit and EHR code, this algorithm assigns the earliest of the two dates as the event date (UK Biobank 2018). Within the UK Biobank, all-cause dementia and its subtypes are exclusively algorithmically identified. In a previous study of this algorithm, positive predictive values (PPV) across all sources were found to be 82.5% for all-cause dementia, 71.4% for AD, and 43.8% for VAD. Due to the small number, Wilkinson et al. (2019) combined FTD cases were combined with the “other dementias” category that included Parkinson’s disease dementia and Dementia with Lewy bodies, which resulted in a PPV of 66.7%.
Time to incident dementia (including AD, VAD, and FTD), and follow-up time was reported from the initial start of follow-up (January 1, 2010) to censor event date (i.e. death, last hospitalization, or loss to follow-up). In this analysis, we used 2010 as the initial follow-up year because that is the year in which the air pollution measures are reported. To calculate follow-up time, the earliest date of death, hospitalization or loss to follow-up was used as the censor event. Last available records for hospital admissions dates varied by country and for England was March 31, 2017; October 31, 2016 for Scotland; and in Wales was February 29, 2016. The most recent data was made available and downloaded in September 2019. We used complete cases for this analysis and excluded all participants who had missing data for the covariates (including APOE-ε4), and/or exposure measures: PM2.5, PM10, PM2.5–10, PM2.5abs, NO2, NOX.
2.4. Genotyping
Genotyping was performed using one of two similar methods, Affymetrix UK BiLEVE Axiom array or the UK Biobank Axiom array (Affymetrix Inc., Santa Clara, CA). More details regarding genotyping can be found elsewhere (Bycroft et al. 2017). We categorized individuals into three different APOE haplotypes based on the rs429358 and rs7412 SNPs. Individuals with CC homozygous genotypes at both of these SNPs were classified as homozygous for the ε4 allele. Those with homozygous CC genotypes at only one of the SNPs and heterozygous at the other were classified as ε4 heterozygous. Approximately 10,000 individuals were excluded since they were heterozygous at both SNPs and could thus not be assigned a haplotype status. An additive model was assumed and APOE genotype was coded as “0” for those with zero ε4 alleles, “1” for those with one ε4 allele, and “2” for those with two ε4 alleles.
2.5. Covariates
Several covariates considered for adjustment were selected a priori using directed acyclic graphs (DAGs) to ensure that confounding pathways were blocked or resolved, and to mitigate possible bias from mediators and colliders (Greenland, 1999) (Supplementary Figure S2). Potential adjustment was considered for the variables: age, sex, APOE-ε4, BMI, household income, Townsend Deprivation Index, college degree, smoking, and urban/rural residence. Income was the total household income before tax. Income was ordinally categorized as less than £18,000, £18,000 to £51,999, and greater than £52,000. College was defined as yes or no to having a university degree or other professional degree. Smoking status was categorized as current, never, and past smokers. To control for participants living in various regions at the start of follow-up, urban residence was dichotomized into yes or no, based on home area population density. Urban designation was ascribed to postcodes that fall within areas of 10,000 or more individuals in England and Wales, and ≥3,000 in Scotland. We categorized research centers according to city/country. In our final model we included age (years) at start of follow-up sex, household income, college education, smoking status, urban residence, research center site, and APOE-ε4. Based on this fully adjusted model, cardiovascular risk factors (coded as self-reported medical diagnosis of diabetes, hypertension, and stroke/angina/heart attack) were added to estimate the main effect for dementia.
2.6. Statistical analysis
Cox proportional hazard ratios (HR) and 95% Confidence Intervals (CIs) were calculated to estimate the associations of air pollutants PM2.5, PM2.5–10, PM2.5abs, PM10, NO2, NOX, with risk of dementia in single pollutant models. Separate models were developed for each possible pair of dementia outcome and air pollutant. For all air pollutants, we evaluated associations with IQRs and with quartiles. Models were tested by using Schoenfeld residuals and log-log plots to ensure that the proportional hazards model assumptions were met; and no violations were detected (p>0.05). Influential observations were checked using deviance residuals. Deviance residuals revealed that air pollution outliers (high leverage) represented <0.016% of observations. To assess the joint effects of APOE-ε4 status and each air pollutant, we assessed the interaction between APOE-ε4 and pollutants independently for each model. The likelihood ratio test was used to assess all interactions with an alpha threshold of p<0.05.
We conducted several sensitivity analyses. These included evaluating the interaction between air pollution and sex, and a three-way interaction between APOE-ε4 status, sex, and pollution. We also restricted participants to only those that identified as British/Irish white ethnicity, to account for the possibility of genetic heterogeneity. We also changed our censor date to exclude those who died before hospitalization. In a subgroup analysis we examined all individuals with a EHR diagnosis of FTD and did not impose an age restriction of >60 years, as it is accepted that FTD cases may have an earlier age at-onset. We also used participant’s date of enrollment (versus initial follow-update: January 1, 2010) to observe whether risk of dementia changed. An additional analysis excluded participants who developed any type of dementia within three years of the initial follow-up date. Lastly, we restricted our analysis to participants who lived less than 3 years in their reported baseline address to evaluate if estimates remained the same after excluding individuals who moved.
To address missing data, we conducted multiple imputation of covariates in our survival models as recommended by White et al. (2011). We assumed that data was missing at random (MAR) and used multiple imputation by chained equations “mice package” in R to generate 5 datasets and 5 iterations to obtain a pooled dataset according to Rubin’s Rule (van Buuren and Groothuis-Oudshoorn 2011; Rubin 2004). All statistical analyses were performed using R Version 3.5.2 (R CoreTeam 2020).
3. Results
3.1. Characteristics of participants
There were 216,506 participants eligible for the study, and 29,312 were excluded due to missingness. Individuals missing air pollution exposures, APOE-ε4 genotype, college education, income, BMI, smoking status, or urban residence were found to be comparable to the final analytic cohort (Supplementary Table S1). Of the 187,194 participants, who had complete case data and aged 60 years or older, they had a mean of 7.04 years (2.84 SD) of follow-up time. The mean age was 64.1 years, women made up approximately half of the sample, and 97% were White (British and Irish ethnicity). Over this period, 1,742 participants were diagnosed with incident all-cause dementia (680 with AD, 377 with VAD, 63 with FTD, 622 with unspecified dementia). Compared to those without dementia, dementia cases were more likely to be male, to not have a college degree (82.1% versus 74.4% of non-dementia) and to belong to the lowest income bracket (36.0% versus 27.5%). Table 1 shows the distribution of the exposures and covariates in this sample stratified by all-cause dementia. In 2010, the median ambient exposures of PM2.5, PM2.5–10, PM2.5abs, PM10, NO2, NOX were 9.86 μg/m3, 6.09 μg/m3, 1.11 μg/m3, 15.98 μg/m3, 25.45 μg/m3, and 41.20 μg/m3, respectively. In this study, median particulate matter and nitrogen dioxide concentrations were well below the air quality standards put forth by the UK DEFRA and EU Ambient Air Quality Directive. However, the median exposure level for NOX was above the 30 μg/m3 standard, although this is not necessarily a human health standard (DEFRA 2020). Assuming a constant incidence rate during the 10-year period, the incidence for all-cause dementia was 11.8 per 10,000 person-years (95% CI: 11.2, 12.3), 4.6 per 10,000 person-years for AD (95% CI: 4.3, 5.0), 2.6 per 10,000 person-years for VAD (95% CI: 2.3, 2.8), and for FTD 0.5 per 10,000 person-years (95% CI: 0.33, 0.55).
Table 1:
Baseline characteristics of UK Biobank participants (N=187,194) aged 60 years or older by incident dementia status (including Alzheimer’s Disease, Frontotemporal, and Vascular)
| Any Dementia (n=1,742) | No Dementia (n=185,452) | |
|---|---|---|
|
| ||
| Sociodemographic variables | Mean ± SD or N (%) | Mean ± SD or N (%) |
|
| ||
| Age (years) | 65.81 ± 2.71 | 64.10 ± 2.84 |
| Sex (Female) | 778 (44.7%) | 97,681 (52.7%) |
| College degree or higher | 311 (17.9%) | 47,462 (25.6%) |
| Income | ||
| Less than £18,000 | 627 (36.0%) | 51,083 (27.5%) |
| £18,000 to £51,999 | 573 (32.9%) | 81,257 (43.8%) |
| £Greater than £52,000 | 76 (4.4%) | 18,815 (10.1%) |
| Missing (Decline/Do not know) | 466 (26.8%) | 34,297 (18.5) |
| White (British/Irish) | 1,662 (95.9%) | 179,257 (97.0%) |
| APOE-ε4 | ||
| 0 risk alleles | 831 (47.7%) | 137,205 (74.0%) |
| 1 risk allele | 698 (40.1%) | 44,003 (23.7%) |
| 2 risk alleles | 213 (12.2%) | 4,244 (2.3%) |
| BMI (kg/m2) | 27.7 ± 5.0 | 27.6 ± 4.5 |
| Smoking Status | ||
| Never | 770 (44.2%) | 92,540 (49.9%) |
| Previous | 783 (44.9%) | 77,369 (41.7%) |
| Current | 171 (9.8%) | 14,591 (7.9%) |
| Declined | 18 (1.0%) | 952 (0.5%) |
| Urban residency | 1,640 (94.1%) | 170,925 (92.2%) |
|
| ||
| Air Pollutants (μg/m3) | Median (IQR) | Median (IQR) |
|
| ||
| PM2.5 | 9.98 (1.25) | 9.86 (1.25) |
| PM2.5–10 | 6.10 (0.76) | 6.09 (0.77) |
| PM2.5 absorbance | 1.13 (0.29) | 1.11 (0.29) |
| PM10 | 16.06 (1.74) | 15.98 (1.76) |
| NO2 | 26.72 (9.39) | 25.45 (9.47) |
| NOx | 43.04 (16.02) | 41.20 (15.88) |
Abbreviations: IQR, Interquartile range; APOE- ε4, apolipoprotein E.
3.2. Associations with air pollutants and dementia-related outcomes
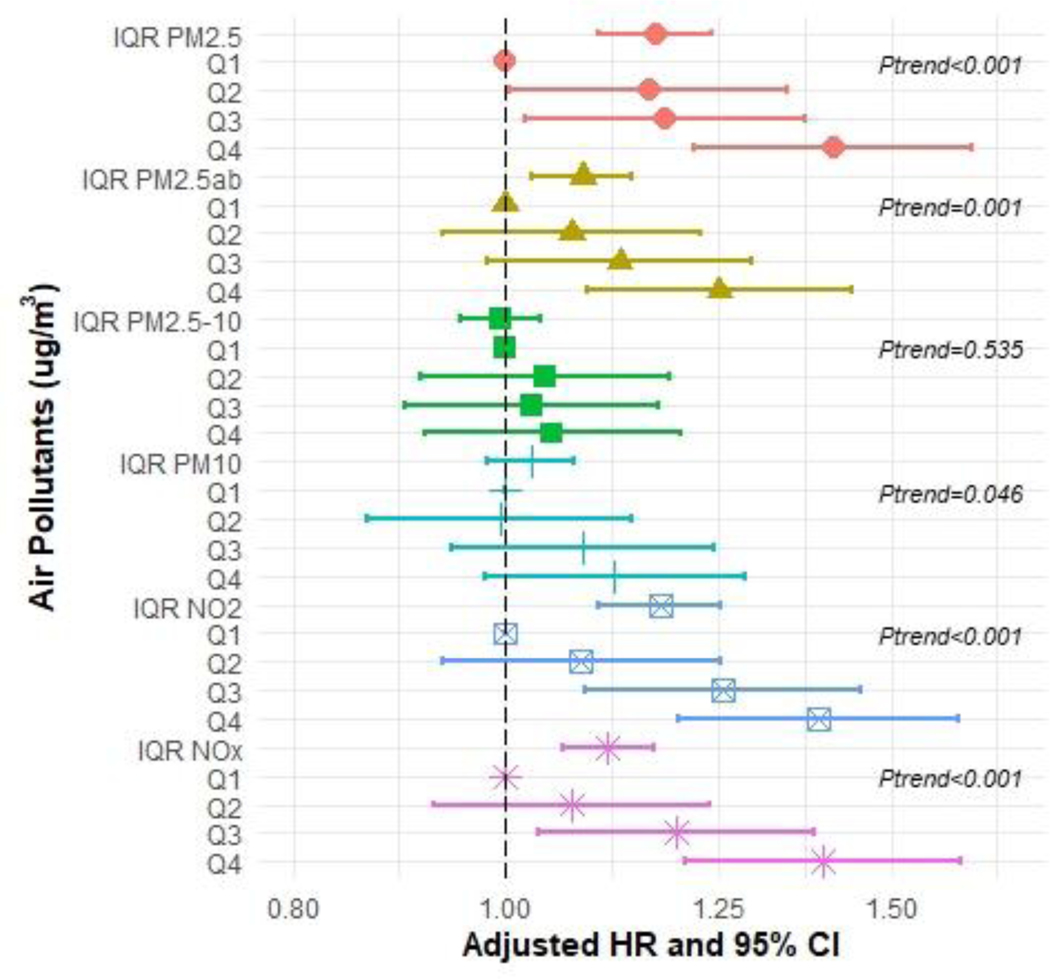
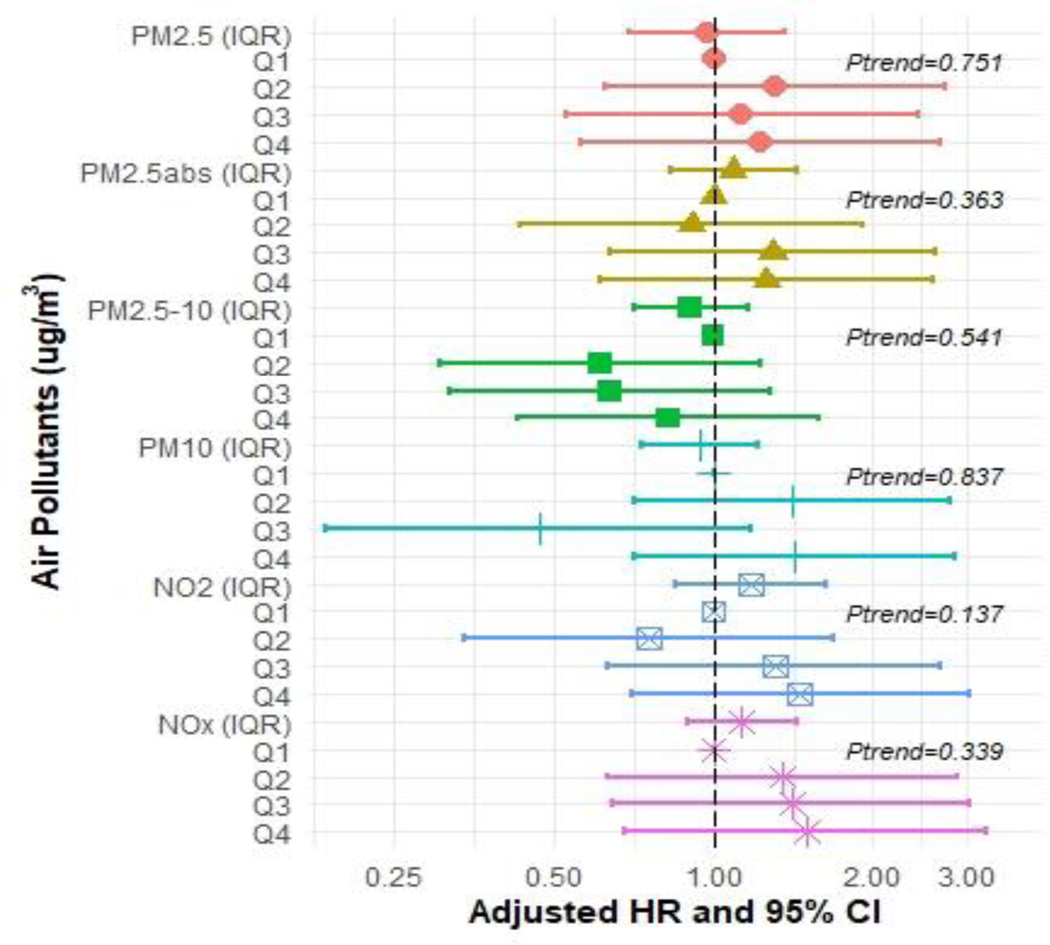
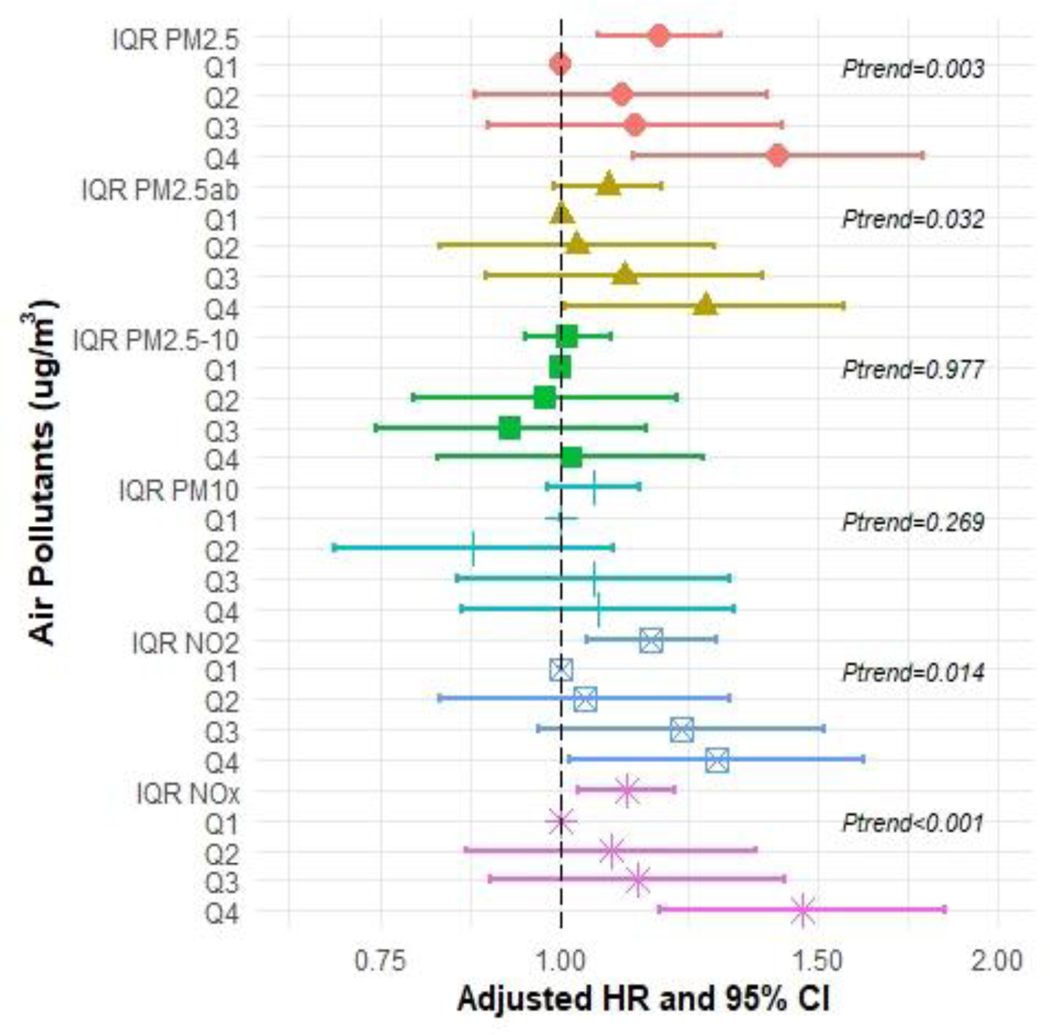
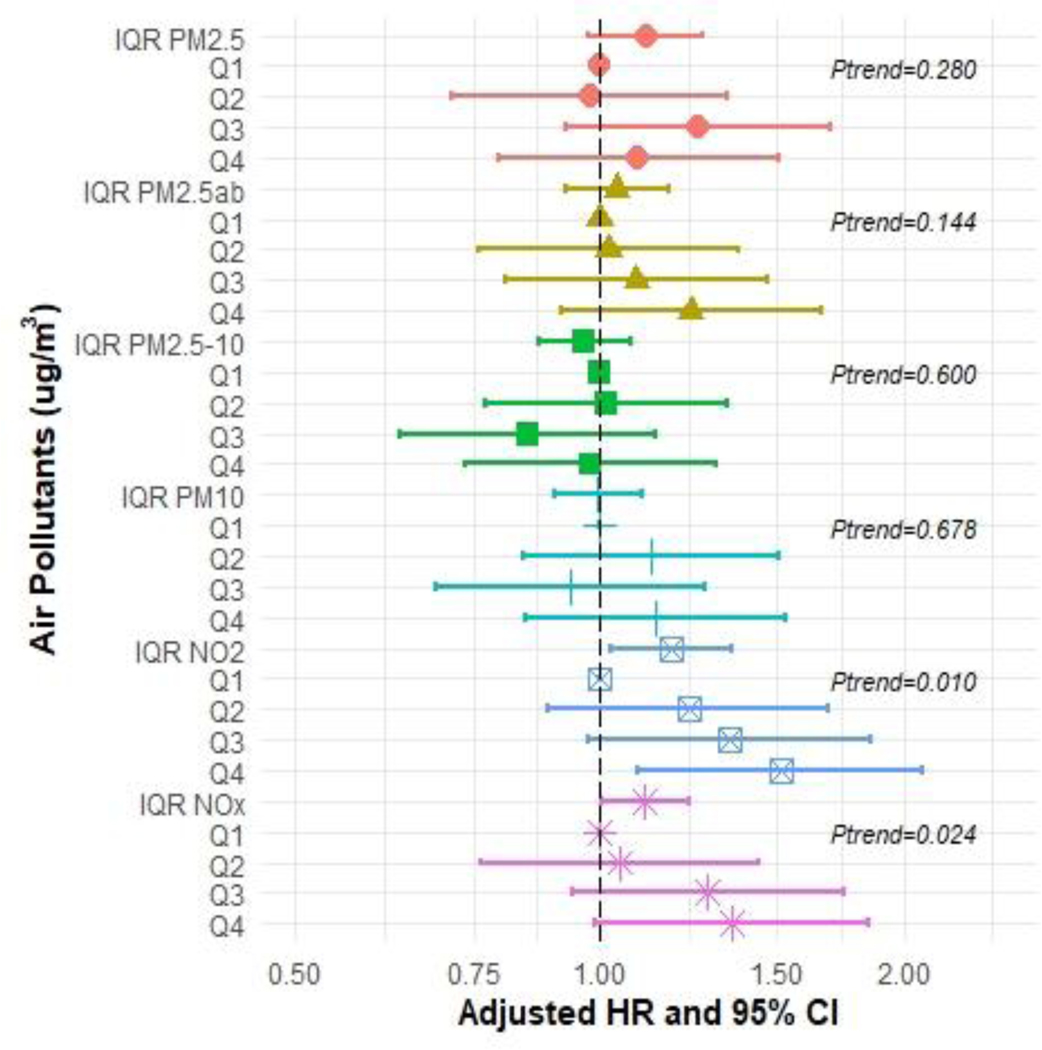
In the Cox proportional hazard models for each air pollutant and each dementia type pair (including AD, FTD, and VAD), we observed positive associations of PM2.5, NO2, and NOX exposure with all measures of incident dementia except for FTD (Figures 2–5 and Supplementary Tables S2–S5). In the fully adjusted models, continuous IQR measures include any incident dementia with a HR for 1 IQR increase in PM2.5=1.17 (95% CI: 1.10, 1.24); PM2.5abs=1.09 (95% CI: 1.03, 1.14); NO2=1.18 (95% CI: 1.10, 1.25); NOx=1.11 (95% CI: 1.06, 1.17) (Figure 2 and Supplementary Table S2), incident AD for PM2.5=1.17 (95% CI 1.06, 1.29); NO2 =1.15; (95% CI: 1.04, 1.28); NOx=1.11 (95% CI 1.03, 1.20) (Figure 3 and Supplementary Table S3), and incident VAD, NO2=1.18; (95% CI: 1.03, 1.35); NOx =1.11, (95% CI: 1.03, 1.23) (Figure 4 and Supplementary Table S4). Associations with FTD did not reach significance (Figure 5 and Supplementary Table S5).
Figure 2:
Hazard ratios (HR) and 95% confidence intervals (CIs) for incident all-cause dementia associated with IQR (interquartile range) and quartile increments (compared to Q1 referent) in air pollutant levels (μg/m3), with adjustment baseline age, sex, college enrolled, smoking status, income, urban, research center by country and APOE-ε4.
Figure 5:
Hazard ratios (HR) and 95% confidence intervals (CIs) for incident Frontotemporal dementia (FTD) associated with IQR (interquartile range) and quartile increments (compared to Q1 referent) in air pollutant levels (μg/m3), with adjustment baseline age, sex, college enrolled, smoking status, income, urban, research center by country and APOE-ε4.
Figure 3:
Hazard ratios (HR) and 95% confidence intervals (CIs) for incident Alzheimer’s Disease (AD) associated with IQR (interquartile range) and quartile increments (compared to Q1 referent) in air pollutant levels (μg/m3), with adjustment for baseline age, sex, college enrolled, smoking status, income, urban, research center and APOE-ε4.
Figure 4:
Hazard ratios (HR) and 95% confidence intervals (CIs) for incident Vascular dementia (VAD) associated with IQR (interquartile range) and quartile increments (compared to Q1 referent) in air pollutant levels (μg/m3), with adjustment baseline age, sex, college enrolled, smoking status, income, urban, research center by country and APOE-ε4.
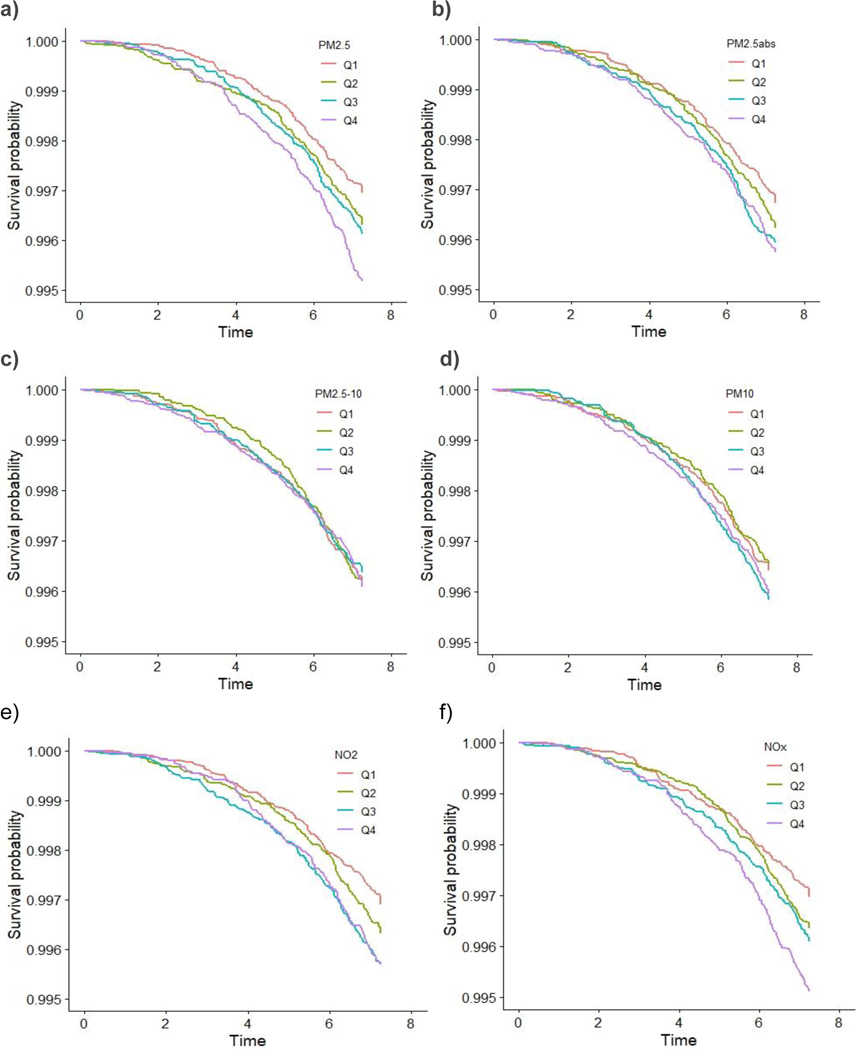
These associations were generally consistent with results from analyses comparing across quartiles, which showed a dose-response trend. For instance, compared to individuals in the first quartile, those in the highest quartile of exposure for PM2.5 had a HR for all-cause dementia of 1.41 [(95% CI: 1.22, 1.64), p-trend across quartiles <0.001]. Similarly, individuals with the highest exposure to PM2.5abs had a HR for all-cause dementia of 1.25 (95% CI: 1.09, 1.44), p-trend=0.001]. For NO2 the HR for all-cause dementia was 1.39 [(95% CI: 1.20, 1.61), p-trend <0.001], and for NOX, the HR for all-cause dementia was 1.40 [(95% CI: 1.21, 1.61), p-trend <0.001]. We did observe that the risk of developing AD increased by approximately 40% at the highest levels of exposures (Quartile 4 compared to Quartile 1) for PM2.5 and NOX even after adjustment [HR=1.41 (95% CI: 1.12, 1.78) and HR=1.47 (95% CI: 1.17, 1.84), respectively]. Associations for the fourth quartile of NO2 with AD were also elevated [HR=1.28 (95% CI: 1.01, 1.62)]. We also observed associations for PM2.5abs with all-cause dementia for both IQRs and the quartiles analysis. We did not observe associations for PM2.5–10 with any dementia. The Kaplan-Meier curves stratified by pollutant for AD (Figure 6) and for the other dementia types are depicted in Supplementary Figures S2–S4.
Figure 6:
Kaplan-Meier survival curves by (a) PM2.5 (b) PM2.5abs (c)PM2.5–10 (d) PM10 (e) NO2 (f) NOX quartiles for Alzheimer’s Disease (AD).
3.3. Effect Modification by APOE-ε4
APOE-ε4 did not modify the association between any air particulates and dementia risk (Supplementary Tables S6–S9). Additionally, in our unadjusted measures we did not find air pollution exposure to vary by APOE-ε4 status (Supplementary Figure S5).
3.4. Sensitivity Analysis
In a sub-analysis to assess the modifying effect of sex on APOE-ε4 and air pollution we introduced an interaction term in our models. We did not find any two-way or three-way interactions with APOE-ε4, air pollutants, and sex in our Cox regression analysis. Restricting the cohort to only those of British/Irish white ancestry also resulted in no changes to the results. In our sensitivity analysis of FTD with no age restriction (n=90) we found the effect sizes for every pollutant were attenuated toward the null, with no significant associations (Supplementary Table S10). For the other sensitivity analyses (a) comparing results of enrollment date (versus initial follow-up date) and (b) excluding individuals with diagnosis dates within 3 years of initial follow-up date, our results mostly remained the same (Supplementary Table S11). Compared to our main HR models, using the exact enrollment date resulted in similar effect estimates, apart from PM2.5abs for AD that reached statistical significance [HR=1.10 (95%CI: 1.01, 1.20)]. Upon exclusion of those individuals diagnosed with all-cause dementia within three years of enrollment there was a total of n=1,452 incident cases, and the association of PM2.5 with VAD strengthened towards significance [HR=1.16, (95%CI: 1.00, 1.34)]. We also tested the interaction between air pollution and cardiovascular risk factors. Further adjustment for cardiovascular risk factors did not change the main findings for any dementia outcome. Our findings indicate that excluding individuals (n=9,996) who lived less than 3 years at their residence reported at the baseline visit did not change the main findings for any dementia outcomes (Supplementary Table S12). Compared to the results from our complete case analysis, the imputed data produced similar estimates and interpretations for all pollutants will all-cause dementia, and all pollutants with Alzheimer’s disease. For incident VAD and FTD, interpretations for most pollutants remained the same, however, estimates for NO2 and NOx were no longer significant (Supplementary Table S12).
4. Discussion
4.1. Main Findings
In this sample of adults aged 60 years and older, we observed strong associations of PM2.5, NO2, and NOX with all-cause dementia, AD, VAD, but not FTD. The associations of ambient exposures to PM2.5abs and PM10 with dementia were weaker for specific dementia types, but still present for all-cause dementia. These associations were not modified by APOE-ε4. Our results corroborate the body of literature that strongly suggests that ambient PM2.5, NO2, and NOX are associated with an increased risk of developing incident dementia and Alzheimer’s disease (Killin et al. 2016; Peters et al. 2019; Power et al. 2016; Weuve et al. 2021). Here, we extend this body of evidence by investigating different types of dementia in addition to all-cause dementia, and the potential effect modification by APOE-ε4.
4.2. Background
According to our results, AD risk was positively associated with ambient exposure to PM2.5, NO2, and NOX. For PM2.5abs, a positive association was found among those with the highest quartile (Q4) of exposures. We observed a consistent dose-response relationship for nearly every pollutant apart from PM2.5–10 and PM10. Namely, individuals living in areas with the highest PM2.5, NOx and NO2 were at increased risk of developing dementia and AD. Recently, several European studies have shown an association between chronic air pollution and dementia, including AD and VAD. In metropolitan London, Carey et al. reported the incident risk of dementia increased by 6%, and 10% for AD and VAD per 0.95 μg/m3 IQR change of PM2.5 (2018). In this study, the strongest associations were found between all-cause dementia and NO2 (HR=1.16, 95% CI: 1.05,1.27). Our findings are congruent to results from a population-based sample of 7,066 urban French adults, who were followed for approximately 12 years. Researchers demonstrated that in this prospective study, PM2.5 was associated with a 20% increased hazard for both all-cause dementia and AD, and a 33% increase for VAD, but no dementia outcomes were associated with NO2 (Mortamais et al. 2021). In Sweden, with lower ambient air pollution concentrations and over a 5-year period, associations of PM2.5 and NOx with all-cause dementia were HR=1.54 (95% CI: 1.33, 1.78) and HR=1.14 (95% CI: 1.01, 1.29), respectively (Grande et al. 2020). This result was comparable to other Swedish longitudinal studies with 15-year follow-up (Andersson et al. 2018; Oudin et al. 2016; Oudin et al. 2018). Compared to our results from the UK Biobank, a substantially higher risk of VAD (66%) due to PM2.5 exposure was reported by Grande et al. (2020).
Some of the largest and most robust longitudinal cohort studies of air pollution and dementia are from Canada, which also has one of the lowest air pollution levels in the world (Chen et al. 2017a). In a study of over 2 million older individuals with 250,000 incident dementia cases over a 13-year follow-up period, they report weak evidence for associations between pollutants (PM2.5 and NO2) and neurodegenerative disease. Adjusted HRs for every IQR increase in exposure to PM2.5 (IQR = 4.8 μg/m3) and NO2 (IQR = 14.2 ppb) and dementia were 1.04 and 1.10. Weak positive associations of PM2.5 and NO2 with dementia were also observed in three other Canadian studies (Ilango et al. 2020; Smargiassi et al. 2020; Yuchi et al. 2020). Yet, a similar effect size of the association between incident dementia and PM2.5 (HR=1.16; 95% CI: 1.03, 1.31) were reported among ageing urban residents of Seattle, WA, who also had an 11% higher risk for AD associated with PM2.5 (95% CI: 0.97, 1.27) (Shaffer et al. 2021b). In a recent analysis of the Women’s Health Initiative (WHI), the authors found that PM2.5 increased the risk of AD (HR=1.28, 95% CI: 1.02,1.61), and furthermore, this HR was two-times higher among Blacks versus Whites, even after adjusting for factors related to socioeconomic status, lifestyle, hormone therapy, and CVD risk profiles (Younan et al. 2021). In Taiwan, an increased risk of incident AD of 138% was attributed to chronic PM2.5 exposure for the highest quartiles (95% CI: 2.21, 2.56) (Jung et al. 2015). These discrepant results between the aforementioned studies could be attributed to different study designs, air pollution levels at or below current air quality guidelines, varying characteristics of the study sample (e.g. age and health behaviors), and differences in PM components, and regulations across countries.
Contrary to our results, a longitudinal study (n>350,000) found negative associations for air pollution exposure and incident hospitalization admission for dementia (Cerza et al. 2019). Specifically, dementia was negatively associated with NO2 (HR=0.98, 95% CI: 0.96, 0.99 for each 10 μg/m3 increase in pollutant) and PM2.5–10 (HR=0.98, 95% CI: 0.96, 1.00 for each 5 μg/m3 increase in pollutant). Interestingly, every air pollutant measured was negatively associated with AD, and positively associated with VAD (HRs ranging from 1.02 to 1.15). However, we detected strong positive associations of NO2 and NOX with VAD. One possible reason for the VAD divergence is that the outcome used by Cerza et al. (2019) was first-dementia-hospitalizations, while we and others have used more sources to identify dementia cases, which may reduce outcome misclassification. Conversely, a study in the United States also examined longitudinal hospital administrative data for over 9 million Medicaid enrollees in 50 cities to assess associations between annual air pollution fluctuations and first dementia hospitalizations (Kioumourtzoglou et al. 2016). Positive associations were reported for time to hospitalization for dementia and AD with PM2.5 (HR=1.08, HR=1.15 per μg/m3, respectively). It is important to note that comparisons within and between countries must be considered in the light of varying air pollution standards, chemical composition of air pollutants, and meteorological conditions.
The Betula project in Sweden is one of the few prospective studies of long-term air pollution exposure on dementia that have considered pollution sources (Andersson et al. 2018; Oudin et al. 2016; Oudin et al. 2018). Wood burning and traffic exhaust of PM2.5 were linked to risk of dementia (Oudin et al. 2018). There is also evidence from other studies that living close to traffic and major roads is linked to incident dementia (Chen et al. 2017b, Yuchi et al. 2020). However, null findings for traffic were reported in London using a dispersion model for NO2 (Carey et al. 2018). A separate analysis of the Betula cohort revealed a positive association of the highest quartiles of NOX exposure from traffic pollution with dementia (HR= 1.71, 95% CI: 1.08, 2.73), but this did not reach significance for AD (HR=1.38, 95% CI: 0.87, 2.19) or VAD (HR=1.47, 95% CI: 0.83, 2.61) (Oudin et al. 2016). We found similar effect sizes that did reach significance, likely due to our larger sample size, which decreased standard errors and increased the power to detect an association. As in our study, researchers observed a positive dose-response relationship across NOX quartiles for dementia, AD, and VAD.
Older individuals homozygous for the APOE-e4 alleles are highly susceptible to developing dementia and AD. Only a handful of studies have investigated the role of APOE-ε4 in the context of air pollution and cognitive decline (Cacciottolo et al. 2017; Kulick et al. 2020; Schikowski et al. 2015) and even fewer have investigated APOE-ε4 as a potential modifier of the relationship between air pollution and dementia (Mortamais et al. 2021; Oudin et al. 2019; Shaffer et al. 2021b; Wu et al. 2015). Our results showed no modifying effect by APOE-ε4 in a region where median levels of all air pollutants fall below air quality standards for human health. Previous studies on cognitive outcomes have found the estimates of putative pollution effects to be more pronounced among carriers of APOE-ε4 alleles. Within these studies, exposures were stratified further by sex (Cacciottolo et al. 2017; Schikowski et al. 2015) and race/ethnicity (Kulick et al. 2020). The air-pollution associated risk of dementia was found to be higher among older female carriers of APOE-ε4 in highly polluted environments. This finding highlights the need for more sex-stratified and racially diverse cohorts on the influence of traffic-related air pollution. Nevertheless, we found no modifying effect of sex and/or APOE-ε4 on dementia. This is consistent with other studies in less polluted regions that included both sexes, and they found that APOE did not interact synergistically with air pollutants. Specifically, associations with PM2.5 and PM10 did not differ according to APOE-ε4 status across geographically diverse ageing cohorts with similar or lower air pollution exposure levels, like in France (Mortamais et al. 2021), Sweden (Oudin et al. 2019), USA (Shaffer et al. 2021b) and Taiwan (Wu et al. 2015).
4.3. Limitations
Our study had several strengths and limitations. We used an EHR-based algorithm and a death registry to identify dementia cases. The diagnosis of dementia (or its individual types) was determined by a compilation of neurological, laboratory, and clinical exams, which can lead to incomplete patient data or the underreporting of dementia. Yet the gold standard for dementia diagnosis and its subtypes can be challenging for large cohort studies such as the UK Biobank, as it can often require repeated clinical, neuropsychological, brain imaging, genetic, and/or fluid biomarker assessments over time, together with expert adjudication. A recent validation study of UK Biobank’s dementia outcomes from EHR data, including primary care data in combination with hospital and death sources, found that for a subset of UK Biobank participants with dementia and AD diagnosed through adjudication by clinical experts, PPV was 82.5% and 71.4%, respectively (Wilkinson et al 2019). PPV was deemed lower for VAD (43.8%) and FTD and other dementias (66.7%), such that this level of accuracy may reduce our power to detect an association. The numbers of incident FTD cases are likely underrepresented in this cohort potentially due to misdiagnosis (Bang et al. 2015). When we did not restrict incident FTD on age, we observed a total of 90 cases versus 62, but this did not affect our results. It is likely that in this cohort we did not have sufficient power to detect an effect given the rather small number of cases and potentially due to misclassification of the outcome. Larger and more robust studies with better diagnostic criteria are needed to investigate whether any FTD cases may have been missed by the algorithm-based diagnostic approach. Next, exposures were estimated for residential addresses in 2010, which likely reflects chronic long-term exposures. It is likely that some participants may have moved between 2010 and their dementia diagnosis. Individuals with early dementia or those with deteriorating brain function might move more to care homes or with relatives over time. Conversely, more able-bodied retirees may be less likely to move residential locations and age in place. Although we could not test this assumption for the time period after 2010 due to missing data, we observed that our estimates were similar when restricting to non-movers or the time period before 2010. These findings that excluded people who lived in their baseline home for less than 3 years support a prior study using ESCAPE data, which determined that individuals often moved to areas with similar exposure levels (Oudin et al. 2012). Another limitation is that we cannot account for cumulative lifetime exposures or non-residential sources of exposure that might affect long-term respiratory health, such as occupational exposures. Although we adjusted for other important covariates (e.g. smoking), we cannot rule out residual confounding from unmeasured covariates. Other environmental risk factors that were not considered were time spent outdoors, light at night, traffic noise, or access to green space (Andersson et al. 2018; Crous-Bou et al. 2020; Yu et al. 2020; Yuchi et al. 2020). Additionally, external validity may be somewhat limited as evidenced by the predominately White, healthier, and urbanite sample that makes up the UK Biobank (Fry et al. 2017). Selection bias may have influenced the effect measures in this volunteer-based cohort of UK Biobank participants. More research is therefore needed to quantify the extent to which these results are generalizable to the broader UK population and to populations outside of the UK.
4.4. Strengths
A strength of this study is the large sample size from the UK Biobank of a high-risk sample of persons 60 years or older, albeit a generally healthy group of volunteers (Fry et al 2017). We were sufficiently powered to detect weak associations for VAD, a relatively rare outcome. Second, our analysis considered important individual-level confounders (e.g. smoking, socioeconomic status) from a rich dataset that allowed us to approximate risk of dementia and modification by APOE-ε4 gene prospectively. Third, we were able to examine associations prospectively across six different air pollutant measures. Fourth, our large sample size of all-cause incident dementia included three sub-types of dementia outcomes. Further, case ascertainment was obtained through validated methods using medical and death records with a previously validated algorithm. In summary, even though air pollution levels in this cohort are below air quality guidelines, relatively higher levels were associated with increased dementia risk. Our finding suggest that the effects of air pollution may be even more of a public health hazard in countries with higher exposure levels.
5. Conclusion
While air pollution exposure limits in the UK Biobank cohort are below air quality standards, we found strong associations with higher incidence of dementia, AD and VAD in this sample of individuals ≥60 years of age. We did not find any evidence of effect modification of air pollutants by APOE-ε4 or sex on dementia-related outcomes. Our results revealed consistent findings with a growing body of recent evidence that indicates risk of incident dementia among older-aged individuals is associated with long-term exposure to PM2.5, NO2, and NOX. Researchers have yet to completely understand the complex biological and environmental relationships between air pollution and neurotoxicity. More longitudinal studies that account for long-term and non-residential exposures to air pollutants are needed to examine the risk of dementia across ethnically and regionally diverse populations.
Supplementary Material
Acknowledgements
The authors would like to thank the UK Biobank study participants, funders, and organizers for providing a rich epidemiological resource for this analysis. This research was conducted under UK Biobank application number 21259.
Funding
The authors would like to acknowledge support from National Heart, Lung, and Blood Institute (R01-HL136528), National Institute of Environmental Health Sciences (R00-ES028743, P30ES006694), National Institute on Aging (P30AG072980, P30AG019610, R56AG067200, R01AG049464), the state of Arizona and Arizona Department of Health Services, and the McKnight Brain Research Foundation.
Human Subjects
All participants provided written informed consent. Ethical approval of the UK Biobank study was given by the North West Multicentre Research Ethics Committee, the National Information Governance Board for Health & Social Care, and the Community Health Index Advisory Group.
Abbreviations:
- AD
Alzheimer’s disease
- FTD
frontotemporal dementia
- VAD
vascular dementia
- APOE- ε4
apolipoprotein E
Footnotes
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alemany S, Crous-Bou M, Vilor-Tejedor N, Milà-Alomà M, Suárez-Calvet M, Salvadó G, et al. 2021. Associations between air pollution and biomarkers of Alzheimer’s disease in cognitively unimpaired individuals. Environ Int 157:106864, PMID: 34537521, DOI: 10.1016/j.envint.2021. [DOI] [PubMed] [Google Scholar]
- Andersson J, Oudin A, Sundstrom A, Forsberg B, Adolfsson R, Nordin M. 2018. Road traffic noise, air pollution, and risk of dementia - results from the betula project. Environ Res 166:334–339, PMID: 29909174, DOI: 10.1016/j.envres.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Bang J, Spina S, Miller B. 2015. Frontotemporal dementia. Lancet 386:1672–1682, PMID: 26595641, DOI: 10.1016/S0140-6736(15)00461-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelen R, Hoek G, Vienneau D, Eeftens M, Dimakopoulou K, Pedeli X, et al. 2013. Development of no2 and nox land use regression models for estimating air pollution exposure in 36 study areas in europe - the ESCAPE project. Atmos Environ 72:10–23, 10.1016/j.atmosenv.2013.02.037. [DOI] [Google Scholar]
- Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. 2018. The UK Biobank resource with deep phenotyping and genomic data. Nature 562(7726), 203–209, PMID: 30305743, DOI: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J, et al. 2017. Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiatry 7:e1022, PMID: 28140404, DOI: 10.1038/tp.2016.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvin CM, Wilkinson T, Starr JM, Sudlow C, Hagenaars SP, Harris SE, et al. 2019. Predicting incident dementia 3–8 years after brief cognitive tests in the UK Biobank prospective study of 500,000 people. Alzheimers Dement 15(12):1546–1557, PMID: 31619348, DOI: 10.1016/j.jalz.2019.07.014.. [DOI] [PubMed] [Google Scholar]
- Carey I, Anderson H, Atkinson R, Beevers S, Cook D, Strachan D, et al. 2018. Are noise and air pollution related to the incidence of dementia? A cohort study in London, England. BMJ Open 8:e022404, PMID: 30206085, DOI: 10.1136/bmjopen-2018-022404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerza F, Renzi M, Gariazzo C, Davoli M, Michelozzi P, Forastiere F, et al. 2019. Long-term exposure to air pollution and hospitalization for dementia in the Rome longitudinal study. Environ Health 18:72, PMID: 31399053, DOI: 10.1186/s12940-019-0511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Hystad P, van Donkelaar A, Tu K, et al. 2017a. Exposure to ambient air pollution and the incidence of Dementia: A population-based cohort study. Environ Int 108:271–277, PMID: 28917207, DOI: 10.1016/j.envint.2017.08.020. [DOI] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, et al. 2017b. Living near major roads and the incidence of Dementia, Parkinson’s Disease, and Multiple Sclerosis: A population-based cohort study. Lancet 389:718–726, PMID: 28063597, DOI: 10.1016/S0140-6736(16)32399-6. [DOI] [PubMed] [Google Scholar]
- Clifford A, Lang L, Chen R, Anstey K, Seaton A. 2016. Exposure to air pollution and cognitive functioning across the life course--a systematic literature review. Environ Res 147:383–398, PMID: 26945620, DOI: 10.1016/j.envres.2016.01.018. [DOI] [PubMed] [Google Scholar]
- Corder E, Saunders A, Strittmatter W, Schmechel D, Gaskell P, Small G, et al. 1993. Gene dose of apolipoprotein e type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261(5123):921–923, PMID: 8346443, DOI: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crous-Bou M, Gascon M, Gispert JD, Cirach M, Sánchez-Benavides G, Falcon C, et al. 2020. Impact of urban environmental exposures on cognitive performance and brain structure of healthy individuals at risk for Alzheimer’s dementia. Environ Int 138:105546, PMID: 32151419, DOI: 10.1016/j.envint.2020.105546. [DOI] [PubMed] [Google Scholar]
- Cui P, Huang Y, Han J, Song F, Chen K. 2015. Ambient particulate matter and lung cancer incidence and mortality: A meta-analysis of prospective studies. E J Public Health 25(2):324–329, PMID: 25201901, DOI: 10.1093/eurpub/cku145. [DOI] [PubMed] [Google Scholar]
- Cullen B, Newby D, Lee D, Lyall DM, Nevado-Holgado AJ, Evans JJ, et al. 2018. Cross-sectional and longitudinal analyses of outdoor air pollution exposure and cognitive function in UK Biobank. Sci Rep 8:12089, PMID: 30108252, DOI: 10.1038/s41598-018-30568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEFRA. 2020. Uk air: Air information resource. Available: https://uk-air.defra.gov.uk/air-pollution/uk-eu-limits [accessed July 2020].
- Dimakakou E, Johnston HJ, Streftaris G, Cherrie JW. 2020. Is Environmental and Occupational Particulate Air Pollution Exposure Related to Type-2 Diabetes and Dementia? A Cross-Sectional Analysis of the UK Biobank. Int J Environ Res Public Health 21;17(24):9581, PMID: 33371391, DOI: 10.3390/ijerph17249581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eeftens M, Tsai M-Y, Ampe C, Anwander B, Beelen R, Bellander T, et al. 2012. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PM coarse concentrations between and within 20 European study areas and the relationship with NO2 -- results of the ESCAPE project. Atmos Environ 62:303–317, 10.1016/j.atmosenv.2012.08.038. [DOI] [Google Scholar]
- Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, Collins R, Allen NE. 2017. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. AJE. 186(9):1026–34. PMID: 28641372, DOI: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S, Zadeoglulari Z, Fuss S, Genc K. 2012. The adverse effects of air pollution on the nervous system. J Toxicol 2012:782462, PMID: 22523490, DOI: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande G, Ljungman PLS, Eneroth K, Bellander T, Rizzuto D. 2020. Association Between Cardiovascular Disease and Long-term Exposure to Air Pollution With the Risk of Dementia. JAMA Neurol 1;77(7):801–809, PMID: 32227140, DOI: 10.1001/jamaneurol.2019.4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango SD, Chen H, Hystad P, van Donkelaar A, Kwong JC, Tu K, et a. 2020. The role of cardiovascular disease in the relationship between air pollution and incident dementia: a population-based cohort study. Int J Epidemiol 1;49(1):36–44, PMID: 31347651, DOI: 10.1093/ije/dyz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Stewart L, Kuo H, McGilberry W, Wall S, Liang B, et al. 2019. Cyclic O3 exposure synergizes with aging leading to memory impairment in male APOE ε3, but not APOE ε4, targeted replacement mice. Neurobiol Aging 81:9–21, PMID: 31207469, DOI: 10.1016/j.neurobiolaging.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CR, Lin YT, Hwang BF. 2015. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J Alzheimers Dis 44:573–584, PMID: 25310992, DOI: 10.3233/JAD-140855. [DOI] [PubMed] [Google Scholar]
- Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, et al. 2016. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the multi-ethnic study of atherosclerosis and air pollution): A longitudinal cohort study. Lancet 388(10045):696–704, PMID: 27233746, DOI: 10.1016/S0140-6736(16)00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killin LO, Starr JM, Shiue IJ, Russ TC. 2016. Environmental risk factors for Dementia: A systematic review. BMC Geriatr 16:175, PMID: 27729011, DOI: 10.1186/s12877-016-0342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Schwartz JD, Weisskopf MG, Melly SJ, Wang Y, Dominici F, et al. 2016. Long-term PM2.5 exposure and neurological hospital admissions in the northeastern United States. Environ Health Perspect 124(1):23–29, PMID: 2597870, DOI: 10.1289/ehp.1408973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulick E, Elkind M, Boehme A, Joyce N, Schupf N, Kaufman J, et al. 2020. Long-term exposure to ambient air pollution, APOE-ε4 status, and cognitive decline in a cohort of older adults in northern Manhattan. Environ Int 136:105440, PMID: 31926436, DOI: 10.1016/j.envint.2019.105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt OK, Zhang J, Pinkerton KE. 2016. Pulmonary health effects of air pollution. Curr Opin Pulm Med 22(2):138–143, PMID: 26761628, DOI: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan P, Fuller R, Acosta N, Adeyi O, Arnold R, Basu N, et al. 2018. The Lancet Commission on Pollution and Health. Lancet 391(10119):462–512, PMID: 29056410, DOI: 10.1016/S0140-6736(17)32345-0. [DOI] [PubMed] [Google Scholar]
- Liu CC, Takahisa K, Huaxi X, Guojun B. 2013. Apolipoprotein E and Alzheimer disease: Risk, mechanisms, and therapy. Nat Rev Neurol 9(2):106–118, PMID: 23296339, DOI: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortamais M, Gutierrez LA, de Hoogh K, Chen J, Vienneau D, Carrière et al. 2021. Long-term exposure to ambient air pollution and risk of dementia: Results of the prospective Three-City Study. Environ Int 148:106376, PMID: 33484961, DOI: 10.1016/j.envint.2020. [DOI] [PubMed] [Google Scholar]
- Oudin A, Forsberg B, Strömgren M, Beelen R, Modig L. 2012. Impact of residential mobility on exposure assessment in longitudinal air pollution studies: A sensitivity analysis within the ESCAPE project. The ScientificWorldJournal 2012:125818, PMID: 23251098, DOI: 10.1100/2012/125818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Forsberg B, Adolfsson AN, Lind N, Modig L, Nordin M, et al. 2016. Traffic-related air pollution and Dementia incidence in northern Sweden: A longitudinal study. Environ Health Perspect 124:306–312, PMID: 26305859, DOI: 10.1289/ehp.1408322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Segersson D, Adolfsson R, Forsberg B. 2018. Association between air pollution from residential wood burning and Dementia incidence in a longitudinal study in northern Sweden. PLoS One 13:e0198283, PMID: 29897947, DOI: 10.1371/journal.pone.0198283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudin A, Andersson J, Sundström A, Nordin AA, Oudin ÅD, Adolfsson R, et al. 2019. Traffic-related air pollution as a risk factor for Dementia: No clear modifying effects of APOEɛ4 in the Betula cohort. J Alzheimers Dis 71(3):733–740, PMID: 31450491, DOI: 10.3233/JAD-181037. [DOI] [PubMed] [Google Scholar]
- Peters R, Ee N, Peters J, Booth A, Mudway I, Anstey K. 2019. Air pollution and Dementia: A systematic review. J Alzheimers Dis 70(s1), S145–S163, PMID: 30775976, DOI: 10.3233/JAD180631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power MC, Adar SD, Yanosky JD, Weuve J. 2016. Exposure to air pollution as a potential contributor to cognitive function, cognitive decline, brain imaging, and Dementia: A systematic review of epidemiologic research. Neurotoxicology 56:235–253, PMID: 27328897, DOI: 10.1016/j.neuro.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri C. 2013. The global prevalence of Dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75.e2, PMID: 23305823, DOI: 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- R Core Team 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/. [Google Scholar]
- Rajagopalan S, Al-Kindi S, Brook R. 2018. Air pollution and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol 72(17):2054–2070, PMID: 30336830, DOI: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- Rubin DB 2004. Multiple imputation for nonresponse in surveys (Vol. 81). John Wiley & Sons. [Google Scholar]
- Schikowski T, Vossoughi M, Vierkötter A, Schulte T, Teichert T, Sugiri D, et al. 2015. Association of air pollution with cognitive functions and its modification by APOE gene variants in elderly women. Environ Res 142:10–16, PMID: 26092807, DOI: 10.1016/j.envres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Shaffer RM, Li G, Adar SD, Dirk Keene C, Latimer CS, Crane PK, et al. 2021a. Fine Particulate Matter and Markers of Alzheimer’s Disease Neuropathology at Autopsy in a Community-Based Cohort. J Alzheimers Dis 79(4):1761–1773, PMID: 33459717, DOI: 10.3233/JAD-201005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer RM, Blanco MN, Li G, Adar SD, Carone M, Szpiro AA, et al. 2021b. Fine Particulate Matter and Dementia Incidence in the Adult Changes in Thought Study. Environ Health Perspect 129(8):87001, PMID: 34347531, DOI: 10.1289/EHP9018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smargiassi A, Sidi EA, Robert LE, Plante C, Haddad M, Gamache P, Burnett R, Goudreau S, Liu L, Fournier M, Pelletier E. 2020. Exposure to ambient air pollutants and the onset of dementia in Québec, Canada. Environ Res 190:109870, PMID: 32739624, DOI: 10.1016/j.envres.2020.109870. [DOI] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. 2015. UK Biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 12(3):e1001779, PMID: 25826379, DOI: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Biobank. 2018. Definitions of Dementia and the Major Diagnostic Pathologies, UK Biobank Phase 1 Outcomes Adjudication. Available: http://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/alg_outcome_dementia.pdf, [accessed July 2020].
- Van Buuren S, & Groothuis-Oudshoorn K. 2011. Multivariate imputation by chained equations in R. J Stat Software, 45, 1–67. [Google Scholar]
- Veronesi B, Makwana O, Pooler M, Chen L. 2005. Effects of subchronic exposures to concentrated ambient particles. VII. Degeneration of dopaminergic neurons in Apo E−/− mice. Inhal Toxicol 17(4–5):235–241, PMID: 15804941, DOI: 10.1080/08958370590912888. [DOI] [PubMed] [Google Scholar]
- Weuve J, Bennett EE, Ranker L, Gianattasio KZ, Pedde M, Adar SD, et al. 2021. Exposure to Air Pollution in Relation to Risk of Dementia and Related Outcomes: An Updated Systematic Review of the Epidemiological Literature. Environ Health Perspect 129(9):96001, PMID: 34558969, DOI: 10.1289/EHP8716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White IR, Royston P, Wood AM. 2011. Multiple imputation using chained equations: Issues and guidance for practice. Stat Med 20;30(4):377–99, PMID: 21225900, DOI: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- Wilkinson T, Schnier C, Bush K, Rannikmäe K, Henshall D, Lerpiniere C, et al. 2019. Identifying Dementia outcomes in UK Biobank: A validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol 34(6):557–565, PMID: 30806901, DOI: 10.1007/s10654-01900499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC, Pakbin P, Saffari A, Shirmohammadi F, Haghani A, Sioutas C, et al. 2017. Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol Aging 53:48–58, PMID: 28212893, DOI: 10.1016/j.neurobiolaging.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Lin YC, Yu HL, Chen JH, Chen TF, Sun Y, et al. 2015. Association between air pollutants and Dementia risk in the elderly. Alzheimers Dement 1:220–228, PMID: 27239507, DOI: 10.1016/j.dadm.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yitshak-Sade M, Nethery R, Schwartz JD, Mealli F, Dominici F, Di Q, Abu Awad Y, Ifergane G, Zanobetti A. PM2.5 and hospital admissions among Medicare enrollees with chronic debilitating brain disorders. Sci Total Environ. 2021 Feb 10;755(Pt 2):142524. doi: 10.1016/j.scitotenv.2020.142524. Epub 2020 Oct 3. PMID: 33065503; PMCID: PMC7749824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younan D, Wang X, Gruenewald T, Gatz M, Serre ML, Vizuete W, et al. 2021. Racial/Ethnic Disparities in Alzheimer’s Disease Risk: Role of Exposure to Ambient Fine Particles. J Gerontol A Biol Sci Med Sci PMID: 34383042, DOI: 10.1093/gerona/glab231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Haan M, Paul KC, Mayeda ER, Jerrett M, Wu J, et al. 2020. Metabolic dysfunction modifies the influence of traffic-related air pollution and noise exposure on late-life dementia and cognitive impairment: A cohort study of older Mexican-Americans. Environ Epidemiol 4(6):e122, PMID: 33778355, DOI: 10.97/EE9.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuchi W, Sbihi H, Davies H, Tamburic L, Brauer M. 2020. Road proximity, air pollution, noise, green space and neurologic disease incidence: A population-based cohort study. Environ Health 19(1):8, PMID: 31964412, DOI: 10.1186/s12940-020-0565-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.