Abstract
Purpose:
Small cell lung cancer is an exceptionally lethal form of lung cancer with limited treatment options. Delta-like ligand 3 (DLL3) is an attractive therapeutic target as surface expression is almost exclusive to tumor cells.
Experimental Design:
We radiolabeled the anti-DLL3 monoclonal antibody SC16 with the therapeutic radioisotope, Lutetium-177. [177Lu]Lu-DTPA-CHX-A”-SC16 binds to DLL3 on SCLC cells and delivers targeted radiotherapy while minimizing radiation to healthy tissue.
Results:
[177Lu]Lu-DTPA-CHX-A”-SC16 demonstrated high tumor uptake with DLL3-target specificity in tumor xenografts. Dosimetry analyses of biodistribution studies suggested that the blood and liver were most at risk for toxicity from treatment with high doses of [177Lu]Lu-DTPA-CHX-A”-SC16. In the radioresistant NCI-H82 model, survival studies showed that 500 μCi and 750 μCi doses of [177Lu]Lu-DTPA-CHX-A”-SC16 led to prolonged survival over controls, and three of the eight mice that received high doses of [177Lu]Lu-DTPA-CHX-A”-SC16 had pathologically confirmed complete responses. In the patient-derived xenograft model Lu149, all doses of [177Lu]Lu-DTPA-CHX-A”-SC16 markedly prolonged survival. At the 250 μCi and 500 μCi doses, 5/10 and 7/9 mice demonstrated pathologically confirmed complete responses, respectively. Four of ten mice that received 750 μCi of [177Lu]Lu-DTPA-CHX-A”-SC16 demonstrated petechiae severe enough to warrant euthanasia, but the remaining six mice demonstrated pathologically confirmed complete responses. IHC on residual tissues from partial responses confirmed retained DLL3 expression. Hematologic toxicity was dose-dependent and transient, with full recovery within 4 weeks. Hepatotoxicity was not observed.
Conclusions:
Together, the compelling antitumor efficacy, pathologic complete responses, and mild and transient toxicity profile demonstrate strong potential for clinical translation of [177Lu]Lu-DTPA-CHX-A”-SC16.
INTRODUCTION
Small cell lung cancer (SCLC) represents approximately 15% of lung cancer diagnoses and is exceptionally lethal, accounting for over 25,000 deaths annually in the United States (1–4). Median overall survival of patients with extensive stage disease is approximately one year, and the 5-year survival rate is <5%. SCLC is difficult to treat for a variety of reasons. At initial diagnosis, 75–80% of patients present with extrathoracic metastases, necessitating systemic treatment (5). While patients typically respond well to first-line therapy (usually a combination of a platinum agent, etoposide and anti-PD-L1 immune checkpoint blockade), most patients relapse within months and responses to second- and third-line treatments are infrequent and almost universally transient.
Delta-like Ligand 3 (DLL3) is a cell surface protein that has emerged as a promising candidate for targeted therapy in high-grade neuroendocrine tumors, including SCLC (6). DLL3 is notable for its tumor-selective expression profile, its high prevalence in SCLC, and its pathobiological role in this disease. DLL3 expression is induced by ASCL1, a critical transcription factor in SCLC, and has been implicated in promoting clonogenic, tumorigenic, and metastatic capacity (7–10). DLL3 expression in healthy adult tissues is almost completely intracellular, primarily confined to the Golgi apparatus (11,12). However, it is markedly upregulated in SCLC tumors and is aberrantly trafficked to the cell surface, creating an opportunity for tumor-specific targeting (6). Approximately 72% of treatment-naïve SCLC and up to 85% of treatment-refractory SCLC stain positively for DLL3 (6)(13–15). The high prevalence of DLL3 expression makes DLL3-targeted therapies widely applicable to SCLC patients.
Three DLL3-targeted therapeutic agents have been tested clinically: (1) the antibody-drug conjugate rovalpituzumab-tesirine (Rova-T or SC16LD6.5), (2) the bi-specific T-cell engager (BiTE) AMG 757, and (3) the chimeric antigen receptor T cell (CAR-T) AMG 119 (16). Results of the initial clinical trials of AMG 757 and AMG 119 have not yet been published; however, Rova-T has completed phase II and phase III clinical testing in patients with SCLC (17,18). While Rova-T initially demonstrated promising anti-cancer activity and validated DLL3 as a therapeutic target in SCLC, its development was ultimately stopped due to toxicity concerns that precluded repetitive dosing (17–21). The anti-DLL3 monoclonal antibody of Rova-T (SC16) demonstrates high affinity and specificity but has no evident therapeutic activity against DLL3-expressing pre-clinical models without the cytotoxic warhead (6).
SCLC is an exceptionally radiosensitive disease. Approximately 25% of patients with limited stage disease, confined to one hemithorax and one radiation port, are cured with external beam gamma radiation with concomitant radiosensitizing chemotherapy (22). Limited stage SCLC is the only solid tumor in which prophylactic cranial irradiation is routinely deployed to treat the possibility of microscopic and undetected disease in the brain; prophylactic cranial radiation in this context improves survival (23,24).
In contrast to limited stage SCLC, extensive stage (metastatic) disease is essentially never cured by chemotherapy alone. The tumor-specific expression of DLL3 provides an opportunity to focus delivery of radiation to sites of disease in patients with SCLC, regardless of disease stage. Instead of using SC16 to deliver the pyrrolobenzodiazepine warhead to tumor cells, herein we explored the potential of using 177Lu-labeled SC16 to deliver targeted radiotherapy to SCLC tumors in vivo. This work builds on our prior report demonstrating successful delineation of metastatic SCLC in various murine models of the disease using 89Zr-labeled SC16 (25). By substituting Zr-89 (a PET isotope) with a beta-emitting radionuclide, Lu-177, we aim to specifically direct therapeutic radiation to tumor sites throughout the body while minimizing radiation exposure to healthy tissues, something that is not feasible with conventional external beam radiation. Lu-177 is particularly well-suited to anti-cancer treatment in a disease with a predilection for widespread metastasis. Its short tissue penetration makes it an ideal candidate for ablation of micrometastases while minimizing off-target radiation (26,27). Herein, we report the preclinical efficacy and toxicity of 177Lu-labeled SC16 ([177Lu]Lu-DTPA-CHX-A”-SC16) for treatment of human SCLC in tumor-bearing mice.
MATERIALS AND METHODS
For details regarding antibody functionalization and radiolabeling, cell binding assays, stability studies, SPECT imaging, biodistribution studies, and dosimetry calculations please refer to the Supplementary Information.
In vivo therapy in subcutaneous xenograft models
Mice were randomized into one of eight therapy groups (n=8–10 animals per group). One day after randomization, mice were injected with vehicle (0.9% sterile saline, 50 μL via intra-peritoneal injection), unmodified SC16 (60 μg via intra-peritoneal injection), a single dose of antibody-drug conjugate (0.3 mg/kg SC16LD6.5 or IgGLD6.5 via intra-peritoneal injection for the H82 model, 0.4 mg/kg SC16LD6.5 via tail vein injection for the Lu149 model), or increasing doses of the radioimmunoconjugate (~250, 500, or 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 or [177Lu]Lu-DTPA-CHX-A”-IgG, 150 μL) via tail vein. While the activity of the radioimmunoconjugate increased between groups, the antibody mass was kept at 60 μg. Tumor volumes were measured twice weekly for 100 days post-treatment in the H82 model and 70 days post-treatment in the Lu149 model, or until mice reached a set endpoint (severe petechiae, >20% weight loss, or tumor volume > 2000 mm3).
Pathology of residual tissues at tumor site
Any residual tissue remaining at the site of tumor implantation was collected upon euthanasia. Tissues were fixed in 10% neutral buffered formalin and stored in 70% ethanol at 4°C for a minimum of 10 half-lives, 67 days post-administration. Tissues were then paraffin embedded, sliced, and mounted on slides. Slides were either stained with hematoxylin and eosin (H&E) or immunohistochemistry (IHC) was performed. Please refer to the Supplementary Information for further details regarding IHC. Stained tissue slides were analyzed by a board-certified thoracic pathologist blinded to the treatment groups (WDT).
Toxicity studies
Mice were monitored for outward signs of toxicity, including lethargy, loss of appetite, and petechiae. Mice were weighed twice weekly to monitor weight loss, and blood samples for hematologic analysis were collected once weekly via retro-orbital blood draws. White blood cells, red blood cells, and platelets were determined using a Hemavet 950 (Drew Scientific) or an Element HT5 (Heska Corporation) and compared with both normal range estimates for female athymic mice in the literature and baseline values that were measured prior to administration of the radiotherapeutic.
Hepatotoxicity was assessed by terminal serum hepatic enzyme levels and histologic appearance. Blood was collected at euthanasia and allowed to clot at room temperature for >30 minutes, then centrifuged at 2000g for 10 minutes. The supernatant (serum) was collected and frozen at −20°C for a minimum of 10 half-lives, 67 days. The serum alanine transaminase (ALT), aspartate transaminase (AST), and bilirubin levels were measured by the Memorial Sloan Kettering Anti-Tumor Assessment Core using a Heska DC7000 Dri-chem analyzer (Heska Corporation). Livers were collected and fixed in 10% neutral buffered formalin (Fisher Scientific) for 48h and transferred to 70% ethanol for storage until they were processed, paraffin embedded, sliced and mounted on slides. Sections of livers stained with H&E were analyzed for toxicity by a board-certified veterinary pathologist blinded to the treatment groups (AP).
Statistical analyses
All data are shown as mean values ± standard deviation (unless otherwise noted) and sample sizes are detailed in figure legends. Statistical analyses were performed using GraphPad Prism version 8.3.1. A P-value <0.05 was considered significant. The Bonferroni correction for multiple hypothesis testing was applied where appropriate in order to minimize false positive reporting.
Data availability
All data generated during this study are included in this published article and its supplementary information files. Original data is available upon request from the corresponding authors.
RESULTS
[177Lu]Lu-DTPA-CHX-A”-SC16 demonstrates favorable biodistribution in mouse models of SCLC
To assess the potential of using SC16 as the targeting vector for radioimmunotherapy, unmodified SC16 antibody was bioconjugated to p-SCN-Bn-CHX-A”-DTPA and radiolabeled with Lu-177 (Figure 1A). The chelate p-SCN-Bn-CHX-A”-DTPA was chosen instead of a DOTA analog to avoid the high temperature otherwise required for radiolabeling, which could disrupt antibody stability. After bioconjugation of the p-SCN-Bn-CHX-A”-DTPA to SC16, the immunoconjugates were analyzed using MALDI-TOF MS/MS. The calculated average number of DTPA chelates per SC16 antibody was 1.4 ± 0.1 (Figure S1). Radiolabeling the SC16-CHX-A”-DTPA conjugates yielded high radiochemical yield (≥95%) and specific activity (13–18 mCi/mg) – an outcome indicative of favorable bioconjugation (Figure S2A). [177Lu]Lu-DTPA-CHX-A”-SC16 radioimmunoconjugate was isolated with high radiochemical purity (≥99%) and demonstrated ≥95% serum stability when incubated in human serum at 37°C for seven days (Figures S2B and S3).
Figure 1.
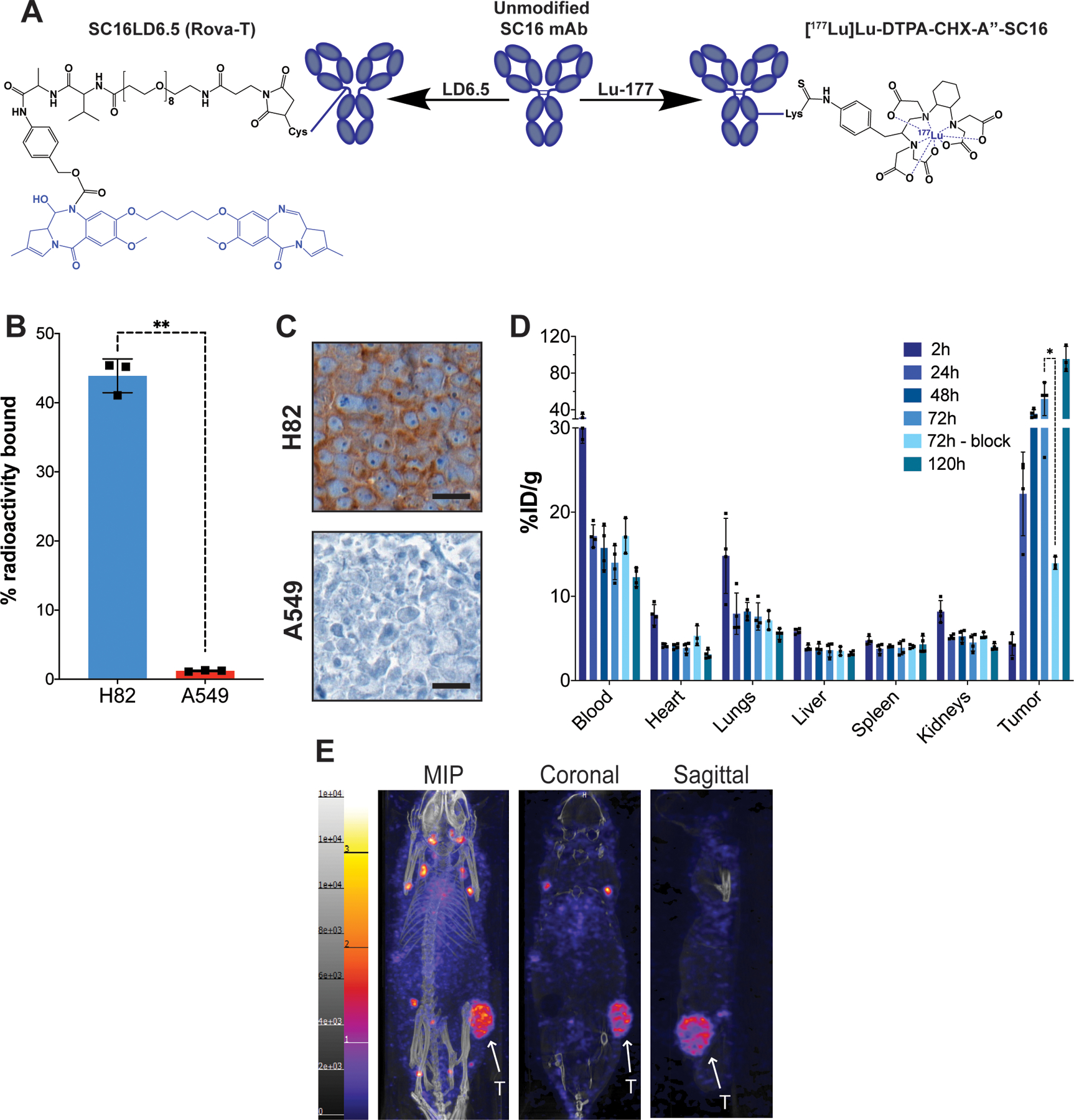
A) Representative structures of the unmodified SC16 antibody, the antibody-drug conjugate SC16LD6.5 (Rovalpituzumab tesirine), and the radioimmunoconjugate [177Lu]Lu-DTPA-CHX-A”-SC16. The cytotoxic moieties of each therapeutic are highlighted in blue. B) A radioligand binding assay of [177Lu]Lu-DTPA-CHX-A”-SC16 in H82 and A549 cells. Error bars represent SD (n=3). An unpaired, two-tailed t-test was performed (p-value = 0.001) C) DLL3 immunohistochemistry on subcutaneous tumor xenografts. Scale bar: 20 μm. See Figure S4 Mouse 108 for the lower magnification image of the H82 tumor DLL3 IHC and corresponding histology. D) The ex vivo biodistribution data of select organs from athymic mice bearing subcutaneous H82 xenografts after the administration of [177Lu]Lu-DTPA-CHX-A”-SC16 (10–30 μCi; 3 μg in 150 uL PBS; n=3 mice per time point) via tail vein. The tumor uptake at 72h was sufficiently blocked using 100-fold excess of unlabeled SC16 (p-value = 0.0212). See Figure S9 for the uptake in all organs collected. E) Representative whole body SPECT/CT images of athymic mice bearing subcutaneous H82 xenografts 120h after the administration of [177Lu]Lu-DTPA-CHX-A”-SC16 (1050–1100 μCi; 60–70 μg in 150 uL PBS) via tail vein. The tumors (indicated by white arrows) can clearly be delineated in the maximum intensity projection (MIP), coronal, and sagittal images.
Having confirmed reproducible production of high specific activity [177Lu]Lu-DTPA-CHX-A”-SC16, we sought to confirm the immunoreactivity of the radioimmunoconjugate using the same cell lines that would be used for in vivo studies. The SCLC NCI-H82 line was selected as a DLL3-positive line and A549 cells were used as a negative control. Previous studies by our group have shown that H82 cells have median DLL3 expression among a cohort of SCLC lines, and we had successfully imaged H82 xenograft tumors in vivo using 89Zr-labeled SC16 (25). H82 was derived from the pleural fluid of a patient with recurrent and progressive disease after prior chemotherapy and chest RT, a similar context in which radioimmunotherapy might be applied clinically (28). Furthermore, H82 xenografts demonstrate rapid growth in athymic nude mice and are notably radioresistant, setting a high bar for in vivo efficacy (29).
To confirm the immunoreactivity of the SC16 antibody for DLL3 post-bioconjugation and radiolabeling, cell binding assays were performed using H82 and A549 cells. [177Lu]Lu-DTPA-CHX-A”-SC16 demonstrated approximately 40-fold differential binding favoring H82, with some (1.2%) non-specific uptake in A549 cells (Figure 1B). To assess maintenance and homogeneity of DLL3 expression, H82 and A549 cells were subcutaneously xenografted into athymic nude mice and allowed to grow to a volume of 2000 mm3, and sections of FFPE tumors were stained for DLL3 expression. DLL3 immunohistochemistry confirmed strong staining in the H82 tumors and minimal staining in the A549 tumors (Figure 1C). Notably, H82 tumors showed membrane-localized staining that colocalized with the viable SCLC cells seen in the H&E and Ki67 IHC (Figures S4).
Ex vivo biodistribution profiles of the SC16 versus isotype control radioimmunoconjugates were assessed in non-tumor-bearing mice as well as mice bearing subcutaneous H82 and A549 xenografts by injection of either [177Lu]Lu-DTPA-CHX-A”-SC16 or [177Lu]Lu-DTPA-CHX-A”-IgG (10–30 μCi; 3 μg in 150 μL PBS; n=3). In non-tumor-bearing mice, organ uptake of [177Lu]Lu-DTPA-CHX-A”-SC16 decreased over time, with the highest radioactivity concentrations in the blood and liver (10.1±2.2 and 12.5±1.9 %ID/g at 120h, respectively; Figure S5A). The biodistribution of [177Lu]Lu-DTPA-CHX-A”-IgG in non-tumor-bearing mice followed similar trends but had lower uptake in the liver and spleen (Figure S5B). To evaluate non-specific tumor uptake, the biodistribution of [177Lu]Lu-DTPA-CHX-A”-SC16 and [177Lu]Lu-DTPA-CHX-A”-IgG was profiled in mice bearing DLL3-negative A549 subcutaneous xenografts. The biodistribution profiles were consistent with those observed in non-tumor-bearing mice, and non-specific uptake in the tumor only reached 4.2 ± 0.9 and 6.4 ± 1.9 %ID/g for [177Lu]Lu-DTPA-CHX-A”-SC16 and [177Lu]Lu-DTPA-CHX-A”-IgG, respectively (Figure S6A and B). Finally, the biodistribution profiles were compared in mice bearing DLL3-positive H82 subcutaneous xenografts. [177Lu]Lu-DTPA-CHX-A”-SC16 showed high tumor uptake in mice bearing H82 subcutaneous xenografts, with uptake reaching 95±14 %ID/g at 120 h post injection (p.i.) (Figure 1D; see Figure S7A for full biodistribution data). Specificity of uptake was demonstrated by co-dosing [177Lu]Lu-DTPA-CHX-A”-SC16 with a 100-fold excess of unlabeled SC16 antibody and euthanizing the mice at 72 h p.i. to evaluate blockade of DLL3-mediated accretion of radioactivity in the tumor. Qualitative SPECT images of mice bearing H82 tumors confirmed the ex vivo biodistribution data showing high tumor uptake and minimal uptake in nontarget organs (Figure 1E). The focal enrichment of [177Lu]Lu-DTPA-CHX-A”-SC16 outside of the tumor that is seen in the MIP is nonspecific lymph node uptake; this is also seen in the MIPs when performing immunoPET imaging using the [89Zr]Zr-DFO-SC16 construct, and it is consistent across various subcutaneous xenograft models in athymic mice. Notably, this nonspecific lymph node uptake is not seen when using [89Zr]Zr-DFO-SC16 to image DLL3 expression in human SCLC patients (NCT04199741), and thus should not pose a risk for radioimmunotherapy. The ex vivo biodistribution of isotype control [177Lu]Lu-DTPA-CHX-A”-IgG in mice bearing H82 xenografts was also assessed, and non-specific tumor uptake peaked at 7.5±1.6 %ID/g at 72 h p.i. (Figure S7B).
Mouse dosimetry indicates blood and liver are the highest concerns for toxicity
Dosimetry calculations were performed prior to radiotherapy experiments to guide dose selection and determine which organs would be most at risk for toxicity from radiation exposure. To generate conservative estimates, dosimetry estimates for nontarget organs were calculated using biodistribution data from non-tumor-bearing mice. Because exogenous macromolecules such as antibodies are catabolized in vivo via the reticuloendothelial system, it was unsurprising that the liver had the highest calculated absorbed dose among non-target organs (Table 1). Notably, the liver can tolerate relatively high radiation exposure (~30 Gy), so hepatotoxicity would not be expected in animals receiving lower doses of radiolabeled antibody. Based on dosimetry calculations and the differential radiosensitivity of non-target organs, we chose to dose animals with 250, 500, and 750 μCi of radiolabeled antibody.
Table 1:
Mouse and human dosimetry of [177Lu]Lu-DTPA-CHX-A”-SC16. The absorbed doses for nontarget organs were calculated using the biodistribution data in non-tumor-bearing athymic mice. The absorbed tumor dose was calculated using the biodistribution data in athymic female mice bearing subcutaneous H82 xenografts.
| Mouse | Human | ||
|---|---|---|---|
| Organ | Absorbed dose coefficient (Gy/mCi) | Therapeutic index | Absorbed dose coefficient (mGy/mCi) |
| Red Marrow | 16.7 | 23.0 | 10.5 |
| Lungs | 34.1 | 11.2 | 4.03 |
| Liver | 91.9 | 4.2 | 84.7 |
| Spleen | 51.1 | 7.5 | 27.1 |
| Kidneys | 28.2 | 13.6 | 25.7 |
| Total Body | 31.0 | 12.4 | 10.7 |
| Tumor | 383.3 | - | - |
[177Lu]Lu-DTPA-CHX-A”-SC16 demonstrates robust efficacy in mouse models of SCLC
To assess the therapeutic potential of 177Lu-labeled SC16, we randomized mice bearing subcutaneous H82 xenografts into eight groups (n=8–10 mice per group). Animals were given vehicle (0.9% sterile saline, 50 μL via intra-peritoneal injection), a single dose of antibody-drug conjugate (either 0.3 mg/kg SC16LD6.5 or IgGLD6.5 via intra-peritoneal injection), unmodified SC16 (60 μg, 150 μL via tail vein injection), or radioimmunoconjugate. The radioimmunoconjugate cohorts were 750 μCi of [177Lu]Lu-DTPA-CHX-A”-IgG control (60 μg, 150 μL via tail vein) or increasing doses of the targeting SC16 radioimmunoconjugate (~250, 500, or 750 μCi of [177Lu]Lu-DTPA-CHX-A”-SC16; 60–70 μg, 150 μL via tail vein injection). As anticipated, the tumors in the saline, IgGLD6.5, and unmodified SC16 control cohorts all progressed to 2000 mm3 at similar rates, indicating these treatments had no efficacy (Figure 2A, Figures S8–10). All mice in the [177Lu]Lu-DTPA-CHX-A”-IgG group developed severe petechiae within the first 10 days, and all mice had to be euthanized by 13 days post-treatment (Figure 2A–C, Figure S11). Surprisingly, the tumors in the SC16LD6.5 cohort demonstrated only a minor and statistically insignificant decrease in tumor progression and no significant improvement in survival relative to the saline control cohort (Figures 2A–C, Figure S12). MALDI-TOF MS/MS was performed to confirm that the SC16LD6.5 was pure and intact (Figure S13). An in vitro cytotoxicity assay was also performed and the EC50 of SC16LD6.5 in H82 cells was determined to be 3.7 pM (Figure S14). The low pM in vitro EC50 confirmed that the SC16LD6.5 was intact and functional; the lack of in vivo efficacy could have been a consequence of suboptimal dosing, although this dosing regimen had demonstrated efficacy in other preclinical models (30,31).
Figure 2.

A) The average tumor volume of mice bearing H82 xenografts after treatment (error bars indicate SD; n=8–10). The averages were adjusted by including the final tumor volume of each mouse if the mouse reached its endpoint prior to 100 days post-treatment (e.g. if a mouse reached its endpoint on day 20, the volume of the tumor on day 20 was still included in the averages after day 20 despite the mouse being euthanized). B) Percent survival as a function of time for mice bearing H82 xenografts following the administration of treatment or vehicle. Log-rank tests were performed for comparing each treatment to saline cohort (shading indicates 95% CI; n=8–10; *** p-value = 0.0003, **** p-value < 0.0001). C) The median survival of mice bearing H82 xenografts post-treatment. Log-rank tests were performed for comparing each treatment to saline cohort (n=8–10; “und” = undefined; *** p-value = 0.0003, **** p-value < 0.0001). D) The average tumor volume of mice bearing Lu149 xenografts after treatment (error bars indicate SD; n=9–10). The averages were adjusted by including the final tumor volume of each mouse if the mouse reached its endpoint prior to 100 days post-treatment (e.g. if a mouse reached its endpoint on day 20, the volume of the tumor on day 20 was still included in the averages after day 20 despite the mouse being euthanized). E) Percent survival as a function of time for mice bearing Lu149 xenografts following the administration of treatment or vehicle. Log-rank tests were performed for comparing each treatment to saline cohort (shading indicates 95% CI; n=9–10; * p-value = 0.0108 and 0.0193 for 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 and [177Lu]Lu-DTPA-CHX-A”-IgG, respectively; **** p-value < 0.0001). F) The median survival of mice bearing Lu149 xenografts post-treatment. Log-rank tests were performed for comparing each treatment to saline cohort (n=9–10; “und” = undefined; * p-value = 0.0108 and 0.0193 for 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 and [177Lu]Lu-DTPA-CHX-A”-IgG, respectively; **** p-value < 0.0001).
The [177Lu]Lu-DTPA-CHX-A”-SC16 radiotherapy demonstrated progressive dose-dependent efficacy. Tumors in the lowest activity cohort (250 μCi) reached 2000 mm3 at a rate indistinguishable from SC16LD6.5 and did not show improved survival over saline control (Figures 2A–C, Figure S15). Tumors in animals assigned to the medium and high activity cohorts (500 and 750 μCi, respectively) both demonstrated tumor reduction ~7–13 days post-treatment consistent with anti-tumor efficacy (Figure 2A). Within the 500 μCi cohort, some animals experienced deep but transient partial responses (e.g. mouse 122, Figure S16) while others demonstrated responses that were sustained for the entire 100 days of the study (e.g. mouse 117 Figure S16). The median survival for the 500 μCi cohort was 80 days post-treatment, significantly higher than the saline treatment group (Figures 2B–C; p = 0.0003). At 100 days post-treatment, two of the eight mice in the 500 μCi dose cohort had not reached an endpoint.
The [177Lu]Lu-DTPA-CHX-A”-SC16 cohort that received 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 demonstrated deeper and more durable anti-tumor responses (Figure 2A and S17). Overall survival in this cohort was significantly longer than the saline control, with the median survival not determined as six of the eight mice had not reached a defined endpoint by 100 days post-treatment (Figure 2B–C, Figure S17; p < 0.0001). Of these six mice, three had evident residual tumor at the site of implantation, while the other three had no palpable residual tumor.
Because of the impressive efficacy demonstrated in the radioresistant H82 model and to assess the broader applicability of the radioconjugate, we repeated the therapy experiments in a subcutaneous patient-derived xenograft model to confirm our findings. The PDX Lu149 was selected for its medium DLL3 expression and ability to grow in athymic nude mice (25). Biodistribution studies were performed to confirm tumor uptake and favorable tumor-to-tissue uptake ratios. Tumor uptake of [177Lu]Lu-DTPA-CHX-A”-SC16 was lower in the Lu149 xenografts, but still reached 74±7 %ID/g at 120 h p.i (Figure S19). The blood uptake of the radioconjugate was slightly higher in the Lu149 model than in the H82 model (Figure S20). Cohorts of tumor-bearing mice were treated with vehicle (0.9% sterile saline, 50 μL), a single dose of 0.4 mg/kg SC16LD6.5, or radioimmunoconjugate via tail vein injections (n=9–10 per group). The radioimmunoconjugate cohorts for the Lu149 therapy studies were the same used in the H82 studies; 750 μCi of [177Lu]Lu-DTPA-CHX-A”-IgG control (60 μg, 150 μL via tail vein) or increasing doses of the targeting SC16 radioimmunoconjugate (~250, 500, or 750 μCi of [177Lu]Lu-DTPA-CHX-A”-SC16; 60–70 μg, 150 μL via tail vein injection). Prior publications and our H82 therapy data confirmed that neither unmodified SC16 or IgGLD6.5 demonstrated anti-tumor efficacy, so these two cohorts were not included in the Lu149 therapy studies.
Tumors in the saline-treated cohort rapidly grew to 2000 mm3, resulting in a median survival of 23 days (Figures 2D–F and S21). Consistent with a prior report (6), SC16LD6.5 demonstrated efficacy in the Lu149 model, with slowed tumor progression and improved survival over the saline cohort (median survival 54 days; Figures 2D–F and S22, p < 0.0001). In contrast with the H82 studies, not all mice treated with 750 μCi [177Lu]Lu-DTPA-CHX-A”-IgG developed severe petechiae; three of the nine mice developed petechiae severe enough to warrant euthanasia, but the remaining six mice demonstrated no or very mild petechiae. For the mice that did not demonstrate severe petechiae, treatment with 750 μCi [177Lu]Lu-DTPA-CHX-A”-IgG led to delayed tumor growth curve, and an overall improved median survival of 47 days (Figures 2D–F and S23; p = 0.0193).
The [177Lu]Lu-DTPA-CHX-A”-SC16 radiotherapy demonstrated impressive anti-tumor efficacy in the Lu149 model, even at low and moderate doses. Nine of the ten tumors in the lowest activity cohort (250 μCi) demonstrated complete responses, defined as the tumor volume remaining less than 100 mm3 (to account for residual scar tissue at the implantation site) 70 days p.i. (Figure 2D and S24). One mouse had a deep and sustained partial response, with the tumor only beginning to grow back 50 days p.i. (mouse 406, Figure S24). All mice were still alive at 70 days p.i.: median survival was not defined but was significantly longer than the saline control cohort (Figure 2E–F, p < 0.0001). The tumors in the 500 μCi cohort demonstrated similar results, with all ten mice having complete responses for the duration of the study and a significantly improved survival with an undefined median (Figure 2D–F and S25, p < 0.0001).
The [177Lu]Lu-DTPA-CHX-A”-SC16 cohort that received 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 also demonstrated deep and durable anti-tumor responses (Figure 2D and S26). However, four of the ten mice developed severe petechiae around days 10–13 p.i. and were therefore euthanized. Of the six mice that did not develop severe enough petechiae to warrant euthanasia, all demonstrated complete responses for the duration of the study. The overall survival again was significantly longer than the saline control, with median survival not determined as six of the ten mice had not reached a defined endpoint by 70 days p.i.(Figures 2E–F; p = 0.01).
Histology on residual tissue at tumor implantation site confirms complete responses and demonstrates DLL3 expression in partial responses
At necropsy, tumor beds from all mice were sectioned for H&E staining and Ki67 IHC was performed to assess for residual microscopic tumor (Figure 3A). In the H82-bearing mice treated with 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16, three of the six mice still alive at 100 days did not have palpable tumors. No viable tumor was observed in any of these three animals; the sites of prior tumor growth revealed a combination of fibrosis, necrosis, calcification, and infiltrating histiocytes. In the Lu149 group, nine of the ten mice that were treated with 250 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 had tumors that were either not palpable or remained <100 mm3 for over two months post-treatment. Of these nine tumors, five were pathologically confirmed complete responses with no residual viable tumor tissue. In the Lu149-bearing mice treated with 500 μCi [177Lu]Lu-DTPA-CHX-A”-SC16, the pathologist determined that seven of the nine tumor beds had no viable tumor, confirming complete responses. Finally, in the Lu149-bearing mice that received 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 and did not require euthanasia for heme toxicity, all of the six mice had tumors that were pathologically confirmed complete responses.
Figure 3.
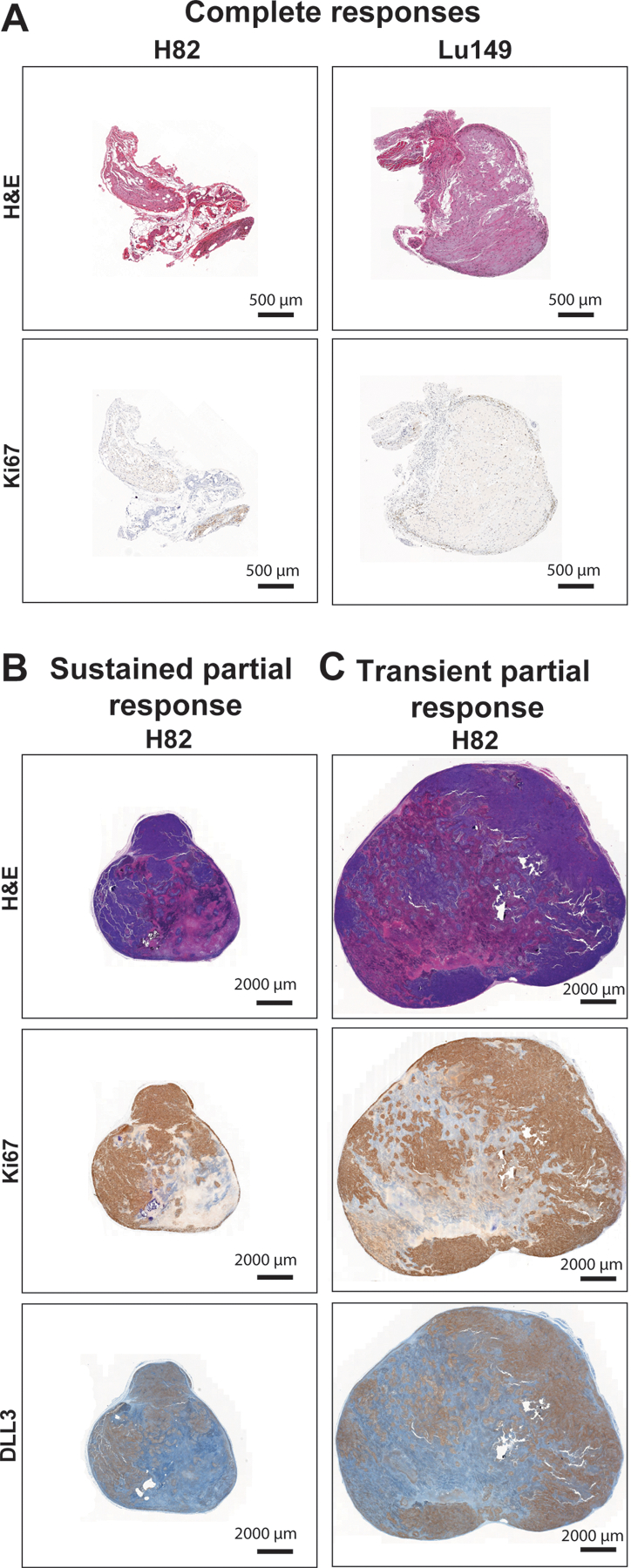
A) Representative H&E staining and Ki67 immunohistochemistry of residual tissues from tumor beds of mice that demonstrated complete responses (H82 mouse 114 and Lu149 mouse 428). Scale bar: 500 μm. B) Representative H&E staining, Ki67 immunohistochemistry and DLL3 immunohistochemistry of the tumor from a mouse that demonstrated a sustained partial response (H82 mouse 126). Scale bar: 2000 μm. C) Representative H&E staining, Ki67 immunohistochemistry and DLL3 immunohistochemistry of the tumor from a mouse that demonstrated a transient partial response (H82 mouse 122). Scale bar: 2000 μm.
While the efficacy experiments were designed to assess the relative benefit of a single dose of therapeutic radioconjugate, repeated dosing should be beneficial in achieving further tumor reduction, and fractionated dosing using lower amounts of activity could ameliorate the toxicity associated with a maximal activity single dose. To determine whether repeated dosing would be feasible in mice demonstrating a partial response to [177Lu]Lu-DTPA-CHX-A”-SC16, we assessed DLL3 target expression on tumors from animals that had experienced partial responses. Examination of tumors at necropsy from these animals demonstrated viable tumor that retained DLL3 expression which colocalized with areas of viability as assessed by Ki67 (Figure 3B–C). These data support the rationale for repeat dosing of [177Lu]Lu-DTPA-CHX-A”-SC16.
[177Lu]Lu-DTPA-CHX-A”-SC16 demonstrates transient heme toxicity and no significant hepatotoxicity
Finally, we wanted to address the concern of the toxicity from [177Lu]Lu-DTPA-CHX-A”-SC16 treatment, as severe toxicity would hinder its translational potential. Mice were weighed twice weekly, and none of the cohorts demonstrated significant weight loss (Figure S28). From dosimetry estimates, we anticipated that blood and liver would be primary concerns for toxicity. To assess heme toxicity, complete blood count analyses were performed weekly for the duration of the study. The pre-treatment ranges for white blood cells (WBCs), red blood cells (RBCs), and platelets (PLTs) were calculated as averages ± one standard deviation (Figure 4A–F; grey box). All three of these parameters demonstrated dose-dependent decreases in both the H82 and Lu149 models. In the H82-bearing mice, all cell types recovered and were consistently within the normal ranges established for female athymic mice (indicated by grey dotted lines, Figure 4A–C) by 4 weeks post-treatment. Notably, none of the H82-bearing mice that received [177Lu]Lu-DTPA-CHX-A”-SC16 exhibited petechiae, despite a transient decrease in platelet counts (Figure 4C). In the Lu149-bearing mice the blood components took longer to recover, with the WBCs, RBCs, and PLTs reaching healthy ranges by eight, seven, and four weeks, respectively (Figure 4D–F). Overall, the hematologic lineages demonstrated dose-dependent and transient decreases with a nadir of 2–3 weeks post dose, and full recovery to the defined normal ranges.
Figure 4.
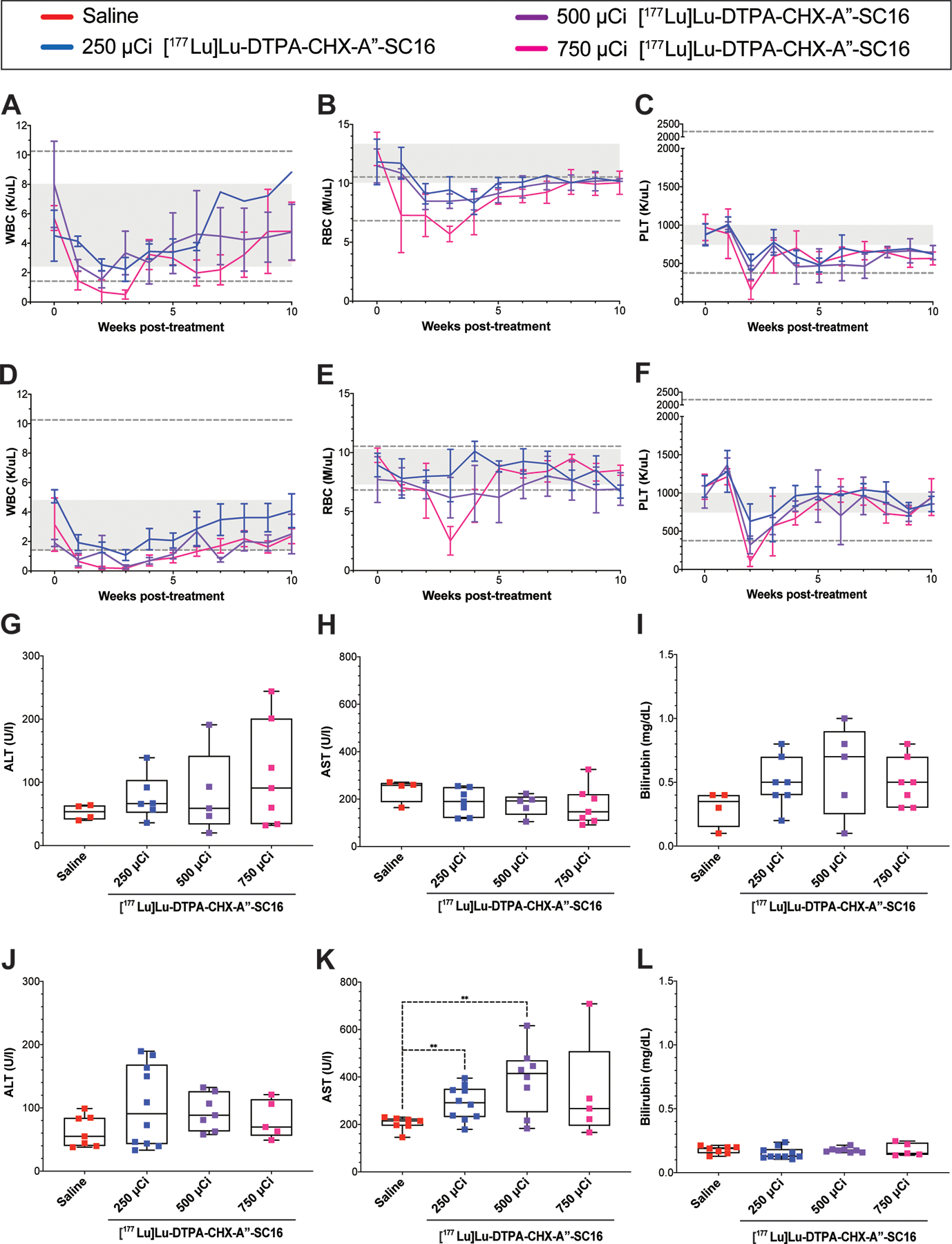
A-C) White blood cell (WBC) counts, red blood cell (RBC) counts, and platelet (PLT) counts of mice bearing H82 xenografts who received [177Lu]Lu-DTPA-CHX-A”-SC16. Complete blood count analyses were performed once a week on a subset of the animals used in the survival study (n=3–4). Twenty parameters were recorded, and three are represented. Values are represented as means with error bars indicating SD. Grey boxes indicate the mean ± SD of values collected from the entire cohort of H82-bearing mice two days prior to therapy administration. Grey dotted lines indicate the healthy range for female athymic mice. D-F) White blood cell (WBC) counts, red blood cell (RBC) counts, and platelet (PLT) counts of mice bearing Lu149 xenografts who received [177Lu]Lu-DTPA-CHX-A”-SC16. Complete blood count analyses were performed once a week on a subset of the animals used in the survival study (n=3–4). Twenty parameters were recorded, and three are represented. Values are represented as means with error bars indicating SD. Grey boxes indicate the mean ± SD of values collected from the entire cohort of Lu149-bearing mice two days prior to therapy administration. Grey dotted lines indicate the healthy range for female athymic mice. H-I) Box plots of the terminal alanine transaminase (ALT), aspartate transaminase (AST) and bilirubin serum levels from H82-bearing mice used in the survival study. Multiple t-tests were performed comparing each radiotherapy cohort to saline, and no significant differences were found (n=4–7). J-L) Box plots of the terminal alanine transaminase (ALT), aspartate transaminase (AST) and bilirubin serum levels from Lu149-bearing mice used in the survival study. Multiple t-tests were performed comparing each radiotherapy cohort to saline (n=5–10; ** p-value = 0.0044 and 0.0067 for 250 and 500 μCi [177Lu]Lu-DTPA-CHX-A”-SC16, respectively).
To evaluate for possible hepatotoxicity, serum and liver were collected for analysis from each mouse bearing an H82 xenograft at euthanasia. Alanine transaminase (ALT), aspartate transaminase (AST), and bilirubin serum levels were measured as markers for hepatotoxicity (Figure 4G–I). Relative to the saline cohort, none of these biomarkers demonstrated increased levels at any of the activity doses of [177Lu]Lu-DTPA-CHX-A”-SC16. The spectrum of morphologic changes observed in the livers of both treated and control animals was consistent with a minimal to mild perivascular (mostly periportal) infiltration of lymphocytes, a common finding in non-SPF mice (Figures S29). Hepatocellular vacuolation and/or hypertrophy were observed rarely (1/6 in the 500 μCi cohort, 1/8 in the 750 μCi cohort) and were hence considered an incidental finding. Minimal hepatocellular degeneration was observed in one of the 250 μCi cohort mice, but is a frequent incidental finding even in experimentally naïve mice (32–34). No correlation was found between the AST and ALT elevation in individual mice with any of these liver changes, together indicating a lack of significant hepatotoxicity at any of the [177Lu]Lu-DTPA-CHX-A”-SC16 doses assessed in the H82-bearing mice.
Serum was also collected at euthanasia from mice bearing Lu149 xenografts, and hepatic enzyme levels were determined to confirm that hepatotoxicity remained negligible. Relative to the saline cohort, AST was the only biomarker that demonstrated significantly increased levels at the 250 and 500 μCi dose cohorts, and none of the biomarkers demonstrated significantly increased levels in the mice treated with 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16 (Figure 4J–L, p = 0.0044 and 0.0067 for 250 and 500 μCi [177Lu]Lu-DTPA-CHX-A”-SC16, respectively). Because a significant increase was only observed in one of the three biomarkers, and an increase was not observed in the highest activity cohort, we concluded that hepatoxicity remained insignificant in the Lu149 xenograft model.
DISCUSSION
The paucity of clinical advances in the treatment of SCLC combined with the exceptionally high mortality rate have led the National Cancer Institute to designate SCLC as one of only two diseases prioritized under the US Recalcitrant Cancer Research Act (35). Clearly, there is an unmet clinical need to develop novel and more durable effective therapeutic approaches for the treatment of SCLC. The nearly universal inactivation or deletion of genes encoding the key cell cycle regulators TP53 and RB1 promotes the rapid and uncontrolled proliferation of this cancer. However, these transformational changes also make it uniquely vulnerable: this tumor is highly sensitive to single and double strand DNA breaks, such as those induced by high energy radiation. While highly effective within the treatment field, a substantial practical limitation of external beam radiation is the inability to address the widespread overt and subclinical metastatic deposits that characterize SCLC. Systemic radioimmunotherapy employing a monoclonal antibody with high specificity for DLL3, a target selectively expressed on the tumor cell surface, can overcome this limitation by delivering targeted radiation to sites of disease. Here we explored this strategy in a proof of principle study in tumor-bearing mice, demonstrating remarkable efficacy in vivo.
[177Lu]Lu-DTPA-CHX-A”-SC16 administered at 500 or 750 μCi doses had substantially higher and more durable antitumor efficacy than the previously clinically active antibody-drug conjugate SC16LD6.5 (rovalpituzumab tesirine) in H82 xenografts, with minimal and transient heme toxicity. Although the SC16LD6.5 dose regimen we selected demonstrated efficacy in other preclinical models, suboptimal dosing could have caused the lack of efficacy shown. Another possible explanation for limited in vivo efficacy of SC16LD6.5 in this xenograft model could be a shared resistance mechanism between the PBD warhead and prior lines of treatment, which for the patient H82 was derived from included high-dose cyclophosphamide, methotrexate, and CCNU followed by vincristine, adriamycin, and procarbazine (28).
In Lu149 xenografts, [177Lu]Lu-DTPA-CHX-A”-SC16 administered at 250, 500 or 750 μCi doses demonstrated even more impressive antitumor efficacy, with concerning heme toxicity only at the highest activity dose. We believe the observed variation in toxicity between the H82 and Lu149 xenografts is attributable to the difference in tumor uptake. Because the average tumor uptake in the Lu149 xenografts is almost 20 %ID/g lower than uptake in the H82 tumors at 120 h p.i., more radioconjugate remains in the blood in the Lu149 model, and this increase in activity in the blood may result in greater heme toxicity. This hypothesis is also supported by the severe petechiae seen in the mice who received the nontargeting [177Lu]Lu-DTPA-CHX-A”-IgG as the nontargeting radioconjugate only has minimal nonspecific intratumoral accumulation.
Despite the heme toxicity seen in some Lu149-bearing mice that received 750 μCi [177Lu]Lu-DTPA-CHX-A”-SC16, single doses at 250 or 500 μCi still led to pathologically confirmed complete responses, indicating that higher activity doses might be unnecessary in more radiosensitive tumors. While both tumor models display aggressive growth in vivo, the radiotherapeutic displayed more impressive antitumor efficacy in the Lu149 xenografts than the H82 xenografts because we specifically chose the H82 xenografts for their radioresistance. As previously discussed, the H82 cell line was derived from a patient that had been heavily pre-treated and was highly resistant to DNA-damaging agents, while the Lu149 xenograft model was derived from a patient that was treatment-naïve. Therefore even though the Lu149 xenografts display lower DLL3 expression than the H82 xenografts (and therefore lower tumor uptake of the radiotherapeutic), the intrinsic sensitivity of each tumor to DNA damage is vastly different, leading to the difference in therapeutic response. Nevertheless, the Lu149 PDX may represent more typical radiosensitivity of SCLC and offers strong evidence for the clinical potential of [177Lu]Lu-DTPA-CHX-A”-SC16.
While it was particularly striking that multiple H82-bearing mice and the majority of Lu149-bearing mice had pathologically confirmed complete responses, complete response to a single dose of the radioconjugate is unlikely in patients with bulkier disease. The initial toxicity profile of [177Lu]Lu-DTPA-CHX-A”-SC16 and retention of DLL3 expression after initial dosing suggests that this agent could be periodically administered in cycles allowing for hematologic recovery. Multiple 177Lu-antibody conjugates have been tested in clinical trials, and their heme toxicity in human patients is well established and manageable, in contrast to SC16LD6.5 for which adverse effects related to the pyrrolobenzodiazepine warhead precluded repetitive dosing in patients (19–21).
The demonstrated efficacy of [177Lu]Lu-DTPA-CHX-A”-SC16 can be considered in context of our previously published studies demonstrating that the SC16 antibody can be repurposed for robust immunoPET imaging of SCLC using 89Zr ([89Zr]Zr-DFO-SC16) (25). 89Zr-immunoPET of SCLC patients to identify those who would benefit from targeted radioimmunotherapy with [177Lu]Lu-DTPA-CHX-A”-SC16 would appear to be a clinically viable approach. An analogous approach is currently being tested clinically using the 5B1 antibody in pancreatic ductal adenocarcinoma (NCT02687230 and NCT03118349). The trace doses of [89Zr]Zr-DFO-SC16 used for PET imaging would not preclude uptake of the therapeutic radioimmunoconjugate. ImmunoPET of SCLC patients with [89Zr]Zr-DFO-SC16 is currently underway (NCT04199741).
The promising efficacy demonstrated here, with multiple pathologically confirmed complete responses and minimal evident toxicity at effective activity doses, contrasts with a recent report by Lakes and colleagues exploring anti-DLL3 radioimmunotherapy using both Lu-177 and the alpha emitter Ac-225 (36). The Lakes study employed NOD-SCID mice and demonstrated dose-limiting hematologic toxicity in the range of 21–64 μCi with a 177Lu-labeled antibody, which is below the effective range defined in our analysis of H82 and Lu149 xenografts. We believe the severity of this toxicity is attributable to the mouse background, as NOD-SCID mice harbor the mutated Prkdc gene which makes them much more sensitive to radiation than athymic mice, or humans (37,38). We too observed dose-dependent hematologic toxicity in our studies, but overt and concerning toxicity (i.e. petechiae) was only observed in a minority of Lu-149 bearing mice in the cohort receiving the highest activity dose of [177Lu]Lu-DTPA-CHX-A”-SC16, while none of the H82-bearing mice demonstrated severe petechiae. The blood components also recovered back to pre-treatment levels, suggesting that the toxicity does not have long-term implications.
In conclusion, the remarkable efficacy, durability of tumor responses, and low toxicity of [177Lu]Lu-DTPA-CHX-A”-SC16 treatment indicates that this is a promising candidate for clinical translation in the treatment of SCLC. While the present work focuses on targeting DLL3 using radioimmunotherapy for SCLC, DLL3 is expressed at comparable levels on the cell surface of other tumor types including neuroendocrine prostate cancer and glioma, implying that this therapeutic approach could be widely applicable for malignancies with aberrant DLL3 expression (30,39).
Supplementary Material
TRANSLATIONAL RELEVANCE.
Small cell lung cancer (SCLC) is a particularly lethal form of lung cancer with a five-year survival rate of less than 5%. Although SCLC is quite radiosensitive, the majority of patients have distant metastases at the time of diagnosis, making them poor candidates for traditional external beam radiation therapy. Delta-like ligand 3 (DLL3) is an attractive target for SCLC as its expression on the cellular surface is almost exclusive to malignant tissue in adults. Herein, we developed a radioimmunotherapy approach for SCLC treatment by radiolabeling the anti-DLL3 antibody SC16 with the beta particle-emitting therapeutic radioisotope Lu-177. [177Lu]Lu-DTPA-CHX-A”-SC16 demonstrated remarkable efficacy in subcutaneous xenograft mouse models of SCLC, with mild and transient heme toxicity. These findings support [177Lu]Lu-DTPA-CHX-A”-SC16 as a candidate for rapid clinical translation for the treatment of SCLC.
ACKNOWLEDGEMENTS
This work was supported in part by NIH grants U01 CA213359 and R01 CA213448 (JTP, CMR, JSL) R35 CA263816 (CMR) and R35 CA232130 (JSL). KMT was supported by the Weill Cornell Graduate School of Medical Sciences T32 GM073546 Predoctoral Training Grant in Pharmacological Sciences. Technical services provided by the MSKCC Small-Animal Imaging Core Facility, supported in part by NIH Cancer Center Support Grant P30 CA998748, are gratefully acknowledged. NIH Shared Instrumentation Grant S10 RR028889, which provided funding support for the purchase of the NanoSPECT/CT Plus, is gratefully acknowledged. Finally, the authors gratefully acknowledge the Memorial Sloan Kettering Cancer Center Anti-Tumor Assessment Core, the Memorial Sloan Kettering Molecular Cytology Core, and the Tri-Institutional Laboratory of Comparative Pathology.
Footnotes
Potential Conflicts of Interest
CMR has consulted regarding oncology drug development with Amgen, AstraZeneca, Celgene, Epizyme, Genentech/Roche, Ipsen, Jazz, Lilly, Pfizer, PharmaMar, Syros, and Vavotek. He serves on the scientific advisory boards of Bridge Medicines, Earli, and Harpoon Therapeutics. JSL has consulted for InVicro, TPG Capital, Longitude Capital, Curie Therapeutics, Earli and Sharp RTx. He serves on the scientific advisory boards of pHLIP, Clarity Pharmaceuticals, Evergreen Theragnostics and Telix Pharmaceuticals Ltd. He is a co-inventor on licensed technology to pHLIP, Elucida Oncology, Samus Therapeutics, CheMatech, Theragnostics, Daiichi Sankyo, Diaprost AB and Sharp RTx. KMT, JTP, CMR, and JSL are inventors on filed patents involving DLL3 antibodies.
References
- 1.Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 2017;17(12):725–37 doi 10.1038/nrc.2017.87. [DOI] [PubMed] [Google Scholar]
- 2.Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol 2017;14(9):549–61 doi 10.1038/nrclinonc.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oronsky B, Reid TR, Oronsky A, Carter CA. What’s New in SCLC? A Review. Neoplasia 2017;19(10):842–7 doi 10.1016/j.neo.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers 2021;7(1):3 doi 10.1038/s41572-020-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell JE. Small cell lung cancer: are we making progress? Am J Med Sci 2010;339(1):68–76 doi 10.1097/MAJ.0b013e3181bccef5. [DOI] [PubMed] [Google Scholar]
- 6.Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, et al. A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015;7(302):302ra136 doi 10.1126/scitranslmed.aac9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borromeo MD, Savage TK, Kollipara RK, He M, Augustyn A, Osborne JK, et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep 2016;16(5):1259–72 doi 10.1016/j.celrep.2016.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuta M, Kikuchi H, Shoji T, Takashima Y, Kikuchi E, Kikuchi J, et al. DLL3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci 2019;110(5):1599–608 doi 10.1111/cas.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan LX, Liu YH, Li Z, Luo DL, Li YF, Yan JH, et al. Prognostic value of delta-like protein 3 combined with thyroid transcription factor-1 in small-cell lung cancer. Oncol Lett 2019;18(3):2254–61 doi 10.3892/ol.2019.10538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudin CM, Poirier JT, Byers LA, Dive C, Dowlati A, George J, et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nat Rev Cancer 2019;19(5):289–97 doi 10.1038/s41568-019-0133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman G, Sparrow DB, Kremmer E, Dunwoodie SL. Notch inhibition by the ligand DELTA-LIKE 3 defines the mechanism of abnormal vertebral segmentation in spondylocostal dysostosis. Hum Mol Genet 2011;20(5):905–16 doi 10.1093/hmg/ddq529. [DOI] [PubMed] [Google Scholar]
- 12.Geffers I, Serth K, Chapman G, Jaekel R, Schuster-Gossler K, Cordes R, et al. Divergent functions and distinct localization of the Notch ligands DLL1 and DLL3 in vivo. J Cell Biol 2007;178(3):465–76 doi 10.1083/jcb.200702009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang RSP, Holmes BF, Powell C, Marati RV, Tyree D, Admire B, et al. Delta-like Protein 3 Prevalence in Small Cell Lung Cancer and DLL3 (SP347) Assay Characteristics. Arch Pathol Lab Med 2019;143(11):1373–7 doi 10.5858/arpa.2018-0497-OA. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Isse K, Fujihira T, Takenoyama M, Saunders L, Bheddah S, et al. Prevalence of Delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer 2018;115:116–20 doi 10.1016/j.lungcan.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Rojo F, Corassa M, Mavroudis D, Oz AB, Biesma B, Brcic L, et al. International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer 2020;147:237–43 doi 10.1016/j.lungcan.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 16.Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbone DP, He K. DLL3: an emerging target in small cell lung cancer. J Hematol Oncol 2019;12(1):61 doi 10.1186/s13045-019-0745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson ML, Zvirbule Z, Laktionov K, Helland A, Cho BC, Gutierrez V, et al. Rovalpituzumab Tesirine as a Maintenance Therapy After First-Line Platinum-Based Chemotherapy in Patients With Extensive-Stage-SCLC: Results From the Phase 3 MERU Study. J Thorac Oncol 2021;16(9):1570–81 doi 10.1016/j.jtho.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Blackhall F, Jao K, Greillier L, Cho BC, Penkov K, Reguart N, et al. Efficacy and Safety of Rovalpituzumab Tesirine Compared With Topotecan as Second-Line Therapy in DLL3-High SCLC: Results From the Phase 3 TAHOE Study. J Thorac Oncol 2021;16(9):1547–58 doi 10.1016/j.jtho.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 19.AbbVie Discontinues Rovalpituzumab Tesirine (Rova-T) Research and Development Program. In: Taveras C, editor: Abbvie.com; 2019.
- 20.Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, et al. Efficacy and Safety of Rovalpituzumab Tesirine in Third-Line and Beyond Patients with DLL3-Expressing, Relapsed/Refractory Small-Cell Lung Cancer: Results From the Phase II TRINITY Study. Clin Cancer Res 2019;25(23):6958–66 doi 10.1158/1078-0432.CCR-19-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poirier JT, George J, Owonikoko TK, Berns A, Brambilla E, Byers LA, et al. New Approaches to SCLC Therapy: From the Laboratory to the Clinic. J Thorac Oncol 2020;15(4):520–40 doi 10.1016/j.jtho.2020.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with cisplatin and etoposide for limited-stage small-cell lung cancer: results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol 2002;20(14):3054–60 doi 10.1200/JCO.2002.12.071. [DOI] [PubMed] [Google Scholar]
- 23.Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med 2007;357(7):664–72 doi 10.1056/NEJMoa071780. [DOI] [PubMed] [Google Scholar]
- 24.Auperin A, Arriagada R, Pignon JP, Le Pechoux C, Gregor A, Stephens RJ, et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N Engl J Med 1999;341(7):476–84 doi 10.1056/NEJM199908123410703. [DOI] [PubMed] [Google Scholar]
- 25.Sharma SK, Pourat J, Abdel-Atti D, Carlin SD, Piersigilli A, Bankovich AJ, et al. Noninvasive Interrogation of DLL3 Expression in Metastatic Small Cell Lung Cancer. Cancer Res 2017;77(14):3931–41 doi 10.1158/0008-5472.CAN-17-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hindie E, Zanotti-Fregonara P, Quinto MA, Morgat C, Champion C. Dose Deposits from 90Y, 177Lu, 111In, and 161Tb in Micrometastases of Various Sizes: Implications for Radiopharmaceutical Therapy. J Nucl Med 2016;57(5):759–64 doi 10.2967/jnumed.115.170423. [DOI] [PubMed] [Google Scholar]
- 27.O’Donoghue JA, Bardies M, Wheldon TE. Relationships between tumor size and curability for uniformly targeted therapy with beta-emitting radionuclides. J Nucl Med 1995;36(10):1902–9. [PubMed] [Google Scholar]
- 28.Phelps RM, Johnson BE, Ihde DC, Gazdar AF, Carbone DP, McClintock PR, et al. NCI-Navy Medical Oncology Branch cell line data base. J Cell Biochem Suppl 1996;24:32–91 doi 10.1002/jcb.240630505. [DOI] [PubMed] [Google Scholar]
- 29.Laird JH, Lok BH, Ma J, Bell A, de Stanchina E, Poirier JT, et al. Talazoparib Is a Potent Radiosensitizer in Small Cell Lung Cancer Cell Lines and Xenografts. Clin Cancer Res 2018;24(20):5143–52 doi 10.1158/1078-0432.CCR-18-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puca L, Gavyert K, Sailer V, Conteduca V, Dardenne E, Sigouros M, et al. Delta-like protein 3 expression and therapeutic targeting in neuroendocrine prostate cancer. Sci Transl Med 2019;11(484) doi 10.1126/scitranslmed.aav0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sano R, Krytska K, Tsang M, Erickson SW, Teicher BA, Saunders L, et al. Abstract LB-136: Pediatric Preclinical Testing Consortium evaluation of a DLL3-targeted antibody drug conjugate rovalpituzumab tesirine, in neuroblastoma. Cancer Research 2018;78(13 Supplement):LB-136-LB- doi 10.1158/1538-7445.Am2018-lb-136. [DOI] [Google Scholar]
- 32.Harada T, Maronpot RR, Enomoto A, Tamano S, Ward JM. Changes in the liver and gallbladder. Pathobiology of the aging mouse 1996;2:207–41. [Google Scholar]
- 33.Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, et al. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol 2010;38(7 Suppl):5S–81S doi 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- 34.Faccini J, Abbott DP, Paulus G. MOUSE HISTOPATHOLOGY: A glossary for use in toxicity and carcinogenicity studies. 1990.
- 35.US Congress. H.R.733 — Recalcitrant Cancer Research Act of 2012. 2012.
- 36.Lakes AL, An DD, Gauny SS, Ansoborlo C, Liang BH, Rees JA, et al. Evaluating (225)Ac and (177)Lu Radioimmunoconjugates against Antibody-Drug Conjugates for Small-Cell Lung Cancer. Mol Pharm 2020;17(11):4270–9 doi 10.1021/acs.molpharmaceut.0c00703. [DOI] [PubMed] [Google Scholar]
- 37.Fulop GM, Phillips RA. The scid mutation in mice causes a general defect in DNA repair. Nature 1990;347(6292):479–82 doi 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- 38.Biedermann KA, Sun JR, Giaccia AJ, Tosto LM, Brown JM. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A 1991;88(4):1394–7 doi 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spino M, Kurz SC, Chiriboga L, Serrano J, Zeck B, Sen N, et al. Cell Surface Notch Ligand DLL3 is a Therapeutic Target in Isocitrate Dehydrogenase-mutant Glioma. Clin Cancer Res 2019;25(4):1261–71 doi 10.1158/1078-0432.CCR-18-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are included in this published article and its supplementary information files. Original data is available upon request from the corresponding authors.


