Abstract
Background:
A Erectile dysfunction (ED) is one of the well-known comorbidities in males with diabetes mellitus (DM), whose pathogenesis might be induced by dysregulation of corpus cavernosum smooth muscle cells. UC-MSCs are multipotent cells that attract considerable interest due to immunoregulatory properties and might be a potential strategy to regulate and recover the functional cells and tissues, including tissue improvement in DMED.
Objective:
This study aims to determine the efficacy of UC-MSCs in improving the erectile function of DMED rats through analyzing the expression of TGF-β, α-SMA, and collagen.
Methods:
Total number of 30 male Sprague-Dawley rats (6 to 8 weeks old) were randomly divided into four groups (negative control group, positive control group, T1 group, and T2 group). After 16 h fast, 24 rats were randomly selected and intraperitoneally injected with streptozotocin to induce DM. At 8 weeks after STZ injection, rats with DMED were identified by unresponsive erectile stimulation within 30 min. PC group received 500 μL; T1 rats treated with 500 μL PBS containing 1x106 UC-MSCs; T2 rats treated with 500 μL PBS containing 3x106 UC-MSCs. After MSCs treatment, the rats were sacrificed and the corpus cavernosum tissues were prepared for histological observations.
Results:
This study resulted in the administration of UC-MSCs could downregulate the expression of TGF-β, α-SMA, and collagen leading to the improvement of DMED.
Conclusion:
UC-MSCs improve the expression of TGF-β, α-SMA, and collagen on erectile dysfunction in streptozotocin-induced diabetic rats.
Keywords: UC-MSC, TGF-β, α-SMA, collagen, DMED
1. BACKGROUND
Erectile dysfunction (ED) is the determined inability to perform an erection hard enough for sexual intercourse (1, 2). Penile erection is induced by various stimuli which leads to the release of nitric oxide (NO) from the nerve finishes causing vasodilation and boosted blood flow into the erectile tissues (3, 4). ED is a more prevalent complication of diabetes mellitus (DM) in men, affecting up to 85% of DM patients (4). Diabetes mellitus-induced erectile dysfunction (DMED), more refractory than ED in nondiabetic patients, is believed to be mainly due to endothelial injury, decreased smooth muscle content, neuropathy, and fibrosis (5, 6). Currently, the use of selective phosphodiesterase type-5 inhibitors (PDE5i) is considered to be the first-line agent for ED, however, it has much lower efficacy to treat patients with DMED (3, 4, 7). In this condition, revealing the novel alternative approach is a major needed to ameliorate the development of DMED.
Over the past years, stem cell therapy is considered to be a potential strategy due to its capability to regulate the inflammatory milieu and recover the functional cells and tissues, including tissue improvement in DMED (4, 8). However, the specific mechanism of MSC in improving DMED remains to be elucidated, especially through the TGF-β, α-sma, and collagen pathways. Therefore, we wanted to investigate the mechanism of MSCs in enhancing mouse DMED via the TGF-β, α-sma, and collagen pathways. Umbilical cord-derived mesenchymal stem cells (UC-MSCs) are ubiquitous fibroblast-like-multipotent cells that attract considerable interest due to they can be easily isolated and abundantly assessed from the UC tissues through standardized procedures and have low immunogenicity potential (9, 10). These cells could be characterized by a high level of surface antigens, such as CD90, CD105, CD73, CD29, and low level of CD31, CD34, CD45, CD14, CD19, and HLA-DR (11, 12). They possess a broad range of capabilities to differentiate into specific somatic cell lineages, such as osteocytes, adipocytes, chondrocytes, and neural cells (13, 14). Other impressive potencies of UC-MSCs are their immunomodulatory and regeneration properties through secreting various trophic factors (15, 16). MSCs also have immunosuppressive features, turning the alteration from inflammation to proliferation, which advances the healing manner (16, 17). MSCs release anti-inflammatory cytokines, such as prostaglandin E2(PGE2), transforming growth factor-β (TGF-β), indoleamine 2,3-dioxygenase (IDO), and interleukin (IL)-10, to regulate the inflammation (18, 19).
A recent study revealed that transplantation of the MSCs into penile tissues of DMED rats can improve the impaired arterial flow and structural changes in erectile function (5). Another previous study also showed that intra-cavernous transplantation of VEGF gene-modified bone marrow-derived MSCs resulted in improved erectile function in diabetic rats (20). The mechanism of this improvement may be via differentiating into endothelial cells and smooth muscle cells, and the secretion by these cells of many kinds of cytokines and other effects (21, 22). In addition, another study also demonstrated that MSCs engineered to express stromal cell-derived factor 1 (SDF-1) have been shown to reduce erectile dysfunction in streptozotocin-induced diabetic rats by increasing levels of neuronal nitric oxide synthase (nNOS), VEGF, and growth factors (23). A recent study revealed that impaired arterial flow and structural changes in cavernosal dysfunction were manifested by elevation of TGF-β and increased collagen accumulation resulting in fibrosis (24). However, studies related to improving erectile function with UC-MSC, especially those related to the expression of TGF-β and α-SMA resulting collagen accumulation have not been studied before.
2. OBJECTIVE
In this study, we aimed to determine the efficacy of UC-MSCs in improving the erectile function of DMED rats through analyzing the expression of TGF-β, α-SMA and collagen.
3. MATERIAL AND METHODS
Animals and Ethical Clearance
Animal experiments were performed according to the regulations by the Ethical Committee of Faculty of Medicine, Universitas Sumatera Utara. This study was approved by Ethical Committee, with the number 370/KEP/USU/2020. A total of 30 male Sprague-Dawley rats (8 weeks-old) weighting 200-250 g were purchased from Lembaga Penelitian dan Pengujian Terpadu (LPPT), Unit IV, Universitas Gadjah Mada. After 16 h fast, 24 rats were randomly selected and intraperitoneally injected with streptozotocin (STZ, 60 mg/kg, Sigma-Aldrich, St. Louis, MO, USA). The remaining 6 rats were employed as an NC group (rats with no DMED received 500 μL phosphate buffer saline (PBS)). Blood glucose levels were analyzed at 3 days after STZ injection using a pharmaceutical-grade glucometer (Accu-Check; Roche, Basel, Switzerland). Of the 24 rats, 21 (87.50%) were diabetic with fasting glucose levels higher than 300 mg/dl. At 8 weeks after STZ injection, apomorphine (APO, 100 μg/kg; Sigma-Aldrich, St. Louis, MO, USA) was used to select the diabetic rats and administrated via neck loose skin. Rats with DMED were present in 18 of the 21 (85.71%) and identified by unresponsive erectile stimulation within 30 min.
Research Design
A total of 24 rats were used in this research. The 6 healthy rats were used as NC group and the 18 selected DMED rats were randomly assigned into three groups (six rats in each group): PC group (DMED rats received 500 μL PBS); T1 (DMED rats treated with 500 μL PBS containing 1x106 UC-MSCs); T2 (DMED rats treated with 500 μL PBS containing 3x106 UC-MSCs).
UC-MSCs Isolation and Culture
UC-MSCs were isolated from UC collected from 19-day pregnant female rats and cultured as per previous standardized methods (25). In brief, the tissue parts of the UC were cut into smaller pieces under aseptic conditions. The section of UC tissues was planted into a culture flask containing Dulbecco’s Modified Eagle Medium (DMEM) (Sigma-Aldrich, Louis St, MO), supported with 1% penicillin (100 U/mL)/streptomycin (100 µg/mL) (Gibco™ Invitrogen, NY, USA), 0,25% amphotericin B (Gibco™ Invitrogen, NY, USA) and 10% fetal bovine serum (FBS) (Gibco™ Invitrogen, NY, USA) and cultured at 37° C in a humid atmosphere consisting of 5% CO2. The culture medium was renewed every 3 days. After reaching approximately 80% confluence, UC-MSCs-like in culture flasks were passage into a new culture flask. UC-MSCs-like at passage 4 were employed for the following experiments.
UC-MSC Phenotype Analysis with Flow cytometry
UC-MSCs-like were characterized by flow cytometry at passage 4. Briefly, after UC-MSCs were passaged and washed, the cells were incubated for 30 min in PBS containing anti-CD90.1-PerCP (cat. # 557266; BD, San Diego, CA, USA), anti-CD29-Alexa fluor 647 (cat. # 562153; BD, San Diego, CA, USA), anti-CD45-FITC (cat. # 554877; BD, San Diego, CA, USA), anti-CD31-PE (cat. # 555027; BD, San Diego, CA, USA) for 30 minutes at room temperature in the dark. The cells then were washed twice using PBS. The flow cytometry and post-acquisition analysis were performed and calculated using a BD Accuri C6 Plus flow cytometer (BD, San Diego, CA, USA) and BD Accuri C6 Plus software (BD, San Diego, CA, USA).
Differentiation of UC-MSCs
We further performed the osteogenic differentiation of UC-MSCs-like in passage 4. The UC-MSCs-like were cultured in standard medium containing DMEM supplemented with 10% FBS, 1% penicillin (100 U/mL)/streptomycin (100 µg/mL) and 0,25% amphotericin B at 37°C and 5% CO2 until reached 95% confluency. Then, the standard medium was replaced using an osteogenic differentiation medium containing MesenCult™ MSC Basal Medium (Mouse) (cat. # 05505; Stem Cell Technologies, Singapore) with 20% MesenCult™ Osteogenic Stimulatory Supplement (mouse) (cat # 05506; Stem Cell Technologies, Singapore), 1% L-Glutamine (Gibco™ Invitrogen, NY, USA), 1% penicillin (100 U/mL)/streptomycin (100 µg/mL) and 0,25% amphotericin B. This medium was replaced every 3 days. Alizarin red (Sigma-Aldrich, Louis St, MO) staining was performed followed by 21 days of osteogenic induction of MSCs. The calcium deposition was shown in red color.
Immunostaining Analysis
After the completion of the APO experiment, five animals from each group were randomly selected, anesthetized and sacrificed by cervical dislocation. Half of the penile tissue from each subject (10 µm) was dehydrated in 30% sucrose and then frozen. After the sections were completely dried, immunostaining staining for α-SMA and TGF-β was performed, using the penile tissue of normal rats as a negative control. At least five random fields per section were examined, and the semi-quantitative evaluations were analyzed with Image-Pro Plus software, version 6.0 (Media Cybernetics, Inc.).
Masson trichrome staining
Penile tissues were fixed in fresh 4% paraformaldehyde. The fixed tissues were embedded in paraffin and cut into 8 μm thick sections before mounting on slides. Masson trichrome staining was employed to evaluate the relative ratio of smooth muscle to collagen as previously described (Yang et al., 2013). Image-Pro Plus 6.0 software (Media Cybernetics Inc, Bethesda, MD, USA) was used to quantitatively analyze smooth muscle content and collagen in five randomly selected specimens per group.
Data analysis
Data are displayed as the means ± standard deviation (SD). The calculations were performed using SPSS 23.0 (IBM Corp., Armonk, NY, USA). The statistical significance of the differences between groups was assessed by the Kruskal-Wallis test, followed by Mann-Whitney posthoc analysis. A p-value of <0.05 and <0.001 was considered significant.
4. RESULTS
Isolation and Characterization of MSCs
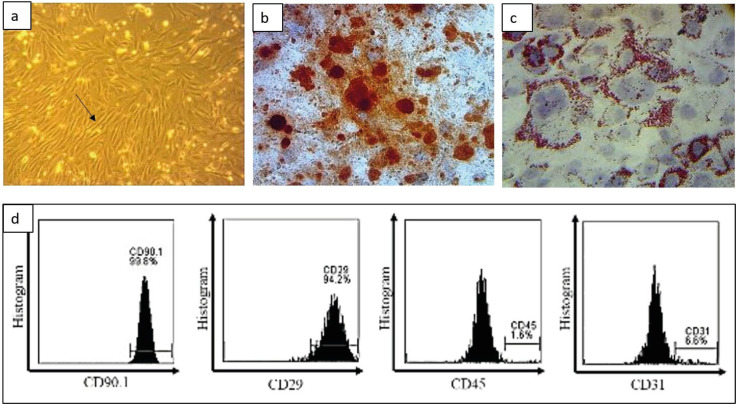
The isolation of MSCs has carried out at the Stem Cell and Cancer Research (SCCR) Laboratory, Faculty of Medicine, Sultan Agung Islamic University, Semarang, using umbilical cords from 21 days pregnant rats. The results of the isolation were then cultured on a flask with DMEM medium with 10% FBS, 1% antibiotic, and 0.25% fungizone. MSCs culture after the fourth passage showed cells adhering to the bottom of the flask with spindle-like cell morphology under microscopic observation (Figure 1a). The osteogenic and adipogenic ability of MSC is also one of the MSC validation methods. MSCs were able to differentiate into osteogenic as indicated by calcium deposits in the form of red staining using Alizarin red staining (Figure 1b). The adipogenic differentiation test was performed by inducing MSCs using a special adipogenic medium that formed adipocytes (Figure 1c). Furthermore, the validation of mouse MSCs markers was carried out using flow cytometry analysis which showed that MSCs were able to express CD90 (99.8%), CD29 (94.2%), and lack of CD45 (1.6%) and CD31 (6.6%) (Figure 1d).
Figure 1. Characteristics of MSCs. (a) The spindle-like morphology of MSC is pointed to by the arrow on the microscope observation (200x magnification), (b) The results of the osteogenic differentiation test show red color representing the results of post-cultured calcium deposits with osteogenic differentiation medium, (c) The adipogenic differentiation test shows that there are fat deposits on the MSCs marked in red using Oil Red O staining (magnification 400x), (d) Flow cytometry analysis of the expression of CD90, CD29, CD45, and CD31.
Measurement of rat’s body weight and blood glucose
The final weight of STZ-induced diabetic rats on PC groups was notably lower than NC rats (P < 0.05). The final blood glucose levels of diabetic rats were remarkably higher compared to PBS-treated NC rats (P < 0.05). The final weight of T1 and T2 groups after received MSC’s administration was significantly higher than PC groups (p<0.05). On another side, the final blood glucose levels of the T1 and T2 groups were also significantly reduced than the PC groups (p<0.05; Table 1).
Table 1. Body weight and blood glucose variable.
| Variable | Sham | Control | T1 | T2 |
|---|---|---|---|---|
| Initial Weight (g) | 213.6±13.8 | 208.8±12.2 | 209.6±13.0 | 211.8±10.3 |
| Final Weight (g) | 346.6±29.3* | 194.2±8.2 | 231.6±22.4* | 293.8±7.3* |
| Initial glucose (mg/dL) | 170.2±11.9 | 156.8±10.5 | 164.7±12.4 | 174.7±8.6 |
| Final glucose (mg/dL) | 140.2±25.9* | 512.8±47.2 | 431.4±27.0* | 265.4±64.0* |
| Values are expressed as mean ± s.d. *P<0.05 when compared with control group. s.d: standard deviation. | ||||
MSCs improve the erectile function of DMED rats
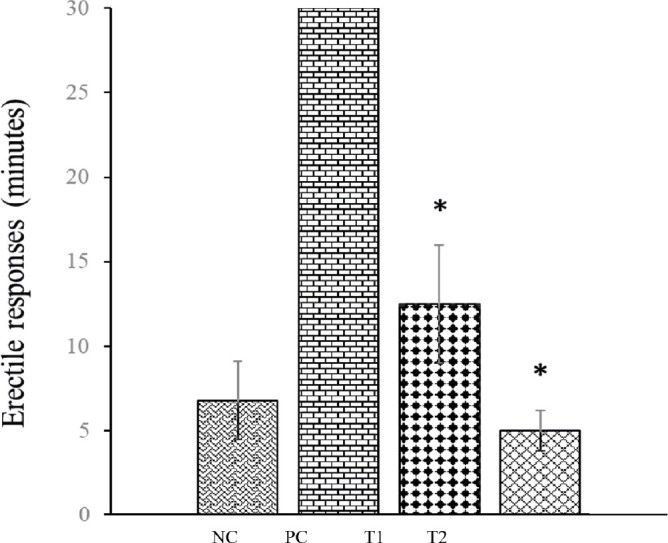
The measurement of erectile function resulted in that streptozotocin-induced DMED caused a significant reduction in erectile response in rats. The rats in T1 and T2 groups after MSCs administration showed a significant increase of erectile response (P<0.05), in which the effective improvement was on the T2 group with a value of 5 ± 1.2 minutes (Figure 2).
Figure 2. Erectile function measurement in rats with DMED. Graph summarizing the quantitative data of erectile responses DMED rats and expressed as mean ± standard deviation. *P < 0.05 compared with control.
UC MSCs regulate TGF-β expression on DMED Rats
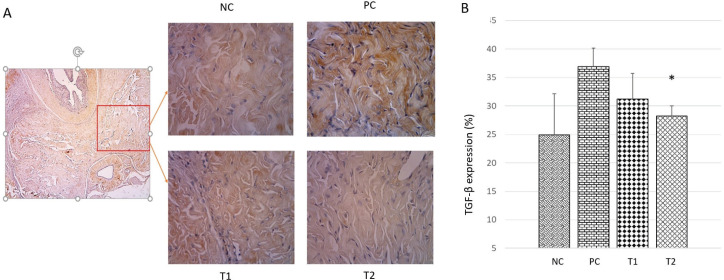
TGF-β plays a role in inducing cell stress in diabetic conditions and plays a role in the initiation of collagen formation in penile tissue. Increased expression of TGF-β is associated with increased collagen synthesis in the corpus cavernosum tissue of streptozotocin-induced diabetic rats and the vasculogenic ED model. Analysis of TGF-β levels in this study was carried out using the immunohistochemical method (Figure 3a). Based on the histological semi-quantitative observation of the TGF-β expression (Figure 3b), the expression of TGF-β in the Positive Control (PC) group (37,09 ± 5,11 %) showed a significant increase (p <0.05) compared to the negative control (NC) group (25,11 ± 2,31 %). While the expression of TGF-β in the treatment groups T1 and T2 have decreased (31,4 ± 3,27 % and 27,87 ± 1,46 %), but only the T2 group was significantly different compared to the PC group.
Figure 3. TGF-β expression in corpus cavernosum tissue in each group. (A) Histological appearance of the corpus cavernosum by immunohistochemical method at 400x magnification. (B) Graph of TGF-β expression percentage in corpus cavernosum tissue. NC: Negative Control; PC: Positive Control; T1: MSCs 1x106; T2: MSCs 1x106.
UC MSCs regulate α-SMA expression on DMED Rats
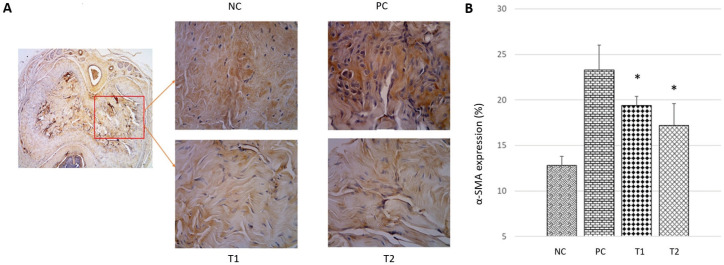
Type I collagen is the main fibrous collagen synthesized by active fibroblasts which are characterized by the expression of α-SMA and are regulated in the process of tissue repair. Analysis of α-SMA expression using immunohistochemical methods was performed to determine the relevance of in vivo collagen production and regulation of ECM by MSCs (Li et al., 2016). The results of the analysis showed that there was a significant decrease in α-SMA expression (p<0.05) in the treatment group compared to the control group. Based on the histological semi-quantitative observation of the α-SMA expression (Figure 4a and 4b), the expression of α-SMA in the Positive Control (PC) group (23,62 ± 2,05 %) showed a significant increase (p <0.05) compared to the negative control (NC) group (13,02 ± 0,94 %). While the expression of α-SMA in the treatment groups T1 and T2 (19,66 ± 0,91 % and 17,14 ± 1,79 %) have decreased but only the T2 group was significantly different compared to the PC group.
Figure 4. The expression of α-SMA in corpus cavernosum tissue in each group. (A) Histological appearance of the corpus cavernosum by immunohistochemical method at 400x magnification. (B) Graph of α-SMA expression percentage in corpus cavernosum tissue. NC: Negative Control; PC: Positive Control; T1: MSCs 1x106; T2: MSCs 1x106.
UC-MSCs regulate collagen deposition on DMED Rats
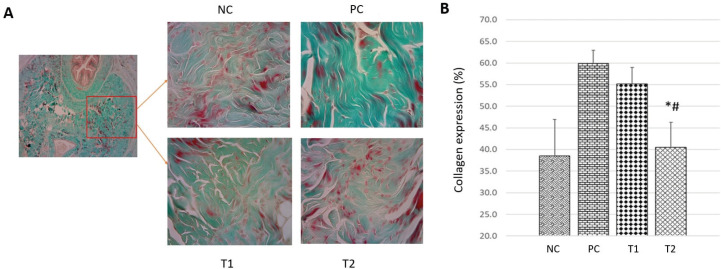
Fibroblast cells in the corpus cavernosum can synthesize collagen, but the excessive amount of collagen causes fibrosis which results in reduced penile contractile function. Measurement of the amount of collagen was carried out using Masson’s Trichome staining method on penile tissue (Siregar et al., 2020). Based on the histological semi-quantitative observation of the collagen deposition (Figure 5), the deposition of collagen in the positive control (PC) group (60,73 ± 4,58 %) showed a significant increase (p <0.05) compared to the negative control (NC) group (38,34 ± 9,83 %). While the deposition of collagen in the treatment groups T1 and T2 have decreased (55,15 ± 4,23 % and 43,9 ± 9,62 %), but only the T2 group was significantly different compared to the PC group.
Figure 5. The expression of collagen in corpus cavernosum tissue in each group. (A) Histological appearance of the corpus cavernosum by Masson-trichome staining method at 400x magnification. (B) Graph of collagen expression percentage in corpus cavernosum tissue. NC: Negative Control; PC: Positive Control; T1: MSCs 1x106; T2: MSCs 1x106.
5. DISCUSSION
Erectile dysfunction (ED), a kind of systemic vascular disease, is one of the well-known comorbidities in males with diabetes mellitus (DM), whose pathogenesis might be induced by dysregulation of corpus cavernosum smooth muscle cells (CCSMCs) (26, 27). CCSMCs have been reported to possess the ability to modulate the phenotype transition from a contractile to a proliferative state under hyperglycemic conditions, which could play an essential role in the pathogenesis of ED (28, 29). Due to the complicated etiology of DMED, the efficacy of currently available treatments is very limited. Therefore, it is necessary to conduct research and development of new therapy for DMED. Recently, stem cell therapy has been added to the treatment options for ED (5, 30, 31). MSCs are now considered an alternative approach to DMED with preliminary studies demonstrating their ability to stimulate smooth muscle cells and regenerate cavernous nerves (5, 28, 32). Another report showed that exosomes secreted from mesenchymal stem cells (MSCs) have been suggested to be conducive to erectile function in streptozotocin-induced (STZ) diabetic rats (33). We show for the first time that UC-MSCs treatment was effective in improving TGF-β expression, α-SMA levels, and collagen deposition in ED of diabetic rats, suggesting a potential protective role for ED treatment against the degenerative structural changes in corpus cavernosum tissue of diabetic animals.
One of the essential findings from our study showed that TGF-β was upregulated in DMED rats indicating system vascular disintegration. Interestingly, after administration of MSCs could reduce the expression of TGF-β, either at low or high doses. Based on this finding, we suggested that MSCs may decrease TGF-β1 expressions in the treatment group can be regulated by MSC administration through the secretion of anti-inflammatory cytokines, especially interleukin 10 (IL-10). As a potent anti-inflammatory cytokine, IL-10 can inhibit fibrosis progression by competitively binding to the TGF- receptor resulting in decreased TGF-β expression (18, 34). The interaction of IL-10 to the TGF-β receptor induces serine/threonine kinase activity leading to induction of the downstream signaling proteins SMAD2 or SMAD3 (35, 36). It is an inhibition of the expression of fibrotic genes such as α-SMA, collagen, and fibronectin (15, 37). This is supported by previous studies that reported an increase in IL-10 induced SMAD7 resulting in downregulation of the SMAD2/SMAD3 pathway (38, 39). Hence, we suppose that preventing the binding of the TGF by receptor by IL-10 secreted by MSCs could be correlated with decreased TGF-β expression leading to an improvement in DMED rats.
The TGF-β1/Smad axis may play an important role in regulating penile homeostasis because TGF-β is up-regulated in the ED of rats with STZ-induced diabetes (40). Normally, TGF-β binds to its receptor, TGF-βRIIβ, which activates the TGF-βRI kinase leading to receptor-regulated phosphorylation of Smads (R-Smads), Smad2, and Smad3 (41). Furthermore, phosphorylated Smad2 and Smad3 will form oligomeric complexes with Smad4 and then translocate into the nucleus to regulating the transcription of target genes, such as α-SMA and ECM production induce genes (41, 42). The increase in TGF-β expression is highly correlated with α-SMA, this is presumably because TGF-β will activate resident fibroblasts to become myofibroblasts which will express α-SMA highly so that the increase in TGF-β expression is in line with the increase in α-SMA expression (42, 43). In line with this statement, the results of this study also showed a decrease in α-SMA expression after MSC administration, both at low and high doses.
The binding of TGF-β to its receptor leads to the phosphorylation of SMAD proteins including SMAD 2 and 3, which binds to SMAD 4 before translocating into the nucleus (42). This drives the initiation of myofibroblasts and matrix deposition, particularly collagen accumulation. The collagen in the corpus cavernosum tissue is predominantly types I, III, and IV (41). Type I collagen, which forms rigid bands of fibrils, is less acquiescent than type III collagen, which is found predominantly in distensible elastic tissue and is essential for normal tensile intensity (44). TGF-β is an important profibrotic cytokine that has been recognized as a key factor associated with corporal fibrotic in ED (29). A previous study demonstrated that TGF-β expression is closely correlated with the accumulation and deposition of ECM, particularly collagen (29, 45). in this study, we also found that collagen accumulation and deposition after MSC administration in DMED rats was seen to decrease at both low and high doses. Thus, we predict that MSCs can regulate the anti-fibrotic responses and due to impaired arterial flow and structural changes of DMED rats through the regulation of the TGF-β pathway. A limitation of this study is that we did not examine IL-10 secreted by MSCs as a sign of anti-inflammatory responses. In addition, we also did not analyze the SMAD levels as fibrosis pathway activation indicator in the DMED rats. Therefore, understanding the role of IL-10 secreted by MSCs to regulate SMAD pathways in DMED rats remains to be explored.
6. CONCLUSION
In conclusion, rat’s UC-MSCs improve the expression of TGF-β, α-SMA, and collagen on erectile dysfunction in streptozotocin-induced diabetic rats. Based on this finding, MSCs present a promising immediate curative effect on the inflammatory reaction and structural improvement in DMED rats.
Acknowledgment:
The authors acknowledge the helpful suggestions concerning this study received from the Stem Cell and Cancer Research (SCCR) Laboratory, the Medical Faculty at Sultan Agung Islamic University (UNISSULA), Semarang, Indonesia, and all who contributed to this research.
Author’s Contributions:
All author made an equal contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspect of the work.
Conflict of interests:
The authors declare no conflicts of interest.
Financial support and sponsorship:
None.
REFERENCES
- 1.Maiorino MI, Bellastella G, Esposito K. Diabetes and sexual dysfunction: Current perspectives. Diabetes, Metab Syndr Obes Targets Ther. 2014;7:95–105. doi: 10.2147/DMSO.S36455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jin H, Kim WJ, Song JS, Choi MJ, Piao S, Shin SH, et al. Functional and Morphologic Characterizations of the Diabetic Mouse Corpus Cavernosum: Comparison of a Multiple Low-Dose and a Single High-Dose Streptozotocin Protocols. J Sex Med. 2009;6:3289–3304. doi: 10.1111/j.1743-6109.2009.01464.x. [DOI] [PubMed] [Google Scholar]
- 3.Davies KP. Development and therapeutic applications of nitric oxide-releasing materials. Vol. 1. Future Science OA. 2015 doi: 10.4155/fso.15.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan H, Ding Y, Lu M. Sexual Medicine Reviews. Elsevier B.V.; 2020. Current Status and Prospects in the Treatment of Erectile Dysfunction by Adipose-Derived Stem Cells in the Diabetic Animal Model. Vol. 8; pp. 486–491. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Zhang K, Ruan Z, Sun D, Zhang H, Lin G, et al. Probucol enhances the therapeutic efficiency of mesenchymal stem cells in the treatment of erectile dysfunction in diabetic rats by prolonging their survival time via Nrf2 pathway. [2021 Jun 9];Stem Cell Res Ther [Internet] 2020 Jul 21;11(1):1–11. doi: 10.1186/s13287-020-01788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34(9):1185–1192. doi: 10.1111/dme.13403. [DOI] [PubMed] [Google Scholar]
- 7.Auffenberg GB, Helfand BT, Mcvary KT. Contemporary Treatment of Erectile Dysfunction. Contemporary Treatment of Erectile Dysfunction. 2011:11–21. [Google Scholar]
- 8.Albersen M, Weyne E, Bivalacqua TJ. Stem cell therapy for erectile dysfunction: Progress and future directions. Sex Med Rev. 2013;1(1):50–64. doi: 10.1002/smrj.5. [DOI] [PubMed] [Google Scholar]
- 9.Marino L, Castaldi MA, Rosamilio R, Ragni E, Vitolo R, Fulgione C, et al. Mesenchymal stem cells from the Wharton’s jelly of the human umbilical cord: Biological properties and therapeutic potential. Int J Stem Cells. 2019;12(2):218–226. doi: 10.15283/ijsc18034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.li Kan X, hua Pan X, Zhao J, He J, min Cai X, qing Pang R, et al. Effect and mechanism of human umbilical cord mesenchymal stem cells in treating allergic rhinitis in mice. Sci Rep. 2020 Dec 1;10(1) doi: 10.1038/s41598-020-76343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Numakura S, Uozaki H, Kikuchi Y, Watabe S, Togashi A, Watanabe M. Mesenchymal stem cell marker expression in gastric cancer stroma. Anticancer Res. 2019;39(1):387–393. doi: 10.21873/anticanres.13124. [DOI] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy [Internet] 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Menocal L, Shareef S, Salgado M, Shabbir A, Van Badiavas E. Role of whole bone marrow, whole bone marrow cultured cells, and mesenchymal stem cells in chronic wound healing. Stem Cell Res Ther. 2015;6(1):1–11. doi: 10.1186/s13287-015-0001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darlan DM, Munir D, Putra A, Jusuf NK. MSCs-released TGFβ1 generate CD4+CD25+Foxp3+ in T-reg cells of human SLE PBMC. J Formos Med Assoc. 2020;(xxxx):1–7. doi: 10.1016/j.jfma.2020.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Lv S, Liu G, Sun A, Wang J, Cheng J, Wang W, et al. Mesenchymal stem cells ameliorate diabetic glomerular fibrosis in vivo and in vitro by inhibiting TGF-β signalling via secretion of bone morphogenetic protein 7. Diabetes Vasc Dis Res. 2014;11(4):251–261. doi: 10.1177/1479164114531300. [DOI] [PubMed] [Google Scholar]
- 16.Fu X, Chen Y, Xie FN, Dong P, Liu WB, Cao Y, et al. Comparison of immunological characteristics of mesenchymal stem cells derived from human embryonic stem cells and bone marrow. Tissue Engineering - Part A. 2015;21:616–626. doi: 10.1089/ten.tea.2013.0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91(1):19–26. doi: 10.1038/icb.2012.56. [DOI] [PubMed] [Google Scholar]
- 18.Peruzzaro ST, Andrews MMM, Al-Gharaibeh A, Pupiec O, Resk M, Story D, et al. Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury 11 Medical and Health Sciences 1109 Neurosciences. J Neuroinflammation. 2019;16(1):1–15. doi: 10.1186/s12974-018-1383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Anna N, et al. The Role of TNF-α Induced MSCs on Suppressive Inflammation.pdf. 2018;6(10):1779–1783. doi: 10.3889/oamjms.2018.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu X, Sun C, Yu W, Lin H, Sun Z, Chen Y, et al. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl. 2012 Jan;33(1):37–44. doi: 10.2164/jandrol.110.012666. [DOI] [PubMed] [Google Scholar]
- 21.Jeon SH, Zhu GQ, Bae WJ, Choi SW, Jeong HC, Cho HJ, et al. Engineered mesenchymal stem cells expressing stromal cell-derived factor-1 improve erectile dysfunction in streptozotocin-induced diabetic rats. [2021 Jun 9];Int J Mol Sci [Internet] 2018 Dec 1;19(12):3730. doi: 10.3390/ijms19123730. Available from: www.mdpi.com/journal/ijms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Zhang K, Ruan Z, Sun D, Zhang H, Lin G, et al. Probucol enhances the therapeutic efficiency of mesenchymal stem cells in the treatment of erectile dysfunction in diabetic rats by prolonging their survival time via Nrf2 pathway. Stem Cell Res Ther. 2020 Jul 21;11(1) doi: 10.1186/s13287-020-01788-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song N, Scholtemeijer M, Shah K. Mesenchymal Stem Cell Immunomodulation: Mechanisms and Therapeutic Potential. Trends Pharmacol Sci [Internet] 2020;xx(xx):1–12. doi: 10.1016/j.tips.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.2020. (No Title) [DOI]
- 25.Putra A, Antari AD, Kustiyah AR, Intan YSN, Sadyah NAC, Wirawan N, et al. Mesenchymal stem cells accelerate liver regeneration in acute liver failure animal model. Biomed Res Ther. 2018;5(11):2802–2810. [Google Scholar]
- 26.Cellek S, Cameron NE, Cotter MA, Muneer A. Pathophysiology of diabetic erectile dysfunction: Potential contribution of vasa nervorum and advanced glycation endproducts. Int J Impot Res. 2013;25(1):1–6. doi: 10.1038/ijir.2012.30. [DOI] [PubMed] [Google Scholar]
- 27.Qiu X, Fandel TM, Ferretti L, Albersen M, Zhang H, Lin G, et al. Both immediate and delayed intracavernous injections of autologous adipose-derived stromal vascular fraction enhance recovery of erectile function in a rat model of cavernous nerve injury. Eur Urol. 2012;62(4):720–727. doi: 10.1016/j.eururo.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huo W, Li Y, Zhang Y, Li H. Mesenchymal stem cells-derived exosomal microRNA-21-5p downregulates PDCD4 and ameliorates erectile dysfunction in a rat model of diabetes mellitus. FASEB J. 2020 Oct 1;34(10):13345–12260. doi: 10.1096/fj.202000102RR. [DOI] [PubMed] [Google Scholar]
- 29.Song J, Sun T, Tang Z, Wang T, Wang S, Liu J. MP84-20 EXOSOMES DERIVED FROM CORPUS CAVERNOSUM SMOOTH MUSCLE CELLS IMPROVE DIABETES-INDUCED ERECTILE DYSFUNCTION VIA ALLEVIATING FIBROSIS AND MODULATING THE TGF-B1/SMAD2/3/CTGF SIGNALING PATHWAYS. J Urol. 2020 Apr;203:e1272. [Google Scholar]
- 30.Qiu X, Sun C, Yu W, Lin H, Sun Z, Chen Y, et al. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl. 2012;33(1):37–44. doi: 10.2164/jandrol.110.012666. [DOI] [PubMed] [Google Scholar]
- 31.He Y, He W, Qin G, Luo J, Xiao M. Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. [2021 Jun 9];Andrologia [Internet] 2014 Jun 1;46(5):479–486. doi: 10.1111/and.12104. [DOI] [PubMed] [Google Scholar]
- 32.Takayanagi A, Sasaki M, Kataoka-Sasaki Y, Kobayashi K, Matsuda Y, Oka S, et al. Intravenous Preload of Mesenchymal Stem Cells Rescues Erectile Function in a Rat Model of Cavernous Nerve Injury. J Sex Med. 2015;12(8):1713–1721. doi: 10.1111/jsm.12957. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang X, Han X, Chen Z, Fang J, Huang X, Wei H. MSC-derived exosomes ameliorate erectile dysfunction by alleviation of corpus cavernosum smooth muscle apoptosis in a rat model of cavernous nerve injury. doi: 10.1186/s13287-018-1003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimabukuro-Vornhagen A, Draube A, Liebig TM, Rothe A, Kochanek M, Von Bergwelt-Baildon MS. The immunosuppressive factors IL-10, TGF-β, and VEGF do not affect the antigen-presenting function of CD40-activated B cells. J Exp Clin Cancer Res. 2012;31(1):1–7. doi: 10.1186/1756-9966-31-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan D, Derynck R. Transforming growth factor-β (TGF-β)-induced up-regulation of TGF-β receptors at the cell surface amplifies the TGF-β response. J Biol Chem. 2019;294(21):8490–8504. doi: 10.1074/jbc.RA118.005763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mao D, Mi J, Pan X, Li F, Rui Y. Suppression of TGF-beta activity with remobilization attenuates immobilization-induced joint contracture in rats. Injury [Internet] 2021;52:434–442. doi: 10.1016/j.injury.2020.12.029. [DOI] [PubMed] [Google Scholar]
- 37.Sapudom J, Wu X, Chkolnikov M, Ansorge M, Anderegg U, Pompe T. Fibroblast fate regulation by time dependent TGF-β1 and IL-10 stimulation in biomimetic 3D matrices. Biomater Sci. 2017;5(9):1858–1867. doi: 10.1039/c7bm00286f. [DOI] [PubMed] [Google Scholar]
- 38.Shi CK, Zhao YP, Ge P, Huang GB. Therapeutic effect of interleukin-10 in keloid fibroblasts by suppression of TGF-β/Smad pathway. Eur Rev Med Pharmacol Sci. 2019;23(20):9085–9092. doi: 10.26355/eurrev_201910_19311. [DOI] [PubMed] [Google Scholar]
- 39.Xu Y, Tang X, Yang M, Zhang S, Li S, Chen Y, et al. Interleukin 10 gene-modified bone marrow-derived dendritic cells attenuate liver fibrosis in mice by inducing regulatory T cells and inhibiting the TGF-β/Smad signaling pathway. Mediators Inflamm. 2019 doi: 10.1155/2019/4652596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel DP, Christensen MB, Hotaling JM, Pastuszak AW. European Urology Focus. Vol. 6. Elsevier B.V.; 2020. [2021 Jun 10]. Erectile Dysfunction and Peyronie’s Disease: Genetic Diseases? [Internet] pp. 572–574. Available from: https://pubmed.ncbi.nlm.nih.gov/31474580/ [DOI] [PubMed] [Google Scholar]
- 41.Lin PS, Chang HH, Yeh CY, Chang MC, Chan CP, Kuo HY, et al. Transforming growth factor beta 1 increases collagen content, and stimulates procollagen I and tissue inhib1. J Formos Med Assoc. 2017;116(5):351–8. doi: 10.1016/j.jfma.2016.07.014. Transforming growth factor beta 1 increases collagen content, and stimulates procolla. [DOI] [PubMed] [Google Scholar]
- 42.Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, et al. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 43.Fathy M, Okabe M, Eldien HMS, Yoshida T. AT-MSCs antifibrotic activity is improved by Eugenol through modulation of TGF-β/Smad signaling pathway in rats. Molecules. 2020;25(2):1–17. doi: 10.3390/molecules25020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shoulders MD, Raines RT. Collagen Structure and Stability Ann Rev Biochemistry. Annu Rev Biochem. 2009;78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao JS, Shan G, Cai SW. Protection against TGF- b 1-induced fibrosis effects of IL-10 on dermal fibroblasts and its potential therapeutics for the reduction of skin scarring. 2013:341–52. doi: 10.1007/s00403-013-1314-0. [DOI] [PubMed] [Google Scholar]