Abstract
Purpose
M1 macrophages can promote corneal allograft rejection (CGR). Inhibiting M1 macrophage polarization by the JAK/STAT1 pathway may be a new strategy to prevent CGR. Tofacitinib, a potent pan-JAK inhibitor, can inhibit JAK/STAT activation. Here, we investigated the inhibitory effects of tofacitinib on M1 macrophage polarization and its therapeutic effect on rat CGR.
Methods
Corneal allograft transplantation was performed and administrated with 0.3% tofacitinib in rats. The corneal allografts were assessed clinically. The corneas were detected for M1 macrophages, lymphatic vessels, and inflammatory cytokine expression using immunohistochemistry and real-time polymerase chain reaction (PCR). Dendritic cells (DCs) in ipsilateral cervical lymph nodes were detected by flow cytometry. The effect and mechanism of tofacitinib on macrophages were explored by real-time PCR, enzyme-linked immunoassay, and western blot analysis in vitro.
Results
The results showed that topical administration of 0.3% tofacitinib significantly prolonged corneal graft survival. Tofacitinib-treated corneal allografts displayed a proportionate decrease in M1 macrophages and reduced lymphatic vessel density with fewer DCs in rat ipsilateral cervical lymph nodes. Tofacitinib reduced the mRNA expression of inflammatory cytokines, including iNOS, MCP-1, TNF-α, IL-6, IL-1β, and VEGF-C, and inhibited STAT1 activation in rat corneal grafts. In addition, tofacitinib suppressed M1 macrophage polarization via STAT1 activation after IFN-γ and lipopolysaccharide stimulation in vitro.
Conclusions
Tofacitinib could suppress M1 macrophage polarization and subsequently delay CGR by inhibiting STAT1 activation. The data indicate that tofacitinib is an effective drug for CGR.
Translational Relevance
This study provided evidence that topical administration of 0.3% tofacitinib may be a novel clinical strategy to prevent CGR.
Keywords: tofacitinib, macrophage, JAK/STAT1, corneal allograft rejection, lymphangiogenesis
Introduction
Corneal transplantation is the major method for treating corneal blindness.1 Approximately 180,000 corneal transplants are performed annually worldwide.1 Corneal allograft rejection (CGR) is the leading reason for transplant failure. It has been reported that the corneal graft survival rate in corneal beds with inflammation and vascularization is less than 35%.2 For such cases over the last few decades, long-term use of corticosteroids and immunosuppressive agents has been necessary to prevent CGR. However, those medications can induce significant systemic and ocular side effects; therefore, we should develop new strategies to delay CGR. Previous studies have shown that macrophages play a key role in CGR.3,4 Macrophage depletion using clodronate liposomes effectively prevented CGR.3 It has been reported that macrophages with a decreased intracellular content of glutathione (icGSH) are regarded as oxidative macrophages (OMps), and those with an increased icGSH are regarded as reductive macrophages (RMps). OMps, but not RMps, can suppress T helper 1 (Th1) cell activation and then delay CGR in mice.5 As we known, macrophages can be classified as M1 macrophages and M2 macrophages depending on diverse stimuli.6 Treatment with interferon gamma (IFN-γ), tumor necrosis factor alpha (TNFα), and lipopolysaccharide (LPS) polarizes resting (M0) macrophages to the M1 phenotype, and these cells secrete pro-inflammatory cytokines, such as TNFα, interleukin (IL)-6 and IL-1β.6,7 In contrast, M2 macrophages play an anti-inflammatory role after stimulation with IL-4 and IL-13.6,7 It was reported that M1 macrophages but not M2 macrophages significantly exacerbated CGR.8,9 Inhibition of M1 macrophage polarization is a good therapeutic strategy to improve corneal graft survival.10
Compared with M0 macrophages and M2 macrophages, M1 macrophages can significantly promote lymphangiogenesis by transdifferentiating into lymphatic endothelial cells (LECs).11 Moreover, TNFα and IL-1β secreted by M1 macrophages can also promote lymphatic endothelial cell proliferation by increasing vascular endothelial growth factor C (VEGF-C) expression in LECs.12,13 During the pathological process of CGR, corneal lymphangiogenesis is the most important mediator of the immune response after transplantation.14 Therefore, M1 macrophages can promote CGR by inducing corneal lymphangiogenesis.
Janus kinases (JAKs) are tyrosine kinases related to cytokine receptors that can activate nuclear signal transducer and activator of transcription (STAT).15 The JAK family consists of JAK-1, JAK-2, JAK-3, and tyrosine kinase. JAK/STAT1 plays a crucial role in macrophage polarization and function.16,17 The canonical role of JAK1/STAT1 signaling drives M0 macrophages to polarize into M1 macrophages.18,19 Pharmacological inhibition of JAK/STAT1 signaling can repress M1 macrophage polarization.20,21 Tofacitinib is a pan-JAK inhibitor that can preferentially inhibit the JAK/STAT1 pathway.22,23 In this study, we explored the effects of tofacitinib on corneal lymphangiogenesis and CGR via the inhibition of M1 macrophage polarization.
Materials and Methods
Animals
This study was authorized by the Institutional Animal Care and Use Committee of the Zhongshan Ophthalmic Centre at Sun Yat-sen University (no. 2015-056). Eight-week-old female Sprague Dawley (SD) rats and Wistar rats were obtained from the Experimental Animal Centre of Guangdong Province. The rats were cared for according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All rats were provided food and water ad libitum and housed on a 12-hour cycle of light and dark in the cages.
Corneal Transplantation Model
Rats were anesthetized by an intraperitoneal injection of pentobarbital (45 mg/kg). Full-thickness donor corneas with diameters of 3.5 mm were acquired from Wistar rats and sutured onto SD rat corneal beds (3 mm in diameter) using eight interrupted 10-0 nylon sutures (Alcon, Fort Worth, TX). Autograft corneal transplantation was performed in SD rats. Tofacitinib eye drops (0.3%, 20 µL/drop, Y-40354; MedChemExpress, Monmouth Junction, NJ) or vehicle alone were administered four times per day postoperatively at the same time points (7:30 AM, 11:30 AM, 3:30 PM, and 7:30 PM). Clinical assessment of corneal allografts was performed using a slit-lamp microscope every 2 days from the third postoperative day.
Clinical Assessment
The corneal grafts were assessed using three parameters: graft opacity, edema, and neovascularization (Table 1).24 Corneal grafts were considered to be rejected when the total score (rejection index) was equal to or greater than 6.
Table 1.
Grading of Clinical Assessment for Corneal Graft Rejection
| Score | Opacity | Edema | Neovascularization |
|---|---|---|---|
| 0 | Transparent | No edema | No vessels |
| 1 | Mild opacity | Slightly edema | Vessels appearing in the graft bed |
| 2 | Some iris texture is invisible | Diffuse graft stromal edema | Vessels appearing in the peripheral part of the graph |
| 3 | Obvious opacity but pupil is still visible | Diffuse edema with small bleb | Vessels appearing in the middle and peripheral part of the graft |
| 4 | Whole opacity with invisible pupil | Diffuse edema with large bleb | Vessels appearing in the center of the graft |
Immunohistochemistry
On postoperative day 7, the eyeballs were excised and embedded in tissue freezing medium after being fixed with 4% paraformaldehyde solution. The sections were prepared (8 µm) and blocked with 5% bovine serum albumin (BSA) for 1 hour. The sections were treated with mouse anti-CD11b (1:200, ab8879; Abcam, Cambridge, UK) and rabbit anti-iNOS (1:200, ab15323; Abcam) primary antibodies overnight at 4°C. Then, the sections were stained with Donkey Anti-Mouse IgG H&L (Alexa Fluor 488) conjugated antibody (1:500, ab150105; Abcam) and Goat Anti-Rabbit IgG H&L (Alexa Fluor 555) preadsorbed antibody (1:500, ab150086; Abcam) for 1 hour. The sections were analyzed under a ZEISS LSM 700 microscope (Carl Zeiss Meditec, Jena, Germany).
Whole-Mount Corneal Immunofluorescence Analysis of Lymphatic Vessels
Whole rat corneas were excised on day 10 postoperatively and fixed with a 4% paraformaldehyde solution. The corneas were digested with a 20-µg/mL proteinase K solution and treated with methanol. The corneas were blocked with 5% BSA and 0.5% Triton X-100 in phosphate buffered saline solution (PBST) overnight and then stained with rabbit anti-rat lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1, 1:200, orb312301; Biorbyt, Cambridge, UK) at 4°C for 24 hours. The corneas were stained with Goat Anti-Rabbit IgG H&L (Alexa Fluor 647) preadsorbed antibody (1:200, ab150083; Abcam) for 2 hours at room temperature.
The corneal tissues were then washed in PBST three times and cut into four slices with a blade. After being flattened, the corneas were scanned by a ZEISS LSM 700 microscope. The areas of lymphatic vessels in the corneal tissues were evaluated using ImageJ (National Institutes of Health, Bethesda, MD).
Flow Cytometry
Rat ipsilateral cervical lymph nodes were obtained and ground on postoperative day 12. The cell suspension was incubated with R-Phycoerythrin (RPE)-conjugated anti-OX62 antibody (MCA 1029G; Bio-Rad AbD Serotec, Oxford, UK) for 30 minutes. The cells were analyzed by flow cytometry (BD Biosciences, San Jose, CA).
Delayed-Type Hypersensitivity Assay
Delayed-type hypersensitivity (DTH) responses to allo-antigens were measured by an ear swelling assay. In brief, spleen single-cell suspensions were obtained from Wistar rats. A suspension of 1 × 106 mitomycin C–treated cells in 25 µL Hanks’ balanced salt solution (HBSS) was injected into the right-ear pinna of SD rats on postoperative day 14. The left-ear pinna of SD rats was injected with 25 µL HBSS. As positive controls, SD rats were immunized by subcutaneous injection with 1 × 107 mitomycin C–treated spleen cells on day 0. Naïve SD rats were regarded as negative controls. Ear thickness was determined using an engineer's micrometer after 24 and 48 hours. The ear swelling was expressed as follows: (24-hour measurement of right ear – 0-hour measurement of right ear) − (24-hour measurement of left ear – 0-hour measurement of left ear).
Cell Treatments
RAW264.7 macrophages (American Type Culture Collection, Rockville, MD) were cultured in 24-well plates (2 × 105 cells/well) with Dulbecco's Modified Eagle's Medium (11995065; Gibco, Carlsbad, CA) containing 10% fetal bovine serum (10099141C; Gibco). Macrophages were incubated with increasing concentrations of tofacitinib (100 µM, 200 µM, and 400 µM) for 4 hours and then stimulated with 10 ng/mL IFN-γ (485-MI; R&D Systems, Minneapolis, MN) and 100 ng/mL LPS (L2630; Sigma-Aldrich, St. Louis, MO). The cells and supernatant were collected for further analysis.
Real-Time PCR
On postoperative day 7, corneal allografts were excised and cut into pieces. RNA was extracted from the corneal grafts and RAW264.7 macrophages and reverse translated into cDNA. Real-time polymerase chain reaction (PCR) was performed using a PrimeScript RT Reagent Kit (Takara Bio, Shiga, Japan). The primers are shown in Table 2.25
Table 2.
Sequence of Primers
| Gene Name | Rat Primer Sequences (5′–3′) | Mouse Primer Sequences (5′–3′) |
|---|---|---|
| GAPDH | ||
| Forward | TCTCTGCTCCTCCCTGTTC | TGAGCAAGAGAGAGGCCCTATC |
| Reverse | ACACCGACCTTCACCATCT | AGGCCCCTCCTGTTATTATG |
| MCP-1 | ||
| Forward | CTGTGCTGACCCCAATAAGGAA | ACCAGCACCAGCCAACTCT |
| Reverse | GAGGTGGTTGTGGAAAAGAGAGTG | TGAATGAGTAGCAGCAGGTGAG |
| TNF-α | ||
| Forward | CCTCTTCTCATTCCTGCTC | CAGGTCACTGTCCCAGCATCT |
| Reverse | CTTCTCCTCCTTGTTGGG | GAGTCCGGGCAGGTCTACTTT |
| IL-6 | ||
| Forward | ATCTGCTCTGGTCTTCTGG | ATACCACTCCCAACAGACC |
| Reverse | TCTGGCTTTGTCTTTCTTGT | CTCATTTCCACGATTTCC |
| IL-1β | ||
| Forward | ATTGTGGCTGTGGAGAAG | CAGGCAGGCAGTATCACTCA |
| Reverse | AAGATGAAGGAAAAGAAGGTG | TGTCCTCATCCTGGAAGGTC |
| VEGF-C | ||
| Forward | GATTCAGGGGTTGATTTCTTG | ACTGCTCCTCCAGGTCTTTGC |
| Reverse | TTTCCTTAATTCATGTGGAGCC | ACTTGCTGTGCTTCTTGTCTC |
Enzyme-Linked Immunoassay
The levels of secreted cytokines were measured using enzyme-linked immunoassay (ELISA) kits (MCP-1 ELISA Kit 88-7391-88, TNF-α ELISA Kit 88-7324-88, IL-6 ELISA Kit 88-7064-88, and IL-1β ELISA Kit 88-7013-88; Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's specifications.
Western Blot Analysis
Proteins were isolated from corneal grafts and RAW264.7 macrophages. Proteins (30–60 µg) were separated on 10% sodium dodecyl sulfate-polyacrylamide gels and then transferred to polyvinylidene fluoride (PVDF) membranes. After being blocked with 5% skim milk for 2 hours, the PVDF membranes were incubated with primary antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:1000, 5174; Cell Signaling Technology, Danvers, MA), STAT1 (1:1000, 14994; Cell Signaling Technology), and phospho-STAT1 (1:1000, AP0054; ABclonal, Wuhan, China) at 4°C for 24 hours followed by incubation with anti-rabbit HRP-linked secondary antibodies (1:5000; Cell Signaling Technology) at 4°C for 1 hour. Chemiluminescence images were collected and analyzed using a molecular imager system (Bio-Rad Laboratories, Hercules, CA).
Statistical Analysis
The survival times of corneal grafts were determined using the Kaplan–Meier estimator and log-rank test. Statistical differences were identified with one-way analysis of variance (ANOVA) using SPSS 20.0 (IBM, Chicago, IL). P < 0.05 was considered statistically significant.
Results
Tofacitinib Significantly Prolonged Corneal Allograft Survival Times
To explore the effects of tofacitinib on corneal rejection, penetrating keratoplasty was performed on rats. Tofacitinib eye drops (0.3%) and vehicle eye drops were administered four times per day postoperatively. All corneal grafts in the vehicle-treated group were rejected within 17 days, with an average survival time of 12.17 ± 2.62 days, whereas the corneal autografts survived until the end of the observation period (Fig. 1A). In the tofacitinib-treated group, six of 12 corneal allografts were rejected from day 9 to day 21, with an average survival time of 15 ± 4.19 days, and six of 12 corneal allografts remained unrejected on postoperative day 30 (Fig. 1A). In comparison with that of the vehicle-treated allografts, tofacitinib treatment significantly prolonged corneal graft survival (Fig. 1A). The average scores of opacity, edema, neovascularization, and rejection index in the tofacitinib-treated group were significantly lower than those in the vehicle-treated group from postoperative day 9 (Figs. 1B–1E). On postoperative days 17 and 30, the vehicle corneal grafts displayed outstanding opacification and edema, whereas six of 12 grafts in the tofacitinib-treated group maintained transparency and mild edema (Fig. 1F).
Figure 1.
Topical administration of 0.3% tofacitinib suppressed allograft corneal rejection and corneal lymphangiogenesis after corneal transplantation in rats. (A) Kaplan–Meier corneal allograft survival curve (n = 12). (B–E) The average scores of opacity, edema, neovascularization, and rejection index in the tofacitinib-treated group were significantly lower than those in the vehicle-treated group from postoperative day 9 (P < 0.05). (F) Anterior segment photography. On postoperative days 17 and 30, corneal allografts in the vehicle-treated group displayed outstanding opacification and edema, whereas six of 12 grafts in the tofacitinib-treated group maintained transparency and mild edema. (G, H) Corneal lymphatic vessels were stained with LYVE-1 (red). In comparison with the vehicle-treated group, tofacitinib significantly suppressed corneal lymphangiogenesis on postoperative day 10 (n = 4). Scale bar: 50 µm. The data are presented as mean ± SD. **P < 0.01 and ***P < 0.001 between the vehicle-treated and tofacitinib-treated groups.
Tofacitinib Inhibited Corneal Lymphangiogenesis
During the pathological process of CGR, corneal lymphangiogenesis is the most important mediator of the immune response after transplantation.14 Corneal lymphatic vessels facilitate the trafficking of recipient antigen-presenting cells to draining lymph nodes and result in T lymphocyte activation, which subsequently induces CGR.26 Inhibiting corneal lymphangiogenesis is a good therapeutic strategy to improve corneal graft survival.27 To explore the role of tofacitinib in corneal lymphangiogenesis, whole-mount corneal lymphatic vessels were stained with the specific marker LYVE-1. On postoperative day 10, we found that lymphatic vessels in the vehicle-treated group expanded from the limbus to the corneal beds. In comparison with that in the vehicle-treated group, tofacitinib significantly decreased the area of lymphatic vessels (n = 4 in each group) (Figs. 1G, 1H). The results indicate that tofacitinib significantly suppressed corneal lymphangiogenesis in rats. Previous studies have demonstrated that corneal lymphatic vessels could play a dominant role in CGR.14,28,29 Our findings indicate that tofacitinib delayed CGR by inhibiting corneal lymphangiogenesis.
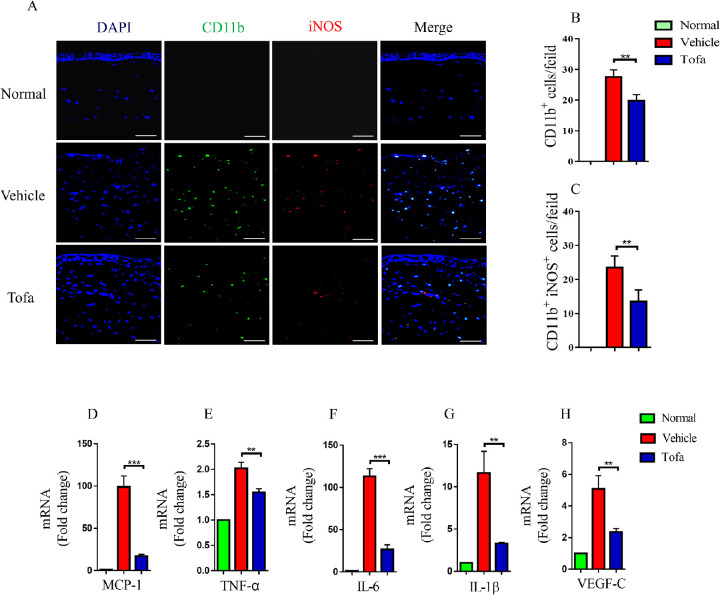
Tofacitinib Suppressed Macrophage Recruitment and M1 Macrophage Polarization in Corneal Grafts
Recent studies have demonstrated that M1 macrophages could promote lymphangiogenesis and corneal rejection.10,11,13 To examine the effects of tofacitinib on macrophage recruitment and polarization, corneal grafts were stained with cluster of differentiation molecule 11b (CD11b) and inducible nitric oxide synthase (iNOS). Normal rat corneas were devoid of CD11b+ macrophages. On postoperative day 7, CD11b+ macrophages infiltrated corneal allografts in the vehicle-treated group, whereas tofacitinib significantly inhibited macrophage recruitment to corneal grafts (Figs. 2A, 2B). Moreover, in comparison with those in the vehicle-treated group, tofacitinib-treated corneal allografts exhibited decreased numbers of CD11b+ iNOS+ M1 macrophages (Figs. 2A, 2C). M1 macrophages could produce inflammatory cytokines, including TNF-α, IL-6, and IL-1β, which are closely related to corneal lymphangiogenesis. Our results showed that tofacitinib inhibited monocyte chemotactic protein-1 (MCP-1), TNF-α, IL-6, IL-1β, and VEGF-C mRNA expression in corneal allografts (Figs. 2D–2H).
Figure 2.
Tofacitinib suppressed macrophage recruitment and M1 macrophage polarization in corneal grafts. (A−C) On postoperative day 7, CD11b+ (green) macrophages infiltrated corneal grafts in the vehicle-treated group, whereas tofacitinib significantly inhibited CD11b+ macrophage recruitment in corneal allografts. In comparison with the vehicle-treated group, tofacitinib-treated corneal grafts displayed decreased numbers of CD11b+ iNOS+ (red) M1 macrophages (n = 4). Scale bar: 50 µm (magnification 400×). In each slide, 10 fields were randomly chosen, and macrophages were observed. (D−H) Compared with the vehicle-treated group, tofacitinib suppressed the mRNA expression of MCP-1, TNF-α, IL-6, IL-1β, and VEGF-C in corneal allografts (n = 3). The data are presented as mean ± SD. **P < 0.01 and ***P < 0.001 between the vehicle-treated and tofacitinib-treated groups.
Tofacitinib Inhibited Dendritic Cell Trafficking to Regional Draining Lymph Nodes
It has been reported that DCs traffic to draining lymph nodes through corneal lymphatic vessels.29,30 After showing that tofacitinib could inhibit corneal lymphangiogenesis, we explored the role of tofacitinib in restricting DC migration from corneal grafts. Cells were obtained from rat ipsilateral cervical lymph nodes and stained with the special marker OX62.31,32 We found proportional increases in OX62+ DCs in rat ipsilateral cervical lymph nodes on postoperative day 12 (Figs. 3A, 3B). In comparison with the vehicle-treated group, tofacitinib significantly inhibited DC migration and accumulation (Figs. 3D, 3E).
Figure 3.
Tofacitinib inhibited the trafficking of dendritic cells (DCs) to regional draining lymph nodes. On postoperative day 12, cells in ipsilateral cervical lymph nodes were analyzed by flow cytometry. In comparison with the vehicle-treated group, the tofacitinib-treated group exhibited decreased proportions and numbers of OX62+ DCs (n = 4). The data are presented as mean ± SD. ***P < 0.001 between the vehicle-treated and tofacitinib-treated groups.
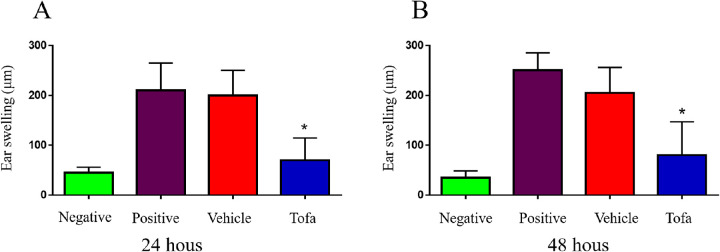
Tofacitinib Inhibited DTH Responses
To assess allo-antigen DTH responses, ear swelling assays were performed in the vehicle-treated and tofacitinib-treated groups. Naïve SD rats served as negative controls, and rats that were immunized with donor spleen cells served as positive controls. Compared with the vehicle-treated group, the tofacitinib-treated rats presented with a reduced ear thickness at 24 and 48 hours after challenge (Fig. 4). The results demonstrated that tofacitinib could significantly inhibit DTH responses to donor allo-antigens in rats.
Figure 4.
Tofacitinib inhibited DTH responses. DTH responses to allo-antigens were measured by an ear swelling assay. Naive SD rats were regarded as negative controls and rats subcutaneously immunized on day 0 were regarded as positive controls. On postoperative day 14, SD rats were challenged with mitomycin C-treated spleen cells. Ear thickness was measured after 24 and 48 hours (n = 5 in each group). The data are presented as mean ± SD. *P < 0.05 between the vehicle-treated and tofacitinib-treated groups.
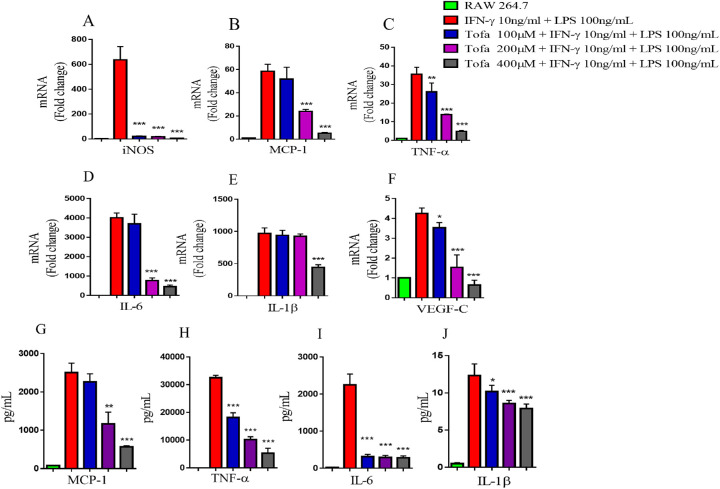
Tofacitinib Suppressed M1 Macrophage Polarization In Vitro
To examine the effect of tofacitinib on macrophage polarization in vitro, RAW264.7 macrophages were incubated with tofacitinib for 4 hours and then treated with 10 ng/mL IFN-γ and 100 ng/mL LPS for 4 hours. IFN-γ and LPS can induce M1 macrophage polarization, which is characterized by the increased expression of M1-associated pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β.33 We observed that tofacitinib significantly inhibited the mRNA expression of iNOS, MCP-1, TNF-α, IL-6, IL-1β, and VEGF-C at concentrations of 100 µM, 200 µM, and 400 µM (Figs. 5A–5F). Consistently, the ELISA results also revealed that tofacitinib treatment significantly inhibited the production of MCP-1, TNF-α, IL-6, and IL-1β in macrophages (Figs. 5G–5J). Therefore, our findings indicate that tofacitinib could inhibit M1 macrophage polarization in vitro.
Figure 5.
Tofacitinib suppressed M1 macrophage polarization in vitro. RAW264.7 macrophages were incubated with increasing concentrations of tofacitinib (100 µM, 200 µM, and 400 µM) for 4 hours and then treated with 10 ng/mL IFN-γ and 100 ng/mL LPS for 4 hours or 24 hours. (A–F) Cytokine mRNA expression was measured by real-time PCR (n = 3). (G–J) The levels of M1-associated cytokines were determined by ELISA (n = 3). The data are presented as the mean ± SD. *P < 0.05, **P < 0.01, and ***P < 0.001 between the IFN-γ/LPS-stimulated and tofacitinib-treated groups.
Tofacitinib Inhibited STAT1 Activation In Vitro and In Vivo
STAT1 activation by IFN-γ has been shown to be responsible for M1 polarization.33–35 The expression of TNF-α, IL-6, and IL-1β in M1 macrophages was repressed by STAT1 inhibition.33 IFN-γ-triggered JAK/STAT1 activation induces inflammation-associated gene expression and M1 macrophage polarization.36,37 In macrophages, we observed that tofacitinib significantly inhibited STAT1 activation after IFN-γ/LPS stimulation (Fig. 6A). In vivo, we found that topical administration of tofacitinib significantly inhibited STAT1 phosphorylation in rat corneal grafts compared with those in the vehicle-treated group on postoperative day 7 (Fig. 6B). Tofacitinib repressed M1 macrophage polarization by inhibiting STAT1 activation.
Figure 6.
Tofacitinib inhibited STAT1 activation in vitro and in vivo. (A) RAW264.7 cells were incubated with increasing concentrations of tofacitinib (100 µM, 200 µM, and 400 µM) for 4 hours and then stimulated with 10 ng/mL IFN-γ and 100 ng/mL LPS. The level of STAT1 phosphorylation in cells was measured using western blot analysis. (B) On postoperative day 7, the level of STAT1 activation in rat corneal grafts was analyzed by western blotting.
Discussion
Macrophages are pivotal mediators of transplant pathology and allograft rejection.8 Pro-inflammatory M1 macrophages are believed to promote CGR; in contrast, M2 macrophages prolong graft survival time.8,10 Previous studies have shown that macrophages polarize to the M1 phenotype by the JAK/STAT1 signaling pathway.18,19,35,37,38 Inhibiting STAT1 activation can suppress M1 macrophage polarization and may become a new strategy to prevent CGR.
Tofacitinib is a potent pan-JAK inhibitor that can restrain JAK/STAT1 pathway activation.39 In this study, we investigated the effects of tofacitinib on CGR. Our results demonstrated that topical administration of 0.3% tofacitinib significantly repressed M1 macrophage polarization by inhibiting STAT1 activation, restrained corneal lymphangiogenesis, and suppressed CGR in rats. In comparison with the vehicle-treated group, tofacitinib-treated corneal allografts displayed a proportionate decrease in M1 macrophages and reduced lymphatic vessel density with fewer DCs in rat ipsilateral cervical lymph nodes.
Pro-inflammatory cytokines are crucial mediators of innate and adaptive immunity via activation of the JAK/STAT pathway, which may be a promising therapeutic target for inflammatory diseases.40,41 As a JAK inhibitor, tofacitinib could alleviate multiple autoimmune diseases, such as rheumatoid arthritis, ulcerative colitis, and psoriasis.42–44 Moreover, tofacitinib significantly alleviated experimental autoimmune uveitis by reducing Th1 cell differentiation.45 We also found that topical application of tofacitinib prevented experimental allergic conjunctivitis by inhibiting JAK3/STAT phosphorylation in mast cells.46 However, the effects of tofacitinib on corneal rejection are not clear. In this study, our findings demonstrated that topical administration of 0.3% tofacitinib could significantly prolong corneal survival time by inhibiting STAT1 phosphorylation. Therefore, tofacitinib may be a promising agent for preventing CGR.
The IFN-γ-induced JAK/STAT1 pathway plays a key role in M1 macrophage activation and polarization.18,19,37,38 IFN-γ was expressed in both syngeneic and allogeneic corneal grafts after transplantation.8 In comparison with the vehicle-treated group, tofacitinib-treated corneal allografts exhibited decreases in CD11b+ macrophages and CD11b+ iNOS+ M1 macrophages. Those results demonstrated that tofacitinib could inhibit M1 macrophage polarization and reduce the expression of pro-inflammatory cytokines, including TNF-α, IL-6, and IL-1β, by restraining STAT1 activation in vivo and in vitro. In addition, MCP-1, mainly produced by monocytes and macrophages, is a potent chemokine for monocyte/macrophage recruitment.47 Tofacitinib inhibited the mRNA expression of MCP-1 in corneal grafts, resulting in the recruitment of fewer macrophages.
Macrophages are the main source of lymphangiogenesis growth factors.48 Macrophage depletion could inhibit corneal lymphangiogenesis and CGR.3,49 Accumulating evidence has demonstrated that modulating lymphangiogenesis by regulating macrophage activation is a potential strategy for treating CGR.48 Currently, it is widely thought that M1 macrophages play a crucial role in corneal lymphangiogenesis.11–13 M1 macrophages can directly transdifferentiate into LECs and incorporate into lymphatic vessels.11 VEGF-C can induce lymphatic endothelial cell proliferation,49 whereas TNF-α, IL-6, and IL-1β can promote cell proliferation by increasing VEGF-C expression in LECs.12,13,50 Despite growing insight into the lymphangiogenic process, there are no effective U.S. Food and Drug Administration–approved drugs for lymphangiogenesis. Our findings demonstrate that tofacitinib could suppress corneal lymphangiogenesis by repressing the activation and polarization of M1 macrophages. Tofacitinib may be a promising JAK inhibitor that targets lymphangiogenesis.
Lymphatic vessels are significantly involved in organ graft rejection, especially corneal rejection.51 It was reported that lymphatic vessels but not blood vessels principally induced corneal rejection.14 Corneal lymphatic vessels serve as the conduit for antigen-presenting cells from the cornea to regional lymph nodes and thus induce CGR.30,51 In this study, we found that the tofacitinib-treated group exhibited a lower density of lymphatic vessels and decreased proportions and numbers of DCs in rat ipsilateral cervical lymph nodes compared with the vehicle-treated group.
In conclusion, our findings demonstrate that topical administration of 0.3% tofacitinib inhibits CGR and promotes graft survival in rats. Tofacitinib could inhibit the recruitment and activation of M1 macrophages by suppressing STAT1 phosphorylation, thus suppressing corneal lymphangiogenesis and CGR in the rat cornea after transplantation (Fig. 7). These data provide evidence that topical administration of 0.3% tofacitinib may be a novel strategy to prevent CGR.
Figure 7.

The molecular mechanisms of tofacitinib inhibition in CGR. The corneal transplantation procedure induces inflammation, which leads to macrophage infiltration and M1 macrophage polarization by JAK/STAT1 activation. M1 macrophages transdifferentiate into LECs and promote the proliferation of LECs by producing VEGF-C, TNF-α, IL-6, and IL-1β, thus accelerating corneal lymphangiogenesis. Moreover, MCP-1 secreted by M1 macrophages further induces macrophage recruitment. Corneal lymphatic vessels transport dendritic cells to cervical lymph nodes and activate T cells, thus inducing and promoting CGR. In brief, tofacitinib inhibits M1 macrophage polarization by repressing STAT1 activation, thus suppressing corneal lymphangiogenesis and CGR after corneal transplantation.
Acknowledgments
Supported by the Science and Technology Cooperation Project of Hong Kong, Macao, and Taiwan (2015DFH30190) and by the Science and Technology Project of Nantong Municipality (JCZ19009).
Disclosure: J. Yu, None; P. Li, None; Z. Li, None; Y. Li, None; J. Luo, None; W. Su, None; D. Liang, None
References
- 1. Gain P, Jullienne R, He Z, et al.. Global survey of corneal transplantation and eye banking. JAMA Ophthalmol. 2016; 134(2): 167–173. [DOI] [PubMed] [Google Scholar]
- 2. Di ZA, Kheirkhah A, Abud TB, Goyal S, Dana R. Management of high-risk corneal transplantation. Surv Ophthalmol. 2017; 62(6): 816–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slegers TP, van Rooijen N, van Rij G, van der Gaag R. Delayed graft rejection in pre-vascularised corneas after subconjunctival injection of clodronate liposomes. Curr Eye Res. 2000; 20(4): 322–324. [PubMed] [Google Scholar]
- 4. Ko JH, Lee HJ, Jeong HJ, et al.. Mesenchymal stem/stromal cells precondition lung monocytes/macrophages to produce tolerance against allo- and autoimmunity in the eye. Proc Natl Acad Sci USA. 2016; 113(1): 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yamada J, Maruyama K, Sano Y, Kinoshita S, Murata Y, Hamuro J. Promotion of corneal allograft survival by the induction of oxidative macrophages. Invest Ophthalmol Vis Sci. 2004; 45(2): 448–454. [DOI] [PubMed] [Google Scholar]
- 6. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020; 877: 173090. [DOI] [PubMed] [Google Scholar]
- 7. Jiang N, Zhang L, Zhao G, et al.. Indoleamine 2,3-dioxygenase regulates macrophage recruitment, polarization and phagocytosis in Aspergillus fumigatus keratitis. Invest Ophthalmol Vis Sci. 2020; 61(8): 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oh JY, Lee HJ, Ko AY, Ko JH, Kim MK, Wee WR. Analysis of macrophage phenotype in rejected corneal allografts. Invest Ophthalmol Vis Sci. 2013; 54(12): 7779–7784. [DOI] [PubMed] [Google Scholar]
- 9. Tian H, Lin S, Wu J, et al.. Kaempferol alleviates corneal transplantation rejection by inhibiting NLRP3 inflammasome activation and macrophage M1 polarization via promoting autophagy. Exp Eye Res. 2021; 208: 108627. [DOI] [PubMed] [Google Scholar]
- 10. Tian H, Wu J, Ma M. Implications of macrophage polarization in corneal transplantation rejection. Transpl Immunol. 2021; 64: 101353. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Zhang C, Li L, et al.. Lymphangiogenesis in renal fibrosis arises from macrophages via VEGF-C/VEGFR3-dependent autophagy and polarization. Cell Death Dis. 2021; 12(1): 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Peppicelli S, Bianchini F, Calorini L. Inflammatory cytokines induce vascular endothelial growth factor-C expression in melanoma-associated macrophages and stimulate melanoma lymph node metastasis. Oncol Lett. 2014; 8(3): 1133–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nihei M, Okazaki T, Ebihara S, et al.. Chronic inflammation, lymphangiogenesis, and effect of an anti-VEGFR therapy in a mouse model and in human patients with aspiration pneumonia. J Pathol. 2015; 235(4): 632–645. [DOI] [PubMed] [Google Scholar]
- 14. Dietrich T, Bock F, Yuen D, et al.. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. J Immunol. 2010; 184(2): 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Assal A, Mapara MY. Janus kinase inhibitors and cell therapy. Front Immunol. 2021; 12: 740847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li L, Wei C, Cai S, Fang L. TRPM7 modulates macrophage polarization by STAT1/STAT6 pathways in RAW264.7 cells. Biochem Biophys Res Commun. 2020; 533(4): 692–697. [DOI] [PubMed] [Google Scholar]
- 17. Wu H, Zheng J, Xu S, et al.. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation. 2021; 18(1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray PJ, Allen JE, Biswas SK, et al.. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014; 41(1): 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ivashkiv LB. IFNγ: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat Rev Immunol. 2018; 18(9): 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding N, Wang Y, Dou C, et al.. Physalin D regulates macrophage M1/M2 polarization via the STAT1/6 pathway. J Cell Physiol. 2019; 234(6): 8788–8796. [DOI] [PubMed] [Google Scholar]
- 21. Guo L, Chen S, Liu Q, et al.. Glutaredoxin 1 regulates macrophage polarization through mediating glutathionylation of STAT1. Thorac Cancer. 2020; 11(10): 2966–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. De Vries LCS, Duarte JM, De Krijger M, et al.. A JAK1 selective kinase inhibitor and tofacitinib affect macrophage activation and function. Inflamm Bowel Dis. 2019; 25(4): 647–660. [DOI] [PubMed] [Google Scholar]
- 23. Yun Y, Chen J, Wang X, et al.. Tofacitinib ameliorates lipopolysaccharide-induced acute kidney injury by blocking the JAK-STAT1/STAT3 signaling pathway. Biomed Res Int. 2021; 2021: 8877056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu YC, Lwin NC, Chan NS, Mehta JS. Use of anterior segment optical coherence tomography to predict corneal graft rejection in small animal models. Invest Ophthalmol Vis Sci. 2014; 55(10): 6736–6741. [DOI] [PubMed] [Google Scholar]
- 25. Yu J, Li Y, Li Z, et al.. Subconjunctival injections of dimethyl fumarate inhibit lymphangiogenesis and allograft rejection in the rat cornea. Int Immunopharmacol. 2021; 96: 107580. [DOI] [PubMed] [Google Scholar]
- 26. Schönberg A, Hamdorf M, Bock F. Immunomodulatory strategies targeting dendritic cells to improve corneal graft survival. J Clin Med. 2020; 9(5): 1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clahsen T, Büttner C, Hatami N, Reis A, Cursiefen C. Role of endogenous regulators of hem- and lymphangiogenesis in corneal transplantation. J Clin Med. 2020; 9(2): 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kataru RP, Jung K, Jang C, et al.. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009; 113(22): 5650–5659. [DOI] [PubMed] [Google Scholar]
- 29. Su W, Han L, Chen X, et al.. Pharmacological inhibition of caspase-8 suppresses inflammation-induced lymphangiogenesis and allograft rejection in the cornea. J Allergy Clin Immunol. 2018; 142(1): 290–294.e9. [DOI] [PubMed] [Google Scholar]
- 30. Pleyer U, Schlickeiser S. The taming of the shrew? The immunology of corneal transplantation. Acta Ophthalmol. 2009; 87(5): 488–497. [DOI] [PubMed] [Google Scholar]
- 31. Friedman AD, Dan O, Drazba JA, Lorenz RR, Strome M. Quantitative analysis of OX62-positive dendritic cell distribution in the rat laryngeal complex. Ann Otol Rhinol Laryngol. 2007; 116(6): 449–456. [DOI] [PubMed] [Google Scholar]
- 32. Nykänen AI, Sandelin H, Krebs R, et al.. Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts. Circulation. 2010; 121(12): 1413–1422. [DOI] [PubMed] [Google Scholar]
- 33. Koh YC, Yang G, Lai CS, Weerawatanakorn M, Pan MH. Chemopreventive effects of phytochemicals and medicines on M1/M2 polarized macrophage role in inflammation-related diseases. Int J Mol Sci. 2018; 19(8): 2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001; 69(4): 598–604. [PubMed] [Google Scholar]
- 35. Zhu X, Guo Q, Zou J, et al.. MiR-19a-3p suppresses M1 macrophage polarization by inhibiting STAT1/IRF1 pathway. Front Pharmacol. 2021; 12: 614044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kovarik P, Stoiber D, Novy M, Decker T. Stat1 combines signals derived from IFN-gamma and LPS receptors during macrophage activation. EMBO J. 1998; 17(13): 3660–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yu T, Zuo Y, Cai R, et al.. SENP1 regulates IFN-γ-STAT1 signaling through STAT3-SOCS3 negative feedback loop. J Mol Cell Biol. 2017; 9(2): 144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang F, Zhang S, Jeon R, et al.. Interferon gamma induces reversible metabolic reprogramming of M1 macrophages to sustain cell viability and pro-inflammatory activity. EBioMedicine. 2018; 30: 303–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014; 57(12): 5023–5038. [DOI] [PubMed] [Google Scholar]
- 40. Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009; 228(1): 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be. Nat Immunol. 2009; 10(4): 356–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Izzo R, Bevivino G, Monteleone G. Tofacitinib for the treatment of ulcerative colitis. Expert Opin Investig Drugs. 2016; 25(8): 991–997. [DOI] [PubMed] [Google Scholar]
- 43. Azevedo A, Torres T. Tofacitinib: a new oral therapy for psoriasis. Clin Drug Investig. 2018; 38(2): 101–112. [DOI] [PubMed] [Google Scholar]
- 44. Fleischmann R. Tofacitinib in the treatment of active rheumatoid arthritis in adults. Immunotherapy. 2018; 10(1): 39–56. [DOI] [PubMed] [Google Scholar]
- 45. Bing SJ, Lyu C, Xu B, et al.. Tofacitinib inhibits the development of experimental autoimmune uveitis and reduces the proportions of Th1 but not of Th17 cells. Mol Vis. 2020; 26: 641–651. [PMC free article] [PubMed] [Google Scholar]
- 46. Li Y, Liu X, Yu J, et al.. Tofacitinib suppresses mast cell degranulation and attenuates experimental allergic conjunctivitis. Int Immunopharmacol. 2020; 86: 106737. [DOI] [PubMed] [Google Scholar]
- 47. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009; 29(6): 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kataru RP, Lee YG, Koh GY. Interactions of immune cells and lymphatic vessels. Adv Anat Embryol Cell Biol. 2014; 214: 107–118. [DOI] [PubMed] [Google Scholar]
- 49. Maruyama K, Ii M, Cursiefen C, et al.. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005; 115(9): 2363–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhao G, Zhu G, Huang Y, et al.. IL-6 mediates the signal pathway of JAK-STAT3-VEGF-C promoting growth, invasion and lymphangiogenesis in gastric cancer. Oncol Rep. 2016; 35(3): 1787–1795. [DOI] [PubMed] [Google Scholar]
- 51. Hos D, Cursiefen C. Lymphatic vessels in the development of tissue and organ rejection. Adv Anat Embryol Cell Biol. 2014; 214: 119–141. [DOI] [PubMed] [Google Scholar]