Abstract
Background
Women living with human immunodeficiency virus (HIV) tend to develop cervical cancer at a younger age than women without HIV. The World Health Organization’s (WHO) 2021 guidelines for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention include a conditional recommendation for initiating screening at age 25 for women living with HIV (WLWH). This recommendation is based on low-certainty evidence, and WHO calls for additional data. We describe the association of age and HIV status with visual inspection with acetic acid (VIA) positivity and cervical intraepithelial neoplasia grade two or higher (CIN2+) in Botswana.
Methods
This was a retrospective cross-sectional study of 5714 participants aged 25 to 49 years who underwent VIA screening in a clinic mainly serving WLWH. VIA-positive women received cryotherapy if eligible or were referred for colposcopy and excisional treatment. Known cervical cancer risk factors, screening outcome, and histological results were extracted from the program database. We compared the proportions and association of VIA positivity and CIN2+ by age and HIV status.
Results
The median age was 35 years [IQR 31–39], and 18% of the women were aged 25–29. Ninety percent were WLWH; median CD4 count was 250 cells/µL [IQR 150–428], and 34.2% were on anti-retroviral treatment (ART). VIA-positivity was associated with younger age (OR 1.48, CI 1.28, 1.72 for 25–29 years vs. 30–49 years), and HIV-positivity (OR 1.85, CI 1.51, 2.28). CIN2+ was only associated with HIV-positivity (OR 6.12, CI 3.39, 11.10), and proportions of CIN2+ were similar for both age groups in WLWH (69.1% vs. 68.3%).
Conclusions
Younger WLWH in Botswana had a significant burden of CIN2+. This finding further supports lowering the screening age for WLWH from 30 to 25.
Keywords: Botswana, Cervical pre-cancer, Cervical cancer screening, Colposcopy, Cryotherapy, Loop electrical excision procedure, See-and-treat, Visual inspection after acetic acid
Background
Low- and middle-income countries (LMICs) carry the highest global burden of cervical cancer incidence and mortality [1]. Cervical cancer is the leading cause of cancer death in women in Southern Africa [2, 3]. While human papillomavirus (HPV) vaccination in young girls offers hope for a significant reduction in cervical cancer in future generations, effective cervical cancer screening services remain essential to reduce morbidity and mortality associated with cervical cancer in women across the globe [4].
Women living with the human immunodeficiency virus (WLWH) have a higher risk of developing pre-invasive cervical disease and cervical cancer [5–7]. Although progression rates from pre-invasive cervical disease to cervical cancer are unknown due to standard intervention for high-grade pre-cancer, cervical cancer is diagnosed at younger ages in WLWH compared to women without HIV [5, 8, 9]. Guidelines for high-income countries (HICs) recommend cervical cancer screening initiation at the early age of 21 [10–12]. Up until recently, guidelines for most LMICs recommended the initiation of cervical cancer screening at the age of 30 despite LMICs having the highest global prevalence of HIV in the reproductive-aged population [13, 14]. The 2021 WHO guidelines have a conditional recommendation based on low-certainty evidence for initiating screening at age 25 for WLWH [15], and call for more data. Further, many LMICs will not be able to change their guidelines immediately due to resource constraints.
Botswana has one of the highest HIV prevalences globally, at 25.1% in women aged 15–49 [16]. Botswana’s national guidelines prioritize screening in the 30 to 49 year-old age group with either cytology or visual inspection with acetic acid (VIA), regardless of HIV status. While practical, these guidelines may not adequately account for the high prevalence of HIV in Botswana and the higher risk of early cervical cancer progression. There is limited published data from Botswana on the prevalence of pre-invasive disease and the role of screening in younger women.
This study describes the association of age and HIV status with VIA positivity and high-grade cervical abnormalities. We aimed to determine how initiating cervical cancer screening at age 25 years, instead of 30 years, in WLWH would improve the identification of high-grade cervical pre-cancer without unduly increasing overtreatment of low-grade cervical pre-cancer. Data presented here could strengthen the evidence for the WHO recommendation on the target age group for cervical cancer screening in WLWH.
Methods
Study design and patient selection
We conducted a retrospective cross-sectional study based on the Botswana Ministry of Health and Wellness (MOHW) National Cervical Cancer Prevention Programme “see-and-treat” pilot programmatic database [17]. The evaluation included women screened between March 2009 and August 2015 with visual inspection after acetic acid (VIA) at Bontleng clinic, a primary care clinic in the capital city Gaborone providing HIV testing and anti-retroviral treatment (ART) for the district. Women with low-grade lesions were offered same-day treatment with cryotherapy for lesions that met eligibility criteria. Women with lesions ineligible for cryotherapy were referred to Princess Marina Hospital (PMH), a regional tertiary hospital located five kilometres away, for colposcopy and excisional procedure. Cervical cancer screening services were provided for WLWH as part of comprehensive HIV care. The services were only extended to women without HIV towards the end of the evaluation period. Screening services were offered free of charge to all Botswana citizens.
Screening services were linked to a physician-led referral colposcopy and loop electrosurgical excision procedure (LEEP) clinic at PMH. Through various channels, women came to screening services, including provider referral, self-referral following sensitization by written materials, and health education talks. Women were excluded from screening if they had previously had a hysterectomy, pelvic radiation for lower genital tract cancer, or a cervical cancer diagnosis. Screening for women who were menstruating heavily, pregnant, or had a persistent vaginal discharge was re-scheduled for after resolution of the condition.
Cervical cancer screening procedures
All patients underwent a speculum examination of the cervix by a nurse who had participated in the Botswana MOHW VIA training program. The women were first assessed for lesions suspicious of cervical cancer (raised, ulcerative lesions with contact bleeding), and where present, women were referred to PMH for further evaluation. Visual assessment was performed after applying 5% acetic acid to the cervix using a cotton swab. The findings were categorized as normal, abnormal with a recommendation for cryotherapy, or abnormal with a recommendation for LEEP. Abnormal lesions were described as: (1) low-grade if they were well-defined and opaque acetowhite, or (2) high-grade if they were dense acetowhite or had abnormal vessels. Women with low-grade lesions covering less than three-quarters of the cervix, and not extending into the endocervical cancel were offered same-day treatment with cryotherapy; these women had no histopathology specimen collected. Women with abnormal lesions ineligible for cryotherapy based on appearance, size, or extension into the endocervical canal, were referred to the colposcopy/LEEP clinic at PMH, and evaluated by a specialist gynecologist or trained medical officers. The colposcopic appearance of lesions determined diagnostic and treatment decisions. Low-grade appearing lesions were treated with cautery after taking a biopsy; high-grade appearing lesions and those extending into the endocervical canal were treated with LEEP. Board-certified pathologists reviewed histopathology specimens at the National Health Laboratory (NHL), a government referral laboratory, offering services to the MOHW and private health facilities in Botswana. The pathologists were blinded to VIA findings. Histopathology results were classified according to the American Society of Colposcopy and Cervical Pathology (ASCCP) and the College of American Pathologists cervical intra-epithelial neoplasia (CIN) criteria. In brief, the specimens were recorded as no CIN, CIN graded one to three based on severity, or invasive cervical cancer (ICC).
HIV procedures
Women with unknown HIV status at the time of screening or with documented HIV negative status more than six months prior were referred to an HIV testing center and requested to share their results. Throughout the study period, the Botswana National HIV program initiated anti-retroviral treatment (ART) at a CD4 count of ≤ 350 cells/µL.
Data collection
All women undergoing VIA screening completed a questionnaire capturing a limited set of patient-level cervical cancer risk factors, including smoking, age of sexual debut, and parity. HIV status was recorded, and for WLWH, CD4 count at the time of HIV diagnosis and whether on ART at the time of screening was documented. VIA screening outcomes were recorded in the programmatic database. Histology results of women referred for colposcopy/LEEP were extracted from the NHL electronic medical record when available and entered into the programmatic database.
Outcomes
The primary outcome was the association of VIA positivity and age, adjusting for cervical cancer risk factors. The secondary outcomes were the association of histopathologically confirmed high-grade abnormality and age, adjusting for cervical cancer risk factors; HIV-status association with VIA positivity and high-grade abnormality; and the proportions of VIA positivity and high-grade abnormality by both age and HIV status.
Data analysis
The analyzed dataset included only women between the ages of 25 and 49. Patient records with missing data for VIA or histopathology that could not be corrected by cross-reference with primary records were excluded from the primary and secondary analysis, respectively. The sample size for the primary outcome was calculated using a 1-sided alpha of 0.05. To attain a 99% power, we assumed VIA positivity to be 30% in women aged 25 to 29 years and 20% in women aged 30 to 49 years based on previous findings [17]. The sample size required to detect a statistically significant difference in VIA-positivity between the two age groups was 2076 women (374 women aged 25 to 29 years and 1702 women aged 30 to 49 years).
The cervical cancer risk factors adjusted for included: HIV status, parity, smoking, and age of sexual debut. CD4 count and ART were included in the analysis of WLWH. Descriptive statistics for these variables are presented as median [interquartile range (IQR)] and proportions. Continuous variables were categorized into binary variables and compared using the chi-square test. Categorical variables included age groups of younger and older women (25 to 29 years; 30 to 49 years), age of sexual debut (≤ 18; > 18 years), parity (≤ 2; > 2), CD4 count (≤ 350 cells/µL; > 350 cells/µL), and histopathology results (benign or CIN 1 [≤ CIN1] for low-grade abnormalities; CIN2+ for high-grade abnormalities being CIN2/3 and ICC). Patterns of missing data were described for the study cohort using percentages.
Logistic regression models computed unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (CI). Only exposure variables with a p-value of less than 0.1 for unadjusted ORs were included in the adjusted regression models [18]. A p-value of less than 0.05 was considered to be statistically significant. We used Stata 14.0 (StataCorp LLC, College Station, Texas).
Results
Overall patient characteristics
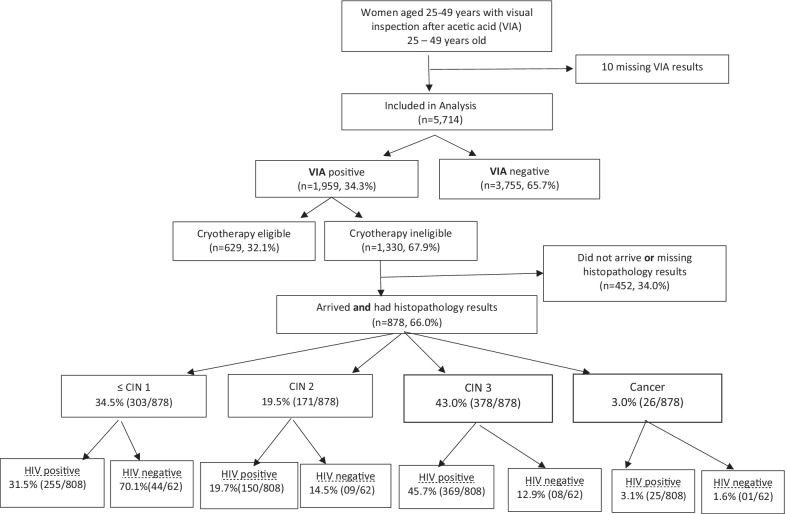
The database included 5724 women aged 25 to 49 years screened with VIA between March 2009 and August 2015 (Fig. 1). Ten women had missing VIA data, leaving 5714 women for the VIA analysis. As shown in Table 1, the median age was 35 years [IQR 31–39], and 1029 (18%) were between 25 and 29 years of age. Smoking was reported by 285 (5%) of the women. The median age of sexual debut was 18 years [IQR 17–20], and the median parity was two [IQR 1–3]. HIV status was known in 5583 (98%), and 5026 (90%) of those with a known status were WLWH. Eight hundred and forty nine (86%) of the women aged 25 to 29 years and 4177 (91%) of the those aged 30 to 49 years were WLWH. Among the WLWH, the median CD4 count was 250 cells/µL [IQR 150–428], and 1628 (34.2%) were on ART. Missing data was ≤ 5% for all the variables except for CD4 count (11%, n = 551). The level of CD4 count missing data was similar for both age groups (10.5% for 25 to 29 year-olds versus 11.1% for 30 to 49 year-olds).
Fig. 1.
Study flow chart
Table 1.
Demographic and clinical characteristics of all study participants
| Variable | All | Age 25–29 years | Age 30–49 years | P-value for χ2 test | |
|---|---|---|---|---|---|
| n (%) | Median [IQR] | na (%) | na (%) | ||
| Age | 5714 | 35 [31, 39] | |||
| Smoking | 5661 | 1018 (18.0) | 4643 (82.0) | 0.008b | |
| Yes | 285 (50.0) | 68 (6.7) | 217 (4.7) | ||
| No | 5376 (94.1) | 950 (93.3) | 4426 (95.3) | ||
| Missing | 53 (1.0) | ||||
| Sexual debut | 5689 | 18 [17, 21] | 1024 (18.0) | 4665 (82.0) | 0.02b |
| ≤ 18 | 3150 (55.3) | 533 (52.1) | 2617 (56.1) | ||
| > 18 | 2539 (44.4) | 491 (47.9) | 2048 (43.9) | ||
| Missing | 25 (0.4) | ||||
| Parity | 5612 | 2 [1, 3] | 1001 (17.8) | 4611 (82.2) | < 0.001b |
| ≤ 2 | 3223 (56.4) | 815 (81.4) | 2408 (52.2) | ||
| > 2 | 2389 (41.8) | 186 (18.6) | 2203 (47.8) | ||
| Missing | 102 (1.8) | ||||
| HIV status | 5583 | 989 (17.7) | 4594 (82.3) | < 0.001b | |
| Negative | 557 (9.7) | 140 (14.2) | 417 (9.1) | ||
| Positive | 5026 (88.0) | 849 (85.8) | 4177 (90.9) | ||
| Missing | 131 (2.3) | ||||
| Initial CD4 at HIV diagnosisc | 4475 | 250 [150, 428] | 760 (16.9) | 3715 (83.1) | < 0.001b |
| ≤ 350 | 2308 (45.9) | 304 (40.0) | 2004 (53.9) | ||
| > 350 | 2167 (43.1) | 456 (60.0) | 1711 (46.1) | ||
| Missing | 551 (11.0) | ||||
| On ART at time of screeningc | 4766 | 802 (16.8) | 3964 (83.2) | < 0.001b | |
| Yes | 1628 (32.4) | 346 (43.1) | 1282 (32.3) | ||
| No | 3138 (62.4) | 456 (56.9) | 2682 (67.7) | ||
| Missing | 260 (5.2) | ||||
| VIA results | 5714 | 1029 (18.0) | 4685 (82.0) | < 0.001b | |
| Positive | 1959 (34.3) | 428 (41.6) | 1531 (32.7) | ||
| Negative | 3755 (65.7) | 601 (58.4) | 3154 (67.3) | ||
ART, anti-retroviral treatment; CIN, cervical intraepithelial neoplasia; IQR, inter-quartile range; VIA, visual inspection after acetic acid
aThe number of women in levels of categorical variable may not add up to total “n” because missing category has been removed
bFor p < 0.05
cFor HIV positive patients only
VIA-positivity
The overall VIA positivity of the study population was 34.3% (n = 1959). The proportion was higher in the 25 to 29 year-olds (41.5%, n = 428) than the 30 to 49 year-olds (32.7%, n = 1531). The WLWH had a higher VIA positivity rate (35.9%, n = 1841) than women without HIV (24.1%, n = 141) (Table 2).
Table 2.
Study participants’ characteristics with bivariate and multivariable odds ratio for VIA positivity
| Variable | All n (%) |
VIA positive na (%) |
VIA negative na (%) |
VIA positivity BVA Odds Ratios (95% CI) |
P-value | VIA Positivity MVA Odds Ratios (95% CI) |
P-value for χ2 test |
|---|---|---|---|---|---|---|---|
| Age Group | 5714 | 1959 (34.3) | 3755 (65.7) | ||||
| 25–29 years | 1029 (18.0) | 428 (41.6) | 601 (58.4) | 1.47 (1.28, 1.69) | < 0.001b | 1.48 (1.28, 1.72) | < 0.001b |
| 30–49 years | 4685 (82.0) | 1531 (32.7) | 3154 (67.3) | Ref | |||
| Smoker | 5661 | 1937 (34.2) | 3724 (65.8) | ||||
| No | 5376 (94.1) | 1825 (33.9) | 3551 (66.1) | Ref | |||
| Yes | 285 (5.0) | 112 (39.3) | 173 (60.7) | 1.27 (1.00, 1.62) | 0.053 | 1.14 (0.89, 1.46) | 0.31 |
| Missing | 53 (0.9) | ||||||
| Age sexual debut | 5689 | 1950 (34.3) | 3739 (65.7) | ||||
| ≤ 18 | 3150 (55.1) | 1075 (34.1) | 2075 (65.9) | 0.99 (0.88, 1.10) | 0.79 | N/A | N/A |
| > 18 | 2539 (44.4) | 875 (34.5) | 1664 (65.5) | Ref | |||
| Missing | 25 (0.5) | ||||||
| Parity | 5612 | 1924 (34.3) | 3688 (65.7) | ||||
| ≤ 2 | 3223 (56.40) | 1151 (35.7) | 2072 (64.3) | Ref | |||
| > 2 | 2389 (41.81) | 773 (32.4) | 1616 (67.6) | 0.86 (0.77, 0.96) | 0.009b | 0.88 (0.79, 0.99) | 0.04b |
| Missing | 102 (1.79) | ||||||
| HIV status | 5583 | 1941 (34.8) | 3642 (65.2) | ||||
| Negative | 557 (9.74) | 134 (24.1) | 423 (75.9) | Ref | |||
| Positive | 5026 (87.96) | 1807 (35.9) | 3219 (64.1) | 1.77 (1.45, 2.17) | < 0.001b | 1.85 (1.51, 2.28) | < 0.001b |
| Missing | 131 (2.30) | ||||||
| CD4 Count at HIV | 4475 | 1620 (36.2) | 2855 (63.8) | ||||
| Diagnosisc | 2308 (45.9) | 809 (35.1) | 1499 (64.9) | 0.90 (0.80, 1.02) | 0.10 | 0.96 (0.83, 1.10) | 0.52 |
| ≤ 350 | 2167 (43.1) | 811 (37.4) | 1356 (62.6) | Ref | |||
| > 350 | 551 (11.0) | ||||||
| Missing | |||||||
| ART at time of screening c | 4766 | 1710 (35.9) | 3056 (64.1) | ||||
| No | 3138 (62.4) | 1096 (34.9) | 2047 (65.1) | 0.89 (0.78, 1.00) | 0.06 | 0.91 (0.78, 1.05) | 0.19 |
| Yes | 1628 (32.4) | 614 (37.7) | 1014 (62.3) | Ref | |||
| Missing | 260 (5.2) |
ART, anti-retroviral therapy; BVA, bivariate analysis; MVA, multivariate analysis; VIA, visual inspection after acetic acid
aThe number of women in levels of categorical variable may not add up to total “n” because missing category has been removed
bFor P < 0.05
cFor HIV positive patients only
In multivariate analyses, VIA positivity was more likely in 25 to 29 year-olds than in 30 to 49 year-olds (OR 1.48, CI 1.28, 1.72), and in WLWH compared to women without HIV (OR 1.85, CI 1.51, 2.28). Among WLWH, VIA positivity was not affected by CD4 count (OR 0.96, CI 0.83, 1.10) or by ART (OR 0.91, CI 0.78, 1.05) (Table 2).
High-grade abnormality
The majority of the VIA-positive lesions were ineligible for treatment with cryotherapy (68%, n = 1330); this was similar for both the 25 to 29 year-olds and the 30 to 49 year-olds (67.1% vs. 68.1%, respectively). Of the 1330 women referred to colposcopy/LEEP, 878 (66%) attended and had recorded histopathology results (58.5% for 25 to 29 year-olds, and 68.1% for 30–49 year-olds). The rates of ≤ CIN1, CIN2, CIN3 and ICC among women with histopathology results were 33.4%, 19.5%, 43.0%, and 3.0%, respectively. Although the CIN 2/3 rates were similar for both age groups, all cancers were in the 30 to 49 age group except for one case recorded in the 25 to 29 age group of WLWH (Fig. 1, Table 3).
Table 3.
VIA and histological outcomes by age group and HIV status
| All participants | HIV positivea | HIV negativeb | ||||
|---|---|---|---|---|---|---|
| 25–29 Age group | 30–49 Age group | 25–29 Age group | 30–49 Age group | 25–29 Age group | 30–49 age group | |
| Number Screened with VIA | N = 1029 | N = 4685 | n = 849 | N = 4177 | N = 140 | N = 417 |
| VIA outcomes | ||||||
| VIA results | ||||||
| Negative | 601 (58.4%) | 3154 (67.3%) | 472 (55.6%) | 2747 (65.8%) | 95 (67.9%) | 328 (78.7%) |
| Positive | 428 (41.6%) | 1531 (32.7%) | 377 (44.4%) | 1430 (34.2%) | 45 (32.1%) | 89 (21.3%) |
| Eligible for cryotherapy | 141 (32.9%) | 488 (31.9%) | 121 (32%) | 477 (33.4%) | 18 (40%) | 34 (38.2%) |
| Not eligible for cryotherapy | 287 (67.1%) | 1043 (68.1%) | 256 (68%) | 953 (66.6%) | 27 (60%) | 55 (61.8% |
| Not eligible for cryotherapy, arrived at colposcopy with histology results | N = 168 | N = 710 | n = 149 | n = 659 | N = 16 | N = 46 |
|---|---|---|---|---|---|---|
| Histology outcomes | ||||||
| ≤ CIN 1 | 56 (33.4%) | 247 (34.8%) | 46 (30.9%) | 209 (31.7%) | 8 (50%) | 36 (78.2%) |
| CIN2 | 36 (21.4%) | 135 (19.0%) | 31 (20.8%) | 128 (19.4%) | 5 (31.3%) | 4 (8.7%) |
| CIN3 | 75 (44.6%) | 303 (42.7%) | 71 (47.6%) | 298 (45.2%) | 3 (18.7%) | 5 (10.9%) |
| Cervical cancer | 1 (0.6%) | 25 (3.5%) | 1 (0.7%) | 24 (3.7%) | 0 (%) | 1 (2.2%) |
CIN, cervical intraepithelial neoplasia; VIA, visual inspection after acetic acid
Figures for a and b do not add up to 5714 due to missing HIV status in 131 records
In multivariate analyses, CIN2 + was associated with a positive HIV status (aOR 6.12, CI 3.39, 11.10), but not with age (OR 1.07, CI 0.75–1.52 for 25 to 29 year-olds compared to 30 to 49 year-olds). In WLWH, neither CD4 count nor ART was associated with CIN2+ (Table 4).
Table 4.
All Study participants’ characteristics with bivariate and multivariable odds ratio for CIN2 +
| Variable | All n (%) |
≥ CIN2+ na (%) |
≤ CIN1 na (%) |
CIN2+ BVA Odds Ratios (95% CI) |
P-value | CIN2+ MVA Odds Ratios (95% CI) |
P-value for χ2 test |
|---|---|---|---|---|---|---|---|
| Age Group | 878 | 575 (65.5) | 303 (34.5) | ||||
| 25–29 years | 168 (19.1) | 112 (66.7) | 56 (33.3) | 1.07 (0.75, 1.52) | 0.72 | N/A | N/A |
| 30–49 years | 710 (80.9) | 463 (65.2) | 247 (34.8) | Ref | |||
| Smoker | 867 | 566 (65.3) | 301 (34.7) | ||||
| No | 817 (93.1) | 530 (64.9) | 287 (35.1) | Ref | |||
| Yes | 50 (5.7) | 36 (72.0) | 14 (28.0) | 1.38 (0.74, 2.62) | 0.30 | N/A | N/A |
| Missing | 11 (1.2) | ||||||
| Age Sexual debut | 873 | 572 (65.5) | 301 (34.5) | ||||
| ≤ 18 | 505 (57.5) | 338 (66.9) | 167 (33.1) | 1.16 (0.87, 1.54) | 0.31 | N/A | N/A |
| > 18 | 368 (41.9) | 234 (63.6) | 134 (36.4) | Ref | |||
| Missing | 5 (0.6) | ||||||
| Parity | 861 | 566 (65.7) | 295 (34.3) | ||||
| ≤ 2 | 517 (58.9) | 328 (63.4) | 189 (36.6) | Ref | |||
| > 2 | 344 (39.2) | 238 (69.2%) | 106 (30.8) | 1.29 (0.97, 1.73) | 0.08 | 1.30 (0.96, 1.75) | 0.09 |
| Missing | 17 (2.0) | ||||||
| HIV Status | 870 | 571 (65.6) | 299 (34.4) | ||||
| Negative | 62 (7.1) | 18 (29.0) | 44 (71.0) | Ref | |||
| Positive | 808 (92.0) | 553 (68.4) | 255 (31.6) | 5.30 (2.96, 9.49) | < 0.001b | 6.12 (3.39, 11.10) | < 0.001b |
| Missing | 8 (0.9) | ||||||
| CD4 Count at HIV | 701 | 494 (70.5) | 207 (29.5) | ||||
| Diagnosisc | 336 (41.6) | 234 (69.6) | 102 (30.4) | 0.93 (0.67, 1.28) | 0.65 | N/A | N/A |
| ≤ 350 | 365 (45.2) | 105 (28.8) | Ref | ||||
| > 350 | 107 (12.2) | ||||||
| Missing | 260 (71.2 | ||||||
| ART at time of screening c | 773 | 527 (68.2) | 246 (31.8) | ||||
| No | 455 (56.3) | 315 (69.2) | 140 (30.8) | 1.13 (0.83, 1.53) | 0.45 | N/A | N/A |
| Yes | 318 (39.4) | 212 (66.7) | 106 (33.3) | Ref | |||
| Missing | 35 (4.3) |
ART, anti-retroviral therapy; BVA, bivariate analysis; MVA, multivariate analysis; VIA, visual inspection after acetic acid
aThe number of women in levels of categorical variable may not add up to total “n” because missing category has been removed
bFor P < 0.05
cFor HIV positive patients only
VIA-positivity and high-grade abnormality by age and HIV-status
In WLWH, the 25 to 29 year-olds were more likely to be VIA positive than the 30 to 49 year-olds (44.4%, n = 377 vs. 34.2%, n = 1430, respectively). We observed a similar pattern for women without HIV (32.1%, n = 45 for 25 to 29 year-olds, compared to 21.3%, n = 89 for 49 year-olds). Among women with histopathology results, the rate of CIN2 + in WLWH was 68.4% (69.1% for the 25 to 29 year-olds and 68.3% for the 30 to 49 year-olds), and the rate of CIN2 + in women without HIV was 29.0% (50% for 25 to 29 year-olds and 21.8% for 30 to 49 year-olds) (Table 3).
Discussion
WLWH aged 25 to 29 years attending routine cervical cancer screening in our national program had the same odds of having high-grade cervical pre-cancer as women aged 30 to 49 years. Prior research has indicated a link between younger age and cervical cancer among WLWH [5, 8, 9]. Our findings confirm the presence of a significant level of cervical cancer precursors requiring intervention in women as young as 25 years, particularly in WLWH, thus supporting the 2021 WHO recommendation to lower the age of initiation of cervical cancer screening from 30 to 25 years in WLWH.
A concern about lowering the cervical cancer screening age has been that clinically insignificant lesions from transient HPV infections would be intervened upon unnecessarily, resulting in overtreatment of young women [19, 20]. Although women in this cohort aged 25 to 29 years had higher rates of VIA positivity than women aged 30 to 49 years, similar proportions were referred for an excisional procedure. The histopathology results indicate that the proportions of CIN2+ detected and appropriately treated were similar for the two age groups in WLWH. If overtreatment did occur, it would primarily have occurred in the group of women treated with cryotherapy, a treatment that ultimately has minimal side effects [21]. As expected, the proportion of CIN2+ was more than three times lower in women without HIV than in WLWH. In the group without HIV, women aged 25 to 29 were twice as likely to have CIN2+ as women aged 30 to 49, a finding that is not in keeping with the general literature. However, it is difficult to draw any conclusion from this finding due to the small number of the women without HIV in the cohort.
We had expected to find a correlation between patient age and high-grade pre-cancer because older women would have had a longer time to progress from HPV infection to cervical pre-cancer without opportunities for intervention [22]. However, our data do not support this hypothesis. Instead, younger women overall and younger WLWH had a similar proportion of CIN2+ compared to older women. Data is limited on HPV progression to cervical pre-cancer and cancer in women aged 20 to 29 years. Adolescent WLWH are more likely to have HPV co-infections and coexisting abnormalities, albeit low-grade, relative to those without HIV [23]. Our finding of high rates of high-grade pre-cancer in 25 to 29 year-old WLWH further supports an accelerated timeframe of progression from HPV infection to high-grade pre-cancer in young WLWH.
Our analysis has limitations. We utilized a programmatic database from a screening programme originally designed to serve only WLWH. Services were only later offered to women without HIV; hence there is a much lower number of women without HIV in this cohort than the general population. Therefore, the findings may not be fully generalizable to all females aged 25–49 years in Botswana. Further, limited patient-level demographic and risk factor data were collected, and not all variables had complete data. For instance, CD4 count had 11% missing data; however, this was similar for both age groups, and we doubt that it would have had a significant effect on the outcomes. Other key HIV-related variables, including viral load and timing of HIV treatment, were not collected. Therefore, the full extent of the immune status of WLWH could not be assessed. Although the rates of VIA positivity and cryotherapy ineligibility were high, similar rates have been observed in other high HIV burden areas and could be related to low rates of prior screening in the population [24]. The high VIA positivity rates in this cohort could also be related to non-HPV-associated cervicitis. High rates of cervicitis have been shown to affect the accuracy of VIA in women in the 30 to 49 age group in a similar population [25]. However, while cervicitis may affect VIA positivity rates in both younger and older age groups, the higher cervicitis rate in the younger group may have accounted for a greater increase in VIA than in the older age group. Over 30% of the women were eligible for cryotherapy without biopsy, which means some CIN2+ lesions might have been treated with ablative treatment without documented histopathology results leading to the underestimation of the CIN2+ rate. Finally, a third of the women with lesions ineligible for cryotherapy did not comply with their referral for colposcopy, and documentation of colposcopy/LEEP referral appointment attendance was not fully recorded. Thus, histopathology results may not represent the entire cohort of women who had or should have had colposcopic evaluation.
Conclusions
Despite the limitations of this study, we present new evidence of the significant burden of CIN2+ in younger WLWH in Botswana. Until the population-level effects of HPV vaccination and universal ART to improve overall immune competence in WLWH are realized [26], the reduction in cervical cancer in LMICs will depend on effective, comprehensive screening programs for WLWH. This additional evidence further supports the current WHO conditional recommendation for initiating screening at age 25.
Acknowledgements
We acknowledge with thanks the contributions of Harvey Friedman, Nicola Zetola, Ari Ho-Foster, Balladia Kizoto, and Kadimo Khutsafalo.
Abbreviations
- ART
Anti-retroviral treatment
- CI
Confidence interval
- CIN
Cervical intraepithelial neoplasia
- HIC
High-income country
- HIV
Human immunodeficiency virus
- HPV
Human papillomavirus
- HRDC
Health research and development committee
- LEEP
Loop electro-surgical excision procedure
- LMIC
Low-middle income county
- IQR
Interquartile range
- MOHW
Ministry of health and wellness
- NHL
National health laboratory
- OR
Odds ratio
- PMH
Princess Marina hospital
- VIA
Visual inspection after acetic acid
- WHO
World Health Organization
- WLHV
Women living with HIV
Authors' contributions
Study conception and design: DRM, RL, SG; Acquisition of data: BM, DRM; Analysis and interpretation of data: DRM, RL, SG, GJH, AM, CM, LG; Drafting of manuscript: DRM, RL, SG; Critical revision: GJH, CM, LBM, AM. All authors approved the manuscript before submission. All authors read and approved the final manuscript.
Funding
The development of the ‘see and treat’ pilot program database was undertaken as part of the PEPFAR-supported Botswana-UPenn Partnership Technical assistance for in-service training for health care providers in Botswana (U2GPS001949).
Availability of data and materials
The dataset generated during the current study is provided.
Declarations
Ethics approval and consent to participate
The Health Research and Development Committee (HRDC), which is the ethical review committee of the Botswana Ministry of Health and Wellness, and the Institutional Review Board of the University of Pennsylvania approved the original programmatic data collection(HPDME-13/181 and Protocol #817775, respectfully). Patient informed consent was obtained for the original data collection ensuring privacy to protect the rights of the participants. The participants were not exposed to any risks or harmed in any way by participating in the study. Permission was granted by the HRDC for this secondary analysis. All research processes were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
No competing interests declared by the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8(2):e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. [DOI] [PubMed]
- 3.Dryden-Peterson S, Bvochora-Nsingo M, Suneja G, et al. HIV infection and survival among women with cervical cancer. J Clin Oncol. 2016;34(31):3749–3757. doi: 10.1200/JCO.2016.67.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peirson L, Fitzpatrick-Lewis D, Ciliska D, et al. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev. 2013;2:35. doi: 10.1186/2046-4053-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabar S, Hleyhel M, Belot A, et al. Invasive cervical cancer in HIV-infected women: risk and survival relative to those of the general population in France. Results from the French Hospital Database on HIV (FHDH)-Agence Nationale de Recherches sur le SIDA et les Hepatites Virales (ANRS) CO4 cohort study. HIV Med. 2019;20(3):222–229. doi: 10.1111/hiv.12703. [DOI] [PubMed] [Google Scholar]
- 6.Stelzle D, Tanaka LF, Lee KK, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. 2021;9(2):e161–e169. doi: 10.1016/S2214-109X(20)30459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu G, Sharma M, Tan N, et al. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018;32(6):795–808. doi: 10.1097/QAD.0000000000001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grover S, Bvochora-Nsingo M, Yeager A, et al. Impact of human immunodeficiency virus infection on survival and acute toxicities from chemoradiation therapy for cervical cancer patients in a limited-resource setting. Int J Radiat Oncol Biol Phys. 2018;101(1):201–210. doi: 10.1016/j.ijrobp.2018.01.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mpunga T, Znaor A, Uwizeye FR, et al. A case-control study of HIV infection and cancer in the era of antiretroviral therapy in Rwanda. Int J Cancer. 2018;143(6):1348–1355. doi: 10.1002/ijc.31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer screening in the European Union. Cancer screening in the European Union. Report on the implementation of the council recommendation on cancer. Screening 2017 [Available from: https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/2017_cancerscreening_2ndreportimplementation_en.pdf.
- 11.Wilt TJ, Harris RP, Qaseem A, et al. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162(10):718–725. doi: 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Practice Guidelines G. Practice Bulletin No. 168: cervical cancer screening and prevention. Obstet Gynecol. 2016;128(4):e111–e30. [DOI] [PubMed]
- 13.South Africa Department of Health Cervical Cancer Screening Programme. South Africa Department of Health Cervical Cancer Screening Programme. National Guidelines for Cervical Cancer Screening Programme. Available from: https://screening.iarc.fr/doc/SAcervical-cancer.pdf.
- 14.World Health Organization. World Health Organization. Cervical cancer. Available from: https://www.who.int/cancer/prevention/diagnosis-screening/cervical-cancer/en/.
- 15.World Health Organization. World Health Organization. Guideline for screening and treatment of cervical pre-cancer lesions for cervical cancer prevention. Available from: https://www.who.int/publications/i/item/9789240030824. [PubMed]
- 16.UNAIDS. UNAIDS. Aidsinfo 2019 data. Available from: http://aidsinfo.unaids.org/.
- 17.Ramogola-Masire D, de Klerk R, Monare B, et al. Cervical cancer prevention in HIV-infected women using the "see and treat" approach in Botswana. J Acquir Immune Defic Syndr. 2012;59(3):308–313. doi: 10.1097/QAI.0b013e3182426227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haque MM RA, Dharma Hagare D, Chowdhury RK. A comparative assessment of variable selection methods in urban water demand forecasting. Water. 2018;10.
- 19.Anderson J, Wysong M, Estep D, et al. Evaluation of cervical cancer screening programs in Cote d'Ivoire, Guyana, and Tanzania: effect of HIV status. PLoS ONE. 2015;10(9):e0139242. doi: 10.1371/journal.pone.0139242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Msyamboza KP, Phiri T, Sichali W, et al. Cervical cancer screening uptake and challenges in Malawi from 2011 to 2015: retrospective cohort study. BMC Public Health. 2016;16(1):806. doi: 10.1186/s12889-016-3530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denny L, Kuhn L, De Souza M, et al. Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294(17):2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 22.Kelly H, Weiss HA, Benavente Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV. 2018;5(1):e45–e58. doi: 10.1016/S2352-3018(17)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moscicki AB, Ellenberg JH, Crowley-Nowick P, et al. Risk of high-grade squamous intraepithelial lesion in HIV-infected adolescents. J Infect Dis. 2004;190(8):1413–1421. doi: 10.1086/424466. [DOI] [PubMed] [Google Scholar]
- 24.Parham GP, Mwanahamuntu MH, Kapambwe S, et al. Population-level scale-up of cervical cancer prevention services in a low-resource setting: development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLoS ONE. 2015;10(4):e0122169. doi: 10.1371/journal.pone.0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Painter H, Erlinger A, Simon B, et al. Impact of cervicitis on performance of cervical cancer screening using HRHPV testing and visual evaluation in women living with HIV in Botswana. Int J Gynaecol Obstet. 2020. [DOI] [PMC free article] [PubMed]
- 26.Makhema J, Wirth KE, Pretorius Holme M, et al. Universal testing, expanded treatment, and incidence of HIV infection in Botswana. N Engl J Med. 2019;381(3):230–242. doi: 10.1056/NEJMoa1812281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset generated during the current study is provided.