Abstract
Background
Thyroid nodules are a challenge in clinical practice and thyroid ultrasonography is essential for assessing the risk of malignancy. The use of ultrasound-based malignancy risk classification systems has been recommended by several scientific societies but radiologist’s adherence to these guidelines may vary. The authors aimed to analyze the quality of the information provided by the thyroid ultrasound report, to assess the malignancy risk of thyroid nodules, in Portugal.
Methods
Multicenter and retrospective study, conducted in three of the five Portuguese NUTS2 corresponding to about 88.3% of the mainland population. We included 344 consecutive unselected participants aged ≥ 18 years who underwent thyroid ultrasonography in 2019. The description of six features of the dominant thyroid nodule was analyzed: maximum size, shape, margins, composition, echogenicity and echogenic foci. A utility score, including these six features, was used as an indicator of the report’s quality. A score of 4 was considered as a minimum value.
Results
Maximum diameter was reported for all nodules. Shape, margins, composition, echogenicity and echogenic foci were reported in 8.1%, 25.0%, 76.5%, 53.2% and 20.9%, respectively. Only 21.8% of the nodules had a score ≥ 4. At least one of four suspicious features, including marked hypoechogenicity, microcalcifications, irregular margins and non-oval shape, was identified in 8.7% of the nodules. Cervical lymph nodes’ status was reported in 93% of the exams. The risk category was only reported in 7.8% of the participants.
Conclusion
The adherence of Portuguese radiologists to a standardized reporting model and to an ultrasound-based malignancy risk stratification system is still low and has implications for the correct characterization of the malignancy risk of nodules and the decision to perform fine-needle aspiration biopsy.
Keywords: Thyroid, Thyroid nodules, Thyroid cancer, Ultrasound, Cancer risk, Clinical guidelines, Fine needle aspiration
Background
Thyroid nodules are very common and its ultrasound (US) prevalence may reach 19–68%, depending on the studied population [1]. Despite their high prevalence, thyroid nodules have a relatively low risk of malignancy, around 3.1% and 4.7% in multinodular goiter and solitary nodule, respectively [2]. Thyroid cancer has a high worldwide incidence and generally has a good prognosis [3]. In Portugal, its incidence and mortality rate is respectively higher and lower than the world data [4]. In addition, the incidence of thyroid cancer has seen a global rise without a proportional increase in mortality [3–5]. The upward trend in the incidence rate of thyroid cancer has been mainly attributed to the diagnosis of asymptomatic cancers, namely microcarcinomas [6]. The increased detection of asymptomatic cancers can be caused by thyroid US “overscreening”, improving accessibility to health care services, and a trend towards an increase in the number of neck imaging studies and fine-needle aspiration biopsy (FNAB) procedures, In addition, the increase in the volume of thyroid surgery and changes in histopathological management guidelines of thyroid histological specimens may also contribute to these numbers [3, 5]. Overdiagnosis of thyroid cancer and its possible overtreatment may have important implications for patients and health care systems.
Clinical management guidelines for thyroid nodules have been changing over the last few years, namely with regard to a better characterization of the US-based malignancy risk and a trend towards a reduction in the number of nodules with indication for FNAB.
Similar to the Breast Imaging Reporting & Data System (BIRADS) classification used in breast US studies, the Thyroid Imaging Reporting & Data System (TIRADS) classification, an US-based malignancy risk stratification system for thyroid nodules, was introduced in 2009 by Horvath and colleagues [7]. In recent years, several scientific societies have proposed their own risk classifications. The US-based classification systems of the American Thyroid Association (ATA) [1], K-TIRADS of the Korean Society of Thyroid Radiology (KSThR) [8], ACR TI-RADS of the American College of Radiology (ACR) [9], and the EU-TIRADS of the European Thyroid Association (ETA) [10], have been widely used. The diagnostic performance comparison of these latest US-based malignancy risk-stratification systems has not shown clear differences between them [11–16]. EU-TIRADS has been widely accepted in Europe and was recently recommended in Portugal by the Thyroid Study Group (GET), of the Portuguese Society of Endocrinology, Diabetes and Metabolism (SPEDM) [17].
Taking into account limitations due to the particularities of each classification system, the cutoff points for FNAB are higher in the ACR TI-RADS and EU-TIRADS systems than in the ATA 2015 or K-TIRADS [16]. This leads to a decrease in the indications for FNAB [18]. Although ACR-TIRADS might have a slightly higher diagnostic accuracy than EU-TIRADS [15, 18], the use of a point scale for nodules classification may be excessively time-consuming in daily clinical practice. On the other hand, EU-TIRADS has shown to have a higher rate of inter-observer agreement than ACR TI-RADS in the classification and selection of nodules for FNAB [14]. Regardless of the classification system used, it is essential that the thyroid US reports, has the minimum necessary information for a correct risk stratification. Thus, the adoption of a standardized thyroid US report model that includes the use of a common lexicon [17, 19] and sufficient information [20, 21] may improve their quality [22]. US reports may, in many cases, not yet be adequate to this new reality [23]. In Portugal, the adherence of board-certified radiologists, who usually do thyroid US exams, to these classification systems seems to be quite low.
The GET intended to contribute to an improvement of the US reports through the development of a project called GET ECO. This initiative included the elaboration and publication of a position statement, based on the review of several international guidelines on the use of a common lexicon and US malignancy risk stratification systems [17]. This document was complemented with the development of an application called GET ECO (which can be consulted at https://get-eco.net), that uses the EU-TIRADS and ACR TI-RADS recommendations to support the drafting of a standardized report. In the context of this project, the authors set out to evaluate the quality of the information provided by the thyroid US report, for the characterization of the thyroid nodules malignancy risk according to EU-TIRADS classification in Portugal.
Methods
The study was conducted in three of the five Portuguese NUTS2 level (Nomenclature of Territorial Units for Statistics 2), including 8,938,358 residents and corresponding to about 88.3% of the mainland population [24]. The following NUTS2 were included in this study: North (NUTS2 1), Center (NUTS2 2) and Lisbon and Tagus Valley (NUTS2 3). The proportion of the population residing in each NUTS2 was 41.4%, 25.7% and 32.9%, respectively.
This study was approved by the Administrative Council of the Portuguese Institute of Oncology of Coimbra Francisco Gentil, Portugal on June 5, 2019 and by its research ethics board on December 5, 2019 (Research work 40/2019).
A convenience, non-probability sampling method was used to select the endocrinologists to be invited for the study. In each NUTS2 region, three endocrinologists with proven experience in thyroid ultrasonography and accredited by GET were invited. The study participants were recruited from their patient lists. Patients aged 18 and over who had performed a thyroid ultrasonography between January 1st, and December 31st, 2019, were included. Most of the thyroid US exams had been ordered by a general practitioner. The endocrinologists involved in the study did not choose the radiologists who performed the thyroid ultrasonography. Patients with nodules smaller than 10 mm or with a past history of thyroid surgery or radioactive iodine treatment were excluded. All participants agreed to provide a copy of their thyroid US report and signed an informed consent. A sample size of 270 participants was estimated. Sample size was calculated considering a population estimate of 8,938,358 residents, a margin of error of 5%, a confidence level of 95% and a population proportion of 50%. Unselected participants, were consecutively included starting from the study start date (June 5, 2019) and until the required number of participants was reached. A copy of each participant's thyroid US report was made with the removal of any identification element. The study was carried out without the processing of personal data that would allow the identification of the participant, the radiologist or the institution that performed the exam. Personal health data of the participants regarding age, sex, NUTS2 of residence and some technical contents of the thyroid US report, were collected, recorded and processed. The reports of the thyroid US exams were retrospectively reviewed by the participating endocrinologists. In addition, all reports were submitted to a second independent review by another endocrinologist experienced in thyroid US. The EU-TIRADS classification was used to assess the malignancy risk [10].
The evaluation of the US report quality focused on the description of the thyroid nodule with the highest reported US-based risk, also called “dominant nodule”.
The thyroid US reports were analyzed to determine whether the description of the nodule features proposed by the EU-TIRADS classification had been included in the report. According to the EU-TIRADS system, the description of six features of the thyroid nodules was considered relevant: maximum diameter, shape, margins, composition, echogenicity and echogenic foci. These features allow to categorize the risk of malignancy of the nodules as well as the indications for FNAB. A utility score, including these six features, was used as an indicator of the report quality [23]. Each of these reported features was assigned as a point. Thus, each nodule had a score from 0 to 6. A score of 4 was considered as a minimum value for calculating the risk of malignancy according to the EU-TIRADS classification.
The reference to the localization, the three-axis diameters, the volume and the vascularity of the thyroid nodule was also searched in the report. Furthermore, the reference to each of the four US features suspected of malignancy according to the EU-TIRADS classification was analyzed: marked hypoechogenicity, microcalcifications, irregular margins and non-oval shape (Fig. 1).
Fig. 1.
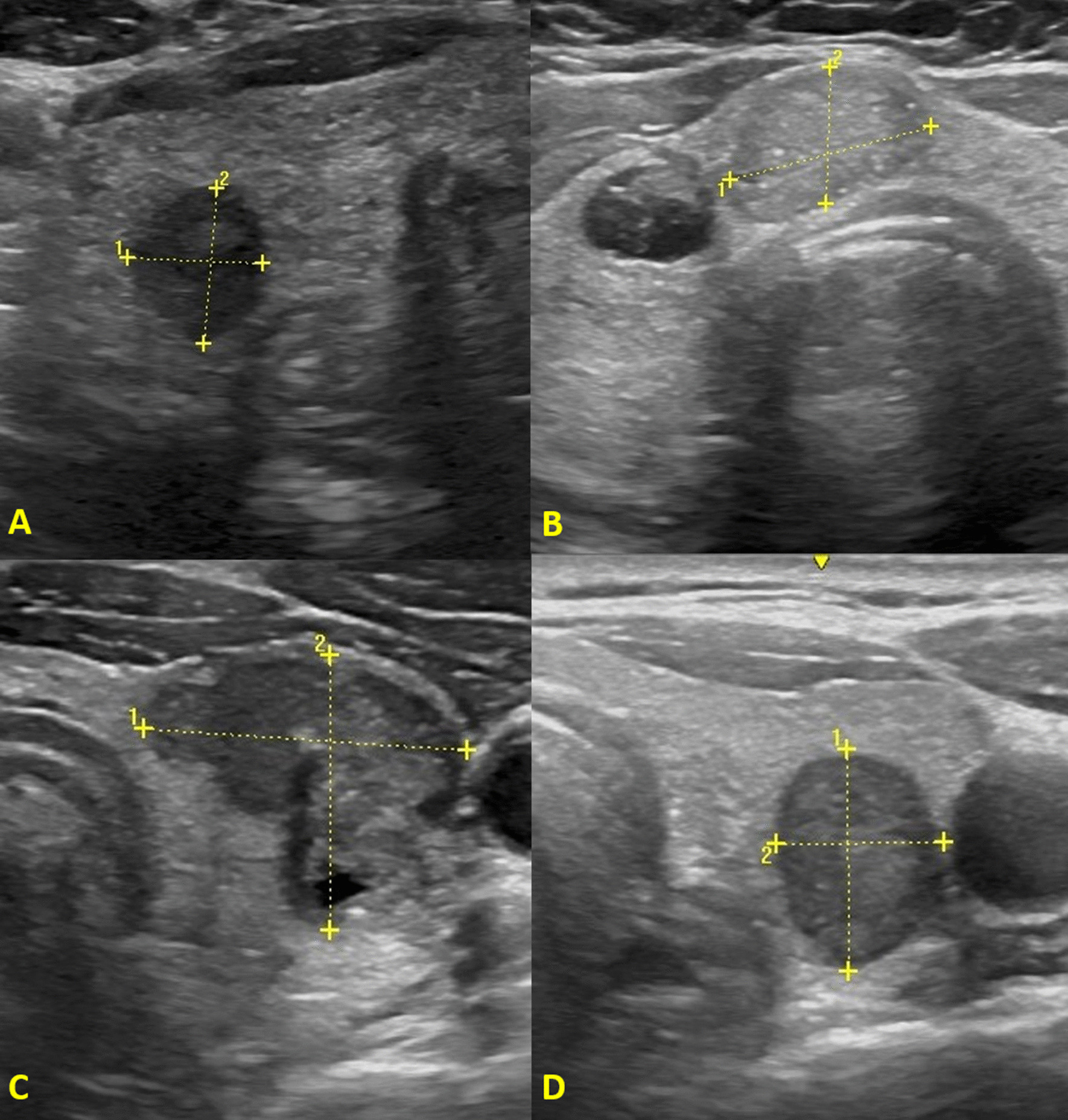
EU-TIRADS suspicious ultrasound features. A Marked hypoechogenicity, B microcalcifications, C irregular margins, D non-oval shape
In addition, reference to a specific malignancy risk classification system was assessed. Finally, the number of non-dominant nodules with indication for FNAB was quantified: EU-TIRADS 3 nodules (low risk) larger than 20 mm; EU-TIRADS 4 nodules (intermediate risk) larger than 15 mm; EU-TIRADS 5 (high risk) nodules larger than 10 mm.
Statistical Analysis
Descriptive statistic was used to report the study variables. Medians (interquartile range) and proportions were generated for continuous and categorical variables, respectively. Categorical variables were compared using the chi-square (χ2) test or Fisher’s exact test. The T Student’s test or the Mann–Whitney’s test were used to compare continuous variables. Statistical tests were 2-tailed. Significance for all statistic tests was set at p < 0.05. All statistical analyses were performed using IBM SPSS Statistic for Windows, version 22.0, Armonk, NY: IBM corp.
Results
Thyroid US reports from 344 participants were analyzed. The median age was 59 years (interquartile range of 22) and there were no statistically significant differences in age distribution by sex (p = 0.623). The proportion of women was 90.1%. The location of the nodule was identified in most cases (99.7%). Maximum size was reported for all nodules. The median of the maximum size was 19 mm (interquartile range of 12). There were no sex statistically significant differences for maximum size (p = 0.370). There was a positive correlation between maximum size and age (p = 0.024). The proportion of nodules > 20 mm was 34.0% and 50.4% in participants aged ≤ 50 years and > 50 years, respectively (p = 0.006). Maximum size was greater than 15 mm and 20 mm in 240 (69.8%) and 156 (45.3%) nodules, respectively. Longitudinal, anteroposterior and transverse diameters were reported in 55 (16.0%), 44 (12.8%) and 29 (8.4%) of the nodules, respectively. The three-axis diameters were only reported in 27 (7.8%) nodules. Longitudinal, anteroposterior and transverse diameters were reported more often in NUTS2 Lisbon and Tagus Valley (24.9%, 23.1% and 14.2%, respectively) than in the other NUTS2. The nodule volume was calculated in only one case. Vascularity in thyroid nodules was reported in 20 (5.8%) of the participants. The proportion of nodules in which the five main US features, have been described, are presented in Table 1. Table 1 also shows the proportion of nodules in which reference was made to each of the four US features suspected of malignancy, according to the EU-TIRADS classification. The presence of at least one of these four suspicious features was identified in 30 (8.7%) nodules. A positive association (p = 0.004) was found between male sex and the presence of at least one of these factors. In addition, Table 1 shows the utility score assigned to the nodules (ranging from 0 to 6). Only 75 (21.8%) of the nodules had a utility score ≥ 4. Taking into account only the five main US features of the nodules, the reference to 0, 1, 2, 3, 4 or 5 of these features was identified in 32 (9.3%), 104 (31.7%), 119 (34.6%), 57 (16.6%), 21 (6.1%) and 6 (1.7%) of the nodules, respectively. There were statistically significant differences between the NUTS2 for composition (p = 0.012), echogenicity (p = 0.001), the utility score (p < 0.001), and score ≥ 4 (p = 0.006). Composition, echogenicity and a score ≥ 4 were reported more frequently in NUTS2 Lisbon and Tagus Valley than in other NUTS2. The risk category for malignancy was reported in only 27 (7.8%) nodules, predominantly in males (p = 0.010) and in NUTS2 North (p = 0.042, for differences between NUTS2). The nodules were classified according to the definitions ATA 2015, ACR (TI-RADS), ETA (EU-TIRADS) or others in 4, 12, 1 and 10 cases, respectively. Non-dominant nodules with indication for FNAB were described in 27 participants, with 14 (4.1%), 12 (3.5%) and 3 (0.9%) nodules in the low (> 20 mm), intermediate (> 15 mm) and high risk (> 10 mm) categories, respectively. Two participants had non-dominant nodules in two different categories. Mention on the cervical lymph nodes (central and lateral compartments) status was reported in 320 (93.0%) of the participants.
Table 1.
Thyroid nodules ultrasound features
| Ultrasound features | Total n (%) |
NUTS2 North n (%) |
NUTS2 Center n (%) |
NUTS2 Lisbon n (%) |
p value |
|---|---|---|---|---|---|
| Number of participants | 344 (100%) | 98 (28.5%) | 77 (22.4%) | 169 (49.1%) | – |
| Shape | 28 (8.1%) | 6 (6.1%) | 11 (14.3%) | 11 (6.5%) | 0.081 |
| Margins | 86 (25.0%) | 22 (22.4%) | 13 (16.9%) | 51 (30.2%) | 0.065 |
| Composition | 263 (76.5%) | 74 (75.5%) | 50 (64.9%) | 139 (82.2%) | 0.012 |
| Echogenicity | 183 (53.2%) | 38 (38.8%) | 39 (50.6%) | 106 (62.7%) | 0.001 |
| Echogenic foci | 72 (20.9%) | 20 (20.4%) | 19 (24.7%) | 33 (19.5%) | 0.647 |
| Marked hypoechogenicity | 4 (1.2%) | 0 | 2 (2.6%) | 2 (1.2%) | 0.282 |
| Microcalcifications | 20 (5.8%) | 7 (7.1%) | 8 (10.4%) | 5 (3.0%) | 0.056 |
| Irregular margins | 12 (3.5%) | 4 (4.1%) | 3 (3.9%) | 5 (3.0%) | 0.869 |
| Non-oval shape | 0 | 0 | 0 | 0 | – |
| Score | |||||
| 0 | 11 (3.2%) | 0 | 9 (11.7%) | 2 (1.2%) | < 0.001 |
| 1 | 39 (11.3%) | 8 (8.2%) | 8 (10.4%) | 23 (13.6%) | |
| 2 | 109 (31.7%) | 44 (44.9%) | 28 (36.4%) | 37 (21.9%) | |
| 3 | 110 (32.0%) | 30 (30.6%) | 22 (28.6%) | 58 (34.3%) | |
| 4 | 51 (14.8%) | 11 (11.2%) | 8 (10.4%) | 32 (18.9%) | |
| 5 | 18 (5.2%) | 5 (5.1%) | 2 (2.6%) | 11 (6.5%) | |
| 6 | 6 (1.7%) | 0 | 0 | 6 (3.6%) | |
| ≥ 4 | 75 (21.8%) | 16 (16.3%) | 10 (13.0%) | 49 (29.0%) | 0.005 |
Discussion
Thyroid nodules are common, with an incidence 2–3 times higher in women than in men [25]. In our sample the men to women ratio was 0.1 but the median age was similar, suggesting that other factors may have contributed to this marked predominance of women. In Portugal, women's use of health services may be greater than in men which may imply greater consumption of health resources. In a recent study including 1339 patients, where the quality of the US report was assessed, the proportion of women was also high (85%) [23].
According to our results maximum size was reported in all nodules. As expected, a positive correlation was found between maximum size and age [26]. Previous studies also found a high reported maximum size feature [23, 27]. Maximum size is sometimes the only criterion used in daily practice to decide whether to perform FNAB or not. However, the size of the nodule alone has little value in assessing the risk of malignancy [28]. Maximum size, although not necessary for calculating the malignancy risk of the nodule, is a very important feature for the decision on the recommendation for FNAB according to the EU-TIRADS and other risk stratification systems [1, 8–10]. The three-axis diameters of the nodules were only mentioned in 7.8% of the participants and only in one case was the nodular volume calculated. Nodule diameters were more frequently reported in NUTS2 Lisbon and Tagus Valley than in the other NUTS2, suggesting some regional variability. In the follow-up of thyroid nodules, US growth criteria according to ATA 2015 and ACR TI-RADS (20% increase in at least two nodule diameters with a minimal increase of 2 mm or more than a 50% change in volume) requires information about the three-axis diameters or alternatively about the volume of the nodule. Furthermore, if the nodule shape is not mentioned, it is necessary to know the nodule diameters to calculate it. Given that the non-oval shape is a malignancy risk feature, high risk nodules may go unnoticed if information about the nodule shape is not available. According to our data, vascularity was only reported in 5.8% of participants. Intra-nodular vascularity has low predictive value, sensitivity and specificity in the diagnosis of malignancy [29, 30]. The EU-TIRADS guidelines states that, although the routine use of Doppler US is not recommended for US malignancy risk stratification it can be used to differentiate solid tissue from thick colloid or to enhance the detection of the limits of a nodule in an isoechogenic parenchyma [10]. In addition, the observation of a “spoke-and-wheel like” peripheral and central pattern of flow in a nodule can predict exceedingly bloody aspirates and non-diagnostic cytological findings [31].
The localization of the nodule is essential for its clinical integration and for a correct identification in future US exams and in preparation for FNAB or other diagnostic or therapeutic procedures. In the present study, the localization of the nodules was reported in the vast majority of cases. Other studies also confirm a high adherence by radiologists to the localization in the US report [23, 27].
Regarding the five main US features of the thyroid nodules, a very low report of shape, margins and echogenic foci was observed, with no significant differences between NUTS2. In contrast, the composition (76.5%) and echogenicity (53.2%) have often been reported, with significant differences between NUTS2. At NUTS2 Lisbon and Tagus Valley, the composition and the echogenicity features were reported more frequently than in the remaining NUTS2. In a previous study on the quality of US exams there was also a low report of the margins (8%) and echogenic foci (14%) and no reference to the shape [22]. Composition and echogenicity were mentioned in 72% and 59% of the reports, respectively. In another study the composition (64.0%) and echogenicity (43.8%) features were also more reported than margins (11.0%), echogenic foci (35.1%) and shape (3.9%) features [23]. A recent Canadian study also showed a higher report of composition (64.0%) and echogenicity (42.0%) than shape (2.0%), echogenic foci (33.0%) and margins (22.0%) features [27].
Dominant nodules classified as EU-TIRADS 5 (with at least one suspicious feature) were reported in 8.7% of participants. In a previous study, which included 1029 nodules 7.4% of the nodules were categorized as EU-TIRADS 5 [32]. In addition the same study showed the presence of marked hypoechogenicity, microcalcifications, irregular margins and non-oval shape in 1.5%, 3.3%, 1.4% and 2.8% of nodules. In another study in which the quality of US reports was analyzed, non-oval shape and marked hypoechogenicity were never reported [22]. In addition, microcalcifications and irregular margin were only reported in 3% and 1% of nodules, respectively. Compared to our results, microcalcifications and irregular margins were less reported in those studies. Our data confirms the low non-oval shape US report. According to our results, malignancy risk features were reported more often in men than in women. Radiologists may be more careful in men due to their increased risk of differentiated thyroid carcinoma (DTC). In fact, men with nodular thyroid disease undergoing FNAB have a higher risk of DTC than women, although the prevalence of the disease and the absolute number of cancers is higher in women [33]. In addition, men with DTC may have a more advanced disease at the time of diagnosis, although male sex is not an independent prognostic factor in itself [34].
According to our results, the utility score of 6 was observed in only 1.7% of the nodules. In addition, only 21.8% of the nodules had a score ≥ 4. Our data is slightly better compared to the results of a previous study in which the score of 6 and the score ≥ 4 were identified in only 0.4% and 13.7%, respectively [23].
Most of the reports (92.2%) did not refer a risk category. The risk category was reported more often in men than in women. As mentioned above, this finding may be due to greater attention from radiologists in men due to its increased risk. The reference to the EU-TIRADS classification system was made in only one participant. These results document the low adherence in Portugal, namely in NUTS2 Center and Lisbon and Tagus Valley to these classification systems in clinical practice. In addition, and although the European-based EU-TIRADS classification, developed by ETA, was published in 2017, its use by Portuguese radiologists is residual. Other studies have shown greater adherence by radiologists to the US-based risk stratification systems. A Canadian study showed that 29.9% of reports cited a risk stratification system [27].
The description of non-dominant nodules with criteria for FNAB was made in only 8.4% of the participants. These results suggest that, in most cases, the report was focused on just one nodule considered to be "dominant".
According to our results, only 7.0% of reports did not mention the presence or absence of cervical lymph nodes. These results are better than those found in another study that included 136 participants and showed that 74% of the US examinations did not mention lymph node status at all [35].
According to several scientific societies, the use of a thyroid imaging and reporting data system is very useful for physicians in their clinical practice [1, 10]. The use of US pattern categories, which include several individual sonographic features, when compared to individual US characteristics, increase the interobserver report reproducibility. In addition, these systems offer a simple communication of results, facilitating decision-making regarding the indication for diagnostic FNAB. Taking into account our results, most reports did not allow the establishment of the nodules’ malignancy risk and US features of aggressive thyroid cancers, thus preventing an informed decision on the recommendation of diagnostic FNAB. It is therefore urgent to make radiologists aware of the need to adhere to these US-based risk stratification systems. Studies like this can contribute to the improvement of clinical practice and to a reduction of unnecessary diagnostic interventions. Finally, there are some strengths and limitations of this study that should be mentioned. It is a multicenter one involving participants from the three largest NUTS2 in mainland Portugal. The thyroid US exams were performed by radiologists in their routine clinical practice as part of their activity in private radiology centers and not in specialized centers. In this way our results show the real life clinical practice. In this study, a non-probabilistic sampling method was used, with convenience selection of participating endocrinologists. For this reason, biases with interference in the representativeness of the results cannot be excluded. However, unselected participants were consecutively included. Furthermore, most of the thyroid US exams had been ordered by a general practitioner and endocrinologists involved in the study did not choose the radiologists who performed the exams. It should also be noted that, despite three endocrinologists from each NUTS2 region being selected, there was no further sampling stratification, namely with regard to the volume of exams performed by each radiologist, as it was not possible to access this information. The retrospective nature of this study did not prevent access to the full content of the reports. The final sample size was determined by the number of participants included within the approved study time. NUTS2 Center did not reach the expected number of participants. In contrast, NUTS2 Lisbon and Tagus Valley included more participants than expected. It was assumed that the non-reference in the report to a nodule feature did not mean the absence of that finding. In some cases, this may have been the radiologist's intention, but the failure to include that information in the report prevented the risk assessment of the nodule.
Conclusions
The adherence of Portuguese radiologists to a standardized reporting model and to US-based malignancy risk stratification systems is still low. Our results also confirm that, when one of these systems was not used, it was not possible, in most cases, to establish the malignancy risk of the nodules. This study highlights the importance of using these classification systems in the characterization of the nodules’ risk and in supporting the informed decision to perform FNAB, thus contributing to a better selection of nodules and reducing unnecessary diagnostic procedures.
Acknowledgements
The authors acknowledge the contributions of the clinical Investigators who participated in the data collection and other members of the Thyroid Study Group of the Portuguese Society of Endocrinology, Diabetes and Metabolism who critically reviewed the study proposal. They also acknowledge the support given to the Thyroid Study Group by the Board of the Portuguese Society of Endocrinology, Diabetes and Metabolism.
Abbreviations
- US
Ultrasound
- FNAB
Fine-needle aspiration biopsy
- BIRADS
Breast Imaging Reporting & Data System
- TIRADS
Thyroid Imaging Reporting & Data System
- ATA
American Thyroid Association
- KSThR
Korean Society of Thyroid Radiology
- ACR
American College of Radiology
- ETA
European Thyroid Association
- GET
Thyroid Study Group (Grupo de Estudo da Tiroide)
- SPEDM
Portuguese Society of Endocrinology, Diabetes and Metabolism (Sociedade Portuguesa de Endocrinologia, Diabetes e Metabolismo)
- NUTS2
Nomenclature of Territorial Units for Statistics 2
- DTC
Differentiated thyroid carcinoma
Author contributions
LR contributed to the work concept and design, to the acquisition and analysis of data for the work and for drafting the work and its critically revising for important intellectual content. He gave his final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. CF contributed to the work conception and design, to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. RM contributed to the work conception and design, to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. She was responsible for submitting study authorization requests to the Administrative Council of the Portuguese Institute of Oncology of Coimbra Francisco Gentil and to his research ethics board. CS contributed to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. IM contributed to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. MJO contributed to the work conception and design, to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. APM contributed to the work conception and design, to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. BM contributed to the acquisition of data for the work and revising it critically for important intellectual content. He gave his final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. GR contributed to the acquisition of data for the work and revising it critically for important intellectual content. He gave his final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TM contributed to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. TA contributed to the acquisition of data for the work and revising it critically for important intellectual content. She gave her final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. FR contributed to the work conception and design and revising it critically for important intellectual content. He gave his final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. He was responsible for submitting study authorization requests to the Administrative Council of the Portuguese Institute of Oncology of Coimbra Francisco Gentil and to his research ethics board. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the World Medical Association Declaration of Helsinki. All participants agreed to provide a copy of their thyroid US report and signed an informed consent. The study was approved by the Administrative Council of the Portuguese Institute of Oncology of Coimbra Francisco Gentil, Portugal on June 5, 2019 and by its research ethics board on December 5, 2019 (Research work 40/2019).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Luís Raposo, Email: luisraposoendo@gmail.com.
Cláudia Freitas, Email: claudiarqfreitas@gmail.com.
Raquel Martins, Email: martins.raquel@hotmail.com.
Catarina Saraiva, Email: catarinamsaraiva@gmail.com.
Isabel Manita, Email: isabelcmanita@gmail.com.
Maria João Oliveira, Email: mjoaooliveira1@sapo.pt.
Ana Paula Marques, Email: marques.apaula@gmail.com.
Bernardo Marques, Email: ber.marques89@gmail.com.
Gustavo Rocha, Email: gumero@gmail.com.
Teresa Martins, Email: teresa.m.martins@live.com.pt.
Teresa Azevedo, Email: tcmfazevedo@gmail.com.
Fernando Rodrigues, Email: fernando.rodrigues@ipocoimbra.min-saude.pt.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26(1):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brito JP, Yarur AJ, Prokop LJ, Mclver B, Murad MH, Montori VM. Prevalence of thyroid cancer in multinodular goiter versus single nodule: a systematic review and meta-analysis. Thyroid. 2013;23(4):449–455. doi: 10.1089/thy.2012.0156. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16(1):17–29. doi: 10.1038/s41574-019-0263-x. [DOI] [PubMed] [Google Scholar]
- 4.Raposo L, Morais S, Oliveira MJ, Marques AP, José Bento M, Lunet N. Trends in thyroid cancer incidence and mortality in Portugal. Eur J Cancer Prev. 2017;26(2):135–143. doi: 10.1097/CEJ.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 5.Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, Ogilvie JB, Terris DJ, Netterville J, Wong RJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology disease state clinical review: the increasing incidence of thyroid cancer. Endocr Pract. 2015;21(6):686–696. doi: 10.4158/EP14466.DSCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jegerlehner S, Bulliard J, Aujesky D, Rodondi N, Germann S, Konzelmann I, Chiolero A, NICER Working Group. Overdiagnosis and overtreatment of thyroid cancer: a population-based temporal trend study. PLoS ONE 2017;12(6):e0179387. 10.1371/journal.pone.0179387. [DOI] [PMC free article] [PubMed]
- 7.Horvath E, Majlis S, Rossi R, Franco C, Niedmann JP, Castro A, Domingez M. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94(5):1748–1751. doi: 10.1210/jc.2008-1724. [DOI] [PubMed] [Google Scholar]
- 8.Shin JH, Baek JH, Chung J, Ha EJ, Kim J-H, Lee YH, Lim HK, Moon W-J, Na DG, Park JS, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J Radiol. 2016;17(3):370–395. doi: 10.3348/kjr.2016.17.3.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tessler FN, Middleton WD, Grant EG, Hoang JK, Berland LL, Teefey SA, Cronan JJ, Beland MD, Desser TS, Frates MC, et al. ACR thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS committee. J Am Coll Radiol. 2017;14(5):587–595. doi: 10.1016/j.jacr.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 10.Russ G, Bonnema SJ, Erdogan MF, Durante C, Ngu R, Leenhardt L. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: the EU-TIRADS. Eur Thyroid J. 2017;6(5):225–237. doi: 10.1159/000478927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middleton WD, Teefey SA, Reading CC, Langer JE, Beland MD, Szabunio MM, Desser TS. Comparison of Performance Characteristics of American College of Radiology TI-RADS, Korean Society of Thyroid Radiology TIRADS, and American Thyroid Association Guidelines. AJR Am J Roentgenol. 2018;210(5):1148–1154. doi: 10.2214/AJR.17.18822. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Xi X, Jiang Y, Yang X, Wang Y, Zhu S, Lai X, Zhang X, Zhao R, Zhang B. Comparison among TIRADS (ACR TI-RADS and KWAK- TI-RADS) and 2015 ATA Guidelines in the diagnostic efficiency of thyroid nodules. Endocrine. 2019;64(1):90–96. doi: 10.1007/s12020-019-01843-x. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi S, Oyekunle T, Jiang X, Scheri R, Perkins J, Stang M, Roman S, Sosa JA. A direct comparison of the ATA and TI-RADS ultrasound scoring systems. Endocr Pract. 2019;25(5):413–422. doi: 10.4158/EP-2018-0369. [DOI] [PubMed] [Google Scholar]
- 14.Grani G, Lamartina L, Cantisani V, Maranghi M, Lucia P, Durante C. Interobserver agreement of various thyroid imaging reporting and data systems. Endocr Connect. 2018;7(1):1–7. doi: 10.1530/EC-17-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grani G, Lamartina L, Ascoli V, Bosco D, Biffoni M, Giacomelli L, Maranghi M, Falcone R, Ramundo V, Cantisani V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: toward the “Right” TIRADS. J Clin Endocrinol Metab. 2019;104(1):95–102. doi: 10.1210/jc.2018-01674. [DOI] [PubMed] [Google Scholar]
- 16.Ha EJ, Na DG, Moon W-J, Lee YH, Choi N. Diagnostic performance of ultrasound-based risk stratification systems for thyroid nodules: comparison of the 2015 American Thyroid Association Guidelines with the 2016 Korean Thyroid Association/Korean Society of Thyroid Radiology and 2017 American College of Radiology Guidelines. Thyroid. 2018;28(11):1532–1537. doi: 10.1089/thy.2018.0094. [DOI] [PubMed] [Google Scholar]
- 17.Raposo L, Oliveira MJ, Marques AP, Capela J, Rodrigues F, Martins T, Ribeiro C, Paiva S, Melo M, Saraiva C, et al. Thyroid Ultrasound Report: A Position Statement of the Thyroid Study Group of the Portuguese Society of Endocrinology, Diabetes and Metabolism. Rev Port Endocrinol Diabetes Metab. 2019;14(2):159–166. 10.26497/na190046
- 18.Xu T, Wu Y, Wu RX, Zhang Y-Z, Gu J-Y, Ye X-H, Tang W, Xu S-H, Liu C, Wu X-H. Validation and comparison of three newly-released Thyroid Imaging Reporting and Data Systems for cancer risk determination. Endocrine. 2019;64:299–307. doi: 10.1007/s12020-018-1817-8. [DOI] [PubMed] [Google Scholar]
- 19.Grant EG, Tessler FN, Hoang JK, Langer JE, Beland MD, Beland LL, Cronan JJ, Desser TS, Frates MC, Hamper UM, et al. Thyroid Ultrasound Reporting Lexicon: White Paper of the ACR Thyroid Imaging, Reporting and Data System (TIRADS) Committee. J Am Coll Radiol. 2015;12(12 Pt A):1272–1279. 10.1016/j.jacr.2015.07.011. [DOI] [PubMed]
- 20.Su HK, Dos Reis LL, Lupo MA, Milas M, Orloff LA, Langer JE, Brett EM, Kazam E, Lee SL, Minkowitz G, et al. Striving toward standardization of reporting of ultrasound features of thyroid nodules and lymph nodes: a multidisciplinary consensus statement. Thyroid. 2014;24(9):1341–1349. doi: 10.1089/thy.2014.0110. [DOI] [PubMed] [Google Scholar]
- 21.Andrioli M, Carzaniga C, Persani L. Standardized ultrasound report for thyroid nodules: the endocrinologist’s viewpoint. Eur Thyroid J. 2013;2(1):37–48. doi: 10.1159/000347144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffin AS, Mitsky J, Rawal U, Bronner AJ, Tessler FN, Hoang JK. Improved quality of thyroid ultrasound reports after implementation of the ACR thyroid imaging reporting and data system nodule lexicon and risk stratification system. J Am Coll Radiol. 2018;15(5):743–748. doi: 10.1016/j.jacr.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 23.Symonds CJ, Seal P, Ghaznavi S, Cheung WY, Paschke R. Thyroid nodule ultrasound reports in routine clinical practice provide insufficient information to estimate risk of malignancy. Endocrine. 2018;61(2):303–307. doi: 10.1007/s12020-018-1634-0. [DOI] [PubMed] [Google Scholar]
- 24.Pordata-Base Dados Portugal Contemporâneo. Municípios. População residente: total e por grandes grupos etários. https://www.pordata.pt/Municipios/Popula%C3%A7%C3%A3o+residente+total+e+por+grandes+grupos+et%C3%A1rios-390. Accessed 11 Feb 2021.
- 25.Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med. 1993;328(8):553–559. doi: 10.1056/NEJM199302253280807. [DOI] [PubMed] [Google Scholar]
- 26.Kwong N, Medici M, Angell TE, Liu X, Marqusee E, Cibas ES, Krane JF, Barletta JÁ, Kim MI, Larsen PR, et al. The influence of patient age on thyroid nodule formation, multinodularity, and thyroid cancer risk. J Clin Endocrinol Metab. 2015;100(12):4434–4440. doi: 10.1210/jc.2015-3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thejeel B, Rahimi B, Seidler M, Al-Agha R, Fung C. Evaluation of thyroid ultrasound report quality and assessing effect of adherence to risk stratification criteria on referral for thyroid nodule biopsy. Can Assoc Radiol J. 2020;846537119900634. 10.1177/0846537119900634. [DOI] [PubMed]
- 28.Kizilgul M, Shrestha R, Radulescu A, Evasovich MR, Burmeister LA. Thyroid nodules over 4 cm do not have higher malignancy or benign cytology false-negative rates. Endocrine. 2019;66(2):249–253. doi: 10.1007/s12020-019-01964-3. [DOI] [PubMed] [Google Scholar]
- 29.Moon HJ, Kwak JY, Kim MJ, Son EJ, Kim EK. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255(1):260–269. doi: 10.1148/radiol.09091284. [DOI] [PubMed] [Google Scholar]
- 30.Brito JP, Gionfriddo MR, Al Nofal A, Boehmer KR, Leppin AL, Reading C, Callstrom M, Elraiyah TA, Prokop LJ, Stan MN, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(4):1253–1263. doi: 10.1210/jc.2013-2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang GCH, Fried KO. Most thyroid cancers detected by sonography lack intranodular vascularity on color doppler imaging: review of the literature and sonographic-pathologic correlations for 698 thyroid neoplasms. J Ultrasound Med. 2017;36(1):89–94. doi: 10.7863/ultra.16.03043. [DOI] [PubMed] [Google Scholar]
- 32.Noto B, Eveslage M, Pixberg M, Carvalho JMG, Schäfers M, Riemann B, Kies P. Prevalence of hyperfunctioning thyroid nodules among those in need of fine needle aspiration cytology according to ATA 2015, EU-TIRADS, and ACR-TIRADS. Eur J Nucl Med Mol Imaging. 2020;47:1518–1526. doi: 10.1007/s00259-020-04740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rago T, Fiore E, Scutari M, Santini F, Di Coscio G, Romani R, Piaggi P, Ugolini C, Basolo F, Miccoli P, et al. Male sex, single nodularity, and young age are associated with the risk of finding a papillary thyroid cancer on fine-needle aspiration cytology in a large series of patients with nodular thyroid disease. Eur J Endocrinol. 2010;162(4):763–770. doi: 10.1530/EJE-09-0895. [DOI] [PubMed] [Google Scholar]
- 34.Nilubol N, Zhang L, Kebebew E. Multivariate analysis of the relationship between male sex, disease-specific survival, and features of tumor aggressiveness in thyroid cancer of follicular cell origin. Thyroid. 2013;23(6):695–702. doi: 10.1089/thy.2012.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carneiro-Pla D, Amin S. Comparison between preconsultation ultrasonography and office surgeon-performed ultrasound in patients with thyroid cancer. World J Surg. 2014;38(3):622–627. doi: 10.1007/s00268-013-2251-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


