Abstract
Over the past half-century, the world has witnessed a steep decline in fertility rates in virtually every country on Earth. This universal decline in fertility is being driven by increasing prosperity largely through the mediation of social factors, the most powerful of which are the education of women and an accompanying shift in life’s purpose away from procreation. In addition, it is clear that environmental and lifestyle factors are also having a profound impact on our reproductive competence particularly in the male where increasing prosperity is associated with a significant rise in the incidence of testicular cancer and a secular decline in semen quality and testosterone levels. On a different timescale, we should also recognize that the increased prosperity associated with the demographic transition greatly reduces the selection pressure on high fertility genes by lowering the rates of infant and childhood mortality. The retention of poor fertility genes within the human population is also being exacerbated by the increased uptake of ART. It is arguable that all of these elements are colluding to drive our species into an infertility trap. If we are to avoid the latter, it will be important to recognize the factors contributing to this phenomenon and adopt the social, political, environmental and lifestyle changes needed to bring this situation under control.
Keywords: population growth, fertility rates, social factors, genetics, sperm counts, testicular cancer, ART
Introduction: the emergence of infertility
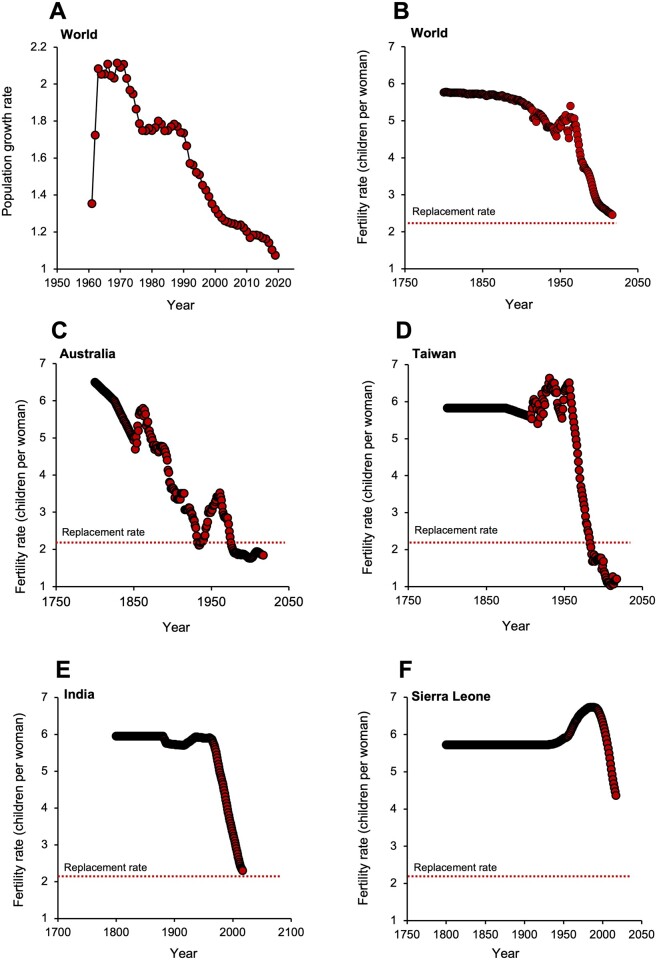
It may seem paradoxical when the consequences of overpopulation are all around us in the form of widespread pollution and climate change, but the demographic tide is about to turn. From its zenith in the 1960s, the rate of human population growth will decline to zero sometime before the end of the century (Fig. 1A), levelling off at a point where there will be around 11 million inhabitants on planet Earth. A subsequent contraction in the size of the global population is foreshadowed by the sustained decline in total fertility rates (TFRs: defined as the total number of children that would be born to each woman if she were to live to the end of her child-bearing years) that has been observed over the past 50 years, particularly in the world’s more developed nations. Global TFR was relatively constant (around 5.7 children per woman) until around 1870, but then exhibited a gradual descent that was rescued by the prosperity that followed World War II, gaining momentum during the 1950s and peaking in 1963. Thereafter there has been a fall in global TFR such that in 2017, it was only just above replacement levels, which is traditionally defined as 2.1 children per women (Fig. 1B) (Craig, 1994; Vollset et al., 2020). In the most developed nations on Earth, exemplified in Fig. 1C and D by Australia and Taiwan, TFR fell rapidly in the mid-20th century and is now stabilizing at levels well below the replacement threshold. This pattern has repeated itself across the globe and is particularly evident in Japan and the Tiger economies of Southeast Asia, which now boast some of the lowest fertility rates on Earth. So powerful is this trend that before the end of the century, 183 out 195 countries will be characterized by TFR values below replacement levels (Vollset et al., 2020).
Figure 1.
Human population dynamics. (A) Changes in the rate of population growth. In the early 1960s, population growth rates approximated to 2.1% per annum. However, beginning in 1970 global population growth rates slowed dramatically and are now just over 1% per annum. Source: http://datacatalog.worldbank.org/ (4 October 2021, date last accessed). (B) Changes in global total fertility rate (TFR) over time; TFR is defined as the number of children born per woman over her lifetime, while replacement rate is defined as 2.1 children per woman. (C) Changes in TFR against time in Australia. (D) Changes in TFR against time in Taiwan as an exemplar of the Tiger economies of SE Asia. (E) Changes in TFR against time in India. (F) Changes in TFR against time in Sierra Leone as an exemplar of sub-Saharan Africa. Source: United Nations—Population Division (2019 revision) accessed via http://ourworldindata.org/fertility-rate. CC-BY (28 September 2021, date last accessed).
The nature and cause of fertility decline
Before discussing the causes of this decline in TFR, it is important to clarify that a reduction in this parameter is not synonymous with a decline in fecundity: the former describes the number of children born per woman, while the latter refers to the capacity of couples to have a child. Whether there is a secular trend in human fecundity is an important question that remains unresolved at present (Smarr et al., 2017). Declining TFRs are however an incontrovertible fact (https://ourworldindata.org/world-population-growth) (18 November 2021, date last accessed). In this article, I shall propose that the short-term decline in human TFR is largely driven by socioeconomic and educational factors that can be readily addressed by changes in policy, support and attitude. However, in parallel, there are long-term changes occurring, driven by a combination of environmental, lifestyle and genetic factors, which could potentially result in permanent damage to the fecundity of our species. Furthermore, the assisted conception industry may, if the uptake of this technology continues to rise at scale, exacerbate the situation by reducing the selection pressure of high fertility genotypes and diverting attention away from resolving the environmental and lifestyle factors that are suppressing TFR in modern industrialized societies.
The immediate impact of immigration
In developed, affluent countries with below-replacement TFR values, the size of the population has traditionally been maintained by the migration of individuals from the less developed corners of the world, lured by the promise of prosperity, religious and political freedom and security. Nations like the USA, UK and Australia would already be suffering the economic and social consequences of rapid depopulation if it were not for liberal immigration policies. However, the international flow of migrants to help bolster population numbers in the world’s most prosperous nations can only be a temporary fix. Even those nations that have historically allowed their citizenry to emigrate to the more affluent corners of the globe are suffering their own fertility decline. For example, India and China are exhibiting exactly the same TFR decrease that we see in the rest of the world. As in many other countries, China showed an increase in TFR in the early 1960’s but this was followed by a spectacular decline to levels that are well below replacement at the present time. Interestingly, the decline in TFR began in the late 1960s/early 1970s and was already clearly evident before the introduction of the one-child-per-family policy in 1979. The rate of fertility decline was also untrammelled by the two-child-policy in January 2016 and will probably not respond to the three-child-policy introduced in May 2021. Whatever is shaping the decline of human fertility, it is not readily responsive to political edicts.
For India, the picture is very similar (Fig. 1E). Starting in 1966, the Indian population exhibited a rapid linear decline in TFR to the point that it is currently poised just above replacement levels at 2.2 births per woman. However, the trend is inexorably downwards and will fall below the replacement threshold in the next year or two (http://ourworldindata.org/fertility-rate) (28 September 2021, date last accessed). Even Africa has experienced a reversal of its traditionally high levels of fertility since 1990, as exemplified by Sierra Leone (Fig. 1F). So, wherever we look, TFRs are falling, with no sign of stabilizing until they are below replacement levels. A rational consequence of this trend is that immigration cannot constitute a long-term solution for the declining TFR observed in affluent societies. The problem can only be addressed once we have a deeper understanding of the mechanisms suppressing fertility rates and develop strategies that address the root cause of this phenomenon, not just its consequences.
The Malthusian paradox
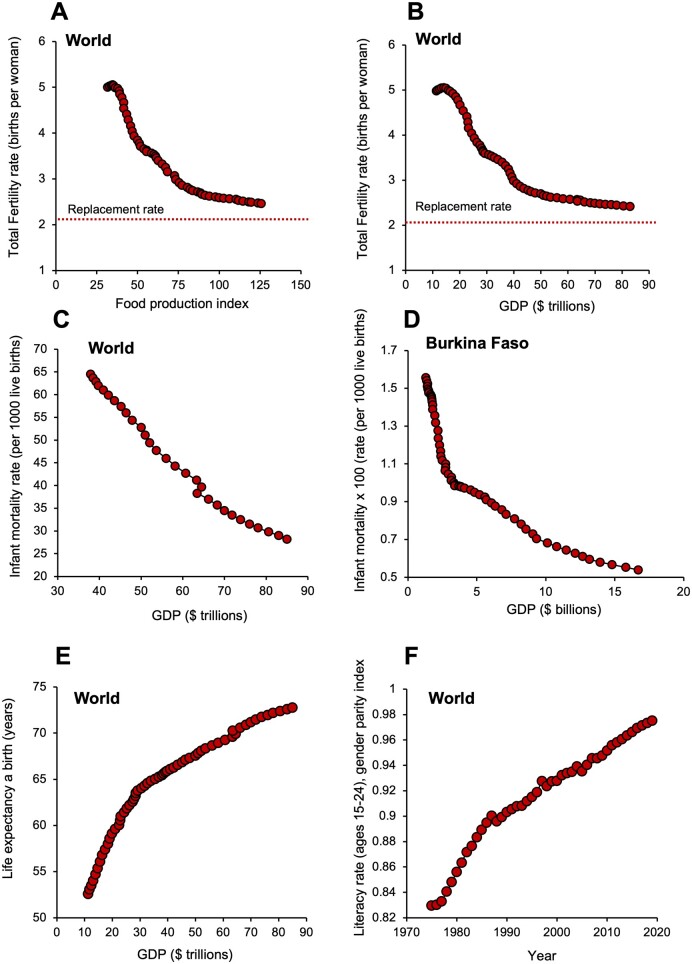
The developing fertility crisis is not, as Thomas Malthus and Paul Ehrlich might have predicted, anything to do with food availability (Malthus, 1798; Ehrlich, 1968). Indeed, our species, which abandoned natural population control mechanisms some time ago, constitutes a Malthusian paradox because food availability has actually increased as TFR values have declined (Fig. 2A). Indeed, it is self-evident that fertility rates are lowest in the most affluent nations on Earth where food is most abundant. If not food, then what other factors might be driving the global decline in human TFR?
Figure 2.
Drivers of fertility decline. (A) The global fall in total fertility rate (TFR) is not related to food availability; as TFR declines, the food production index (covers all food crops that are considered edible and that contain nutrients) actually increases. Source: https://data.worldbank.org/indicator/AG.PRD.FOOD.XD (16 December 2020, date last accessed). (B) The fall in TFR across the globe is associated with an increase in global prosperity, measured in terms of gross domestic product (GDP). (C) As GDP increases, so we see a steady decline in global infant mortality rates. (D) A plot of GDP against infant mortality for Burkina Faso reveals a rapid reduction in mortality rates for just a small increase in GDP. (E) In parallel with the reduction in infant/childhood mortality, the demographic transition is also associated with an increase in life expectancy. (F) In concert with increased prosperity, women gain greater access to education such that global literacy rates in men and women have now almost reached parity. For all panels, GDP is measured in constant 2010 $US; data from the World Bank Open Data (https://data.worldbank.org). CC BY-4.0 (15 September 2021, date last accessed).
Prosperity and the demographic transition
The fundamental cause of human fertility decline is prosperity. A plot of global fertility against gross domestic product (GDP is the total monetary or market value of all the finished goods and services produced within a country’s borders in a specific period of time; Fig. 2B) reveals a clear negative relationship. At the earliest stages of socioeconomic development, we see that just a small increase in national revenue is associated with a very steep decline in TFR. However, the slope of the graph flattens out as we approach the replacement threshold. The decline in family size with increased national prosperity is often referred to as the Demographic Transition (Dyson, 2010) According to this model, countries at the earliest stages of socioeconomic development are characterized by high birth rates and high rates of infant mortality. As national prosperity improves, then TFR values decline in response to a parallel decline in infant deaths. The link between increased socioeconomic development and a decrease in infant mortality rates is certainly robust. It is seen globally (Fig. 2C) as well as in the microcosm of the most impoverished and the most affluent nations on Earth. When nations are poor, like Burkina Faso, (Fig. 2D) just a small increase in GDP can have a major impact of infant mortality as a consequence of improvements in standards of nutrition, primary health care, female education and physical security (Kanté et al., 2016). An additional result of increasing prosperity is a dramatic increase in life expectancy as societies become more socioeconomically advanced (Fig. 2E).
So, as nations become more prosperous their TFR declines in response to the reduction in infant mortality, but population numbers are temporarily buffered by a concomitant increase in life expectancy. An inevitable consequence of these trends is to generate ‘super-aged’ societies like Japan, Finland, Italy or Germany where over 20% of the population is over the age of 65 years (Nakatani, 2019). As a result of these changes, increasing affluence tends to turn the population pyramid on its head. At the earliest stages of socioeconomic development, it has a broad base created by high birth rates rapidly transitioning to a narrow apex as the population succumbs to the impact of time, violence and disease. In some of the world’s most advanced societies, such as those mentioned above, we have the opposite—a narrowing base reflecting the decline in TFR, rising to a broad crown reflecting the survival of the populace into advanced old age. This situation is clearly unsustainable; gaining any measure of control over the powerful changes in fertility precipitated by affluence, will only be achieved once we have gained some clear insights into the underlying mechanisms.
Why are human fertility rates in decline?
Social factors
Of all the forces impacting human TFR, perhaps the most powerful in the short-term are social. Prosperity has meant that strategies designed to support our species during the early stages of its evolution when life was nasty, brutish and short are no longer relevant (Davis, 1986). In modern industrialized societies, there is no need to have large numbers of children to counteract high rates of infant and childhood mortality because more than 99% of newborns will reach puberty (https://ourworldindata.org/child-mortality-in-the-past; last viewed on 17 November 2021). In parallel, as societies become more advanced, they also become more mechanized, again removing the need to have large families as a means of providing cheap manual labour to help with the hunting and gathering or the backbreaking grind of subsistence agriculture. In addition, as societies become more affluent, they become more urbanized and this creates yet more downward pressure on family size. In urban environments, the cost of raising children increases and the means of suppressing fertility, via access to family planning services and contraception, becomes more available (White et al., 2008; Lerch, 2019).
Educational factors
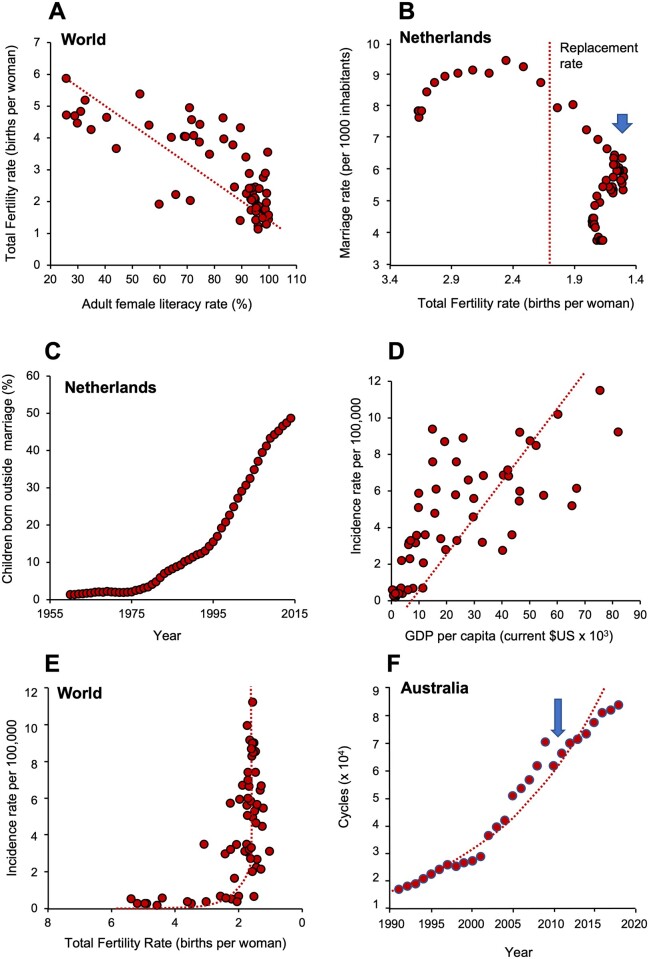
The education of women has probably done more to curtail human fertility than the entire plethora of contraceptive technologies and pronatalist Government policies added together. Using literacy as a measure of education, we are grinding our way towards gender parity at a global level (Fig. 2F). As literacy rates improve, so TFR declines (Fig. 3A). The link between increased female education and reduced fertility is very well established (Liu and Raftery, 2020); however, the reasons behind this powerful association are complex and warrant consideration.
Figure 3.
Determinants of total fertility rate (TFR) decline. (A) A powerful negative correlation between adult female literacy rate and TFR across the world (P < 0.001). (B) Plot of TFR against marriage rate in the Netherlands, as an exemplar of a consistent trend seen in many developed countries. Note that as TFR declines to below replacement levels, marriage rates collapse (arrowed). Data on x-axis plotted in descending order. (C) The number of children born out of wedlock is increasing rapidly with time, again using the Netherlands as the exemplar. (D) A plot of testicular cancer rates against gross domestic product (GDP) per capita (measured in current $US) demonstrates a highly significant correlation (P < 0.001), indicating that the more prosperous a country becomes, the higher the incidence of testicular cancer. (E) When TFR falls to around replacement levels there is a sudden exponential increase in the incidence of testicular cancer, even though it remains a relatively rare condition. Data of x-axis plotted in descending order. (F) Increase in the uptake of ART in Australia (1991–2018). Arrow indicates a sudden, 13% reduction in ART uptake in 2010 when the Australian Government changed its Medicare rebate scheme. Source of data for panel (A): UNICEF (https://data.unicef.org/topic/gender/gender-disparities-in-education). CC BY-4.0 (03 October 2021, date last accessed). Sources of data for panels (B) and (C) from Our World in Data (http://ourworldindata.org/fertility-rate). CC-BY (28 September 2021, date last accessed). Sources of data for panels (D) and (E): Testicular cancers rates by country from GLOBOCAN database accessible at http://gco.iarc.fr/, as part of IARC’s Global Cancer Observatory. Fertility rates from World Bank Open data retrieved from https://data.worldbank.org/ (16 February 2021, date last accessed). Source of data for panel (F): The National Perinatal Epidemiology and Statistics Unit, the University of New South Wales Sydney. https://npesu.unsw.edu.au/ (5 September 2021, date last accessed).
The relationship between female education and fertility clearly constitutes a self-reinforcing cycle in the sense that not only do high levels of educational attainment result in reduced family size but also small families allow for higher levels of education. With increased education, comes increased autonomy; women are more empowered to make decisions about their fertility in the light of multiple factors including peer pressure to conform, perceived self-confidence, anticipated regret, health and the availability of adequate support from within the family, workplace and society at large (Kearney and White, 2016; Nomura et al., 2019). By making women less dependent on their partner’s income and increasing their bargaining power within a relationship, their desire to pursue professional goals rather than commit to a large family becomes heard. Indeed, an increased number of women are choosing childlessness as a lifestyle. No longer is procreation seen as the purpose of existence but, rather, life is viewed as a journey towards prosperity and self-fulfilment. In the USA more than 45% of women aged 15–44 years have never had a child (Martinez et al., 2018), while a YouGov poll released in January 2020 revealed that among Britons who were not already parents, 37% told pollsters they did not want any children, ever (https://yougov.co.uk/topics/education/articles-reports/2020/01/09/why-are-britons-choosing-not-have-children) (11 October 2021, date last accessed). There are many reasons why childlessness is now more socially acceptable than in previous times but some of the dominant factors include—opposition to anthropocentrism, concern about overpopulation and the impact of human populations on the environment, the financial burden imposed by children, the inability to find a suitable partner and professional ambition (Majumdar, 2004; Stegen et al., 2020). Of course, childlessness is a symptom of falling fertility rates, not the solution. If we are to stabilize the fall in TFR associated with female education, an important contribution might come from the provision of families with appropriate incentives in terms of their working conditions and financial support. As ever, the Scandinavian countries lead the way in their enlightened attitude to parental support schemes that help alleviate the tensions between professional development, economic stability and motherhood (Thévenon, 2011).
The education of women clearly enables them to pursue their place in the workforce but this commitment to advanced education and career progression comes at a biological cost. Our species is unusual in that female fertility is lost in midlife starting around the age of 35 years and comes to an abrupt halt in the mid- to late 40s (Eijkemans et al., 2014; Yoldemir, 2016). This is due to a dramatic age-dependent decline in the developmental competence of oocytes as a result of many factors, most important of which is an increase in aneuploidy (Jaswa et al., 2021). One of the inadvertent consequences of female education and career development is that the age at which women contemplate having a family is close to the age at which fertility naturally declines. It is probably no surprise that the average age of women attending IVF clinics is around 36 years, just when fertility is beginning its irrevocable age-dependent decline via mechanisms that ART cannot reverse (Human Fertilization and Embryology Authority, 2021).
The impact of marriage
The changing role of men and women in society, in concert with uncertainty as to life’s fundamental purpose, is also having a major impact on the status of the nuclear family and the institution of marriage. Marriage is no longer seen as relevant to today’s society and, globally, we are seeing fewer and fewer couples becoming married. Plots of marriage rate against TFR for the Netherlands, as an exemplar society, reveal a sudden decline in the incidence of marriage (Fig. 3B, arrowed) as the TFR descends to sub-replacement levels. While at one time there may have been a direct causal relationship between low marriage rates and increased childlessness, especially in higher-income countries (Jones, 2007), it seems likely that this will be a less important driver going forward, as more and more children are born out of wedlock (Fig. 3C).
Environmental and lifestyle factors
While significant, the emergence of powerful social/educational factors that impact human TFR might not overly concern us because, in principle, such trends should be readily addressed with appropriate policies and increased levels of Governmental and societal support. However, in parallel, a variety of lifestyle and environmental factors are also conspiring to suppress human fecundity and, as a result, the impact may be more permanent. Nowhere is this more evident than in the male. Human semen quality is notoriously poor and male infertility is known to be a highly prevalent condition (Agarwal et al., 2015; Aitken, 2020a). Moreover, sperm counts have halved in the past 50 years in both the industrialized West and the East, including China (Levine et al., 2017; Lv et al., 2021; Swan and Colino, 2021). This change is occurring so universally and so rapidly that it cannot have a genetic basis but must be environmentally induced. This phenomenon raises important questions about the nature of the environmental factors involved and whether such changes in sperm number are materially affecting human fertility.
To address the causative mechanisms first, it may be significant that several studies have also recorded a parallel secular decline in circulating testosterone levels. Thus, in Nordic countries (Perheentupa et al., 2013), the USA (Travison et al., 2007) and Israel (Chodick et al., 2020) evidence has been presented indicating that testosterone levels have been declining since the 1970s, in concert with the decline in sperm counts. The situation is complex because serum testosterone levels decline with age and advanced industrialized societies are becoming ‘super-aged’. Nevertheless, even when we take age into account, a decline in testosterone levels is still evident, as clearly illustrated by the Israeli data (Chodick et al., 2020).
The biological consequences of this secular decline in testosterone levels are not known at the present time, but a reasonable hypothesis is that it might underpin the global reduction in sperm counts. The decline in testosterone levels might, in turn, be driven by increased exposure to oestrogenic compounds from multiple sources (Skakkebaek, 2017; Aitken, 2022). For example, clinical conditions such as obesity are known to be associated with low testosterone and elevated oestrogen levels, triggered by a rise in aromatase activity, particularly in patients with long tetranucleotide TTTA repeat polymorphisms in their aromatase gene (Hammoud et al., 2010; Mazur et al., 2013). Dairy products, such as milk and particularly cheese, are also known to contain significant quantities of natural oestrogens and the intake of phytoestrogens via the diet has been shown to lower serum testosterone levels in some, but not all, studies (Gardner-Thorpe et al., 2003; Reed et al., 2021). In addition, exposure to synthetic oestrogens polluting our environment, like ethinyloestradiol or xenoestrogens, including bisphenol A or phthalate esters, is known to be capable of suppressing both gonadotrophin production and testosterone levels in animals and man (Lübbert et al., 1992; Chang et al., 2015; Cariati et al., 2019). Excessive exposure to oestrogens, whether generated via our internal metabolism, associated with our lifestyle, or the inadvertent result of environmental contact, will change the testosterone: oestrogen ratio, drive down testosterone levels via negative feedback effects on the hypothalamo–pituitary axis and disrupt spermatogenesis through the impairment of Sertoli cell function, which is, in turn, heavily dependent on testosterone availability. Such changes could well contribute to the global decline in sperm counts (Mima et al., 2018; Aitken, 2022). Whether the resulting decrease in sperm number is associated with a decline in sperm function, and thus fecundity, is another question.
In the 1990s, research into the application of contraceptive steroids in men suggested that the use of exogenous androgens to induce severe oligozoospermia did not necessarily result in complete infertility (Wallace et al., 1992). It is therefore possible that the suppression of spermatogenesis via the oestrogen-induced inhibition of gonadotrophin output from the pituitary leads to a reduction of sperm number but not sperm function. As a result, the secular decline in sperm counts may not necessarily be accompanied by a parallel decrease in fecundity. However, if current trends continue unabated then ultimately, as Shanna Swan has pointed out, fertility will become compromised by a sheer lack of sperm numbers (Swan and Colino, 2021). Given the potential link between circulating testosterone levels and sperm counts, it will be important to monitor testosterone levels in multiple populations to determine if this association is replicated across the globe and, if this is the case, to develop appropriate countermeasures.
The seriousness of the situation is further emphasized by the changing incidence of testicular cancer which, according to the testicular-dysgenesis-syndrome hypothesis, has a similar origin to the global decline in sperm counts, due to a common dependence on environmental, endocrine disruptors with oestrogen-like activity (Wohlfahrt-Veje et al., 2009; Fénichel and Chevalier, 2019). There is a powerful linear correlation between national GDP and the incidence of testicular cancer (Fig. 3D). Moreover, from a global perspective, testicular cancer increases exponentially as TFR values fall to sub-replacement levels (Fig. 3E). An impact of environmental endocrine disruptors on the development and competence of the male reproductive system therefore seems probable (Ješeta et al., 2021). Furthermore, it should also be acknowledged that such chemical pollutants do not just impact male reproduction. They are also thought to be responsible for a wide variety of reproductive problems in women, from impaired endometrial receptivity and failed embryo implantation to abnormalities of sexual development, complications of pregnancy and birth defects (Caserta et al., 2021; Fucic et al 2021).
Other aspects of modern society that are thought to impair male and female fertility include a variety of environmental and lifestyle factors (diet, smoking, excess alcohol consumption, exposure to industrial pollutants, electromagnetic radiation, stress, sedentary behaviour etc.), many of which are associated with the induction of oxidative stress within the reproductive tract (Aitken and Baker, 2013, 2020; Aitken, 2020a,b). Understanding the various factors that cause oxidative stress in our species and controlling levels of exposure through the imposition of appropriate regulatory frameworks and adoption of relevant protective behaviours, will also be important in keeping this particular cause of human infertility under control (Alonso et al., 2009; De Iuliis et al., 2009; Hajizadeh Maleki et al., 2013; Dai et al., 2015; Durairajanayagam et al., 2015; Rengaraj et al., 2015; Samarasinghe et al., 2018; Bellastella et al., 2019; He et al., 2020; Nguyen-Powanda and Robaire, 2020; Skoracka et al., 2020).
Genetic factors and the IVF industry
In light of the foregoing, it is clear that a variety of social, educational, environmental and lifestyle pressures associated with modern affluent society are suppressing human fertility rates to unprecedentedly low levels. However, in the long term, it may be genetic factors that play the greatest role in determining the fate of the human population by compromising our fundamental fecundity.
During the earliest stages of our existence on this planet, high rates of infant and childhood mortality essentially meant that we were always selecting for high fertility genes. You had to be fertile enough to have five or six children for one or two to survive and pass your genes onto the next generation. Thousands of years of selection resulted in our TFR being optimized to the point that it just exceeded death rates and our numbers slowly expanded (Davis, 1986). Fast forward to our current state of post-transitional development and we are, for all of the reasons given above, no longer selecting for high fertility genotypes; as a consequence, their incidence will decline. This will not happen immediately but something we learn from domestic animals such as dairy cattle, is that if you do not actively select for fertility, you will ultimately lose it (Whitfield, 2020).
The spread of low fertility genotypes will also be encouraged by the IVF industry. The latter has had very little impact on global fertility levels while it has remained a specialized activity responsible for <0.5% of births worldwide. However, in most developed countries, the uptake of ART is increasing rapidly (Fig. 3F), and the reasons for treatment are gradually expanding as more same-sex couples, single women and surrogates are making use of fertility treatment (https://www.hfea.gov.uk/about-us/news-and-press-releases/2019) (28 September 2021, date last accessed). In some countries, like Denmark, where ART is highly subsidized, around 10% of newborn children are conceived using such procedures (https://www.bbc.com/news/world-europe-45512312) (18 November 2021, date last accessed). One of the inadvertent consequences of ART is that, when conducted at scale, it will help poor fertility genes be retained within the population. An obvious example is the treatment of severely oligozoospermic men with AZFc deletions on their Y-chromosome, using ICSI. Naturally, any sons generated using this strategy will exhibit the same infertility as their father and require the same ICSI intervention should they wish to have children in later life (Golin et al., 2021). Preliminary data is already to hand suggesting that males conceived by ICSI have lower sperm counts than their naturally conceived counterparts (Belva et al., 2016). Clearly, these data will need to be substantiated but they are, at the very least, consistent with the powerful ability of ART to promote the vertical transmission of poor fertility genotypes.
For obvious reasons, genetic variants will be rapidly deleted from the population if they cause complete infertility (Aitken and Baker, 2020). However, genotypes associated with subfertility will be encouraged to remain within the population by virtue of the weak selection pressures that modern society imposes on high fecundity. In this complex situation, any mutations that compromise fertility by acting on the quality of gametes and/or fertilization and early development will potentially be encouraged to persist within the population by the widespread practice of ART, particularly ICSI (Chen et al., 2021; Ferreux et al., 2021; Mazaheri Moghaddam et al., 2021). Furthermore, ART may also impact offspring fertility by facilitating the transgenerational transmission of aneuploidies (Belva et al., 2020) and abnormal DNA methylation profiles (Caramaschi et al., 2021) as well as genetic mutations (Aitken et al., 2020). Naturally, the industry is doing everything it can to avoid such problems through the development of sophisticated embryo selection procedures and the widespread adoption of genetic screening protocols (Minasi et al., 2016; Hawkins et al., 2021; Sanders et al., 2021; Sang et al., 2021). Nevertheless, when practiced at scale, ART may still encourage poor fertility genotypes to remain within the population. Furthermore, the increased application of ART may inadvertently encourage a loss of human fecundity by discouraging research into the impacts of environmental and lifestyle factors on human fertility and diminishing the political pressure to address these issues.
Conclusions—an infertility trap?
A crucial question in this debate is whether the changes we are seeing in human TFR in the wake of increasing affluence are readily reversible. The answer to this question is probably a function of how long we allow the current state of affairs to persist. At present, the major causes of falling TFR are socioeconomic and, as such, are potentially resolveable, given appropriate changes in strategy, approach, and assistance. However, such changes will come to nothing if we do not recognize and control the wide range of environmental and lifestyle factors associated with modern living that are impacting the fecundity of our species. Additionally, if the scale at which ART is practiced continues to increase, then it is also possible that this industry may play its own part in enhancing the decline of human fecundity by encouraging the retention of genetic and epigenetic mutations that compromise reproductive efficiency. We have time; these changes will not occur immediately or even within our lifetime. Nevertheless, on an evolutionary timescale, the changes in human fertility are occurring very rapidly and it is important that we are aware of where our current practices might lead us, if nothing is done.
Author’s role
R.J.A. conceived of the work and wrote the manuscript.
Funding
There was no funding for this work.
Conflict of interest
The author declares that he has no conflicts of interests pertaining to this work.
Dedication: The article is dedicated to the memory of the later Professor Roger Valentine Short FRS who first opened my eyes to the wonders of reproductive science.
References
- Agarwal A, Mulgund A, Hamada A, Chyatte MR.. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ. The Infertility Trap; Why Life Choices Impact Your Fertility and Why We Must Act Now. Cambridge, UK: Cambridge University Press, 2022. [Google Scholar]
- Aitken RJ. The male is significantly implicated as the cause of unexplained infertility. Semin Reprod Med 2020a;38:3–20. [DOI] [PubMed] [Google Scholar]
- Aitken RJ. Impact of oxidative stress on male and female germ cells: implications for fertility. Reproduction 2020b;159:R189–R201. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA.. Oxidative stress, spermatozoa and leukocytic infiltration: relationships forged by the opposing forces of microbial invasion and the search for perfection. J Reprod Immunol 2013;100:11–19. [DOI] [PubMed] [Google Scholar]
- Aitken RJ, Baker MA.. The role of genetics and oxidative stress in the etiology of male infertility-a unifying hypothesis? Front Endocrinol (Lausanne) 2020;11:581838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken RJ, De Iuliis GN, Nixon B.. The sins of our forefathers: paternal impacts on de novo mutation rate and development. Annu Rev Genet 2020;54:1–24. [DOI] [PubMed] [Google Scholar]
- Alonso V, Linares V, Bellés M, Albina ML, Sirvent JJ, Domingo JL, Sánchez DJ.. Sulfasalazine induced oxidative stress: a possible mechanism of male infertility. Reprod Toxicol 2009;27:35–40. [DOI] [PubMed] [Google Scholar]
- Bellastella G, Menafra D, Puliani G, Colao A, Savastano S; Obesity Programs of nutrition, Education, Research and Assessment (OPERA) Group. How much does obesity affect the male reproductive function? Int J Obes Suppl 2019;9:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belva F, Bonduelle M, Buysse A, Van den Bogaert A, Hes F, Roelants M, Verheyen G, Tournaye H, Keymolen K.. Chromosomal abnormalities after ICSI in relation to semen parameters: results in 1114 fetuses and 1391 neonates from a single center. Hum Reprod 2020;35:2149–2162. [DOI] [PubMed] [Google Scholar]
- Belva F, Bonduelle M, Roelants M, Michielsen D, Van Steirteghem A, Verheyen G, Tournaye H.. Semen quality of young adult ICSI offspring: the first results. Hum Reprod 2016;31:2811–2820. [DOI] [PubMed] [Google Scholar]
- Caramaschi D, Jungius J, Page CM, Novakovic B, Saffery R, Halliday J, Lewis S, Magnus MC, London SJ, Håberg SE. et al. Association of medically assisted reproduction with offspring cord blood DNA methylation across cohorts. Hum Reprod 2021;36:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariati F, D'Uonno N, Borrillo F, Iervolino S, Galdiero G, Tomaiuolo R.. Bisphenol A: an emerging threat to male fertility. Reprod Biol Endocrinol 2019;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta D, Costanzi F, De Marco MP, Di Benedetto L, Matteucci E, Assorgi C, Pacilli MC, Besharat AR, Bellati F, Ruscito I. et al. Effects of endocrine-disrupting chemicals on endometrial receptivity and embryo implantation: a systematic review of 34 mouse model studies. Int J Environ Res Public Health 2021;18:6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WH, Li SS, Wu MH, Pan HA, Lee CC.. Phthalates might interfere with testicular function by reducing testosterone and insulin-like factor 3 levels. Hum Reprod 2015;30:2658–2670. [DOI] [PubMed] [Google Scholar]
- Chen D, Liang Y, Li J, Zhang X, Zheng R, Wang X, Zhang H, Shen Y.. A novel CCDC39 mutation causes multiple morphological abnormalities of the flagella in a primary ciliary dyskinesia patient. Reprod Biomed Online 2021;43:920–930. [DOI] [PubMed] [Google Scholar]
- Chodick G, Epstein S, Shalev V.. Secular trends in testosterone—findings from a large state-mandate care provider. Reprod Biol Endocrinol 2020;18:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. Replacement level fertility and future population growth. Popul Trends 1994;78:20–22. [PubMed] [Google Scholar]
- Dai JB, Wang ZX, Qiao ZD.. The hazardous effects of tobacco smoking on male fertility. Asian J Androl 2015;17:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis K. Low fertility in evolutionary perspective. Popul Dev Rev 1986;12:48–65. [Google Scholar]
- De Iuliis GN, Newey RJ, King BV, Aitken RJ.. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS One 2009;4:e6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durairajanayagam D, Agarwal A, Ong C.. Causes, effects and molecular mechanisms of testicular heat stress. Reprod Biomed Online 2015;30:14–27. [DOI] [PubMed] [Google Scholar]
- Dyson T. Population and Development: The Demographic Transition. London, UK: Zed Books, 2010. [Google Scholar]
- Ehrlich PR. The Population Bomb. New York, USA: Ballantine Books, 1968. [Google Scholar]
- Eijkemans MJ, van Poppel F, Habbema DF, Smith KR, Leridon H, Te Velde ER.. Too old to have children? Lessons from natural fertility populations. Hum Reprod 2014;29:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fénichel P, Chevalier N.. Is testicular germ cell cancer estrogen dependent? The role of endocrine disrupting chemicals. Endocrinology 2019;160:2981–2989. [DOI] [PubMed] [Google Scholar]
- Ferreux L, Bourdon M, Chargui A, Schmitt A, Stouvenel L, Lorès P, Ray P, Lousqui J, Pocate-Cheriet K, Santulli P. et al. Genetic diagnosis, sperm phenotype and ICSI outcome in case of severe asthenozoospermia with multiple morphological abnormalities of the flagellum. Hum Reprod 2021;36:2848–2860. [DOI] [PubMed] [Google Scholar]
- Fucic A, Duca RC, Galea KS, Maric T, Garcia K, Bloom MS, Andersen HR, Vena JE.. Reproductive health risks associated with occupational and environmental exposure to pesticides. Int J Environ Res Public Health 2021;18:6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner-Thorpe D, O'Hagen C, Young I, Lewis SJ.. Dietary supplements of soya flour lower serum testosterone concentrations and improve markers of oxidative stress in men. Eur J Clin Nutr 2003;57:100–106. [DOI] [PubMed] [Google Scholar]
- Golin AP, Yuen W, Flannigan R.. The effects of Y chromosome microdeletions on in vitro fertilization outcomes, health abnormalities in offspring and recurrent pregnancy loss. Transl Androl Urol 2021;10:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajizadeh Maleki B, Tartibian B, Eghbali M, Asri-Rezaei S.. Comparison of seminal oxidants and antioxidants in subjects with different levels of physical fitness. Andrology 2013;1:607–614. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Griffin J, Meikle AW, Gibson M, Peterson CM, Carrell DT.. Association of aromatase (TTTAn) repeat polymorphism length and the relationship between obesity and decreased sperm concentration. Hum Reprod 2010;25:3146–3151. [DOI] [PubMed] [Google Scholar]
- Hawkins J, Miao X, Cui W, Sun Y.. Biophysical optimization of preimplantation embryo culture: what mechanics can offer ART. Mol Hum Reprod 2021;27:gaaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Zou L, Luo W, Yi Z, Yang P, Yu S, Liu N, Ji J, Guo Y, Liu P. et al. Heavy metal exposure, oxidative stress and semen quality: exploring associations and mediation effects in reproductive-aged men. Chemosphere 2020;244:125498. [DOI] [PubMed] [Google Scholar]
- Human Fertilization and Embryology Authority. Fertility Treatment 2019: Trends and Figures. 2021. https://www.hfea.gov.uk/about-us/publications/research-and-data/fertility-treatment-2019-trends-and-figures (28 September 2021, date last accessed).
- Jaswa EG, McCulloch CE, Simbulan R, Cedars MI, Rosen MP.. Diminished ovarian reserve is associated with reduced euploid rates via preimplantation genetic testing for aneuploidy independently from age: evidence for concomitant reduction in oocyte quality with quantity. Fertil Steril 2021;115:966–973. [DOI] [PubMed] [Google Scholar]
- Ješeta M, Navrátilová J, Franzová K, Fialková S, Kempisty B, Ventruba P, Žáková J, Crha I.. Overview of the mechanisms of action of selected bisphenols and perfluoroalkyl chemicals on the male reproductive axes. Front Genet 2021;12:692897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GW. Delayed marriage and very low fertility in Pacific Asia. Popul Dev Rev 2007;33:453–478. [Google Scholar]
- Kanté AM, Nathan R, Jackson EF, Levira F, Helleringer S, Masanja H, Phillips JF.. Trends in socioeconomic disparities in a rapid under-five mortality transition: a longitudinal study in the United Republic of Tanzania. Bull World Health Organ 2016;94:258–266A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney AL, White KM.. Examining the psychosocial determinants of women's decisions to delay childbearing. Hum Reprod 2016;31:1776–1787. [DOI] [PubMed] [Google Scholar]
- Lerch M. Regional variations in the rural-urban fertility gradient in the global South. PLoS One 2019;14:e0219624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine H, Jørgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH.. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DH, Raftery AE.. How do education and family planning accelerate fertility decline? Popul Dev Rev 2020;46:409–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lübbert H, Leo-Roßberg I, Hammerstein J.. Effects of ethinyl estradiol on semen quality and various hormonal parameters in a eugonadal male. Fertil Steril 1992;58:603–608. [DOI] [PubMed] [Google Scholar]
- Lv MQ, Ge P, Zhang J, Yang YQ, Zhou L, Zhou DX.. Temporal trends in semen concentration and count among 327 373 Chinese healthy men from 1981 to 2019: a systematic review. Hum Reprod 2021;36:1751–1775. [DOI] [PubMed] [Google Scholar]
- Majumdar D. Choosing childlessness: intentions of voluntary childlessness in the United States. MSR 2004;18:108–135. [Google Scholar]
- Martinez GM, Daniels K, Febo-Vazquez I.. Fertility of men and women aged 15-44 in the United States: national survey of family growth, 2011-2015. Natl Health Stat Report 2018;113:1–17. [PubMed] [Google Scholar]
- Malthus TR. An Essay on the Principle of Population. London, UK: J Johnson, 1798. [Google Scholar]
- Mazaheri Moghaddam M, Mazaheri Moghaddam M, Hamzeiy H, Baghbanzadeh A, Pashazadeh F, Sakhinia E.. Genetic basis of acephalic spermatozoa syndrome, and intracytoplasmic sperm injection outcomes in infertile men: a systematic scoping review. J Assist Reprod Genet 2021;38:573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur A, Westerman R, Mueller U.. Is rising obesity causing a secular (age-independent) decline in testosterone among American men? PLoS One 2013;8:e76178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mima M, Greenwald D, Ohlander S.. Environmental toxins and male fertility. Curr Urol Rep 2018;19:50. [DOI] [PubMed] [Google Scholar]
- Minasi MG, Colasante A, Riccio T, Ruberti A, Casciani V, Scarselli F, Spinella F, Fiorentino F, Varricchio MT, Greco E.. Correlation between aneuploidy, standard morphology evaluation and morphokinetic development in 1730 biopsied blastocysts: a consecutive case series study. Hum Reprod 2016;31:2245–2254. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Population aging in Japan: policy transformation, sustainable development goals, universal health coverage, and social determinates of health. Glob Health Med 2019;1:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Powanda P, Robaire B.. Oxidative stress and reproductive function in the aging male. Biology (Basel) 2020;9:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K, Karita K, Araki A, Nishioka E, Muto G, Iwai-Shimada M, Nishikitani M, Inoue M, Tsurugano S, Kitano N. et al. For making a declaration of countermeasures against the falling birth rate from the Japanese Society for Hygiene: summary of discussion in the working group on academic research strategy against an aging society with low birth rate. Environ Health Prev Med 2019;24:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perheentupa A, Mäkinen J, Laatikainen T, Vierula M, Skakkebaek NE, Andersson A-M, Toppari J.. A cohort effect on serum testosterone levels in Finnish men. Eur J Endocrinol 2013;168:227–233. [DOI] [PubMed] [Google Scholar]
- Reed KE, Camargo J, Hamilton-Reeves J, Kurzer M, Messina M.. Neither soy nor isoflavone intake affects male reproductive hormones: An expanded and updated meta-analysis of clinical studies. Reprod Toxicol 2021;100:60–67. [DOI] [PubMed] [Google Scholar]
- Rengaraj D, Kwon WS, Pang MG.. Effects of motor vehicle exhaust on male reproductive function and associated proteins. J Proteome Res 2015;14:22–37. [DOI] [PubMed] [Google Scholar]
- Samarasinghe SVAC, Krishnan K, Naidu R, Megharaj M, Miller K, Fraser B, Aitken RJ.. Parabens generate reactive oxygen species in human spermatozoa. Andrology 2018;6:532–541. [DOI] [PubMed] [Google Scholar]
- Sanders KD, Silvestri G, Gordon T, Griffin DK.. Analysis of IVF live birth outcomes with and without preimplantation genetic testing for aneuploidy (PGT-A): UK Human Fertilisation and Embryology Authority data collection 2016-2018. J Assist Reprod Genet 2021;38:3277–3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Q, Zhou Z, Mu J, Wang L.. Genetic factors as potential molecular markers of human oocyte and embryo quality. J Assist Reprod Genet 2021;38:993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skakkebaek NE. Sperm counts, testicular cancers, and the environment. BMJ 2017;359:j4517. [DOI] [PubMed] [Google Scholar]
- Skoracka K, Eder P, Łykowska-Szuber L, Dobrowolska A, Krela-Kaźmierczak I.. Diet and nutritional factors in male (in)fertility-underestimated factors. JCM 2020;9:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr MM, Sapra KJ, Gemmill A.. Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Hum Reprod 2017;32:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegen H, Swisters L, De Donder L.. Life stories of voluntarily childless older people: a retrospective view on their reasons and experiences. J Fam Issues 2020;42:1536–1558. [Google Scholar]
- Swan SH, Colino S.. Countdown: How Our Modern World is Threatening Sperm Counts, Altering Male and Female Reproductive Development, and Imperiling the Future of the Human Race. New York, USA: Scribner, 2021. [Google Scholar]
- Thévenon O. Family policies in OECD countries: a comparative analysis. Popul Dev Rev 2011;37:57–87. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, O'Donnell AB, Kupelian V, McKinlay JB.. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab 2007;92:196–202. [DOI] [PubMed] [Google Scholar]
- Vollset SE, Goren E, Yuan C-W, Cao J, Smith AE, Hsiao T, Bisignano C, Azhar GS, Castro E, Chalek J. et al. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet 2020;396:1285–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace EM, Aitken RJ, Wu FC.. Residual sperm function in oligozoospermia induced by testosterone enanthate administered as a potential steroid male contraceptive. Int J Androl 1992;15:416–424. [DOI] [PubMed] [Google Scholar]
- White MJ, Muhidin S, Andrzejewski C, Tagoe E, Knight R, Reed H.. Urbanization and fertility: an event-history analysis of coastal Ghana. Demography 2008;45:803–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield L. Milk production, fertility and the modern dairy cow. Livestock 2020;25:72–75. [Google Scholar]
- Wohlfahrt-Veje C, Main KM, Skakkebaek NE.. Testicular dysgenesis syndrome: foetal origin of adult reproductive problems. Clin Endocrinol (Oxf) 2009;71:459–465. [DOI] [PubMed] [Google Scholar]
- Yoldemir T. Fertility in midlife women. Climacteric 2016;19:240–246. [DOI] [PubMed] [Google Scholar]